Abstract
We have shown that multiple tRNA synthetase inhibitors can increase lifespan in both the nematode C. elegans and the budding yeast S. cerevisiae by acting through the conserved transcription factor Gcn4 (yeast)/ATF-4 (worms). To further understand the biology downstream from this conserved transcription factor in the yeast model system, we looked at two different yeast models known to have upregulated Gcn4 and GCN4-dependent increased replicative lifespan. These two models were rpl31aΔ yeast and yeast treated with the tRNA synthetase inhibitor borrelidin. We used both proteomic and RNAseq analysis of a block experimental design that included both of these models to identify GCN4-dependent changes in these two long-lived strains of yeast. Proteomic analysis of these yeast indicate that the long-lived yeast have increased abundances of proteins involved in amino acid biosynthesis. The RNAseq of these same yeast uncovered further regulation of protein degradation, identifying the differential expression of genes associated with autophagy and the ubiquitin–proteasome system (UPS). The data presented here further underscore the important role that GCN4 plays in the maintenance of protein homeostasis, which itself is an important hallmark of aging. In particular, the changes in autophagy and UPS-related gene expression that we have observed could also have wide-ranging implications for the understanding and treatment of diseases of aging that are associated with protein aggregation.
1. Introduction
Many organisms show a decline in their ability to maintain protein homeostasis as they age—a hallmark of aging [1,2]. Many genes and processes influence protein synthesis and protein degradation, both of which are integral for maintaining protein homeostasis [3]. Simple model organisms have repeatedly been found to have increased lifespans under conditions that inhibit protein synthesis [4,5]. In yeast and worms, reducing translation with RNAi or the deletion of ribosomal proteins can lower translation and extend lifespan [4,5,6].
Increased protein degradation, the other leg of protein turnover, has repeatedly been found to increase lifespan in simple model organisms [7,8,9]. One process involved in protein degradation, the ubiquitin–proteasome system (UPS), degrades many proteins in eukaryotic organisms and plays a specific role in degrading dysfunctional, damaged, and misfolded proteins [7,8,10]. Elevated activity of the UPS system has been shown to increase lifespan in multiple model organisms [5,7,11,12,13,14,15]. Another process of protein degradation, namely autophagy, is a bulk-degradation process that degrades cellular components and organelles [16]. Although macroautophagy is often considered in the context of bulk degradation of cellular components, other types of autophagy can play more specific and nuanced roles [9,17,18,19,20,21,22,23,24]. Macroautophagy (hereafter ‘autophagy’) is the bulk degradation process that has been studied the most to date in the context of aging [9]. Specific autophagic genes and their roles in aging and disease phenotypes have recently been reviewed in more detail [25]. Besides aging, increasing autophagy and the UPS have been shown to improve health in animal models with protein aggregate-type diseases [7,26,27,28,29,30].
The transcription factor Gcn4 (general control non-de-repressible kinase 4) in the budding yeast S. cerevisiae regulates gene expression in response to amino acid stress, and its functional ortholog, namely activating transcription factor 4, responds to various stressors in the nematode C. elegans (ATF-4), as well as in mammals (ATF4) [31,32,33]. Gcn4/ATF-4 is necessary for increased lifespan in multiple contexts, including treatment with tRNA synthetase inhibitors in both yeast and worms [34]. Gcn4 is also necessary for increased lifespan in yeast strains lacking ribosomal genes, such as RPL31A and RPL20B (Ribosomal 60S subunit protein large) [6,34,35,36]. The discovery that some ribosomal protein deletions increase the replicative lifespans of yeast through GCN4 first demonstrated GCN4’s role as a gerontological gene [5,6]. Since then, the upregulation of Gcn4/ATF-4 in yeast and worms has been shown to be necessary or sufficient for lifespan extension in several different contexts [34,35,37]. Further, several types of long-lived mice have been shown to have elevated levels of ATF4 relative to those of normal-lived mice [38,39]. The way in which this nutrient-responsive transcription factor extends lifespan has yet to be defined, although our recent work has shown that elevated ATF4 levels increase protein degradation mechanisms in mammalian cells [40].
Gcn4 translation is regulated via four upstream open reading frames (uORFS) in yeast, and this mechanism of translational regulation is conserved through humans, with uORF regulation leading to similarity between worm and mammalian orthologs (Figure 1A) [33,35,41,42]. Reduced translation initiation can lead to the skipping of these regulatory uORFs and the increased translation of Gcn4/ATF-4/ATF4 [31,32,33,40,43,44,45,46].
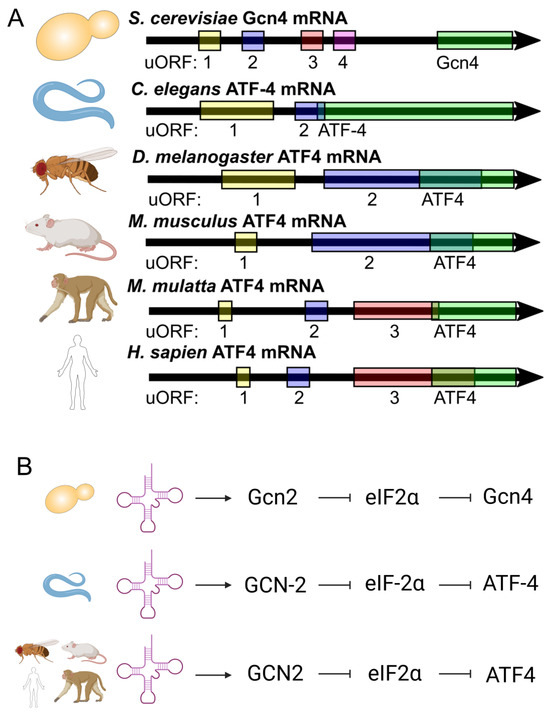
Figure 1.
Gcn4/ATF-4/ATF4 and its highly conserved nature of activation. (A) Gcn4/ATF-4/ATF4 is regulated via upstream open reading frames (uORFs). (B) The uncharged tRNA sensor, namely Gcn2/GCN-2/GCN2, can lead to a signal cascade and the translation of Gcn4/ATF-4/ATF4 in a highly conserved manner.
Our work shows that tRNA synthetase inhibitors can upregulate Gcn4/ATF-4 and increase lifespan in yeast and worms [34]. This increase in Gcn4 translation upon tRNA synthetase inhibitor treatment depends on GCN2 (General control non-de-repressible kinase 2) (Figure 1B) [34]. When Gcn2 senses uncharged tRNA, it phosphorylates eukaryotic initiation factor 2 α (eIF2α), inhibiting translation initiation [36,40,47,48]. While this process lowers most overall translation, due to the uORF regulation of Gcn4 and its orthologs, it leads to the increased translation of Gcn4/ATF-4/ATF4 [31,32,33,40,44].
Given that we now have multiple ways of increasing yeast replicative lifespan through GCN4, we sought to uncover the effects Gcn4 on differential expression in two different long-lived yeast models to try to identify shared signatures of GCN4-dependent increased lifespan.
