Multi-Omics Analysis of Circulating Exosomes in Adherent Long-Term Treated OSA Patients
Abstract
1. Introduction
2. Results
2.1. Subject Characteristics
2.2. Exosome Characterization and Cellular Internalization
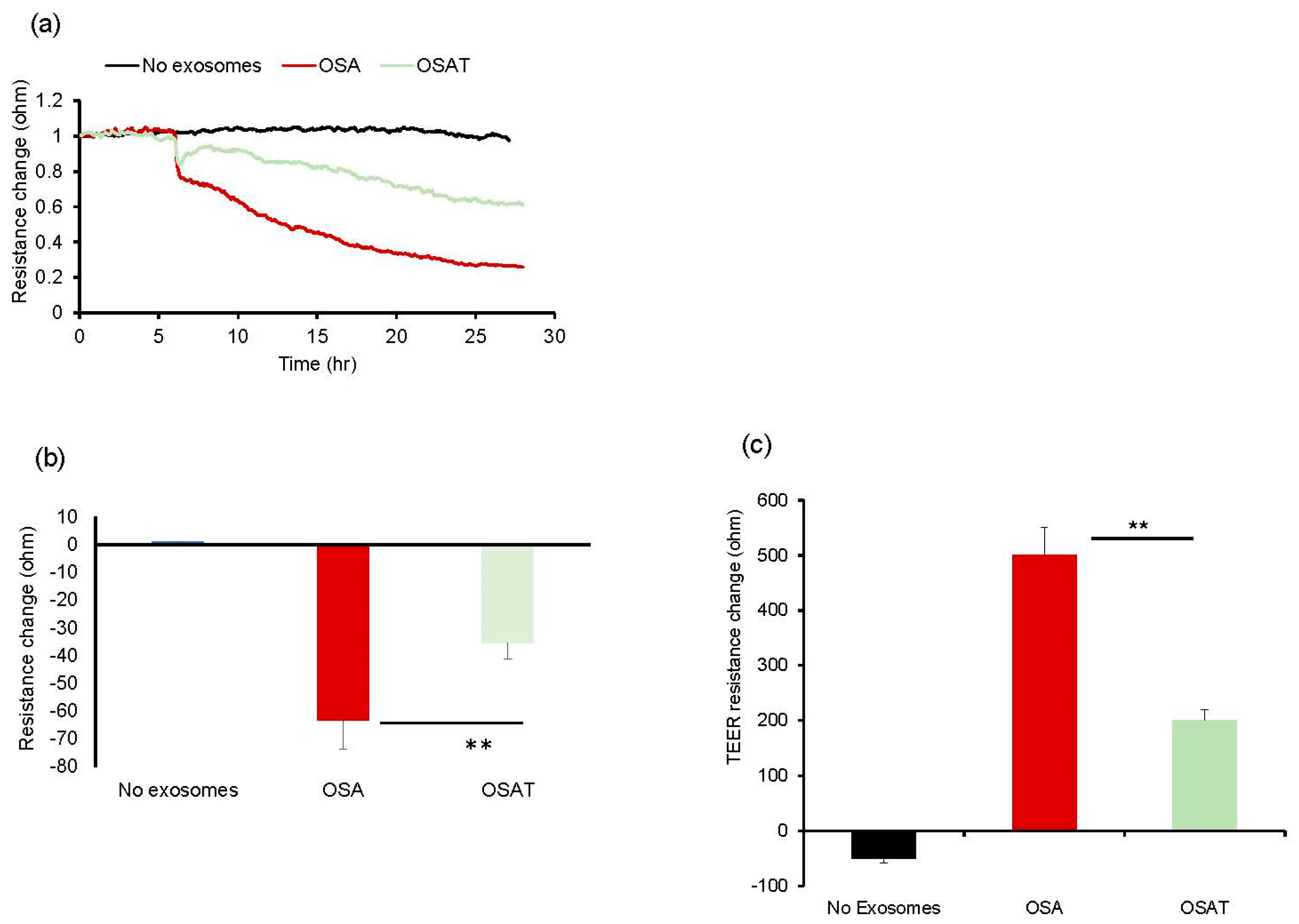
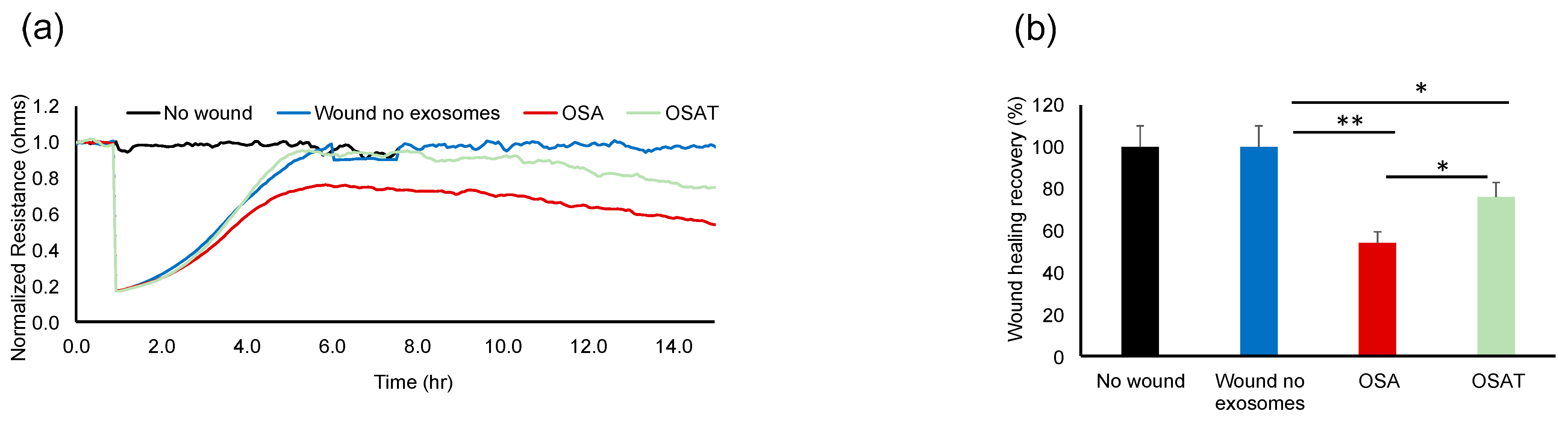
2.3. Endothelial Barrier Integrity and Wound Healing
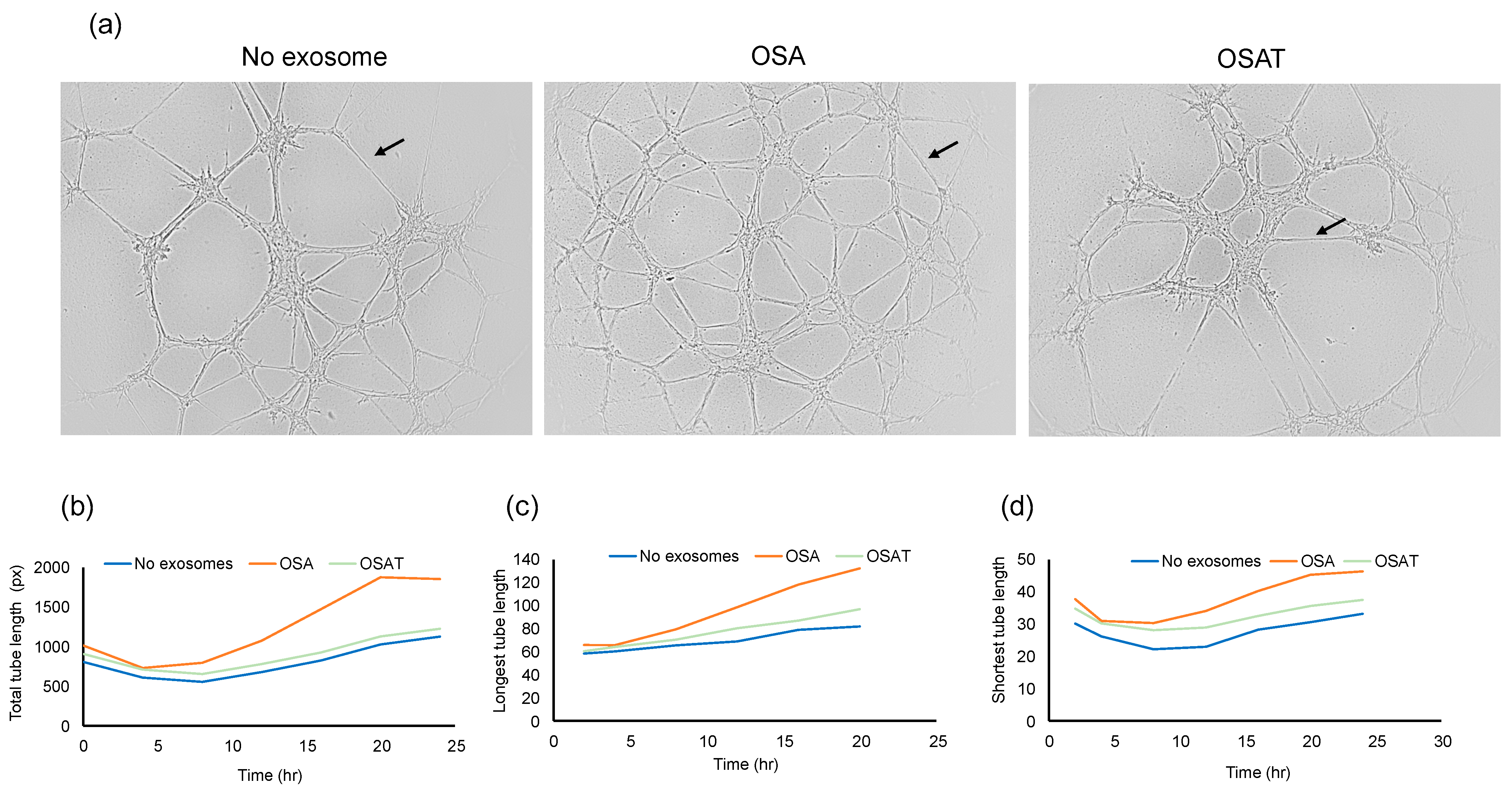
2.4. Angiogenesis (Tube Formation Assay)
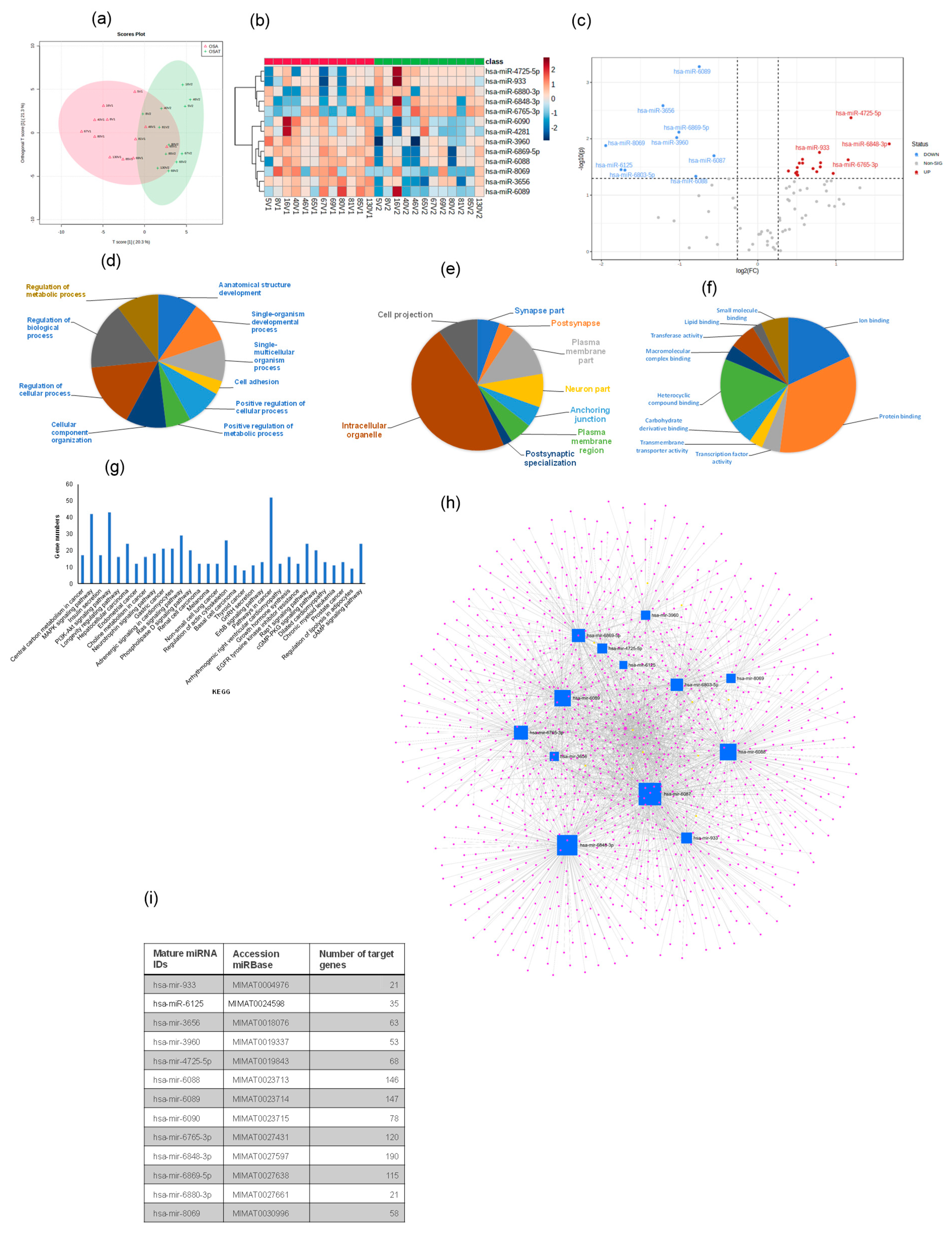
2.5. Exosome Cargos
2.6. Lipidomic Analysis
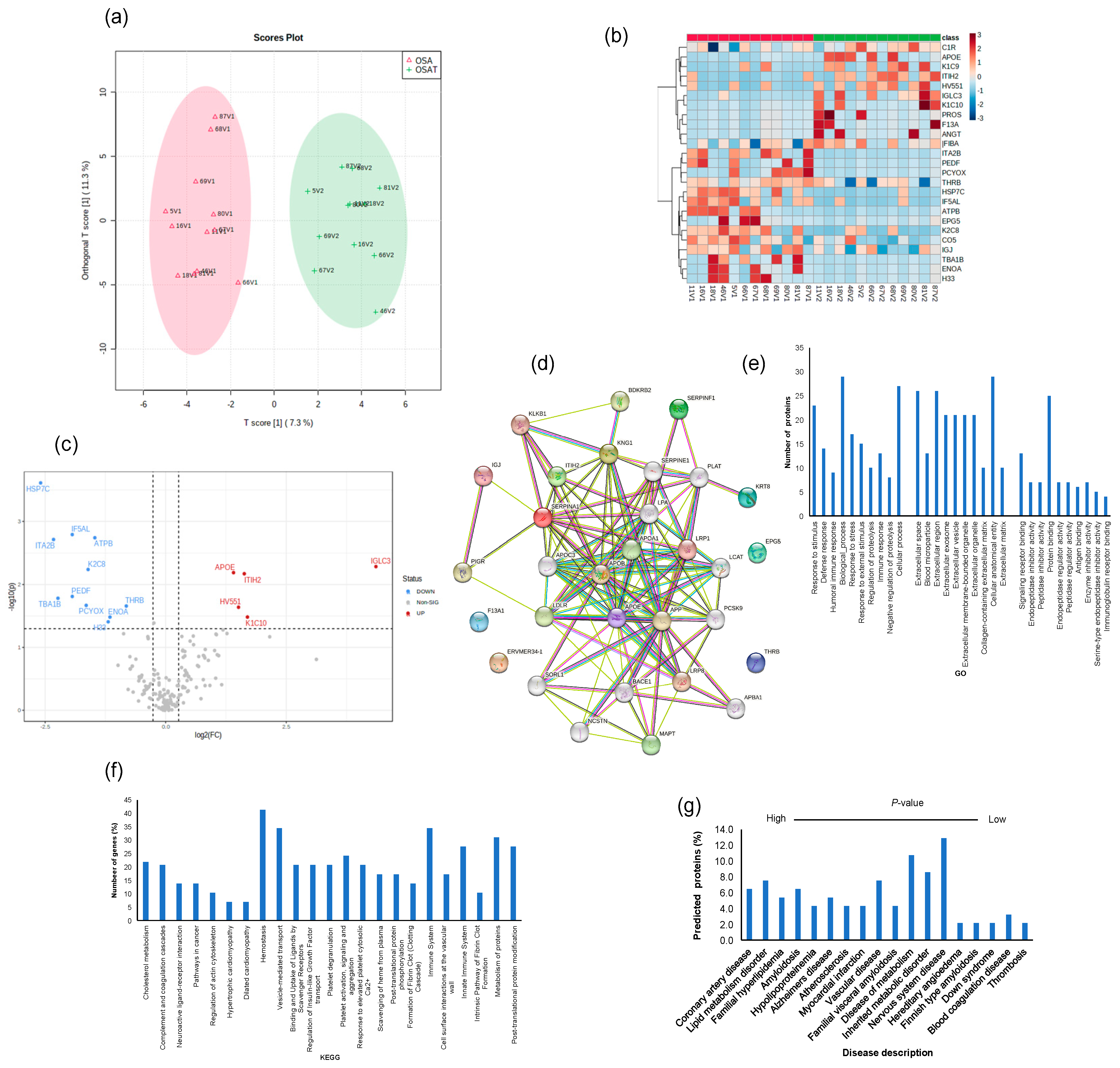
2.7. Exosome Proteomic Analysis
2.8. Exosomal miRNA Profile
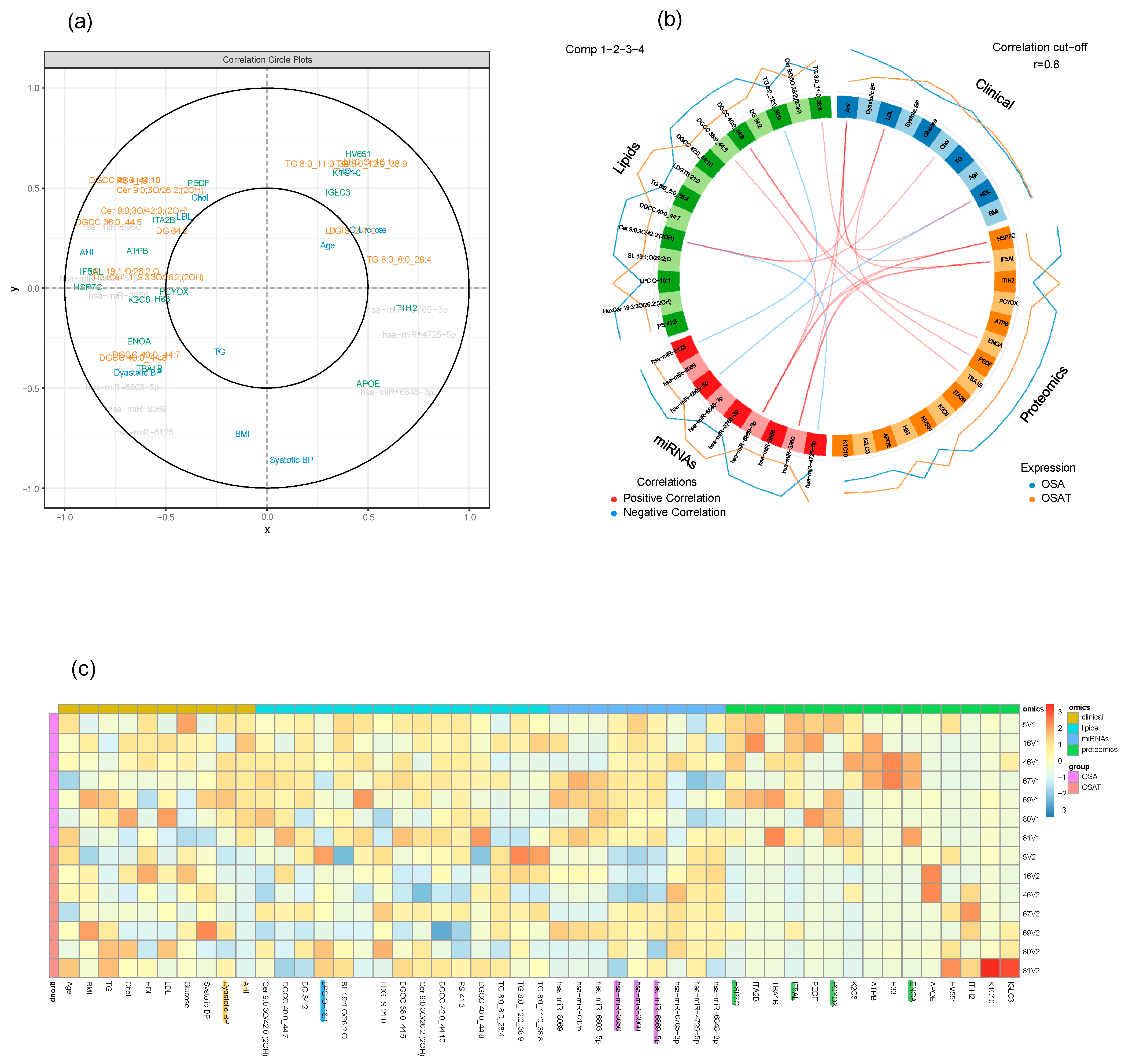
2.9. Multi-Omic Data Integration
3. Discussion
3.1. Functional Effects of Circulating Exosomes on Naïve Endothelial Cells
3.2. Multi-Omic Analysis
3.2.1. Lipid Cargo of Exosomes
3.2.2. Exosome Proteins
3.2.3. Exosome miRNAs
3.3. Data Integration
4. Materials and Methods
4.1. Subject Characteristics
4.2. Exosome Isolation and Characterization
4.3. Exosome Markers Using Flow Cytometry
4.4. Human Endothelial Cells and Exosome Uptake
4.5. Endothelial Cell Barrier Integrity
4.6. Wound-Healing Assay
4.7. Angiogenesis Tube Formation Assay
4.8. Exosome Lipidomics
4.9. Exosome Proteomics
4.10. Exosome miRNAs
4.11. Target Predictions and Functional Annotation
4.12. miRNA qRT-PCR
4.13. Multi-Omics and Multivariate Analyses
4.14. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Benjafield, A.V.; Ayas, N.T.; Eastwood, P.R.; Heinzer, R.; Ip, M.S.M.; Morrell, M.J.; Nunez, C.M.; Patel, S.R.; Penzel, T.; Pepin, J.L.; et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: A literature-based analysis. Lancet Respir. Med. 2019, 7, 687–698. [Google Scholar] [CrossRef]
- Sawunyavisuth, B.; Ngamjarus, C.; Sawanyawisuth, K. A meta-analysis to identify factors associated with CPAP machine purchasing in patients with obstructive sleep apnea. Biomed. Rep. 2022, 16, 45. [Google Scholar] [CrossRef]
- Ding, F.; Cotton-Clay, A.; Fava, L.; Easwar, V.; Kinsolving, A.; Kahn, P.; Rama, A.; Kushida, C. Polysomnographic validation of an under-mattress monitoring device in estimating sleep architecture and obstructive sleep apnea in adults. Sleep Med. 2022, 96, 20–27. [Google Scholar] [CrossRef]
- Drager, L.F.; McEvoy, R.D.; Barbe, F.; Lorenzi-Filho, G.; Redline, S.; Initiative, I. Sleep Apnea and Cardiovascular Disease: Lessons from Recent Trials and Need for Team Science. Circulation 2017, 136, 1840–1850. [Google Scholar] [CrossRef]
- Arnaud, C.; Bochaton, T.; Pepin, J.L.; Belaidi, E. Obstructive sleep apnoea and cardiovascular consequences: Pathophysiological mechanisms. Arch. Cardiovasc. Dis. 2020, 113, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Andre, S.; Andreozzi, F.; Van Overstraeten, C.; Ben Youssef, S.; Bold, I.; Carlier, S.; Gruwez, A.; Bruyneel, A.V.; Bruyneel, M. Cardiometabolic comorbidities in obstructive sleep apnea patients are related to disease severity, nocturnal hypoxemia, and decreased sleep quality. Respir. Res. 2020, 21, 35. [Google Scholar] [CrossRef]
- Khalyfa, A.; Gozal, D.; Masa, J.F.; Marin, J.M.; Qiao, Z.; Corral, J.; Gonzalez, M.; Marti, S.; Kheirandish-Gozal, L.; Egea, C.; et al. Sleep-disordered breathing, circulating exosomes, and insulin sensitivity in adipocytes. Int. J. Obes. 2018, 42, 1127–1139. [Google Scholar] [CrossRef] [PubMed]
- Lavergne, F.; Morin, L.; Armitstead, J.; Benjafield, A.; Richards, G.; Woehrle, H. Atrial fibrillation and sleep-disordered breathing. J. Thorac. Dis. 2015, 7, E575–E584. [Google Scholar] [CrossRef] [PubMed]
- Rana, D.; Torrilus, C.; Ahmad, W.; Okam, N.A.; Fatima, T.; Jahan, N. Obstructive Sleep Apnea and Cardiovascular Morbidities: A Review Article. Cureus 2020, 12, e10424. [Google Scholar] [CrossRef]
- Jurado-Gamez, B.; Guglielmi, O.; Gude, F.; Buela-Casal, G. Workplace accidents, absenteeism and productivity in patients with sleep apnea. Arch. Bronconeumol. 2015, 51, 213–218. [Google Scholar] [CrossRef]
- Veasey, S.C.; Rosen, I.M. Obstructive Sleep Apnea in Adults. N. Engl. J. Med. 2019, 380, 1442–1449. [Google Scholar] [CrossRef]
- Levy, P.; Kohler, M.; McNicholas, W.T.; Barbe, F.; McEvoy, R.D.; Somers, V.K.; Lavie, L.; Pepin, J.L. Obstructive sleep apnoea syndrome. Nat. Rev. Dis. Primers 2015, 1, 15015. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-de-la-Torre, M.; Campos-Rodriguez, F.; Barbe, F. Obstructive sleep apnoea and cardiovascular disease. Lancet Respir. Med. 2013, 1, 61–72. [Google Scholar] [CrossRef]
- Sanchez-de-la-Torre, M.; Cubillos, C.; Veatch, O.J.; Garcia-Rio, F.; Gozal, D.; Martinez-Garcia, M.A. Potential Pathophysiological Pathways in the Complex Relationships between OSA and Cancer. Cancers 2023, 15, 1061. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, P.; Tak, T. Obstructive sleep apnea and cardiovascular disease. Cardiol. Rev. 2011, 19, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Gozal, D.; Almendros, I.; Phipps, A.I.; Campos-Rodriguez, F.; Martinez-Garcia, M.A.; Farre, R. Sleep Apnoea Adverse Effects on Cancer: True, False, or Too Many Confounders? Int. J. Mol. Sci. 2020, 21, 8779. [Google Scholar] [CrossRef]
