Mucopolysaccharidosis IVA: Current Disease Models and Drawbacks
Abstract
:1. Introduction
2. In Vitro MPS IVA Models
2.1. Skin Fibroblast as Models of MPS IVA
2.2. Peripheral Leukocytes
2.3. Chondrocytes
3. In Vivo MPS IVA Models
3.1. MPS IVA Mouse Models
3.2. MPS IVA Rat Model
4. Future Perspectives
- Skin fibroblasts affected with MPS IVA are commonly used in vitro rather than chondrocytes. However, several canonical states observed on chondrocytes, such as resting, proliferative, and hypertrophic states, and their regulatory pathways [73,74] cannot be assessed in fibroblasts. Likewise, critical chondrocyte functions, such as their involvement in endochondral ossification [75,76,77], cannot be evaluated in skin fibroblasts.
- Although some studies reported chondrocytes as an MPS IVA model, some consulted works lack critical information regarding cell culturing, including passages and supplements. It is well known that culturing methods greatly influence cell physiology in primary cultures [78].
- Current mouse models do not display skeletal dysplasia in MPS IVA patients, making it challenging to establish the effectiveness of potential new drugs. Even though Bertolin et al. have shown an improved MPS IVA animal model by using rats able to display some clinical features of the MPS IVA, there is still a high priority for developing large animal models to recapitulate the skeletal dysplasia observed in MPS IVA patients.
- A comprehensive characterization of MPS IVA chondrocytes: The full channelome in healthy chondrocytes has been established [79]. Nevertheless, there is no information on this channelome profile in MPS IVA chondrocytes. We also propose conducting functional studies, such as electrophysiological recordings involving passive and active plasma membrane properties and metabolic profile studies, as they can provide new insights beyond the lack of GALNS or GAG accumulation. These premises have been previously addressed in osteoarthritic chondrocytes (OC) and clearly demonstrate differences in OC compared to healthy chondrocytes [79,80].
- A more realistic microenvironment: Establishing primary chondrocyte culturing by using either iPSC-, MSC-, or surgical specimen-derived sources is strongly recommended. These cultures should be performed in complex culturing systems (i.e., 3D rather than 2D). We also suggest reporting cell culturing conditions, such as passages and supplements, since they can be critical for understanding reported results.
- Organ-on-a-chip (OoC): Developing novel strategies such as organ-on-a-chip (OoC) is implemented to recapitulate relevant physiological conditions [81,82,83,84], including mechanical stimulation. Surfaceome chondrocyte characterization has demonstrated the expression of several ion channels as a response to biomechanical stress [79,85], and early studies have shown that 2D-cultured chondrocytes affect their response to mechanical stimulation [86,87,88]. Therefore, models based on OoC technologies result in better in vitro MPS IVA models to explore the molecular and cellular consequences of the GAG accumulation in chondrocytes under relevant pathophysiological microenvironments.
- Large animal models: All current MPS IVA models are derived from the genetic manipulation of rodents (mouse and rat), and there is no evidence of naturally occurring large animal models as described for MPS, such as MPS I, MPS IIIA, MPS IIIB, MPS IIID, MPS VI, and MPS VII [61]. Therefore, the establishment of new large MPS IVA models, such as non-human primates, could be beneficial for the assessment of conventional (i.e., ERT, PC, classical GT) and novel alternatives (i.e., SDET, CRISPR/Cas9-based GT) for treating MPS IVA, since they can provide a larger blood volume, larger tissues, and more human-like anatomy and physiology [89], compared to rodents.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Selvam, P.; Jain, A.; Abbott, J.; Ahuja, A.S.; Cheema, A.; Bruno, K.A.; Atwal, H.; Forghani, I.; Caulfield, T.; Atwal, P.S. Molecular Modeling and Phenotypic Description of a Patient with a Novel Exonic Deletion of GALNS with Resultant Morquio Syndrome with Two Successful Pregnancies. Mol. Syndromol. 2022, 13, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Frigeni, M.; Rodriguez-Buritica, D.F.; Saavedra, H.; Gunther, K.A.; Hillman, P.R.; Balaguru, D.; Northrup, H. The youngest pair of siblings with Mucopolysaccharidosis type IVA to receive enzyme replacement therapy to date: A case report. Am. J. Med. Genet. Part A 2021, 185, 3510–3516. [Google Scholar] [CrossRef] [PubMed]
- Yi, M.; Wang, Y.; Gao, X.; Han, L.; Qiu, W.; Gu, X.; Maegawa, G.H.; Zhang, H. Investigation of GALNS variants and genotype-phenotype correlations in a large cohort of patients with mucopolysaccharidosis type IVA. J. Inherit. Metab. Dis. 2022, 45, 593–604. [Google Scholar] [CrossRef] [PubMed]