2. Results
2.1. GCN4 Impacts Proteins Related to Amino Acid Biosynthesis in Yeast
Four uORFs in the GCN4 5′ untranslated region regulate GCN4 translation initiation [32,33,45,46]. Yeast treated with borrelidin, a threonyl tRNA synthetase inhibitor, and yeast deleted for RPL31A, which encodes a protein of the large ribosomal subunit, both have increased Gcn4 translation and increased replicative lifespan [5,6,34,49,50]. We designed a proteomic experiment analyzing the GCN4-dependent changes in the yeast proteome, featuring a block-design and considering drug treatment, the RPL31A genotype, and the GCN4 genotype (Figure 2A) [51]. In total, we analyzed 27 samples across these 6 conditions, resulting in 4–5 replicates per condition (Supplementary File S1). Simple volcano plots showing differential protein abundance between the conditions of interest can be seen in Supplementary Figure S2. Based on those analyses, it can be seen that borrelidin-treated yeast only had seven proteins with significantly differential abundance in comparison to either of its two normal-lived counterparts (specifically wild type + vehicle and gcn4Δ + borrelidin) (padj < 0.05) (padj < 0.05) (Supplementary Figure S2A,B). However, a linear model fitted to Gcn4 levels (Design = ~Gcn4 levels + Condition) revealed 44 proteins differentially regulated by Gcn4 (padj < 0.05) (Figure 2B).
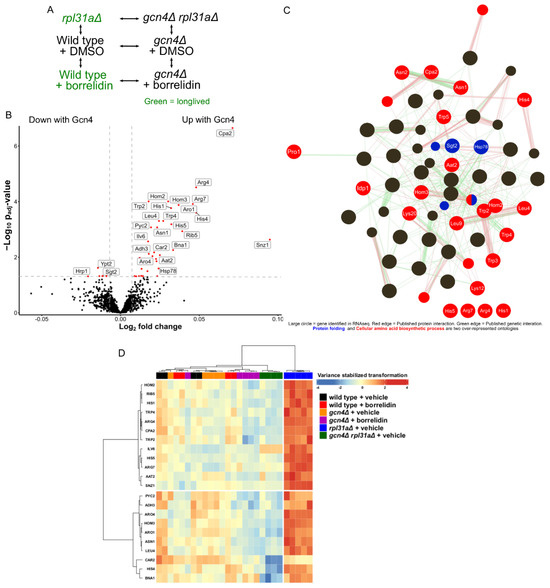
Figure 2.
Proteomic analysis of long-lived yeast. (A) Block design used for the genomic analysis. (B) Volcano plot showing the results of the linear model fitted to Gcn4 translation. Red dots indicate that the gene is significantly differentially expressed from the linear model fitted to reported Gcn4 translation levels (padj < 0.05. Log2 fold change > |0.01|). (C) GeneMania interaction and biological process analysis of differentially abundant proteins (padj < 0.01). (D) Heatmap of the differentially abundant proteins (padj < 0.01) from the linear model fitted to Gcn4 translation.
Gene ontology analysis of proteins whose abundances were linked to the presence of Gcn4 included many biological processes related to cellular amino acid biosynthesis and protein folding (FDR < 1) (Supplementary Figure S1A), as summarized in an edge-node graph of over-represented biological process gene ontology categories (Figure 2C) [52,53]. These data agree with the widely known regulation of amino acid biosynthesis by Gcn4 [31,32,33,54]. The most significantly differentially expressed protein found was Cpa2, which encodes carbamyl phosphate synthetase A, an enzyme involved in the synthesis of the arginine precursor citrulline [55,56]. Other amino acid biosynthesis proteins, namely Aat2, Arg4, Arg7, Asn1, Asn2, His1, His4, His5, Hom2, Hom3, Idp1, Leu4, Lys12, Lys20, Leu9, Trp2, Trp3, Trp4, and Trp5, were upregulated in the long-lived yeast strains (Figure 2D) [57]. In our dataset, we found that Hsp78, which prevents misfolded protein aggregation, and Sgt2, which interacts with prion aggregates and is an amyloid sensor, also showed differential protein abundances in the long-lived yeast strains [58,59,60,61,62,63,64,65]. Altogether, these data suggest that GCN4 impacts the transcription of genes involved in protein synthesis and stabilization, which is in agreement with the results of other studies [34,66,67,68].
2.2. GCN4 Impacts the Transcription of Protein Degradation Mechanisms in Yeast
Next, we sought to increase the coverage of our proteomics experiment and apply the same block design to an RNAseq experiment. Here, we used 96 samples across the same 6 conditions (Figure 2A). Principal component analysis of the samples showed the clustering of samples receiving the same treatment (Figure 3A). Principal component 1 was seemingly able to capture the variances between the different genotypes, while principal component 2 captured the variance in the transcriptomes impacted by translation. Although principal component 2 was able to cleanly capture the variance between the rpl31aΔ and gcn4Δ rpl31aΔ yeast, it was unable to cleanly capture the differences between vehicle- and borrelidin-treated yeast using either the wild-type or gcn4Δ yeast. Single-condition comparisons of particular interest for this RNA sequencing study can be seen in volcano plots in Supplementary Figure S3A–D. We found that 12 genes were consistently differentially expressed between the two long-lived yeast strains and their GCN4-deleted normal-lived counterparts (Supplementary Figure S3E). We also found many genes to be consistently differentially expressed between RPL31A-deleted yeast and its two normal-lived counterparts (wild type and gcn4Δ rpl31aΔ) (Supplementary Figure S3F).
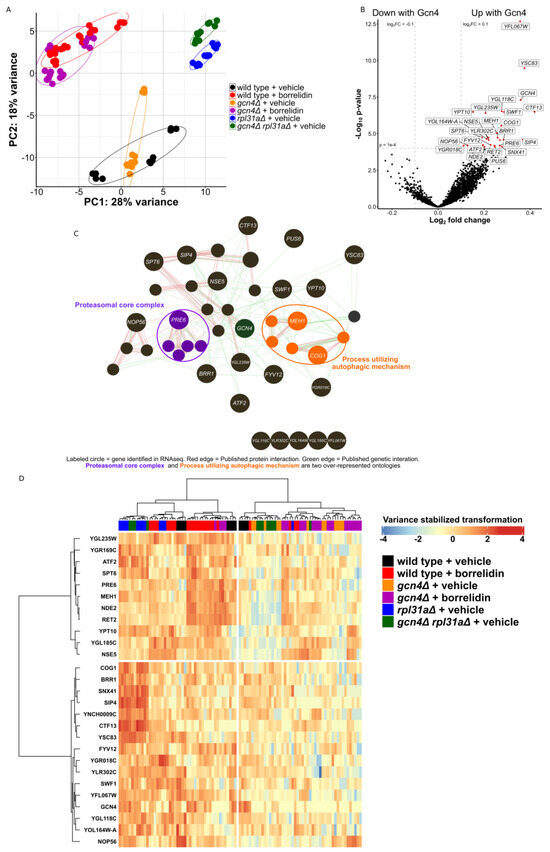
Figure 3.
