Interrogating the Role of miR-125b and Its 3′isomiRs in Protection against Hypoxia
Abstract
:1. Introduction
2. Results
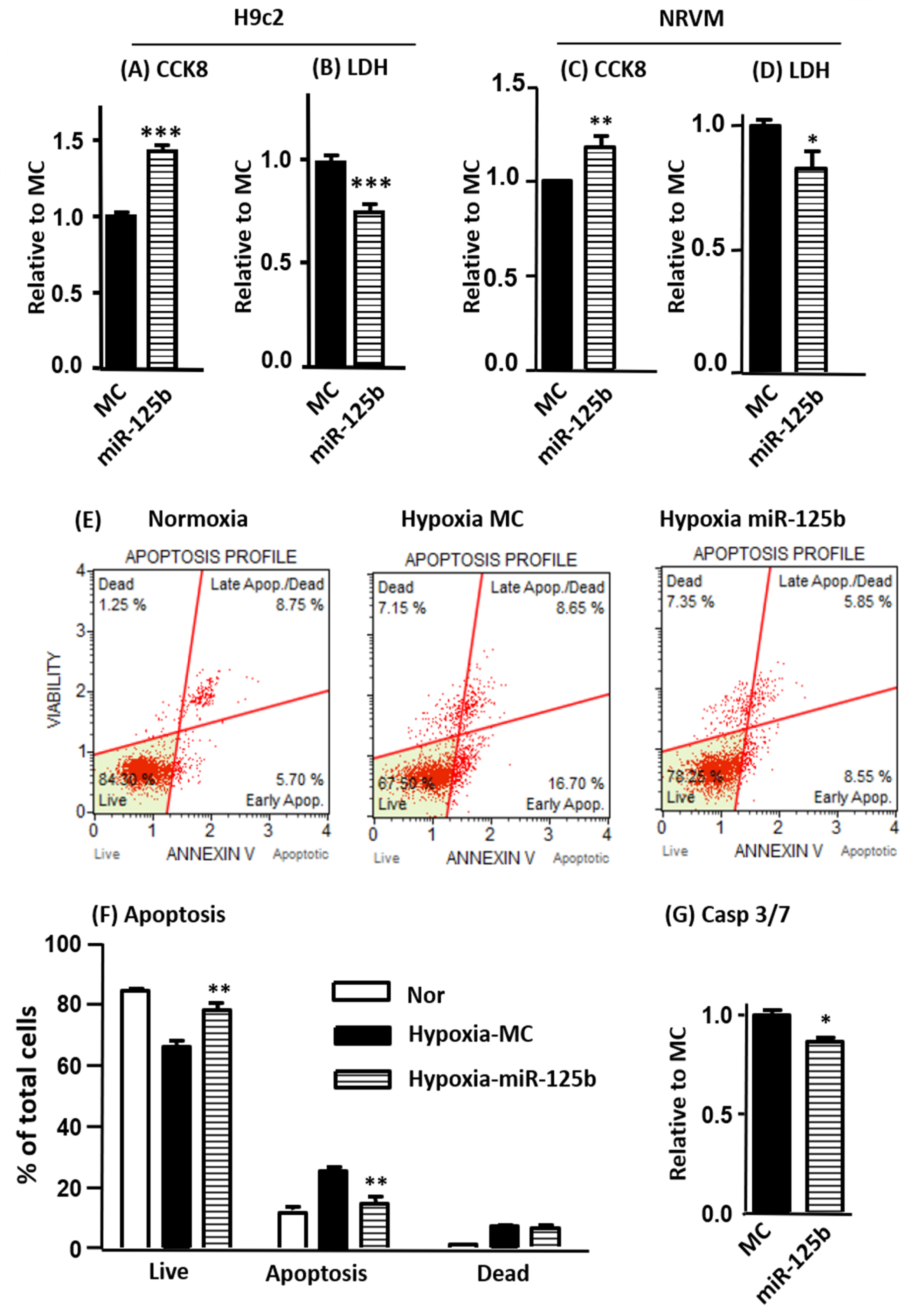
2.1. The Overexpression of miR-125b Reduces Cell Injury and Inhibits Apoptosis
2.2. The Overexpression of miR-125b in H9C2 Cells Down-Regulates Multiple Pro-Apoptotic Genes
2.3. The Assessment of miR-125b isomiRs Using NGS and Validation Using RT-qPCR
2.4. miR-125b and isomiRs Are Protective but Trim AG and Trim AGU Are Less Effective than Others
2.5. Profiling of miR-125b and isomiRs Induced Gene Regulation under Normoxic and Hypoxic Conditions
2.6. Gene Expression Validation Using RT-qPCR
3. Discussion
4. Materials and Methods
4.1. H9C2 Cell Culture, NRVM Isolation, miRNA Mimic Transfection and Hypoxia Treatment
4.2. Cell Injury Assessments with Lactate Dehydrogenase Release, CCK-8, and Apoptosis
4.3. In Vivo Model
4.4. miRNA and mRNA Sequencing
4.5. Analysis of miR-125b isomiRs
4.6. Real-Time qPCR to Detect and Quantify miRNAs, isomiRs and mRNAs
4.7. Western Blot
4.8. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef] [PubMed]
- van Rooij, E.; Olson, E.N. MicroRNA therapeutics for cardiovascular disease: Opportunities and obstacles. Nat. Rev. Drug Discov. 2012, 11, 860–872. [Google Scholar] [CrossRef] [PubMed]
- Barwari, T.; Joshi, A.; Mayr, M. MicroRNAs in Cardiovascular Disease. J. Am. Coll. Cardiol. 2016, 68, 2577–2584. [Google Scholar] [CrossRef] [PubMed]
- Laggerbauer, B.; Engelhardt, S. MicroRNAs as therapeutic targets in cardiovascular disease. J. Clin. Investig. 2022, 132. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A.; Griffiths-Jones, S.; Ashurst, J.L.; Bradley, A. Identification of mammalian microRNA host genes and transcription units. Genome Res. 2004, 14, 1902–1910. [Google Scholar] [CrossRef] [PubMed]
- Marques, F.Z.; Vizi, D.; Khammy, O.; Mariani, J.A.; Kaye, D.M. The transcardiac gradient of cardio-microRNAs in the failing heart. Eur. J. Heart Fail. 2016, 18, 1000–1008. [Google Scholar] [CrossRef]
- Wang, X.; Ha, T.; Zou, J.; Ren, D.; Liu, L.; Zhang, X.; Kalbfleisch, J.; Gao, X.; Williams, D.; Li, C. MicroRNA-125b protects against myocardial ischaemia/reperfusion injury via targeting p53-mediated apoptotic signalling and TRAF6. Cardiovasc. Res. 2014, 102, 385–395. [Google Scholar] [CrossRef]
- Bayoumi, A.S.; Park, K.-M.; Wang, Y.; Teoh, J.-P.; Aonuma, T.; Tang, Y.; Su, H.; Weintraub, N.L.; Kim, I.-M. A carvedilol-responsive microRNA, miR-125b-5p protects the heart from acute myocardial infarction by repressing pro-apoptotic bak1 and klf13 in cardiomyocytes. J. Mol. Cell Cardiol. 2018, 114, 72–82. [Google Scholar] [CrossRef]
