Study on the Treatment of ITP Mice with IVIG Sourced from Distinct Sex-Special Plasma (DSP-IVIG)
Abstract
1. Introduction
2. Results
2.1. Mouse Blood Routine Test
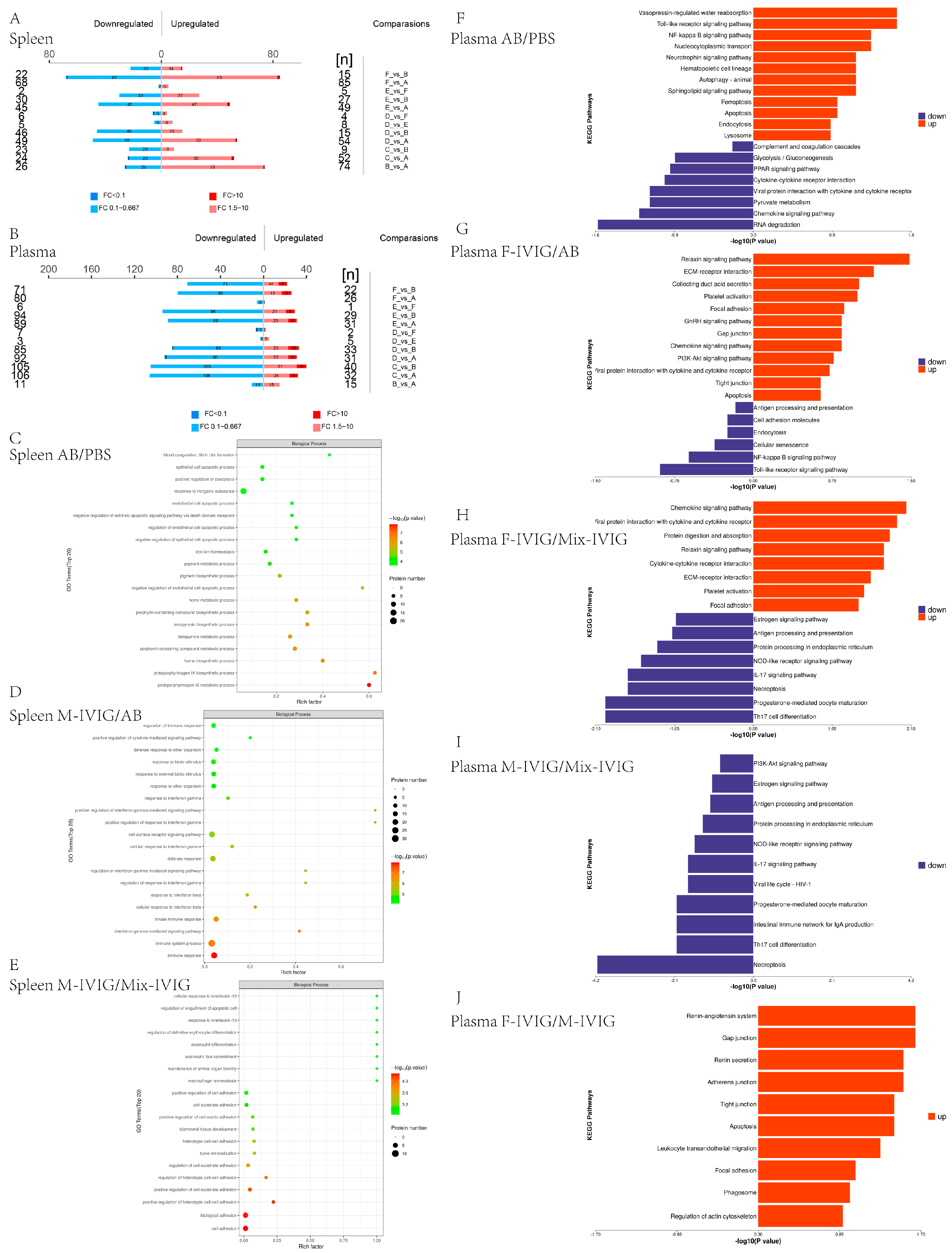
2.2. Proteomics Analysis of Mouse Spleen Tissue and Plasma
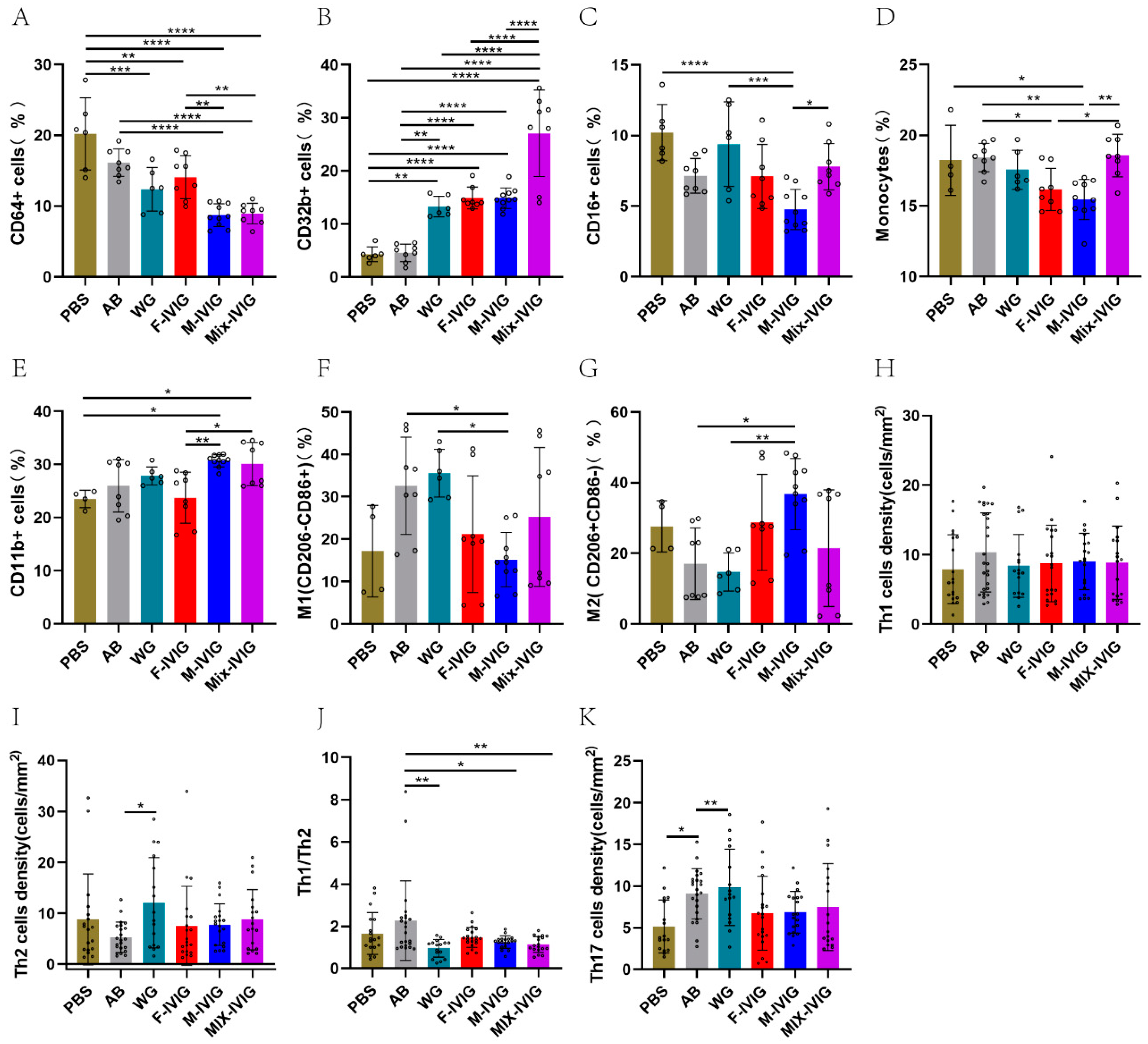
2.3. ITP Mouse Spleen Immune Cells
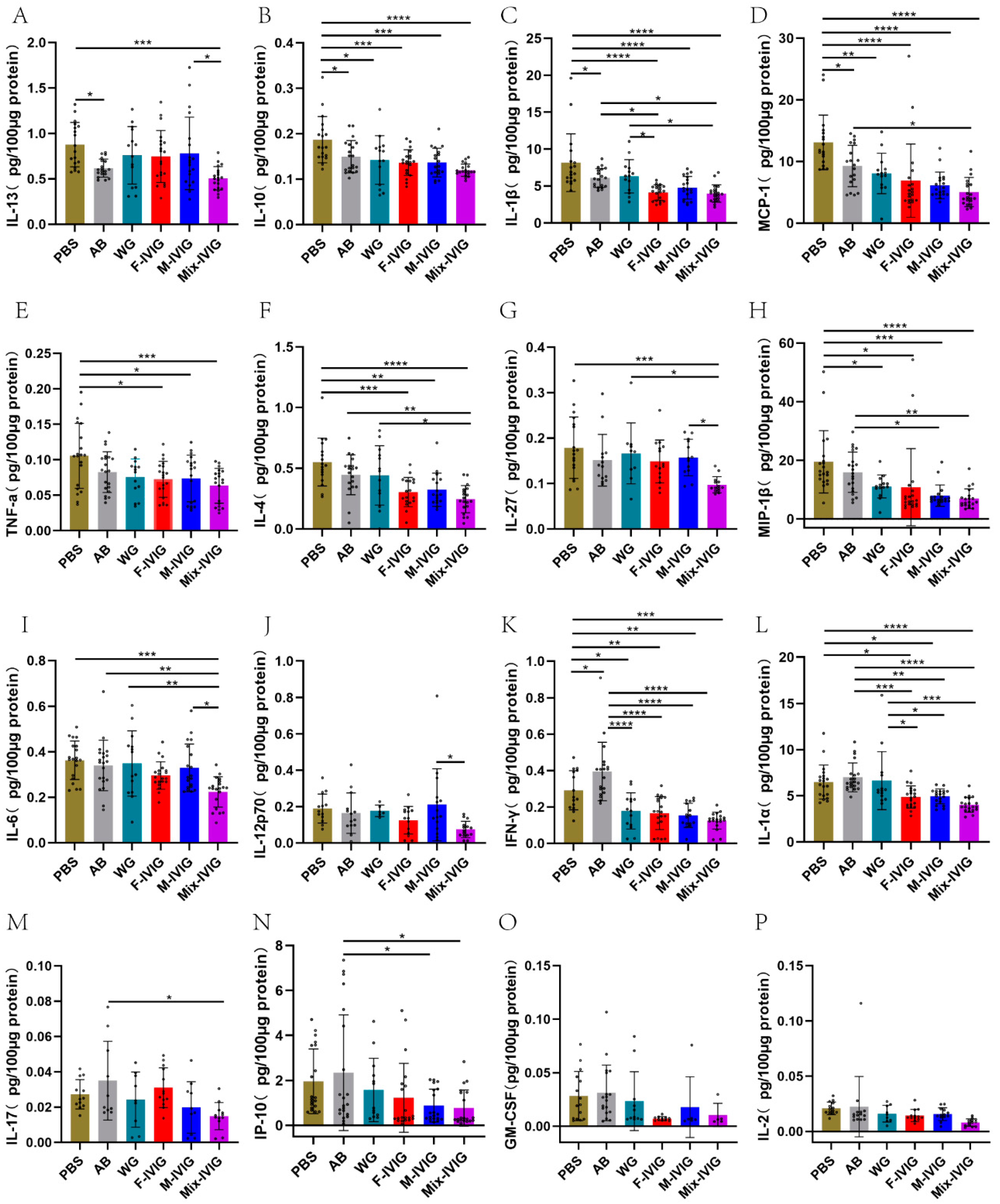
2.4. ITP Mouse Cytokines
2.5. Inhibition of THP-1 and M1/M2 Phagocytosis by DSP-IVIG
2.6. Cell Apoptosis
2.7. M1/M2 Macrophage Markers
2.8. FcγRs Expression
2.9. Complement Activation by the Fc Segment of DSP-IVIG
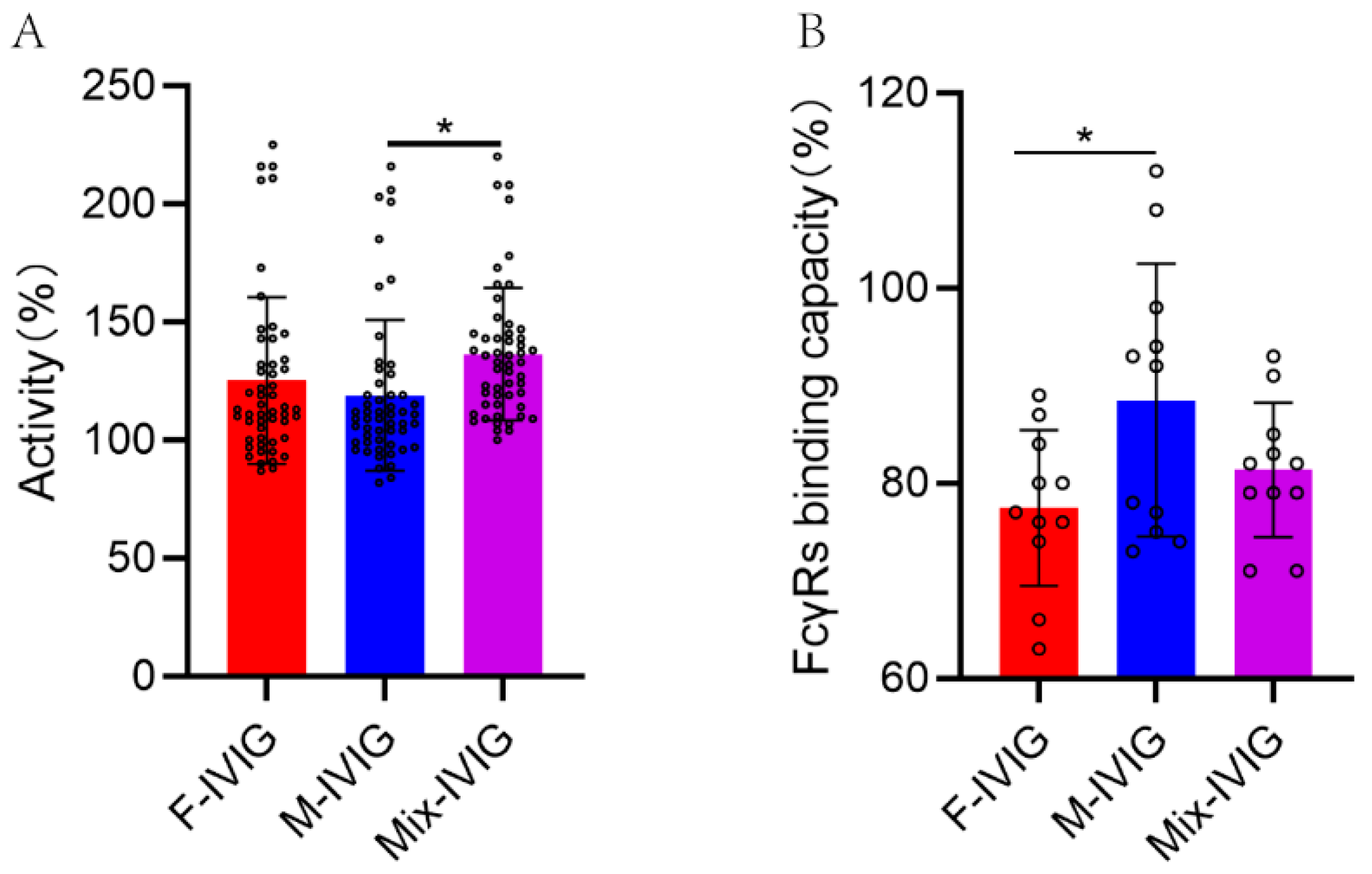
2.10. Binding Capacity of Fc Segment of DSP-IVIG for FcγRs
3. Discussion
4. Materials and Methods
4.1. Mouse Blood Routines
4.2. Proteomics of Mouse Spleen Tissue and Plasma
4.3. Detection of Splenic Immune Cells in Mice by Flow Cytometry
4.4. Detection of Mouse Cytokines
4.5. Inhibition of THP-1 and M1/M2 Phagocytosis by DSP-IVIG
4.6. Cell Apoptosis
4.7. M1/M2 Macrophage Markers
4.8. Cells’ FcγRs Expression
4.9. The Ability of Fc Segment of DSP-IVIG to Active Complement
4.10. The Capacity of Fc Segment of DSP-IVIG to Bind FcγRs
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rodeghiero, F.; Stasi, R.; Gernsheimer, T.; Michel, M.; Provan, D.; Arnold, D.M.; Bussel, J.B.; Cines, D.B.; Chong, B.H.; Cooper, N.; et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: Report from an international working group. Blood 2009, 113, 2386–2393. [Google Scholar] [CrossRef]
- Kapur, R.; Einarsdottir, H.K.; Vidarsson, G. IgG-effector functions: “The good, the bad and the ugly”. Immunol. Lett. 2014, 160, 139–144. [Google Scholar] [CrossRef]
- Ma, L.; Zhang, W.; Hou, M.; Li, D.; Liu, F.; Du, X.; Jiang, P.; Wang, Z.; Zhang, R.; Cao, H.; et al. Analysis of sialic acid levels in Chinese intravenous immunoglobulins by high-performance liquid chromatography with fluorescence detection. Biomed. Chromatogr. BMC 2019, 33, e4452. [Google Scholar] [CrossRef]
- Buhre, J.S.; Becker, M.; Ehlers, M. IgG subclass and Fc glycosylation shifts are linked to the transition from pre- to inflammatory autoimmune conditions. Front. Immunol. 2022, 13, 1006939. [Google Scholar] [CrossRef]
- Trzos, S.; Link-Lenczowski, P.; Sokołowski, G.; Pocheć, E. Changes of IgG N-Glycosylation in Thyroid Autoimmunity: The Modulatory Effect of Methimazole in Graves’ Disease and the Association With the Severity of Inflammation in Hashimoto’s Thyroiditis. Front. Immunol. 2022, 13, 841710. [Google Scholar] [CrossRef]
