Nanoparticles Based on Silver Chloride and Bambusuril[6] for the Fine-Tuning of Biological Activity
Abstract
:1. Introduction
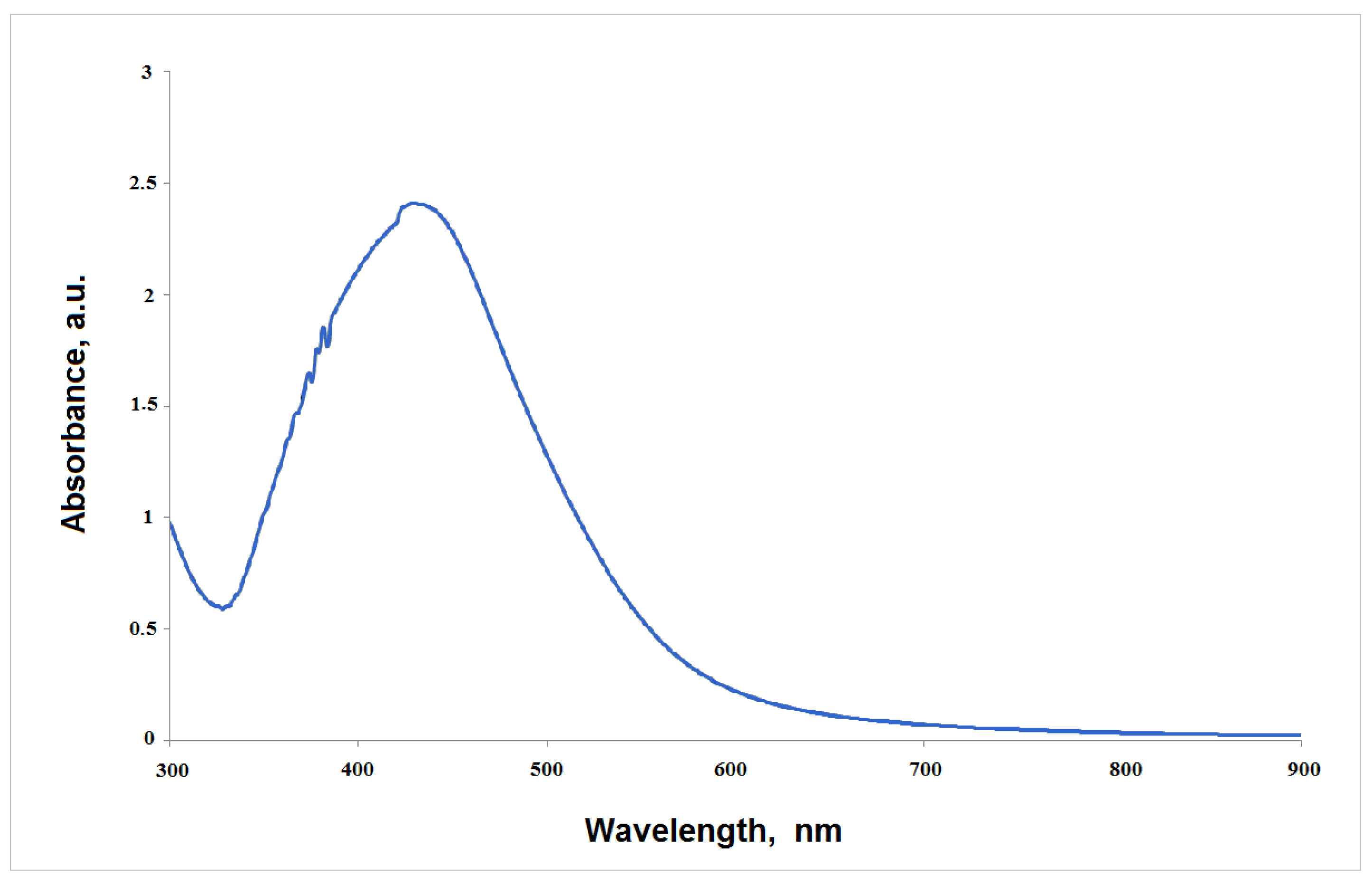
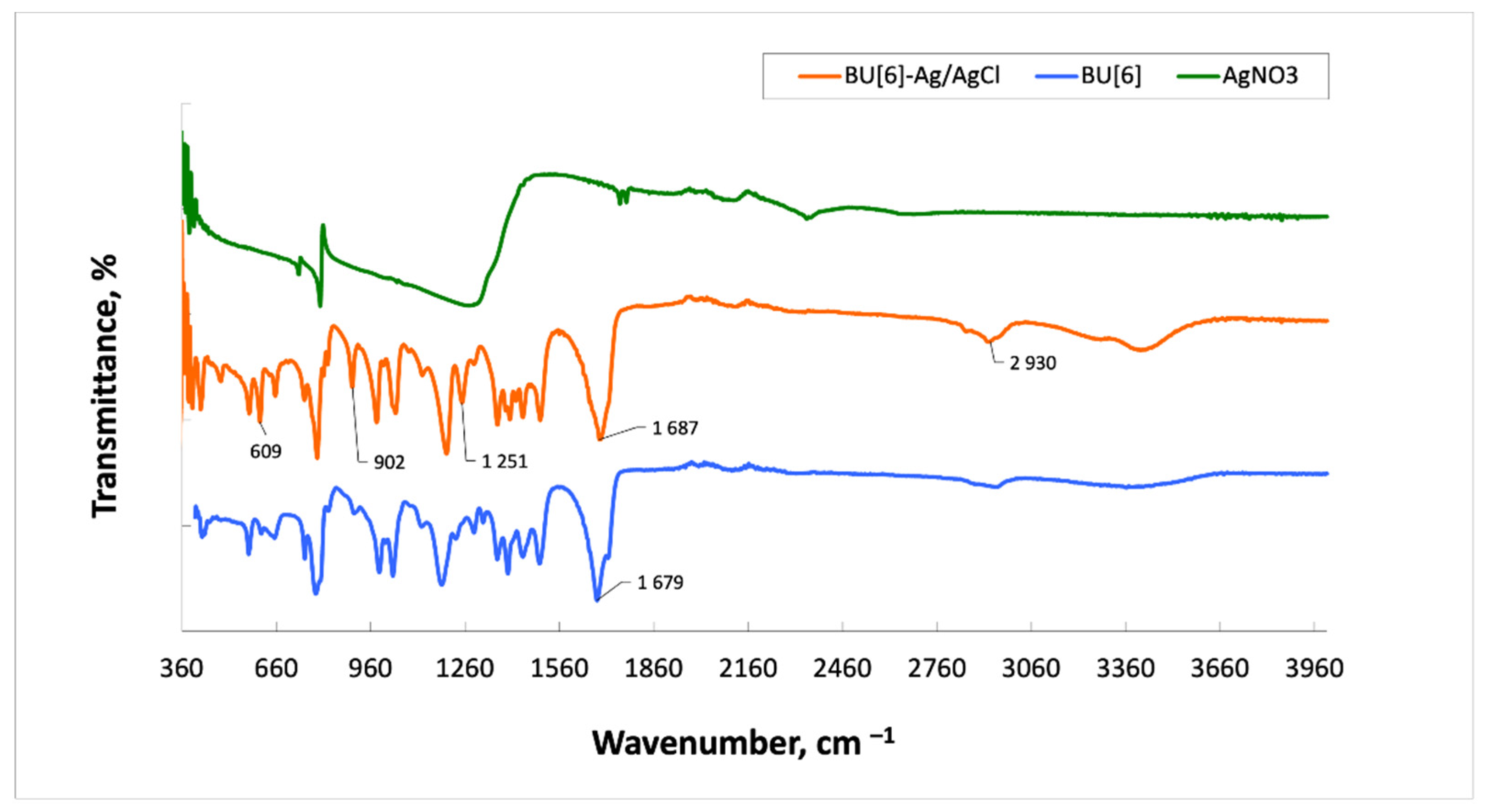
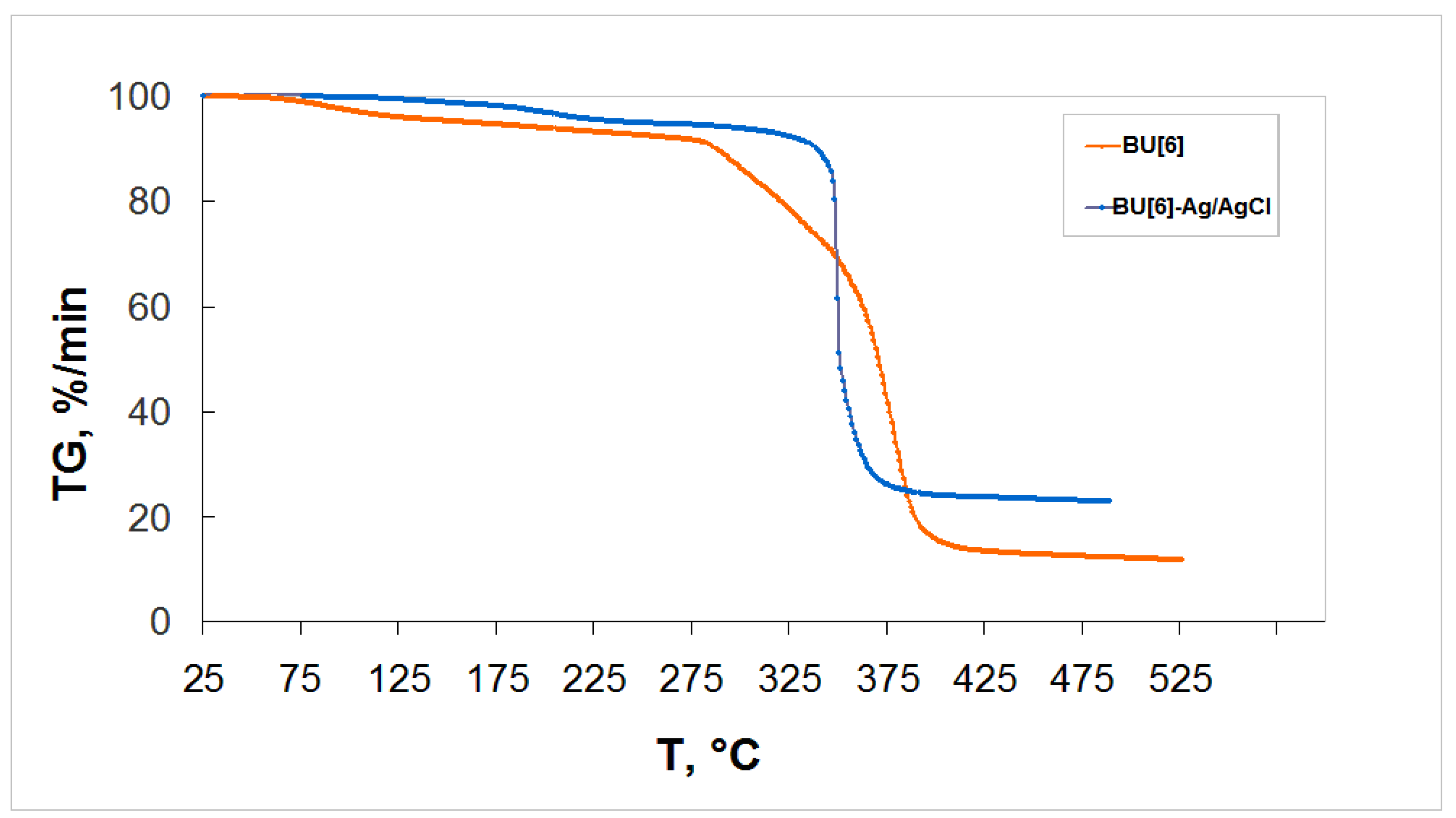
2. Results and Discussion
3. Materials and Methods
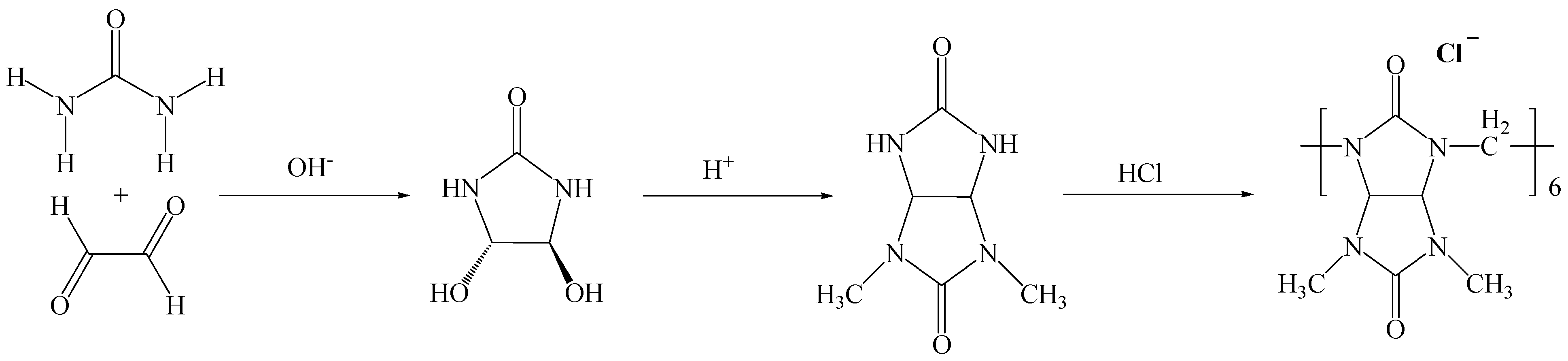
3.1. Bambusuril[6] Synthesis
3.2. Synthesis of 4,5-Dihydroxy Imidazolidin-2-One
3.3. Synthesis of 2,4-Dimethylglycoluril
3.4. Synthesis of BU[6]-Ag/AgCl NPs
3.5. Infrared Spectroscopy
3.6. NMR Spectroscopy
3.7. Thermogravimetric Analysis (TGA)
3.8. Antibacterial Analysis
3.9. MTT Test
3.10. Hemolysis
3.11. XRD
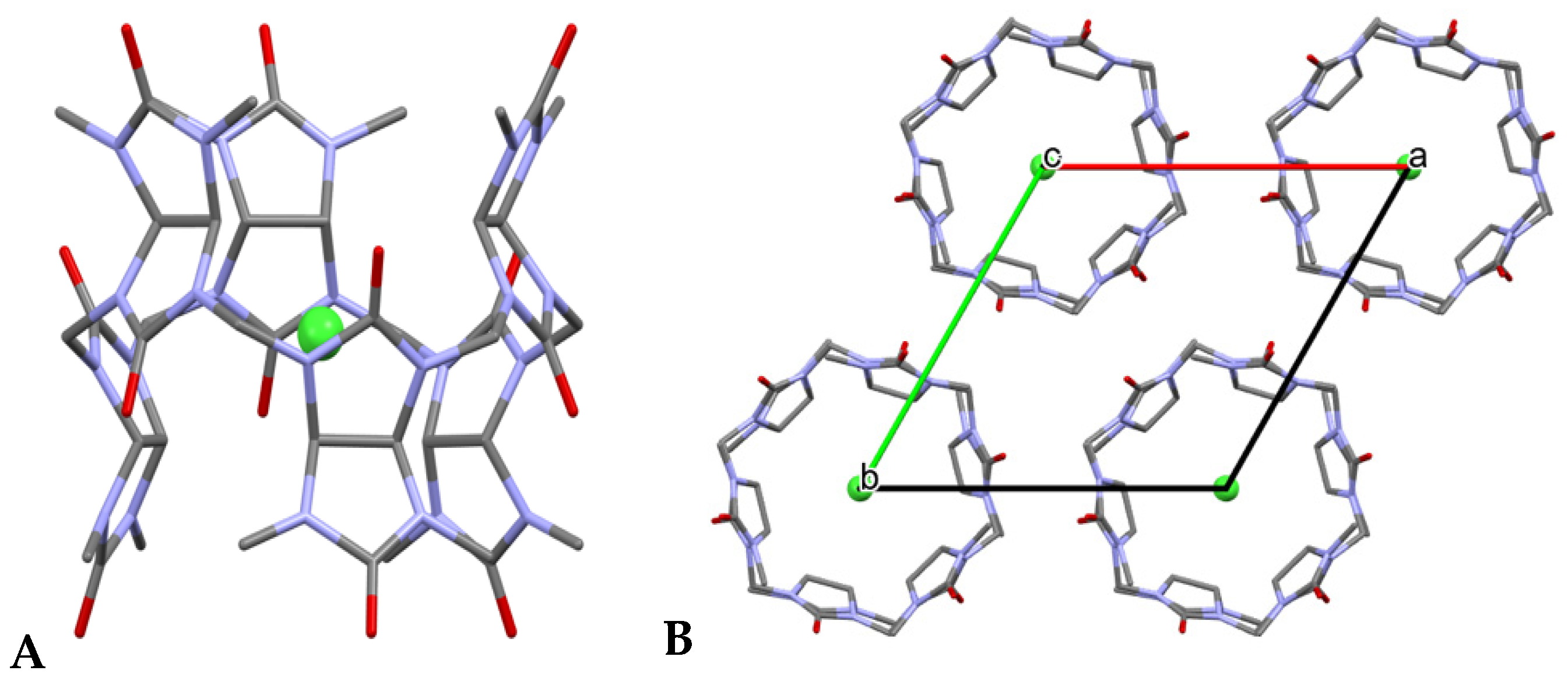
3.12. X-ray Crystallography
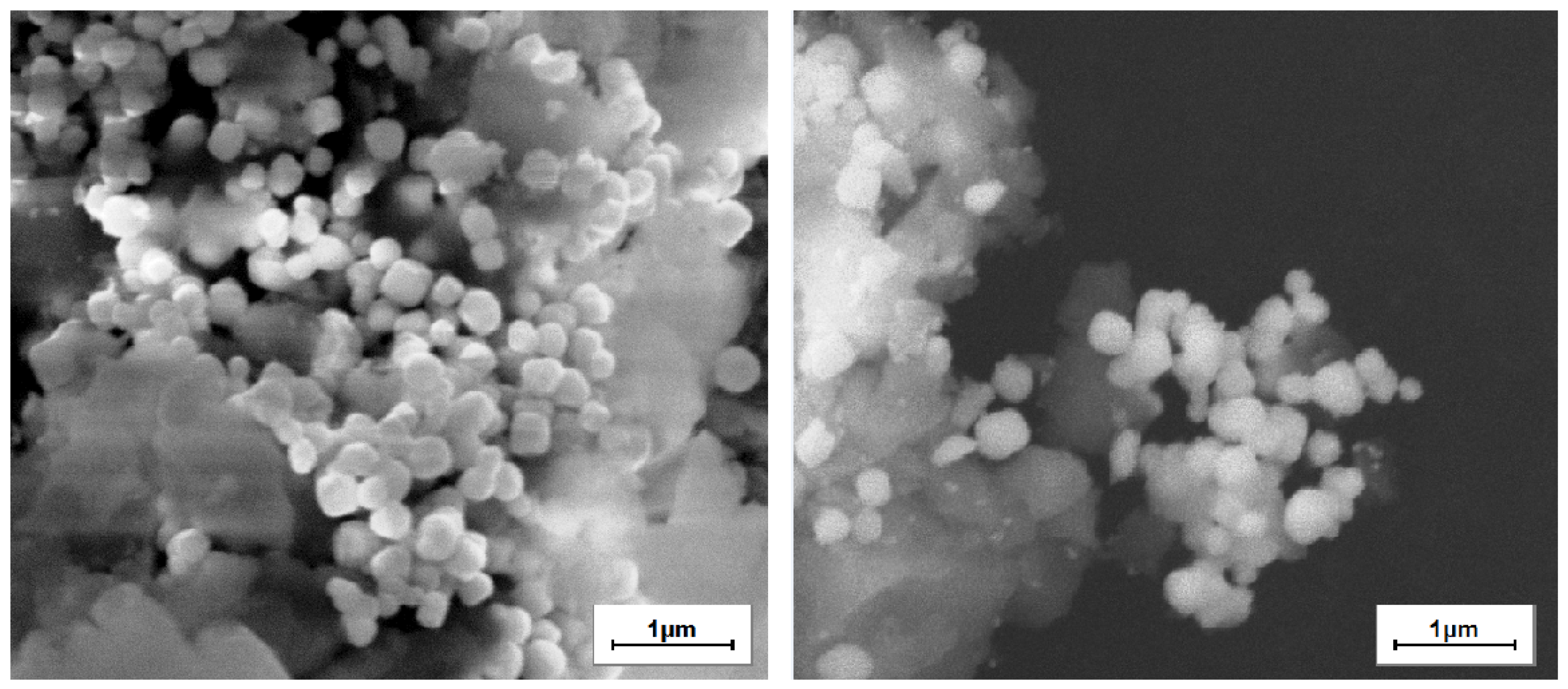
3.13. SEM
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cassano, D.; Mapanao, A.-K.; Summa, M.; Vlamidis, Y.; Giannone, G.; Santi, M.; Guzzolino, E.; Pitto, L.; Poliseno, L.; Bertorelli, R.; et al. Biosafety and Biokinetics of Noble Metals: The Impact of Their Chemical Nature. ACS Appl. Bio Mater. 2019, 2, 4464–4470. [Google Scholar] [CrossRef]
- Sondi, I.; Salopek-Sondi, B. Silver Nanoparticles as Antimicrobial Agent: A Case Study on E. coli as a Model for Gram-Negative Bacteria. J. Colloid Interface Sci. 2004, 275, 177–182. [Google Scholar] [CrossRef]
