Application of Luteolin in Neoplasms and Nonneoplastic Diseases
Abstract
:1. Introduction
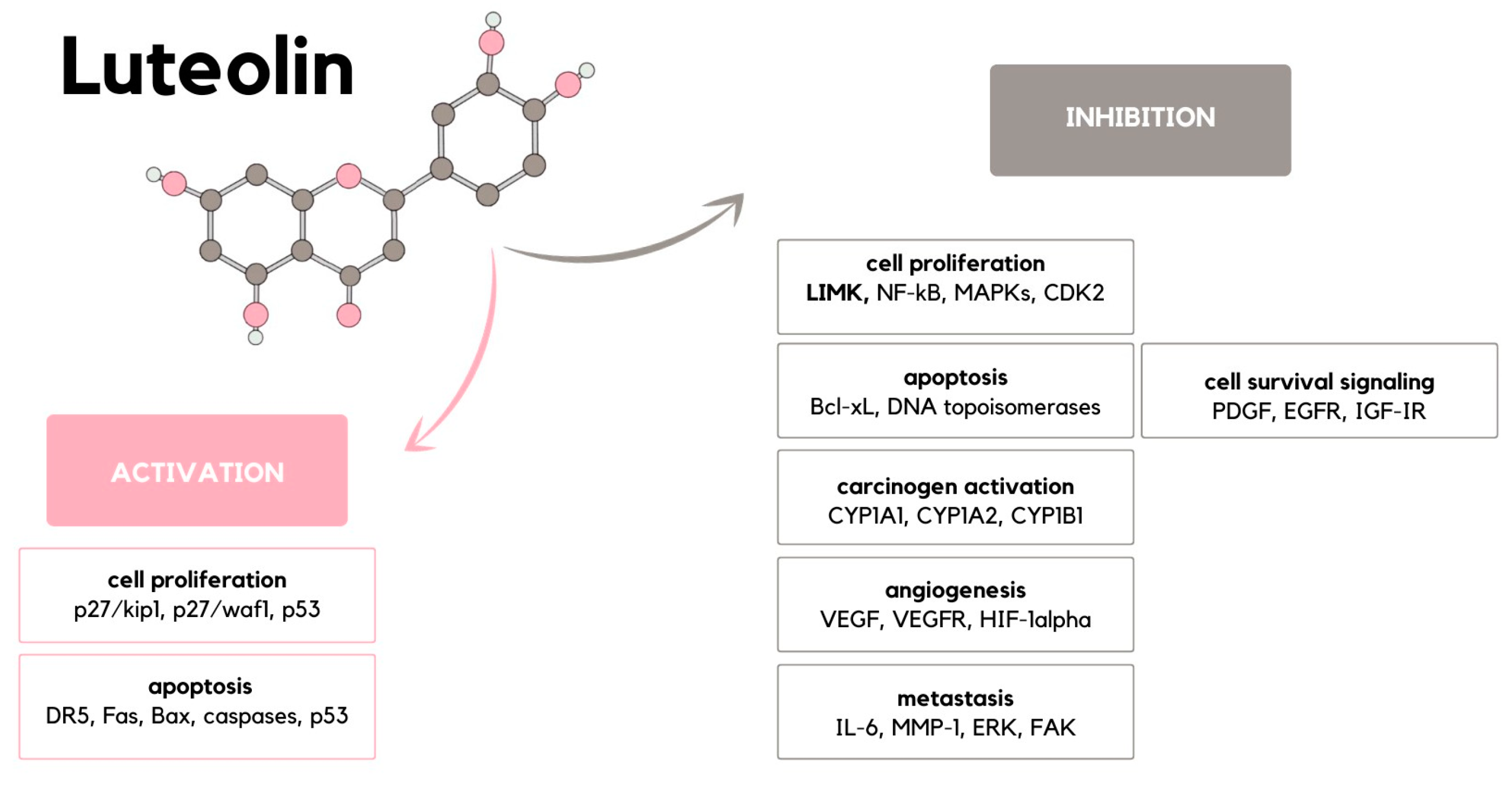
2. Carcinogenesis
3. Cell Proliferation
4. Apoptosis Induction
5. Carcinogen Activation
6. Angiogenesis
7. Metastasis
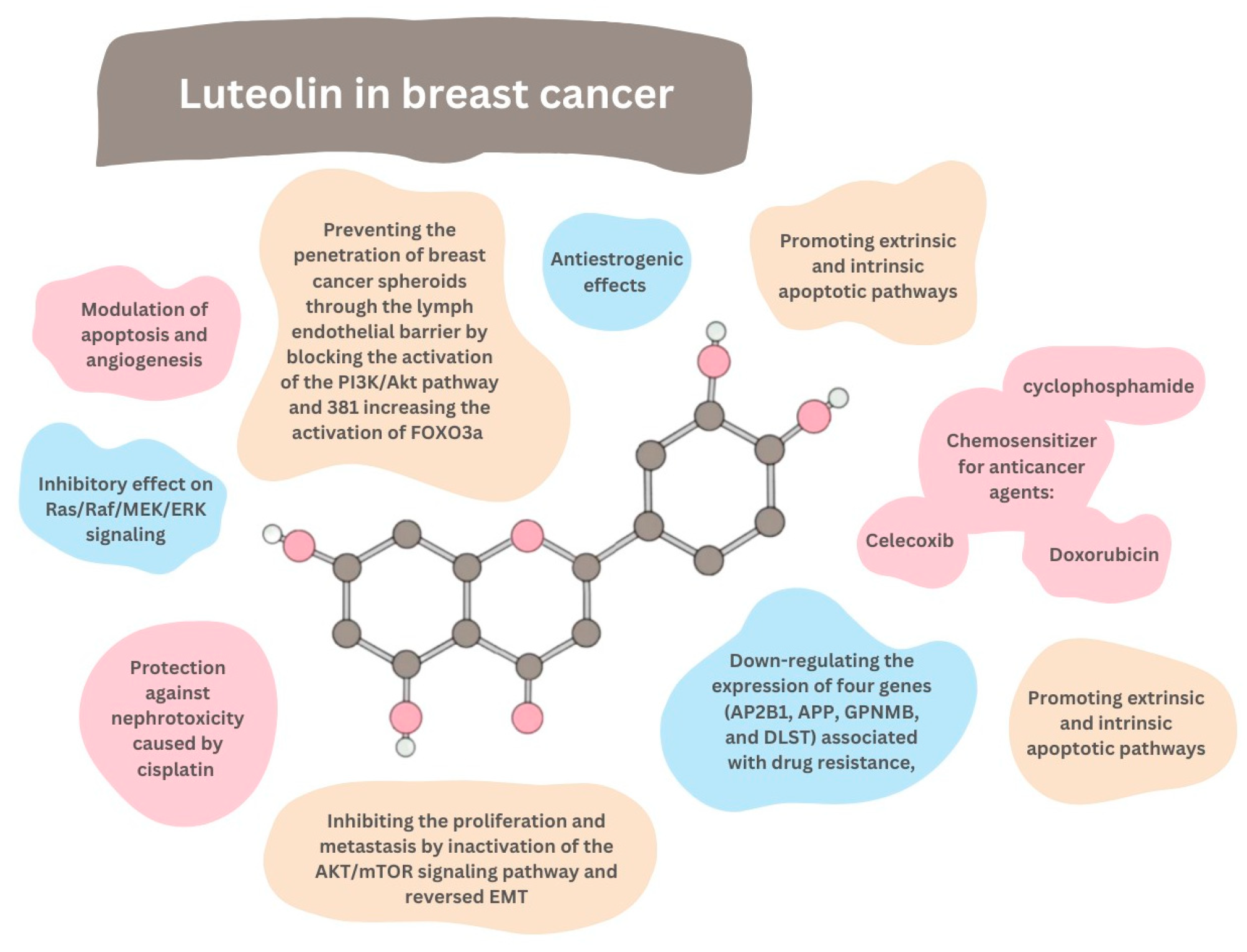
8. Breast Cancer
- Block the proliferation of IGF-1-stimulated luminal A subtype ERα-positive MCF-7 cells;
- Suppress the growth of triple-negative/basal-like ERα-negative MDA-MB-231 cells;
- Decrease the viability of breast cancer cells MCF7/6 and MDA-MB231-1833;
- Reduce the tumor burden in nude mice inoculated with MDA-MB-231 cells;
- Inhibit the migration and invasion of highly metastatic triple-negative breast cancer (TNBC) cell lines MDA-MB-231 and BT-549;
- Suppress the formation of lung metastases in breast cancer xenograft tumors originating from MDA-MD-231 cells;
- Inhibit the migration of ERα-positive MCF-7 cells;
- Inhibit the migration and viability of human MDA-MB-435 and MDA-MB-231 cells;
- Reverse the epithelial–mesenchymal transition (EMT) of MDA-MB-231 and BT5-49;
8.1. MicroRNAs
8.2. IGF-1 Pathways
8.3. Kinases
9. Chemosensitization
9.1. Doxorubicin
9.2. Celecoxib
9.3. Cyclophosphamide
10. Colon Cancer
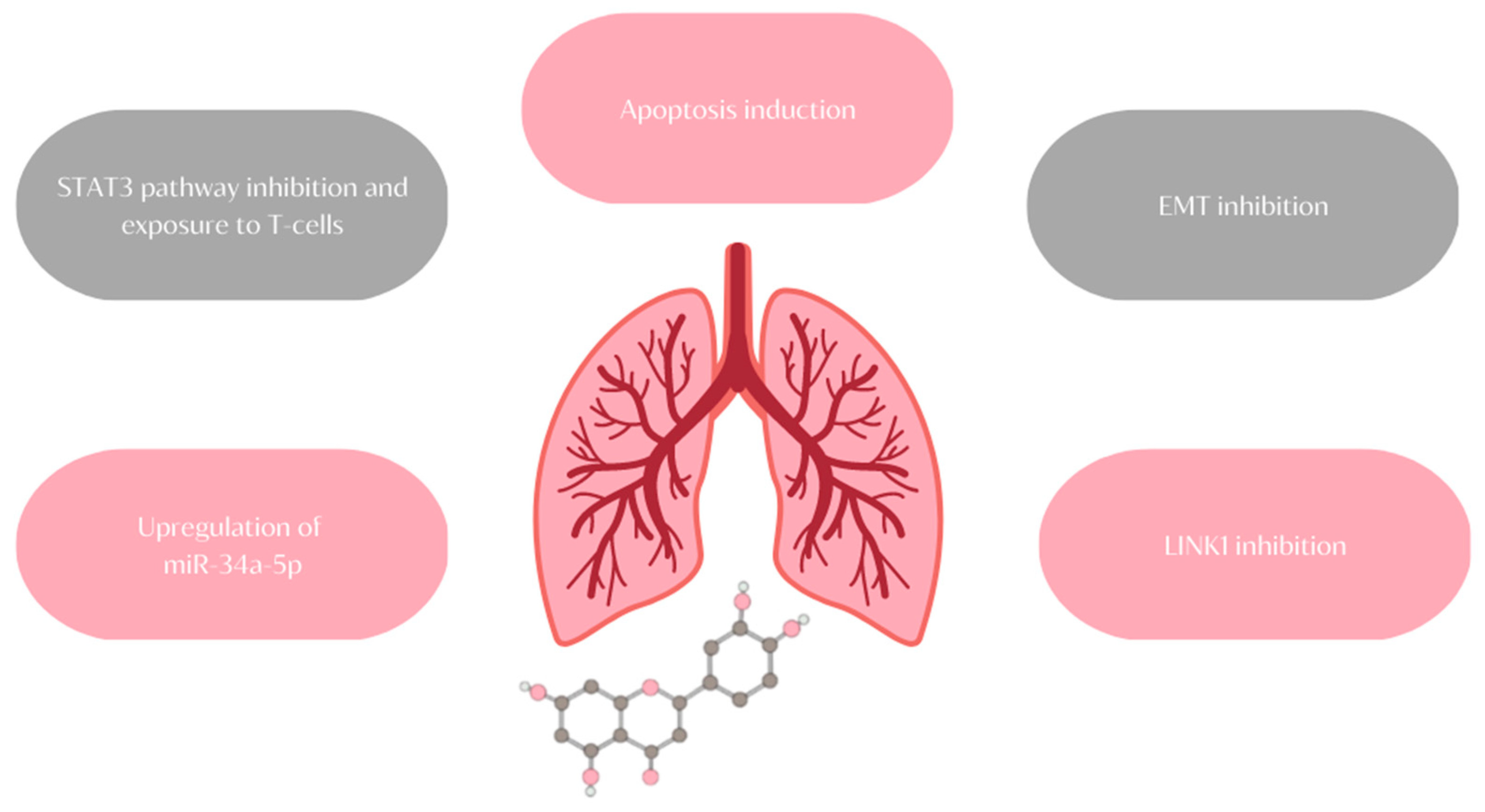
11. Lung Cancer
12. Inflammatory Skin Diseases
13. Psoriasis
14. Atopic and Contact Dermatitis
15. Diabetes Mellitus
16. COVID-19
17. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Wen, K.; Fang, X.; Yang, J.; Yao, Y.; Nandakumar, K.S.; Salem, M.L.; Cheng, K. Recent Research on Flavonoids and their Biomedical Applications. Curr. Med. Chem. 2021, 28, 1042–1066. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.C.; Chen, C.-C.; Lin, K.-H.; Chao, P.-Y.; Lin, H.-H.; Huang, M.-Y. Bioactive Compounds, Antioxidants, and Health Benefits of Sweet Potato Leaves. Molecules 2021, 26, 1820. [Google Scholar] [CrossRef] [PubMed]
- Criste, A.; Urcan, A.C.; Bunea, A.; Furtuna, F.R.P.; Olah, N.K.; Madden, R.H.; Corcionivoschi, N. Phytochemical Composition and Biological Activity of Berries and Leaves from Four Romanian Sea Buckthorn (Hippophae rhamnoides L.) Varieties. Molecules 2020, 25, 1170. [Google Scholar] [CrossRef] [PubMed]
- Manzoor, M.F.; Ahmad, N.; Ahmed, Z.; Siddique, R.; Zeng, X.; Rahaman, A.; Aadil, R.M.; Wahab, A. Novel extraction techniques and pharmaceutical activities of luteolin and its derivatives. J. Food Biochem. 2019, 43, e12974. [Google Scholar] [CrossRef]
- Cao, Y.; Xie, L.; Liu, K.; Liang, Y.; Dai, X.; Wang, X.; Lu, J.; Zhang, X.; Li, X. The antihypertensive potential of flavonoids from Chinese Herbal Medicine: A review. Pharmacol. Res. 2021, 174, 105919. [Google Scholar] [CrossRef]
- Slika, H.; Mansour, H.; Wehbe, N.; Nasser, S.A.; Iratni, R.; Nasrallah, G.; Shaito, A.; Ghaddar, T.; Kobeissy, F.; Eid, A.H. Therapeutic potential of flavonoids in cancer: ROS-mediated mechanisms. Biomed. Pharmacother. 2022, 146, 112442. [Google Scholar] [CrossRef]
- Prasher, P.; Sharma, M.; Singh, S.K.; Gulati, M.; Chellappan, D.K.; Zacconi, F.; De Rubis, G.; Gupta, G.; Sharifi-Rad, J.; Cho, W.C.; et al. Luteolin: A flavonoid with a multifaceted anticancer potential. Cancer Cell Int. 2022, 22, 386. [Google Scholar] [CrossRef]
- Wang, Z.; Zeng, M.; Wang, Z.; Qin, F.; Chen, J.; He, Z. Dietary Luteolin: A Narrative Review Focusing on Its Pharmacokinetic Properties and Effects on Glycolipid Metabolism. J. Agric. Food Chem. 2021, 69, 1441–1454. [Google Scholar] [CrossRef]
- Tesio, A.Y.; Robledo, S.N. Analytical determinations of luteolin. BioFactors 2021, 47, 141–164. [Google Scholar] [CrossRef]
- Nabavi, S.F.; Braidy, N.; Gortzi, O.; Sobarzo-Sanchez, E.; Daglia, M.; Skalicka-Woźniak, K.; Nabavi, S.M. Luteolin as an anti-inflammatory and neuroprotective agent: A brief review. Brain Res. Bull. 2015, 119, 1–11. [Google Scholar] [CrossRef]
- Ali, F.; Siddique, Y.H. Bioavailability and Pharmaco-therapeutic Potential of Luteolin in Overcoming Alzheimer’s Disease. CNS Neurol. Disord. Drug Targets 2019, 18, 352–365. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chen, Q.; Zhu, L.; Li, Q.; Zeng, X.; Lu, L.; Hu, M.; Wang, X.; Liu, Z. Metabolic Disposition of Luteolin Is Mediated by the Interplay of UDP-Glucuronosyltransferases and Catechol-O-Methyltransferases in Rats. Drug Metab. Dispos. 2017, 45, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Daily, J.W.; Kang, S.; Park, S. Protection against Alzheimer’s disease by luteolin: Role of brain glucose regulation, anti-inflammatory activity, and the gut microbiota-liver-brain axis. BioFactors 2021, 47, 218–231. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Gao, C.; Tian, X.; Chao, B.; Wang, F.; Zhang, Y.; Zou, J.; Liu, D. Pharmacokinetics, tissue distribution and excretion of luteolin and its major metabolites in rats: Metabolites predominate in blood, tissues and are mainly excreted via bile. J. Funct. Foods 2017, 35, 332–340. [Google Scholar] [CrossRef]
- Hussain, Y.; Cui, J.H.; Khan, H.; Aschner, M.; Batiha, G.E.-S.; Jeandet, P. Luteolin and cancer metastasis suppression: Focus on the role of epithelial to mesenchymal transition. Med. Oncol. 2021, 38, 66. [Google Scholar] [CrossRef]
- El Gueder, D.; Maatouk, M.; Kalboussi, Z.; Daouefi, Z.; Chaaban, H.; Ioannou, I.; Ghedira, K.; Ghedira, L.C.; Luis, J. Heat processing effect of luteolin on anti-metastasis activity of human glioblastoma cells U87. Environ. Sci. Pollut. Res. Int. 2018, 25, 36545–36554. [Google Scholar] [CrossRef]
- Zou, Y.; Luo, X.; Feng, Y.; Fang, S.; Tian, J.; Yu, B.; Li, J. Luteolin prevents THP-1 macrophage pyroptosis by suppressing ROS production via Nrf2 activation. Chem. Biol. Interact. 2021, 345, 109573. [Google Scholar] [CrossRef]
- Mittler, R. ROS Are Good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef]
