Transcriptome Analysis Revealed the Advantages of Room Temperature Preservation of Concentrated Oocystis borgei Cultures for Use in Aquaculture
Abstract
:1. Introduction
2. Results
2.1. Effect of Storage Temperature on Polysaccharide and Lipid Contents in O. borgei
2.2. Survival Rate and Resuscitation of O. borgei
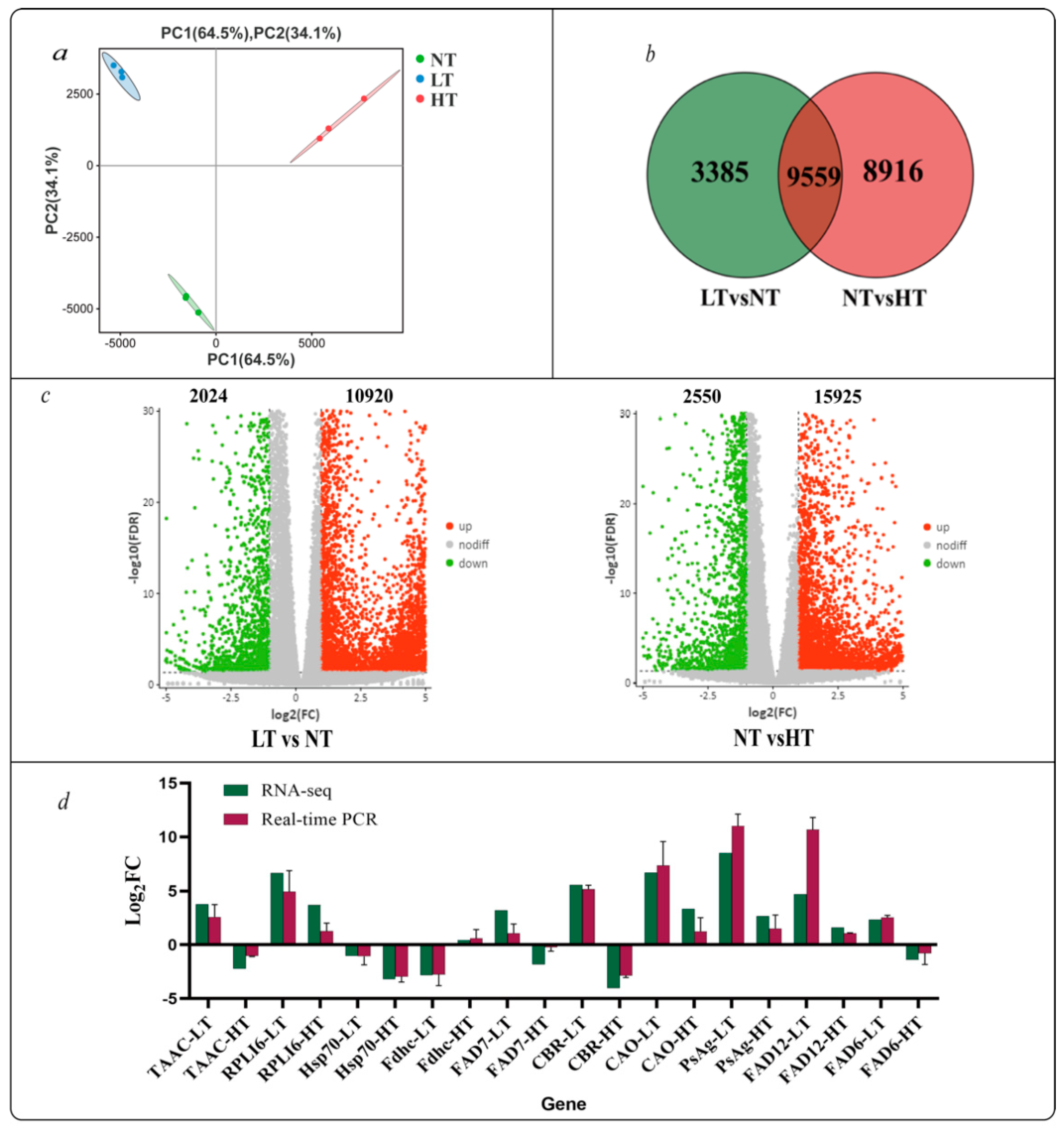
2.3. RNA-Seq Quality Control and Unigene Functional Annotation
2.4. DEG Analysis
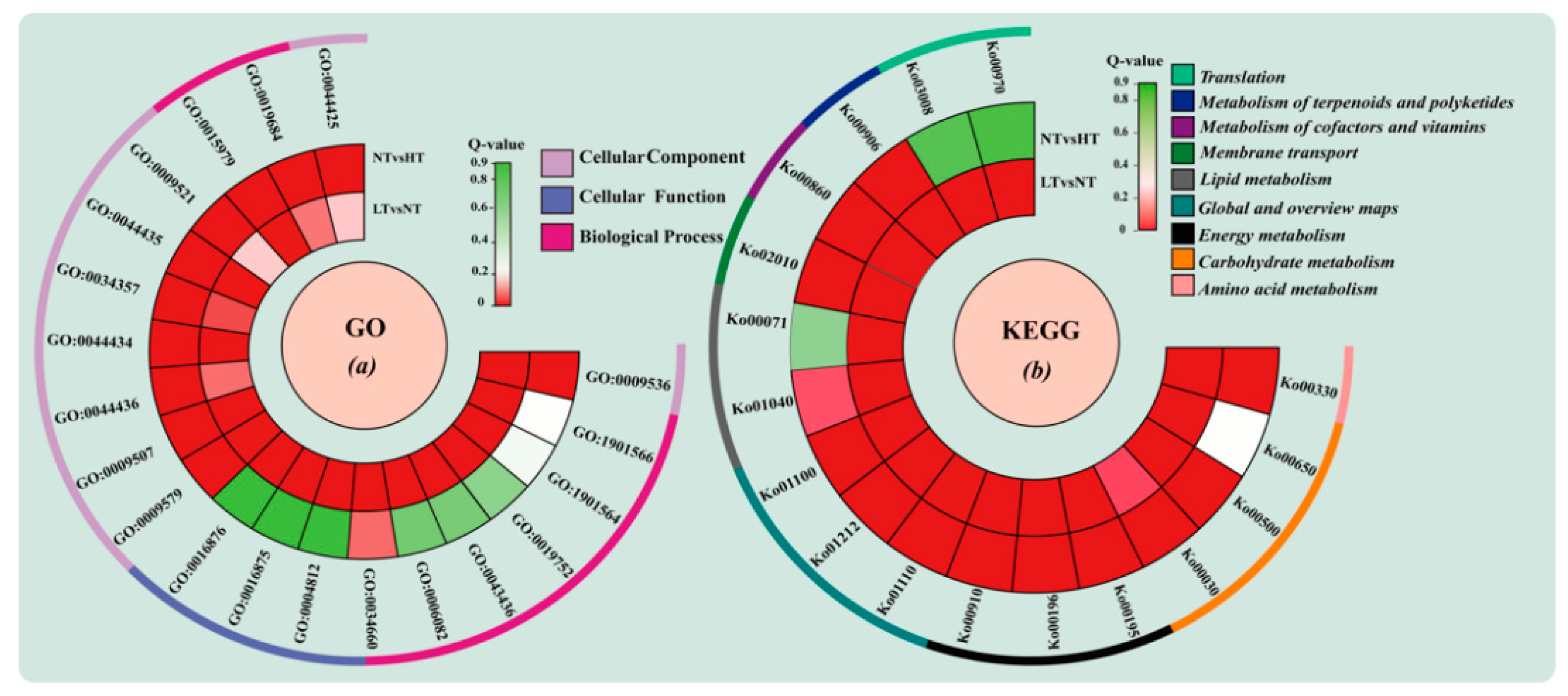
2.5. GO and KEGG Enrichment Analysis
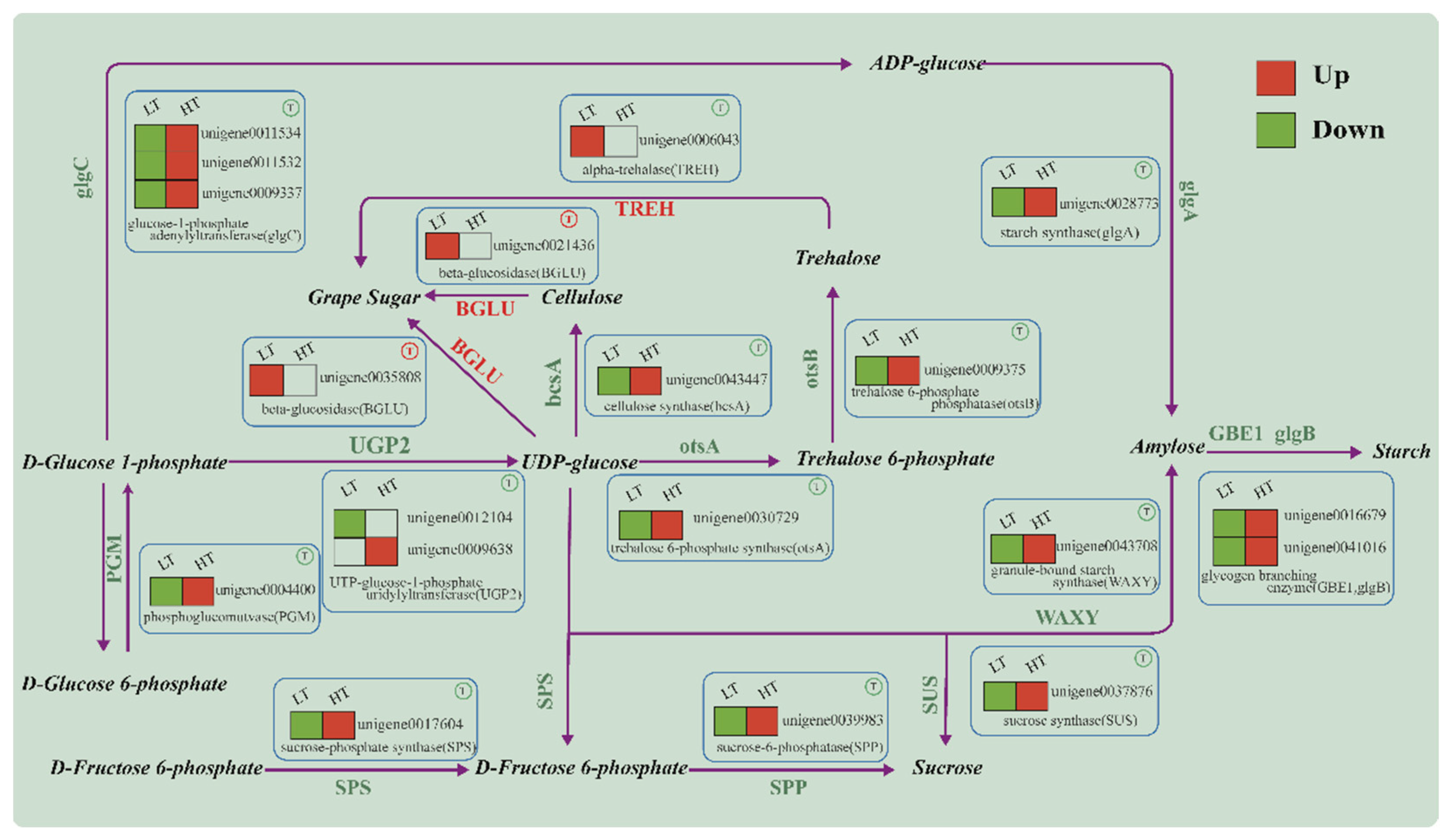
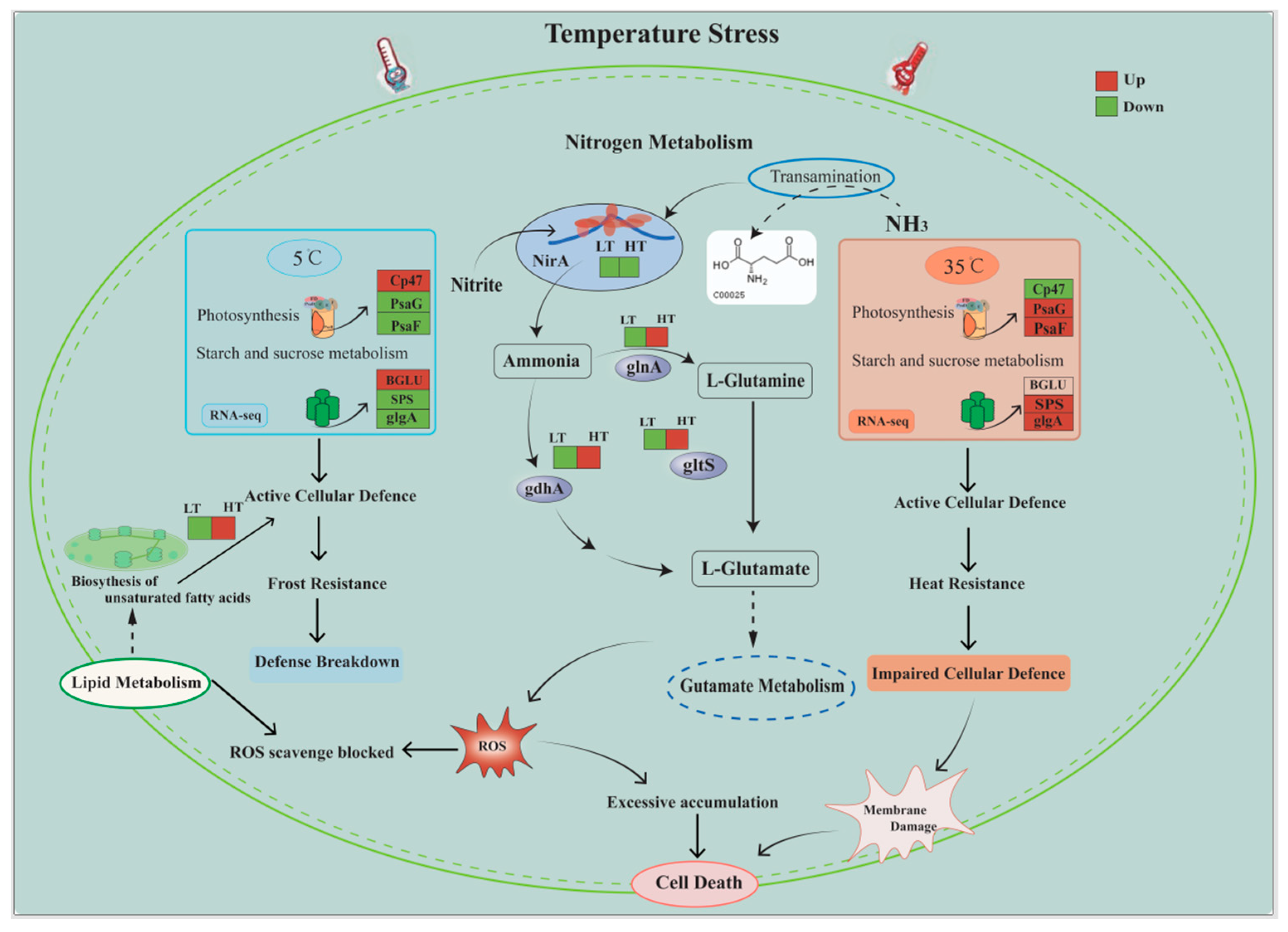
3. Discussion
4. Materials and Methods
4.1. Cultivation, Concentration, and Preservation of O. borgei
4.2. Determination of Polysaccharide and Lipid Contents
4.3. Determination of the Cell Survival Rate and Recovery Efficacy
4.4. RNA Extraction, cDNA Library Construction, and Sequencing
4.5. RNA-Seq Analysis
4.6. Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR) Validation
4.7. Statistic Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Han, P.; Lu, Q.; Fan, L.; Zhou, W. A review on the use of microalgae for sustainable aquaculture. Appl. Sci. 2019, 9, 2377. [Google Scholar] [CrossRef]
- Chang, Z.; Neori, A.; He, Y.; Li, J.; Qiao, L.; Preston, S.I.; Liu, P.; Li, J. Development and current state of seawater shrimp farming, with an emphasis on integrated multi-trophic pond aquaculture farms, in China—A Review. Rev. Aquac. 2020, 12, 2544–2558. [Google Scholar] [CrossRef]
- Patil, P.K.; Geetha, R.; Ravisankar, T.; Avunje, S.; Solanki, H.G.; Abraham, T.J.; Vinoth, S.P.; Jithendran, K.P.; Alavandi, S.V.; Vijayan, K.K. Economic loss due to diseases in indian shrimp farming with special reference to Enterocytozoon Hepatopenaei (EHP) and White Spot Syndrome Virus (WSSV). Aquaculture 2021, 533, 736231. [Google Scholar] [CrossRef]