- Matzdorff, A.; Meyer, O.; Ostermann, H.; Kiefel, V.; Eberl, W.; Kühne, T.; Pabinger, I.; Rummel, M. Immune Thrombocytopenia—Current Diagnostics and Therapy: Recommendations of a Joint Working Group of DGHO, ÖGHO, SGH, GPOH, and DGTI. Oncol. Res. Treat. 2018, 41 (Suppl. 5), 1–30. [Google Scholar] [CrossRef]
- Feng, Q.; Xu, M.; Yu, Y.Y.; Hou, Y.; Mi, X.; Sun, Y.X.; Ma, S.; Zuo, X.Y.; Shao, L.L.; Hou, M.; et al. High-dose dexamethasone or all-trans-retinoic acid restores the balance of macrophages towards M2 in immune thrombocytopenia. J. Thromb. Haemost. JTH 2017, 15, 1845–1858. [Google Scholar] [CrossRef]
- Zufferey, A.; Kapur, R.; Semple, J.W. Pathogenesis and Therapeutic Mechanisms in Immune Thrombocytopenia (ITP). J. Clin. Med. 2017, 6, 16. [Google Scholar] [CrossRef]
- Gilardin, L.; Bayry, J.; Kaveri, S.V. Intravenous immunoglobulin as clinical immune-modulating therapy. CMAJ Can. Med. Assoc. J. 2015, 187, 257–264. [Google Scholar] [CrossRef]
- Sun, C.; Yang, J.; Wang, M.; Pan, L.; Yao, J.; Wang, S.; Guo, N.; Li, C.; Zhang, G. Icaritin Improves Antibody-Induced Thrombocytopenia in a Mouse Model by Regulating T-cell Polarization. Planta Medica 2018, 84, 168–175. [Google Scholar] [CrossRef]
- Chen, Y.; Hu, J.; Chen, Y. Platelet desialylation and TFH cells-the novel pathway of immune thrombocytopenia. Exp. Hematol. Oncol. 2021, 10, 21. [Google Scholar] [CrossRef]
- Audia, S.; Rossato, M.; Trad, M.; Samson, M.; Santegoets, K.; Gautheron, A.; Bekker, C.; Facy, O.; Cheynel, N.; Ortega-Deballon, P.; et al. B cell depleting therapy regulates splenic and circulating T follicular helper cells in immune thrombocytopenia. J. Autoimmun. 2017, 77, 89–95. [Google Scholar] [CrossRef]
- Xie, J.; Cui, D.; Liu, Y.; Jin, J.; Tong, H.; Wang, L.; Ruan, G.; Lu, Y.; Yuan, H. Changes in follicular helper T cells in idiopathic thrombocytopenic purpura patients. Int. J. Biol. Sci. 2015, 11, 220–229. [Google Scholar] [CrossRef]
- Yao, X.; Li, C.; Yang, J.; Wang, G.; Li, C.; Xia, Y. Differences in frequency and regulation of T follicular helper cells between newly diagnosed and chronic pediatric immune thrombocytopenia. Blood Cells Mol. Dis. 2016, 61, 26–36. [Google Scholar] [CrossRef]
- Rossi, F.; Tortora, C.; Palumbo, G.; Punzo, F.; Argenziano, M.; Casale, M.; Di Paola, A.; Locatelli, F.; Perrotta, S. CB2 Receptor Stimulation and Dexamethasone Restore the Anti-Inflammatory and Immune-Regulatory Properties of Mesenchymal Stromal Cells of Children with Immune Thrombocytopenia. Int. J. Mol. Sci. 2019, 20, 1049. [Google Scholar] [CrossRef]