- Abud, R.; Salgueiro, M.; Drake, L.; Reyes, T.; Jorquera, J.; Labarca, G. Efficacy of continuous positive airway pressure (CPAP) preventing type 2 diabetes mellitus in patients with obstructive sleep apnea hypopnea syndrome (OSAHS) and insulin resistance: A systematic review and meta-analysis. Sleep Med. 2019, 62, 14–21. [Google Scholar] [CrossRef]
- Marin, J.M.; Carrizo, S.J.; Vicente, E.; Agusti, A.G. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: An observational study. Lancet 2005, 365, 1046–1053. [Google Scholar] [CrossRef]
- Labarca, G.; Dreyse, J.; Drake, L.; Jorquera, J.; Barbe, F. Efficacy of continuous positive airway pressure (CPAP) in the prevention of cardiovascular events in patients with obstructive sleep apnea: Systematic review and meta-analysis. Sleep Med. Rev. 2020, 52, 101312. [Google Scholar] [CrossRef]
- Mashaqi, S.; Gozal, D. The impact of obstructive sleep apnea and PAP therapy on all-cause and cardiovascular mortality based on age and gender-a literature review. Respir. Investig. 2020, 58, 7–20. [Google Scholar] [CrossRef]
- Gomez-Olivas, J.D.; Campos-Rodriguez, F.; Nagore, E.; Martorell, A.; Garcia-Rio, F.; Cubillos, C.; Hernandez, L.; Banuls, J.; Arias, E.; Ortiz, P.; et al. Role of Sleep Apnea and Long-Term Cpap Treatment in the Prognosis of Patients with Melanoma. Chest 2023, 23, 1–7. [Google Scholar] [CrossRef]
- Peracaula, M.; Torres, D.; Poyatos, P.; Luque, N.; Rojas, E.; Obrador, A.; Orriols, R.; Tura-Ceide, O. Endothelial Dysfunction and Cardiovascular Risk in Obstructive Sleep Apnea: A Review Article. Life 2022, 12, 537. [Google Scholar] [CrossRef]
- Khalyfa, A.; Kheirandish-Gozal, L.; Gozal, D. Circulating exosomes in obstructive sleep apnea as phenotypic biomarkers and mechanistic messengers of end-organ morbidity. Respir. Physiol. Neurobiol. 2018, 256, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Khalyfa, A.; Zhang, C.; Khalyfa, A.A.; Foster, G.E.; Beaudin, A.E.; Andrade, J.; Hanly, P.J.; Poulin, M.J.; Gozal, D. Effect on Intermittent Hypoxia on Plasma Exosomal Micro RNA Signature and Endothelial Function in Healthy Adults. Sleep 2016, 39, 2077–2090. [Google Scholar] [CrossRef] [PubMed]
- Cai, P.Y.; Zheng, Y.L.; Zhou, Y.F.; Wang, W.D.; Li, M.M.; Shi, Y.C.; Lin, H.L.; Lin, S. Research progress on the role of exosomes in obstructive sleep apnea-hypopnea syndrome-related atherosclerosis. Sleep Med. Rev. 2022, 66, 101696. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Rubio, D.; Khalyfa, A.; Qiao, Z.; Ullate, J.; Marin, J.M.; Kheirandish-Gozal, L.; Gozal, D. Cell-Selective Altered Cargo Properties of Extracellular Vesicles Following In Vitro Exposures to Intermittent Hypoxia. Int. J. Mol. Sci. 2021, 22, 5604. [Google Scholar] [CrossRef] [PubMed]
- Khalyfa, A.; Ericsson, A.; Qiao, Z.; Almendros, I.; Farre, R.; Gozal, D. Circulating exosomes and gut microbiome induced insulin resistance in mice exposed to intermittent hypoxia: Effects of physical activity. EBioMedicine 2021, 64, 103208. [Google Scholar] [CrossRef] [PubMed]
- Khalyfa, A.; Castro-Grattoni, A.L.; Gozal, D. Cardiovascular morbidities of obstructive sleep apnea and the role of circulating extracellular vesicles. Ther. Adv. Respir. Dis. 2019, 13, 1753466619895229. [Google Scholar] [CrossRef]
- Willms, E.; Cabanas, C.; Mager, I.; Wood, M.J.A.; Vader, P. Extracellular Vesicle Heterogeneity: Subpopulations, Isolation Techniques, and Diverse Functions in Cancer Progression. Front. Immunol. 2018, 9, 738. [Google Scholar] [CrossRef]
- Khalyfa, A.; Gozal, D. Exosomal miRNAs as potential biomarkers of cardiovascular risk in children. J. Transl. Med. 2014, 12, 162. [Google Scholar] [CrossRef]
- Garcia-Martin, R.; Brandao, B.B.; Thomou, T.; Altindis, E.; Kahn, C.R. Tissue differences in the exosomal/small extracellular vesicle proteome and their potential as indicators of altered tissue metabolism. Cell Rep. 2022, 38, 110277. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.S.; Kim, D.K.; Kim, Y.K.; Gho, Y.S. Proteomics, transcriptomics and lipidomics of exosomes and ectosomes. Proteomics 2013, 13, 1554–1571. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.I.; Ma, J.; Carter, C.L.; Loudig, O. Circulating Exosome Cargoes Contain Functionally Diverse Cancer Biomarkers: From Biogenesis and Function to Purification and Potential Translational Utility. Cancers 2022, 14, 3350. [Google Scholar] [CrossRef] [PubMed]
- Di Sario, G.; Rossella, V.; Famulari, E.S.; Maurizio, A.; Lazarevic, D.; Giannese, F.; Felici, C. Enhancing clinical potential of liquid biopsy through a multi-omic approach: A systematic review. Front. Genet. 2023, 14, 1152470. [Google Scholar] [CrossRef]
- Hinzman, C.P.; Singh, B.; Bansal, S.; Li, Y.; Iliuk, A.; Girgis, M.; Herremans, K.M.; Trevino, J.G.; Singh, V.K.; Banerjee, P.P.; et al. A multi-omics approach identifies pancreatic cancer cell extracellular vesicles as mediators of the unfolded protein response in normal pancreatic epithelial cells. J. Extracell. Vesicles 2022, 11, e12232. [Google Scholar] [CrossRef]