- Tomasello, L.; Distefano, R.; Nigita, G.; Croce, C.M. The MicroRNA Family Gets Wider: The IsomiRs Classification and Role. Front. Cell Dev. Biol. 2021, 9, 668648. [Google Scholar] [CrossRef]
- Cloonan, N.; Wani, S.; Xu, Q.; Gu, J.; Lea, K.; Heater, S.; Barbacioru, C.; Steptoe, A.L.; Martin, H.C.; Nourbakhsh, E.; et al. MicroRNAs and their isomiRs function cooperatively to target common biological pathways. Genome Biol. 2011, 12, R126. [Google Scholar] [CrossRef]
- Haseeb, A.; Makki, M.S.; Khan, N.M.; Ahmad, I.; Haqqi, T.M. Deep sequencing and analyses of miRNAs, isomiRs and miRNA induced silencing complex (miRISC)-associated miRNome in primary human chondrocytes. Sci. Rep. 2017, 7, 15178. [Google Scholar] [CrossRef] [PubMed]
- Olejniczak, M.; Kotowska-Zimmer, A.; Krzyzosiak, W. Stress-induced changes in miRNA biogenesis and functioning. Cell Mol. Life Sci. 2018, 75, 177–191. [Google Scholar] [CrossRef] [PubMed]
- van der Kwast, R.; Woudenberg, T.; Quax, P.H.A.; Nossent, A.Y. MicroRNA-411 and Its 5′-IsomiR Have Distinct Targets and Functions and Are Differentially Regulated in the Vasculature under Ischemia. Mol. Ther. 2020, 28, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Yang, H.D.; Seo, J.W.; Nam, J.W.; Nam, S.W. hnRNPC induces isoform shifts in miR-21-5p leading to cancer development. Exp. Mol. Med. 2022, 54, 812–824. [Google Scholar] [CrossRef] [PubMed]
- Kingston, E.R.; Bartel, D.P. Global analyses of the dynamics of mammalian microRNA metabolism. Genome Res. 2019, 29, 1777–1790. [Google Scholar] [CrossRef] [PubMed]
- Neilsen, C.T.; Goodall, G.J.; Bracken, C.P. IsomiRs—The overlooked repertoire in the dynamic microRNAome. Trends Genet. 2012, 28, 544–549. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Pillman, K.A.; Neilsen, C.T.; Toubia, J.; Lawrence, D.M.; Tsykin, A.; Gantier, M.P.; Callen, D.F.; Goodall, G.J.; Bracken, C.P. Naturally existing isoforms of miR-222 have distinct functions. Nucleic Acids Res. 2017, 45, 11371–11385. [Google Scholar] [CrossRef]
- Wong, L.L.; Rademaker, M.T.; Saw, E.L.; Lew, K.S.; Ellmers, L.J.; Charles, C.J.; Richards, A.M.; Wang, P. Identification of novel microRNAs in the sheep heart and their regulation in heart failure. Sci. Rep. 2017, 7, 8250. [Google Scholar] [CrossRef]
- Zhou, Y.; Richards, A.M.; Wang, P. MicroRNA-221 Is Cardioprotective and Anti-fibrotic in a Rat Model of Myocardial Infarction. Mol. Ther. Nucleic Acids 2019, 17, 185–197. [Google Scholar] [CrossRef]