- Ogawara, H.; Handa, H.; Morita, K.; Hayakawa, M.; Kojima, J.; Amagai, H.; Tsumita, Y.; Kaneko, Y.; Tsukamoto, N.; Nojima, Y.; et al. High Th1/Th2 ratio in patients with chronic idiopathic thrombocytopenic purpura. Eur. J. Haematol. 2003, 71, 283–288. [Google Scholar] [CrossRef]
- Mithoowani, S.; Arnold, D.M. First-Line Therapy for Immune Thrombocytopenia. Hamostaseologie 2019, 39, 259–265. [Google Scholar] [CrossRef]
- Provan, D.; Arnold, D.M.; Bussel, J.B.; Chong, B.H.; Cooper, N.; Gernsheimer, T.; Ghanima, W.; Godeau, B.; González-López, T.J.; Grainger, J.; et al. Updated international consensus report on the investigation and management of primary immune thrombocytopenia. Blood Adv. 2019, 3, 3780–3817. [Google Scholar] [CrossRef]
- Samson, M.; Fraser, W.; Lebowitz, D. Treatments for Primary Immune Thrombocytopenia: A Review. Cureus 2019, 11, e5849. [Google Scholar] [CrossRef]
- Leontyev, D.; Katsman, Y.; Branch, D.R. Mouse background and IVIG dosage are critical in establishing the role of inhibitory Fcγ receptor for the amelioration of experimental ITP. Blood 2012, 119, 5261–5264. [Google Scholar] [CrossRef]
- Schwab, I.; Mihai, S.; Seeling, M.; Kasperkiewicz, M.; Ludwig, R.J.; Nimmerjahn, F. Broad requirement for terminal sialic acid residues and FcγRIIB for the preventive and therapeutic activity of intravenous immunoglobulins in vivo. Eur. J. Immunol. 2014, 44, 1444–1453. [Google Scholar] [CrossRef] [PubMed]
- Silliman, C.C.; Dzieciatkowska, M.; Moore, E.E.; Kelher, M.R.; Banerjee, A.; Liang, X.; Land, K.J.; Hansen, K.C. Proteomic analyses of human plasma: Venus versus Mars. Transfusion 2012, 52, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Benson, A.B. Pulmonary complications of transfused blood components. Crit. Care Nurs. Clin. N. Am. 2012, 24, 403–418. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Eder, A.F.; Notari, E.P.t.; Dodd, R.Y. Do reactions after whole blood donation predict syncope on return donation? Transfusion 2012, 52, 2570–2576. [Google Scholar] [CrossRef]
- Chapman, C.E.; Stainsby, D.; Jones, H.; Love, E.; Massey, E.; Win, N.; Navarrete, C.; Lucas, G.; Soni, N.; Morgan, C.; et al. Ten years of hemovigilance reports of transfusion-related acute lung injury in the United Kingdom and the impact of preferential use of male donor plasma. Transfusion 2009, 49, 440–452. [Google Scholar] [CrossRef]