- Xiao, H.; Bartoszek, K.; Lio, P. Multi-omic analysis of signalling factors in inflammatory comorbidities. BMC Bioinform. 2018, 19, 439. [Google Scholar] [CrossRef]
- Chella Krishnan, K.; Kurt, Z.; Barrere-Cain, R.; Sabir, S.; Das, A.; Floyd, R.; Vergnes, L.; Zhao, Y.; Che, N.; Charugundla, S.; et al. Integration of Multi-omics Data from Mouse Diversity Panel Highlights Mitochondrial Dysfunction in Non-alcoholic Fatty Liver Disease. Cell Syst. 2018, 6, 103–115.e107. [Google Scholar] [CrossRef]
- Hasin, Y.; Seldin, M.; Lusis, A. Multi-omics approaches to disease. Genome Biol. 2017, 18, 83. [Google Scholar] [CrossRef]
- Khalyfa, A.; Marin, J.M.; Qiao, Z.; Rubio, D.S.; Kheirandish-Gozal, L.; Gozal, D. Plasma exosomes in OSA patients promote endothelial senescence: Effect of long-term adherent continuous positive airway pressure. Sleep 2020, 43, zsz217. [Google Scholar] [CrossRef]
- Keese, C.R.; Wegener, J.; Walker, S.R.; Giaever, I. Electrical wound-healing assay for cells in vitro. Proc. Natl. Acad. Sci. USA 2004, 101, 1554–1559. [Google Scholar] [CrossRef]
- Redline, S.; Azarbarzin, A.; Peker, Y. Obstructive sleep apnoea heterogeneity and cardiovascular disease. Nat. Rev. Cardiol. 2023, 20, 560–573. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Tromp, J.; Li, M. PatternHunter: Faster and more sensitive homology search. Bioinformatics 2002, 18, 440–445. [Google Scholar] [CrossRef] [PubMed]
- Franceschini, A.; Szklarczyk, D.; Frankild, S.; Kuhn, M.; Simonovic, M.; Roth, A.; Lin, J.; Minguez, P.; Bork, P.; von Mering, C.; et al. STRING v9.1: Protein-protein interaction networks, with increased coverage and integration. Nucleic Acids Res. 2013, 41, D808–D815. [Google Scholar] [CrossRef]
- Chang, L.; Zhou, G.; Soufan, O.; Xia, J. miRNet 2.0: Network-based visual analytics for miRNA functional analysis and systems biology. Nucleic Acids Res. 2020, 48, W244–W251. [Google Scholar] [CrossRef]
- Linz, D.; McEvoy, R.D.; Cowie, M.R.; Somers, V.K.; Nattel, S.; Levy, P.; Kalman, J.M.; Sanders, P. Associations of Obstructive Sleep Apnea with Atrial Fibrillation and Continuous Positive Airway Pressure Treatment: A Review. JAMA Cardiol. 2018, 3, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Senaratna, C.V.; Perret, J.L.; Lodge, C.J.; Lowe, A.J.; Campbell, B.E.; Matheson, M.C.; Hamilton, G.S.; Dharmage, S.C. Prevalence of obstructive sleep apnea in the general population: A systematic review. Sleep Med. Rev. 2017, 34, 70–81. [Google Scholar] [CrossRef]
- Thompson, C.; Legault, J.; Moullec, G.; Baltzan, M.; Cross, N.; Dang-Vu, T.T.; Martineau-Dussault, M.E.; Hanly, P.; Ayas, N.; Lorrain, D.; et al. A portrait of obstructive sleep apnea risk factors in 27,210 middle-aged and older adults in the Canadian Longitudinal Study on Aging. Sci. Rep. 2022, 12, 5127. [Google Scholar] [CrossRef]
- Labarca, G.; Gower, J.; Lamperti, L.; Dreyse, J.; Jorquera, J. Chronic intermittent hypoxia in obstructive sleep apnea: A narrative review from pathophysiological pathways to a precision clinical approach. Sleep Breath. 2020, 24, 751–760. [Google Scholar] [CrossRef]
- Bushi, G.; Padhi, B.K.; Shabil, M.; Satapathy, P.; Rustagi, S.; Pradhan, K.B.; Al-Qaim, Z.H.; Khubchandani, J.; Sah, R.; Sah, S.; et al. Cardiovascular Disease Outcomes Associated with Obstructive Sleep Apnea in Diabetics: A Systematic Review and Meta-Analysis. Diseases 2023, 11, 103. [Google Scholar] [CrossRef]
- Unnikrishnan, D.; Jun, J.; Polotsky, V. Inflammation in sleep apnea: An update. Rev. Endocr. Metab. Disord. 2015, 16, 25–34. [Google Scholar] [CrossRef]
- Aardoom, J.J.; Loheide-Niesmann, L.; Ossebaard, H.C.; Riper, H. Effectiveness of eHealth Interventions in Improving Treatment Adherence for Adults with Obstructive Sleep Apnea: Meta-Analytic Review. J. Med. Internet Res. 2020, 22, e16972. [Google Scholar] [CrossRef]
- Cattazzo, F.; Pengo, M.F.; Giontella, A.; Soranna, D.; Bilo, G.; Zambon, A.; Karalliedde, J.; Gnudi, L.; Martinez-Garcia, M.A.; Minuz, P.; et al. Effect of Continuous Positive Airway Pressure on Glucose and Lipid Profiles in Patients with Obstructive Sleep Apnoea: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Arch. Bronconeumol. 2023, 59, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Alberti, A.; Sarchielli, P.; Gallinella, E.; Floridi, A.; Floridi, A.; Mazzotta, G.; Gallai, V. Plasma cytokine levels in patients with obstructive sleep apnea syndrome: A preliminary study. J. Sleep Res. 2003, 12, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Ciftci, T.U.; Kokturk, O.; Bukan, N.; Bilgihan, A. The relationship between serum cytokine levels with obesity and obstructive sleep apnea syndrome. Cytokine 2004, 28, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Crewe, C.; Joffin, N.; Rutkowski, J.M.; Kim, M.; Zhang, F.; Towler, D.A.; Gordillo, R.; Scherer, P.E. An Endothelial-to-Adipocyte Extracellular Vesicle Axis Governed by Metabolic State. Cell 2018, 175, 695–708. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, A.; Kim, H.S.; Bojmar, L.; Gyan, K.E.; Cioffi, M.; Hernandez, J.; Zambirinis, C.P.; Rodrigues, G.; Molina, H.; Heissel, S.; et al. Extracellular Vesicle and Particle Biomarkers Define Multiple Human Cancers. Cell 2020, 182, 1044–1061. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef]
