Skeletal Morphogenesis and Anomalies in Gilthead Seabream: A Comprehensive Review
Abstract
1. Introduction
2. Skeletogenesis
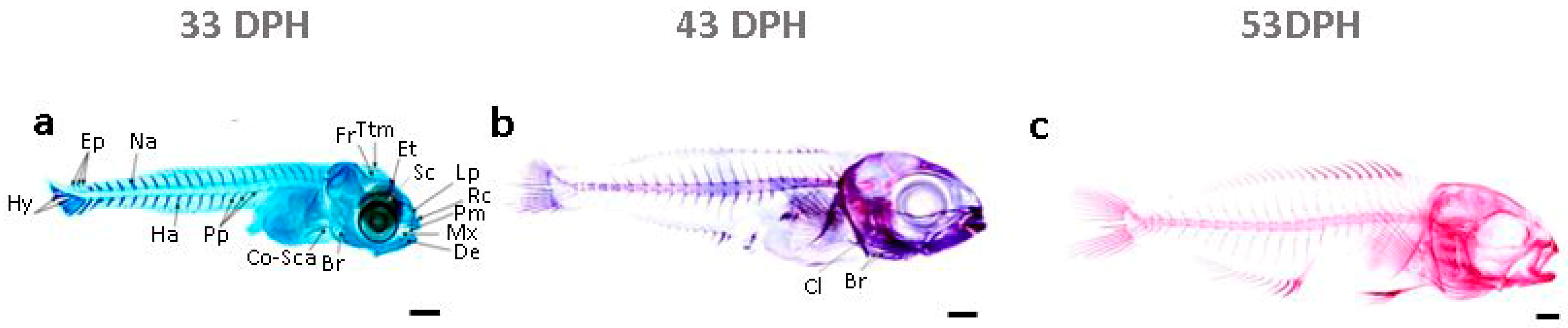
2.1. The Ossification Pattern
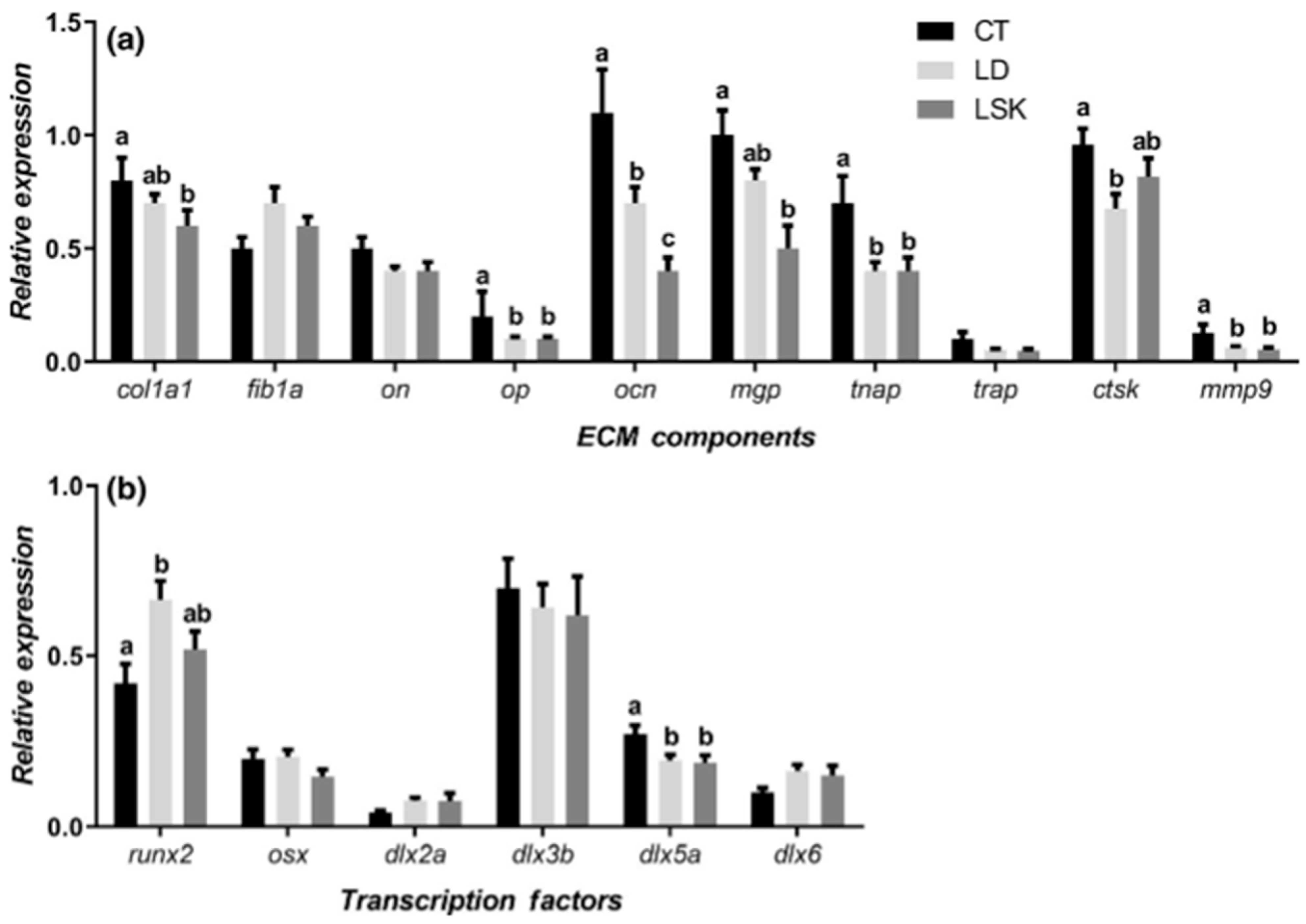
2.2. The Genetics of Skeletogenesis
3. Bone Deformities
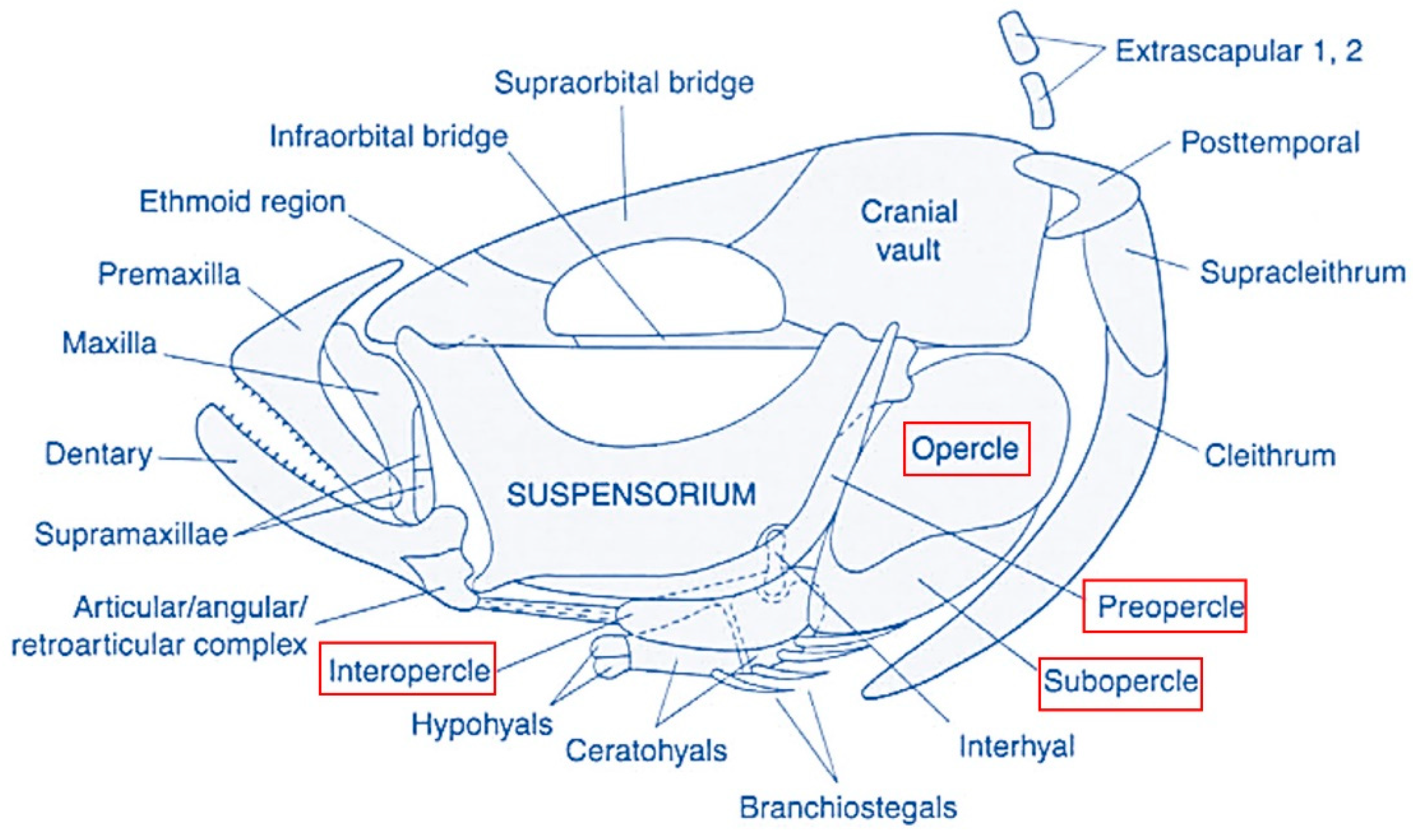
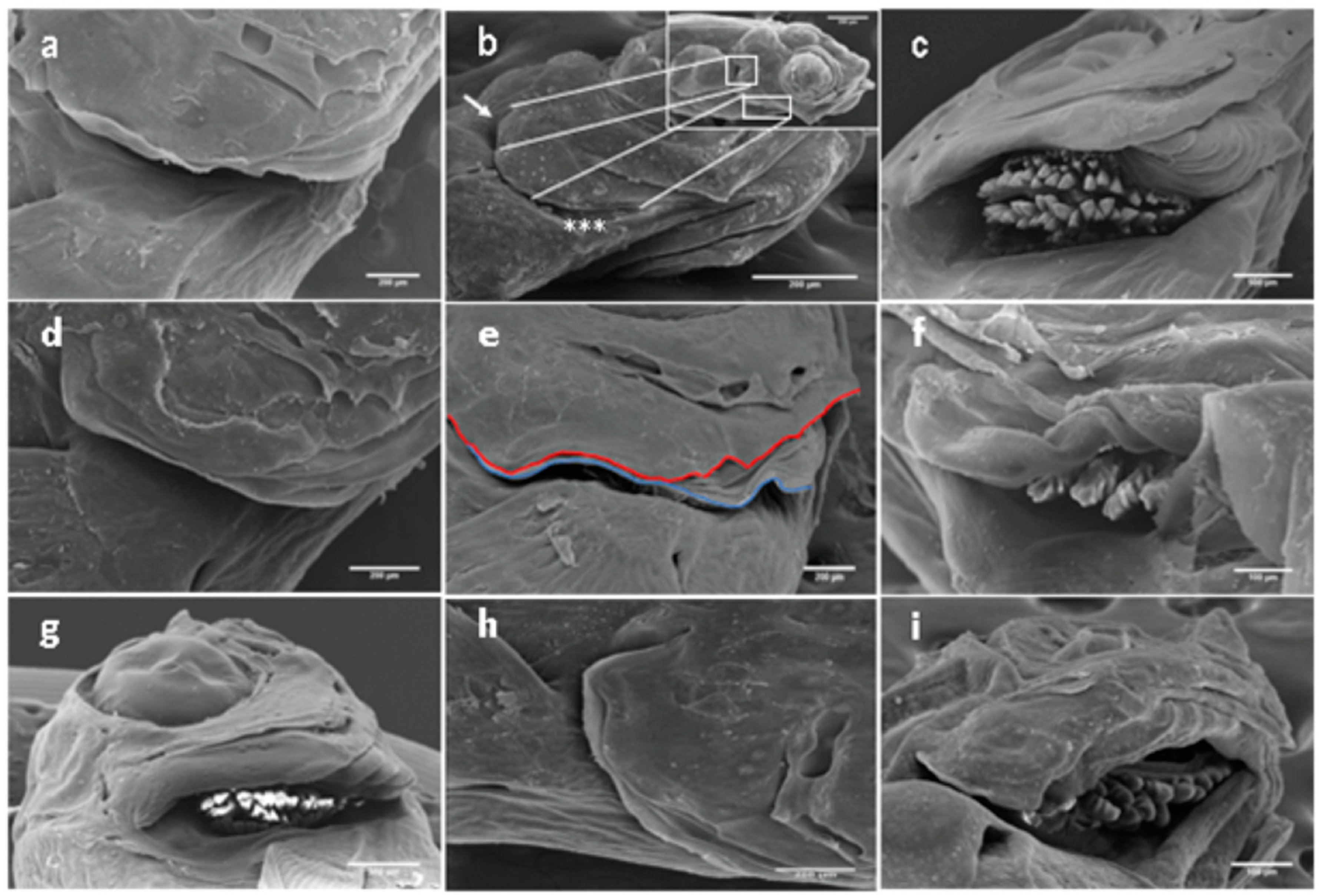
3.1. The Opercular Complex Deformities
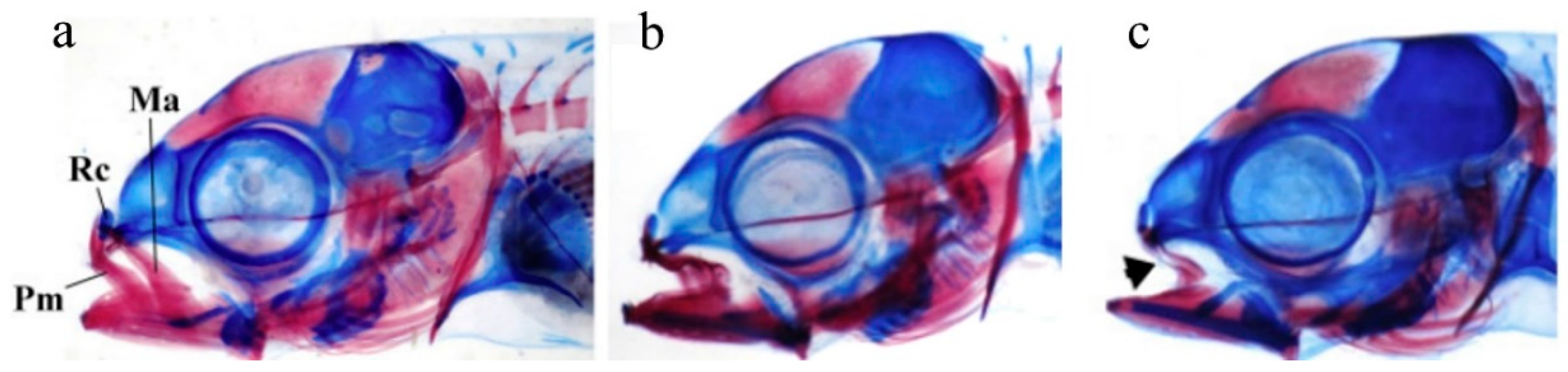
3.2. Other Cranial Deformities
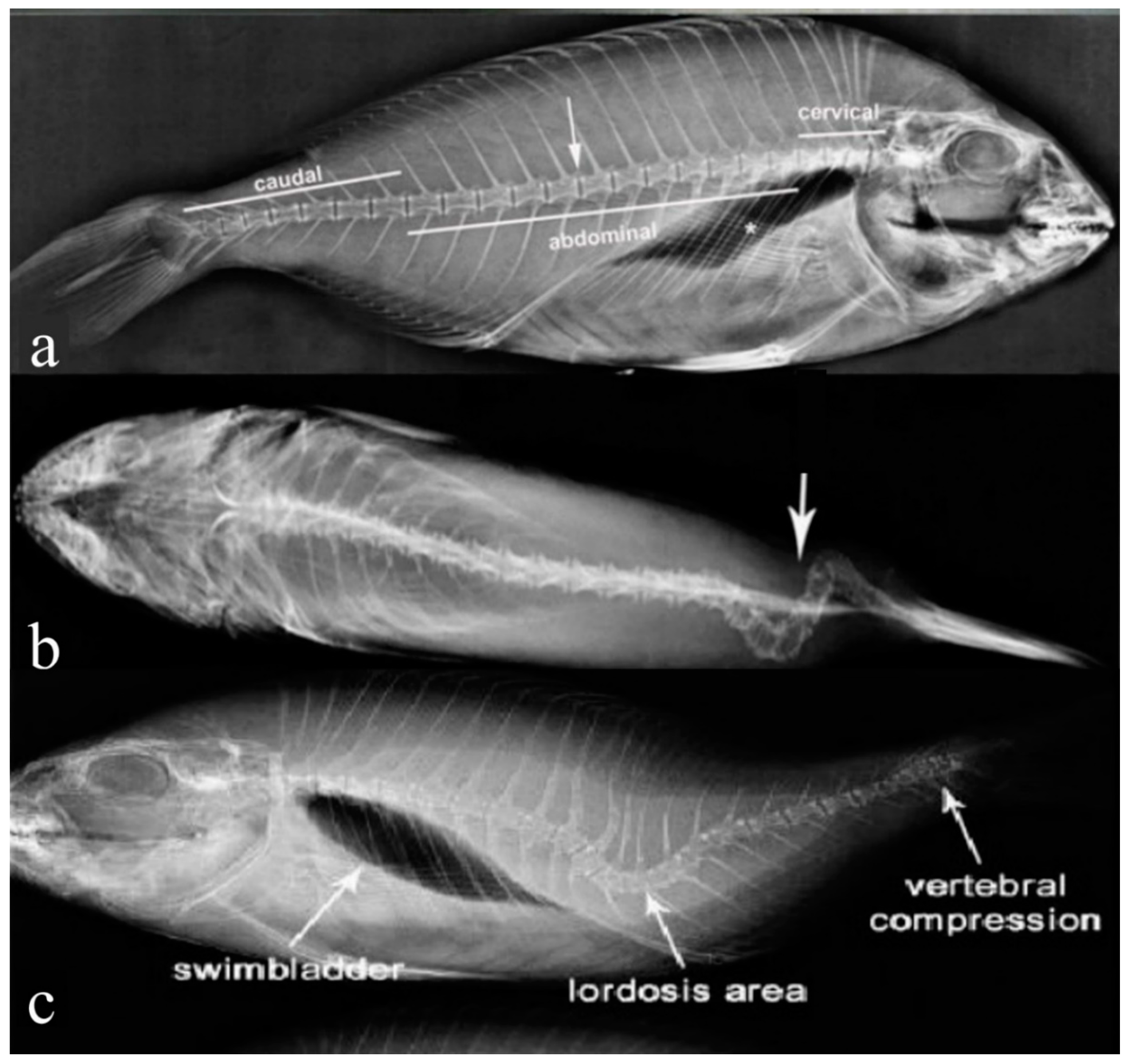
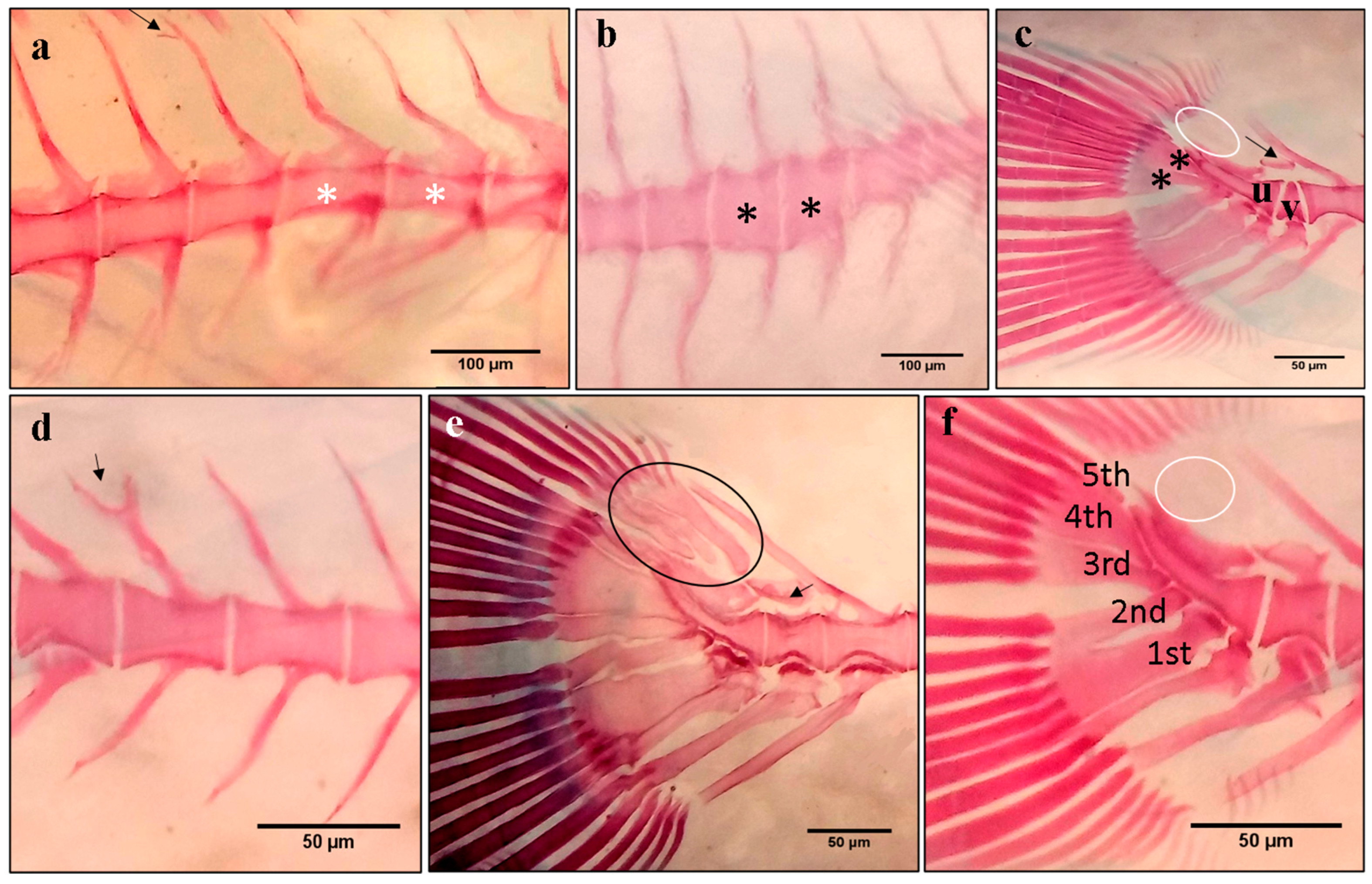
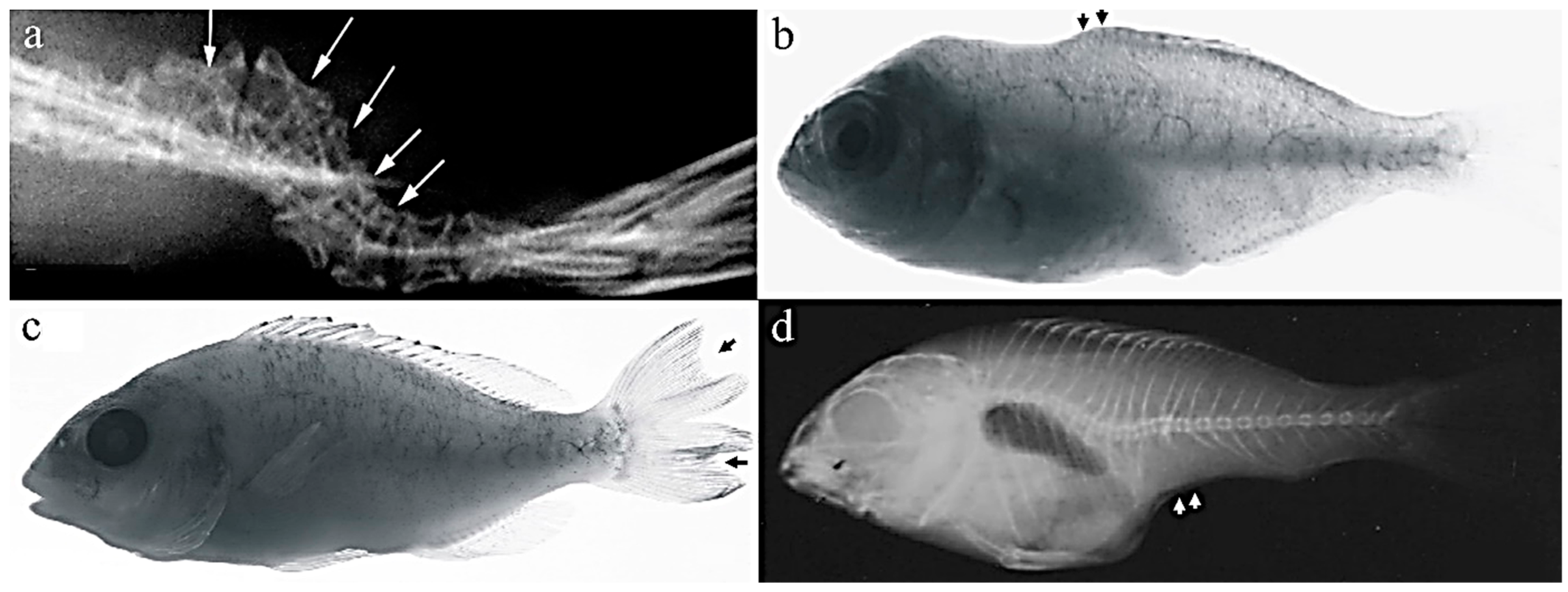
3.3. Spinal Deformity
3.4. Caudal Fin Deformity
3.5. Body Shape Deformities
4. Cause of Deformities
5. Effect of Deformities on Production and Welfare
6. The Genetic Component of Bone Deformities
7. Mitigating Skeletal Abnormalities in Gilthead Seabream: Strategies for a Sustainable Aquaculture
8. Perspective for Investigating Bone Defects