- Sharma, V.K.; Yngard, R.A.; Lin, Y. Silver Nanoparticles: Green Synthesis and Their Antimicrobial Activities. Adv. Colloid Interface Sci. 2009, 145, 83–96. [Google Scholar] [CrossRef]
- Gurunathan, S.; Kalishwaralal, K.; Vaidyanathan, R.; Venkataraman, D.; Pandian, S.R.K.; Muniyandi, J.; Hariharan, N.; Eom, S.H. Biosynthesis, Purification and Characterization of Silver Nanoparticles Using Escherichia coli. Colloids Surf. B Biointerfaces 2009, 74, 328–335. [Google Scholar] [CrossRef]
- Panáček, A.; Kolář, M.; Večeřová, R.; Prucek, R.; Soukupová, J.; Kryštof, V.; Hamal, P.; Zbořil, R.; Kvítek, L. Antifungal Activity of Silver Nanoparticles against Candida spp. Biomaterials 2009, 30, 6333–6340. [Google Scholar] [CrossRef]
- Zodrow, K.; Brunet, L.; Mahendra, S.; Li, D.; Zhang, A.; Li, Q.; Alvarez, P.J.J. Polysulfone Ultrafiltration Membranes Impregnated with Silver Nanoparticles Show Improved Biofouling Resistance and Virus Removal. Water Res. 2009, 43, 715–723. [Google Scholar] [CrossRef]
- Aboody, M.S. Silver/silver chloride (Ag/AgCl) nanoparticles synthesized from Azadirachta indica lalex and its antibiofilm activity against fluconazole resistant Candida tropicalis. Artif. Cells Nanomed. Biotechnol. 2019, 47, 2107–2113. [Google Scholar] [CrossRef]