- Liu, X.; Wu, D.; Liu, J.; Li, G.; Zhang, Z.; Chen, C.; Zhang, L.; Li, J. Characterization of xanthine oxidase inhibitory activities of phenols from pickled radish with molecular simulation. Food Chem. X 2022, 14, 100343. [Google Scholar] [CrossRef]
- Ren, P.; Cao, J.-L.; Lin, P.-L.; Cao, B.-Y.; Chen, J.-L.; Gao, K.; Zhang, J. Molecular mechanism of luteolin regulating lipoxygenase pathway against oxygen-glucose deprivation/reperfusion injury in H9c2 cardiomyocytes based on molecular docking. Zhongguo Zhong Yao Za Zhi 2021, 46, 5665–5673. [Google Scholar] [CrossRef]
- Rungsung, S.; Singh, T.U.; Rabha, D.J.; Kumar, T.; Lingaraju, M.C.; Parida, S.; Paul, A.; Sahoo, M.; Kumar, D. Luteolin attenuates acute lung injury in experimental mouse model of sepsis. Cytokine 2018, 110, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, P.; Jagadeesan, R.; Sekaran, S.; Dhanasekaran, A.; Vimalraj, S. Flavonoids: Classification, Function, and Molecular Mechanisms Involved in Bone Remodelling. Front. Endocrinol. 2021, 12, 779638. [Google Scholar] [CrossRef] [PubMed]
- Eren-Guzelgun, B.; Ince, E.; Gurer-Orhan, H. In vitro antioxidant/prooxidant effects of combined use of flavonoids. Nat. Prod. Res. 2018, 32, 1446–1450. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Torres, I.; Guarner-Lans, V.; Rubio-Ruiz, M.E. Reductive Stress in Inflammation-Associated Diseases and the Pro-Oxidant Effect of Antioxidant Agents. Int. J. Mol. Sci. 2017, 18, 2098. [Google Scholar] [CrossRef]
- Cai, W.; Xiong, Y.; Han, M.; Li, Z.; Peng, L.; Zhang, H.; Zou, Q.; Wu, L.; Ye, Q.; Liao, L. Characterization and Quantification of Luteolin-Metal Complexes in Aqueous Extract of Lonicerae Japonicae Flos and Huangshan Wild Chrysanthemum. Int. J. Anal. Chem. 2021, 2021, 6677437. [Google Scholar] [CrossRef]
- Liu, W.-N.; Shi, J.; Fu, Y.; Zhao, X.-H. The Stability and Activity Changes of Apigenin and Luteolin in Human Cervical Cancer Hela Cells in Response to Heat Treatment and Fe2+/Cu2+ Addition. Foods 2019, 8, 346. [Google Scholar] [CrossRef]
- Ou, H.-C.; Pandey, S.; Hung, M.-Y.; Huang, S.-H.; Hsu, P.-T.; Day, C.-H.; Pai, P.; Viswanadha, V.P.; Kuo, W.-W.; Huang, C.-Y. Luteolin: A Natural Flavonoid Enhances the Survival of HUVECs against Oxidative Stress by Modulating AMPK/PKC Pathway. Am. J. Chin. Med. 2019, 47, 541–557. [Google Scholar] [CrossRef]
- You, Y.; Wang, R.; Shao, N.; Zhi, F.; Yang, Y. Luteolin suppresses tumor proliferation through inducing apoptosis and autophagy via MAPK activation in glioma. OncoTargets Ther. 2019, 12, 2383–2396. [Google Scholar] [CrossRef]
- Ye, Y.; Huang, Z.; Chen, M.; Mo, Y.; Mo, Z. Luteolin Potentially Treating Prostate Cancer and COVID-19 Analyzed by the Bioinformatics Approach: Clinical Findings and Drug Targets. Front. Endocrinol. 2022, 12, 802447. [Google Scholar] [CrossRef]
- Lee, H.-S.; Park, B.-S.; Kang, H.-M.; Kim, J.-H.; Shin, S.-H.; Kim, I.-R. Role of Luteolin-Induced Apoptosis and Autophagy in Human Glioblastoma Cell Lines. Medicina 2021, 57, 879. [Google Scholar] [CrossRef]
- Zhou, Y.-S.; Cui, Y.; Zheng, J.-X.; Quan, Y.-Q.; Wu, S.-X.; Xu, H.; Han, Y. Luteolin relieves lung cancer-induced bone pain by inhibiting NLRP3 inflammasomes and glial activation in the spinal dorsal horn in mice. Phytomedicine 2022, 96, 153910. [Google Scholar] [CrossRef] [PubMed]
- Gendrisch, F.; Esser, P.R.; Schempp, C.M.; Wölfle, U. Luteolin as a modulator of skin aging and inflammation. BioFactors 2021, 47, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Ntalouka, F.; Tsirivakou, A. Luteolin: A promising natural agent in management of pain in chronic conditions. Front. Pain Res. 2023, 4, 1114428. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Chen, T.; Wang, L.; Liu, R.; Niu, Y.; Sun, L.; Yao, B.; Wang, Y.; Yang, W.; Liu, Q.; et al. CXCR4 mediates matrix stiffness-induced downregulation of UBTD1 driving hepatocellular carcinoma progression via YAP signaling pathway. Theranostics 2020, 10, 5790–5801. [Google Scholar] [CrossRef]
- Galati, G.; O’Brien, P.J. Potential toxicity of flavonoids and other dietary phenolics: Significance for their chemopreventive and anticancer properties. Free Radic. Biol. Med. 2004, 37, 287–303. [Google Scholar] [CrossRef] [PubMed]
- JKhan, J.; Deb, P.K.; Priya, S.; Medina, K.D.; Devi, R.; Walode, S.G.; Rudrapal, M. Dietary Flavonoids: Cardioprotective Potential with Antioxidant Effects and Their Pharmacokinetic, Toxicological and Therapeutic Concerns. Molecules 2021, 26, 4021. [Google Scholar] [CrossRef]
- Skibola, C.F.; Smith, M.T. Potential health impacts of excessive flavonoid intake. Free Radic. Biol. Med. 2000, 29, 375–383. [Google Scholar] [CrossRef]
- Lin, Y.; Shi, R.; Wang, X.; Shen, H.-M. Luteolin, a flavonoid with potentials for cancer prevention and therapy. Curr. Cancer Drug Targets 2008, 8, 634. [Google Scholar] [CrossRef]
- Yi, C.; Li, G.; Ivanov, D.N.; Wang, Z.; Velasco, M.X.; Hernández, G.; Kaundal, S.; Villarreal, J.; Gupta, Y.K.; Qiao, M.; et al. Luteolin inhibits Musashi1 binding to RNA and disrupts cancer phenotypes in glioblastoma cells. RNA Biol. 2018, 15, 1420–1432. [Google Scholar] [CrossRef]
- Al-Megrin, W.A.; Alkhuriji, A.F.; Yousef, A.O.S.; Metwally, D.M.; Habotta, O.A.; Kassab, R.B.; Abdel Moneim, A.E.; El-Khadragy, M.F. Antagonistic Efficacy of Luteolin against Lead Acetate Exposure-Associated with Hepatotoxicity is Mediated via Antioxidant, Anti-Inflammatory, and Anti-Apoptotic Activities. Antioxidants 2020, 9, 10. [Google Scholar] [CrossRef]
- Taliou, A.; Zintzaras, E.; Lykouras, L.; Francis, K. An open-label pilot study of a formulation containing the anti-inflammatory flavonoid luteolin and its effects on behavior in children with autism spectrum disorders. Clin. Ther. 2013, 35, 592–602. [Google Scholar] [CrossRef] [PubMed]