- Semova, I.; Carten, J.D.; Stombaugh, J.; Mackey, L.C.; Knight, R.; Farber, S.A.; John, F. Rawls microbiota regulate intestinal absorption and metabolism of fatty acids in the Zebrafish. Cell Host Microbe 2012, 12, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Mohsen, M.; Lin, C.; Hamouda, H.I.; Al-Zayat, A.M.; Yang, H. Plastic-associated microbial communities in aquaculture areas. Front. Mar. Sci. 2022, 9, 895611. [Google Scholar] [CrossRef]
- Ge, H.; Li, J.; Chang, Z.; Chen, P.; Shen, M.; Zhao, F. Effect of microalgae with semicontinuous harvesting on water quality and zootechnical performance of white shrimp reared in the zero water exchange system. Aquac. Eng. 2016, 72–73, 70–76. [Google Scholar] [CrossRef]
- Huang, X.; Li, F. Directional cultivation of microalgae in prawn pond. J. Biol. 2021, 38, 18–21. (In Chinese) [Google Scholar] [CrossRef]
- Zeng, G.; Huang, C.; Jiang, X.; Zhang, P.; Peng, R.; Chen, T. Effects of directional cultivation of Thalassiosira pseudonana on growth and survival of Penaeus vannamei and water quality. J. Ningbo Univ. (Nat. Sci. Eng. Ed.) 2021, 34, 1–7. (In Chinese) [Google Scholar] [CrossRef]
- Yao, D.; Zhang, D.; Zhang, S.; Zhang, Y. Effects of green alga Chlorella vulgaris on microalga community and its relationship with environmental factors in Pacific White Shrimp Litopenaeus vannamei culture ponds. Fish. Sci. 2022, 41, 581–588. (In Chinese) [Google Scholar] [CrossRef]
- Nasir, N.M.; Jusoh, A.; Manan, H.; Kasan, N.A.; Kamaruzzan, A.S.; Wan Abdul Karim Ghani, W.A.; Kurniawan, S.B.; Lananan, F. Utilization of Microalgae, Chlorella Sp. UMT LF2 for bioremediation of Litopenaeus Vannamei culture system and harvesting using Bio-flocculant, aspergillus niger. Biocatal. Agric. Biotechnol. 2023, 47, 102596. [Google Scholar] [CrossRef]