RNA sequencing of long-lived yeast. (A) Principal component (PC) analysis of the sequenced and analyzed samples. (B) Volcano plot showing the result of the linear model fitted to Gcn4 translation. Red dots indicate that the gene is significantly differentially expressed from the linear model fitted to reported Gcn4 translation levels (padj < 1 × 10−4, Log2 fold change > |0.1|). (C) GeneMania interaction and biological process analysis of these differentially expressed genes (padj < 1 × 10−4). (D) Heatmap showing the mRNA abundance of the 27 genes found to be differentially expressed from the linear model fitted to Gcn4 translation (padj < 1 × 10−4).
Using a linear model fitted to Gcn4 levels, identical to that used in the proteomics experiment, we were able to uncover 304 genes either up- or downregulated under changed Gcn4 levels (padj < 0.01, Figure 2B). YFL067W, the most significantly differentially expressed gene, encodes a protein of unknown function and is not known to be conserved to other organisms [69]. YSC83, the second most significantly differentially expressed gene, is a non-essential mitochondrial protein of unknown function, although it is known to change expression during meiosis and located proximally to ARG4 in the yeast genome [70]. ALD6 is significantly downregulated in yeast with high Gcn4 translation and known to negatively regulate autophagy and impact vacuolar acidity [71,72]. PRB1 and TPS2 are also downregulated in yeast with increased Gcn4 translation, and these genes are known to be involved with the stress response, protein aggregation, proteolysis, and autophagy [73,74,75,76,77]. Although these genes are known to have positive and negative influences on autophagic flux in different contexts, it is clear that Gcn4 is impacting the differential expression of genes relating to autophagy. The heat-shock protein chaperones HSP26, HSP42, and HSP12 are downregulated in the yeast strains with higher Gcn4 translation (Figure 3B) [78]. This further indicates the change in how the yeast strain is handling proteins after their translation.
The genes upregulated (FDR < 1) in response to increased Gcn4 translation were then plotted on an edge-node graph using GeneMania (Figure 3C) [52,53]. The proteasomal core complex and process utilizing autophagic mechanism were two of the most highly over-represented biological process ontology categories in this analysis. Among these, MEH1 is known to stimulate microautophagy and positively impact vacuolar acidification [79]. COG1 is known to impact protein trafficking through the fusion of transport vesicles [80,81,82]. PRE6, a highly conserved gene found here to be greatly increased with high Gcn4 translation, is a structural component of the yeast 20S proteasome [83]. Notably, PRE6 transcripts were upregulated in the borrelidin-treated wild-type yeast in comparison to either vehicle-treated wild-type yeast or treated yeast without GCN4. Similarly, rpl31aΔ yeast had increased PRE6 mRNA levels in comparison to gcn4Δ rpl31aΔ yeast, as is the case with a number of genes depicted in the heatmap provided (Figure 3D).
Overall, there were nine genes with significantly different abundances of both mRNA and protein from their respective linear models fitted to Gcn4 translation (Supplementary Figure S4). ARO4 (AROmatic amino acid requiring 4), ILV6 (IsoLeucine Valine 6), HIS4 (HIStidine requiring), HOM3 (HOMoserine requiring), IDP1 (Isocitrate dehydrogenase NADP-specific), LYS20 (LYSine requiring), and HOM2 (HOMoserine requiring) were the genes with both increased transcript levels (padj < 0.05) and increased protein abundance (padj < 0.05) in these studies, further confirming the known role of Gcn4 in regulating amino acid biosynthesis. The other two genes, namely HSP78 (Heat Shock Protein) and HIS1 (HIStidine), had significantly decreased mRNA in the RNAseq and increased protein abundance in the proteomics from their linear model fits, respectively.
2.3. Conserved Genes Involved in Protein Degradation Are Differentially Expressed in This Yeast Dataset
We recently found that proteasomal activity and autophagy are upregulated in response to borrelidin treatment in mouse embryonic fibroblasts (MEFs) [40]. Wipi2 was among many autophagy genes recently shown to be strongly upregulated with increased ATF4 translation in MEFs, and its yeast ortholog (ATG21) is shown to be greatly upregulated in this study (padj = 0.007). This gene is known to be important in the induction of autophagy, and its differential expression in both of these datasets is consistent with the possibility that autophagy may be regulated similarly downstream of GCN4 in yeast and Atf4 in mammals [84,85,86,87,88,89].
Several yeast genes related to proteasomal degradation, including RAD28, HSE1, VPS27, DFM1, and RMD5, are differentially expressed in this yeast dataset with increased Gcn4 translation (padj < 0.01), and they also have orthologs that have previously been shown to be differentially expressed in MEFs with increased Atf4 translation (padj < 1 × 10−10). RAD28 deleted yeast appear to have increased mutations upon UV treatment [90]. HSE1 and VPS27 form a complex and sort ubiquitinated membrane proteins for degradation [91,92]. DFM1 is a required component of the ER-associated protein degradation pathway that removes aggregated proteins [93,94]. RMD5 is a glucose-induced degradation deficient protein, a part of an E3 ubiquitin ligase [95,96]. Altogether, these data suggest that the protein degradation regulation downstream of increased GCN4/Atf4 may show conserved patterns of gene regulation in yeast and mammals.
3. Discussion
In order to further understand the shared mechanisms of GCN4-dependent delayed aging, we measured both proteomic and transcriptomic changes correlated with increased Gcn4 translation in parallel with two different Gcn4-dependent long-lived yeast models. Gcn4 and its orthologs are known to impact protein translation and amino acid biosynthesis, so it is unsurprising that we observed differential abundances of proteins related to these processes [34,40,66]. Our novel finding of significant changes in Hsp78 and Spt2 expression suggest that these long-lived yeast may have altered responses to protein aggregation.
Aligning with the published findings that autophagy is necessary for extended lifespan in a GCN4-over-expressing yeast strain and elevated ATF4 increases autophagy in mammalian cell cultures, it is not surprising that we found the differential expression of genes known to impact autophagy [37,40,97]. Our datasets identify potential genetic activators of autophagy under conditions of increased Gcn4 translation, especially ATG21, COG1, and MEH1. Elevated UPS activity has been associated with increased lifespan, and genes involved in proteasomal activity have been shown to change significantly via the upregulation of Gcn4’s functional mammalian ortholog, namely ATF4, so further inquiry into how the UPS changes downstream of Gcn4 is warranted [5,7,8,12,13,14,28,40].