- Zand, R.S.R.; Jenkins, D.J.; Diamandis, E.P. Steroid hormone activity of flavonoids and related compounds. Breast Cancer Res. Treat. 2000, 62, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Han, D.-H.; Denison, M.S.; Tachibana, H.; Yamada, K. Relationship between estrogen receptor-binding and estrogenic activities of environmental estrogens and suppression by flavonoids. Biosci. Biotechnol. Biochem. 2002, 66, 1479–1487. [Google Scholar] [CrossRef] [PubMed]
- Nordeen, S.K.; Bona, B.J.; Jones, D.N.; Lambert, J.R.; Jackson, T.A. Endocrine Disrupting Activities of the Flavonoid Nutraceuticals Luteolin and Quercetin. Horm. Cancer 2013, 4, 293. [Google Scholar] [CrossRef] [PubMed]
- Karrasch, T.; Kim, J.-S.; Jang, B.I.; Jobin, C. The Flavonoid Luteolin Worsens Chemical-Induced Colitis in NF-κBEGFP Transgenic Mice through Blockade of NF-κB-Dependent Protective Molecules. PLoS ONE 2007, 2, e596. [Google Scholar] [CrossRef]
- Fishbein, A.; Hammock, B.D.; Serhan, C.N.; Panigrahy, D. Carcinogenesis: Failure of resolution of inflammation? Pharmacol. Ther. 2020, 218, 107670. [Google Scholar] [CrossRef]
- Goodenow, D.; Emmanuel, F.; Berman, C.; Sahyouni, M.; Richardson, C. Bioflavonoids cause DNA double-strand breaks and chromosomal translocations through topoisomerase II-dependent and -independent mechanisms. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2020, 849, 503144. [Google Scholar] [CrossRef]
- Hsuan, C.-F.; Lu, Y.-C.; Tsai, I.-T.; Houng, J.-Y.; Wang, S.-W.; Chang, T.-H.; Chen, Y.-L.; Chang, C.-C. Glossogyne tenuifolia Attenuates Proliferation and Migration of Vascular Smooth Muscle Cells. Molecules 2020, 25, 5832. [Google Scholar] [CrossRef]
- Santes-Palacios, R.; Marroquín-Pérez, A.L.; Hernández-Ojeda, S.L.; Camacho-Carranza, R.; Govezensky, T.; Espinosa-Aguirre, J.J. Human CYP1A1 inhibition by flavonoids. Toxicol. Vitr. 2020, 62, 104681. [Google Scholar] [CrossRef]
- Zhang, Z.-T.; Zhang, D.-Y.; Xie, K.; Wang, C.-J.; Xu, F. Luteolin activates Tregs to promote IL-10 expression and alleviating caspase-11-dependent pyroptosis in sepsis-induced lung injury. Int. Immunopharmacol. 2021, 99, 107914. [Google Scholar] [CrossRef]
- Wang, S.; Chen, Y.; Chow, J.; Chien, M.; Yang, S.; Wen, Y.; Lee, W.; Tseng, T. Stimulation of Fas/FasL-mediated apoptosis by luteolin through enhancement of histone H3 acetylation and c-Jun activation in HL-60 leukemia cells. Mol. Carcinog. 2018, 57, 866–877. [Google Scholar] [CrossRef] [PubMed]
- Raina, R.; Pramodh, S.; Rais, N.; Haque, S.; Shafarin, J.; Bajbouj, K.; Hamad, M.; Hussain, A. Luteolin inhibits proliferation, triggers apoptosis and modulates Akt/mTOR and MAP kinase pathways in HeLa cells. Oncol. Lett. 2021, 21, 192. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.S.; Won, S.B.; Kwon, Y.H. Luteolin Induces Apoptosis and Autophagy in HCT116 Colon Cancer Cells via p53-Dependent Pathway. Nutr. Cancer 2022, 74, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Pai, J.-T.; Hsu, M.-W.; Leu, Y.-L.; Chang, K.-T.; Weng, M.-S. Induction of G2/M Cell Cycle Arrest via p38/p21Waf1/Cip1-Dependent Signaling Pathway Activation by Bavachinin in Non-Small-Cell Lung Cancer Cells. Molecules 2021, 26, 5161. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, R.; Tian, J.; Song, M.; Zhao, R.; Liu, K.; Zhu, F.; Shim, J.; Dong, Z.; Lee, M. Targeting LIMK1 with luteolin inhibits the growth of lung cancer in vitro and in vivo. J. Cell. Mol. Med. 2021, 25, 5560–5571. [Google Scholar] [CrossRef]
- Jang, C.H.; Moon, N.; Oh, J.; Kim, J.-S. Luteolin Shifts Oxaliplatin-Induced Cell Cycle Arrest at G₀/G₁ to Apoptosis in HCT116 Human Colorectal Carcinoma Cells. Nutrients 2019, 11, 770. [Google Scholar] [CrossRef]
- Wang, F.; Gao, F.; Pan, S.; Zhao, S.; Xue, Y. Luteolin induces apoptosis, G0/G1 cell cycle growth arrest and mitochondrial membrane potential loss in neuroblastoma brain tumor cells. Drug Res. 2015, 65, 91–95. [Google Scholar] [CrossRef]
- Ma, J.; Chen, X.; Zhu, X.; Pan, Z.; Hao, W.; Li, D.; Zheng, Q.; Tang, X. Luteolin potentiates low-dose oxaliplatin-induced inhibitory effects on cell proliferation in gastric cancer by inducing G2/M cell cycle arrest and apoptosis. Oncol. Lett. 2021, 23, 16. [Google Scholar] [CrossRef]
- Huang, W.-C.; Liou, C.-J.; Shen, S.-C.; Hu, S.; Hsiao, C.-Y.; Wu, S.-J. Luteolin Attenuates IL-1 β-Induced THP-1 Adhesion to ARPE-19 Cells via Suppression of NF-κB and MAPK Pathways. Mediat. Inflamm. 2020, 2020, 9421340. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, H.; Jia, Y.; Ding, H.; Zhang, L.; Pan, H. Luteolin reduces migration of human glioblastoma cell lines via inhibition of the p-IGF-1R/PI3K/AKT/mTOR signaling pathway. Oncol. Lett. 2017, 14, 3545–3551. [Google Scholar] [CrossRef]
- Morana, O.; Wood, W.; Gregory, C.D. The Apoptosis Paradox in Cancer. Int. J. Mol. Sci. 2022, 23, 1328. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, D.; Garg, V.K.; Goel, N. Intrinsic and extrinsic pathways of apoptosis: Role in cancer development and prognosis. Adv. Protein Chem. Struct. Biol. 2021, 125, 73–120. [Google Scholar] [CrossRef] [PubMed]
- Anson, D.M.; Wilcox, R.M.; Huseman, E.D.; Stump, T.A.; Paris, R.L.; Darkwah, B.O.; Lin, S.; Adegoke, A.O.; Gryka, R.J.; Jean-Louis, D.S.; et al. Luteolin Decreases Epidermal Growth Factor Receptor-Mediated Cell Proliferation and Induces Apoptosis in Glioblastoma Cell Lines. Basic Clin. Pharmacol. Toxicol. 2018, 123, 678–686. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Xiong, J.; Zhou, Y.; Wu, Y.; Song, Y.; Wang, N.; Chen, L.; Zhang, J. Luteolin enhances TRAIL sensitivity in non-small cell lung cancer cells through increasing DR5 expression and Drp1-mediated mitochondrial fission. Arch. Biochem. Biophys. 2020, 692, 108539. [Google Scholar] [CrossRef]
