Novel Peritoneal Sclerosis Rat Model Developed by Administration of Bleomycin and Lansoprazole
Abstract
:1. Introduction
2. Results
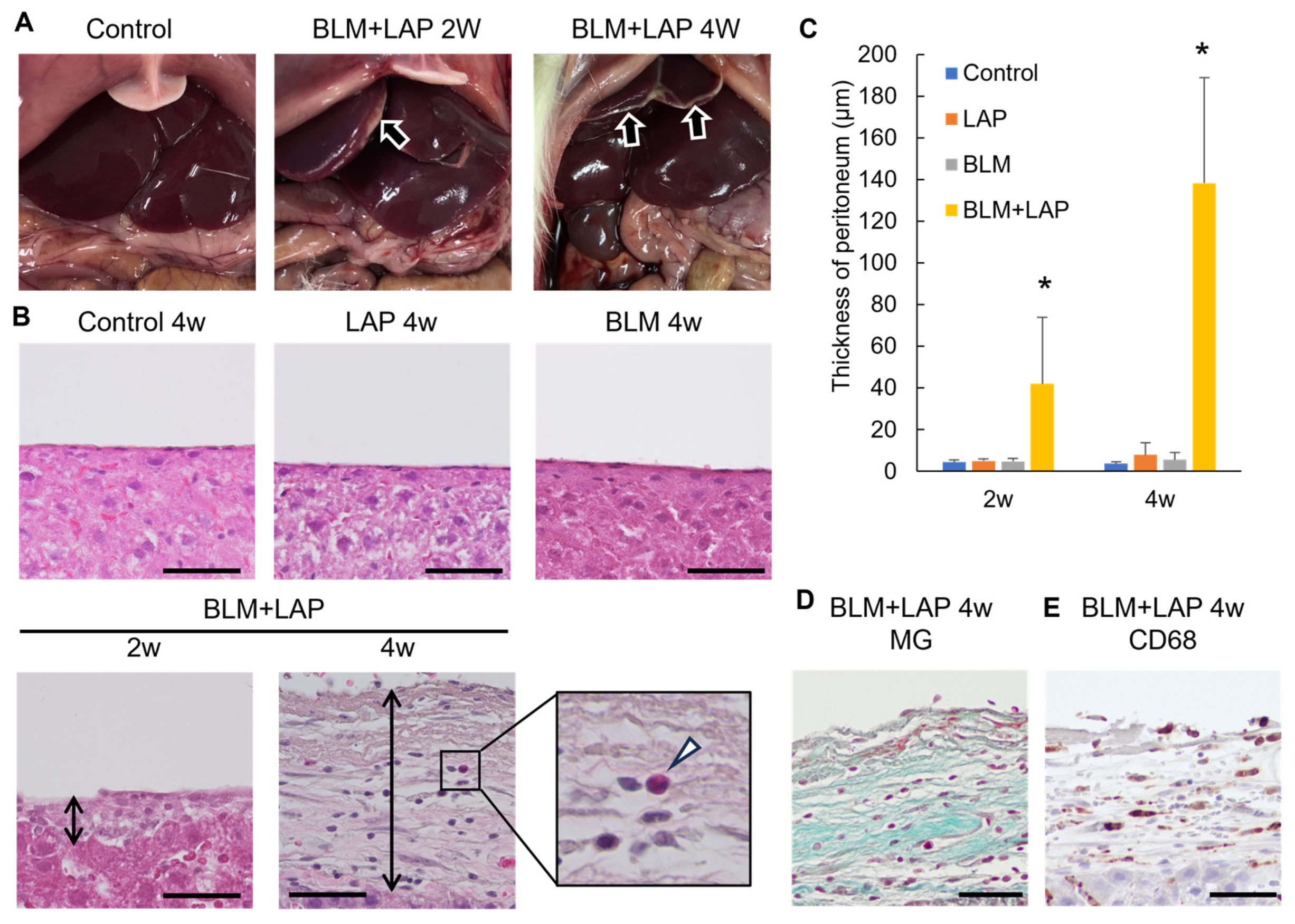
2.1. Peritoneal Changes around the Livers of Rats Given BLM + LAP
2.1.1. Histological Changes in the Peritoneal Tissue around the Liver
2.1.2. Effect of BLM + LAP on Rat Liver Injuries
2.1.3. Expression Changes of Genes Associated with Fibrosis in the Peritoneal Tissue around the Liver
2.1.4. Expression Changes of Genes Associated with Migration of Macrophages and Eosinophils in the Peritoneal Tissue around the Liver