- Desai, M.K.; Brinton, R.D. Autoimmune Disease in Women: Endocrine Transition and Risk Across the Lifespan. Front. Endocrinol. 2019, 10, 265. [Google Scholar] [CrossRef]
- Ngo, S.T.; Steyn, F.J.; McCombe, P.A. Gender differences in autoimmune disease. Front. Neuroendocrinol. 2014, 35, 347–369. [Google Scholar] [CrossRef]
- El-Shanawany, T.; Jolles, S. Intravenous immunoglobulin and autoimmune disease. Ann. N. Y. Acad. Sci. 2007, 1110, 507–515. [Google Scholar] [CrossRef]
- Wasserman, R.L.; Garcia, D.; Greener, B.N.; Kestenberg, K.; Pinkert, A.; Mond, J.; Grossman, A. Manufacturing process optimization of ADMA Biologics’ intravenous immunoglobulin products, BIVIGAM(®) and ASCENIV™. Immunotherapy 2019, 11, 1423–1433. [Google Scholar] [CrossRef]
- Mikolajczyk, M.G.; Concepcion, N.F.; Wang, T.; Frazier, D.; Golding, B.; Frasch, C.E.; Scott, D.E. Characterization of antibodies to capsular polysaccharide antigens of Haemophilus influenzae type b and Streptococcus pneumoniae in human immune globulin intravenous preparations. Clin. Diagn. Lab. Immunol. 2004, 11, 1158–1164. [Google Scholar]
- Orange, J.S.; Du, W.; Falsey, A.R. Therapeutic Immunoglobulin Selected for High Antibody Titer to RSV also Contains High Antibody Titers to Other Respiratory Viruses. Front. Immunol. 2015, 6, 431. [Google Scholar] [CrossRef] [PubMed]
- Peter, J.G.; Chapel, H. Immunoglobulin replacement therapy for primary immunodeficiencies. Immunotherapy 2014, 6, 853–869. [Google Scholar] [CrossRef] [PubMed]
- Tzekou, A.; Fehlings, M.G. Treatment of spinal cord injury with intravenous immunoglobulin G: Preliminary evidence and future perspectives. J. Clin. Immunol. 2014, 34 (Suppl. 1), S132–S138. [Google Scholar] [CrossRef]
- Spycher, M.; Matozan, K.; Minnig, K.; Zehnder, R.; Miescher, S.; Hoefferer, L.; Rieben, R. In vitro comparison of the complement-scavenging capacity of different intravenous immunoglobulin preparations. Vox Sang. 2009, 97, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Frauger, E.; Grassi, J.; Pradel, V.; Bornet, C.; Rouby, F.; Delorme, J.; Ousset, S.; Braguer, D.; Azulay, J.P.; Penot-Ragon, C.; et al. Use of intravenous immunoglobulins in clinical practice: Data from three French university hospitals. Fundam. Clin. Pharmacol. 2011, 25, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Zhang, W.; Du, X.; Zhu, L.; Xu, J.; Cheng, L.; Zhou, X.; Shi, Z.; Liu, Y.; Xie, T.; et al. Demographic Characteristics and Lifestyle Habits of Chinese Plasma Donors: A Multicenter Study. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2021, 27, e931471. [Google Scholar] [CrossRef]