- Ansari, F.A.; Guldhe, A.; Gupta, S.K.; Rawat, I.; Bux, F. Improving the feasibility of aquaculture feed by using microalgae. Environmental Science and Pollution Research 2021, 28, 43234–43257. [Google Scholar] [CrossRef]
- Aragão, C.; Conceição, L.E.C.; Dinis, M.T.; Fyhn, H.-J. Amino acid pools of rotifers and artemia under different conditions: Nutritional implications for fish larvae. Aquaculture 2004, 234, 429–445. [Google Scholar] [CrossRef]
- Coutteau, P.; Sorgeloos, P. The use of algal substitutes and the requirement for live algae in the hatchery and nursery rearing of bivalve molluscs: An international survey. J. Shellfish Res. 1992, 11, 467. [Google Scholar]
- Duy, N.D.Q.; Francis, D.S.; Southgate, P.C. The nutritional value of live and concentrated micro-algae for early juveniles of sandfish, holothuria Scabra. Aquaculture 2017, 473, 97–104. [Google Scholar] [CrossRef]
- Cheng, P.; Zhou, C.; Chu, R.; Chang, T.; Xu, J.; Ruan, R.; Chen, P.; Yan, X. Effect of microalgae diet and culture system on the rearing of bivalve mollusks: Nutritional properties and potential cost improvements. Algal Res. 2020, 51, 102076. [Google Scholar] [CrossRef]
- Tang, Y.; Wang, R.; Tan, L.; Guo, L.; Duan, Y.; Yang, L.; Jiang, S.; Zhou, F.; Jiang, S.; Huang, J. Effects of live microalgae and algae powder on microbial community, survival, metamorphosis and digestive enzyme activity of penaeus monodon larvae at different growth stages. Aquaculture 2020, 526, 735344. [Google Scholar] [CrossRef]
- Borowitzka, M.A. Microalgae for aquaculture: Opportunities and constraints. J. Appl. Phycol. 1997, 9, 393–401. [Google Scholar] [CrossRef]
