Synergic Action of Systemic Risedronate and Local Rutherpy in Peri-implantar Repair of Ovariectomized Rats: Biomechanical and Molecular Analysis
Abstract
:1. Introduction
2. Results
2.1. Clinical Data
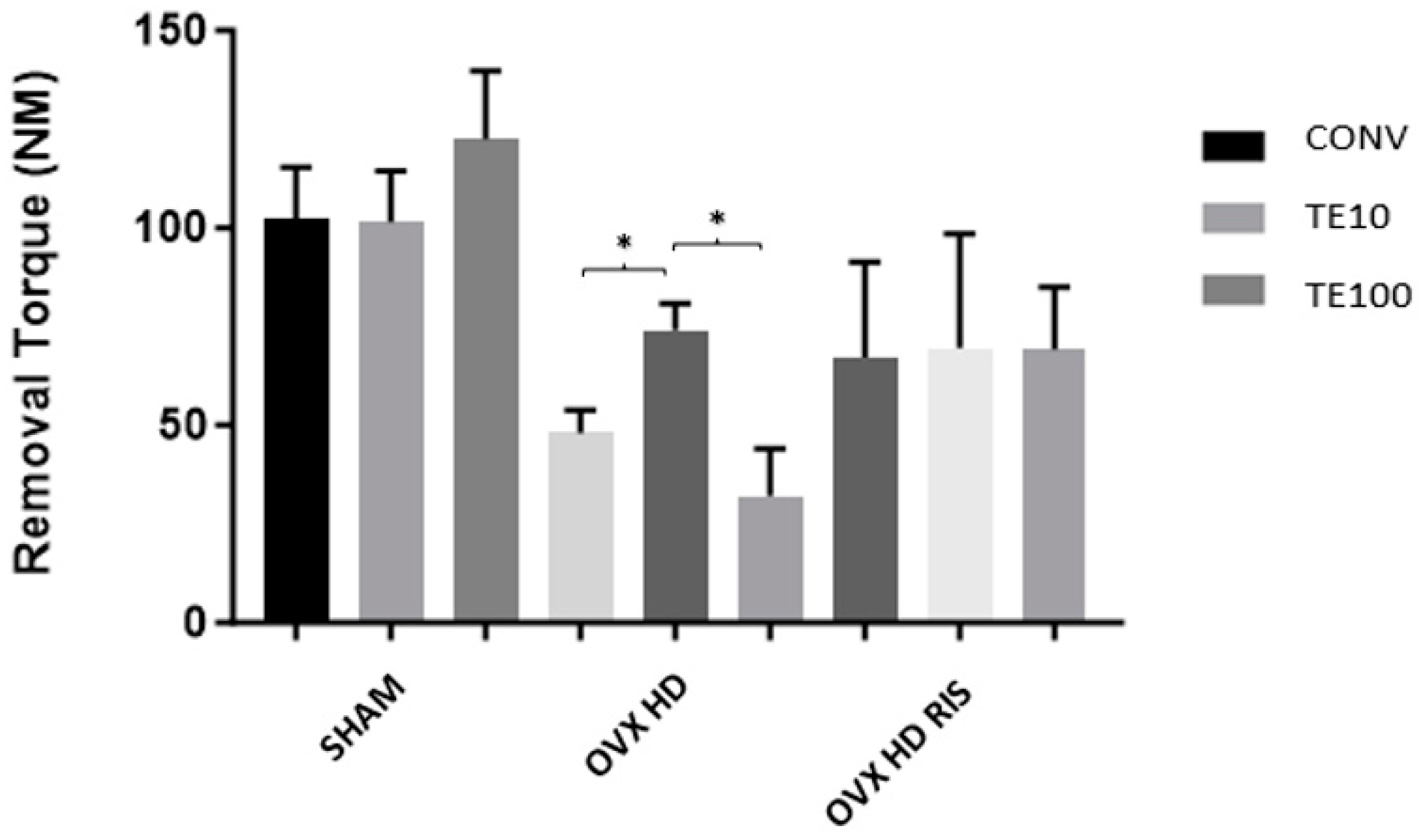
2.2. Biomechanical Analysis (Counter-Torque)
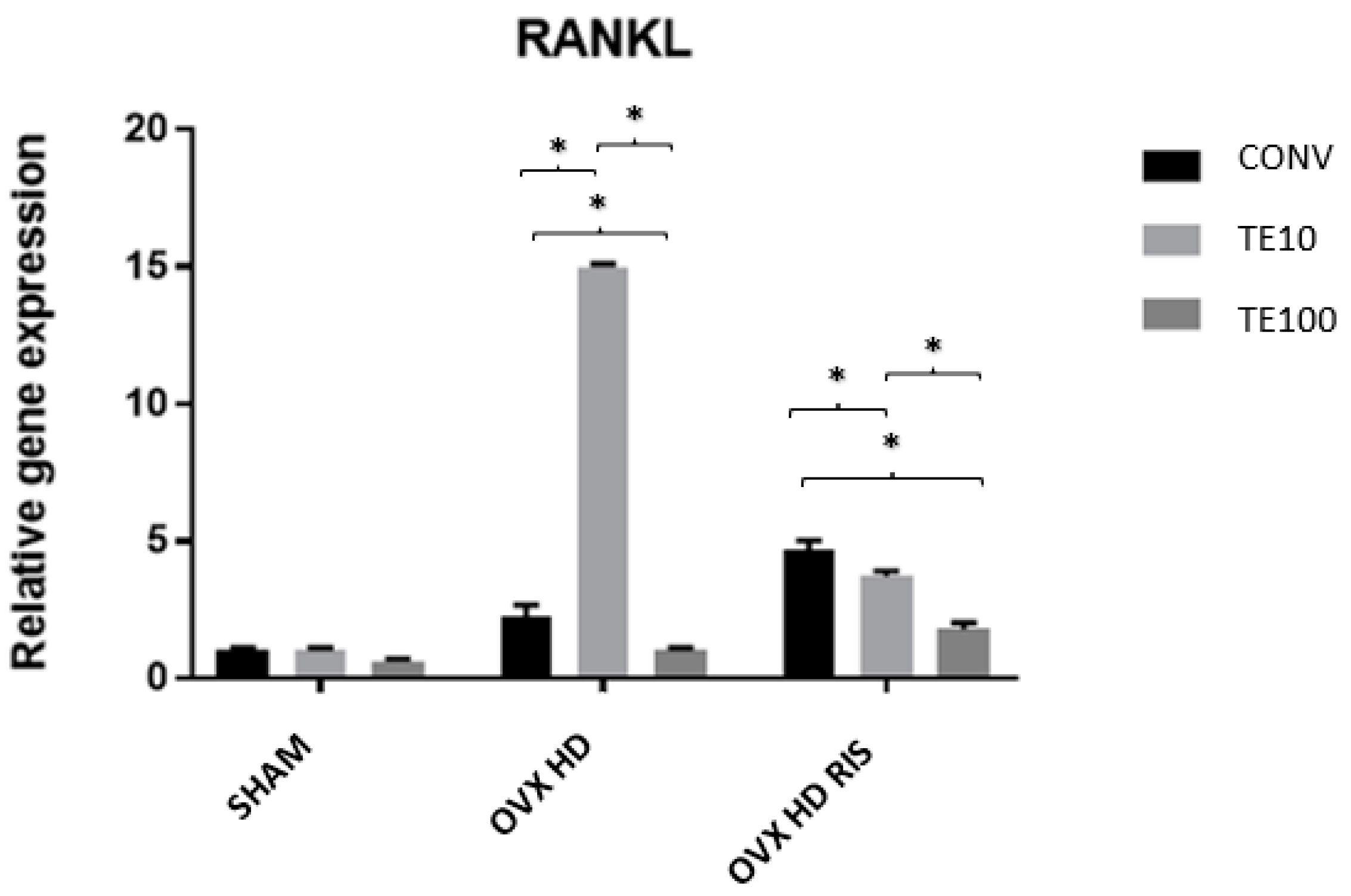
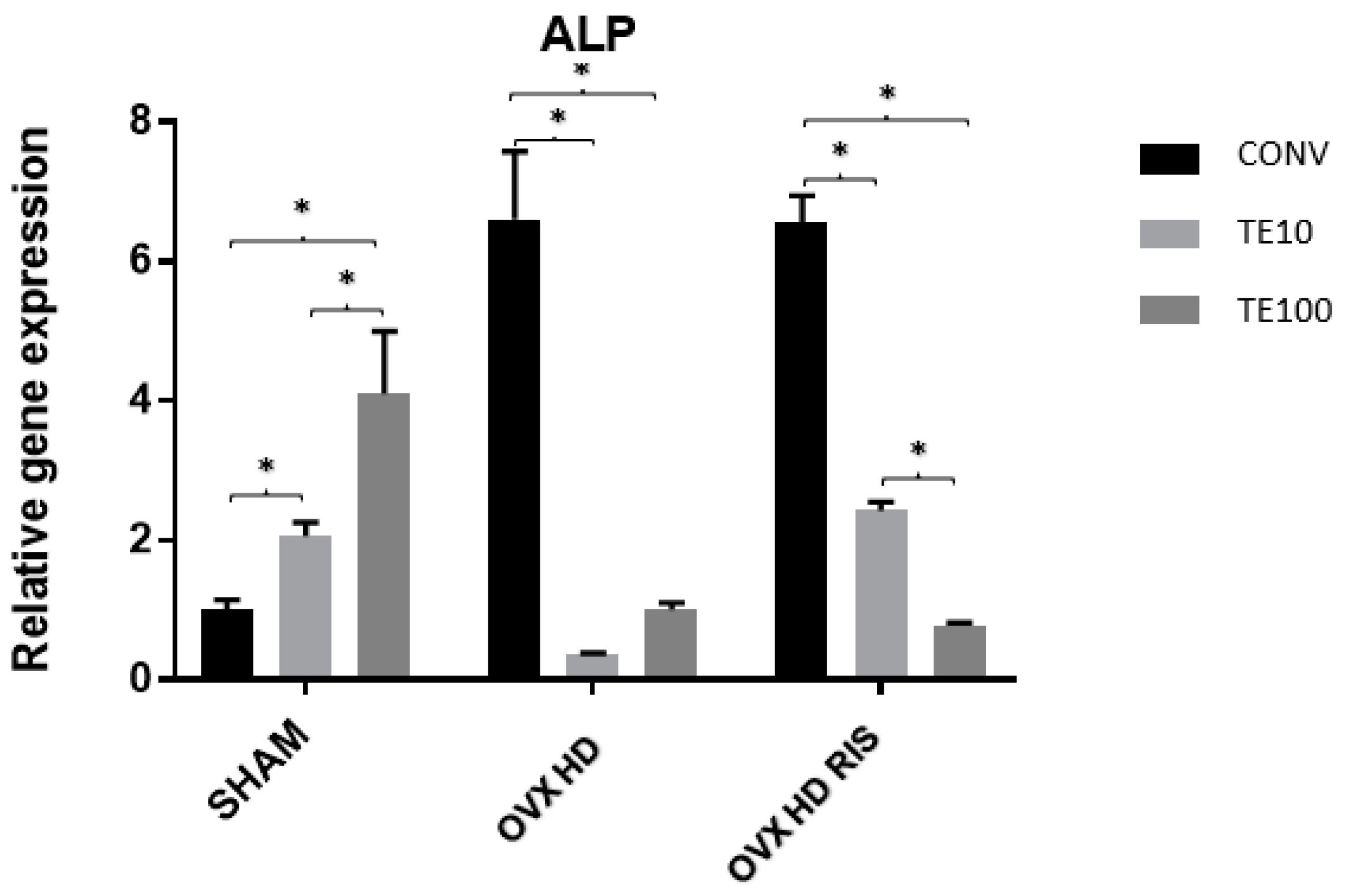
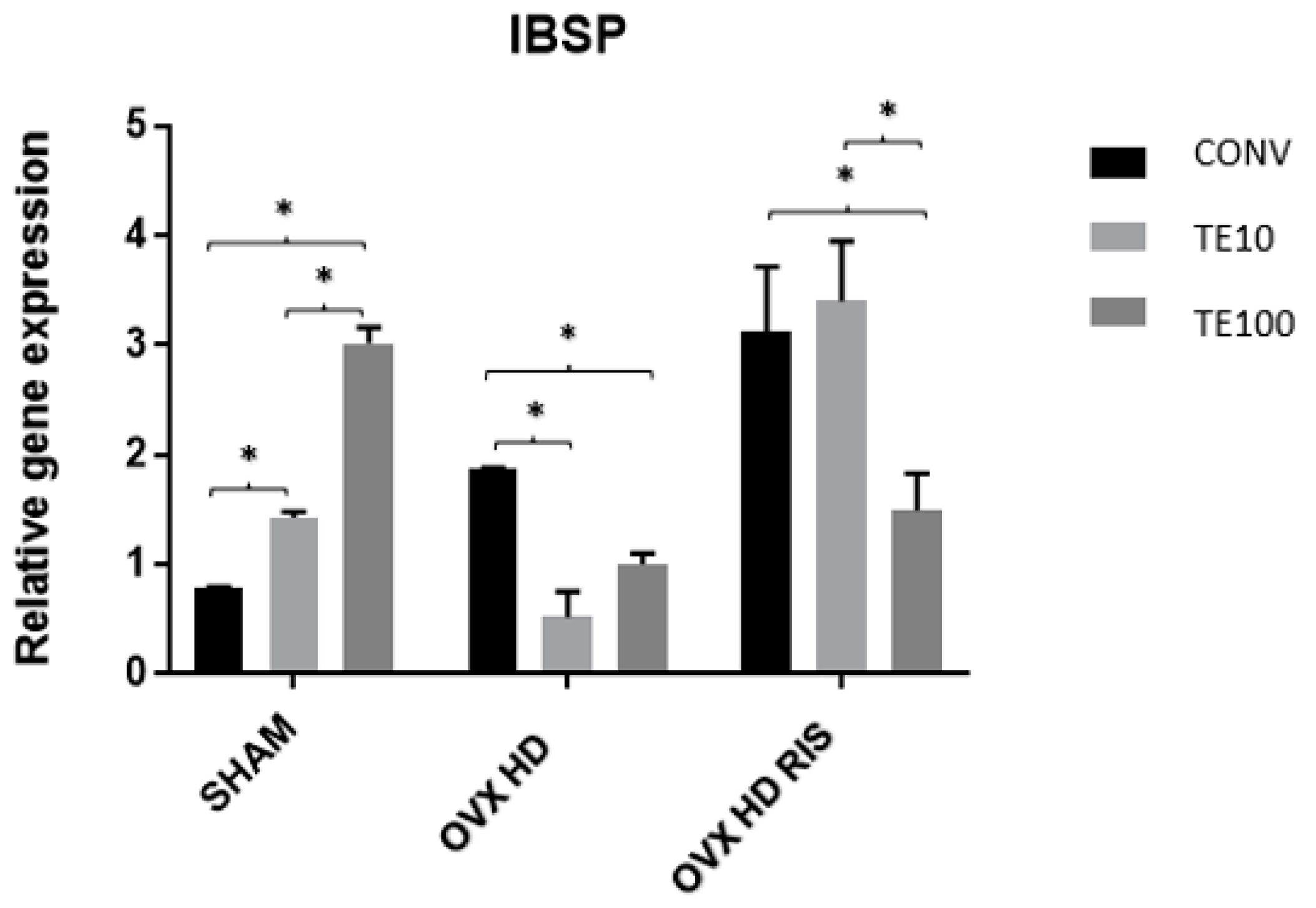
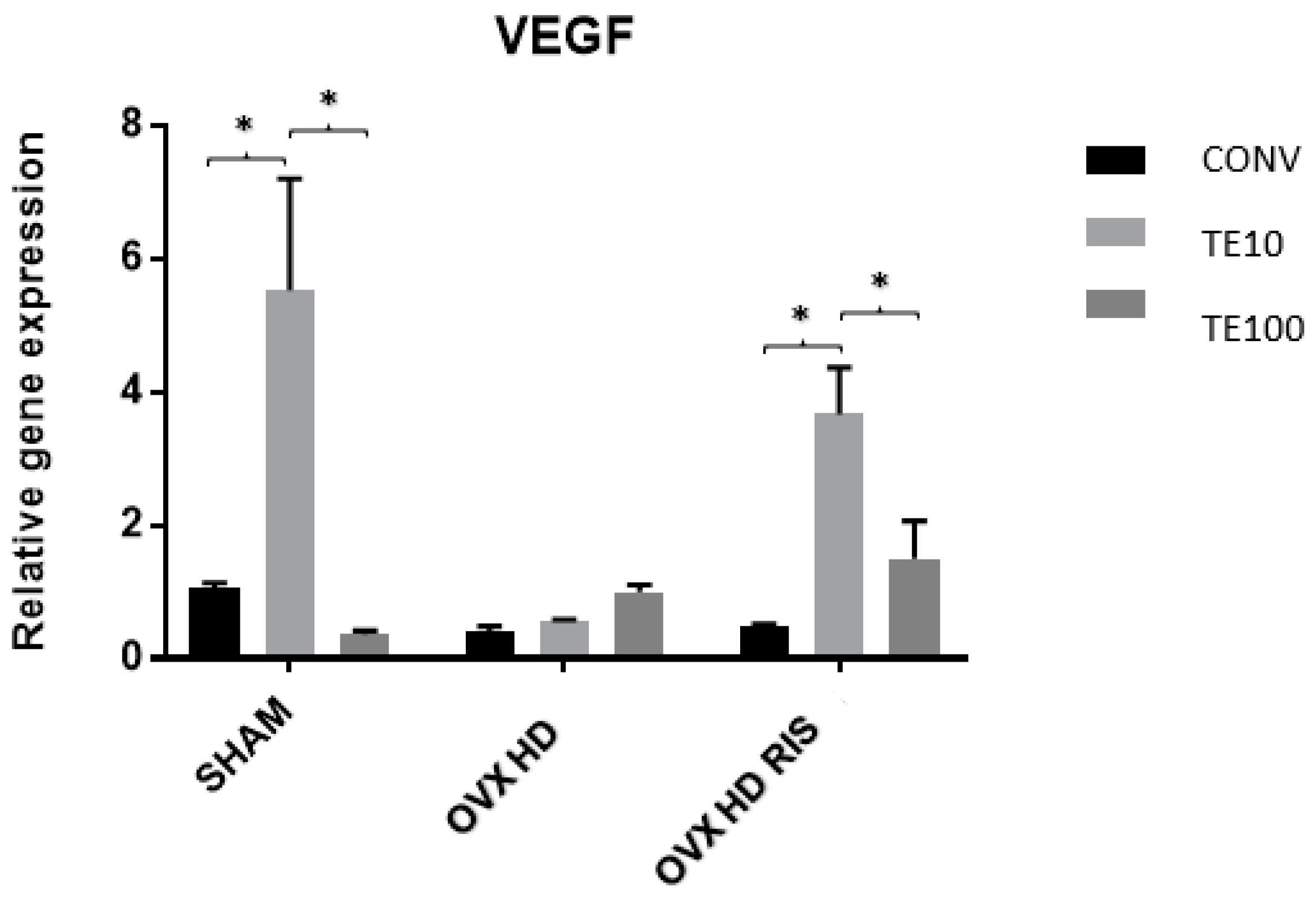