Our transcriptomic analysis uncovered differentially expressed genes involved in amino acid biosynthesis, autophagy, the structure of the proteasome, protein folding, and protein aggregation, likely as a response to the sensed depletion of amino acids and needed protein maintenance [40,98]. Notably, a handful of genes in the yeast ubiquitin-dependent protein catabolic biological process ontology category were differentially expressed in both this dataset and a published borrelidin-treated mammalian cell RNAseq dataset [40]. ATG21 and its mammalian ortholog Wipi2 (which is a gene involved in the induction of autophagy) are also upregulated with increased Gcn4 and ATF4 translation in both the yeast RNAseq dataset shown here and the published borrelidin mammalian cell dataset. These data suggest that both yeast Gcn4 and mammalian ATF4 genetically impact the transcription of genes involved in both the UPS and autophagy, two major protein degradation processes.
Further studies exploring the impacts of these GCN4 targets on protein homeostasis in the context of aging are warranted. Although some genes and proteins with differential abundance in these long-lived yeast strains were identified, we still do not know which processes downstream of Gcn4 are responsible for the increased lifespan. Our interpretation of these data assumes that borrelidin, as well as the long-lived phenotype associated with it, are associated with its ability to upregulate Gcn4 translation and downstream transcriptional activity, in part due to the GCN4 dependence of increased lifespan in borrelidin-treated yeast. Although these global studies were conducted with high sample numbers for each condition in both our proteomics and RNA sequencing studies, further studies confirming individual gene and protein hits of particular interest using standard methods, including qPCR or Western blot, are suggested.
Overall, these data indicate that drugs which increase Gcn4 and have been shown elsewhere to increase its orthologs could be used to improve protein turnover. This suggests the possibility that these drugs have potential to treat diseases characterized by protein aggregation, such as Huntington’s disease, Parkinson’s disease, and Alzheimer’s disease.
4. Materials and Methods
4.1. Yeast Culture
Yeast strains were pulled from the YKO deletion collection and grown at 30 °C in sterile YPD medium (1% yeast extract (BD Biosciences, San Jose, CA, USA, Cat No. 212730), 2% peptone (ThermoFisher Scientific, Detroit, MI, USA, Cat No. 211820), 2% dextrose (VWR, Solon, OH, USA, Cat. No. 97061-172)) via orbital shaking at 250 RPM with flasks of sterile water to maintain humidity for all protein and RNA extractions, as outlined below.
4.2. Protein Extraction, Mass Spectrometry, and Proteome Analysis
Yeast protein was extracted from yeast found to have an OD600 of between 0.3 and 0.9, with further guidelines taken from the Clontech Yeast Protocols Handbook [99]. These yeast should, thus, have a replicative age of ~1 generation, with the majority having divided zero or one times. The concentrations of proteins were confirmed via the Bradford assay using the Coomassie Plus Kit (Thermo Scientific, Detroit, MI, USA, Cat. No. 23236). Protein samples were sent to the University of Arkansas for Medical Science’s (UAMS) Proteomics Core Facility as part of an IDEA National Resource for Quantitative Proteomics Nationwide Voucher Program Award. The proteomics analysis of mass spectrometry data from the 27 samples that passed quality control involved BiocManager’s DEP package (Supplementary File S1) [100].
4.3. RNA Extraction, Sequencing, and Analysis
RNA was extracted using the Yeast RNA miniprep kit acquired from Zymo Research (Cat# R1002) in accordance with the manufacturer’s protocols during log-phase growth between an OD600 nm of 0.6–0.8, as measured via a Victor NIVO [101]. DMSO was used as the vehicle in all cases for drug-treated yeast. In total, 5 μM and 10 μM measurements of borrelidin were used in the borrelidin-treated samples, in accordance with the upregulation of Gcn4, as previously published [34]. Transcriptomes were sequenced with the Illumina NextSeq 500 at the Center for Evolutionary and Theoretical Immunology. We first used fastp for quality control and the removal of forward and reverse adapters [102]. After that step, we used HISAT2 and featurecounts to align and quantify these trimmed files [103,104]. Data analysis and differential expression utilized R programming and associated libraries, such as limma and DESEQ2 [100,105,106,107,108].
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms242216163/s1.
Author Contributions
Conceptualization, B.L.M. and M.A.M.; Formal analysis, B.L.M. and J.P.; Funding acquisition, M.A.M.; Investigation, M.A.M.; Methodology, B.L.M., D.P.F., R.J.C., J.P. and M.A.M.; Software, B.L.M.; Supervision, M.A.M.; Visualization, B.L.M.; Writing—original draft, B.L.M. and J.P.; Writing—review and editing, B.L.M., D.P.F., J.P. and M.A.M. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the National Institutes of Health grant P20GM121176, the National Institutes of Health grant R01AG07077601, the Longevity Impetus Grant, the Glenn Foundation for Medical Research/American Federation for Aging Research Junior Investigator Grant, the American Federation for Aging Research Reboot Award, the IDEA National Resource for Quantitative Proteomics Nationwide Voucher Program Award, the University of New Mexico School of Medicine Research Allocation Committee New Investigator Award, and the Japan Agency for Medical Research and Development/New York Academy of Sciences Longevity Interstellar Initiative Award.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All genomic data described in this study are available in the supplemental materials or the Gene Expression Omnibus (GEO) with accession number #GSE242739.
Conflicts of Interest
The authors declare no competing interests.