- Gui, Y.; Zamora, L.; Dunphy, B.J.; Jeffs, A.G. Evaluation of the formulated diet myspat for feeding hatchery-reared spat of the Green-Lipped Mussel, Perna Canaliculus (Gmelin, 1791). Aquac. Res. 2016, 47, 3907–3912. [Google Scholar] [CrossRef]
- Welladsen, H.; Kent, M.; Mangott, A.; Li, Y. Shelf-life assessment of microalgae concentrates: Effect of cold preservation on microalgal nutrition profiles. Aquaculture 2014, 430, 241–247. [Google Scholar] [CrossRef]
- Heasman, M.; Diemar, J.; O’connor, W.; Sushames, T.; Foulkes, L. Development of extended shelf-life microalgae concentrate diets harvested by centrifugation for bivalve molluscs—A summary. Aquac. Res. 2000, 31, 637–659. [Google Scholar] [CrossRef]
- Camacho-Rodríguez, J.; Cerón-García, M.C.; Macías-Sánchez, M.D.; Fernández-Sevilla, J.M.; López-Rosales, L.; Molina-Grima, E. Long-term preservation of concentrated Nannochloropsis Gaditana cultures for use in aquaculture. J. Appl. Phycol. 2016, 28, 299–312. [Google Scholar] [CrossRef]
- Seychelles, L.H.; Audet, C.; Tremblay, R.; Fournier, R.; Pernet, F. Essential fatty acid enrichment of cultured rotifers (brachionus plicatilis, müller) using frozen-concentrated microalgae. Aquac. Nutr. 2009, 15, 431–439. [Google Scholar] [CrossRef]
- Diouf, D.; Tremblay, R.; Fournier, R.; Pernet, F. Preservation of lipid content in microalgae concentrates from ultrafiltration process. In Proceedings of the Aquaculture Canada 2008. In Proceedings of the Contributed Papers of the 25th Annual Meeting of the Aquaculture Association of Canada, Saint John, NB, USA, 11–14 May 2008; Aquaculture Association of Canada: Toronto, Canada, 2009; pp. 36–38. [Google Scholar]
- Wang, J.; Tang, W.; Chen, L.; Liu, W.; Liu, T. Simple method for preserving large quantity of high viability red biomass of Haematococcus in the medium term (3~6 months). J. Appl. Phycol. 2019, 31, 1607–1613. [Google Scholar] [CrossRef]