9. Gilthead Seabream as a Model for Understanding Skeletogenesis
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abbate, F.; Guerrera, M.C.; Levanti, M.; Laurà, R.; Aragona, M.; Mhalhel, K.; Montalbano, G.; Germanà, A. Morphological Characteristics of the Blackspot Seabream (Pagellus bogaraveo) Tongue: A Structural and Immunohistochemical Study. Anat. Histol. Embryol. 2022, 51, 103–111. [Google Scholar] [CrossRef]
- Dellacqua, Z.; Di Biagio, C.; Costa, C.; Pousão-Ferreira, P.; Ribeiro, L.; Barata, M.; Gavaia, P.J.; Mattei, F.; Fabris, A.; Izquierdo, M.; et al. Distinguishing the Effects of Water Volumes versus Stocking Densities on the Skeletal Quality during the Pre-Ongrowing Phase of Gilthead Seabream (Sparus aurata). Animals 2023, 13, 557. [Google Scholar] [CrossRef] [PubMed]
- Espírito-Santo, C.; Guardiola, F.A.; Ozório, R.O.A.; Magnoni, L.J. Short-Term Swimming up-Regulates pro-Inflammatory Mediators and Cytokines in Gilthead Seabream (Sparus aurata). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2023, 284, 111487. [Google Scholar] [CrossRef]
- Guerrera, M.C.; Aragona, M.; Briglia, M.; Porcino, C.; Mhalhel, K.; Cometa, M.; Abbate, F.; Montalbano, G.; Laurà, R.; Levanti, M.; et al. The Alimentary Tract of African Bony-Tongue, Heterotis Niloticus (Cuvier, 1829): Morphology Study. Animals 2022, 12, 1565. [Google Scholar] [CrossRef]
- Mhalhel, K.; Montalbano, G.; Giurdanella, G.; Abbate, F.; Laurà, R.; Guerrera, M.C.; Germanà, A.; Levanti, M. Histological and Immunohistochemical Study of Gilthead Seabream Tongue from the Early Stage of Development: TRPV4 Potential Roles. Ann. Anat. 2022, 244, 151985. [Google Scholar] [CrossRef]
- Sola, L.; Moretti, A.; Crosetti, D.; Karaiskou, N.; Magoulas, A.; Rossi, A.R.; Rye, M.; Triantafyllidis, A.; Tsigenopoulos, C.S. Gilthead Seabream—Sparus aurata. Genet. Impact Aquac. Act. Nativ. Popul. 2007, 47–54. Available online: https://www.vliz.be/imisdocs/publications/318461.pdf (accessed on 2 July 2023).