2.3. Molecular Analysis (Real-Time PCR)
3. Discussion
4. Methods and Materials
4.1. Animals
4.2. High-Fat Diet
4.3. Clinical Data Collection
4.4. Fictitious Surgery and Bilateral Ovariectomy Surgery
4.5. Systemic and Local Drug Treatments
4.5.1. Systemic Treatment with Risedronate Sodium
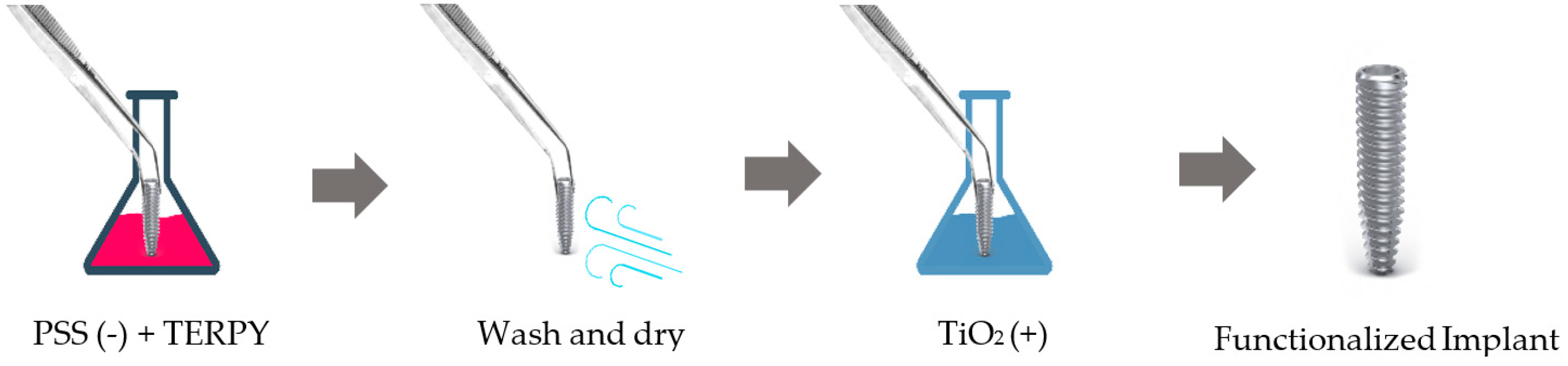
4.5.2. Implant Surface Functionalization with TERPY
4.6. Implant Installation
4.7. Euthanasia
4.8. Proposed Analyses
4.8.1. Biomechanical Analysis (Removal Torque)
4.8.2. Molecular Analysis (Real-Time PCR)
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Harvey, N.; Dennison, E.; Cooper, C. Osteoporosis: Impact on health and economics. Nat. Rev. Rheumatol. 2012, 6, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Darcey, J.; Horner, K.; Walsh, T.; Southern, H.; Marjanovic, E.J.; Devlin, H. Tooth loss and osteoporosis: To assess the association between osteoporosis status and tooth number. Br. Dent. J. 2013, 214, E10. [Google Scholar] [CrossRef] [PubMed]
- Drake, M.T.; Khosla, S. Male Osteoporosis. Endocrinol. Metab. Clin. N. Am. 2012, 41, 629–641. [Google Scholar] [CrossRef] [PubMed]
- International Osteoporis Foundation. IOF Osteoporosis Risk Check. Available online: https://www.osteoporosis.foundation/patients/about-osteoporosis (accessed on 12 March 2023).