- Quijada-Fraile, P.; Canales, E.A.; Martín-Hernández, E.; Ballesta-Martínez, M.J.; Guillén-Navarro, E.; Pintos-Morell, G.; Moltó-Abad, M.; Moreno-Martínez, D.; Morillo, S.G.; Blasco-Alonso, J.; et al. Clinical features and health-related quality of life in adult patients with mucopolysaccharidosis IVA: The Spanish experience. Orphanet J. Rare Dis. 2021, 16, 464. [Google Scholar] [CrossRef] [PubMed]
- Akyol, M.U.; MPS Consensus Programme Steering Committee; Alden, T.D.; Amartino, H.; Ashworth, J.; Belani, K.; Berger, K.I.; Borgo, A.; Braunlin, E.; Eto, Y.; et al. Recommendations for the management of MPS IVA: Systematic evidence- and consensus-based guidance. Orphanet J. Rare Dis. 2019, 14, 137. [Google Scholar] [CrossRef]
- Solanki, G.A.; Martin, K.W.; Theroux, M.C.; Lampe, C.; White, K.K.; Shediac, R.; Lampe, C.G.; Beck, M.; Mackenzie, W.G.; Hendriksz, C.J.; et al. Spinal involvement in mucopolysaccharidosis IVA (Morquio-Brailsford or Morquio A syndrome): Presentation, diagnosis and management. J. Inherit. Metab. Dis. 2013, 36, 339–355. [Google Scholar] [CrossRef]
- DeLong, K.; Feigenbaum, A.; Pollard, L.; Lay, A.; Wood, T. Characterization of a novel exonic deletion in the GALNS gene causing Morquio A syndrome. Mol. Genet. Metab. Rep. 2022, 33, 100920. [Google Scholar] [CrossRef]
- Cárdenas, J.M.; Vergara, D.; Witting, S.; Balut, F.; Guerra, P.; Mesa, J.T.; Silva, S.; Tello, J.; Retamales, A.; Barrios, A.; et al. Genotype and Phenotype Characterization of Patients with Mucopolysaccharidosis IV-A in Chile. Mol. Syndromol. 2023, 14, 416–427. [Google Scholar] [CrossRef]
- Cozma, C.; Eichler, S.; Wittmann, G.; Bonet, A.F.; Kramp, G.J.; Giese, A.-K.; Rolfs, A. Diagnosis of Morquio Syndrome in Dried Blood Spots Based on a New MRM-MS Assay. PLoS ONE 2015, 10, e0131228. [Google Scholar] [CrossRef]
- Pachajoa, H.; Acosta, M.A.; Alméciga-Díaz, C.J.; Ariza, Y.; Diaz-Ordoñez, L.; Caicedo-Herrera, G.; Cuartas, D.; Nastasi-Catanese, J.A.; Ramírez-Montaño, D.; Silva, Y.K.; et al. Molecular characterization of mucopolysaccharidosis type IVA patients in the Andean region of Colombia. Am. J. Med. Genet. Part C Semin. Med. Genet. 2021, 187, 388–395. [Google Scholar] [CrossRef]
- Rivera-Colón, Y.; Schutsky, E.K.; Kita, A.Z.; Garman, S.C. The structure of human GALNS reveals the molecular basis for mucopolysaccharidosis IV A. J. Mol. Biol. 2012, 423, 736–751. [Google Scholar] [CrossRef] [PubMed]
- Zanetti, A.; D’Avanzo, F.; AlSayed, M.; Brusius-Facchin, A.C.; Chien, Y.; Giugliani, R.; Izzo, E.; Kasper, D.C.; Lin, H.; Lin, S.; et al. Molecular basis of mucopolysaccharidosis IVA (Morquio A syndrome): A review and classification of GALNS gene variants and reporting of 68 novel variants. Hum. Mutat. 2021, 42, 1384–1398. [Google Scholar] [CrossRef]
- Morrone, A.; Caciotti, A.; Atwood, R.; Davidson, K.; Du, C.; Francis-Lyon, P.; Harmatz, P.; Mealiffe, M.; Mooney, S.; Oron, T.R.; et al. Morquio A syndrome-associated mutations: A review of alterations in the GALNS gene and a new locus-specific database. Hum. Mutat. 2014, 35, 1271–1279. [Google Scholar] [CrossRef] [PubMed]
- Montaño, A.M.; Sukegawa, K.; Kato, Z.; Carrozzo, R.; Di Natale, P.; Christensen, E.; Tomatsu, S. Effect of ‘attenuated’ mutations in mucopolysaccharidosis IVA on molecular phenotypes of N-acetylgalactosamine-6-sulfate sulfatase. J. Inherit. Metab. Dis. 2007, 30, 758–767. [Google Scholar] [CrossRef]
- Tomatsu, S.; Montaño, A.M.; Nishioka, T.; Gutierrez, M.A.; Peña, O.M.; Firescu, G.G.T.; Lopez, P.; Yamaguchi, S.; Noguchi, A.; Orii, T. Mutation and polymorphism spectrum of the GALNS gene in mucopolysaccharidosis IVA (Morquio A). Hum. Mutat. 2005, 26, 500–512. [Google Scholar] [CrossRef]
- Morrone, A.; Tylee, K.; Al-Sayed, M.; Brusius-Facchin, A.; Caciotti, A.; Church, H.; Coll, M.; Davidson, K.; Fietz, M.; Gort, L.; et al. Molecular testing of 163 patients with Morquio A (Mucopolysaccharidosis IVA) identifies 39 novel GALNS mutations. Mol. Genet. Metab. 2014, 112, 160–170. [Google Scholar] [CrossRef]
- Tapiero-Rodriguez, S.M.; Guio, J.C.A.; Porras-Hurtado, G.L.; García, N.; Solano, M.; Pachajoa, H.; Velasco, H.M. Determination of genotypic and clinical characteristics of Colombian patients with mucopolysaccharidosis IVA. Appl. Clin. Genet. 2018, 11, 45–57. [Google Scholar] [CrossRef]
- Schweighardt, B.; Tompkins, T.; Lau, K.; Jesaitis, L.; Qi, Y.; Musson, D.G.; Farmer, P.; Haller, C.; Shaywitz, A.J.; Yang, K.; et al. Immunogenicity of Elosulfase Alfa, an Enzyme Replacement Therapy in Patients With Morquio A Syndrome: Results from MOR-004, a Phase III Trial. Clin. Ther. 2015, 37, 1012–1021.e6. [Google Scholar] [CrossRef]