- Foo, S.C.; Mok, C.Y.; Ho, S.Y.; Khong, N.M.H. Microalgal culture preservation: Progress, trends and future developments. Algal Res. 2023, 71, 103007. [Google Scholar] [CrossRef]
- Wang, P.; Gong, Q.; Mai, K.; Zhao, M. Research on results of condensation and conservation of two kinds of arine micro algae. Trans. Oceanol. Limnol. 2001, 4, 12–19. (In Chinese) [Google Scholar] [CrossRef]
- Zhu, B.; Pan, K.; Lin, L. The effect of storage of 2 varieties of golden algaes at different temperatures. Mar. Sci. 2006, 10, 70–74. (In Chinese) [Google Scholar] [CrossRef]
- He, Z.; Huang, X.; Li, C.; Chen, X.; Lin, E. Effect of Rhodopseudomonas palustris on the storage of Oocystis borgei at normal temperature. S. China Fish. Sci. 2013, 9, 50–55. (In Chinese) [Google Scholar] [CrossRef]
- Huang, X.; Li, C.; Liu, C.; Zeng, D. Studies on the ecological factors of Oocystis Borgei. J. Guangdong Ocean Univ. 2002, 3, 8–12. (In Chinese) [Google Scholar] [CrossRef]
- Chen, H.; Deng, C.; Liu, Y.; Huang, X.; Li, F.; Zhang, Y.; Li, C.; Zhang, N. Effects of Oocystis borgei culture on growth performance, antioxidant capacity, and intestinal microflora of Litopenaeus vannamei. J. Guangdong Ocean Univ. 2023, 43, 67–74. (In Chinese) [Google Scholar] [CrossRef]
- Liu, M.; Huang, X.; Zhang, R.; Li, C.; Gu, B. Uptake of urea nitrogen by Oocystis borgei in prawn (Litopenaeus vannamei) aquaculture ponds. Bull. Environ. Contam. Toxicol. 2018, 101, 586–591. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Huang, X.; Li, C.; Gu, B. Study on the uptake of dissolved nitrogen by Oocystis borgei in prawn (Litopenaeus vannamei) aquaculture ponds and establishment of uptake model. Aquac. Int. 2020, 28, 1445–1458. [Google Scholar] [CrossRef]