- Eastell, R.; O’Neill, T.W.; Hofbauer, L.C.; Langdahl, B.; Reid, I.R.; Gold, D.T.; Cummings, S.R. Postmenopausal osteoporosis. Nat. Rev. Dis. Primers 2016, 2, 16069. [Google Scholar] [CrossRef]
- Cauble, M.A.; Muckley, M.J.; Fang, M.; Fessler, J.A.; Welch, K.; Rothman, E.D.; Orr, B.G.; Duong, L.T.; Holl, M.M.B. Estrogen depletion and drug treatment alter the microstructure of type I collagen in bone. Bone Rep. 2016, 5, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Gaspard, U.J.; Gottal, J.M.; Van den Brûle, F.A. Postmenopausal changes of lipid and glucose metabolism: A review of their main aspects. Maturitas 1995, 21, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Ali, D.; Figeac, F.; Caci, A.; Ditzel, N.; Schmal, C.; Kerckhofs, G.; Havelund, J.; Faergeman, N.; Rauch, A.; Tencerova, M.; et al. High-fat diet-induced obesity augments the deleterious effects of estrogen deficiency on bone: Evidence from ovariectomized mice. Aging Cell 2022, 21, e13726. [Google Scholar] [CrossRef]
- Wong, S.K.; Chin, K.Y.; Suhaimi, F.H.; Ahmad, F.; Ima-Nirwana, S. The Relationship between Metabolic Syndrome and Osteoporosis: A Review. Nutrients 2016, 8, 347. [Google Scholar] [CrossRef]
- Ko, S.H.; Jung, Y. Energy Metabolism Changes and Dysregulated Lipid Metabolism in Postmenopausal Women. Nutrients 2021, 13, 4556. [Google Scholar] [CrossRef]
- Tawfik, S.H.; Mahmoud, B.F.; Saad, M.I.; Shehata, M.; Kamel, M.A.; Helmy, M.H. Similar and Additive Effects of Ovariectomy and Diabetes on Insulin Resistance and Lipid Metabolism. Biochem. Res. Int. 2015, 2015, 567945. [Google Scholar] [CrossRef]
- Wong, S.K.; Mahmoud, B.F.; Saad, M.I.; Shehata, M.; Kamel, M.A.; Helmy, M.H. Osteoporosis is associated with metabolic syndrome induced by high-carbohydrate high-fat diet in a rat model. Biomed. Pharmacother. 2018, 98, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhang, Q.; Yuan, X.; Wang, J.; Li, C.; Sheng, H.; Qu, S.; Li, H. Association between metabolic syndrome and osteoporosis: A meta-analysis. Bone 2013, 57, 30–35. [Google Scholar] [CrossRef]
- Abbasi, M.; Farzam, S.A.; Mamaghani, Z.; Yazdi, Z. Relationship between metabolic syndrome and its components with bone densitometry in postmenopausal women. Diabetes Metab. Syndr. 2017, 11, S73–S76. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Tandon, V.R.; Mahajan, S.; Mahajan, V.; Majahan, A. Obesity: Friend or foe for osteoporosis. J. Mid-life Health 2014, 5, 6. [Google Scholar] [CrossRef] [PubMed]
- Zong, Q.; Bundkirchen, K.; Neunaber, C.; Noack, S. Are the Properties of Bone Marrow-Derived Mesenchymal Stem Cells Influenced by Overweight and Obesity? Int. J. Mol. Sci. 2023, 24, 4831. [Google Scholar] [CrossRef]
- Rolvien, T.; Amling, M. Disuse Osteoporosis: Clinical and Mechanistic Insights. Calcif. Tissue Int. 2021, 110, 592–604. [Google Scholar] [CrossRef]
- Calder, P.C.; Ahluwalia, N.; Brouns, F.; Buetler, T.; Clement, K.; Cunningham, K.; Esposito, K.; Jönsson, L.S.; Kolb, H.; Lansink, M.; et al. Dietary factors and low-grade inflammation in relation to overweight and obesity. Br. J. Nutr. 2011, 106, S5–S78. [Google Scholar] [CrossRef]
- Kinjo, M.; Setoguchi, S.; Solomon, D.H. Bone Mineral Density in Adults with the Metabolic Syndrome: Analysis in a Population-Based U.S. Sample. J. Clin. Endocrinol. Metab. 2007, 92, 4161–4164. [Google Scholar] [CrossRef]
- Campos, R.M.; de Piano, A.; da Silva, P.L.; Carnier, J.; Sanches, P.L.; Corgosinho, F.C.; Masquio, D.C.; Lazaretti-Castro, M.; Oyama, L.M.; Nascimento, C.M.; et al. The role of pro/anti-inflammatory adipokines on bone metabolism in NAFLD obese adolescents: Effects of long-term interdisciplinary therapy. Endocrine 2012, 42, 146–156. [Google Scholar] [CrossRef]
- Dias-Junior, C.A.; Cau, S.B.D.A.; Tanus-Santos, J.E. Papel do óxido nítrico na regulação da circulação pulmonar: Implicações fisiológicas, fisiopatológicas e terapêuticas. J. Bras. Pneumol. 2008, 34, 412–419. [Google Scholar] [CrossRef]
- Saltiel, A.R.; Olefsky, J.M. Inflammatory mechanisms linking obesity and metabolic disease. J. Clin. Investig. 2017, 127, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Izaola, O.; Luis, D.; Sajoux, I.; Domingo, J.C.; Vidal, M. Inflamación y obesidad (lipoinflamación). Nutr. Hosp. 2015, 31, 2352–2358. [Google Scholar] [PubMed]