- Gimbrone, M.A., Jr.; Garcia-Cardena, G. Endothelial Cell Dysfunction and the Pathobiology of Atherosclerosis. Circ. Res. 2016, 118, 620–636. [Google Scholar] [CrossRef]
- Bister, N.; Pistono, C.; Huremagic, B.; Jolkkonen, J.; Giugno, R.; Malm, T. Hypoxia and extracellular vesicles: A review on methods, vesicular cargo and functions. J. Extracell. Vesicles 2020, 10, e12002. [Google Scholar] [CrossRef]
- Huber, H.J.; Holvoet, P. Exosomes: Emerging roles in communication between blood cells and vascular tissues during atherosclerosis. Curr. Opin. Lipidol. 2015, 26, 412–419. [Google Scholar] [CrossRef]
- Carter, N.; Mathiesen, A.H.; Miller, N.; Brown, M.; Colunga Biancatelli, R.M.L.; Catravas, J.D.; Dobrian, A.D. Endothelial cell-derived extracellular vesicles impair the angiogenic response of coronary artery endothelial cells. Front. Cardiovasc. Med. 2022, 9, 923081. [Google Scholar] [CrossRef]
- Khalyfa, A.; Gozal, D.; Chan, W.C.; Andrade, J.; Prasad, B. Circulating plasma exosomes in obstructive sleep apnoea and reverse dipping blood pressure. Eur. Respir. J. 2020, 55, 1901072. [Google Scholar] [CrossRef] [PubMed]
- Khalyfa, A.; Kheirandish-Gozal, L.; Khalyfa, A.A.; Philby, M.F.; Alonso-Alvarez, M.L.; Mohammadi, M.; Bhattacharjee, R.; Teran-Santos, J.; Huang, L.; Andrade, J.; et al. Circulating Plasma Extracellular Microvesicle MicroRNA Cargo and Endothelial Dysfunction in Children with Obstructive Sleep Apnea. Am. J. Respir. Crit. Care Med. 2016, 194, 1116–1126. [Google Scholar] [CrossRef] [PubMed]
- Khalyfa, A.; Gaddameedhi, S.; Crooks, E.; Zhang, C.; Li, Y.; Qiao, Z.; Trzepizur, W.; Kay, S.A.; Andrade, J.; Satterfield, B.C.; et al. Circulating Exosomal miRNAs Signal Circadian Misalignment to Peripheral Metabolic Tissues. Int. J. Mol. Sci. 2020, 21, 6396. [Google Scholar] [CrossRef]
- Bhattacharjee, R.; Khalyfa, A.; Khalyfa, A.A.; Mokhlesi, B.; Kheirandish-Gozal, L.; Almendros, I.; Peris, E.; Malhotra, A.; Gozal, D. Exosomal Cargo Properties, Endothelial Function and Treatment of Obesity Hypoventilation Syndrome: A Proof of Concept Study. J. Clin. Sleep Med. 2018, 14, 797–807. [Google Scholar] [CrossRef] [PubMed]
- Donoso-Quezada, J.; Ayala-Mar, S.; Gonzalez-Valdez, J. The role of lipids in exosome biology and intercellular communication: Function, analytics and applications. Traffic 2021, 22, 204–220. [Google Scholar] [CrossRef] [PubMed]
- Skotland, T.; Sandvig, K.; Llorente, A. Lipids in exosomes: Current knowledge and the way forward. Prog. Lipid Res. 2017, 66, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Lebkuchen, A.; Carvalho, V.M.; Venturini, G.; Salgueiro, J.S.; Freitas, L.S.; Dellavance, A.; Martins, F.C.; Lorenzi-Filho, G.; Cardozo, K.H.M.; Drager, L.F. Metabolomic and lipidomic profile in men with obstructive sleep apnoea: Implications for diagnosis and biomarkers of cardiovascular risk. Sci. Rep. 2018, 8, 11270. [Google Scholar] [CrossRef] [PubMed]
- Kawai, M.; Kirkness, J.P.; Yamamura, S.; Imaizumi, K.; Yoshimine, H.; Oi, K.; Ayuse, T. Increased phosphatidylcholine concentration in saliva reduces surface tension and improves airway patency in obstructive sleep apnoea. J. Oral. Rehabil. 2013, 40, 758–766. [Google Scholar] [CrossRef]
- Wang, M.; Han, X. Advanced Shotgun Lipidomics for Characterization of Altered Lipid Patterns in Neurodegenerative Diseases and Brain Injury. Methods Mol. Biol. 2016, 1303, 405–422. [Google Scholar] [CrossRef]
- Stuffers, S.; Sem Wegner, C.; Stenmark, H.; Brech, A. Multivesicular endosome biogenesis in the absence of ESCRTs. Traffic 2009, 10, 925–937. [Google Scholar] [CrossRef]
- Trajkovic, K.; Hsu, C.; Chiantia, S.; Rajendran, L.; Wenzel, D.; Wieland, F.; Schwille, P.; Brugger, B.; Simons, M. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 2008, 319, 1244–1247. [Google Scholar] [CrossRef] [PubMed]
- Djekic, D.; Pinto, R.; Repsilber, D.; Hyotylainen, T.; Henein, M. Serum untargeted lipidomic profiling reveals dysfunction of phospholipid metabolism in subclinical coronary artery disease. Vasc. Health Risk Manag. 2019, 15, 123–135. [Google Scholar] [CrossRef]
- Paapstel, K.; Kals, J.; Eha, J.; Tootsi, K.; Ottas, A.; Piir, A.; Jakobson, M.; Lieberg, J.; Zilmer, M. Inverse relations of serum phosphatidylcholines and lysophosphatidylcholines with vascular damage and heart rate in patients with atherosclerosis. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, C.; Sandin, M.; Sampaio, J.L.; Almgren, P.; Narkiewicz, K.; Hoffmann, M.; Hedner, T.; Wahlstrand, B.; Simons, K.; Shevchenko, A.; et al. Plasma lipid composition and risk of developing cardiovascular disease. PLoS ONE 2013, 8, e71846. [Google Scholar] [CrossRef]