- Verhaegen, Y.; Adriaens, D.; Wolf, T.D.; Dhert, P.; Sorgeloos, P. Deformities in Larval Gilthead Sea Bream (Sparus aurata): A Qualitative and Quantitative Analysis Using Geometric Morphometrics. Aquaculture 2007, 268, 156–168. [Google Scholar] [CrossRef]
- Sadek, S.; Osman, M.F.; Mansour, M.A. Growth, Survival and Feed Conversion Rates of Sea Bream (Sparus aurata) Cultured in Earthen Brackish Water Ponds Fed Different Feed Types. Aquac. Int. 2004, 12, 409–421. [Google Scholar] [CrossRef]
- Kır, M. Thermal Tolerance and Standard Metabolic Rate of Juvenile Gilthead Seabream (Sparus aurata) Acclimated to Four Temperatures. J. Therm. Biol. 2020, 93, 102739. [Google Scholar] [CrossRef] [PubMed]
- Manchado, M.; Planas, J.V.; Cousin, X.; Rebordinos, L.; Claros, M.G. 8-Current Status in Other Finfish Species: Description of Current Genomic Resources for the Gilthead Seabream (Sparus aurata) and Soles (Solea Senegalensis and Solea Solea). In Genomics in Aquaculture; MacKenzie, S., Jentoft, S., Eds.; Academic Press: San Diego, CA, USA, 2016; pp. 195–221. ISBN 978-0-12-801418-9. [Google Scholar]
- Beraldo, P.; Canavese, B. Recovery of Opercular Anomalies in Gilthead Sea Bream, Sparus aurata L.: Morphological and Morphometric Analysis. J. Fish Dis. 2011, 34, 21–30. [Google Scholar] [CrossRef]
- Mhalhel, K.; Germanà, A.; Abbate, F.; Guerrera, M.C.; Levanti, M.; Laurà, R.; Montalbano, G. The Effect of Orally Supplemented Melatonin on Larval Performance and Skeletal Deformities in Farmed Gilthead Seabream (Sparus aurata). Int. J. Mol. Sci. 2020, 21, 9597. [Google Scholar] [CrossRef] [PubMed]
- Negrín-Báez, D.; Navarro, A.; Lee-Montero, I.; Soula, M.; Afonso, J.M.; Zamorano, M.J. Inheritance of Skeletal Deformities in Gilthead Seabream (Sparus aurata)-Lack of Operculum, Lordosis, Vertebral Fusion and LSK Complex. J. Anim. Sci. 2015, 93, 53–61. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ortiz-Delgado, J.B.; Fernández, I.; Sarasquete, C.; Gisbert, E. Normal and Histopathological Organization of the Opercular Bone and Vertebrae in Gilthead Sea Bream Sparus aurata. Aquat. Biol. 2014, 21, 67–84. [Google Scholar] [CrossRef]
- Sfakianakis, D.G.; Katharios, P.; Tsirigotakis, N.; Doxa, C.K.; Kentouri, M. Lateral Line Deformities in Wild and Farmed Sea Bass (Dicentrarchus labrax, L.) and Sea Bream (Sparus aurata, L.). J. Appl. Ichthyol. 2013, 29, 1015–1021. [Google Scholar] [CrossRef]
- Al-Harbi, A.H. Skeletal Deformities in Cultured Common Carp Cyprinus carpio L. Asian Fish. Sci. 2001, 14, 247–254. [Google Scholar] [CrossRef]
- Galeotti, M.; Beraldo, P.; de Dominis, S.; D’Angelo, L.; Ballestrazzi, R.; Musetti, R.; Pizzolito, S.; Pinosa, M. A Preliminary Histological and Ultrastructural Study of Opercular Anomalies in Gilthead Sea Bream Larvae (Sparus aurata). Fish Physiol. Biochem. 2000, 22, 151–157. [Google Scholar] [CrossRef]
- Berillis, P. Skeletal Deformities in Seabreams. Understanding the Genetic Origin Can Improve Production? J. Fish. Com. 2017, 11, 57. [Google Scholar] [CrossRef]
- Faustino, M.; Power, D.M. Osteologic Development of the Viscerocranial Skeleton in Sea Bream: Alternative Ossification Strategies in Teleost Fish. J. Fish Biol. 2001, 58, 537–572. [Google Scholar] [CrossRef]
- Vermeylen, V.; Kegel, B.; De Wolf, T.; Adriaens, D. Skeletal Deformities in Gilthead Seabream (Sparus aurata): Exploring the Association between Mechanical Loading and Opercular Deformation. Belg. J. Zool. 2023, 153, 81–104. [Google Scholar] [CrossRef]
- Shahar, R.; Dean, M.N. The Enigmas of Bone without Osteocytes. Bonekey Rep. 2013, 2, 343. [Google Scholar] [CrossRef]
- Ytteborg, E.; Baeverfjord, G.; Torgersen, J.; Hjelde, K.; Takle, H. Molecular Pathology of Vertebral Deformities in Hyperthermic Atlantic Salmon (Salmo Salar). BMC Physiol. 2010, 10, 12. [Google Scholar] [CrossRef]
- Boglione, C.; Gisbert, E.; Gavaia, P.E.; Witten, P.; Moren, M.; Fontagné, S.; Koumoundouros, G. Skeletal Anomalies in Reared European Fish Larvae and Juveniles. Part 2: Main Typologies, Occurrences and Causative Factors. Rev. Aquac. 2013, 5, S121–S167. [Google Scholar] [CrossRef]
- Mahamid, J.; Sharir, A.; Addadi, L.; Weiner, S. Amorphous Calcium Phosphate Is a Major Component of the Forming Fin Bones of Zebrafish: Indications for an Amorphous Precursor Phase. Proc. Natl. Acad. Sci. USA 2008, 105, 12748–12753. [Google Scholar] [CrossRef]
- Saka; Şahin; Çoban, D.; Kamacı, H.O.; Süzer, C.; Fırat, K. Early Development of Cephalic Skeleton in Hatchery-Reared Gilthead Seabream, Sparus aurata. Turk. J. Fish. Aquat. Sci. 2008, 8, 341–345. [Google Scholar]
- Prestinicola, L.; Boglione, C.; Makridis, P.; Spanò, A.; Rimatori, V.; Palamara, E.; Scardi, M.; Cataudella, S. Environmental Conditioning of Skeletal Anomalies Typology and Frequency in Gilthead Seabream (Sparus aurata L., 1758) Juveniles. PLoS ONE 2013, 8, e55736. [Google Scholar] [CrossRef] [PubMed]
- Gavaia, P.J.; Sarasquete, C.; Cancela, M.L. Detection of Mineralized Structures in Early Stages of Development of Marine Teleostei Using a Modified Alcian Blue-Alizarin Red Double Staining Technique for Bone and Cartilage. Biotech. Histochem. 2000, 75, 79–84. [Google Scholar] [CrossRef]
- Fernández, I.; Darias, M.; Andree, K.B.; Mazurais, D.; Zambonino-Infante, J.L.; Gisbert, E. Coordinated Gene Expression during Gilthead Sea Bream Skeletogenesis and Its Disruption by Nutritional Hypervitaminosis A. BMC Dev. Biol. 2011, 11, 7. [Google Scholar] [CrossRef]
- Sarropoulou, E.; Kotoulas, G.; Power, D.M.; Geisler, R. Gene Expression Profiling of Gilthead Sea Bream during Early Development and Detection of Stress-Related Genes by the Application of cDNA Microarray Technology. Physiol. Genom. 2005, 23, 182–191. [Google Scholar] [CrossRef]
- Tiago, D.M.; Laizé, V.; Bargelloni, L.; Ferraresso, S.; Romualdi, C.; Cancela, M.L. Global Analysis of Gene Expression in Mineralizing Fish Vertebra-Derived Cell Lines: New Insights into Anti-Mineralogenic Effect of Vanadate. BMC Genom. 2011, 12, 310. [Google Scholar] [CrossRef]
- Harris, M.P.; Henke, K.; Hawkins, M.B.; Witten, P.E. Fish Is Fish: The Use of Experimental Model Species to Reveal Causes of Skeletal Diversity in Evolution and Disease. J. Appl. Ichthyol. 2014, 30, 616–629. [Google Scholar] [CrossRef]
- Lorenzo-Felipe, Á.; Shin, H.S.; León-Bernabeu, S.; Pérez-García, C.; Zamorano, M.J.; Pérez-Sánchez, J.; Afonso-López, J.M. The Effect of the Deformity Genetic Background of the Breeders on the Spawning Quality of Gilthead Seabream (Sparus aurata L.). Front. Mar. Sci. 2021, 8, 656901. [Google Scholar] [CrossRef]