References
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The Hallmarks of Aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [PubMed]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of aging: An expanding universe. Cell 2023, 186, 243–278. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, T.; Cohen, E. Organismal Protein Homeostasis Mechanisms. Genetics 2020, 215, 889–901. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.; Taubert, S.; Crawford, D.; Libina, N.; Lee, S.; Kenyon, C. Lifespan extension by conditions that inhibit translation in Caenorhabditis elegans. Aging Cell 2007, 6, 95–110. [Google Scholar] [CrossRef]
- McCormick, M.A.; Delaney, J.R.; Tsuchiya, M.; Tsuchiyama, S.; Shemorry, A.; Sim, S.; Chou, A.C.-Z.; Ahmed, U.; Carr, D.; Murakami, C.J.; et al. A Comprehensive Analysis of Replicative Lifespan in 4,698 Single-Gene Deletion Strains Uncovers Conserved Mechanisms of Aging. Cell Metab. 2015, 22, 895–906. [Google Scholar] [CrossRef]
- Steffen, K.K.; MacKay, V.L.; Kerr, E.O.; Tsuchiya, M.; Hu, D.; Fox, L.A.; Dang, N.; Johnston, E.D.; Oakes, J.A.; Tchao, B.N.; et al. Yeast Life Span Extension by Depletion of 60S Ribosomal Subunits Is Mediated by Gcn4. Cell 2008, 133, 292–302. [Google Scholar] [CrossRef]
- Chondrogianni, N.; Sakellari, M.; Lefaki, M.; Papaevgeniou, N.; Gonos, E.S. Proteasome activation delays aging in vitro and in vivo. Free Radic. Biol. Med. 2014, 71, 303–320. [Google Scholar] [CrossRef]
- Jana, N.R. Protein homeostasis and aging: Role of ubiquitin protein ligases. Neurochem. Int. 2012, 60, 443–447. [Google Scholar] [CrossRef]
- Rubinsztein, D.C.; Marino, G.; Kroemer, G. Autophagy and Aging. Cell 2011, 146, 682–695. [Google Scholar] [CrossRef]
- Coux, O.; Tanaka, K.; Goldberg, A.L. Structure and functions of the 20S and 26S proteasomes. Annu. Rev. Biochem. 1996, 65, 801–847. [Google Scholar] [CrossRef]
- Nguyen, N.N.; Rana, A.; Goldman, C.; Moore, R.; Tai, J.; Hong, Y.; Shen, J.; Walker, D.W.; Hur, J.H. Proteasome β5 subunit overexpression improves proteostasis during aging and extends lifespan in Drosophila melanogaster. Sci. Rep. 2019, 9, 3170. [Google Scholar] [CrossRef] [PubMed]
- Vilchez, D.; Morantte, I.; Liu, Z.; Douglas, P.M.; Merkwirth, C.; Rodrigues, A.P.C.; Manning, G.; Dillin, A. RPN-6 determines C. elegans longevity under proteotoxic stress conditions. Nature 2012, 489, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Kruegel, U.; Robison, B.; Dange, T.; Kahlert, G.; Delaney, J.R.; Kotireddy, S.; Tsuchiya, M.; Tsuchiyama, S.; Murakami, C.J.; Schleit, J.; et al. Elevated Proteasome Capacity Extends Replicative Lifespan in Saccharomyces cerevisiae. PLoS Genet. 2011, 7, e1002253. [Google Scholar] [CrossRef] [PubMed]
- Ghazi, A.; Henis-Korenblit, S.; Kenyon, C. Regulation of Caenorhabditis elegans lifespan by a proteasomal E3 ligase complex. Proc. Natl. Acad. Sci. USA 2007, 104, 5947–5952. [Google Scholar] [CrossRef]
- Jensen, M.B.; Jasper, H. Mitochondrial Proteostasis in the Control of Aging and Longevity. Cell Metab. 2014, 20, 214–225. [Google Scholar] [CrossRef]
- Nakatogawa, H.; Suzuki, K.; Kamada, Y.; Ohsumi, Y. Dynamics and diversity in autophagy mechanisms: Lessons from yeast. Nat. Rev. Mol. Cell Biol. 2009, 10, 458–467. [Google Scholar] [CrossRef]
- Wong, S.Q.; Kumar, A.V.; Mills, J.; Lapierre, L.R. Autophagy in aging and longevity. Hum. Genet. 2020, 139, 277–290. [Google Scholar] [CrossRef]
- Cebollero, E.; Reggiori, F.; Kraft, C. Reticulophagy and Ribophagy: Regulated Degradation of Protein Production Factories. Int. J. Cell Biol. 2012, 2012, e182834. [Google Scholar] [CrossRef]
- Tolkovsky, A.M. Mitophagy. Biochim. Biophys. Acta-Mol. Cell Res. 2009, 1793, 1508–1515. [Google Scholar] [CrossRef]
- Zaffagnini, G.; Martens, S. Mechanisms of Selective Autophagy. J. Mol. Biol. 2016, 428, 1714–1724. [Google Scholar] [CrossRef]
- Klionsky, D.J.; Abdel-Aziz, A.K.; Abdelfatah, S.; Abdellatif, M.; Abdoli, A.; Abel, S.; Abeliovich, H.; Abildgaard, M.H.; Abudu, Y.P.; Acevedo-Arozena, A.; et al. Guidelines for the use and interpretation of assays for monitoring autophagy (4th edition)1. Autophagy 2021, 17, 1–382. [Google Scholar] [CrossRef] [PubMed]
- Ponpuak, M.; Mandell, M.A.; Kimura, T.; Chauhan, S.; Cleyrat, C.; Deretic, V. Secretory autophagy. Curr. Opin. Cell Biol. 2015, 35, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Pilli, M.; Arko-Mensah, J.; Ponpuak, M.; Roberts, E.; Master, S.; Mandell, M.A.; Dupont, N.; Ornatowski, W.; Jiang, S.; Bradfute, S.B.; et al. TBK-1 promotes autophagy-mediated antimicrobial defense by controlling autophagosome maturation. Immunity 2012, 37, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Mandell, M.A.; Jain, A.; Arko-Mensah, J.; Chauhan, S.; Kimura, T.; Dinkins, C.; Silvestri, G.; Münch, J.; Kirchhoff, F.; Simonsen, A.; et al. TRIM Proteins Regulate Autophagy and Can Target Autophagic Substrates by Direct Recognition. Dev. Cell 2014, 30, 394–409. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Zhang, S.; Mizushima, N. Autophagy genes in biology and disease. Nat. Rev. Genet. 2023, 24, 382–400. [Google Scholar] [CrossRef]
- Seo, H.; Sonntag, K.-C.; Kim, W.; Cattaneo, E.; Isacson, O. Proteasome Activator Enhances Survival of Huntington’s Disease Neuronal Model Cells. PLoS ONE 2007, 2, e238. [Google Scholar] [CrossRef]
- Yazgili, A.S.; Ebstein, F.; Meiners, S. The Proteasome Activator PA200/PSME4: An Emerging New Player in Health and Disease. Biomolecules 2022, 12, 1150. [Google Scholar] [CrossRef]
- Jung, R.; Lechler, M.C.; Fernandez-Villegas, A.; Chung, C.W.; Jones, H.C.; Choi, Y.H.; Thompson, M.A.; Rödelsperger, C.; Röseler, W.; Schierle, G.S.K.; et al. A safety mechanism enables tissue-specific resistance to protein aggregation during aging in C. elegans. PLoS Biol. 2023, 21, e3002284. [Google Scholar] [CrossRef]