- Magner, M.; Almássy, Z.; Gucev, Z.; Kieć-Wilk, B.; Plaiasu, V.; Tylki-Szymańska, A.; Zafeiriou, D.; Zaganas, I.; Lampe, C. Consensus statement on enzyme replacement therapy for mucopolysaccharidosis IVA in Central and South-Eastern European countries. Orphanet J. Rare Dis. 2022, 17, 190. [Google Scholar] [CrossRef]
- Ficicioglu, C.; Matalon, D.R.; Luongo, N.; Menello, C.; Kornafel, T.; Degnan, A.J. Diagnostic journey and impact of enzyme replacement therapy for mucopolysaccharidosis IVA: A sibling control study. Orphanet J. Rare Dis. 2020, 15, 336. [Google Scholar] [CrossRef]
- Lee, C.L.; Chuang, C.K.; Chiu, H.C.; Tu, R.Y.; Lo, Y.T.; Chang, Y.H.; Lin, H.Y. Clinical Utility of Elosulfase Alfa in the Treatment of Morquio A Syndrome. Drug Des. Dev. Ther. 2022, 16, 143–154. [Google Scholar]
- Cleary, M.; Davison, J.; Gould, R.; Geberhiwot, T.; Hughes, D.; Mercer, J.; Morrison, A.; Murphy, E.; Santra, S.; Jarrett, J.; et al. Impact of long-term elosulfase alfa treatment on clinical and patient-reported outcomes in patients with mucopolysaccharidosis type IVA: Results from a Managed Access Agreement in England. Orphanet J. Rare Dis. 2021, 16, 38. [Google Scholar] [CrossRef] [PubMed]
- Giannitsi, S.; Bougiakli, M.; Bechlioulis, A.; Kotsia, A.; Michalis, L.K.; Naka, K.K. 6-minute walking test: A useful tool in the management of heart failure patients. Ther. Adv. Cardiovasc. Dis. 2019, 13, 1753944719870084. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Kim, H.Y.; Cho, T.J.; Kim, H.; Ko, J.M. Clinical characteristics and effects of enzyme replacement therapy with elosulfase alfa in Korean patients with mucopolysaccharidosis type IVA. Mol. Genet. Metab. Rep. 2022, 31, 100869. [Google Scholar] [CrossRef] [PubMed]
- Bilginer Gurbuz, B.; Aypar, E.; Coskun, T.; Alehan, D.; Dursun, A.; Tokatli, A.; Sivri, H.S. The effectiveness of enzyme replacement therapy on cardiac findings in patients with mucopolysaccharidosis. J. Pediatr. Endocrinol. Metab. 2019, 32, 1049–1053. [Google Scholar] [CrossRef] [PubMed]
- Stevens, B.; Kenny, T.; Thomas, S.; Morrison, A.; Jarrett, J.; Jain, M. Elosulfase alfa in the treatment of mucopolysaccharidosis type IVA: Insights from the first managed access agreement. Orphanet J. Rare Dis. 2021, 16, 394. [Google Scholar] [CrossRef]
- Donida, B.; Marchetti, D.P.; Biancini, G.B.; Deon, M.; Manini, P.R.; da Rosa, H.T.; Moura, D.J.; Saffi, J.; Bender, F.; Burin, M.G.; et al. Oxidative stress and inflammation in mucopolysaccharidosis type IVA patients treated with enzyme replacement therapy. Biochim. Biophys. Acta 2015, 1852, 1012–1019. [Google Scholar] [CrossRef]
- Alméciga-Diaz, C.J.; Hidalgo, O.A.; Olarte-Avellaneda, S.; Rodríguez-López, A.; Guzman, E.; Garzón, R.; Pimentel-Vera, L.N.; Puentes-Tellez, M.A.; Rojas-Rodriguez, A.F.; Gorshkov, K.; et al. Identification of Ezetimibe and Pranlukast as Pharmacological Chaperones for the Treatment of the Rare Disease Mucopolysaccharidosis Type IVA. J. Med. Chem. 2019, 62, 6175–6189. [Google Scholar] [CrossRef]
- Sawamoto, K.; Tomatsu, S. Development of Substrate Degradation Enzyme Therapy for Mucopolysaccharidosis IVA Murine Model. Int. J. Mol. Sci. 2019, 20, 4139. [Google Scholar] [CrossRef]
- Leal, A.F.; Alméciga-Díaz, C.J. Efficient CRISPR/Cas9 nickase-mediated genome editing in an in vitro model of mucopolysaccharidosis IVA. Gene Ther. 2022, 30, 107–114. [Google Scholar] [CrossRef]
- Sawamoto, K.; Karumuthil-Melethil, S.; Khan, S.; Stapleton, M.; Bruder, J.T.; Danos, O.; Tomatsu, S. Liver-Targeted AAV8 Gene Therapy Ameliorates Skeletal and Cardiovascular Pathology in a Mucopolysaccharidosis IVA Murine Model. Mol. Ther. Methods Clin. Dev. 2020, 18, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Leal, A.F.; Cifuentes, J.; Torres, C.E.; Suárez, D.; Quezada, V.; Gómez, S.C.; Cruz, J.C.; Reyes, L.H.; Espejo-Mojica, A.J.; Alméciga-Díaz, C.J. Delivery and assessment of a CRISPR/nCas9-based genome editing system on in vitro models of mucopolysaccharidoses IVA assisted by magnetite-based nanoparticles. Sci. Rep. 2022, 12, 15045. [Google Scholar] [CrossRef] [PubMed]
- Puentes-Tellez, M.A.; Sánchez, O.F.; Rojas-Rodriguez, F.; Benincore-Flórez, E.; Barbosa, H.; Díaz, C.J.A. Evaluation of HIV-1 derived lentiviral vectors as transductors of Mucopolysaccharidosis type IV a fibroblasts. Gene 2021, 780, 145527. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, V.J.; Bravo, S.B.; Chantada-Vazquez, M.P.; Colón, C.; De Castro, M.J.; Morales, M.; Couce, M.L. Characterization of New Proteomic Biomarker Candidates in Mucopolysaccharidosis Type IVA. Int. J. Mol. Sci. 2020, 22, 226. [Google Scholar] [CrossRef]