- Gok, Z.G.; Karayel, M.; Yiğitoğlu, M. Synthesis of carrageenan coated silver nanoparticles by an easy green method and their characterization and antimicrobial activities. Res. Chem. Intermed. 2021, 47, 1843–1864. [Google Scholar] [CrossRef]
- Patil, M.P.; Palma, J.; Simeon, N.C.; Jin, X.; Liu, X.; Ngabire, D.; Kim, N.H.; Tarte, N.H.; Kim, G.D. Sasa borealis leaf extract-mediated green synthesis of silver-silver chloride nanoparticles and their antibacterial and anticancer activities. New J. Chem. 2017, 41, 1363–1371. [Google Scholar] [CrossRef]
- Li, S.; Zhang, H.; Cong, B.; He, P.; Liu, W.; Liu, S. A Novel Ag-AgCl Nanoparticle Synthesized by Arctic Marine Bacterium: Characterization, Activity and Mechanism. Int. J. Mol. Sci. 2022, 23, 15558. [Google Scholar] [CrossRef]
- Morais, M.; Teixeira, A.L.; Dias, F.; Machado, V.; Medeiros, R.; Prior, J.A.V.J. Cytotoxic Effect of Silver Nanoparticles Synthesized by Green Methods in Cancer. Med. Chem. 2020, 63, 14308–14335. [Google Scholar] [CrossRef]
- Ramírez-Hernández, M.; Valera-Zaragoza, M.; Viñas-Bravo, O.; Huerta-Heredia, A.; Peña-Rico, M. In search of cytotoxic selectivity on cancer cells with biogenically synthesized Ag/AgCl nanoparticles. Beilstein J. Nanotechnol. 2022, 13, 1505–1519. [Google Scholar] [CrossRef]