- Wang, Y.; Tan, J.; Wang, L.; Pei, G.; Cheng, H.; Zhang, Q.; Wang, S.; He, C.; Fu, C.; Wei, Q. MiR-125 Family in Cardiovascular and Cerebrovascular Diseases. Front. Cell Dev. Biol. 2021, 9, 799049. [Google Scholar] [CrossRef]
- Ambros, V.; Horvitz, H.R. Heterochronic mutants of the nematode Caenorhabditis elegans. Science 1984, 226, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Wightman, B.; Ha, I.; Ruvkun, G. Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans. Cell 1993, 75, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Sempere, L.F.; Freemantle, S.; Pitha-Rowe, I.; Moss, E.; Dmitrovsky, E.; Ambros, V. Expression profiling of mammalian microRNAs uncovers a subset of brain-expressed microRNAs with possible roles in murine and human neuronal differentiation. Genome Biol. 2004, 5, R13. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Liu, T.; He, Y.; Yan, Q.; Chen, X.; Wang, H. MiR-125b promotes proliferation and migration of type II endometrial carcinoma cells through targeting TP53INP1 tumor suppressor in vitro and in vivo. BMC Cancer 2011, 11, 425. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Richards, A.M.; Wang, P. Characterization and Standardization of Cultured Cardiac Fibroblasts for Ex Vivo Models of Heart Fibrosis and Heart Ischemia. Tissue Eng. Part C Methods 2017, 23, 422–433. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, D.; Gao, X.; Lew, K.; Richards, A.M.; Wang, P. mTORC2 phosphorylation of Akt1: A possible mechanism for hydrogen sulfide-induced cardioprotection. PLoS ONE 2014, 9, e99665. [Google Scholar] [CrossRef]
- Telonis, A.G.; Loher, P.; Jing, Y.; Londin, E.; Rigoutsos, I. Beyond the one-locus-one-miRNA paradigm: MicroRNA isoforms enable deeper insights into breast cancer heterogeneity. Nucleic Acids Res. 2015, 43, 9158–9175. [Google Scholar] [CrossRef]
- Starega-Roslan, J.; Witkos, T.M.; Galka-Marciniak, P.; Krzyzosiak, W.J. Sequence features of Drosha and Dicer cleavage sites affect the complexity of isomiRs. Int. J. Mol. Sci. 2015, 16, 8110–8127. [Google Scholar] [CrossRef]
- Wyman, S.K.; Knouf, E.C.; Parkin, R.K.; Fritz, B.R.; Lin, D.W.; Dennis, L.M.; Krouse, M.A.; Webster, P.J.; Tewari, M. Post-transcriptional generation of miRNA variants by multiple nucleotidyl transferases contributes to miRNA transcriptome complexity. Genome Res. 2011, 21, 1450–1461. [Google Scholar] [CrossRef]
- Wu, C.W.; Evans, J.M.; Huang, S.; Mahoney, D.W.; Dukek, B.A.; Taylor, W.R.; Yab, T.C.; Smyrk, T.C.; Jen, J.; Kisiel, J.B.; et al. A Comprehensive Approach to Sequence-oriented IsomiR annotation (CASMIR): Demonstration with IsomiR profiling in colorectal neoplasia. BMC Genom. 2018, 19, 401. [Google Scholar] [CrossRef]