- Lee, S.-J.; Lee, D.-E.; Choi, S.-Y.; Kwon, O.-S. OSMI-1 Enhances TRAIL-Induced Apoptosis through ER Stress and NF-κB Signaling in Colon Cancer Cells. Int. J. Mol. Sci. 2021, 22, 11073. [Google Scholar] [CrossRef]
- Chen, T.; Zhang, X.; Zhu, G.; Liu, H.; Chen, J.; Wang, Y.; He, X. Quercetin inhibits TNF-α induced HUVECs apoptosis and inflammation via downregulating NF-κB and AP-1 signaling pathway in vitro. Medicine 2020, 99, e22241. [Google Scholar] [CrossRef]
- Hussain, S.; Gupta, G.; Goyal, A.; Thapa, R.; Almalki, W.H.; Kazmi, I.; Alzarea, S.I.; Fuloria, S.; Meenakshi, D.U.; Jakhmola, V.; et al. From nature to therapy: Luteolin’s potential as an immune system modulator in inflammatory disorders. J. Biochem. Mol. Toxicol. 2023, e23482. [Google Scholar] [CrossRef]
- Ma, J.; Pan, Z.; Du, H.; Chen, X.; Zhu, X.; Hao, W.; Zheng, Q.; Tang, X. Luteolin induces apoptosis by impairing mitochondrial function and targeting the intrinsic apoptosis pathway in gastric cancer cells. Oncol. Lett. 2023, 26, 327. [Google Scholar] [CrossRef]
- Wilsher, N.E.; Arroo, R.R.; Matsoukas, M.; Tsatsakis, A.M.; Spandidos, D.A.; Androutsopoulos, V.P. Cytochrome P450 CYP1 metabolism of hydroxylated flavones and flavonols: Selective bioactivation of luteolin in breast cancer cells. Food Chem. Toxicol. 2017, 110, 383–394. [Google Scholar] [CrossRef]
- Chen, Y.-F.; Wu, S.; Li, X.; Chen, M.; Liao, H.-F. Luteolin Suppresses Three Angiogenesis Modes and Cell Interaction in Uveal Melanoma in Vitro. Curr. Eye Res. 2022, 47, 1590–1599. [Google Scholar] [CrossRef]
- Li, X.; Chen, M.; Lei, X.; Huang, M.; Ye, W.; Zhang, R.; Zhang, D. Luteolin inhibits angiogenesis by blocking Gas6/Axl signaling pathway. Int. J. Oncol. 2017, 51, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.-T.; Lin, J.; Liu, Y.-E.; Chen, H.-F.; Hsu, K.-W.; Lin, S.-H.; Peng, K.-Y.; Lin, K.-J.; Hsieh, C.-C.; Chen, D.-R. Luteolin suppresses androgen receptor-positive triple-negative breast cancer cell proliferation and metastasis by epigenetic regulation of MMP9 expression via the AKT/mTOR signaling pathway. Phytomedicine 2021, 81, 153437. [Google Scholar] [CrossRef]
- Cayetano-Salazar, L.; Nava-Tapia, D.A.; Astudillo-Justo, K.D.; Arizmendi-Izazaga, A.; Sotelo-Leyva, C.; Herrera-Martinez, M.; Villegas-Comonfort, S.; Navarro-Tito, N. Flavonoids as regulators of TIMPs expression in cancer: Consequences, opportunities, and challenges. Life Sci. 2022, 308, 120932. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Zheng, T.; Hou, Z.; Lv, C.; Xue, A.; Han, T.; Han, B.; Sun, X.; Wei, Y. Luteolin, an aryl hydrocarbon receptor ligand, suppresses tumor metastasis in vitro and in vivo. Oncol. Rep. 2020, 44, 2231–2240. [Google Scholar] [CrossRef]
- Lin, D.; Kuang, G.; Wan, J.; Zhang, X.; Li, H.; Gong, X.; Li, H. Luteolin suppresses the metastasis of triple-negative breast cancer by reversing epithelial-to-mesenchymal transition via downregulation of β-catenin expression. Oncol. Rep. 2017, 37, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, J.; Gong, X.; Wen, X.; Gu, X. Luteolin: Anti-breast Cancer Effects and Mechanisms. J. Explor. Res. Pharmacol. 2018, 3, 85–90. [Google Scholar]
- Fasoulakis, Z.; Koutras, A.; Syllaios, A.; Schizas, D.; Garmpis, N.; Diakosavvas, M.; Angelou, K.; Tsatsaris, G.; Pagkalos, A.; Ntounis, T.; et al. Breast Cancer Apoptosis and the Therapeutic Role of Luteolin. Chirurgia 2021, 116, 170–177. [Google Scholar] [CrossRef]
- Ahmed, S.; Khan, H.; Fratantonio, D.; Hasan, M.M.; Sharifi, S.; Fathi, N.; Ullah, H.; Rastrelli, L. Apoptosis induced by luteolin in breast cancer: Mechanistic and therapeutic perspectives. Phytomedicine 2019, 59, 152883. [Google Scholar] [CrossRef]
- Wang, S.-H.; Wu, C.-H.; Tsai, C.-C.; Chen, T.-Y.; Tsai, K.-J.; Hung, C.-M.; Hsu, C.-Y.; Wu, C.-W.; Hsieh, T.-H. Effects of Luteolin on Human Breast Cancer Using Gene Expression Array: Inferring Novel Genes. Curr. Issues Mol. Biol. 2022, 44, 2107–2121. [Google Scholar] [CrossRef]
- Kang, K.P.; Park, S.K.; Kim, D.H.; Sung, M.J.; Jung, Y.J.; Lee, A.S.; Lee, J.E.; Ramkumar, K.M.; Lee, S.; Park, M.H.; et al. Luteolin ameliorates cisplatin-induced acute kidney injury in mice by regulation of p53-dependent renal tubular apoptosis. Nephrol. Dial. Transplant. 2011, 26, 814–822. [Google Scholar] [CrossRef]
- Sun, D.-W.; Zhang, H.-D.; Mao, L.; Mao, C.-F.; Chen, W.; Cui, M.; Ma, R.; Cao, H.-X.; Jing, C.-W.; Wang, Z.; et al. Luteolin Inhibits Breast Cancer Development and Progression In Vitro and In Vivo by Suppressing Notch Signaling and Regulating MiRNAs. Cell. Physiol. Biochem. 2015, 37, 1693–1711. [Google Scholar] [CrossRef]
- Gao, G.; Ge, R.; Li, Y.; Liu, S. Luteolin exhibits anti-breast cancer property through up-regulating miR-203. Artif. cells, nanomedicine, Biotechnol. 2019, 47, 3265–3271. [Google Scholar] [CrossRef]
- Ludwig, J.A.; Lamhamedi-Cherradi, S.-E.; Lee, H.-Y.; Naing, A.; Benjamin, R. Dual Targeting of the Insulin-Like Growth Factor and Collateral Pathways in Cancer: Combating Drug Resistance. Cancers 2011, 3, 3029–3054. [Google Scholar] [CrossRef]
- Walsh, L.A.; Damjanovski, S. IGF-1 increases invasive potential of MCF 7 breast cancer cells and induces activation of latent TGF-β1 resulting in epithelial to mesenchymal transition. Cell Commun. Signal. 2011, 9, 10. [Google Scholar] [CrossRef]
- Wang, L.-M.; Xie, K.-P.; Huo, H.-N.; Shang, F.; Zou, W.; Xie, M.-J. Luteolin inhibits proliferation induced by IGF-1 pathway dependent ERα in human breast cancer MCF-7 cells. Asian Pac. J. Cancer Prev. 2012, 13, 1431–1437. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Moten, A.; Lin, H.-K. Akt: A new activation mechanism. Cell Res. 2014, 24, 785–786. [Google Scholar] [CrossRef] [PubMed]