- Graf, C.; Vossen, D.L.J.; Imhof, A.; van Blaaderen, A. A General Method to Coat Colloidal Particles with Silica. Langmuir 2003, 19, 6693–6700. [Google Scholar] [CrossRef]
- Albanese, A.; Tang, P.S.; Chan, W.C.W. The Effect of Nanoparticle Size, Shape, and Surface Chemistry on Biological Systems. Annu. Rev. Biomed. Eng. 2012, 14, 1–16. [Google Scholar] [CrossRef]
- Saravanan, M.; Barik, S.K.; MubarakAli, D.; Prakash, P.; Pugazhendhi, A. Synthesis of Silver Nanoparticles from Bacillus brevis (NCIM 2533) and Their Antibacterial Activity against Pathogenic Bacteria. Microb. Pathog. 2018, 116, 221–226. [Google Scholar] [CrossRef]
- Shah, A.; Lutfullah, G.; Ahmad, K.; Khalil, A.T.; Maaza, M. Daphne Mucronata-Mediated Phytosynthesis of Silver Nanoparticles and Their Novel Biological Applications, Compatibility and Toxicity Studies. Green Chem. Lett. Rev. 2018, 11, 318–333. [Google Scholar] [CrossRef]
- Ilangovan, R.; Subha, V.; Ravindran, R.S.E.; Kirubanandan, S.; Renganathan, S. Chapter 2—Nanomaterials: Synthesis, Physicochemical Characterization, and Biopharmaceutical Applications. In Nanoscale Processing; Thomas, S., Balakrishnan, P., Eds.; Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2021; pp. 33–70. [Google Scholar] [CrossRef]