- Tickner, J.A.; Urquhart, A.J.; Stephenson, S.A.; Richard, D.J.; O’Byrne, K.J. Functions and therapeutic roles of exosomes in cancer. Front. Oncol. 2014, 4, 127. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, S.; Weber, J.; Baxter, D.; Galas, D.J. Export of microRNAs and microRNA-protective protein by mammalian cells. Nucleic Acids Res. 2010, 38, 7248–7259. [Google Scholar] [CrossRef] [PubMed]
- Record, M.; Silvente-Poirot, S.; Poirot, M.; Wakelam, M.J.O. Extracellular vesicles: Lipids as key components of their biogenesis and functions. J. Lipid Res. 2018, 59, 1316–1324. [Google Scholar] [CrossRef] [PubMed]
- Tkach, M.; Thery, C. Communication by Extracellular Vesicles: Where We Are and Where We Need to Go. Cell 2016, 164, 1226–1232. [Google Scholar] [CrossRef]
- Lamichhane, T.N.; Raiker, R.S.; Jay, S.M. Exogenous DNA Loading into Extracellular Vesicles via Electroporation is Size-Dependent and Enables Limited Gene Delivery. Mol. Pharm. 2015, 12, 3650–3657. [Google Scholar] [CrossRef]
- Conte, L.; Greco, M.; Toraldo, D.M.; Arigliani, M.; Maffia, M.; De Benedetto, M. A review of the “OMICS” for management of patients with obstructive sleep apnoea. Acta Otorhinolaryngol. Ital. 2020, 40, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Sun, H.; Du, Y.; Li, L.; Lv, Q.; Yu, H.; Li, F.; Wang, Y.; Jiao, X.; Hu, C.; et al. Comprehensive Metabolomics and Machine Learning Identify Profound Oxidative Stress and Inflammation Signatures in Hypertensive Patients with Obstructive Sleep Apnea. Antioxidants 2022, 11, 1946. [Google Scholar] [CrossRef] [PubMed]
- Kowal, J.; Arras, G.; Colombo, M.; Jouve, M.; Morath, J.P.; Primdal-Bengtson, B.; Dingli, F.; Loew, D.; Tkach, M.; Thery, C. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc. Natl. Acad. Sci. USA 2016, 113, E968–E977. [Google Scholar] [CrossRef] [PubMed]
- Jeppesen, D.K.; Fenix, A.M.; Franklin, J.L.; Higginbotham, J.N.; Zhang, Q.; Zimmerman, L.J.; Liebler, D.C.; Ping, J.; Liu, Q.; Evans, R.; et al. Reassessment of Exosome Composition. Cell 2019, 177, 428–445.e418. [Google Scholar] [CrossRef]
- Abuyassin, B.; Badran, M.; Ayas, N.T.; Laher, I. Intermittent hypoxia causes histological kidney damage and increases growth factor expression in a mouse model of obstructive sleep apnea. PLoS ONE 2018, 13, e0192084. [Google Scholar] [CrossRef]
- Cederberg, K.L.J.; Hanif, U.; Peris Sempere, V.; Hedou, J.; Leary, E.B.; Schneider, L.D.; Lin, L.; Zhang, J.; Morse, A.M.; Blackman, A.; et al. Proteomic Biomarkers of the Apnea Hypopnea Index and Obstructive Sleep Apnea: Insights into the Pathophysiology of Presence, Severity, and Treatment Response. Int. J. Mol. Sci. 2022, 23, 7983. [Google Scholar] [CrossRef]
- Cheng, H.; Jin, S.; Huang, S.; Hu, T.; Zhao, M.; Li, D.; Wu, B. Serum Proteomic Analysis by Tandem Mass Tag-Based Quantitative Proteomics in Pediatric Obstructive Sleep Apnea. Front. Mol. Biosci. 2022, 9, 762336. [Google Scholar] [CrossRef]
- Kundel, V.; Cohen, O.; Khan, S.; Patel, M.; Kim-Schulze, S.; Kovacic, J.; Suarez-Farinas, M.; Shah, N.A. Advanced Proteomics and Cluster Analysis for Identifying Novel Obstructive Sleep Apnea Subtypes before and after Continuous Positive Airway Pressure Therapy. Ann. Am. Thorac. Soc. 2023, 20, 1038–1047. [Google Scholar] [CrossRef]
- Goetzl, L.; Merabova, N.; Darbinian, N.; Martirosyan, D.; Poletto, E.; Fugarolas, K.; Menkiti, O. Diagnostic Potential of Neural Exosome Cargo as Biomarkers for Acute Brain Injury. Ann. Clin. Transl. Neurol. 2018, 5, 4–10. [Google Scholar] [CrossRef]
- Li, D.; Huang, Z.; Dai, Y.; Guo, L.; Lin, S.; Liu, X. Bioinformatic identification of potential biomarkers and therapeutic targets in carotid atherosclerosis and vascular dementia. Front. Neurol. 2022, 13, 1091453. [Google Scholar] [CrossRef]
- Teixeira, A.R.; Ferreira, V.V.; Pereira-da-Silva, T.; Ferreira, R.C. The role of miRNAs in the diagnosis of stable atherosclerosis of different arterial territories: A critical review. Front. Cardiovasc. Med. 2022, 9, 1040971. [Google Scholar] [CrossRef]
- Trajkovski, M.; Hausser, J.; Soutschek, J.; Bhat, B.; Akin, A.; Zavolan, M.; Heim, M.H.; Stoffel, M. MicroRNAs 103 and 107 regulate insulin sensitivity. Nature 2011, 474, 649–653. [Google Scholar] [CrossRef]
- Schroen, B.; Heymans, S. Small but smart—microRNAs in the centre of inflammatory processes during cardiovascular diseases, the metabolic syndrome, and ageing. Cardiovasc. Res. 2012, 93, 605–613. [Google Scholar] [CrossRef]
- Vickers, K.C.; Remaley, A.T. MicroRNAs in atherosclerosis and lipoprotein metabolism. Curr. Opin. Endocrinol. Diabetes Obes. 2010, 17, 150–155. [Google Scholar] [CrossRef]
- Marquart, T.J.; Allen, R.M.; Ory, D.S.; Baldan, A. miR-33 links SREBP-2 induction to repression of sterol transporters. Proc. Natl. Acad. Sci. USA 2010, 107, 12228–12232. [Google Scholar] [CrossRef]