- Di Paola, A.; Palumbo, G.; Merli, P.; Argenziano, M.; Tortora, C.; Strocchio, L.; Roberti, D.; Santoro, C.; Perrotta, S.; Rossi, F. Effects of Eltrombopag on In Vitro Macrophage Polarization in Pediatric Immune Thrombocytopenia. Int. J. Mol. Sci. 2020, 22, 97. [Google Scholar] [CrossRef]
- Xu, P.; Jiang, Y.; Zuo, H.; Liu, X.; Xia, T.; Zhou, R.; Chen, B.; Ouyang, J. Vincristine-loaded platelets coated with anti-CD41 mAbs: A new macrophage targeting proposal for the treatment of immune thrombocytopenia. Biomater. Sci. 2019, 7, 4568–4577. [Google Scholar] [CrossRef]
- Shao, X.; Wu, B.; Cheng, L.; Li, F.; Zhan, Y.; Liu, C.; Ji, L.; Min, Z.; Ke, Y.; Sun, L.; et al. Distinct alterations of CD68(+)CD163(+) M2-like macrophages and myeloid-derived suppressor cells in newly diagnosed primary immune thrombocytopenia with or without CR after high-dose dexamethasone treatment. J. Transl. Med. 2018, 16, 48. [Google Scholar] [CrossRef]
- Sun, L.; Li, L.; Sun, T.; Zhang, L.; Li, C.; Xu, M.; Hou, Y.; Peng, J.; Hou, M.; Wang, L. Antihuman CD44 antibody BJ18 inhibits platelet phagocytosis by correcting aberrant FcɣR expression and M1 polarization in immune thrombocytopenia. Int. Immunopharmacol. 2021, 95, 107502. [Google Scholar] [CrossRef]
- Bournazos, S.; Gupta, A.; Ravetch, J.V. The role of IgG Fc receptors in antibody-dependent enhancement. Nat. Rev. Immunol. 2020, 20, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.J.; Brown, E.J. CR3 (Mac-1, alpha M beta 2, CD11b/CD18) and Fc gamma RIII cooperate in generation of a neutrophil respiratory burst: Requirement for Fc gamma RIII and tyrosine phosphorylation. J. Cell Biol. 1994, 125, 1407–1416. [Google Scholar] [CrossRef] [PubMed]
- Coxon, A.; Cullere, X.; Knight, S.; Sethi, S.; Wakelin, M.W.; Stavrakis, G.; Luscinskas, F.W.; Mayadas, T.N. Fc gamma RIII mediates neutrophil recruitment to immune complexes. a mechanism for neutrophil accumulation in immune-mediated inflammation. Immunity 2001, 14, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Green, J.M.; Schreiber, A.D.; Brown, E.J. Role for a glycan phosphoinositol anchor in Fc gamma receptor synergy. J. Cell Biol. 1997, 139, 1209–1217. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.J.; Song, Y.; Guo, X.Q.; Wei, D.; Cao, X.T.; Sun, Y.; Xu, Y.G.; Hu, X.M. The Construction of ITP Diagnostic Modeling Based on the Expressions of Hub Genes Associated with M1 Polarization of Macrophages. J. Inflamm. Res. 2022, 15, 5905–5915. [Google Scholar] [CrossRef]