- Koumoundouros, G.; Oran, G.; Divanach, P.; Stefanakis, S.; Kentouri, M. The Opercular Complex Deformity in Intensive Gilthead Sea Bream (Sparus aurata L.) Larviculture. Moment of Apparition and Description. Aquaculture 1997, 156, 165–177. [Google Scholar] [CrossRef]
- Boglione, C.; Gavaia, P.; Koumoundouros, G.; Gisbert, E.; Moren, M.; Fontagné, S.; Witten, P.E. Skeletal Anomalies in Reared European Fish Larvae and Juveniles. Part 1: Normal and Anomalous Skeletogenic Processes. Rev. Aquac. 2013, 5, S99–S120. [Google Scholar] [CrossRef]
- Moretti, A.; Pedini Fernandez-Criado, M.; Cittolin, G.; Guidastri, R. Manual on Hatchery Production of Seabass and Gilthead Seabream; FAO: Rome, Italy, 1999; Volume 1. [Google Scholar]
- Peruzzi, S.; Westgaard, J.-I.; Chatain, B. Genetic Investigation of Swimbladder Inflation Anomalies in the European Sea Bass, Dicentrarchus labrax L. Aquaculture 2007, 265, 102–108. [Google Scholar] [CrossRef]
- Izquierdo, M.S.; Scolamacchia, M.; Betancor, M.; Roo, J.; Caballero, M.J.; Terova, G.; Witten, P.E. Effects of Dietary DHA and α-Tocopherol on Bone Development, Early Mineralisation and Oxidative Stress in Sparus aurata (Linnaeus, 1758) Larvae. Br. J. Nutr. 2013, 109, 1796–1805. [Google Scholar] [CrossRef] [PubMed]
- Fernández, I.; Hontoria, F.; Ortiz-Delgado, J.B.; Kotzamanis, Y.; Estévez, A.; Zambonino-Infante, J.L.; Gisbert, E. Larval Performance and Skeletal Deformities in Farmed Gilthead Sea Bream (Sparus aurata) Fed with Graded Levels of Vitamin A Enriched Rotifers (Brachionus plicatilis). Aquaculture 2008, 283, 102–115. [Google Scholar] [CrossRef]
- Roo, J.; Socorro, J.; Izquierdo, M.S. Effect of Rearing Techniques on Skeletal Deformities and Osteological Development in Red Porgy Pagrus Pagrus (Linnaeus, 1758) Larvae. J. Appl. Ichthyol. 2010, 26, 372–376. [Google Scholar] [CrossRef]
- Georgakopoulou, E.; Katharios, P.; Divanach, P.; Koumoundouros, G. Effect of Temperature on the Development of Skeletal Deformities in Gilthead Seabream (Sparus aurata Linnaeus, 1758). Aquaculture 2010, 308, 13–19. [Google Scholar] [CrossRef]
- Huysseune, A. Chapter 18-Skeletal System. In The Laboratory Fish; Ostrander, G.K., Ed.; Academic Press: London, UK, 2000; pp. 307–317. ISBN 1874480X. [Google Scholar]
- Andrades, J.A.; Becerra, J.; Fernández-Llebrez, P. Skeletal Deformities in Larval, Juvenile and Adult Stages of Cultured Gilthead Sea Bream (Sparus aurata L.). Aquaculture 1996, 141, 1–11. [Google Scholar] [CrossRef]
- Beraldo, P.; Pinosa, M.; Tibaldi, E.; Canavese, B. Abnormalities of the Operculum in Gilthead Sea Bream (Sparus aurata): Morphological Description. Aquaculture 2003, 220, 89–99. [Google Scholar] [CrossRef]
- Fragkoulis, S.; Batargias, C.; Kolios, P.; Koumoundouros, G. Genetic Parameters of the Upper-Jaw Abnormalities in Gilthead Seabream Sparus aurata. Aquaculture 2018, 497, 226–233. [Google Scholar] [CrossRef]
- Loizides, M.; Georgiou, A.N.; Somarakis, S.; Witten, P.E.; Koumoundouros, G. A New Type of Lordosis and Vertebral Body Compression in Gilthead Sea Bream, Sparus aurata L.: Aetiology, Anatomy and Consequences for Survival. J. Fish Dis. 2014, 37, 949–957. [Google Scholar] [CrossRef]
- Afonso, J.M.; Montero, D.; Robaina, L.; Astorga, N.; Izquierdo, M.S.; Gines, R. Association of a Lordosis-Scoliosis-Kyphosis Deformity in Gilthead Seabream (Sparus aurata) with Family Structure. Fish Physiol. Biochem. 2000, 22, 159–163. [Google Scholar] [CrossRef]
- Kranenbarg, S.; Waarsing, J.H.; Muller, M.; Weinans, H.; van Leeuwen, J.L. Lordotic Vertebrae in Sea Bass (Dicentrarchus labrax L.) Are Adapted to Increased Loads. J. Biomech. 2005, 38, 1239–1246. [Google Scholar] [CrossRef] [PubMed]
- Boursiaki, V.; Theochari, C.; Zaoutsos, S.P.; Mente, E.; Vafidis, D.; Apostologamvrou, C.; Berillis, P. Skeletal Deformity of Scoliosis in Gilthead Seabreams (Sparus aurata): Association with Changes to Calcium-Phosphor Hydroxyapatite Salts and Collagen Fibers. Water 2019, 11, 257. [Google Scholar] [CrossRef]
- Berillis, P. Factors that can lead to the development of skeletal deformities in fishes: A review. J. Fisheriessci. Com. 2015, 9, 17–23. [Google Scholar]
- Roo, J.; Hernandez-Cruz, M.C.; Socorro, J.A.; Fernández-Palacios, H.; Izquierdo, M.S. Development of Skeletal Deformities in Gilthead Sea Bream (Sparus aurata) Reared under Different Larval Culture and Dietary Conditions; University of Ghent: Gent, Belgium, 2005. [Google Scholar]
- Koumoundouros, G. Morpho-Anatomical Abnormalities in Mediterranean Marine Aquaculture. Recent Adv. Aquac. Res. 2010, 661, 125–148. [Google Scholar]
- Fragkoulis, S.; Printzi, A.; Geladakis, G.; Katribouzas, N.; Koumoundouros, G. Recovery of Haemal Lordosis in Gilthead Seabream (Sparus aurata L.). Sci. Rep. 2019, 9, 9832. [Google Scholar] [CrossRef] [PubMed]
- Koumoundouros, G.; Gagliardi, F.; Divanach, P.; Boglione, C.; Cataudella, S.; Kentouri, M. Normal and Abnormal Osteological Development of Caudal Fin in Sparus aurata L. Fry. Aquaculture 1997, 149, 215–226. [Google Scholar] [CrossRef]
- Fragkoulis, S.; Economou, I.; Moukas, G.; Koumoundouros, G.; Batargias, C. Caudal Fin Abnormalities in Gilthead Seabream (Sparus aurata L.) Have a Strong Genetic Variance Component. J. Fish Dis. 2020, 43, 825–828. [Google Scholar] [CrossRef]
- Fragkoulis, S.; Kerasovitis, D.; Batargias, C.; Koumoundouros, G. Body-Shape Trajectories and Their Genetic Variance Component in Gilthead Seabream (Sparus aurata L.). Sci. Rep. 2021, 11, 16964. [Google Scholar] [CrossRef]
- Fragkoulis, S.; Christou, M.; Karo, R.; Ritas, C.; Tzokas, C.; Batargias, C.; Koumoundouros, G. Scaling of Body-Shape Quality in Reared Gilthead Seabream Sparus aurata L. Consumer Preference Assessment, Wild Standard and Variability in Reared Phenotype. Aquac. Res. 2017, 48, 2402–2410. [Google Scholar] [CrossRef]
- Noble, C.; Cañon Jones, H.A.; Damsgård, B.; Flood, M.J.; Midling, K.Ø.; Roque, A.; Sæther, B.-S.; Cottee, S.Y. Injuries and Deformities in Fish: Their Potential Impacts upon Aquacultural Production and Welfare. Fish Physiol. Biochem. 2012, 38, 61–83. [Google Scholar] [CrossRef]
- Branson, E.J.; Turnbull, T. Welfare and Deformities in Fish. In Fish Welfare; Wiley Online Library: Hoboken, NJ, USA, 2008; pp. 202–216. ISBN 978-0-470-69761-0. [Google Scholar]
- Gjerde, B.; Refstie, T. The Effect of Fin-Clipping on Growth Rate, Survival and Sexual Maturity of Rainbow Trout. Aquaculture 1988, 73, 383–389. [Google Scholar] [CrossRef]