2.2. BLM + LAP Increased the Expression of Genes Associated with the Migration and Proliferation of Fibroblasts
2.3. BLM + LAP Increased the Number of Mesothelial Cells Indicating Immunoreactivity against Cytokeratin and αSMA.
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Animals
4.3. Drug Administration
4.4. Cell Culture
4.5. Histology
4.6. Quantitative PCR
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bermo, M.S.; Koppula, B.; Kumar, M.; Leblond, A.; Matesan, M.C. The Peritoneum: What Nuclear Radiologists Need to Know. Semin. Nucl. Med. 2020, 50, 405–418. [Google Scholar] [CrossRef]
- do Amaral, R.; Arcanjo, K.D.; El-Cheikh, M.C.; de Oliveira, F.L. The Peritoneum: Health, Disease, and Perspectives regarding Tissue Engineering and Cell Therapies. Cells Tissues Organs 2017, 204, 211–217. [Google Scholar] [CrossRef]
- Jagirdar, R.M.; Bozikas, A.; Zarogiannis, S.G.; Bartosova, M.; Schmitt, C.P.; Liakopoulos, V. Encapsulating Peritoneal Sclerosis: Pathophysiology and Current Treatment Options. Int. J. Mol. Sci. 2019, 20, 5765. [Google Scholar] [CrossRef]
- Masola, V.; Bonomini, M.; Borrelli, S.; Di Liberato, L.; Vecchi, L.; Onisto, M.; Gambaro, G.; Palumbo, R.; Arduini, A. Fibrosis of Peritoneal Membrane as Target of New Therapies in Peritoneal Dialysis. Int. J. Mol. Sci. 2022, 23, 4831. [Google Scholar] [CrossRef]
- Williams, J.D.; Craig, K.J.; Topley, N.; Von Ruhland, C.; Fallon, M.; Newman, G.R.; Mackenzie, R.K.; Williams, G.T. Morphologic changes in the peritoneal membrane of patients with renal disease. J. Am. Soc. Nephrol. 2002, 13, 470–479. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, L.; He, W.; Zhu, C.; Yang, J.; Sheng, M. Astragalus membranaceus inhibits peritoneal fibrosis via monocyte chemoattractant protein (MCP)-1 and the transforming growth factor-beta1 (TGF-beta1) pathway in rats submitted to peritoneal dialysis. Int. J. Mol. Sci. 2014, 15, 12959–12971. [Google Scholar] [CrossRef]
- Sachs, G.; Shin, J.M.; Briving, C.; Wallmark, B.; Hersey, S. The pharmacology of the gastric acid pump: The H+,K+ ATPase. Annu. Rev. Pharmacol. Toxicol. 1995, 35, 277–305. [Google Scholar] [CrossRef]
- Yamashita, Y.; Ueyama, T.; Nishi, T.; Yamamoto, Y.; Kawakoshi, A.; Sunami, S.; Iguchi, M.; Tamai, H.; Ueda, K.; Ito, T.; et al. Nrf2-inducing anti-oxidation stress response in the rat liver--new beneficial effect of lansoprazole. PLoS ONE 2014, 9, e97419. [Google Scholar] [CrossRef]
- Khaleel, S.A.; Alzokaky, A.A.; Raslan, N.A.; Alwakeel, A.I.; Abd El-Aziz, H.G.; Abd-Allah, A.R. Lansoprazole halts contrast induced nephropathy through activation of Nrf2 pathway in rats. Chem. Biol. Interact. 2017, 270, 33–40. [Google Scholar] [CrossRef]
- Yoda, Y.; Amagase, K.; Kato, S.; Tokioka, S.; Murano, M.; Kakimoto, K.; Nishio, H.; Umegaki, E.; Takeuchi, K.; Higuchi, K. Prevention by lansoprazole, a proton pump inhibitor, of indomethacin -induced small intestinal ulceration in rats through induction of heme oxygenase-1. J. Physiol. Pharmacol. 2010, 61, 287–294. [Google Scholar]