- Álvarez, J.V.; Bravo, S.B.; García-Vence, M.; De Castro, M.J.; Luzardo, A.; Colón, C.; Couce, M.L. Proteomic Analysis in Morquio A Cells Treated with Immobilized Enzymatic Replacement Therapy on Nanostructured Lipid Systems. Int. J. Mol. Sci. 2019, 20, 4610. [Google Scholar] [CrossRef]
- Dvorak-Ewell, M.; Wendt, D.; Hague, C.; Christianson, T.; Koppaka, V.; Crippen, D.; Kakkis, E.; Vellard, M. Enzyme replacement in a human model of mucopolysaccharidosis IVA in vitro and its biodistribution in the cartilage of wild type mice. PLoS ONE 2010, 5, e12194. [Google Scholar] [CrossRef]
- Guadalupe-Sierra, V. Development of a Cellular Model for Morquio A Syndrome; Dominican University of California: San Rafael, CA, USA, 2013. [Google Scholar]
- Alméciga-Díaz, C.J.; Montaño, A.M.; Tomatsu, S.; Barrera, L.A. Adeno-associated virus gene transfer in Morquio A disease-effect of promoters and sulfatase-modifying factor 1. FEBS J. 2010, 277, 3608–3619. [Google Scholar] [CrossRef]
- Alméciga-Díaz, C.J.; Montaño, A.M.; Barrera, L.A.; Tomatsu, S. Tailoring the AAV2 capsid vector for bone-targeting. Pediatr. Res. 2018, 84, 545–551. [Google Scholar] [CrossRef]
- Bertolin, J.; Sánchez, V.; Ribera, A.; Jaén, M.L.; Garcia, M.; Pujol, A.; Sánchez, X.; Muñoz, S.; Marcó, S.; Pérez, J.; et al. Treatment of skeletal and non-skeletal alterations of Mucopolysaccharidosis type IVA by AAV-mediated gene therapy. Nat. Commun. 2021, 12, 5343. [Google Scholar] [CrossRef]
- Tomatsu, S.; Montaño, A.M.; Gutierrez, M.; Grubb, J.H.; Oikawa, H.; Dung, V.C.; Ohashi, A.; Nishioka, T.; Yamada, M.; Yamada, M.; et al. Characterization and pharmacokinetic study of recombinant human N-acetylgalactosamine-6-sulfate sulfatase. Mol. Genet. Metab. 2007, 91, 69–78. [Google Scholar] [CrossRef]
- Tomatsu, S.; Montaño, A.M.; Ohashi, A.; Gutierrez, M.A.; Oikawa, H.; Oguma, T.; Dung, V.C.; Nishioka, T.; Orii, T.; Sly, W.S. Enzyme replacement therapy in a murine model of Morquio A syndrome. Hum. Mol. Genet. 2008, 17, 815–824. [Google Scholar] [CrossRef]
- Toietta, G.; Severini, G.M.; Traversari, C.; Tomatsu, S.; Sukegawa, K.; Fukuda, S.; Kondo, N.; Tortora, P.; Bordignon, C. Various cells retrovirally transduced with N-acetylgalactosoamine-6-sulfate sulfatase correct Morquio skin fibroblasts in vitro. Hum. Gene Ther. 2001, 12, 2007–2016. [Google Scholar] [CrossRef] [PubMed]
- Gaffke, L.; Pierzynowska, K.; Podlacha, M.; Hoinkis, D.; Rintz, E.; Brokowska, J.; Cyske, Z.; Wegrzyn, G. Underestimated Aspect of Mucopolysaccharidosis Pathogenesis: Global Changes in Cellular Processes Revealed by Transcriptomic Studies. Int. J. Mol. Sci. 2020, 21, 1204. [Google Scholar] [CrossRef]
- Donida, B.; Marchetti, D.P.; Jacques, C.E.D.; Ribas, G.; Deon, M.; Manini, P.; da Rosa, H.T.; Moura, D.J.; Saffi, J.; Giugliani, R.; et al. Oxidative profile exhibited by Mucopolysaccharidosis type IVA patients at diagnosis: Increased keratan urinary levels. Mol. Genet. Metab. Rep. 2017, 11, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Bank, R.A.; Groener, J.E.; van Gemund, J.J.; Maaswinkel, P.D.; Hoeben, K.A.; Schut, H.A.; Everts, V. Deficiency in N-acetylgalactosamine-6-sulfate sulfatase results in collagen perturbations in cartilage of Morquio syndrome A patients. Mol. Genet. Metab. 2009, 97, 196–201. [Google Scholar] [CrossRef] [PubMed]
- De Franceschi, L.; Roseti, L.; Desando, G.; Facchini, A.; Grigolo, B. A molecular and histological characterization of cartilage from patients with Morquio syndrome. Osteoarthr. Cartil. 2007, 15, 1311–1317. [Google Scholar] [CrossRef]
- Chen, H.; Tan, X.-N.; Hu, S.; Liu, R.-Q.; Peng, L.-H.; Li, Y.-M.; Wu, P. Molecular Mechanisms of Chondrocyte Proliferation and Differentiation. Front. Cell Dev. Biol. 2021, 9, 664168. [Google Scholar] [CrossRef]
- Gosset, M.; Berenbaum, F.; Thirion, S.; Jacques, C. Primary culture and phenotyping of murine chondrocytes. Nat. Protoc. 2008, 3, 1253–1260. [Google Scholar] [CrossRef]
- Mellor, L.F.; Baker, T.L.; Brown, R.J.; Catlin, L.W.; Oxford, J.T. Optimal 3D culture of primary articular chondrocytes for use in the rotating wall vessel bioreactor. Aviat. Space Environ. Med. 2014, 85, 798–804. [Google Scholar] [CrossRef]
- Caron, M.M.J.; Emans, P.J.; Coolsen, M.M.E.; Voss, L.; Surtel, D.A.M.; Cremers, A.; van Rhijn, L.W.; Welting, T.J.M. Redifferentiation of dedifferentiated human articular chondrocytes: Comparison of 2D and 3D cultures. Osteoarthr. Cartil. 2012, 20, 1170–1178. [Google Scholar] [CrossRef]
- Li, X.; Chen, S.; Li, J.; Wang, X.; Zhang, J.; Kawazoe, N.; Chen, G. 3D Culture of Chondrocytes in Gelatin Hydrogels with Different Stiffness. Polymers 2016, 8, 269. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Zhang, W.; Li, X.; Zhong, D.; Li, Y.; Li, J.; Jin, R. Strategies to Modulate the Redifferentiation of Chondrocytes. Front. Bioeng. Biotechnol. 2021, 9, 764193. [Google Scholar] [CrossRef] [PubMed]