- Nicola, S.J.; Cordone, A.J. Effects of Fin Removal on Survival and Growth of Rainbow Trout (Salmo Gairdneri) in a Natural Environment. Trans. Am. Fish. Soc. 1973, 102, 753–758. [Google Scholar] [CrossRef]
- Zymonas, N.D.; McMahon, T.E. Effect of Pelvic Fin Ray Removal on Survival and Growth of Bull Trout. N. Am. J. Fish. Manag. 2006, 26, 953–959. [Google Scholar] [CrossRef]
- Chatain, B. Abnormal Swimbladder Development and Lordosis in Sea Bass (Dicentrarchus labrax) and Sea Bream (Sparus auratus). Aquaculture 1994, 119, 371–379. [Google Scholar] [CrossRef]
- Sneddon, L.U. Evolution of Nociception and Pain: Evidence from Fish Models. Philos. Trans. R. Soc. B Biol. Sci. 2019, 374, 20190290. [Google Scholar] [CrossRef]
- Riera-Heredia, N.; Vélez, E.J.; Gutiérrez, J.; Navarro, I.; Capilla, E. Gene Expression Analyses in Malformed Skeletal Structures of Gilthead Sea Bream (Sparus aurata). J. Fish Dis. 2019, 42, 1169–1180. [Google Scholar] [CrossRef]
- Georgiou, S.; Makridis, P.; Dimopoulos, D.; Power, D.M.; Mamuris, Z.; Moutou, K.A. Myosin Light Chain 2 Isoforms in Gilthead Sea Bream (Sparus aurata L.): Molecular Growth Markers at Early Stages. Aquaculture 2014, 432, 434–442. [Google Scholar] [CrossRef]
- Abbink, W.; Flik, G. Parathyroid Hormone-Related Protein in Teleost Fish. Gen. Comp. Endocrinol. 2007, 152, 243–251. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kwong, R.W.M.; Perry, S.F. An Essential Role for Parathyroid Hormone in Gill Formation and Differentiation of Ion-Transporting Cells in Developing Zebrafish. Endocrinology 2015, 156, 2384–2394. [Google Scholar] [CrossRef] [PubMed]
- Ingleton, P.M.; Power, D.M.; Keenan, L.J.; Morton, J.A.; Danks, J.A.; Brown, B.L.; Canario, A.V.M. Parathyroid Hormone-Related Protein (PTHRP) and PTHRP Gene Expression in Normal and Dystrophic Sea Bream. In Proceedings of the Third European Marine Science and Technology Conference, Lisbon, Portugal, 23–27 May 1998; Office for Official Publications of the European Communities: Luxembourg, 1999; p. 199. [Google Scholar]
- De Pedro, N.; Martínez-Álvarez, R.M.; Delgado, M.J. Melatonin Reduces Body Weight in Goldfish (Carassius Auratus): Effects on Metabolic Resources and Some Feeding Regulators. J. Pineal Res. 2008, 45, 32–39. [Google Scholar] [CrossRef]
- Porter, M.J.R.; Randall, C.F.; Bromage, N.R.; Thorpe, J.E. The Role of Melatonin and the Pineal Gland on Development and Smoltification of Atlantic Salmon (Salmo salar) Parr. Aquaculture 1998, 168, 139–155. [Google Scholar] [CrossRef]
- Taylor, J.F.; North, B.P.; Porter, M.J.R.; Bromage, N.R.; Migaud, H. Photoperiod Can Be Used to Enhance Growth and Improve Feeding Efficiency in Farmed Rainbow Trout, Oncorhynchus Mykiss. Aquaculture 2006, 256, 216–234. [Google Scholar] [CrossRef]
- Georgiou, A.N.; Georga, I.; Stamopoulou, A.; Tzokas, K.; Koumoundouros, G.; Flytzanis, C.N. High Expression Levels of the Genes Cyclin-A2 and Glucocorticoid Receptor Are Associated with High-Quality Embryos in Gilthead Sea Bream (Sparus aurata L.). Aquac. J. 2022, 2, 51–58. [Google Scholar] [CrossRef]
- Mhalhel, K.; Levanti, M.; Abbate, F.; Laurà, R.; Guerrera, M.C.; Aragona, M.; Porcino, C.; Briglia, M.; Germanà, A.; Montalbano, G. Review on Gilthead Seabream (Sparus aurata) Aquaculture: Life Cycle, Growth, Aquaculture Practices and Challenges. J. Mar. Sci. Eng. 2023, 11, 2008. [Google Scholar] [CrossRef]
- Lall, S.P.; Lewis-McCrea, L.M. Role of Nutrients in Skeletal Metabolism and Pathology in Fish-An Overview. Aquaculture 2007, 267, 3–19. [Google Scholar] [CrossRef]
- Fernández, I.; Ortiz-Delgado, J.B.; Sarasquete, C.; Gisbert, E. Vitamin A Effects on Vertebral Bone Tissue Homeostasis in Gilthead Sea Bream (Sparus aurata) Juveniles. J. Appl. Ichthyol. 2012, 28, 419–426. [Google Scholar] [CrossRef]
- Dominguez, D.; Montero, D.; Zamorano, M.J.; Castro, P.; Fontanillas, R.; Antony Jesu Prabhu, P.; Izquierdo, M. Effects of Vitamin D3 Supplementation in Gilthead Seabream (Sparus aurata) Juveniles Fed Diets High in Plant Based Feedstuffs. Aquaculture 2021, 543, 736991. [Google Scholar] [CrossRef]
- Sivagurunathan, U.; Dominguez, D.; Tseng, Y.; Eryalçın, K.M.; Roo, J.; Boglione, C.; Prabhu, P.A.J.; Izquierdo, M. Effects of Dietary Vitamin D3 Levels on Survival, Mineralization, and Skeletal Development of Gilthead Seabream (Sparus aurata) Larvae. Aquaculture 2022, 560, 738505. [Google Scholar] [CrossRef]
- Sivagurunathan, U.; Dominguez, D.; Tseng, Y.; Zamorano, M.J.; Philip, A.J.P.; Izquierdo, M. Interaction between Dietary Vitamin D(3) and Vitamin K(3) in Gilthead Seabream Larvae (Sparus aurata) in Relation to Growth and Expression of Bone Development-Related Genes. Aquac. Nutr. 2023, 2023, 3061649. [Google Scholar] [CrossRef]
- Saleh, R.; Betancor, M.B.; Roo, J.; Benítez-Santana, T.; Zamorano, M.J.; Izquierdo, M. Biomarkers of Bone Development and Oxidative Stress in Gilthead Sea Bream Larvae Fed Microdiets with Several Levels of Polar Lipids and α-Tocopherol. Aquac. Nutr. 2015, 21, 341–354. [Google Scholar] [CrossRef]
- Sivagurunathan, U.; Dominguez, D.; Tseng, Y.; Zamorano, M.J.; Prabhu, A.J.; Izquierdo, M. Deficiency and Excess in Dietary Vitamin K3 Negatively Affect Gilthead Seabream (Sparus aurata) Larvae Performance and Bone Health. Aquaculture 2023, 574, 739646. [Google Scholar] [CrossRef]
- Dominguez, D.; Castro, P.; Lall, S.; Montero, D.; Zamorano, M.J.; Fontanillas, R.; Izquierdo, M. Effects of Menadione Sodium Bisulphite (Vitamin K3) Supplementation of the Diets Based on Plant Feed Ingredients on Growth and Bone Health of Gilthead Seabream (Sparus aurata) Fingerlings. Aquac. Nutr. 2022, 2022, 1613030. [Google Scholar] [CrossRef]
- Balbuena-Pecino, S.; Riera-Heredia, N.; Vélez, E.J.; Gutiérrez, J.; Navarro, I.; Riera-Codina, M.; Capilla, E. Temperature Affects Musculoskeletal Development and Muscle Lipid Metabolism of Gilthead Sea Bream (Sparus aurata). Front. Endocrinol. 2019, 10, 173. [Google Scholar] [CrossRef] [PubMed]
- Villamizar, N.; García-Alcazar, A.; Sánchez-Vázquez, F.J. Effect of Light Spectrum and Photoperiod on the Growth, Development and Survival of European Sea Bass (Dicentrarchus labrax) Larvae. Aquaculture 2009, 292, 80–86. [Google Scholar] [CrossRef]