- Klasen, H.J. Historical Review of the Use of Silver in the Treatment of Burns. I. Early Uses. Burns 2000, 26, 117–130. [Google Scholar] [CrossRef]
- Gurunathan, S.; Han, J.W.; Eppakayala, V.; Jeyaraj, M.; Kim, J.-H. Cytotoxicity of Biologically Synthesized Silver Nanoparticles in MDA-MB-231 Human Breast Cancer Cells. BioMed Res. Int. 2013, 2013, 535796. [Google Scholar] [CrossRef]
- Gurunathan, S.; Kang, M.-H.; Qasim, M.; Kim, J.-H. Nanoparticle-Mediated Combination Therapy: Two-in-One Approach for Cancer. Int. J. Mol. Sci. 2018, 19, 3264. [Google Scholar] [CrossRef]
- Firdhouse, M.J.; Lalitha, P. Biosynthesis of Silver Nanoparticles Using the Extract of Alternanthera Sessilis—Antiproliferative Effect against Prostate Cancer Cells. Cancer Nanotechnol. 2013, 4, 137–143. [Google Scholar] [CrossRef]
- Cameron, S.J.; Hosseinian, F.; Willmore, W.G. A Current Overview of the Biological and Cellular Effects of Nanosilver. Int. J. Mol. Sci. 2018, 19, 2030. [Google Scholar] [CrossRef]
- Trojan-Horse Mechanism in the Cellular Uptake of Silver Nanoparticles Verified by Direct Intra- and Extracellular Silver Speciation Analysis|Environmental Science & Technology. Available online: https://pubs.acs.org/doi/abs/10.1021/es504705p (accessed on 29 June 2023).