- Black, D.M.; Rosen, C.J. Postmenopausal Osteoporosis. N. Engl. J. Med. 2016, 374, 254–262. [Google Scholar] [CrossRef]
- Aspray, T.J.; Francis, R.M. Treatment of osteoporosis in women intolerant of oral bisphosphonates. Maturitas 2012, 71, 76–78. [Google Scholar] [CrossRef]
- Yun, K.J.; Lee, J.G.; Kim, S.S.; Kim, B.H.; Kim, S.J.; Kim, Y.K.; Kim, I.J. Association between bone mineral density and metabolic syndrome in pre- and postmenopausal women. Endocr. J. 2011, 58, 87–93. [Google Scholar]
- Malavasi, M.; Louro, R.; Barros, M.B.; Teixeira, L.N.; Peruzzo, D.C.; Joly, J.C.; Martinez, E.F.; Napimoga, M.H. Effects of risedronate on osteoblastic cell cultures. Arch. Oral Biol. 2016, 68, 43–47. [Google Scholar] [CrossRef]
- Wang, G.; Zhu, Z.; Lei, C.; Li, M.; Liu, F.; Mao, Y.; Yu, Z.; Liu, M.; Zhao, X.; Tang, T. Low-dose Risedronate Sodium Protects Bone Cells after Abrupt Oestrogen Withdrawal. J. Int. Med. Res. 2012, 40, 1761–1774. [Google Scholar] [CrossRef]
- D’Amelio, P.; Grimaldi, A.; Di Bella, S.; Tamone, C.; Brianza, S.Z.M.; Ravazzoli, M.G.A.; Bernabei, P.; Cristofaro, M.A.; Pescarmona, G.P.; Isaia, G. Risedronate Reduces Osteoclast Precursors and Cytokine Production in Postmenopausal Osteoporotic Women. J. Bone Miner. Res. 2007, 23, 373–379. [Google Scholar] [CrossRef]
- Ruggiero, S.L.; Dodson, T.B.; Fantasia, J.; Goodday, R.; Aghaloo, T.; Mehrotra, B.; O’Ryan, F. American Association of Oral and Maxillofacial Surgeons position paper on medication-related osteonecrosis of the jaw—2014 update. J. Oral Maxillofac. Surg. 2014, 72, 1938–1956. [Google Scholar] [CrossRef]
- Ramalho-Ferreira, G.; Faverani, L.P.; Prado, F.B.; Garcia, I.R., Jr.; Okamoto, R. Raloxifene enhances peri-implant bone healing in osteoporotic rats. Int. J. Oral Maxillofac. Surg. 2015, 44, 798–805. [Google Scholar] [CrossRef]
- Luvizuto, E.R.; Queiroz, T.P.; Dias, S.M.D.; Okamoto, T.; Dornelles, R.C.M.; Garcia, I.R., Jr.; Okamoto, R. Histomorphometric analysis and immunolocalization of RANKL and OPG during the alveolar healing process in female ovariectomized rats treated with oestrogen or raloxifene. Arch. Oral Biol. 2010, 55, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Faverani, L.P.; Polo, T.O.B.; Ramalho-Ferreira, G.; Momesso, G.A.C.; Hassumi, J.S.; Rossi, A.C.; Freire, A.R.; Prado, F.B.; Luvizuto, E.R.; Gruber, R.; et al. Raloxifene but not alendronate can compensate the impaired osseointegration in osteoporotic rats. Clin. Oral Investig. 2018, 22, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Tu, K.N.; Lie, J.D.; Wan, C.K.V.; Cameron, M.; Austel, A.G.; Nguyen, J.K.; Van, K.; Hyun, D. Osteoporosis: A Review of Treatment Options. Pharm. Ther. 2018, 43, 92–104. [Google Scholar]
- Gomes-Ferreira, P.H.S.; Micheletti, C.; Frigério, P.B.; de Souza Batista, F.R.; Monteiro, N.G.; Bim-júnior, O.; Okamoto, R. PTH 1-34-functionalized bioactive glass improves peri-implant bone repair in orchiectomized rats: Microscale and ultrastructural evaluation. Biomater. Adv. 2022, 134, 112688. [Google Scholar] [CrossRef] [PubMed]
- Delmas, P.D. Treatment of postmenopausal osteoporosis. Lancet 2002, 359, 2018–2026. [Google Scholar] [CrossRef] [PubMed]
- Trovas, G.P.; Lyritis, G.P.; Galanos, A.; Raptou, P.; Constantelou, E. A Randomized Trial of Nasal Spray Salmon Calcitonin in Men With Idiopathic Osteoporosis: Effects on Bone Mineral Density and Bone Markers. J. Bone Miner. Res. 2002, 17, 521–527. [Google Scholar] [CrossRef]
- de Oliveira, J.H.A.; Bracco, O.L.; Kayath, M.; Guarniero, R. Teriparatida (PTH[1-34]rh): Uma nova perspectiva no tratamento da osteoporose. Acta Ortop. Bras. 2003, 11, 184–189. [Google Scholar] [CrossRef]
- Andrews, E.B.; Gilsenan, A.W.; Midkiff, K.; Sherrill, B.; Wu, Y.; Mann, B.H.; Masica, D. The US postmarketing surveillance study of adult osteosarcoma and teriparatide: Study design and findings from the first 7 years. J. Bone Miner. Res. 2012, 27, 2429–2437. [Google Scholar] [CrossRef]
- Wang, P.G.; Xian, M.; Tang, X.; Wu, X.; Wen, Z.; Cai, T.; Janczuk, A.J. Nitric oxide donors: Chemical activities and biological applications. Chem. Rev. 2002, 102, 1091–1134. [Google Scholar] [CrossRef]
- Bettache, N.; Carter, T.; Corrie, J.E.; Ogden, D.; Trentham, D.R. Photolabile donors of nitric oxide: Ruthenium nitrosyl chlorides as caged nitric oxide. Methods Enzymol. 1996, 268, 266–281. [Google Scholar]