- Farhan, M.; Wang, H.; Gaur, U.; Little, P.J.; Xu, J.; Zheng, W. FOXO Signaling Pathways as Therapeutic Targets in Cancer. Int. J. Biol. Sci. 2017, 13, 815. [Google Scholar] [CrossRef]
- Lin, C.-H.; Chang, C.-Y.; Lee, K.-R.; Lin, H.-J.; Chen, T.-H.; Wan, L. Flavones inhibit breast cancer proliferation through the Akt/FOXO3a signaling pathway. BMC Cancer 2015, 15, 958. [Google Scholar] [CrossRef]
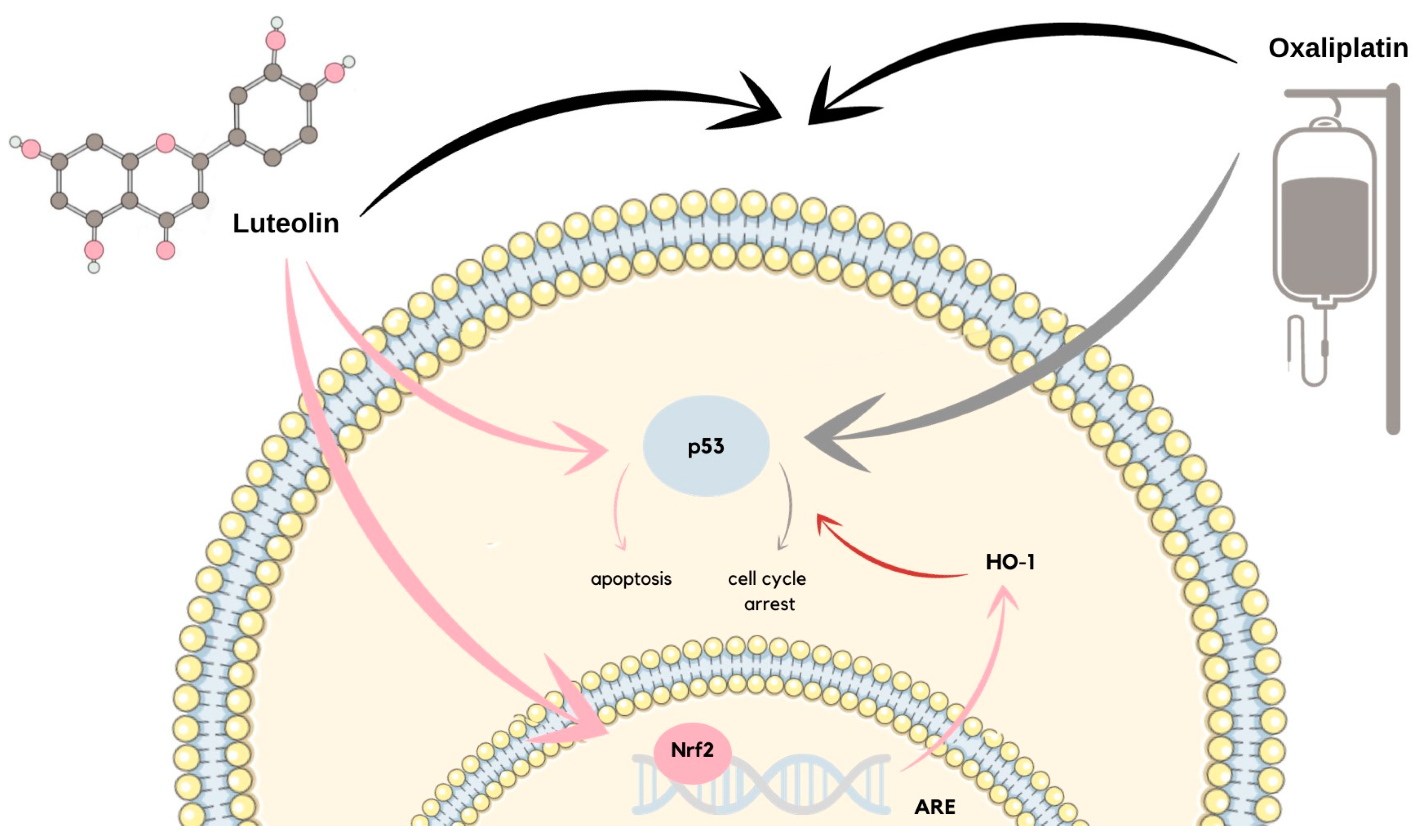
- Tsai, K.-J.; Tsai, H.-Y.; Tsai, C.-C.; Chen, T.-Y.; Hsieh, T.-H.; Chen, C.-L.; Mbuyisa, L.; Huang, Y.-B.; Lin, M.-W. Luteolin Inhibits Breast Cancer Stemness and Enhances Chemosensitivity through the Nrf2-Mediated Pathway. Molecules 2021, 26, 6452. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Sasaki, N.; Saito, M.; Endo, N.; Kugawa, F.; Ueno, A. Luteolin attenuates doxorubicin-induced cytotoxicity to MCF-7 human breast cancer cells. Biol. Pharm. Bull. 2015, 38, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Du, G.-J.; Song, Z.-H.; Lin, H.-H.; Han, X.-F.; Zhang, S.; Yang, Y.-M. Luteolin as a glycolysis inhibitor offers superior efficacy and lesser toxicity of doxorubicin in breast cancer cells. Biochem. Biophys. Res. Commun. 2008, 372, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.-W.; Suh, Y.J. Synergistic apoptotic effect of celecoxib and luteolin on breast cancer cells. Oncol. Rep. 2013, 29, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Jin, K.; Lan, H. Luteolin inhibits cell cycle progression and induces apoptosis of breast cancer cells through downregulation of human telomerase reverse transcriptase. Oncol. Lett. 2019, 17, 3842–3850. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lee, I.-M.; Zhang, S.M.; Blumberg, J.B.; Buring, J.E.; Sesso, H.D. Dietary intake of selected flavonols, flavones, and flavonoid-rich foods and risk of cancer in middle-aged and older women. Am. J. Clin. Nutr. 2009, 89, 905–912. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, Z.Z. Interpretation of global colorectal cancer statistics. Zhonghua Liu Xing Bing Xue Za Zhi 2021, 42, 149–152. [Google Scholar] [CrossRef]
- Jiang, J.; Zhu, F.; Zhang, H.; Sun, T.; Fu, F.; Chen, X.; Zhang, Y. Luteolin suppresses the growth of colon cancer cells by inhibiting the IL-6/STAT3 signaling pathway. J. Gastrointest. Oncol. 2022, 13, 1722–1732. [Google Scholar] [CrossRef]
- Hu, Z.; Luo, D.; Wang, D.; Ma, L.; Zhao, Y.; Li, L. IL-17 Activates the IL-6/STAT3 Signal Pathway in the Proliferation of Hepatitis B Virus-Related Hepatocellular Carcinoma. Cell. Physiol. Biochem. 2017, 43, 2379–2390. [Google Scholar] [CrossRef]
- Pucci, M.; Raimondo, S.; Urzì, O.; Moschetti, M.; Di Bella, M.A.; Conigliaro, A.; Caccamo, N.; La Manna, M.P.; Fontana, S.; Alessandro, R. Tumor-Derived Small Extracellular Vesicles Induce Pro-Inflammatory Cytokine Expression and PD-L1 Regulation in M0 Macrophages via IL-6/STAT3 and TLR4 Signaling Pathways. Int. J. Mol. Sci. 2021, 22, 12118. [Google Scholar] [CrossRef]
- Sun, J.; Shigemi, H.; Cao, M.; Qin, E.; Tang, J.; Shen, J.; Iwasaki, H. Minocycline Induces Autophagy and Inhibits Cell Proliferation in LPS-Stimulated THP-1 Cells. BioMed Res. Int. 2020, 2020, 5459209. [Google Scholar] [CrossRef]
- Nakayama, M.; Oshima, M. Mutant p53 in colon cancer. J. Mol. Cell Biol. 2019, 11, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Potočnjak, I.; Šimić, L.; Gobin, I.; Vukelić, I.; Domitrović, R. Antitumor activity of luteolin in human colon cancer SW620 cells is mediated by the ERK/FOXO3a signaling pathway. Toxicol. Vitr. 2020, 66, 104852. [Google Scholar] [CrossRef] [PubMed]
- Pai, S.G.; Carneiro, B.A.; Mota, J.M.; Costa, R.; Leite, C.A.; Barroso-Sousa, R.; Kaplan, J.B.; Chae, Y.K.; Giles, F.J. Wnt/beta-catenin pathway: Modulating anticancer immune response. J. Hematol. Oncol. 2017, 10, 101. [Google Scholar] [CrossRef] [PubMed]
- Duchartre, Y.; Kim, Y.-M.; Kahn, M. The Wnt signaling pathway in cancer. Crit. Rev. Oncol. Hematol. 2016, 99, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ao, X.; Ding, W.; Ponnusamy, M.; Wu, W.; Hao, X.; Yu, W.; Wang, Y.; Li, P.; Wang, J. Critical role of FOXO3a in carcinogenesis. Mol. Cancer 2018, 17, 104. [Google Scholar] [CrossRef]
- Monti, E.; Marras, E.; Prini, P.; Gariboldi, M.B. Luteolin impairs hypoxia adaptation and progression in human breast and colon cancer cells. Eur. J. Pharmacol. 2020, 881, 173210. [Google Scholar] [CrossRef]
- Vadde, R.; Vemula, S.; Jinka, R.; Merchant, N.; Bramhachari, P.V.; Nagaraju, G.P. Role of hypoxia-inducible factors (HIF) in the maintenance of stemness and malignancy of colorectal cancer. Crit. Rev. Oncol. Hematol. 2017, 113, 22–27. [Google Scholar] [CrossRef]
- Johansson, E.; Grassi, E.S.; Pantazopoulou, V.; Tong, B.; Lindgren, D.; Berg, T.J.; Pietras, E.J.; Axelson, H.; Pietras, A. CD44 Interacts with HIF-2α to Modulate the Hypoxic Phenotype of Perinecrotic and Perivascular Glioma Cells. Cell Rep. 2017, 20, 1641–1653. [Google Scholar] [CrossRef]
- Zhang, D.; Yang, L.; Liu, X.; Gao, J.; Liu, T.; Yan, Q.; Yang, X. Hypoxia modulates stem cell properties and induces EMT through N-glycosylation of EpCAM in breast cancer cells. J. Cell. Physiol. 2020, 235, 3626–3633. [Google Scholar] [CrossRef]
- Torre, L.A.; Siegel, R.L.; Jemal, A. Lung cancer statistics. Adv. Exp. Med. Biol. 2016, 893, 1–19. [Google Scholar]
- Rodriguez-Canales, J.; Parra-Cuentas, E.; Wistuba, I.I. Diagnosis and Molecular Classification of Lung Cancer. Cancer Treat. Res. 2016, 170, 25–46. [Google Scholar] [CrossRef] [PubMed]
- Nooreldeen, R.; Bach, H. Current and Future Development in Lung Cancer Diagnosis. Int. J. Mol. Sci. 2021, 22, 8661. [Google Scholar] [CrossRef]
- American Lung Association Lung: Cancer Fact Sheet; State of Lung Cancer, Lung.org: Chicago, IL, USA, 2022; pp. 1–16.