- Ding, B.; Liu, L.; Dai, Y.; Li, M.; Xia, A.; Song, X.; Liu, J.; Wang, X.; Song, Y.; Zhou, H. Identification and verification of differentially expressed key genes in peripheral blood-derived T cells between chronic immune thrombocytopenia patients and healthy controls. Bioengineered 2022, 13, 13587–13595. [Google Scholar] [CrossRef]
- Segú-Vergés, C.; Caño, S.; Calderón-Gómez, E.; Bartra, H.; Sardon, T.; Kaveri, S.; Terencio, J. Systems biology and artificial intelligence analysis highlights the pleiotropic effect of IVIg therapy in autoimmune diseases with a predominant role on B cells and complement system. Front. Immunol. 2022, 13, 901872. [Google Scholar] [CrossRef]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage plasticity, polarization, and function in health and disease. J. Cell. Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef]
- Snyder, R.J.; Lantis, J.; Kirsner, R.S.; Shah, V.; Molyneaux, M.; Carter, M.J. Macrophages: A review of their role in wound healing and their therapeutic use. Wound Repair Regen. Off. Publ. Wound Heal. Soc. Eur. Tissue Repair Soc. 2016, 24, 613–629. [Google Scholar] [CrossRef]
- Audia, S.; Santegoets, K.; Laarhoven, A.G.; Vidarsson, G.; Facy, O.; Ortega-Deballon, P.; Samson, M.; Janikashvili, N.; Saas, P.; Bonnotte, B.; et al. Fcγ receptor expression on splenic macrophages in adult immune thrombocytopenia. Clin. Exp. Immunol. 2017, 188, 275–282. [Google Scholar] [CrossRef]
- Okamoto, N.; Homma, M.; Kawaguchi, Y.; Kabasawa, N.; Uto, Y.; Hattori, N.; Yamamoto, S.; Shiozawa, E.; Yamochi, T.; Tate, G.; et al. Increased expression of interleukin-17 is associated with macrophages in chronic immune thrombocytopenia. Int. J. Clin. Exp. Pathol. 2018, 11, 2419–2429. [Google Scholar] [PubMed]
- Bournazos, S.; Ravetch, J.V. Fcγ receptor pathways during active and passive immunization. Immunol. Rev. 2015, 268, 88–103. [Google Scholar] [CrossRef] [PubMed]
- Bournazos, S.; DiLillo, D.J.; Ravetch, J.V. The role of Fc-FcγR interactions in IgG-mediated microbial neutralization. J. Exp. Med. 2015, 212, 1361–1369. [Google Scholar] [CrossRef]