- Lunardi, C.N.; Da Silva, R.S.; Bendhack, L.M. New nitric oxide donors based on ruthenium complexes. Braz. J. Med. Biol. Res. 2009, 42, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Schwentker, A.; Billiar, T.R. Nitric oxide and wound repair. Surg. Clin. N. Am. 2003, 83, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Koyama, A.; Otsuka, E.; Inpue, A.; Hirose, S.; Hagiwara, H. Nitric oxide accelerates the ascorbic acid-induced osteoblastic differentiation of mouse stromal ST2 cells by stimulating the production of prostaglandin E(2). Eur. J. Pharmacol. 2000, 391, 225–231. [Google Scholar] [CrossRef]
- Afzal, F.; Polak, J.M.; Lee, D.K. Buttery. Endothelial nitric oxide synthase in the control of osteoblastic mineralizing activity and bone integrity. J. Pathol. 2004, 202, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Chae, H.J.; Park, R.K.; Chung, H.T.; Kang, J.S.; Kim, M.S.; Choi, D.Y.; Bang, B.G.; Kim, H.R. Nitric Oxide is a Regulator of Bone Remodelling. J. Pharm. Pharmacol. 1997, 49, 897–902. [Google Scholar] [CrossRef] [PubMed]
- Stefanska, A.; Bergmann, K.; Sypniewska, G. Metabolic Syndrome and Menopause. Adv. Clin. Chem. 2015, 72, 1–75. [Google Scholar]
- Perry, A.; Wang, X.; Goldberg, R.; Ross, R.; Jackson, L. Androgenic sex steroids contribute to metabolic risk beyond intra-abdominal fat in overweight/obese black and white women. Obesity 2013, 21, 1618–1624. [Google Scholar] [CrossRef]
- Janssen, I.; Powell, L.H.; Kazlauskaite, R.; Dugan, S.A. Testosterone and Visceral Fat in Midlife Women: The Study of Women’s Health Across the Nation (SWAN) Fat Patterning Study. Obesity 2009, 18, 604–610. [Google Scholar] [CrossRef]
- Caceres, M.; Teran, C.G.; Rodriguez, S.; Medina, M. Prevalence of insulin resistance and its association with metabolic syndrome criteria among Bolivian children and adolescents with obesity. BMC Pediatr. 2008, 8, 31. [Google Scholar] [CrossRef]
- Almeida, M.E.F.; Santos, V.S.; Simão, A.A.; Corrêa, A.D. Dieta de cafeteria com chocolate, amendoim e biscoito: Eficácia na indução do excesso de peso e da dislipidemia em ratos. SaBios-Rev. Saúde Biol. 2015, 10, 15–24. [Google Scholar]
- Mellado-Valero, A.; Ferrer-García, J.C.; Calvo-Catalá, J.; Labaig-Rueda, C. Implant treatment in patients with osteoporosis. Med. Oral Patol. Oral Cir. Bucal 2009, 15, e52–e57. [Google Scholar] [CrossRef] [PubMed]
- Szulc, P.; Seeman, E.; Duboef, F.; Sornay-Rendi, E.; Delmas, P.D. Bone Fragility: Failure of Periosteal Apposition to Compensate for Increased Endocortical Resorption in Postmenopausal Women. J. Bone Miner. Res. 2006, 21, 1856–1863. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, K.K.; Macdonald, H.M.; Buie, H.R.; Hanley, D.A.; Boyd, S.K. Postmenopausal Women with Osteopenia Have Higher Cortical Porosity and Thinner Cortices at the Distal Radius and Tibia Than Women with Normal aBMD: An In Vivo HR-pQCT Study. J. Bone Miner. Res. 2009, 25, 882–890. [Google Scholar] [CrossRef]
- Payne, J.B.; Zachs, N.R.; Reinhardt, R.A.; Nummikoski, P.V.; Patil, K. The association between estrogen status and alveolar bone density changes in postmenopausal women with a history of periodontitis. J. Periodontol. 1997, 68, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, D.; Hassumi, J.S.; Gomes-Ferreira, P.H.S.; Polo, T.O.B.; Ramalho-Ferreira, G.; Faverani, L.P.; Okamoto, R. Short Term Sodium Alendronate Administration Improves the Peri-Implant Bone Quality in Osteoporotic Animals. J. Appl. Oral Sci. 2017, 25, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Jin, D. [Risedronate inhibits rat bone marrow adipogenesis and reduces RANKL expression in adipocytes]. Nan Fang Yi Ke Da Xue Xue Bao J. South. Med. Univ. 2019, 39, 987–992. [Google Scholar]
- Li, M.; Aveyard, J.; Fleming, G.; Curran, J.M.; McBride, F.; Raval, R.; D’sa, R.A. Nitric Oxide Releasing Titanium Surfaces for Antimicrobial Bone-Integrating Orthopedic Implants. ACS Appl. Mater. Interfaces 2020, 12, 22433–22443. [Google Scholar] [CrossRef]
- Anastasio, A.T.; Paniagua, A.; Diamond, C.; Ferlauto, H.R.; Fernandez-Moure, J.S. Nanomaterial Nitric Oxide Delivery in Traumatic Orthopedic Regenerative Medicine. Front. Bioeng. Biotechnol. 2021, 8, 592008. [Google Scholar] [CrossRef]