- Park, E.-J.; Yi, J.; Kim, Y.; Choi, K.; Park, K. Silver Nanoparticles Induce Cytotoxicity by a Trojan-Horse Type Mechanism. Toxicol. Vitr. 2010, 24, 872–878. [Google Scholar] [CrossRef]
- Kovács, D.; Igaz, N.; Gopisetty, M.K.; Kiricsi, M. Cancer Therapy by Silver Nanoparticles: Fiction or Reality? Int. J. Mol. Sci. 2022, 23, 839. [Google Scholar] [CrossRef]
- Ariga, K.; Kunitake, T. Supramolecular Chemistry—Fundamentals and Applications: Advanced Textbook; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Schneider, H.-J. Applications of Supramolecular Chemistry; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Wang, F.; Zhang, J.; Ding, X.; Dong, S.; Liu, M.; Zheng, B.; Li, S.; Wu, L.; Yu, Y.; Gibson, H.W.; et al. Metal Coordination Mediated Reversible Conversion between Linear and Cross-Linked Supramolecular Polymers. Angew. Chem. Int. Ed. 2010, 49, 1090–1094. [Google Scholar] [CrossRef]
- Zhang, Z.; Luo, Y.; Chen, J.; Dong, S.; Yu, Y.; Ma, Z.; Huang, F. Formation of Linear Supramolecular Polymers That Is Driven by C—H⋯π Interactions in Solution and in the Solid State. Angew. Chem. Int. Ed. 2011, 50, 1397–1401. [Google Scholar] [CrossRef]
- Wang, F.; Han, C.; He, C.; Zhou, Q.; Zhang, J.; Wang, C.; Li, N.; Huang, F. Self-Sorting Organization of Two Heteroditopic Monomers to Supramolecular Alternating Copolymers. J. Am. Chem. Soc. 2008, 130, 11254–11255. [Google Scholar] [CrossRef]
- Yu, J.; Qi, D.; Li, J. Design, Synthesis and Applications of Responsive Macrocycles. Commun. Chem. 2020, 3, 189. [Google Scholar] [CrossRef]
- Harada, A.; Kobayashi, R.; Takashima, Y.; Hashidzume, A.; Yamaguchi, H. Macroscopic Self-Assembly through Molecular Recognition. Nat. Chem. 2011, 3, 34–37. [Google Scholar] [CrossRef]
- Liu, J.; Lan, Y.; Yu, Z.; Tan, C.S.Y.; Parker, R.M.; Abell, C.; Scherman, O.A. Cucurbit[n]Uril-Based Microcapsules Self-Assembled within Microfluidic Droplets: A Versatile Approach for Supramolecular Architectures and Materials. Acc. Chem. Res. 2017, 50, 208–217. [Google Scholar] [CrossRef]
- Svec, J.; Necas, M.; Sindelar, V. Bambus[6]Uril. Angew. Chem. Int. Ed. 2010, 49, 2378–2381. [Google Scholar] [CrossRef]
- Lizal, T.; Sindelar, V. Bambusuril Anion Receptors. Isr. J. Chem. 2018, 58, 326–333. [Google Scholar] [CrossRef]
- Yawer, M.A.; Havel, V.; Sindelar, V. A Bambusuril Macrocycle That Binds Anions in Water with High Affinity and Selectivity. Angew. Chem. Int. Ed. 2015, 54, 276–279. [Google Scholar] [CrossRef]
- Svec, J.; Dusek, M.; Fejfarova, K.; Stacko, P.; Klán, P.; Kaifer, A.E.; Li, W.; Hudeckova, E.; Sindelar, V. Anion-Free Bambus[6]Uril and Its Supramolecular Properties. Chem.–Eur. J. 2011, 17, 5605–5612. [Google Scholar] [CrossRef] [PubMed]
- Valkenier, H.; Akrawi, O.; Jurček, P.; Sleziaková, K.; Lízal, T.; Bartik, K.; Šindelář, V. Fluorinated Bambusurils as Highly Effective and Selective Transmembrane Cl−/HCO3− Antiporters. Chem 2019, 5, 429–444. [Google Scholar] [CrossRef]
- Hamacek, J.; Sokolov, J.; Šindelář, V. Bambusuril Macrocycles as Mediators of Supramolecular Interactions: Application to the Europium Cage Helicate. Chem.–Eur. J. 2021, 27, 5492–5497. [Google Scholar] [CrossRef] [PubMed]
- Rando, C.; Vázquez, J.; Sokolov, J.; Kokan, Z.; Nečas, M.; Šindelář, V. Highly Efficient and Selective Recognition of Dicyanoaurate(I) by a Bambusuril Macrocycle in Water. Angew. Chem. 2022, 134, e202210184. [Google Scholar] [CrossRef]