- Norris, P.A.A.; Segel, G.B.; Burack, W.R.; Sachs, U.J.; Lissenberg-Thunnissen, S.N.; Vidarsson, G.; Bayat, B.; Cserti-Gazdewich, C.M.; Callum, J.; Lin, Y.; et al. FcγRI and FcγRIII on splenic macrophages mediate phagocytosis of anti-glycoprotein IIb/IIIa autoantibody-opsonized platelets in immune thrombocytopenia. Haematologica 2021, 106, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Muppidi, S.; Utsugisawa, K.; Benatar, M.; Murai, H.; Barohn, R.J.; Illa, I.; Jacob, S.; Vissing, J.; Burns, T.M.; Kissel, J.T.; et al. Long-term safety and efficacy of eculizumab in generalized myasthenia gravis. Muscle Nerve 2019, 60, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Eid, L.; Coste-Verdier, V.; Longueville, E.; Ribeiro, E.; Nicolescu-Catargi, B.; Korobelnik, J.F. The effects of Rituximab on Graves’orbitopathy: A retrospective study of 14 patients. Eur. J. Ophthalmol. 2020, 30, 1008–1013. [Google Scholar] [CrossRef] [PubMed]
- Dalakas, M.C.; Alexopoulos, H.; Spaeth, P.J. Complement in neurological disorders and emerging complement-targeted therapeutics. Nat. Rev. Neurol. 2020, 16, 601–617. [Google Scholar] [CrossRef]
- Basta, M.; Dalakas, M.C. High-dose intravenous immunoglobulin exerts its beneficial effect in patients with dermatomyositis by blocking endomysial deposition of activated complement fragments. J. Clin. Investig. 1994, 94, 1729–1735. [Google Scholar] [CrossRef]
- Dalakas, M.C. Mechanistic effects of IVIg in neuroinflammatory diseases: Conclusions based on clinicopathologic correlations. J. Clin. Immunol. 2014, 34 (Suppl. 1), S120–S126. [Google Scholar] [CrossRef]
- Dalakas, M.C. Intravenous immunoglobulin in autoimmune neuromuscular diseases. JAMA 2004, 291, 2367–2375. [Google Scholar] [CrossRef]
- Lünemann, J.D.; Nimmerjahn, F.; Dalakas, M.C. Intravenous immunoglobulin in neurology—Mode of action and clinical efficacy. Nat. Rev. Neurol. 2015, 11, 80–89. [Google Scholar] [CrossRef]
- Brennan, F.H.; Kurniawan, N.D.; Vukovic, J.; Bartlett, P.F.; Käsermann, F.; Arumugam, T.V.; Basta, M.; Ruitenberg, M.J. IVIg attenuates complement and improves spinal cord injury outcomes in mice. Ann. Clin. Transl. Neurol. 2016, 3, 495–511. [Google Scholar] [CrossRef] [PubMed]
- Leontyev, D.; Neschadim, A.; Branch, D.R. Cytokine profiles in mouse models of experimental immune thrombocytopenia reveal a lack of inflammation and differences in response to intravenous immunoglobulin depending on the mouse strain. Transfusion 2014, 54, 2871–2879. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lu, J.; Wei, G.; Tong, H.; Zhou, J.; Ding, Y.; Zhang, S.; Xu, X.; Lai, R.; Luo, Q.; et al. Tacrolimus ameliorates thrombocytopenia in an ITP mouse model. Ann. Hematol. 2020, 99, 2315–2322. [Google Scholar] [CrossRef] [PubMed]