- David, D. Aging and the aggregating proteome. Front. Genet. 2012, 3, 247. [Google Scholar] [CrossRef]
- David, D.C.; Ollikainen, N.; Trinidad, J.C.; Cary, M.P.; Burlingame, A.L.; Kenyon, C. Widespread Protein Aggregation as an Inherent Part of Aging in C. elegans. PLoS Biol. 2010, 8, e1000450. [Google Scholar] [CrossRef]
- Hinnebusch, A.G.; Natarajan, K. Gcn4p, a Master Regulator of Gene Expression, Is Controlled at Multiple Levels by Diverse Signals of Starvation and Stress. Eukaryot. Cell 2002, 1, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Hinnebusch, A.G. Mechanisms of gene regulation in the general control of amino acid biosynthesis in Saccharomyces cerevisiae. Microbiol. Rev. 1988, 52, 248–273. [Google Scholar] [CrossRef] [PubMed]
- Hinnebusch, A.G. Translational regulation of GCN4 and the general amino acid control of yeast. Annu. Rev. Microbiol. 2005, 59, 407–450. [Google Scholar] [CrossRef] [PubMed]
- Robbins, C.E.; Patel, B.; Sawyer, D.L.; Wilkinson, B.; Kennedy, B.K.; McCormick, M.A. Cytosolic and mitochondrial tRNA synthetase inhibitors increase lifespan in a GCN4/atf-4-dependent manner. iScience 2022, 25, 105410. [Google Scholar] [CrossRef]
- Statzer, C.; Meng, J.; Venz, R.; Bland, M.; Robida-Stubbs, S.; Patel, K.; Petrovic, D.; Emsley, R.; Liu, P.; Morantte, I.; et al. ATF-4 and hydrogen sulfide signalling mediate longevity in response to inhibition of translation or mTORC1. Nat. Commun. 2022, 13, 967. [Google Scholar] [CrossRef]
- Molenaars, M.; Janssens, G.E.; Williams, E.G.; Jongejan, A.; Lan, J.; Rabot, S.; Joly, F.; Moerland, P.D.; Schomakers, B.V.; Lezzerini, M.; et al. A Conserved Mito-Cytosolic Translational Balance Links Two Longevity Pathways. Cell Metab. 2020, 31, 549–563.e7. [Google Scholar] [CrossRef]
- Hu, Z.; Xia, B.; Postnikoff, S.D.; Shen, Z.-J.; Tomoiaga, A.S.; Harkness, T.A.; Seol, J.H.; Li, W.; Chen, K.; Tyler, J.K. Ssd1 and Gcn2 suppress global translation efficiency in replicatively aged yeast while their activation extends lifespan. eLife 2018, 7, e35551. [Google Scholar] [CrossRef]
- Li, W.; Li, X.; Miller, R.A. ATF4 activity: A common feature shared by many kinds of slow-aging mice. Aging Cell 2014, 13, 1012–1018. [Google Scholar] [CrossRef]
- Li, W.; Miller, R.A. Elevated ATF4 Function in Fibroblasts and Liver of Slow-Aging Mutant Mice. J. Gerontol. Ser. A 2015, 70, 263–272. [Google Scholar] [CrossRef]
- Mariner, B.L.; Rodriguez, A.S.; Heath, O.C.; McCormick, M.A. Induction of proteasomal activity in mammalian cells by lifespan-extending tRNA synthetase inhibitors. In GeroScience; Springer: Berlin/Heidelberg, Germany, 2023. [Google Scholar] [CrossRef]
- Lu, P.D.; Harding, H.P.; Ron, D. Translation reinitiation at alternative open reading frames regulates gene expression in an integrated stress response. J. Cell Biol. 2004, 167, 27–33. [Google Scholar] [CrossRef]
- Harding, H.P.; Novoa, I.; Zhang, Y.; Zeng, H.; Wek, R.; Schapira, M.; Ron, D. Regulated Translation Initiation Controls Stress-Induced Gene Expression in Mammalian Cells. Mol. Cell 2000, 6, 1099–1108. [Google Scholar] [CrossRef]
- Rolfes, R.J.; Hinnebusch, A.G. Translation of the yeast transcriptional activator GCN4 is stimulated by purine limitation: Implications for activation of the protein kinase GCN2. Mol. Cell. Biol. 1993, 13, 5099–5111. [Google Scholar] [CrossRef]
- Hinnebusch, A.G. The Scanning Mechanism of Eukaryotic Translation Initiation. Annu. Rev. Biochem. 2014, 83, 779–812. [Google Scholar] [CrossRef]
- Hinnebusch, A.G. Molecular mechanism of scanning and start codon selection in eukaryotes. Microbiol. Mol. Biol. Rev. 2011, 75, 434–467. [Google Scholar] [CrossRef]
- Hinnebusch, A.G.; Dever, T.E.; Asano, K. Mechanism of Translation Initiation in the Yeast Saccharomyces cerevisiae. Cold Spring Harb. Monogr. Arch. 2007, 48, 225–268. [Google Scholar]
- Pakos-Zebrucka, K.; Koryga, I.; Mnich, K.; Ljujic, M.; Samali, A.; Gorman, A.M. The integrated stress response. EMBO Rep. 2016, 17, 1374–1395. [Google Scholar] [CrossRef]
- Postnikoff, S.D.L.; Johnson, J.E.; Tyler, J.K. The integrated stress response in budding yeast lifespan extension. Microb. Cell 2017, 4, 368–375. [Google Scholar] [CrossRef]
- Habibi, D.; Ogloff, N.; Jalili, R.B.; Yost, A.; Weng, A.P.; Ghahary, A.; Ong, C.J. Borrelidin, a small molecule nitrile-containing macrolide inhibitor of threonyl-tRNA synthetase, is a potent inducer of apoptosis in acute lymphoblastic leukemia. Investig. New Drugs 2012, 30, 1361–1370. [Google Scholar] [CrossRef]
- Steffen, K.K.; McCormick, M.A.; Pham, K.M.; MacKay, V.L.; Delaney, J.R.; Murakami, C.J.; Kaeberlein, M.; Kennedy, B.K. Ribosome Deficiency Protects Against ER Stress in Saccharomyces cerevisiae. Genetics 2012, 191, 107–118. [Google Scholar] [CrossRef]
- McCormick, M.; Chen, K.; Ramaswamy, P.; Kenyon, C. New genes that extend Caenorhabditis elegans’ lifespan in response to reproductive signals. Aging Cell 2012, 11, 192–202. [Google Scholar] [CrossRef]
- Montojo, J.; Zuberi, K.; Rodriguez, H.; Kazi, F.; Wright, G.; Donaldson, S.L.; Morris, Q.; Bader, G.D. GeneMANIA Cytoscape plugin: Fast gene function predictions on the desktop. Bioinformatics 2010, 26, 2927–2928. [Google Scholar] [CrossRef]
- Mostafavi, S.; Ray, D.; Warde-Farley, D.; Grouios, C.; Morris, Q. GeneMANIA: A real-time multiple association network integration algorithm for predicting gene function. Genome Biol. 2008, 9, S4. [Google Scholar] [CrossRef]
- Pavlova, N.N.; King, B.; Josselsohn, R.H.; Violante, S.; Macera, V.L.; Vardhana, S.A.; Cross, J.R.; Thompson, C.B. Translation in amino-acid-poor environments is limited by tRNAGln charging. eLife 2020, 9, e62307. [Google Scholar] [CrossRef]