- Sokolov, J.; Štefek, A.; Šindelář, V. Functionalized Chiral Bambusurils: Synthesis and Host-Guest Interactions with Chiral Carboxylates. ChemPlusChem 2020, 85, 1307–1314. [Google Scholar] [CrossRef]
- Premkumar, T.; Lee, Y.; Geckeler, K.E. Macrocycles as a Tool: A Facile and One-Pot Synthesis of Silver Nanoparticles Using Cucurbituril Designed for Cancer Therapeutics. Chem.–Eur. J. 2010, 16, 11563–11566. [Google Scholar] [CrossRef]
- Marchenko, E.; Luchsheva, V.; Baigonakova, G.; Bakibaev, A.; Vorozhtsov, A. Functionalization of the Surface of Porous Nickel–Titanium Alloy with Macrocyclic Compounds. Materials 2023, 16, 66. [Google Scholar] [CrossRef]
- Luchsheva, V.R.; Bakibaev, A.A.; Marchenko, E.S. Development of bioactive composite material based on bambusuril and porous titanium nickelide. Vopr. Materialoved. 2023, 4, 35–42. [Google Scholar] [CrossRef]
- Heath, J.R. Size-Dependent Surface-Plasmon Resonances of Bare Silver Particles. Phys. Rev. B 1989, 40, 9982–9985. [Google Scholar] [CrossRef] [PubMed]
- Gün Gök, Z.; Günay, K.; Arslan, M.; Yiğitoğlu, M.; Vargel, İ. Coating of Modified Poly(Ethylene Terephthalate) Fibers with Sericin-Capped Silver Nanoparticles for Antimicrobial Application. Polym. Bull. 2020, 77, 1649–1665. [Google Scholar] [CrossRef]
- Aramwit, P.; Bang, N.; Ratanavaraporn, J.; Ekgasit, S. Green Synthesis of Silk Sericin-Capped Silver Nanoparticles and Their Potent Anti-Bacterial Activity. Nanoscale Res. Lett. 2014, 9, 79. [Google Scholar] [CrossRef]
- Devi, T.B.; Ahmaruzzaman, M.; Begum, S. A rapid, facile and green synthesis of Ag@AgCl nanoparticles for the effective reduction of 2,4-dinitrophenyl hydrazine. N. J. Chem. 2016, 40, 1497–1506. [Google Scholar] [CrossRef]
- Ali, H.; Azad, A.K.; Khan, K.A.; Rahman, O.; Chakma, U.; Kumer, A. Analysis of Crystallographic Structures and Properties of Silver Nanoparticles Synthesized Using PKL Extract and Nanoscale Characterization Techniques. ACS Omega 2023, 8, 28133–28142. [Google Scholar] [CrossRef] [PubMed]
- Ali, M. Etching of photon energy into binding energy in depositing carbon films at different chamber pressures. J. Mater. Sci. Mater. Electron. 2023, 34, 1209. [Google Scholar] [CrossRef]
- Ayala-Nunez, V.; Villegas, L.; Ixtepan, C.; Turrent, L. Silver nanoparticles toxicity and bactericidal effect against methicillin-resistant Staphylococcus aureus: Nanoscale does matter. Nanobiotechnology 2009, 5, 2–9. [Google Scholar] [CrossRef]
- ISO 10993-4:2017; Biological Evaluation of Medical Devices—Part 4: Selection of Tests for Interactions with Blood—Technical Committee: ISO/TC 194. ISO: Geneva, Switzerland, 2017; p. 69.
- Buranaamnuay, K. The MTT Assay Application to Measure the Viability of Spermatozoa: A Variety of the Assay Protocols. Open Vet. J. 2021, 11, 251–269. [Google Scholar] [CrossRef]
- Popova, A.; Kzhyshkowska, J.; Nurgazieva, D.; Goerdt, S.; Gratchev, A. Pro- and Anti-Inflammatory Control of M-CSF-Mediated Macrophage Differentiation. Immunobiology 2011, 216, 164–172. [Google Scholar] [CrossRef]
- Gamal-Eldin, M.A.; Macartney, D.H. Selective Molecular Recognition of Methylated Lysines and Arginines by Cucurbit[6]Uril and Cucurbit[7]Uril in Aqueous Solution. Org. Biomol. Chem. 2012, 11, 488–495. [Google Scholar] [CrossRef]