- Isyar, M.; Yilmaz, I.; Sirin, D.Y.; Yalcin, S.; Guler, O.; Mahirogullari, M. A practical way to prepare primer human chondrocyte culture. J. Orthop. 2016, 13, 162–167. [Google Scholar] [CrossRef]
- Zito, E.; Fraldi, A.; Pepe, S.; Annunziata, I.; Kobinger, G.; Di Natale, P.; Ballabio, A.; Cosma, M.P. Sulphatase activities are regulated by the interaction of sulphatase-modifying factor 1 with SUMF2. EMBO Rep. 2005, 6, 655–660. [Google Scholar] [CrossRef] [PubMed]
- Poetsch, M.S.; Strano, A.; Guan, K. Human Induced Pluripotent Stem Cells: From Cell Origin, Genomic Stability, and Epigenetic Memory to Translational Medicine. Stem Cells 2022, 40, 546–555. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Pei, D. The magic of four: Induction of pluripotent stem cells from somatic cells by Oct4, Sox2, Myc and Klf4. Cell Res. 2007, 17, 578–580. [Google Scholar] [CrossRef]
- Wang, X.; Li, Z.; Wang, C.; Bai, H.; Wang, Z.; Liu, Y.; Bao, Y.; Ren, M.; Liu, H.; Wang, J. Enlightenment of Growth Plate Regeneration Based on Cartilage Repair Theory: A Review. Front. Bioeng. Biotechnol. 2021, 9, 654087. [Google Scholar] [CrossRef]
- Richard, D.; Pregizer, S.; Venkatasubramanian, D.; Raftery, R.M.; Muthuirulan, P.; Liu, Z.; Capellini, T.D.; Craft, A.M. Lineage-specific differences and regulatory networks governing human chondrocyte development. eLife 2023, 12, e79925. [Google Scholar] [CrossRef]
- Li, R.; Baskfield, A.; Beers, J.; Zou, J.; Liu, C.; Alméciga-Díaz, C.J.; Zheng, W. Generation of an induced pluripotent stem cell line (TRNDi005-A) from a Mucopolysaccharidosis Type IVA (MPS IVA) patient carrying compound heterozygous p.R61W and p.WT405del mutations in the GALNS gene. Stem Cell Res. 2019, 36, 101408. [Google Scholar] [CrossRef]
- Gurda, B.L.; Vite, C.H. Large animal models contribute to the development of therapies for central and peripheral nervous system dysfunction in patients with lysosomal storage diseases. Hum. Mol. Genet. 2019, 28, R119–R131. [Google Scholar] [CrossRef]
- Favret, J.M.; Weinstock, N.I.; Feltri, M.L.; Shin, D. Pre-clinical Mouse Models of Neurodegenerative Lysosomal Storage Diseases. Front. Mol. Biosci. 2020, 7, 57. [Google Scholar] [CrossRef] [PubMed]
- Vuolo, D.; Do Nascimento, C.C.; D’Almeida, V. Reproduction in Animal Models of Lysosomal Storage Diseases: A Scoping Review. Front. Mol. Biosci. 2021, 8, 773384. [Google Scholar] [CrossRef] [PubMed]
- Ribitsch, I.; Baptista, P.M.; Lange-Consiglio, A.; Melotti, L.; Patruno, M.; Jenner, F.; Schnabl-Feichter, E.; Dutton, L.C.; Connolly, D.J.; van Steenbeek, F.G.; et al. Large Animal Models in Regenerative Medicine and Tissue Engineering: To Do or Not to Do. Front. Bioeng. Biotechnol. 2020, 8, 972. [Google Scholar] [CrossRef]
- Szabo, M.; Akusjärvi, S.S.; Saxena, A.; Liu, J.; Janebjer, G.C.; Kitambi, S.S. Cell and small animal models for phenotypic drug discovery. Drug Des. Dev. Ther. 2017, 11, 1957–1967. [Google Scholar] [CrossRef]
- Singh, V.K.; Seed, T.M. How necessary are animal models for modern drug discovery? Expert Opin. Drug Discov. 2021, 16, 1391–1397. [Google Scholar] [CrossRef] [PubMed]
- Swearengen, J.R. Choosing the right animal model for infectious disease research. Anim. Models Exp. Med. 2018, 1, 100–108. [Google Scholar] [CrossRef]
- Tomatsu, S.; Orii, K.O.; Vogler, C.; Nakayama, J.; Levy, B.; Grubb, J.H.; Sly, W.S. Mouse model of N-acetylgalactosamine-6-sulfate sulfatase deficiency (Galns-/-) produced by targeted disruption of the gene defective in Morquio A disease. Hum. Mol. Genet. 2003, 12, 3349–3358. [Google Scholar] [CrossRef]
- McLellan, M.A.; Rosenthal, N.A.; Pinto, A.R. Cre-loxP-Mediated Recombination: General Principles and Experimental Considerations. Curr. Protoc. Mouse Biol. 2017, 7, 1–12. [Google Scholar] [CrossRef]
- Tomatsu, S.; Vogler, C.; Montaño, A.M.; Gutierrez, M.; Oikawa, H.; Dung, V.C.; Sly, W.S. Murine model (Galns(tm(C76S)slu)) of MPS IVA with missense mutation at the active site cysteine conserved among sulfatase proteins. Mol. Genet. Metab. 2007, 91, 251–258. [Google Scholar] [CrossRef]
- Tomatsu, S.; Gutierrez, M.; Nishioka, T.; Yamada, M.; Yamada, M.; Tosaka, Y.; Laybauer, L. Development of MPS IVA mouse (Galnstm(hC79S.mC76S)slu) tolerant to human N-acetylgalactosamine-6-sulfate sulfatase. Hum. Mol. Genet. 2005, 14, 3321–3335. [Google Scholar] [CrossRef]
- FNIH. The Foundation for the National Institutes of Health Announces Selection of Eight Rare Diseases for the Bespoke Gene Therapy Consortium Clinical Trial Portfolio. 2023. Available online: https://fnih.org/news/the-foundation-for-the-national-institutes-of-health-announces-selection-of-eight-rare-diseases-for-the-bespoke-gene-therapy-consortium-clinical-trial-portfolio/ (accessed on 8 August 2023).