- Hassanein, E.H.M.; Ali, F.E.M.; Mohammedsaleh, Z.M.; Atwa, A.M.; Elfiky, M. The involvement of Nrf2/HO-1/cytoglobin and Ang-II/NF-kappaB signals in the cardioprotective mechanism of lansoprazole against cisplatin-induced heart injury. Toxicol. Mech. Methods 2023, 33, 316–326. [Google Scholar] [CrossRef]
- Nishi, T.; Yamamoto, Y.; Yamagishi, N.; Iguchi, M.; Tamai, H.; Ito, T.; Tsuruo, Y.; Ichinose, M.; Kitano, M.; Ueyama, T. Lansoprazole prevents the progression of liver fibrosis in non-alcoholic steatohepatitis model rats. J. Pharm. Pharmacol. 2018, 70, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.N.; Lee, J.; Yang, H.S.; Cho, J.W.; Kwon, S.; Kim, Y.B.; Her, J.D.; Cho, K.H.; Song, C.W.; Lee, K. Dose-response Effects of Bleomycin on Inflammation and Pulmonary Fibrosis in Mice. Toxicol. Res. 2010, 26, 217–222. [Google Scholar] [CrossRef]
- Yang, H.Z.; Cui, B.; Liu, H.Z.; Chen, Z.R.; Yan, H.M.; Hua, F.; Hu, Z.W. Targeting TLR2 attenuates pulmonary inflammation and fibrosis by reversion of suppressive immune microenvironment. J. Immunol. 2009, 182, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.J.; Ye, H.; Zhang, Q.; Li, F.Z.; Song, L.J.; Yang, J.; Mu, Q.; Rao, S.S.; Cai, P.C.; Xiang, F.; et al. Bleomycin induced epithelial-mesenchymal transition (EMT) in pleural mesothelial cells. Toxicol. Appl. Pharmacol. 2015, 283, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Ju, N.; Hayashi, H.; Shimamura, M.; Baba, S.; Yoshida, S.; Morishita, R.; Rakugi, H.; Nakagami, H. Prevention of bleomycin-induced pulmonary fibrosis by a RANKL peptide in mice. Sci. Rep. 2022, 12, 12474. [Google Scholar] [CrossRef]
- Braun, N.; Fritz, P.; Ulmer, C.; Latus, J.; Kimmel, M.; Biegger, D.; Ott, G.; Reimold, F.; Thon, K.P.; Dippon, J.; et al. Histological criteria for encapsulating peritoneal sclerosis—A standardized approach. PLoS ONE 2012, 7, e48647. [Google Scholar] [CrossRef]
- Marchant, V.; Tejera-Munoz, A.; Marquez-Exposito, L.; Rayego-Mateos, S.; Rodrigues-Diez, R.R.; Tejedor, L.; Santos-Sanchez, L.; Egido, J.; Ortiz, A.; Valdivielso, J.M.; et al. IL-17A as a Potential Therapeutic Target for Patients on Peritoneal Dialysis. Biomolecules 2020, 10, 1361. [Google Scholar] [CrossRef]
- Si, M.; Wang, Q.; Li, Y.; Lin, H.; Luo, D.; Zhao, W.; Dou, X.; Liu, J.; Zhang, H.; Huang, Y.; et al. Inhibition of hyperglycolysis in mesothelial cells prevents peritoneal fibrosis. Sci. Transl. Med. 2019, 11, eaav5341. [Google Scholar] [CrossRef]
- Pein, M.; Insua-Rodriguez, J.; Hongu, T.; Riedel, A.; Meier, J.; Wiedmann, L.; Decker, K.; Essers, M.A.G.; Sinn, H.P.; Spaich, S.; et al. Metastasis-initiating cells induce and exploit a fibroblast niche to fuel malignant colonization of the lungs. Nat. Commun. 2020, 11, 1494. [Google Scholar] [CrossRef]
- Szentes, V.; Gazdag, M.; Szokodi, I.; Dezsi, C.A. The Role of CXCR3 and Associated Chemokines in the Development of Atherosclerosis and during Myocardial Infarction. Front. Immunol. 2018, 9, 1932. [Google Scholar] [CrossRef] [PubMed]