- Rayner, K.J.; Suarez, Y.; Davalos, A.; Parathath, S.; Fitzgerald, M.L.; Tamehiro, N.; Fisher, E.A.; Moore, K.J.; Fernandez-Hernando, C. MiR-33 contributes to the regulation of cholesterol homeostasis. Science 2010, 328, 1570–1573. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Ni, Y.-Q.; Liu, Y.-S. Mechanisms of Action of MiRNAs and LncRNAs in Extracellular Vesicle in Atherosclerosis. Front. Cardiovasc. Med. 2021, 8, 733985. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Chen, Z.; Qin, Y.; Wei, Y. MiR-664a-3p expression in patients with obstructive sleep apnea: A potential marker of atherosclerosis. Medicine 2018, 97, e9813. [Google Scholar] [CrossRef] [PubMed]
- Freitas, L.S.; Silveira, A.C.; Martins, F.C.; Costa-Hong, V.; Lebkuchen, A.; Cardozo, K.H.M.; Bernardes, F.M.; Bortolotto, L.A.; Lorenzi-Filho, G.; Oliveira, E.M.; et al. Severe obstructive sleep apnea is associated with circulating microRNAs related to heart failure, myocardial ischemia, and cancer proliferation. Sleep Breath. 2020, 24, 1463–1472. [Google Scholar] [CrossRef]
- Gongol, B.; Shang, F.; He, M.; Zhao, Y.; Shi, W.; Cheng, M.; Shyy, J.Y.; Wang, L.; Malhotra, A.; Bhattacharjee, R. Serum miR-92a is Elevated in Children and Adults with Obstructive Sleep Apnea. J. Mol. Biomark. Diagn. 2020, 11, 1–16. [Google Scholar]
- Shang, F.; Wang, S.C.; Gongol, B.; Han, S.Y.; Cho, Y.; Schiavon, C.R.; Chen, L.; Xing, Y.; Zhao, Y.; Ning, M.; et al. Obstructive Sleep Apnea-induced Endothelial Dysfunction Is Mediated by miR-210. Am. J. Respir. Crit. Care Med. 2023, 207, 323–335. [Google Scholar] [CrossRef]
- Javaheri, S.; Barbe, F.; Campos-Rodriguez, F.; Dempsey, J.A.; Khayat, R.; Javaheri, S.; Malhotra, A.; Martinez-Garcia, M.A.; Mehra, R.; Pack, A.I.; et al. Sleep Apnea: Types, Mechanisms, and Clinical Cardiovascular Consequences. J. Am. Coll. Cardiol. 2017, 69, 841–858. [Google Scholar] [CrossRef] [PubMed]
- Zietzer, A.; Breitruck, N.; Dusing, P.; Bohle, S.; Klussmann, J.P.; Al-Kassou, B.; Goody, P.R.; Hosen, M.R.; Nickenig, G.; Nachtsheim, L.; et al. The lncRNA MRPL20-AS1 is associated with severe OSAS and downregulated upon hypoxic injury of endothelial cells. Int. J. Cardiol. 2022, 369, 65–68. [Google Scholar] [CrossRef]
- Graw, S.; Chappell, K.; Washam, C.L.; Gies, A.; Bird, J.; Robeson, M.S., II; Byrum, S.D. Multi-omics data integration considerations and study design for biological systems and disease. Mol. Omics 2021, 17, 170–185. [Google Scholar] [CrossRef]
- Khalyfa, A.; Kheirandish-Gozal, L.; Gozal, D. Exosome and Macrophage Crosstalk in Sleep-Disordered Breathing-Induced Metabolic Dysfunction. Int. J. Mol. Sci. 2018, 19, 3383. [Google Scholar] [CrossRef] [PubMed]
- Skotland, T.; Ekroos, K.; Kauhanen, D.; Simolin, H.; Seierstad, T.; Berge, V.; Sandvig, K.; Llorente, A. Molecular lipid species in urinary exosomes as potential prostate cancer biomarkers. Eur. J. Cancer 2017, 70, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Melo, S.A.; Luecke, L.B.; Kahlert, C.; Fernandez, A.F.; Gammon, S.T.; Kaye, J.; LeBleu, V.S.; Mittendorf, E.A.; Weitz, J.; Rahbari, N.; et al. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature 2015, 523, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Otero-Ortega, L.; Laso-Garcia, F.; Gomez-de Frutos, M.; Fuentes, B.; Diekhorst, L.; Diez-Tejedor, E.; Gutierrez-Fernandez, M. Role of Exosomes as a Treatment and Potential Biomarker for Stroke. Transl. Stroke Res. 2019, 10, 241–249. [Google Scholar] [CrossRef]
- Cohn, W.; Melnik, M.; Huang, C.; Teter, B.; Chandra, S.; Zhu, C.; McIntire, L.B.; John, V.; Gylys, K.H.; Bilousova, T. Multi-Omics Analysis of Microglial Extracellular Vesicles from Human Alzheimer’s Disease Brain Tissue Reveals Disease-Associated Signatures. Front. Pharmacol. 2021, 12, 766082. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Liu, H.; Tang, W.H. Exosomes: Biogenesis, biologic function and clinical potential. Cell Biosci. 2019, 9, 19. [Google Scholar] [CrossRef]
- Chaput, N.; Thery, C. Exosomes: Immune properties and potential clinical implementations. Semin. Immunopathol. 2011, 33, 419–440. [Google Scholar] [CrossRef]
- de Miguel Perez, D.; Rodriguez Martinez, A.; Ortigosa Palomo, A.; Delgado Urena, M.; Garcia Puche, J.L.; Robles Remacho, A.; Exposito Hernandez, J.; Lorente Acosta, J.A.; Ortega Sanchez, F.G.; Serrano, M.J. Extracellular vesicle-miRNAs as liquid biopsy biomarkers for disease identification and prognosis in metastatic colorectal cancer patients. Sci. Rep. 2020, 10, 3974. [Google Scholar] [CrossRef]
- Wu, J.; Shen, Z. Exosomal miRNAs as biomarkers for diagnostic and prognostic in lung cancer. Cancer Med. 2020, 9, 6909–6922. [Google Scholar] [CrossRef] [PubMed]
- Mokhlesi, B.; Ham, S.A.; Gozal, D. The effect of sex and age on the comorbidity burden of OSA: An observational analysis from a large nationwide US health claims database. Eur. Respir. J. 2016, 47, 1162–1169. [Google Scholar] [CrossRef] [PubMed]
- Marin, J.M.; Artal, J.; Martin, T.; Carrizo, S.J.; Andres, M.; Martin-Burriel, I.; Bolea, R.; Sanz, A.; Varona, L.; Godino, J.; et al. Epigenetics modifications and Subclinical Atherosclerosis in Obstructive Sleep Apnea: The EPIOSA study. BMC Pulm. Med. 2014, 14, 114. [Google Scholar] [CrossRef]