- Polanco, D.; Pinilla, L.; Gracia-Lavedan, E.; Mas, A.; Bertran, S.; Fierro, G.; Seminario, A.; Gómez, S.; Barbé, F. Prognostic value of symptoms at lung cancer diagnosis: A three-year observational study. J. Thorac. Dis. 2021, 13, 1485. [Google Scholar] [CrossRef] [PubMed]
- Lemjabbar-Alaoui, H.; Hassan, O.U.; Yang, Y.-W.; Buchanan, P. Lung cancer: Biology and treatment options. Biochim. Biophys. Acta 2015, 1856, 189. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Rauf, A.; Abu-Izneid, T.; Nadeem, M.; Shariati, M.A.; Khan, I.A.; Imran, A.; Orhan, I.E.; Rizwan, M.; Atif, M.; et al. Luteolin, a flavonoid, as an anticancer agent: A review. Biomed. Pharmacother. 2019, 112, 108612. [Google Scholar] [CrossRef]
- Jiang, Z.-B.; Wang, W.-J.; Xu, C.; Xie, Y.-J.; Wang, X.-R.; Zhang, Y.-Z.; Huang, J.-M.; Huang, M.; Xie, C.; Liu, P.; et al. Luteolin and its derivative apigenin suppress the inducible PD-L1 expression to improve anti-tumor immunity in KRAS-mutant lung cancer. Cancer Lett. 2021, 515, 36–48. [Google Scholar] [CrossRef]
- Tsoukalas, N.; Aravantinou-Fatorou, E.; Tolia, M.; Giaginis, C.; Galanopoulos, M.; Kiakou, M.; Kostakis, I.D.; Dana, E.; Vamvakaris, I.; Korogiannos, A.; et al. Epithelial-Mesenchymal Transition in Non Small-cell Lung Cancer. Anticancer. Res. 2017, 37, 1773–1778. [Google Scholar] [CrossRef]
- Lu, G.; Zhou, Y.; Zhang, C.; Zhang, Y. Upregulation of LIMK1 Is Correlated With Poor Prognosis and Immune Infiltrates in Lung Adenocarcinoma. Front. Genet. 2021, 12, 889. [Google Scholar] [CrossRef]
- Chen, K.-C.; Chen, C.-Y.; Lin, C.-J.; Yang, T.-Y.; Chen, T.-H.; Wu, L.-C.; Wu, C.-C. Luteolin attenuates TGF-β1-induced epithelial–mesenchymal transition of lung cancer cells by interfering in the PI3K/Akt–NF-κB–Snail pathway. Life Sci. 2013, 93, 924–933. [Google Scholar] [CrossRef]
- Chen, X.; Xu, B.; Li, Q.; Xu, X.; Li, X.; You, X.; Yu, Z. Genetic profile of non-small cell lung cancer (NSCLC): A hospital-based survey in Jinhua. Mol. Genet. Genom. Med. 2020, 8, e1398. [Google Scholar] [CrossRef]
- Watterson, A.; Coelho, M.A. Cancer immune evasion through KRAS and PD-L1 and potential therapeutic interventions. Cell Commun. Signal. 2023, 21, 45. [Google Scholar] [CrossRef] [PubMed]
- Ardekani, A.M.; Naeini, M.M. The Role of MicroRNAs in Human Diseases. Avicenna J. Med. Biotechnol. 2010, 2, 161. [Google Scholar]
- Wani, J.A.; Majid, S.; Imtiyaz, Z.; Rehman, M.U.; Alsaffar, R.M.; Shah, N.N.; Alshehri, S.; Ghoneim, M.M.; Imam, S.S. MiRNAs in Lung Cancer: Diagnostic, Prognostic, and Therapeutic Potential. Diagnostics 2022, 12, 1610. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liao, Y.; Tang, L. MicroRNA-34 family: A potential tumor suppressor and therapeutic candidate in cancer. J. Exp. Clin. Cancer Res. 2019, 38, 53. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Liu, C.; Liu, X.; Tang, D.G.; Wang, J. The microRNA miR-34a Inhibits Non-Small Cell Lung Cancer (NSCLC) Growth and the CD44hi Stem-Like NSCLC Cells. PLoS ONE 2014, 9, e90022. [Google Scholar] [CrossRef]
- Jiang, Z.-Q.; Li, M.-H.; Qin, Y.-M.; Jiang, H.-Y.; Zhang, X.; Wu, M.-H. Luteolin Inhibits Tumorigenesis and Induces Apoptosis of Non-Small Cell Lung Cancer Cells via Regulation of MicroRNA-34a-5p. Int. J. Mol. Sci. 2018, 19, 447. [Google Scholar] [CrossRef]
- Çetinkaya, M.; Baran, Y. Therapeutic Potential of Luteolin on Cancer. Vaccines 2023, 11, 554. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A Review of Programmed Cell Death. Toxicol. Pathol. 2007, 35, 495. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, S.; Chen, J.; Zhang, Q.; Lin, S.; Chen, Z.; Jiang, J. Luteolin Induces Mitochondria-dependent Apoptosis in Human Lung Adenocarcinoma Cell. Nat. Prod. Commun. 2012, 7, 29–32. [Google Scholar] [CrossRef]
- Ju, W.; Wang, X.; Shi, H.; Chen, W.; Belinsky, S.A.; Lin, Y. A critical role of luteolin-induced reactive oxygen species in blockage of tumor necrosis factor-activated nuclear factor-kappaB pathway and sensitization of apoptosis in lung cancer cells. Mol. Pharmacol. 2007, 71, 1381–1388. [Google Scholar] [CrossRef]
- D’antonio, C.; Passaro, A.; Gori, B.; Del Signore, E.; Migliorino, M.R.; Ricciardi, S.; Fulvi, A.; de Marinis, F. Therapeutic Advances in Medical Oncology Bone and brain metastasis in lung cancer: Recent advances in therapeutic strategies. Ther. Adv. Med. Oncol. 2014, 6, 101–114. [Google Scholar] [CrossRef] [PubMed]
- Rawlings, J.S.; Rosler, K.M.; Harrison, D.A. The JAK/STAT signaling pathway. J. Cell Sci. 2004, 117 Pt 8, 1281–1283. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.-Y.; Wang, C.-J.; Chen, N.-F.; Ho, W.-H.; Lu, F.-J.; Tseng, T.-H. Luteolin enhances paclitaxel-induced apoptosis in human breast cancer MDA-MB-231 cells by blocking STAT3. Chem. Biol. Interact. 2014, 213, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Ye, J.; Xia, Z.; Cheng, B. Effect of luteolin on apoptosis, MAPK and JNK signaling pathways in guinea pig chondrocyte with osteoarthritis. Cell. Mol. Biol. 2019, 65, 91–95. [Google Scholar] [CrossRef]
- Herster, F.; Bittner, Z.; Archer, N.K.; Dickhöfer, S.; Eisel, D.; Eigenbrod, T.; Knorpp, T.; Schneiderhan-Marra, N.; Löffler, M.W.; Kalbacher, H.; et al. Neutrophil extracellular trap-associated RNA and LL37 enable self-amplifying inflammation in psoriasis. Nat. Commun. 2020, 11, 105. [Google Scholar] [CrossRef]
- Kwon, E.-Y.; Choi, M.-S. Luteolin Targets the Toll-Like Receptor Signaling Pathway in Prevention of Hepatic and Adipocyte Fibrosis and Insulin Resistance in Diet-Induced Obese Mice. Nutrients 2018, 10, 1415. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Kong, X.; Wang, M.; Li, J.; Chen, W.; Jiang, D. Luteolin Partially Inhibits LFA-1 Expression in Neutrophils Through the ERK Pathway. Inflammation 2019, 42, 365–374. [Google Scholar] [CrossRef]
- Yang, S.-C.; Chen, P.-J.; Chang, S.-H.; Weng, Y.-T.; Chang, F.-R.; Chang, K.-Y.; Chen, C.-Y.; Kao, T.-I.; Hwang, T.-L. Luteolin attenuates neutrophilic oxidative stress and inflammatory arthritis by inhibiting Raf1 activity. Biochem. Pharmacol. 2018, 154, 384–396. [Google Scholar] [CrossRef]
- Ye, S.; Liu, H.; Chen, Y.; Qiu, F.; Liang, C.-L.; Zhang, Q.; Huang, H.; Wang, S.; Zhang, Z.-D.; Lu, W.; et al. A Novel Immunosuppressant, Luteolin, Modulates Alloimmunity and Suppresses Murine Allograft Rejection. J. Immunol. 2019, 203, 3436–3446. [Google Scholar] [CrossRef]
- Wang, T.; Pan, D.; Zhang, Y.; Li, D.; Zhang, Y.; Xu, T.; Luo, Y.; Ma, Y. Luteolin antagonizes angiotensin II-dependent proliferation and collagen synthesis of cultured rat cardiac fibroblasts. Curr. Pharm. Biotechnol. 2015, 16, 430–439. [Google Scholar] [CrossRef]
- Verbeek, R.; Plomp, A.C.; Van Tol, E.; Van Noort, J.M. The flavones luteolin and apigenin inhibit in vitro antigen-specific proliferation and interferon-gamma production by murine and human autoimmune T cells. Biochem. Pharmacol. 2004, 68, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Zhou, D.; Wang, Y.; Sun, W.; Zhang, C.; Xu, J.; Yang, H.; Zhou, T.; Li, P. Effects of luteolin on treatment of psoriasis by repressing HSP90. Int. Immunopharmacol. 2020, 79, 106070. [Google Scholar] [CrossRef] [PubMed]
- Palombo, R.; Savini, I.; Avigliano, L.; Madonna, S.; Cavani, A.; Albanesi, C.; Mauriello, A.; Melino, G.; Terrinoni, A. Luteolin-7-glucoside inhibits IL-22/STAT3 pathway, reducing proliferation, acanthosis, and inflammation in keratinocytes and in mouse psoriatic model. Cell Death Dis. 2016, 7, e2344. [Google Scholar] [CrossRef] [PubMed]