- Boniakowski, A.E.; Kimball, A.S.; Joshi, A.; Schaller, M.; Davis, F.M.; denDekker, A.; Obi, A.T.; Moore, B.B.; Kunkel, S.L.; Gallagher, K.A. Murine macrophage chemokine receptor CCR2 plays a crucial role in macrophage recruitment and regulated inflammation in wound healing. Eur. J. Immunol. 2018, 48, 1445–1455. [Google Scholar] [CrossRef] [PubMed]
- Xuan, Y.; Chen, C.; Wen, Z.; Wang, D.W. The Roles of Cardiac Fibroblasts and Endothelial Cells in Myocarditis. Front. Cardiovasc. Med. 2022, 9, 882027. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Liu, C.; Wang, Q.; Liu, S.; Min, J. CC Chemokines in Idiopathic Pulmonary Fibrosis: Pathogenic Role and Therapeutic Potential. Biomolecules 2023, 13, 333. [Google Scholar] [CrossRef] [PubMed]
- Unver, N. Macrophage chemoattractants secreted by cancer cells: Sculptors of the tumor microenvironment and another crucial piece of the cancer secretome as a therapeutic target. Cytokine Growth Factor. Rev. 2019, 50, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Vatrella, A.; Maglio, A.; Pelaia, C.; Ciampo, L.; Pelaia, G.; Vitale, C. Eosinophilic inflammation: An Appealing Target for Pharmacologic Treatments in Severe Asthma. Biomedicines 2022, 10, 2181. [Google Scholar] [CrossRef]
- Kubota, A.; Frangogiannis, N.G. Macrophages in myocardial infarction. Am. J. Physiol. Cell Physiol. 2022, 323, C1304–C1324. [Google Scholar] [CrossRef]
- Lenga, Y.; Koh, A.; Perera, A.S.; McCulloch, C.A.; Sodek, J.; Zohar, R. Osteopontin expression is required for myofibroblast differentiation. Circ. Res. 2008, 102, 319–327. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, B.; Cao, B.; Wei, F.; Yu, X.; Li, G.F.; Chen, H.; Wei, L.Q.; Wang, P.L. Synergistic protection of Schizandrin B and Glycyrrhizic acid against bleomycin-induced pulmonary fibrosis by inhibiting TGF-beta1/Smad2 pathways and overexpression of NOX4. Int. Immunopharmacol. 2017, 48, 67–75. [Google Scholar] [CrossRef]
- Ceccato, T.L.; Starbuck, R.B.; Hall, J.K.; Walker, C.J.; Brown, T.E.; Killgore, J.P.; Anseth, K.S.; Leinwand, L.A. Defining the Cardiac Fibroblast Secretome in a Fibrotic Microenvironment. J. Am. Heart Assoc. 2020, 9, e017025. [Google Scholar] [CrossRef]
- Gibb, A.A.; Lazaropoulos, M.P.; Elrod, J.W. Myofibroblasts and Fibrosis: Mitochondrial and Metabolic Control of Cellular Differentiation. Circ. Res. 2020, 127, 427–447. [Google Scholar] [CrossRef] [PubMed]
- Ruan, H.; Gao, S.; Li, S.; Luan, J.; Jiang, Q.; Li, X.; Yin, H.; Zhou, H.; Yang, C. Deglycosylated Azithromycin Attenuates Bleomycin-Induced Pulmonary Fibrosis via the TGF-beta1 Signaling Pathway. Molecules 2021, 26, 2820. [Google Scholar] [CrossRef] [PubMed]
- Anwar, M.A.; Basith, S.; Choi, S. Negative regulatory approaches to the attenuation of Toll-like receptor signaling. Exp. Mol. Med. 2013, 45, e11. [Google Scholar] [CrossRef] [PubMed]