- Berry, R.B.; Budhiraja, R.; Gottlieb, D.J.; Gozal, D.; Iber, C.; Kapur, V.K.; Marcus, C.L.; Mehra, R.; Parthasarathy, S.; Quan, S.F.; et al. Rules for scoring respiratory events in sleep: Update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J. Clin. Sleep Med. 2012, 8, 597–619. [Google Scholar] [CrossRef]
- Thery, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Wang, Y.; Wang, H.; Zhu, Z.; Xiao, Z. Visualizing of the cellular uptake and intracellular trafficking of exosomes by live-cell microscopy. J. Cell. Biochem. 2010, 111, 488–496. [Google Scholar] [CrossRef]
- Chen, L.; Xiang, B.; Wang, X.; Xiang, C. Exosomes derived from human menstrual blood-derived stem cells alleviate fulminant hepatic failure. Stem Cell Res. Ther. 2017, 8, 9. [Google Scholar] [CrossRef] [PubMed]
- Khalyfa, A.; Youssefnia, N.; Foster, G.E.; Beaudin, A.E.; Qiao, Z.; Pialoux, V.; Pun, M.; Hanly, P.J.; Kheirandish-Gozal, L.; Poulin, M.J.; et al. Plasma Exosomes and Improvements in Endothelial Function by Angiotensin 2 Type 1 Receptor or Cyclooxygenase 2 Blockade following Intermittent Hypoxia. Front. Neurol. 2017, 8, 709. [Google Scholar] [CrossRef]
- Giaever, I.; Keese, C.R. A morphological biosensor for mammalian cells. Nature 1993, 366, 591–592. [Google Scholar] [CrossRef]
- Khalyfa, A.; Gozal, D.; Kheirandish-Gozal, L. Plasma Extracellular Vesicles in Children with OSA Disrupt Blood-Brain Barrier Integrity and Endothelial Cell Wound Healing In Vitro. Int. J. Mol. Sci. 2019, 20, 6233. [Google Scholar] [CrossRef]
- Carpentier, G.; Berndt, S.; Ferratge, S.; Rasband, W.; Cuendet, M.; Uzan, G.; Albanese, P. Angiogenesis Analyzer for ImageJ—A comparative morphometric analysis of “Endothelial Tube Formation Assay” and “Fibrin Bead Assay”. Sci. Rep. 2020, 10, 11568. [Google Scholar] [CrossRef] [PubMed]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Kind, T.; Liu, K.H.; Lee, D.Y.; DeFelice, B.; Meissen, J.K.; Fiehn, O. LipidBlast in silico tandem mass spectrometry database for lipid identification. Nat. Methods 2013, 10, 755–758. [Google Scholar] [CrossRef]
- Sticht, C.; De La Torre, C.; Parveen, A.; Gretz, N. miRWalk: An online resource for prediction of microRNA binding sites. PLoS ONE 2018, 13, e0206239. [Google Scholar] [CrossRef] [PubMed]
- Dennis, C. Genomics: Compare and contrast. Nature 2003, 426, 750–751. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kohl, M.; Wiese, S.; Warscheid, B. Cytoscape: Software for visualization and analysis of biological networks. Methods Mol. Biol. 2011, 696, 291–303. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Rohart, F.; Gautier, B.; Singh, A.; Le Cao, K.A. mixOmics: An R package for ‘omics feature selection and multiple data integration. PLoS Comput. Biol. 2017, 13, e1005752. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Gu, L.; Eils, R.; Schlesner, M.; Brors, B. circlize Implements and enhances circular visualization in R. Bioinformatics 2014, 30, 2811–2812. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, X. miRDB: An online database for prediction of functional microRNA targets. Nucleic Acids Res. 2020, 48, D127–D131. [Google Scholar] [CrossRef] [PubMed]
- UniProt, C. UniProt: The Universal Protein Knowledgebase in 2023. Nucleic Acids Res 2023, 51, D523–D531. [Google Scholar] [CrossRef]
- Raudvere, U.; Kolberg, L.; Kuzmin, I.; Arak, T.; Adler, P.; Peterson, H.; Vilo, J. g:Profiler: A web server for functional enrichment analysis and conversions of gene lists (2019 update). Nucleic Acids Res. 2019, 47, W191–W198. [Google Scholar] [CrossRef]
- Pang, Z.; Chong, J.; Zhou, G.; de Lima Morais, D.A.; Chang, L.; Barrette, M.; Gauthier, C.; Jacques, P.E.; Li, S.; Xia, J. MetaboAnalyst 5.0: Narrowing the gap between raw spectra and functional insights. Nucleic Acids Res. 2021, 49, W388–W396. [Google Scholar] [CrossRef]

| Term | OSA | OSAT |
|---|---|---|
| Age | 41.11 ± 8.12 | 42.13 ± 6.0 |
| BMI, kg/m2 | 30.21 ± 0.51 | 32.12 ± 3.22 |
| AHI, events/hour | 70.03 ± 16.08 * | 2.71 ± 2.05 ** |
| Triglycerides (mg/dL) | 222.14 ± 74. 18 | 148.15 ± 78.17 ** |
| Total cholesterol (mg/dL) | 234.07 ± 20.14 | 212.28 ± 33.32 * |
| HDL cholesterol (mg/dL) | 47.01 ± 10.06 | 42.12 ± 7.13 * |
| LDL cholesterol (mg/dL) | 135.29 ± 13.18 | 151.16 ± 33.36 |
| Glucose (mmol/L) | 101.12 ± 11.16 | 94.09 ± 14.28 * |
| SysBP | 126.17 ± 10.05 | 125.22 ± 15.15 |
| DyBP | 82.11 ± 8.13 | 73.16 ± 10.05 * |
| SpO2 during wake (%) | 90.50 ± 2.87 | 94.21 ± 1.07 ** |
| Items | FC | log2 (FC) | p-Value | =−LOG10 (p-Value) |
|---|---|---|---|---|
| LPC O-16:1 | 0.06 | −4.1391 | 0.003138 | 2.5034 |
| TG 8:0_8:0_28:4 | 0.28 | −1.8468 | 0.015079 | 1.8216 |
| LDGTS 21:0 | 0.37 | −1.4297 | 0.008058 | 2.0938 |
| TG 8:0_12:0_38:9 | 0.48 | −1.0739 | 0.018855 | 1.7246 |
| TG 8:0_11:0_38:8 | 0.49 | −1.0182 | 0.041437 | 1.3826 |
| AHexCer (O-20:5)16:1;2O/14:0;O | 0.51 | −0.96081 | 0.019799 | 1.7034 |
| PC 9:0_42:6 | 0.53 | −0.91807 | 0.043307 | 1.3634 |