- Van Belle, A.B.; de Heusch, M.; Lemaire, M.M.; Hendrickx, E.; Warnier, G.; Dunussi-Joannopoulos, K.; Fouser, L.A.; Renauld, J.-C.; Dumoutier, L. IL-22 is required for imiquimod-induced psoriasiform skin inflammation in mice. J. Immunol. 2012, 188, 462–469. [Google Scholar] [CrossRef]
- Patel, A.B.; Tsilioni, I.; Weng, Z.; Theoharides, T.C. TNF stimulates IL-6, CXCL8 and VEGF secretion from human keratinocytes via activation of mTOR, inhibited by tetramethoxyluteolin. Exp. Dermatol. 2018, 27, 135–143. [Google Scholar] [CrossRef]
- Vijayalakshmi, A.; Madhira, G. Anti-psoriatic activity of flavonoids from Cassia tora leaves using the rat ultraviolet B ray photodermatitis model. Rev. Bras. Farm. 2014, 24, 322–329. [Google Scholar] [CrossRef]
- Caporali, S.; De Stefano, A.; Calabrese, C.; Giovannelli, A.; Pieri, M.; Savini, I.; Tesauro, M.; Bernardini, S.; Minieri, M.; Terrinoni, A. Anti-Inflammatory and Active Biological Properties of the Plant-Derived Bioactive Compounds Luteolin and Luteolin 7-Glucoside. Nutrients 2022, 14, 1155. [Google Scholar] [CrossRef]
- Esser, P.R.; Martin, S.F. Pathomechanisms of Contact Sensitization. Curr. Allergy Asthma Rep. 2017, 17, 83. [Google Scholar] [CrossRef]
- Schempp, C.M.; Meinke, M.C.; Lademann, J.; Ferrari, Y.; Brecht, T.; Gehring, W. Topical antioxidants protect the skin from chemical-induced irritation in the repetitive washing test: A placebo-controlled, double-blind study. Contact Dermat. 2012, 67, 234–237. [Google Scholar] [CrossRef]
- Casetti, F.; Jung, W.; Wölfle, U.; Reuter, J.; Neumann, K.; Gilb, B.; Wähling, A.; Wagner, S.; Merfort, I.; Schempp, C. Topical application of solubilized Reseda luteola extract reduces ultraviolet B-induced inflammation in vivo. J. Photochem. Photobiol. B 2009, 96, 260–265. [Google Scholar] [CrossRef]
- Esser, P.R.; Wölfle, U.; Dürr, C.; von Loewenich, F.D.; Schempp, C.M.; Freudenberg, M.A.; Jakob, T.; Martin, S.F. Contact sensitizers induce skin inflammation via ROS production and hyaluronic acid degradation. PLoS ONE 2012, 7, e41340. [Google Scholar] [CrossRef] [PubMed]
- Chibli, L.A.; Rodrigues, K.C.; Gasparetto, C.M.; Pinto, N.C.; Fabri, R.L.; Scio, E.; Alves, M.S.; Del-Vechio-Vieira, G.; Sousa, O.V. Anti-inflammatory effects of Bryophyllum pinnatum (Lam.) Oken ethanol extract in acute and chronic cutaneous inflammation. J. Ethnopharmacol. 2014, 154, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Dudeck, A.; Dudeck, J.; Scholten, J.; Petzold, A.; Surianarayanan, S.; Köhler, A.; Peschke, K.; Vöhringer, D.; Waskow, C.; Krieg, T.; et al. Mast cells are key promoters of contact allergy that mediate the adjuvant effects of haptens. Immunity 2011, 34, 973–984. [Google Scholar] [CrossRef] [PubMed]
- Weng, Z.; Patel, A.B.; Panagiotidou, S.; Theoharides, T.C. The novel flavone tetramethoxyluteolin is a potent inhibitor of human mast cells. J. Allergy Clin. Immunol. 2015, 135, 1044–1052.e5. [Google Scholar] [CrossRef]
- Kim, S.-H.; Saba, E.; Kim, B.-K.; Yang, W.-K.; Park, Y.-C.; Shin, H.J.; Han, C.K.; Lee, Y.C.; Rhee, M.H. Luteolin attenuates airway inflammation by inducing the transition of CD4+CD25- to CD4+CD25+ regulatory T cells. Eur. J. Pharmacol. 2018, 820, 53–64. [Google Scholar] [CrossRef]
- Chiurchiù, V.; Rapino, C.; Talamonti, E.; Leuti, A.; Lanuti, M.; Gueniche, A.; Jourdain, R.; Breton, L.; Maccarrone, M. Anandamide Suppresses Proinflammatory T Cell Responses In Vitro through Type-1 Cannabinoid Receptor-Mediated mTOR Inhibition in Human Keratinocytes. J. Immunol. 2016, 197, 3545–3553. [Google Scholar] [CrossRef]
- Patel, A.B.; Theoharides, T.C. Methoxyluteolin Inhibits Neuropeptide-stimulated Proinflammatory Mediator Release via mTOR Activation from Human Mast Cells. J. Pharmacol. Exp. Ther. 2017, 361, 462–471. [Google Scholar] [CrossRef]
- Góngora, L.; Giner, R.M.; Máñez, S.; Recio, M.d.C.; Ríos, J.L. Phagnalon rupestre as a source of compounds active on contact hypersensitivity. Planta Med. 2002, 68, 561–564. [Google Scholar] [CrossRef]
- Jo, B.-G.; Park, N.-J.; Jegal, J.; Choi, S.; Lee, S.W.; Yi, L.W.; Kim, S.-N.; Yang, M.-H. Stellera chamaejasme and Its Main Compound Luteolin 7-O-Glucoside Alleviates Skin Lesions in Oxazolone- and 2,4-Dinitrochlorobenzene-Stimulated Murine Models of Atopic Dermatitis. Planta Med. 2019, 85, 583–590. [Google Scholar] [CrossRef]
- Jegal, J.; Kim, T.-Y.; Park, N.-J.; Jo, B.-G.; Jo, G.-A.; Choi, H.-S.; Kim, S.-N.; Yang, M.H. Inhibitory Effects of Luteolin 7-Methyl Ether Isolated from Wikstroemia ganpi on Tnf-A/Ifn-Γ Mixture-Induced Inflammation in Human Keratinocyte. Nutrients 2021, 13, 4387. [Google Scholar] [CrossRef]
- Liang, K.-L.; Yu, S.-J.; Huang, W.-C.; Yen, H.-R. Luteolin Attenuates Allergic Nasal Inflammation via Inhibition of Interleukin-4 in an Allergic Rhinitis Mouse Model and Peripheral Blood From Human Subjects With Allergic Rhinitis. Front. Pharmacol. 2020, 11, 291. [Google Scholar] [CrossRef] [PubMed]
- Cole, J.B.; Florez, J.C. Genetics of diabetes mellitus and diabetes complications. Nat. Rev. Nephrol. 2020, 16, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Ceriello, A.; Prattichizzo, F. Variability of risk factors and diabetes complications. Cardiovasc. Diabetol. 2021, 20, 101. [Google Scholar] [CrossRef] [PubMed]
- Luc, K.; Schramm-Luc, A.; Guzik, T.J.; Mikolajczyk, T.P. Oxidative stress and inflammatory markers in prediabetes and diabetes. J. Physiol. Pharmacol. 2019, 70, 70. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, X.-B.; Lu, H.-E.; Wang, F.; Fan, X.-H. Effect of luteoin in delaying cataract in STZ-induced diabetic rats. Arch. Pharm. Res. 2017, 40, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Torres, I.; Castrejón-Téllez, V.; Soto, M.E.; Rubio-Ruiz, M.E.; Manzano-Pech, L.; Guarner-Lans, V. Oxidative Stress, Plant Natural Antioxidants, and Obesity. Int. J. Mol. Sci. 2021, 22, 1786. [Google Scholar] [CrossRef]
- Malone, J.I.; Hansen, B.C. Does obesity cause type 2 diabetes mellitus (T2DM)? Or is it the opposite? Pediatr. Diabetes 2019, 20, 5–9. [Google Scholar] [CrossRef]
- Kanaley, J.A.; Colberg, S.R.; Corcoran, M.H.; Malin, S.K.; Rodriguez, N.R.; Crespo, C.J.; Kirwan, J.P.; Zierath, J.R. Exercise/Physical Activity in Individuals with Type 2 Diabetes: A Consensus Statement from the American College of Sports Medicine. Med. Sci. Sports Exerc. 2022, 54, 353–368. [Google Scholar] [CrossRef]
- Aune, D.; Norat, T.; Leitzmann, M.; Tonstad, S.; Vatten, L.J. Physical activity and the risk of type 2 diabetes: A systematic review and dose-response meta-analysis. Eur. J. Epidemiol. 2015, 30, 529–542. [Google Scholar] [CrossRef]
- Flores-Opazo, M.; McGee, S.L.; Hargreaves, M. Exercise and GLUT4. Exerc. Sport Sci. Rev. 2020, 48, 110–118. [Google Scholar] [CrossRef]
- Prattichizzo, F.; de Candia, P.; Ceriello, A. Diabetes and kidney disease: Emphasis on treatment with SGLT-2 inhibitors and GLP-1 receptor agonists. Metabolism 2021, 120, 154799. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, M.P.; Pratley, R.E. GLP-1 Analogs and DPP-4 Inhibitors in Type 2 Diabetes Therapy: Review of Head-to-Head Clinical Trials. Front. Endocrinol. 2020, 11, 178. [Google Scholar] [CrossRef] [PubMed]
- Maselli, D.B.; Camilleri, M. Effects of GLP-1 and Its Analogs on Gastric Physiology in Diabetes Mellitus and Obesity. Adv. Exp. Med. Biol. 2021, 1307, 171–192. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Chen, Z.; Wang, L.; Wang, G.; Wang, Z.; Dong, X.; Wen, B.; Zhang, Z. The Pathogenesis of Diabetes Mellitus by Oxidative Stress and Inflammation: Its Inhibition by Berberine. Front. Pharmacol. 2018, 9, 782. [Google Scholar] [CrossRef]