- Hallett, S.A.; Matsushita, Y.; Ono, W.; Sakagami, N.; Mizuhashi, K.; Tokavanich, N.; Ono, N. Chondrocytes in the resting zone of the growth plate are maintained in a Wnt-inhibitory environment. eLife 2021, 10, e64513. [Google Scholar] [CrossRef] [PubMed]
- Hallett, S.A.; Ono, W.; Ono, N. Growth Plate Chondrocytes: Skeletal Development, Growth and Beyond. Int. J. Mol. Sci. 2019, 20, 6009. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Lin, Y.-L.; Yan, M.; Li, T.; Wu, E.Y.; Zimmel, K.; Qureshi, O.; Falck, A.; Sherman, K.M.; Huggins, S.S.; et al. Hyaline cartilage differentiation of fibroblasts in regeneration and regenerative medicine. Development 2022, 149, 200249. [Google Scholar] [CrossRef]
- Fujii, Y.; Liu, L.; Yagasaki, L.; Inotsume, M.; Chiba, T.; Asahara, H. Cartilage Homeostasis and Osteoarthritis. Int. J. Mol. Sci. 2022, 23, 6316. [Google Scholar] [CrossRef]
- Rim, Y.A.; Nam, Y.; Ju, J.H. The Role of Chondrocyte Hypertrophy and Senescence in Osteoarthritis Initiation and Progression. Int. J. Mol. Sci. 2020, 21, 2358. [Google Scholar] [CrossRef] [PubMed]
- Cantor, J.R. The Rise of Physiologic Media. Trends Cell Biol. 2019, 29, 854–861. [Google Scholar] [CrossRef] [PubMed]
- Mobasheri, A.; Matta, C.; Uzielienè, I.; Budd, E.; Martín-Vasallo, P.; Bernotiene, E. The chondrocyte channelome: A narrative review. Jt. Bone Spine 2019, 86, 29–35. [Google Scholar] [CrossRef]
- Defois, A.; Bon, N.; Charpentier, A.; Georget, M.; Gaigeard, N.; Blanchard, F.; Hamel, A.; Waast, D.; Armengaud, J.; Renoult, O.; et al. Osteoarthritic chondrocytes undergo a glycolysis-related metabolic switch upon exposure to IL-1b or TNF. Cell Commun. Signal. 2023, 21, 137. [Google Scholar] [CrossRef]
- Cao, U.M.N.; Zhang, Y.; Chen, J.; Sayson, D.; Pillai, S.; Tran, S.D. Microfluidic Organ-on-A-chip: A Guide to Biomaterial Choice and Fabrication. Int. J. Mol. Sci. 2023, 24, 3232. [Google Scholar] [CrossRef]
- Ingber, D.E. Human organs-on-chips for disease modelling, drug development and personalized medicine. Nat. Rev. Genet. 2022, 23, 467–491. [Google Scholar] [CrossRef]