| DGGA 27:0_17:1 | 0.56 | −0.8414 | 0.015464 | 1.8107 |
| TG 8:0_12:0_38:7 | 0.56 | −0.83895 | 0.034252 | 1.4653 |
| TG 8:0_10:0_38:7 | 0.57 | −0.81719 | 0.015201 | 1.8181 |
| TG 8:0_9:0_36:4 | 0.6 | −0.74876 | 0.007062 | 2.1511 |
| TG 8:0_10:0_38:5 | 0.61 | −0.70639 | 0.017472 | 1.7577 |
| TG 8:0_8:0_38:4 | 0.63 | −0.65961 | 0.004549 | 2.342 |
| TG 8:0_8:0_38:5 | 0.63 | −0.6593 | 0.014651 | 1.8341 |
| TG 54:4|TG 18:1_18:1_18:2 | 0.64 | −0.6345 | 0.000793 | 3.1007 |
| TG 8:0_8:0_38:6 | 0.64 | −0.65175 | 0.024064 | 1.6186 |
| TG 8:0_9:0_38:4 | 0.65 | −0.61458 | 0.000224 | 3.6501 |
| PS 41:4 | 0.66 | −0.59339 | 0.009325 | 2.0303 |
| DGGA 17:0_27:0 | 0.66 | −0.60012 | 0.024245 | 1.6154 |
| TG 8:0_12:0_38:6 | 0.67 | −0.57631 | 0.039363 | 1.4049 |
| SM 32:6;2O | 0.7 | −0.51904 | 0.010348 | 1.9851 |
| TG 8:0_8:0_36:3 | 0.72 | −0.47121 | 0.045847 | 1.3387 |
| TG 8:0_9:0_28:2 | 1.41 | 0.49281 | 0.034692 | 1.4598 |
| PC 84:7 | 1.44 | 0.52454 | 0.029241 | 1.534 |
| TG 17:1_18:1_18:2 | 1.44 | 0.52957 | 0.043477 | 1.3617 |
| TG 15:0_15:0_17:2 | 1.47 | 0.55505 | 0.032567 | 1.4872 |
| SL 21:0;O/26:2;O | 1.47 | 0.55466 | 0.049341 | 1.3068 |
| SL 21:0;O/26:0;O | 1.48 | 0.56277 | 0.047757 | 1.321 |
| TG 47:2|TG 14:0_15:0_18:2 | 1.49 | 0.57647 | 0.029857 | 1.525 |
| TG 51:0|TG 16:0_17:0_18:0 | 1.51 | 0.59701 | 0.032805 | 1.4841 |
| TG 48:2|TG 14:0_16:0_18:2 | 1.53 | 0.61758 | 0.046616 | 1.3315 |
| TG 46:3|TG 10:0_17:1_19:2 | 1.54 | 0.62159 | 0.032146 | 1.4929 |
| TG O-16:1_18:0_18:0 | 1.54 | 0.62365 | 0.036821 | 1.4339 |
| TG 45:1|TG 12:0_15:0_18:1 | 1.56 | 0.64287 | 0.030752 | 1.5121 |
| TG 10:0_18:2_18:2 | 1.57 | 0.65003 | 0.037827 | 1.4222 |
| TG 49:0|TG 16:0_16:0_17:0 | 1.58 | 0.65533 | 0.006496 | 2.1874 |
| TG 13:0_13:0_18:2 | 1.59 | 0.6695 | 0.040482 | 1.3927 |
| TG 46:2|TG 12:0_16:0_18:2.1 | 1.59 | 0.66815 | 0.041077 | 1.3864 |
| TG 46:2|TG 12:0_16:0_18:2 | 1.6 | 0.67826 | 0.035556 | 1.4491 |
| TG 42:0|TG 12:0_14:0_16:0 | 1.6 | 0.67606 | 0.035651 | 1.4479 |
| TG 42:1|TG 10:0_16:0_16:1 | 1.6 | 0.67972 | 0.044905 | 1.3477 |
| TG 8:0_9:0_26:1 | 1.61 | 0.68498 | 0.01263 | 1.8986 |
| TG 8:0_8:0_26:2 | 1.65 | 0.7221 | 0.020791 | 1.6821 |
| TG 48:0|TG 14:0_16:0_18:0 | 1.65 | 0.72383 | 0.039058 | 1.4083 |
| TG 8:0_9:0_28:1 | 1.66 | 0.72772 | 0.031111 | 1.5071 |
| TG 48:0|TG 16:0_16:0_16:0 | 1.66 | 0.73223 | 0.035803 | 1.4461 |
| TG 42:2|TG 8:0_16:0_18:2 | 1.67 | 0.7407 | 0.017513 | 1.7566 |
| SM 39:2;3O | 1.67 | 0.7361 | 0.042669 | 1.3699 |
| TG 8:0_9:0_28:3 | 1.69 | 0.75753 | 0.002915 | 2.5354 |
| TG 44:0|TG 12:0_14:0_18:0 | 1.69 | 0.75964 | 0.047626 | 1.3222 |
| TG 12:0_14:0_18:1 | 1.69 | 0.75294 | 0.04945 | 1.3058 |
| TG 46:1|TG 12:0_16:0_18:1 | 1.69 | 0.7595 | 0.049784 | 1.3029 |
| SL 21:1;O/26:2;O | 1.7 | 0.76579 | 0.045348 | 1.3434 |
| TG 8:0_9:0_30:3 | 1.72 | 0.77893 | 0.033386 | 1.4764 |
| DGCC 40:0_44:8.1 | 1.77 | 0.82628 | 0.026113 | 1.5831 |
| TG 8:0_8:0_26:1 | 1.79 | 0.84235 | 0.021518 | 1.6672 |
| TG 44:2|TG 10:0_16:0_18:2 | 1.8 | 0.84756 | 0.024438 | 1.6119 |
| TG 43:1|TG 9:0_16:0_18:1 | 1.8 | 0.84461 | 0.037674 | 1.424 |
| PC 80:2 | 1.87 | 0.90004 | 0.031744 | 1.4983 |
| DGCC 42:0_44:6 | 1.94 | 0.95621 | 0.034975 | 1.4562 |
| TG 40:1|TG 8:0_16:0_16:1 | 1.96 | 0.97235 | 0.016498 | 1.7826 |
| SL 17:0;O/26:2;O | 1.98 | 0.98487 | 0.004897 | 2.3101 |
| Cer 9:0;3O/26:2;(2OH) | 2.02 | 1.0143 | 0.010273 | 1.9883 |
| DGCC 40:0_44:8 | 2.05 | 1.0387 | 0.014613 | 1.8353 |
| SL 19:1;O/26:2;O | 2.09 | 1.065 | 0.005659 | 2.2473 |
| PS 41:3 | 2.17 | 1.1178 | 0.013763 | 1.8613 |
| DGCC 40:0_44:7 | 2.2 | 1.1372 | 0.002424 | 2.6154 |
| DGCC 42:0_44:10 | 2.24 | 1.1666 | 0.010602 | 1.9746 |
| Cer 9:0;3O/42:0;(2OH) | 2.4 | 1.263 | 0.001415 | 2.8492 |
| DG 34:2 | 2.52 | 1.3321 | 0.002642 | 2.5781 |
| DGCC 38:0_44:5 | 2.98 | 1.5758 | 0.008464 | 2.0724 |
| HexCer 19:3;3O/26:2;(2OH) | 4.06 | 2.0217 | 0.006126 | 2.2128 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalyfa, A.; Marin, J.M.; Sanz-Rubio, D.; Lyu, Z.; Joshi, T.; Gozal, D. Multi-Omics Analysis of Circulating Exosomes in Adherent Long-Term Treated OSA Patients. Int. J. Mol. Sci. 2023, 24, 16074. https://doi.org/10.3390/ijms242216074
Khalyfa A, Marin JM, Sanz-Rubio D, Lyu Z, Joshi T, Gozal D. Multi-Omics Analysis of Circulating Exosomes in Adherent Long-Term Treated OSA Patients. International Journal of Molecular Sciences. 2023; 24(22):16074. https://doi.org/10.3390/ijms242216074
Chicago/Turabian StyleKhalyfa, Abdelnaby, Jose M. Marin, David Sanz-Rubio, Zhen Lyu, Trupti Joshi, and David Gozal. 2023. "Multi-Omics Analysis of Circulating Exosomes in Adherent Long-Term Treated OSA Patients" International Journal of Molecular Sciences 24, no. 22: 16074. https://doi.org/10.3390/ijms242216074
APA StyleKhalyfa, A., Marin, J. M., Sanz-Rubio, D., Lyu, Z., Joshi, T., & Gozal, D. (2023). Multi-Omics Analysis of Circulating Exosomes in Adherent Long-Term Treated OSA Patients. International Journal of Molecular Sciences, 24(22), 16074. https://doi.org/10.3390/ijms242216074