- Huang, X. Directional cultivation experiment of Oocystis borgei in shrimp ponds. China Fish. 2005, 10, 84–86. (In Chinese) [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Li, C.; Huang, X.; Li, F.; Wang, X.; Zhang, N. Allelopathic effect of Oocystis borgei culture on microcystis aeruginosa. Environ. Technol. 2022, 43, 1662–1671. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Li, C.; Liu, C.; Zhen, L.; Wang, R. Effects of the immobilized microalgae on the quantity dynamics of vibrio in the shrimp ponds. Acta Hydrobiol. Sin. 2005, 6, 80–90. (In Chinese) [Google Scholar] [CrossRef]
- Xie, L.; Zhang, N.; Li, C.; Huang, X. Effects of sodium nitrate on growth, biochemical components and sedimentation of Oocystis Borgei. J. Guangdong Ocean Univ. 2020, 40, 48–55. (In Chinese) [Google Scholar] [CrossRef]
- Low, C.; Toledo, M.I. Assessment of the shelf life of Nannochloropsis oculata flocculates stored at different temperatures. Lat. Am. J. Aquat. Res. 2015, 43, 315–321. [Google Scholar] [CrossRef]
- Yang, S.; Guarnieri, M.T.; Smolinski, S.; Ghirardi, M.; Pienkos, P.T. De novo transcriptomic analysis of hydrogen production in the green alga Chlamydomonas moewusii through RNA-Seq. Biotechnol Biofuels 2013, 6, 118. [Google Scholar] [CrossRef] [PubMed]
- Alsenani, F.; Wass, T.J.; Ma, R.; Eltanahy, E.; Netzel, M.E.; Schenk, P.M. Transcriptome-wide analysis of chlorella reveals auxin-induced carotenogenesis pathway in green microalgae. Algal Res. 2019, 37, 320–335. [Google Scholar] [CrossRef]
- Azaman, S.N.A.; Wong, D.C.J.; Tan, S.W.; Yusoff, F.M.; Nagao, N.; Yeap, S.K. De novo transcriptome analysis of chlorella sorokiniana: Effect of glucose assimilation, and moderate light intensity. Scitific Rep. 2020, 10, 17331. [Google Scholar] [CrossRef] [PubMed]