- García-Celdrán, M.; Ramis, G.; Manchado, M.; Estévez, A.; Afonso, J.M.; María-Dolores, E.; Peñalver, J.; Armero, E. Estimates of Heritabilities and Genetic Correlations of Growth and External Skeletal Deformities at Different Ages in a Reared Gilthead Sea Bream (Sparus aurata L.) Population Sourced from Three Broodstocks along the Spanish Coasts. Aquaculture 2015, 445, 33–41. [Google Scholar] [CrossRef]
- Fernandes, T.; Herlin, M.; Belluga, M.D.L.; Ballón, G.; Martinez, P.; Toro, M.A.; Fernández, J. Estimation of Genetic Parameters for Growth Traits in a Hatchery Population of Gilthead Sea Bream (Sparus aurata L.). Aquac. Int. 2017, 25, 499–514. [Google Scholar] [CrossRef]
- Saura, M.; Caballero, A.; Santiago, E.; Fernández, A.; Morales-González, E.; Fernández, J.; Cabaleiro, S.; Millán, A.; Martínez, P.; Palaiokostas, C.; et al. Estimates of Recent and Historical Effective Population Size in Turbot, Seabream, Seabass and Carp Selective Breeding Programmes. Genet. Sel. Evol. 2021, 53, 85. [Google Scholar] [CrossRef] [PubMed]
- Loukovitis, D.; Sarropoulou, E.; Batargias, C.; Apostolidis, A.P.; Kotoulas, G.; Tsigenopoulos, C.S.; Chatziplis, D. Quantitative Trait Loci for Body Growth and Sex Determination in the Hermaphrodite Teleost Fish Sparus aurata L. Anim. Genet. 2012, 43, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Loukovitis, D.; Batargias, C.; Sarropoulou, E.; Apostolidis, A.P.; Kotoulas, G.; Magoulas, A.; Tsigenopoulos, C.S.; Chatziplis, D. Quantitative Trait Loci Affecting Morphology Traits in Gilthead Seabream (Sparus aurata L.). Anim. Genet. 2013, 44, 480–483. [Google Scholar] [CrossRef]
- Loukovitis, D.; Sarropoulou, E.; Tsigenopoulos, C.S.; Batargias, C.; Magoulas, A.; Apostolidis, A.P.; Chatziplis, D.; Kotoulas, G. Quantitative Trait Loci Involved in Sex Determination and Body Growth in the Gilthead Sea Bream (Sparus aurata L.) through Targeted Genome Scan. PLoS ONE 2011, 6, e16599. [Google Scholar] [CrossRef]
- Boulton, K.; Massault, C.; Houston, R.D.; de Koning, D.J.; Haley, C.S.; Bovenhuis, H.; Batargias, C.; Canario, A.V.M.; Kotoulas, G.; Tsigenopoulos, C.S. QTL Affecting Morphometric Traits and Stress Response in the Gilthead Seabream (Sparus aurata). Aquaculture 2011, 319, 58–66. [Google Scholar] [CrossRef]
- Negrín-Báez, D.; Navarro, A.; Afonso, J.M.; Ginés, R.; Zamorano, M.J. Detection of QTL Associated with Three Skeletal Deformities in Gilthead Seabream (Sparus aurata L.): Lordosis, Vertebral Fusion and Jaw Abnormality. Aquaculture 2015, 448, 123–127. [Google Scholar] [CrossRef]
- McGonnell, I.M.; Fowkes, R.C. Fishing for Gene Function--Endocrine Modelling in the Zebrafish. J. Endocrinol. 2006, 189, 425–439. [Google Scholar] [CrossRef]
- Witten, P.E.; Harris, M.P.; Huysseune, A.; Winkler, C. Small Teleost Fish Provide New Insights into Human Skeletal Diseases. Methods Cell Biol. 2017, 138, 321–346. [Google Scholar] [CrossRef] [PubMed]
- Mhalhel, K.; Sicari, M.; Pansera, L.; Chen, J.; Levanti, M.; Diotel, N.; Rastegar, S.; Germanà, A.; Montalbano, G. Zebrafish: A Model Deciphering the Impact of Flavonoids on Neurodegenerative Disorders. Cells 2023, 12, 252. [Google Scholar] [CrossRef]
- Montalbano, G.; Mhalhel, K.; Briglia, M.; Levanti, M.; Abbate, F.; Guerrera, M.C.; D’Alessandro, E.; Laurà, R.; Germanà, A. Zebrafish and Flavonoids: Adjuvants against Obesity. Molecules 2021, 26, 3014. [Google Scholar] [CrossRef]
- Mhalhel, K.; Briglia, M.; Aragona, M.; Porcino, C.; Abbate, F.; Guerrera, M.C.; Laurà, R.; Krichen, Y.; Guerbej, H.; Germanà, A.; et al. Nothobranchius as a Model for Anorexia of Aging Research: An Evolutionary, Anatomical, Histological, Immunohistochemical, and Molecular Study. Ann. Anat.-Anat. Anz. 2023, 250, 152116. [Google Scholar] [CrossRef] [PubMed]
- Lleras-Forero, L.; Winkler, C.; Schulte-Merker, S. Zebrafish and Medaka as Models for Biomedical Research of Bone Diseases. Dev. Biol. 2020, 457, 191–205. [Google Scholar] [CrossRef] [PubMed]
- Bergen, D.J.M.; Kague, E.; Hammond, C.L. Zebrafish as an Emerging Model for Osteoporosis: A Primary Testing Platform for Screening New Osteo-Active Compounds. Front. Endocrinol. 2019, 10, 6. [Google Scholar] [CrossRef] [PubMed]
- Witten, P.E.; Huysseune, A. A Comparative View on Mechanisms and Functions of Skeletal Remodelling in Teleost Fish, with Special Emphasis on Osteoclasts and Their Function. Biol. Rev. 2009, 84, 315–346. [Google Scholar] [CrossRef] [PubMed]
- Vieira, F.A.; Thorne, M.A.S.; Stueber, K.; Darias, M.; Reinhardt, R.; Clark, M.S.; Gisbert, E.; Power, D.M. Comparative Analysis of a Teleost Skeleton Transcriptome Provides Insight into Its Regulation. Gen. Comp. Endocrinol. 2013, 191, 45–58. [Google Scholar] [CrossRef] [PubMed]
- Riera-Heredia, N.; Martins, R.; Mateus, A.P.; Costa, R.A.; Gisbert, E.; Navarro, I.; Gutiérrez, J.; Power, D.M.; Capilla, E. Temperature Responsiveness of Gilthead Sea Bream Bone; an in Vitro and in Vivo Approach. Sci. Rep. 2018, 8, 11211. [Google Scholar] [CrossRef]
- Kamacı, H.O.; Saka, Ş.; Fırat, K. The Cleavage and Embryonic Phase of Gilthead Sea Bream (Sparus aurata Linnaeus, 1758) Eggs. Su Ürünleri Derg. 2005, 22, 205–209. [Google Scholar]
- Seginer, I. Growth Models of Gilthead Sea Bream (Sparus aurata L.) for Aquaculture: A Review. Aquac. Eng. 2016, 70, 15–32. [Google Scholar] [CrossRef]
- Canario, A.V.M.; Bargelloni, L.; Volckaert, F.; Houston, R.D.; Massault, C.; Guiguen, Y. Genomics Toolbox for Farmed Fish. Rev. Fish. Sci. 2008, 16, 3–15. [Google Scholar] [CrossRef]
- Laizé, V.; Pombinho, A.R.; Cancela, M.L. Characterization of Sparus aurata Osteonectin cDNA and in Silico Analysis of Protein Conserved Features: Evidence for More than One Osteonectin in Salmonidae. Biochimie 2005, 87, 411–420. [Google Scholar] [CrossRef][Green Version]
- Louro, B.; Passos, A.L.S.; Souche, E.L.; Tsigenopoulos, C.; Beck, A.; Lagnel, J.; Bonhomme, F.; Cancela, L.; Cerdà, J.; Clark, M.S.; et al. Gilthead Sea Bream (Sparus Auratus) and European Sea Bass (Dicentrarchus labrax) Expressed Sequence Tags: Characterization, Tissue-Specific Expression and Gene Markers. Mar. Genom. 2010, 3, 179–191. [Google Scholar] [CrossRef]
- Adzhubei, A.A.; Vlasova, A.V.; Hagen-Larsen, H.; Ruden, T.A.; Laerdahl, J.K.; Høyheim, B. Annotated Expressed Sequence Tags (ESTs) from Pre-Smolt Atlantic Salmon (Salmo Salar) in a Searchable Data Resource. BMC Genom. 2007, 8, 209. [Google Scholar] [CrossRef]