- Zhiyanov, A.; Nersisyan, S.; Tonevitsky, A. Hairpin sequence and structure is associated with features of isomiR biogenesis. RNA Biol. 2021, 18, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Loher, P.; Londin, E.R.; Rigoutsos, I. IsomiR expression profiles in human lymphoblastoid cell lines exhibit population and gender dependencies. Oncotarget 2014, 5, 8790–8802. [Google Scholar] [CrossRef] [PubMed]
- Telonis, A.G.; Magee, R.; Loher, P.; Chervoneva, I.; Londin, E.; Rigoutsos, I. Knowledge about the presence or absence of miRNA isoforms (isomiRs) can successfully discriminate amongst 32 TCGA cancer types. Nucleic Acids Res. 2017, 45, 2973–2985. [Google Scholar] [CrossRef] [PubMed]
- Tan, G.C.; Chan, E.; Molnar, A.; Sarkar, R.; Alexieva, D.; Isa, I.M.; Robinson, S.; Zhang, S.; Ellis, P.; Langford, C.F.; et al. 5′isomiR variation is of functional and evolutionary importance. Nucleic Acids Res. 2014, 42, 9424–9435. [Google Scholar] [CrossRef] [PubMed]
- Martí, E.; Pantano, L.; Bañez-Coronel, M.; Llorens, F.; Miñones-Moyano, E.; Porta, S.; Sumoy, L.; Ferrer, I.; Estivill, X. A myriad of miRNA variants in control and Huntington’s disease brain regions detected by massively parallel sequencing. Nucleic Acids Res. 2010, 38, 7219–7235. [Google Scholar] [CrossRef]
- Boele, J.; Persson, H.; Shin, J.W.; Ishizu, Y.; Newie, I.S.; Søkilde, R.; Hawkins, S.M.; Coarfa, C.; Ikeda, K.; Takayama, K.-I.; et al. PAPD5-mediated 3′ adenylation and subsequent degradation of miR-21 is disrupted in proliferative disease. Proc. Natl. Acad. Sci. USA 2014, 111, 11467–11472. [Google Scholar] [CrossRef]
- Gutiérrez-Vázquez, C.; Enright, A.J.; Rodríguez-Galán, A.; Pérez-García, A.; Collier, P.; Jones, M.R.; Benes, V.; Mizgerd, J.P.; Mittelbrunn, M.; Ramiro, A.R.; et al. 3′ Uridylation controls mature microRNA turnover during CD4 T-cell activation. RNA 2017, 23, 882–891. [Google Scholar] [CrossRef]
- Jones, M.R.; Quinton, L.J.; Blahna, M.T.; Neilson, J.R.; Fu, S.; Ivanov, A.R.; Wolf, D.A.; Mizgerd, J.P. Zcchc11-dependent uridylation of microRNA directs cytokine expression. Nat. Cell Biol. 2009, 11, 1157–1163. [Google Scholar] [CrossRef]
- Yang, A.; Bofill-De Ros, X.; Shao, T.-J.; Jiang, M.; Li, K.; Villanueva, P.; Dai, L.; Gu, S. 3′ Uridylation Confers miRNAs with Non-canonical Target Repertoires. Mol. Cell 2019, 75, 511–522.e4. [Google Scholar] [CrossRef]
- Fotakis, G.; Timbrell, J.A. In vitro cytotoxicity assays: Comparison of LDH, neutral red, MTT and protein assay in hepatoma cell lines following exposure to cadmium chloride. Toxicol. Lett. 2006, 160, 171–177. [Google Scholar] [CrossRef]