- Ma, C.; Peng, Y.; Li, H.; Chen, W. Organ-on-a-Chip: A New Paradigm for Drug Development. Trends Pharmacol. Sci. 2021, 42, 119–133. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Liu, J.; Wang, X.; Feng, L.; Wu, J.; Zhu, X.; Wen, W.; Gong, X. Organ-on-a-chip: Recent breakthroughs and future prospects. Biomed. Eng. Online 2020, 19, 9. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Erickson, A.; Dudley, A.T.; Ryu, S. Mechanical stimulation of growth plate chondrocytes: Previous approaches and future directions. Exp. Mech. 2019, 59, 1261–1274. [Google Scholar] [CrossRef]
- Das, R.; Jahr, H.; Verhaar, J.; van der Linden, J.; van Osch, G.; Weinans, H. In vitro expansion affects the response of chondrocytes to mechanical stimulation. Osteoarthr. Cartil. 2008, 16, 385–391. [Google Scholar] [CrossRef]
- Xu, W.; Zhu, J.; Hu, J.; Xiao, L. Engineering the biomechanical microenvironment of chondrocytes towards articular cartilage tissue engineering. Life Sci. 2022, 309, 121043. [Google Scholar] [CrossRef] [PubMed]
- Du, G.; Li, L.; Zhang, X.; Liu, J.; Hao, J.; Zhu, J.; Wu, H.; Chen, W.; Zhang, Q. Roles of TRPV4 and piezo channels in stretch-evoked Ca2+ response in chondrocytes. Exp. Biol. Med. 2020, 245, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Vallender, E.J.; Hotchkiss, C.E.; Lewis, A.D.; Rogers, J.; Stern, J.A.; Peterson, S.M.; Ferguson, B.; Sayers, K. Nonhuman primate genetic models for the study of rare diseases. Orphanet J. Rare Dis. 2023, 18, 20. [Google Scholar] [CrossRef]
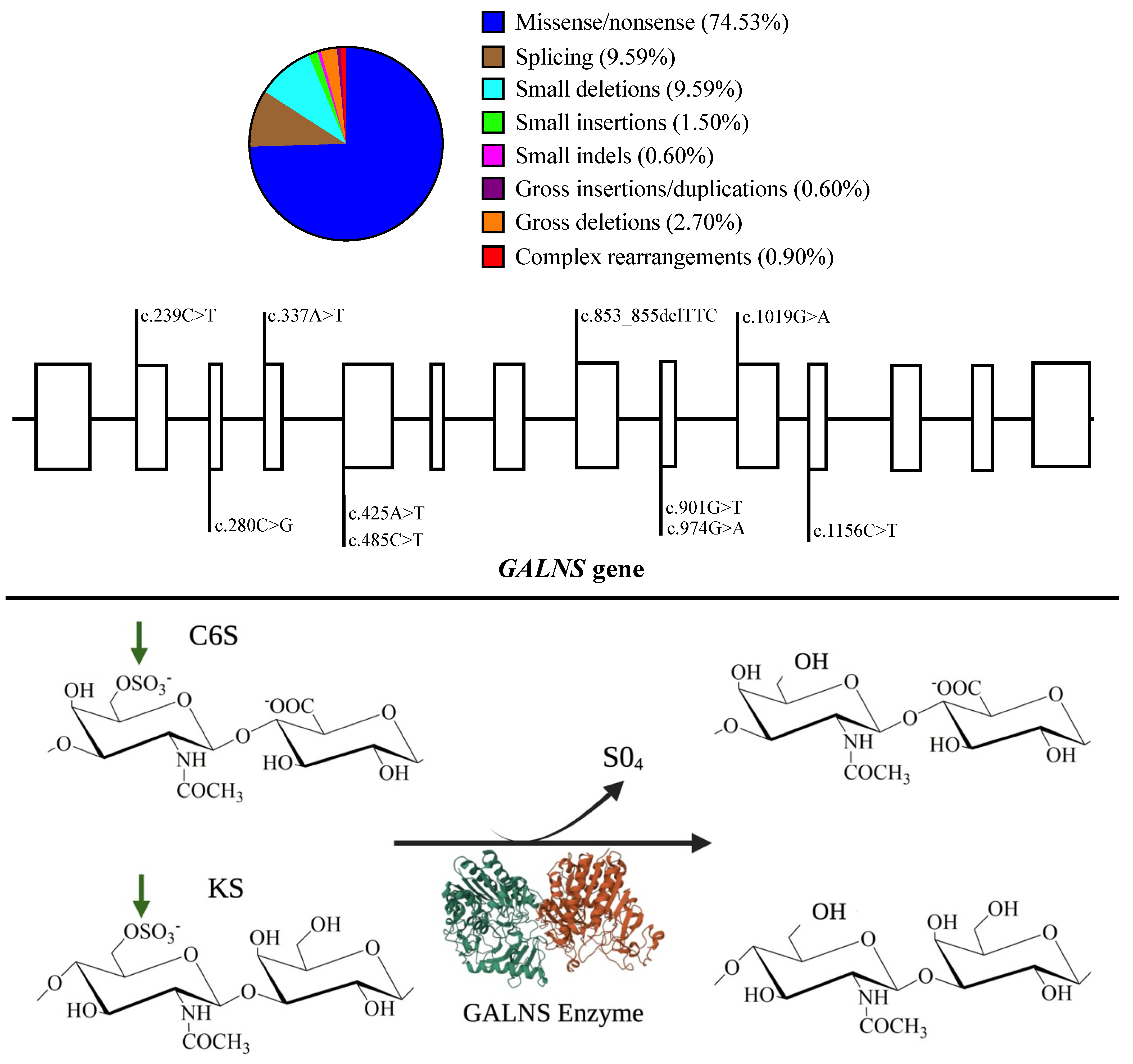
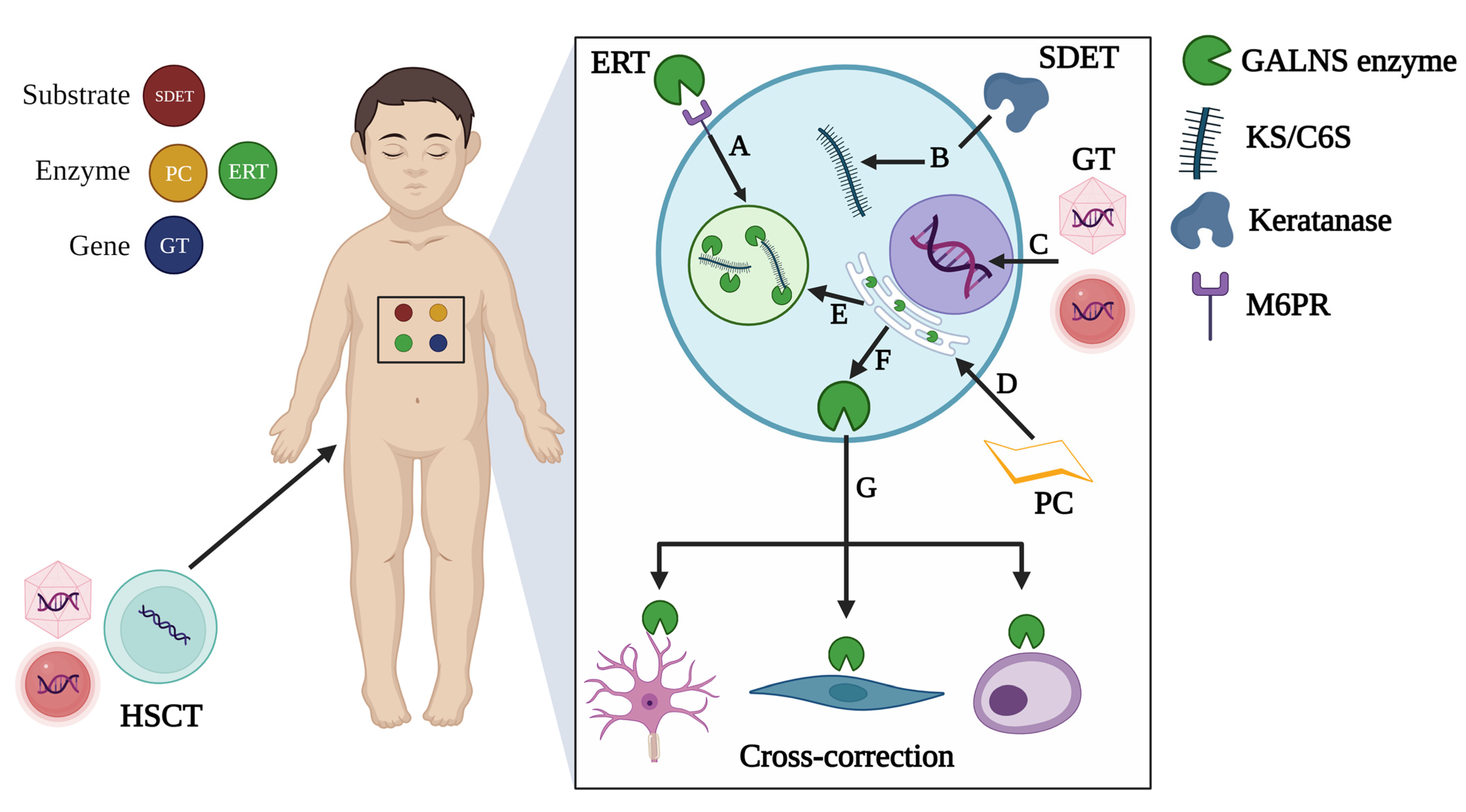
| Approach | Model | Human | Mouse | Rat |
|---|---|---|---|---|
| In vitro | Fibroblasts | X | X | |
| Chondrocytes | X | X | ||
| Leukocytes | X | |||
| iPSC-MSC-derived chondrocytes | X | |||
| In vivo | GALNS knock-out | X | ||
| GALNS missense mutation | X | X |
| Purpose | MPS IVA Model | * Plat. | Media | Suppl. | P | Refs. |
|---|---|---|---|---|---|---|
| Proteomics | Human fibroblast | 2D | McCoy 5A | 10% FBS, 1% P/S | ND | [35] |
| Human leukocytes | NA | NA | NA | NA | [34] | |
| ERT | Human chondrocytes | 2D | 2D: CGMTM | 3D: Ascorbic acid | ND | [36,41] |
| 3D | 3D: CDMTM | |||||
| Human skin fibroblasts | 2D | DMEM | 15% FBS, 1% P/S | ND | [41,42] | |
| iPSC-MSC-derived human chondrocytes | 3D | MEM | TGFβ3 | P1 * | [37] | |
| PC | Human fibroblast | 2D | DMEM | 15% FBS, 1% P/S | ND | [28] |
| SDET | Human chondrocytes | 3D | CBM™ Basal Medium | NA | ND | [29] |
| Retrovirus | Fibroblast | 2D | DMEM | 10% FCS | ND | [43] |
| PBLs | 2D | IMDM and RPMI | IMDM: 10% FCS, PHA, IL-2RPMI: HS | ND | ||