- Mishima, K.; Kitoh, H.; Ohkawara, B.; Okuno, T.; Ito, M.; Masuda, A.; Ishiguro, N.; Ohno, K. Lansoprazole Upregulates Polyubiquitination of the TNF Receptor-Associated Factor 6 and Facilitates Runx2-mediated Osteoblastogenesis. EBioMedicine 2015, 2, 2046–2061. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, A.; Yanez-Mo, M.; Selgas, R.; Sanchez-Madrid, F.; Lopez-Cabrera, M. Epithelial to mesenchymal transition as a triggering factor of peritoneal membrane fibrosis and angiogenesis in peritoneal dialysis patients. Curr. Opin. Investig. Drugs 2005, 6, 262–268. [Google Scholar]
- Wilson, R.B.; Archid, R.; Reymond, M.A. Reprogramming of Mesothelial-Mesenchymal Transition in Chronic Peritoneal Diseases by Estrogen Receptor Modulation and TGF-beta1 Inhibition. Int. J. Mol. Sci. 2020, 21, 4158. [Google Scholar] [CrossRef]
- Huang, J.; Tong, X.; Zhang, L.; Zhang, Y.; Wang, L.; Wang, D.; Zhang, S.; Fan, H. Hyperoside Attenuates Bleomycin-Induced Pulmonary Fibrosis Development in Mice. Front. Pharmacol. 2020, 11, 550955. [Google Scholar] [CrossRef]
- Xiong, C.; Liu, N.; Shao, X.; Sharif, S.; Zou, H.; Zhuang, S. Delayed administration of suramin attenuates peritoneal fibrosis in rats. BMC Nephrol. 2019, 20, 411. [Google Scholar] [CrossRef]






| Symbol | Forward | Reverse |
|---|---|---|
| Col1a1 | AGGCTGGTGTGATGGGATT | AGGGCCTTGTTCACCTCTCT |
| Tgfb1 | GACCGCAACAACGCAAT | GGCACTGCTTCCCGAAT |
| HO-1 | ACAGGGTGACAGAAGAGGCTAA | CTGTGAGGGACTCTGGTCTTTG |
| Cat | GCCTGTGTGAGAACATTGC | CCTGTACGTAGGTGTGAATTG |
| Gsta2 | CTTCTCCTCTATGTTGAAGAGTTTG | TTTTGCATCCACGGGAA |
| Nqo1 | CAGCGGCTCCATGTACT | GACCTGGAAGCCACAGAAG |
| Gpx1 | GGACTACACCGAAATGAATGAT | CTCGCACTTCTCAAACAATG |
| Mcp1 | TTG TCA CCA AGC TCA AGA GA | CAC ATT CAA AGG TGC TGA AG |
| Mcp3 | GCATGGAAGTCTGTGCTGAA | CGTTCCTACCCCTTAGGAC |
| Ip-10 | TCCTGCAAGTCTATCCTGTC | TGGCTTCTCTCTAGTTACGG |
| Mip-1a | GCGAGTACCAGTCCCTTCTC | GGTGCTGAGCAGGTAACAGA |
| Rantes | TCGTCTTTGTCACTCGAAGG | GAGCAAGCAATGACAGGAAA |
| Opn | AGTGGTTTGCCTTTGCCTGTT | TCAGCCAAGTGGCTACAGCAT |
| Actb | GGAGATTACTGCCCTGGCTCCTA | GACTCATCGTACTCCTGCTTGCTG |
| Gapdh | AGGTTGTCTCCTGTGACTTC | CTGTTGCTGTAGCCATATTC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kunitatsu, K.; Yamamoto, Y.; Nasu, S.; Taniji, A.; Kawashima, S.; Yamagishi, N.; Ito, T.; Inoue, S.; Kanai, Y. Novel Peritoneal Sclerosis Rat Model Developed by Administration of Bleomycin and Lansoprazole. Int. J. Mol. Sci. 2023, 24, 16108. https://doi.org/10.3390/ijms242216108
Kunitatsu K, Yamamoto Y, Nasu S, Taniji A, Kawashima S, Yamagishi N, Ito T, Inoue S, Kanai Y. Novel Peritoneal Sclerosis Rat Model Developed by Administration of Bleomycin and Lansoprazole. International Journal of Molecular Sciences. 2023; 24(22):16108. https://doi.org/10.3390/ijms242216108
Chicago/Turabian StyleKunitatsu, Kosei, Yuta Yamamoto, Shota Nasu, Akira Taniji, Shuji Kawashima, Naoko Yamagishi, Takao Ito, Shigeaki Inoue, and Yoshimitsu Kanai. 2023. "Novel Peritoneal Sclerosis Rat Model Developed by Administration of Bleomycin and Lansoprazole" International Journal of Molecular Sciences 24, no. 22: 16108. https://doi.org/10.3390/ijms242216108