| IsomiR RT-qPCR Primer Sequences | ||
|---|---|---|
| IsomiR | Forward Sequence (5′–3′) | Reverse Sequence (5′–3′) |
| U6 | CAGCACATATACTAAAATTGGAACG | ACGAATTTGCGTGTCATCC |
| hsa-miR-125b-5p mimic | ACTGATAAATCCCTGAGACCCTAAC | TATGGTTTTGACGACTGTGTGAT |
| hsa-miR-125b-5p mimic A 3prime | TGCGTCCCTGAGACCCTAACT | TATGGTTGTTGACGACTGGTTGAC |
| hsa-miR-125b-5p mimic trim1 | AATCGCTCCCTGAGACCCTAA | TATGGTTGTTGACGACTGGTTGAC |
| hsa-miR-125b-5p mimic trim2 | CAGCGTTCCCTGAGACCCTA | TATGGTTGTTGACGACTGGTTGAC |
| hsa-miR-125b-5p mimic trim3 | CTACGGAATCCCTGAGACCCT | TATGGTTGTTGACGACTGGTTGAC |
| mRNA RT-qPCR primer sequences | ||
| Gene | Forward sequence | Reverse sequence |
| β-actin | GTACAACCTTCTTGCAGCTCCTC | TGACCCATACCCACCATCAC |
| Bak1 | GCCTACGAACTCTTCACCAAG | CACGCTGGTAGACATACAGG |
| Bim | AGACGAGTTCAATGAGACTTACAC | CGGAAGATGAATCGTAACAGTTG |
| Bmf | TTGCAGACCAGTTCCATCG | CCCTTCCCTGTTTTCTCGTC |
| Traf6 | CCTCATAAGAGAACAGATGCCT | CGTGCCAAGTGATTCCTCT |
| HIF1an | ACATTGAGAAGATGCTTGGAGAG | CATGTGGACAGGATAGCAGTC |
| Tnfrsf1b | PrimeTime® qPCR Primer Assay (Assay ID: Rn.PT.58.35970749; Integrated DNA Technologies, Inc., Singapore) | |
| Tp53 | PrimeTime® qPCR Primer Assay (Assay ID: Rn.PT.58.35888325; Integrated DNA Technologies, Inc., Singapore) | |
| Tp53inp1 | TCCTGGTCTCAGTGAAGCTA | ACAGCAGTGAATGTGCGT |
| Tp53inp2 | CACCTTCCCCTCACCCT | CCTTCTCCAGCAGCACAG |
| Dram2 | ATTGCCTTACATCAGCGACA | GGTTCTCTTCAGGGTTCAGTG |
| Puma | GCAGTACGAGCGGCGGAGACAAGAAGAGC | CCCTGGGTAAGGGGAGGAGTCCCATGAAGAG |
| Casp2 | GTCCAAGTCTACAGAACAAACCA | CAGCATCACTCTCCTCACATC |
| Tnnt2 | CGAGCAGCAGCGTATTCGC | CAGCCTTCCTCCTGTTCTCCTC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wong, L.L.; Fadzil, A.B.; Chen, Q.; Rademaker, M.T.; Charles, C.J.; Richards, A.M.; Wang, P. Interrogating the Role of miR-125b and Its 3′isomiRs in Protection against Hypoxia. Int. J. Mol. Sci. 2023, 24, 16015. https://doi.org/10.3390/ijms242116015
Wong LL, Fadzil AB, Chen Q, Rademaker MT, Charles CJ, Richards AM, Wang P. Interrogating the Role of miR-125b and Its 3′isomiRs in Protection against Hypoxia. International Journal of Molecular Sciences. 2023; 24(21):16015. https://doi.org/10.3390/ijms242116015
Chicago/Turabian StyleWong, Lee Lee, Azizah Binti Fadzil, Qiying Chen, Miriam T. Rademaker, Christopher J. Charles, Arthur Mark Richards, and Peipei Wang. 2023. "Interrogating the Role of miR-125b and Its 3′isomiRs in Protection against Hypoxia" International Journal of Molecular Sciences 24, no. 21: 16015. https://doi.org/10.3390/ijms242116015