| Lymphoblastoid | 2D | DMEM | 10% FCS | ND | ||
| LV GT | Human fibroblast | 2D | DMEM | 10% FBS 1% P/S | ND | [33] |
| AAV GT | Human fibroblasts | 2D | NA | NA | ND | [39] |
| Murine chondrocytes | 3D | NA | NA | ND | [38] | |
| CRISPR/nCas9 GT | Fibroblast | 2D | DMEM | 15% FBS 1% P/S | P3-P7 | [30,32] |
| Parameter | Mouse Models | Rat Model | ||
|---|---|---|---|---|
| MKC | C2 | MTOL | ||
| GALNS gene | * KO | mGALNS: C79S | mGALNS: C79S hGALNS: C76S | rGALNS: R388C |
| GALNS activity | UD | UD | UD | UD |
| Body weight | UA | UA | UA | 50% reduction |
| Skeletal dysplasia | No | No | No | Yes |
| Total GAGs | Urine: 6-fold | Urine: UA | Urine: 1.3-fold | NA |
| Cornea: 2.3-fold | Cornea: NA | Cornea: NA | ||
| KS | Urine: UD | NA | Urine: NA | Serum: ~3-fold |
| Cornea: 1.7-fold | Cornea: UA | Femur: 4-fold | ||
| Bone | GP: UA | GP: NA | GP: Irregular structure | GP: Short |
| Chon: UA | Chon: Vacuo. | Chon: Vacuo. | Chond: Vacuo. | |
| Osteob: UA | Osteob: UA | Osteob: Vacuo. | Osteob: NA | |
| Osteoc: UA | Osteoc: UA | Osteoc: Vacuo. | Osteoc: NA | |
| Kidney EC in glomeruli | Vacuo. | Vacuo. | Vacuo. | NA |
| Heart Valves | Vacuo. | Vacuo. | Vacuo. | Vacuo. |
| Liver Kupffer cells | Vacuo. | Vacuo. | Vacuo. | Vacuo. |
| Other non-GALNS sulfatases | NA | ARSB: Increased | ARSB: Decreased | NA |
| IDS: Increased excepting bone | IDS: Decreased | |||
| Sulfa: Increased | Sulfa: Decreased | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leal, A.F.; Alméciga-Díaz, C.J.; Tomatsu, S. Mucopolysaccharidosis IVA: Current Disease Models and Drawbacks. Int. J. Mol. Sci. 2023, 24, 16148. https://doi.org/10.3390/ijms242216148
Leal AF, Alméciga-Díaz CJ, Tomatsu S. Mucopolysaccharidosis IVA: Current Disease Models and Drawbacks. International Journal of Molecular Sciences. 2023; 24(22):16148. https://doi.org/10.3390/ijms242216148
Chicago/Turabian StyleLeal, Andrés Felipe, Carlos Javier Alméciga-Díaz, and Shunji Tomatsu. 2023. "Mucopolysaccharidosis IVA: Current Disease Models and Drawbacks" International Journal of Molecular Sciences 24, no. 22: 16148. https://doi.org/10.3390/ijms242216148
APA StyleLeal, A. F., Alméciga-Díaz, C. J., & Tomatsu, S. (2023). Mucopolysaccharidosis IVA: Current Disease Models and Drawbacks. International Journal of Molecular Sciences, 24(22), 16148. https://doi.org/10.3390/ijms242216148