- Oguntibeju, O.O. Type 2 diabetes mellitus, oxidative stress and inflammation: Examining the links. Int. J. Physiol. Pathophysiol. Pharmacol. 2019, 11, 45. [Google Scholar]
- Benhar, M. Roles of mammalian glutathione peroxidase and thioredoxin reductase enzymes in the cellular response to nitrosative stress. Free Radic. Biol. Med. 2018, 127, 160–164. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180. [Google Scholar] [CrossRef]
- Volpe, C.M.O.; Villar-Delfino, P.H.; Dos Anjos, P.M.F.; Nogueira-Machado, J.A. Cellular death, reactive oxygen species (ROS) and diabetic complications. Cell Death Dis. 2018, 9, 119. [Google Scholar] [CrossRef]
- Tsalamandris, S.; Antonopoulos, A.S.; Oikonomou, E.; Papamikroulis, G.-A.; Vogiatzi, G.; Papaioannou, S.; Deftereos, S.; Tousoulis, D. The Role of Inflammation in Diabetes: Current Concepts and Future Perspectives. Eur. Cardiol. Rev. 2019, 14, 50. [Google Scholar] [CrossRef]
- Wu, Y.; Hu, Y.L.; Liu, W.; Sun, B.J.; Zhang, C.F.; Wu, L.L.; Liu, T.H. Meta-analysis Flavonoids from traditional Chinese herbs for diabetes in rats: A network Meta-analysi. J. Tradit. Chin. Med. Chung I Tsa Chih Ying Wen Pan 2022, 42, 1–8. [Google Scholar] [CrossRef]
- Rienks, J.; Barbaresko, J.; Oluwagbemigun, K.; Schmid, M.; Nöthlings, U. Polyphenol exposure and risk of type 2 diabetes: Dose-response meta-analyses and systematic review of prospective cohort studies. Am. J. Clin. Nutr. 2018, 108, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Castellino, G.; Nikolic, D.; Magán-Fernández, A.; Malfa, G.A.; Chianetta, R.; Patti, A.M.; Amato, A.; Montalto, G.; Toth, P.P.; Banach, M.; et al. Altilix® Supplement Containing Chlorogenic Acid and Luteolin Improved Hepatic and Cardiometabolic Parameters in Subjects with Metabolic Syndrome: A 6 Month Randomized, Double-Blind, Placebo-Controlled Study. Nutrients 2019, 11, 2580. [Google Scholar] [CrossRef] [PubMed]
- Terzo, S.; Amato, A.; Magán-Fernández, A.; Castellino, G.; Calvi, P.; Chianetta, R.; Giglio, R.V.; Patti, A.M.; Nikolic, D.; Firenze, A.; et al. A Nutraceutical Containing Chlorogenic Acid and Luteolin Improves Cardiometabolic Parameters in Subjects with Pre-Obesity: A 6-Month Randomized, Double-Blind, Placebo-Controlled Study. Nutrients 2023, 15, 462. [Google Scholar] [CrossRef] [PubMed]
- Egbuna, C.; Awuchi, C.G.; Kushwaha, G.; Rudrapal, M.; Patrick-Iwuanyanwu, K.C.; Singh, O.; Odoh, U.E.; Khan, J.; Jeevanandam, J.; Kumarasamy, S.; et al. Bioactive Compounds Effective Against Type 2 Diabetes Mellitus: A Systematic Review. Curr. Top. Med. Chem. 2021, 21, 1067–1095. [Google Scholar] [CrossRef]
- LMousavi, L.; Salleh, R.M.; Murugaiyah, V. Phytochemical and bioactive compounds identification of Ocimum tenuiflorum leaves of methanol extract and its fraction with an antidiabetic potential. Int. J. Food Prop. 2018, 21, 2390–2399. [Google Scholar] [CrossRef]
- Chen, L.-Y.; Cheng, H.-L.; Liao, C.-K.; Kuan, Y.-H.; Liang, T.-J.; Tseng, T.-J.; Lin, H.-C. Luteolin improves nephropathy in hyperglycemic rats through anti-oxidant, anti-inflammatory, and anti-apoptotic mechanisms. J. Funct. Foods 2023, 102, 105461. [Google Scholar] [CrossRef]
- Li, L.; Luo, W.; Qian, Y.; Zhu, W.; Qian, J.; Li, J.; Jin, Y.; Xu, X.; Liang, G. Luteolin protects against diabetic cardiomyopathy by inhibiting NF-κB-mediated inflammation and activating the Nrf2-mediated antioxidant responses. Phytomedicine 2019, 59, 152774. [Google Scholar] [CrossRef]
- Ma, K.; Tian, C.; Guo, C.; Li, M. The efficacy of Chinese patent medicine intervention on blood glucose and lipid in prediabetes: A meta-analysis. Heliyon 2022, 8, e12112. [Google Scholar] [CrossRef]
- Ambasta, R.K.; Gupta, R.; Kumar, D.; Bhattacharya, S.; Sarkar, A.; Kumar, P. Can luteolin be a therapeutic molecule for both colon cancer and diabetes? Brief. Funct. Genom. 2018, 18, 230–239. [Google Scholar] [CrossRef]
- Di Stadio, A.; D’ascanio, L.; Vaira, L.A.; Cantone, E.; De Luca, P.; Cingolani, C.; Motta, G.; De Riu, G.; Vitelli, F.; Spriano, G.; et al. Ultramicronized Palmitoylethanolamide and Luteolin Supplement Combined with Olfactory Training to Treat Post-COVID-19 Olfactory Impairment: A Multi-Center Double-Blinded Randomized Placebo-Controlled Clinical Trial. Curr. Neuropharmacol. 2022, 20, 2001. [Google Scholar] [CrossRef]
- Kempuraj, D.; Thangavel, R.; Kempuraj, D.D.; Ahmed, M.E.; Selvakumar, G.P.; Raikwar, S.P.; Zaheer, S.A.; Iyer, S.S.; Govindarajan, R.; Chandrasekaran, P.N.; et al. Neuroprotective effects of flavone luteolin in neuroinflammation and neurotrauma. BioFactors 2021, 47, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Jo, M.H.; Ikram, M.; Khan, A.; Kim, M.O. Deciphering the Potential Neuroprotective Effects of Luteolin against Aβ1-42-Induced Alzheimer’s Disease. Int. J. Mol. Sci. 2021, 22, 9583. [Google Scholar] [CrossRef] [PubMed]
- Debiaggi, M.; Tateo, F.; Pagani, L.; Luini, M.; Romero, E. Effects of propolis flavonoids on virus infectivity and replication. Microbiologica 1990, 13, 207–213. [Google Scholar] [PubMed]
- Rahman, N.; Basharat, Z.; Yousuf, M.; Castaldo, G.; Rastrelli, L.; Khan, H. Virtual Screening of Natural Products against Type II Transmembrane Serine Protease (TMPRSS2), the Priming Agent of Coronavirus 2 (SARS-CoV-2). Molecules 2020, 25, 2271. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Cholevas, C.; Polyzoidis, K.; Politis, A. Long-COVID syndrome-associated brain fog and chemofog: Luteolin to the rescue. BioFactors 2021, 47, 232. [Google Scholar] [CrossRef]
- Marshall, J.S.; Portales-Cervantes, L.; Leong, E. Mast Cell Responses to Viruses and Pathogen Products. Int. J. Mol. Sci. 2019, 20, 4241. [Google Scholar] [CrossRef]
- Theoharides, T.C. Potential association of mast cells with coronavirus disease 2019. Ann. Allergy. Asthma Immunol. 2021, 126, 217–218. [Google Scholar] [CrossRef]
- Theoharides, T.C. Luteolin supplements: All that glitters is not gold. BioFactors 2021, 47, 242–244. [Google Scholar] [CrossRef]
- Angeloni, C.; Malaguti, M.; Barbalace, M.C.; Hrelia, S. Bioactivity of Olive Oil Phenols in Neuroprotection. Int. J. Mol. Sci. 2017, 18, 2230. [Google Scholar] [CrossRef]
- Mukai, K.; Tsai, M.; Saito, H.; Galli, S.J. Mast cells as sources of cytokines, chemokines, and growth factors. Immunol. Rev. 2018, 282, 121–150. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rakoczy, K.; Kaczor, J.; Sołtyk, A.; Szymańska, N.; Stecko, J.; Sleziak, J.; Kulbacka, J.; Baczyńska, D. Application of Luteolin in Neoplasms and Nonneoplastic Diseases. Int. J. Mol. Sci. 2023, 24, 15995. https://doi.org/10.3390/ijms242115995
Rakoczy K, Kaczor J, Sołtyk A, Szymańska N, Stecko J, Sleziak J, Kulbacka J, Baczyńska D. Application of Luteolin in Neoplasms and Nonneoplastic Diseases. International Journal of Molecular Sciences. 2023; 24(21):15995. https://doi.org/10.3390/ijms242115995
Chicago/Turabian StyleRakoczy, Katarzyna, Justyna Kaczor, Adam Sołtyk, Natalia Szymańska, Jakub Stecko, Jakub Sleziak, Julita Kulbacka, and Dagmara Baczyńska. 2023. "Application of Luteolin in Neoplasms and Nonneoplastic Diseases" International Journal of Molecular Sciences 24, no. 21: 15995. https://doi.org/10.3390/ijms242115995
APA StyleRakoczy, K., Kaczor, J., Sołtyk, A., Szymańska, N., Stecko, J., Sleziak, J., Kulbacka, J., & Baczyńska, D. (2023). Application of Luteolin in Neoplasms and Nonneoplastic Diseases. International Journal of Molecular Sciences, 24(21), 15995. https://doi.org/10.3390/ijms242115995