- Piérard, A.; Schröter, B. Structure-Function Relationships in the Arginine Pathway Carbamoylphosphate Synthase of Saccharomyces cerevisiae. J. Bacteriol. 1978, 134, 167–176. [Google Scholar] [CrossRef]
- Lusty, C.J.; Widgren, E.E.; Broglie, K.E.; Nyunoya, H. Yeast carbamyl phosphate synthetase. Structure of the yeast gene and homology to Escherichia coli carbamyl phosphate synthetase. J. Biol. Chem. 1983, 258, 14466–14477. [Google Scholar] [CrossRef]
- Penn, M.D.; Galgoci, B.; Greer, H. Identification of AAS genes and their regulatory role in general control of amino acid biosynthesis in yeast. Proc. Natl. Acad. Sci. USA 1983, 80, 2704–2708. [Google Scholar] [CrossRef]
- von Janowsky, B.; Major, T.; Knapp, K.; Voos, W. The disaggregation activity of the mitochondrial ClpB homolog Hsp78 maintains Hsp70 function during heat stress. J. Mol. Biol. 2006, 357, 793–807. [Google Scholar] [CrossRef]
- Moczko, M.; Schönfisch, B.; Voos, W.; Pfanner, N.; Rassow, J. The mitochondrial ClpB homolog Hsp78 cooperates with matrix Hsp70 in maintenance of mitochondrial function. J. Mol. Biol. 1995, 254, 538–543. [Google Scholar] [CrossRef]
- Röttgers, K.; Zufall, N.; Guiard, B.; Voos, W. The ClpB homolog Hsp78 is required for the efficient degradation of proteins in the mitochondrial matrix. J. Biol. Chem. 2002, 277, 45829–45837. [Google Scholar] [CrossRef]
- Ishikawa, Y.; Nishino, S.; Fukuda, S.; Nguyet, V.T.A.; Izawa, S. Severe ethanol stress induces the preferential synthesis of mitochondrial disaggregase Hsp78 and formation of DUMPs in Saccharomyces cerevisiae. Biochimica Et Biophysica Acta. Gen. Subj. 2022, 1866, 130147. [Google Scholar] [CrossRef]
- Kiktev, D.A.; Patterson, J.C.; Müller, S.; Bariar, B.; Pan, T.; Chernoff, Y.O. Regulation of chaperone effects on a yeast prion by cochaperone Sgt2. Mol. Cell. Biol. 2012, 32, 4960–4970. [Google Scholar] [CrossRef]
- Wang, F.; Brown, E.C.; Mak, G.; Zhuang, J.; Denic, V. A chaperone cascade sorts proteins for posttranslational membrane insertion into the endoplasmic reticulum. Mol. Cell 2010, 40, 159–171. [Google Scholar] [CrossRef]
- Huh, W.-K.; Falvo, J.V.; Gerke, L.C.; Carroll, A.S.; Howson, R.W.; Weissman, J.S.; O’Shea, E.K. Global analysis of protein localization in budding yeast. Nature 2003, 425, 686–691. [Google Scholar] [CrossRef]
- Tkach, J.M.; Yimit, A.; Lee, A.Y.; Riffle, M.; Costanzo, M.; Jaschob, D.; Hendry, J.A.; Ou, J.; Moffat, J.; Boone, C.; et al. Dissecting DNA damage response pathways by analysing protein localization and abundance changes during DNA replication stress. Nat. Cell Biol. 2012, 14, 966–976. [Google Scholar] [CrossRef]
- Mittal, N.; Guimaraes, J.C.; Gross, T.; Schmidt, A.; Vina-Vilaseca, A.; Nedialkova, D.D.; Aeschimann, F.; Leidel, S.A.; Spang, A.; Zavolan, M. The Gcn4 transcription factor reduces protein synthesis capacity and extends yeast lifespan. Nat. Commun. 2017, 8, 457. [Google Scholar] [CrossRef]
- Harding, H.P.; Zhang, Y.; Bertolotti, A.; Zeng, H.; Ron, D. Perk Is Essential for Translational Regulation and Cell Survival during the Unfolded Protein Response. Mol. Cell 2000, 5, 897–904. [Google Scholar] [CrossRef]
- Tameire, F.; Verginadis, I.I.; Leli, N.M.; Polte, C.; Conn, C.S.; Ojha, R.; Salinas, C.S.; Chinga, F.; Monroy, A.M.; Fu, W.; et al. ATF4 couples MYC-dependent translational activity to bioenergetic demands during tumour progression. Nat. Cell Biol. 2019, 21, 889–899. [Google Scholar] [CrossRef]
- Yofe, I.; Weill, U.; Meurer, M.; Chuartzman, S.; Zalckvar, E.; Goldman, O.; Ben-Dor, S.; Schütze, C.; Wiedemann, N.; Knop, M.; et al. One library to make them all: Streamlining the creation of yeast libraries via a SWAp-Tag strategy. Nat. Methods 2016, 13, 371–378. [Google Scholar] [CrossRef]
- Rocco, V.; Daly, M.J.; Matre, V.; Lichten, M.; Nicolas, A. Identification of two divergently transcribed genes centromere-proximal to the ARG4 locus on chromosome VIII of Saccharomyces cerevisiae. Yeast 1993, 9, 1111–1120. [Google Scholar] [CrossRef]
- Onodera, J.; Ohsumi, Y. Ald6p Is a Preferred Target for Autophagy in Yeast, Saccharomyces cerevisiae. J. Biol. Chem. 2004, 279, 16071–16076. [Google Scholar] [CrossRef]
- Delorme-Axford, E.; Wen, X.; Klionsky, D.J. The yeast transcription factor Stb5 acts as a negative regulator of autophagy by modulating cellular metabolism. Autophagy 2023, 19, 2719–2732. [Google Scholar] [CrossRef] [PubMed]
- Takeshige, K.; Baba, M.; Tsuboi, S.; Noda, T.; Ohsumi, Y. Autophagy in yeast demonstrated with proteinase-deficient mutants and conditions for its induction. J. Cell Biol. 1992, 119, 301–311. [Google Scholar] [CrossRef]
- Pereira, M.D.; Eleutherio, E.C.; Panek, A.D. Acquisition of tolerance against oxidative damage in Saccharomyces cerevisiae. BMC Microbiol. 2001, 1, 11. [Google Scholar] [CrossRef]
- Kim, B.; Lee, Y.; Choi, H.; Huh, W.-K. The trehalose-6-phosphate phosphatase Tps2 regulates ATG8 transcription and autophagy in Saccharomyces cerevisiae. Autophagy 2021, 17, 1013–1027. [Google Scholar] [CrossRef]
- Michaillat, L.; Mayer, A. Identification of Genes Affecting Vacuole Membrane Fragmentation in Saccharomyces cerevisiae. PLoS ONE 2013, 8, e54160. [Google Scholar] [CrossRef]
- Lang, T.; Reiche, S.; Straub, M.; Bredschneider, M.; Thumm, M. Autophagy and the cvt Pathway Both Depend on AUT9. J. Bacteriol. 2000, 182, 2125–2133. [Google Scholar] [CrossRef]
- Verghese, J.; Abrams, J.; Wang, Y.; Morano, K.A. Biology of the Heat Shock Response and Protein Chaperones: Budding Yeast (Saccharomyces cerevisiae) as a Model System. Microbiol. Mol. Biol. Rev. 2012, 76, 115–158. [Google Scholar] [CrossRef]
- Eising, S.; Esch, B.; Wälte, M.; Duarte, P.V.; Walter, S.; Ungermann, C.; Bohnert, M.; Fröhlich, F. A lysosomal biogenesis map reveals the cargo spectrum of yeast vacuolar protein targeting pathways. J. Cell Biol. 2022, 221, e202107148. [Google Scholar] [CrossRef]