- Holt, J.; Hertzberg, B.; Weinhold, P.; Storm, W.; Schoenfisch, M.; Dahners, L. Decreasing Bacterial Colonization of External Fixation Pins Through Nitric Oxide Release Coatings. J. Orthop. Trauma 2011, 25, 432–437. [Google Scholar] [CrossRef]
- Aguirre, J.; Buttery, L.; O’Shaughnessy, M.; Afzal, F.; de Marticorena, I.F.; Hukkanen, M.; Huang, P.; MacIntyre, I.; Polak, J. Endothelial Nitric Oxide Synthase Gene-Deficient Mice Demonstrate Marked Retardation in Postnatal Bone Formation, Reduced Bone Volume, and Defects in Osteoblast Maturation and Activity. Am. J. Pathol. 2001, 158, 247–257. [Google Scholar] [CrossRef]
- Otsuka, E.; Hirano, K.; Matsushita, S.; Inoue, A.; Hirose, S.; Yamaguchi, A.; Hagiwara, H. Effects of nitric oxide from exogenous nitric oxide donors on osteoblastic metabolism. Eur. J. Pharmacol. 1998, 349, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Jamal, S.A.; Hamilton, C.J.; Black, D.; Cummings, S.R. The effects of organic nitrates on osteoporosis: A randomized controlled trial [ISRCTN94484747]. Trials 2006, 7, 10–14. [Google Scholar] [CrossRef]
- Mancini, L.; Moradi_Bidhendi, N.; Becherrini, L.; Martineti, V.; MacIntyre, I. The biphasic effects of nitric oxide in primary rat osteoblasts are cGMP dependent. Biochem. Biophys. Res. Commun. 2000, 274, 477–481. [Google Scholar] [CrossRef]
- Kaneko, K.; Miyamoto, Y.; Ida, T.; Morita, M.; Yoshimura, K.; Nagasaki, K.; Toba, K.; Sugisaki, R.; Motohashi, H.; Akaike, T.; et al. 8-Nitro-cGMP suppresses mineralization by mouse osteoblasts. J. Clin. Biochem. Nutr. 2022, 71, 191–197. [Google Scholar] [CrossRef]
- Saura, M.; Tarin, C.; Zaragoza, C. Recent Insights into the Implication of Nitric Oxide in Osteoblast Differentiation and Proliferation during Bone Development. J. Clin. Biochem. Nutr. 2010, 10, 624–632. [Google Scholar] [CrossRef]
- Kaneko, K.; Miyamoto, Y.; Tsukuura, R.; Sasa, K.; Akaike, T.; Fujii, S.; Yoshimura, K.; Nagayama, K.; Hoshino, M.; Inoue, S.; et al. 8-Nitro-cGMP is a promoter of osteoclast differentiation induced by RANKL. Nitric Oxide 2018, 72, 46–51. [Google Scholar] [CrossRef]


| Groups | Type of Implant | Description |
|---|---|---|
| SHAM | CONV (n = 8) | Rats subjected to fictitious ovariectomy surgery |
| TERPY 10 μM (n = 8) | ||
| TERPY 100 μM (n = 8) | ||
| OVX + HD | CONV (n = 8) | Rats subjected to ovariectomy surgery and hypercaloric diet |
| TERPY 10 μM (n = 8) | ||
| TERPY 100 μM (n = 8) | ||
| OVX + HD + RIS | CONV (n = 8) | Rats subjected to ovariectomy surgery and hypercaloric diet and treated with sodium risedronate 0.35 mg/kg |
| TERPY 10 μM (n = 8) | ||
| TERPY100 μM (n = 8) |
| Food | Quantity (g/rat/Day) |
|---|---|
| Stuffed Cracker | 10 |
| Wafer | 10 |
| Corn Chips | 10 |
| Water + sucrose (12%) | 24 g + 200 mL of water |
| Gene | Gene Name | Identification |
|---|---|---|
| OPG | Tnfrsf11b | Rn00563499_m1 |
| RANKL | Tnfrsf11 | Rn00589289_m1 |
| ALP | ALPL | Rn00564931_m1 |
| IBSP | IBSP | Rn00561414_m1 |
| VEGF | VEGFA | Rn01511602_m1 |
| B2M | B2M | Rn00560865_m1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inoue, B.K.N.; Paludetto, L.V.; Monteiro, N.G.; Batista, F.R.d.S.; Kitagawa, I.L.; da Silva, R.S.; Antoniali, C.; Lisboa Filho, P.N.; Okamoto, R. Synergic Action of Systemic Risedronate and Local Rutherpy in Peri-implantar Repair of Ovariectomized Rats: Biomechanical and Molecular Analysis. Int. J. Mol. Sci. 2023, 24, 16153. https://doi.org/10.3390/ijms242216153
Inoue BKN, Paludetto LV, Monteiro NG, Batista FRdS, Kitagawa IL, da Silva RS, Antoniali C, Lisboa Filho PN, Okamoto R. Synergic Action of Systemic Risedronate and Local Rutherpy in Peri-implantar Repair of Ovariectomized Rats: Biomechanical and Molecular Analysis. International Journal of Molecular Sciences. 2023; 24(22):16153. https://doi.org/10.3390/ijms242216153
Chicago/Turabian StyleInoue, Bruna Kaori Namba, Laura Vidoto Paludetto, Naara Gabriela Monteiro, Fábio Roberto de Souza Batista, Igor Lebedenco Kitagawa, Roberto Santana da Silva, Cristina Antoniali, Paulo Noronha Lisboa Filho, and Roberta Okamoto. 2023. "Synergic Action of Systemic Risedronate and Local Rutherpy in Peri-implantar Repair of Ovariectomized Rats: Biomechanical and Molecular Analysis" International Journal of Molecular Sciences 24, no. 22: 16153. https://doi.org/10.3390/ijms242216153