- Calduch-Giner, J.A.; Echasseriau, Y.; Crespo, D.; Baron, D.; Planas, J.V.; Prunet, P.; Pérez-Sánchez, J. Transcriptional Assessment by Microarray Analysis and Large-Scale Meta-Analysis of the Metabolic Capacity of Cardiac and Skeletal Muscle Tissues to Cope with Reduced Nutrient Availability in Gilthead Sea Bream (Sparus aurata L.). Mar. Biotechnol. 2014, 16, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Sáez-Arteaga, A.; Wu, Y.; Silva-Marrero, J.I.; Rashidpour, A.; Almajano, M.P.; Fernández, F.; Baanante, I.V.; Metón, I. Gene Markers of Dietary Macronutrient Composition and Growth in the Skeletal Muscle of Gilthead Sea Bream (Sparus aurata). Aquaculture 2022, 555, 738221. [Google Scholar] [CrossRef]
- Rafael, M.S.; Marques, C.L.; Parameswaran, V.; Cancela, M.L.; Laizé, V. Fish Bone-Derived Cell Lines: An Alternative in Vitro Cell System to Study Bone Biology. J. Appl. Ichthyol. 2010, 26, 230–234. [Google Scholar] [CrossRef]
- Vijayakumar, P.; Laizé, V.; Cardeira, J.; Trindade, M.; Cancela, M.L. Development of an in Vitro Cell System from Zebrafish Suitable to Study Bone Cell Differentiation and Extracellular Matrix Mineralization. Zebrafish 2013, 10, 500–509. [Google Scholar] [CrossRef] [PubMed]
- Pombinho, A.R.; Laizé, V.; Molha, D.M.; Marques, S.M.P.; Cancela, M.L. Development of Two Bone-Derived Cell Lines from the Marine Teleost Sparus aurata; Evidence for Extracellular Matrix Mineralization and Cell-Type-Specific Expression of Matrix Gla Protein and Osteocalcin. Cell Tissue Res. 2004, 315, 393–406. [Google Scholar] [CrossRef]
| Identified Genes | GO: Biological Processes/Molecular Function | FCM/FCMV Score |
|---|---|---|
| Photoreceptor outer segment all-trans retinol dehydrogenase [IPIAcc:IPI00024598] | metabolic process/oxidoreductase activity | 240.1 |
| AMBP protein precursor [Uniprot Acc:P02760] | -/- | 131.5 |
| Ependymin related protein-1 precursor [IPIAcc:IPI00554718] | cell-matrix adhesion/calcium ion binding | 111.8 |
| Xaa-Pro aminopeptidase 2 precursor [Uniprot Acc:O43895] | proteolysis, creatine metabolic process/metalloexopeptidase activity, hydrolase activity, creatinase activity | 110.2 |
| Microtubule-associated proteins 1A/1B light chain 3C precursor [Uniprot Acc:Q9BXW4] | -/- | 89.63 |
| Cell death activator CIDE-3 [Uniprot Acc:Q96AQ7] | apoptosis/protein binding | 45.71 |
| PREDICTED: hypothetical protein [Danio rerio] | pore complex biogenesis/channel activity | 35.20 |
| Hypothetical protein LOC447879 [RefSeq_peptideAcc:NP_001004618] | integrin-mediated signaling pathway/- | 32.64 |
| Granulocyte-macrophage colony-stimulating factor receptor alpha chain precursor [Uniprot Acc:P15509] | -/- | 29.83 |
| IgGFc-binding protein precursor [Uniprot Acc:Q9Y6R7] | cell adhesion/- | 23.12 |
| Hydroxyacid oxidase 2 [Uniprot Acc:Q9NYQ3] | metabolic process, electron transport/oxidoreductase activity | 21.38 |
| Phosphatase and actin regulator 4 isoform 1 [RefSeq_peptideAcc:NP_001041648] | -/- | 18.08 |
| Ependymin-related protein-1 precursor [IPIAcc:IPI00554718] | cell–matrix adhesion/calcium ion binding | 18.02 |
| Glucose-6-phosphate 1-dehydrogenase [Uniprot Acc:P11413] | glucose metabolic process/glucose-6-phosphate dehydrogenase activity | 16.05 |
| Glutamate–cysteine ligase catalytic subunit [Uniprot Acc:P48506] | -/- | 16.04 |
| Complement factor I precursor [Uniprot Acc:P05156] | proteolysis/catalytic activity, serine-type endopeptidase activity, hydrolase activity, scavenger receptor activity | 15.91 |
| Hypothetical protein LOC406276 [RefSeq_peptideAcc:NP_998168] | -/calcium ion binding | 12.54 |
| Hypothetical protein LOC336637 [RefSeq_peptideAcc:NP_956317] | cell redox homeostasis/- | 12.17 |
| Actin filament-associated protein 1-like 2. [Uniprot Acc:Q8N4X5] | -/- | 11.79 |
| Transcribed locus, weakly similar to NP_175293.1 [UniGeneAcc:Tru.931] | metabolic process/methyltransferase activity | 11.69 |
| Ornithine carbamoyltransferase, mitochondrial precursor [Uniprot Acc:P00480] | -/ornithine carbamoyltransferase activity, amino acid binding | 11.38 |
| CDNA FLJ31025 fis, clone HLUNG2000501. [Uniprot/SPTREMBLAcc:Q96ND9] | -/- | 11.25 |
| Stromal cell-derived factor 1 precursor [Uniprot Acc:P48061] | -/- | 10.36 |
| ADM precursor [Uniprot Acc:P35318] | -/- | 10.10 |
| Beta-2-glycoprotein 1 precursor [Uniprot Acc:P02749] | -/- | 10.05 |
| Signal peptide, CUB and EGF-like domain-containing protein 2 precursor [Uniprot Acc:Q9NQ36] | -/calcium ion binding | 78.01 |
| Actin, alpha skeletal muscle 1. [Uniprot Acc:P68140] | -/structural molecule activity, ATP binding, protein binding | 38.39 |
| Inter-alpha globulIn InhIbItor H3 [IPIAcc:IPI00028413] | hyaluronan metabolic process/serine-type endopeptidase inhibitor activity | 24.67 |
| Hypothetical protein LOC553753 [RefSeq_peptideAcc:NP_001018560] | -/protein binding | 21.14 |
| Tenascin precursor [Uniprot Acc:P24821] | signal transduction/receptor binding | 20.56 |
| Prolargin precursor [Uniprot Acc:P51888] | -/- | 18.89 |
| Eukaryotic translation initiation factor 4E-1A-binding protein [Uniprot Acc:Q98TT6] | negative regulation of translational initiation/eukaryotic initiation factor 4E binding | 12.02 |
| WNT1-inducible-signaling pathway protein 1 precursor [Uniprot Acc:O95388] | regulation of cell growth/insulin-like growth factor binding | 10.77 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mhalhel, K.; Levanti, M.; Abbate, F.; Laurà, R.; Guerrera, M.C.; Aragona, M.; Porcino, C.; Pansera, L.; Sicari, M.; Cometa, M.; et al. Skeletal Morphogenesis and Anomalies in Gilthead Seabream: A Comprehensive Review. Int. J. Mol. Sci. 2023, 24, 16030. https://doi.org/10.3390/ijms242216030
Mhalhel K, Levanti M, Abbate F, Laurà R, Guerrera MC, Aragona M, Porcino C, Pansera L, Sicari M, Cometa M, et al. Skeletal Morphogenesis and Anomalies in Gilthead Seabream: A Comprehensive Review. International Journal of Molecular Sciences. 2023; 24(22):16030. https://doi.org/10.3390/ijms242216030
Chicago/Turabian StyleMhalhel, Kamel, Maria Levanti, Francesco Abbate, Rosaria Laurà, Maria Cristina Guerrera, Marialuisa Aragona, Caterina Porcino, Lidia Pansera, Mirea Sicari, Marzio Cometa, and et al. 2023. "Skeletal Morphogenesis and Anomalies in Gilthead Seabream: A Comprehensive Review" International Journal of Molecular Sciences 24, no. 22: 16030. https://doi.org/10.3390/ijms242216030
APA StyleMhalhel, K., Levanti, M., Abbate, F., Laurà, R., Guerrera, M. C., Aragona, M., Porcino, C., Pansera, L., Sicari, M., Cometa, M., Briglia, M., Germanà, A., & Montalbano, G. (2023). Skeletal Morphogenesis and Anomalies in Gilthead Seabream: A Comprehensive Review. International Journal of Molecular Sciences, 24(22), 16030. https://doi.org/10.3390/ijms242216030