- Whyte, J.R.; Munro, S. The Sec34/35 Golgi transport complex is related to the exocyst, defining a family of complexes involved in multiple steps of membrane traffic. Dev. Cell 2001, 1, 527–537. [Google Scholar] [CrossRef]
- Loh, E.; Hong, W. The binary interacting network of the conserved oligomeric Golgi tethering complex. J. Biol. Chem. 2004, 279, 24640–24648. [Google Scholar] [CrossRef]
- Corbacho, I.; Olivero, I.; Hernández, L.M. Identification of low-dye-binding (ldb) mutants of Saccharomyces cerevisiae. FEMS Yeast Res. 2004, 4, 437–444. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Heinemeyer, W.; Troendle, N.; Albrecht, G.; Wolf, D.H. PRE5 and PRE6, the last missing genes encoding 20S proteasome subunits from yeast? Indication for a set of 14 different subunits in the eukaryotic proteasome core. Biochemistry 1994, 33, 12229–12237. [Google Scholar] [CrossRef] [PubMed]
- Dooley, H.C.; Razi, M.; Polson, H.E.; Girardin, S.E.; Wilson, M.I.; Tooze, S.A. WIPI2 links LC3 conjugation with PI3P, autophagosome formation, and pathogen clearance by recruiting Atg12-5-16L1. Mol. Cell 2014, 55, 238–252. [Google Scholar] [CrossRef]
- Mercer, T.J.; Gubas, A.; Tooze, S.A. A molecular perspective of mammalian autophagosome biogenesis. J. Biol. Chem. 2018, 293, 5386–5395. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.I.; Dooley, H.C.; Tooze, S.A. WIPI2b and Atg16L1: Setting the stage for autophagosome formation. Biochem. Soc. Trans. 2014, 42, 1327–1334. [Google Scholar] [CrossRef] [PubMed]
- Strømhaug, P.E.; Reggiori, F.; Guan, J.; Wang, C.-W.; Klionsky, D.J. Atg21 is a phosphoinositide binding protein required for efficient lipidation and localization of Atg8 during uptake of aminopeptidase I by selective autophagy. Mol. Biol. Cell 2004, 15, 3553–3566. [Google Scholar] [CrossRef]
- Meiling-Wesse, K.; Barth, H.; Voss, C.; Eskelinen, E.-L.; Epple, U.D.; Thumm, M. Atg21 is required for effective recruitment of Atg8 to the preautophagosomal structure during the Cvt pathway. J. Biol. Chem. 2004, 279, 37741–37750. [Google Scholar] [CrossRef]
- Meijer, W.H.; van der Klei, I.J.; Veenhuis, M.; Kiel, J.A.K.W. ATG genes involved in non-selective autophagy are conserved from yeast to man, but the selective Cvt and pexophagy pathways also require organism-specific genes. Autophagy 2007, 3, 106–116. [Google Scholar] [CrossRef]
- Bhatia, P.K.; Verhage, R.A.; Brouwer, J.; Friedberg, E.C. Molecular cloning and characterization of Saccharomyces cerevisiae RAD28, the yeast homolog of the human Cockayne syndrome A (CSA) gene. J. Bacteriol. 1996, 178, 5977–5988. [Google Scholar] [CrossRef]
- Bilodeau, P.S.; Urbanowski, J.L.; Winistorfer, S.C.; Piper, R.C. The Vps27p Hse1p complex binds ubiquitin and mediates endosomal protein sorting. Nat. Cell Biol. 2002, 4, 534–539. [Google Scholar] [CrossRef]
- Bilodeau, P.S.; Winistorfer, S.C.; Kearney, W.R.; Robertson, A.D.; Piper, R.C. Vps27-Hse1 and ESCRT-I complexes cooperate to increase efficiency of sorting ubiquitinated proteins at the endosome. J. Cell Biol. 2003, 163, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Stolz, A.; Schweizer, R.S.; Schäfer, A.; Wolf, D.H. Dfm1 forms distinct complexes with Cdc48 and the ER ubiquitin ligases and is required for ERAD. Traffic 2010, 11, 1363–1369. [Google Scholar] [CrossRef] [PubMed]
- Kandel, R.; Jung, J.; Syau, D.; Kuo, T.; Songster, L.; Horn, C.; Chapman, C.; Aguayo, A.; Duttke, S.; Benner, C.; et al. Yeast derlin Dfm1 employs a chaperone-like function to resolve misfolded membrane protein stress. PLoS Biol. 2023, 21, e3001950. [Google Scholar] [CrossRef]
- Santt, O.; Pfirrmann, T.; Braun, B.; Juretschke, J.; Kimmig, P.; Scheel, H.; Hofmann, K.; Thumm, M.; Wolf, D.H. The yeast GID complex, a novel ubiquitin ligase (E3) involved in the regulation of carbohydrate metabolism. Mol. Biol. Cell 2008, 19, 3323–3333. [Google Scholar] [CrossRef] [PubMed]
- Menssen, R.; Schweiggert, J.; Schreiner, J.; Kušević, D.; Reuther, J.; Braun, B.; Wolf, D.H. Exploring the topology of the Gid complex, the E3 ubiquitin ligase involved in catabolite-induced degradation of gluconeogenic enzymes. J. Biol. Chem. 2012, 287, 25602–25614. [Google Scholar] [CrossRef]
- Shen, Z.-J.; Postnikoff, S.; Tyler, J.K. Is Gcn4-induced autophagy the ultimate downstream mechanism by which hormesis extends yeast replicative lifespan? Curr. Genet. 2019, 65, 717–720. [Google Scholar] [CrossRef] [PubMed]
- Patil, C.K.; Li, H.; Walter, P. Gcn4p and Novel Upstream Activating Sequences Regulate Targets of the Unfolded Protein Response. PLoS Biol. 2004, 2, e246. [Google Scholar] [CrossRef]
- Yeast Protocols Handbook PT3024–1; CLONTECH: Mountain View, CA, USA, 2009.
- Sepulveda, J.L. Using R and Bioconductor in Clinical Genomics and Transcriptomics. J. Mol. Diagn. 2020, 22, 3–20. [Google Scholar] [CrossRef]
- Small, E.M.; Felker, D.P.; Heath, O.C.; Cantergiani, R.J.; Robbins, C.E.; Osley, M.A.; McCormick, M.A. SPOCK, an R based package for high-throughput analysis of growth rate, survival, and chronological lifespan in yeast. Transl. Med. Aging 2020, 4, 141–148. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. Fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Smyth, G.K.; Shi, W. Feature Counts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Law, C.W.; Chen, Y.; Shi, W.; Smyth, G.K. voom: Precision weights unlock linear model analysis tools for RNA-seq read counts. Genome Biol. 2014, 15, R29. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Liu, S.; Wang, Z.; Zhu, R.; Wang, F.; Cheng, Y.; Liu, Y. Three Differential Expression Analysis Methods for RNA Sequencing: Limma, EdgeR, DESeq2. JOVE 2021, 175, e62528. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).