- Neschadim, A.; Branch, D.R. Mouse Models for Immune-Mediated Platelet Destruction or Immune Thrombocytopenia (ITP). Curr. Protoc. Immunol. 2016, 113, 15.30.1–15.30.13. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, P.; Liu, H.; Liu, X.; Xie, S.; Wang, X.; Wu, Y.; Chang, J.; Ma, L. Assessment of Th17/Treg cells and Th cytokines in an improved immune thrombocytopenia mouse model. Hematology 2017, 22, 493–500. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, G.; Liu, X.; Liu, H.; Yang, P.; Ma, L. Mesenchymal stem cells improve platelet counts in mice with immune thrombocytopenia. J. Cell. Biochem. 2019, 120, 11274–11283. [Google Scholar] [CrossRef]
- Whelan, S.A.; Hendricks, N.; Dwight, Z.L.; Fu, Q.; Moradian, A.; Van Eyk, J.E.; Mockus, S.M. Assessment of a 60-Biomarker Health Surveillance Panel (HSP) on Whole Blood from Remote Sampling Devices by Targeted LC/MRM-MS and Discovery DIA-MS Analysis. Anal. Chem. 2023, 95, 11007–11018. [Google Scholar] [CrossRef]
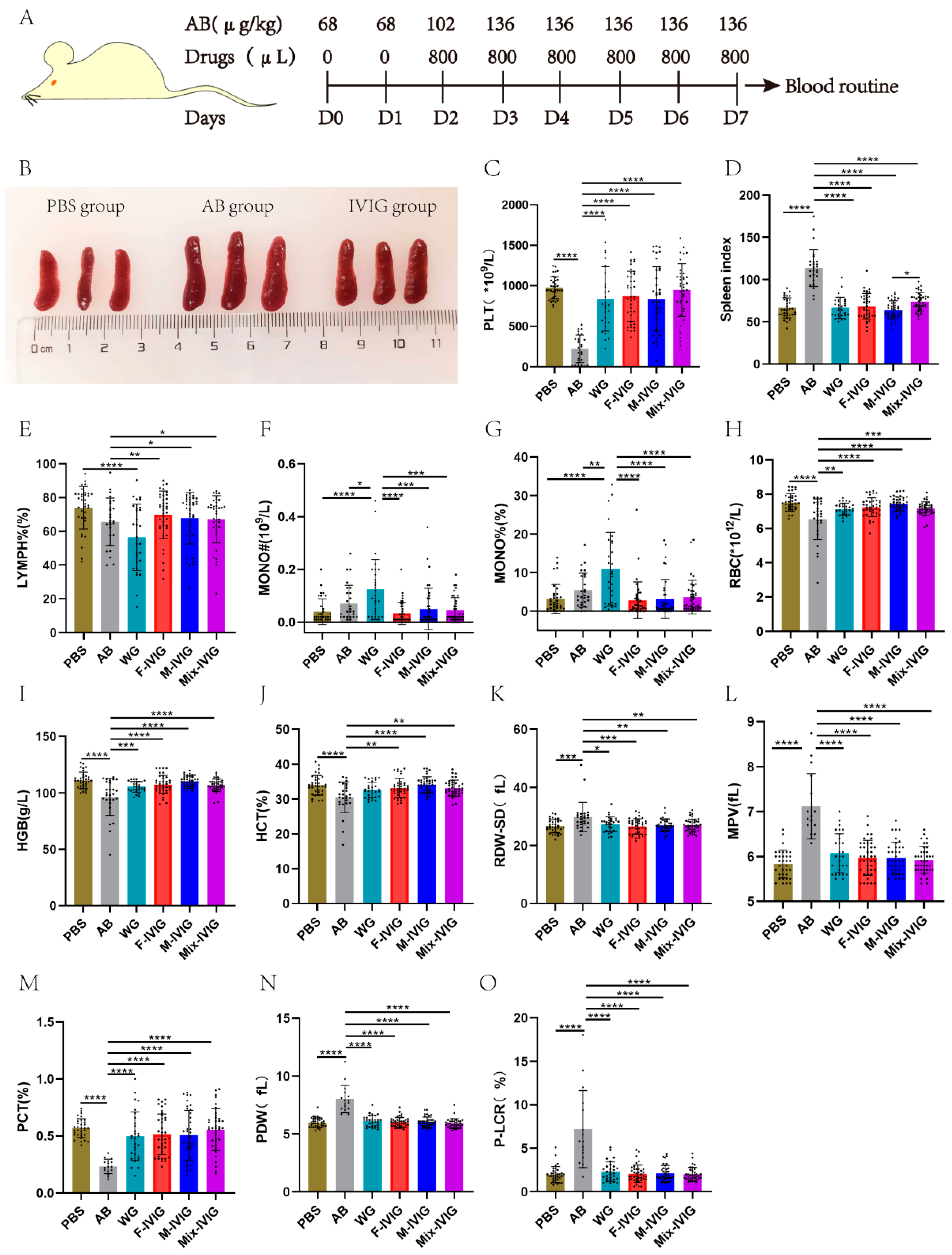


| Groups | ITP Model | Treatment |
|---|---|---|
| PBS | PBS | Albumin |
| AB | MWReg30 | Albumin |
| WG | MWReg30 | WG IVIG |
| F-IVIG | MWReg30 | F-IVIG |
| M-IVIG | MWReg30 | M-IVIG |
| Mix-IVIG | MWReg30 | Mix-IVIG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Yuan, X.; Wang, Z.; Xu, J.; Ye, S.; Jiang, P.; Du, X.; Liu, F.; Lin, F.; Zhang, R.; et al. Study on the Treatment of ITP Mice with IVIG Sourced from Distinct Sex-Special Plasma (DSP-IVIG). Int. J. Mol. Sci. 2023, 24, 15993. https://doi.org/10.3390/ijms242115993
Zhang W, Yuan X, Wang Z, Xu J, Ye S, Jiang P, Du X, Liu F, Lin F, Zhang R, et al. Study on the Treatment of ITP Mice with IVIG Sourced from Distinct Sex-Special Plasma (DSP-IVIG). International Journal of Molecular Sciences. 2023; 24(21):15993. https://doi.org/10.3390/ijms242115993
Chicago/Turabian StyleZhang, Wei, Xin Yuan, Zongkui Wang, Jixuan Xu, Shengliang Ye, Peng Jiang, Xi Du, Fengjuan Liu, Fangzhao Lin, Rong Zhang, and et al. 2023. "Study on the Treatment of ITP Mice with IVIG Sourced from Distinct Sex-Special Plasma (DSP-IVIG)" International Journal of Molecular Sciences 24, no. 21: 15993. https://doi.org/10.3390/ijms242115993
APA StyleZhang, W., Yuan, X., Wang, Z., Xu, J., Ye, S., Jiang, P., Du, X., Liu, F., Lin, F., Zhang, R., Ma, L., & Li, C. (2023). Study on the Treatment of ITP Mice with IVIG Sourced from Distinct Sex-Special Plasma (DSP-IVIG). International Journal of Molecular Sciences, 24(21), 15993. https://doi.org/10.3390/ijms242115993