- Bailey, D.M.; Hennig, A.; Uzunova, V.D.; Nau, W.M. Supramolecular Tandem Enzyme Assays for Multiparameter Sensor Arrays and Enantiomeric Excess Determination of Amino Acids. Chem. Weinh. Bergstr. Ger. 2008, 14, 6069–6077. [Google Scholar] [CrossRef] [PubMed]
- Chinai, J.M.; Taylor, A.B.; Ryno, L.M.; Hargreaves, N.D.; Morris, C.A.; Hart, P.J.; Urbach, A.R. Molecular Recognition of Insulin by a Synthetic Receptor. J. Am. Chem. Soc. 2011, 133, 8810–8813. [Google Scholar] [CrossRef] [PubMed]
- Cáceres, J.; Robinson-Duggon, J.; Tapia, A.; Paiva, C.; Gómez, M.; Bohne, C.; Fuentealba, D. Photochemical Behavior of Biosupramolecular Assemblies of Photosensitizers, Cucurbit[n]Urils and Albumins. Phys. Chem. Chem. Phys. 2017, 19, 2574–2582. [Google Scholar] [CrossRef] [PubMed]
- Lei, W.; Jiang, G.; Zhou, Q.; Zhang, B.; Wang, X. Greatly Enhanced Binding of a Cationic Porphyrin towards Bovine Serum Albumin by Cucurbit[8]Uril. Phys. Chem. Chem. Phys. 2010, 12, 13255–13260. [Google Scholar] [CrossRef] [PubMed]


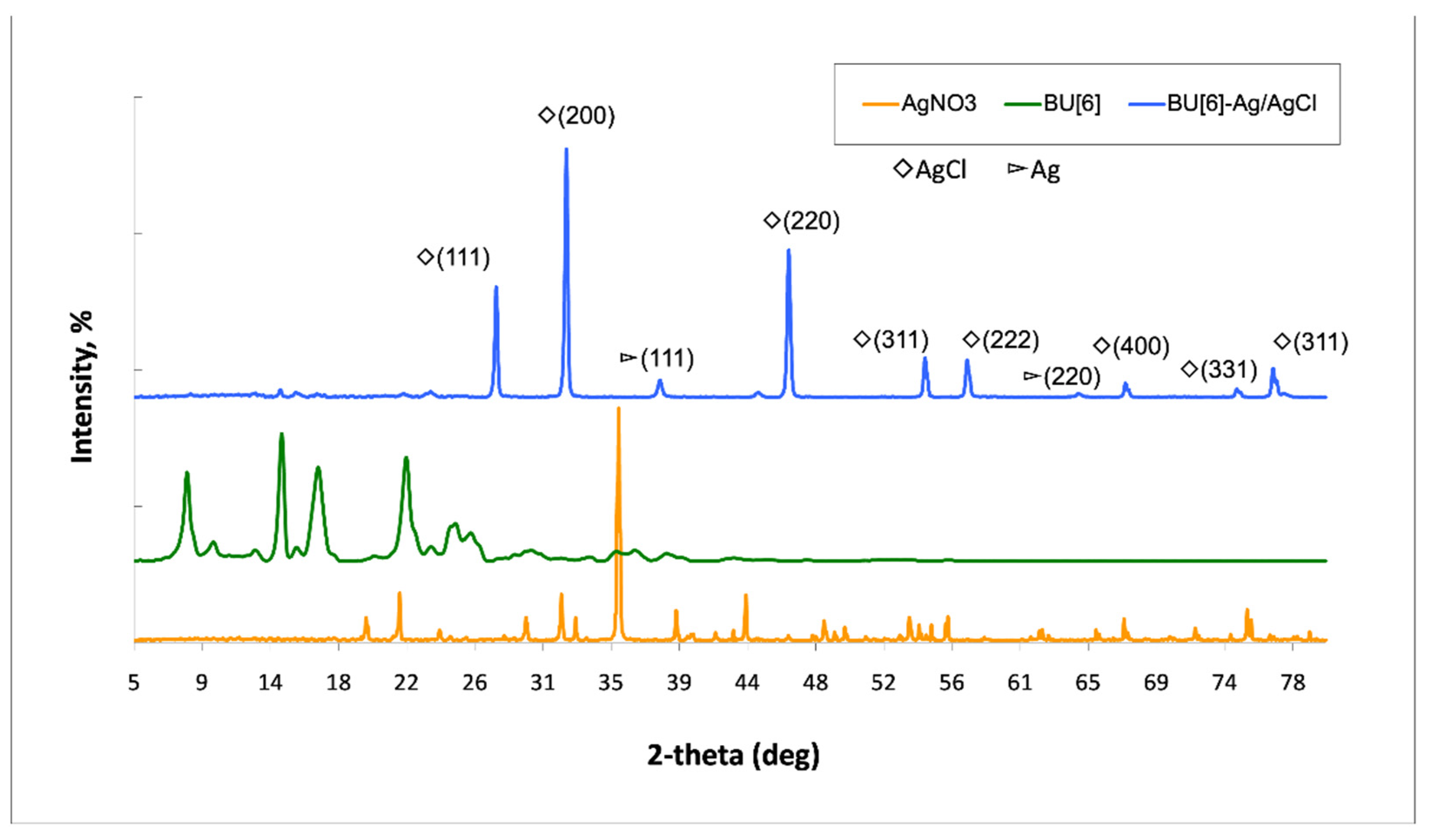



| Miller Indices (hkl) | 2θ of the Intense Peak (°) | θ of the Intense Peak (Radian) | FWHM, β (°) | FWHM, β (Radian) | Crystallite Diameter, D (nm) |
|---|---|---|---|---|---|
| 111 (AgCl) | 27.71 | 0.241077 | 0.25 | 0.004363 | 31.40 |
| 200 (AgCl) | 32.11 | 0.283692 | 0.41 | 0.007156 | 19.14 |
| 220 (AgCl) | 46.33 | 0.409326 | 0.35 | 0.006109 | 22.43 |
| 311 (AgCl) | 54.93 | 0.485307 | 0.25 | 0.004363 | 31.40 |
| 222 (AgCl) | 57.6 | 0.508896 | 0.35 | 0.006109 | 22.43 |
| 111 (Ag) | 38.13 | 0.336879 | 0.45 | 0.007854 | 17.44 |
| Samples | Inhibition Zone of E. coli (mm) | Inhibition Zone of S. aureus (mm) |
|---|---|---|
| Negative control (NaCl) | 0 | 0 |
| 10 mg/mL Bambusuril[6] | 0 | 0 |
| 1 mg/mL BU[6]-Ag/AgCl NPs | 0 | 0 |
| 2 mg/mL BU[6]-Ag/AgCl NPs | 12.4 | 12.1 |
| 4 mg/mL BU[6]-Ag/AgCl NPs | 13.9 | 13.4 |
| 8 mg/mL BU[6]-Ag/AgCl NPs | 17.5 | 17.4 |
| MIC | 2 mg/mL | |
| Samples | Hemolysis, % |
|---|---|
| BU[6] | 0.3 |
| CTRL (plasma) | 0 |
| Samples | Name | Concentration, mg/mL |
|---|---|---|
| BU[6] | D1 | 10 |
| BU[6] | D2 | 5 |
| BU[6] | D3 | 2.5 |
| BU[6]-Ag/AgCl | D4 | 10 |
| BU[6]-Ag/AgCl | D5 | 5 |
| BU[6]-Ag/AgCl | D6 | 2.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turebayeva, P.; Luchsheva, V.; Fedorishin, D.; Yerkassov, R.; Bakibaev, A.; Bolysbekova, S.; Tugambayeva, T.; Sergazina, S.; Nurmukhanbetova, N. Nanoparticles Based on Silver Chloride and Bambusuril[6] for the Fine-Tuning of Biological Activity. Int. J. Mol. Sci. 2023, 24, 16126. https://doi.org/10.3390/ijms242216126
Turebayeva P, Luchsheva V, Fedorishin D, Yerkassov R, Bakibaev A, Bolysbekova S, Tugambayeva T, Sergazina S, Nurmukhanbetova N. Nanoparticles Based on Silver Chloride and Bambusuril[6] for the Fine-Tuning of Biological Activity. International Journal of Molecular Sciences. 2023; 24(22):16126. https://doi.org/10.3390/ijms242216126
Chicago/Turabian StyleTurebayeva, Pana, Venera Luchsheva, Dmitriy Fedorishin, Rakhmetulla Yerkassov, Abdigali Bakibaev, Saltanat Bolysbekova, Tokzhan Tugambayeva, Samal Sergazina, and Nurgul Nurmukhanbetova. 2023. "Nanoparticles Based on Silver Chloride and Bambusuril[6] for the Fine-Tuning of Biological Activity" International Journal of Molecular Sciences 24, no. 22: 16126. https://doi.org/10.3390/ijms242216126