- Sharma, T.; Chauhan, R.S. Comparative transcriptomics reveals molecular components associated with differential lipid accumulation between microalgal sp., scenedesmus dimorphus and scenedesmus quadricauda. Algal Res. 2016, 19, 109–122. [Google Scholar] [CrossRef]
- Chen, W.; Zhou, P.; Zhang, M.; Zhu, Y.; Wang, X.; Luo, X.; Bao, Z.; Yu, L. Transcriptome analysis reveals that up-regulation of the fatty acid synthase gene promotes the accumulation of docosahexaenoic acid in Schizochytrium sp. S056 when glycerol is used. Algal Res. 2016, 15, 83–92. [Google Scholar] [CrossRef]
- Peng, H.; Wei, D.; Chen, G.; Chen, F. Transcriptome analysis reveals global regulation in response to co2 supplementation in oleaginous microalga coccomyxa subellipsoidea c-169. Biotechnol. Biofuels 2016, 9, 151. [Google Scholar] [CrossRef] [PubMed]
- Xing, G.; Yuan, H.; Yang, J.; Li, J.; Gao, Q.; Li, W.L.; Wang, E. Integrated analyses of transcriptome, proteome, and fatty acid profilings of the oleaginous microalga Auxenochlorella protothecoides UTEX 2341 reveal differential reprogramming of fatty acid metabolism in response to low and high temperatures. Algal Res. 2018, 33, 16–27. [Google Scholar] [CrossRef]
- Blanc, G.; Duncan, G.; Agarkova, I.; Borodovsky, M.; Gurnon, J.; Kuo, A.; Lindquist, E.; Lucas, S.; Pangilinan, J.; Polle, J.; et al. The chlorella variabilis nc64a genome reveals adaptation to photosymbiosis, coevolution with viruses, and cryptic sex. Plant Cell 2010, 22, 2943–2955. [Google Scholar] [CrossRef]
- Devireddy, A.R.; Tschaplinski, T.J.; Tuskan, G.A.; Muchero, W.; Chen, J.-G. Role of reactive oxygen species and hormones in plant responses to temperature changes. Int. J. Mol. Sci. 2021, 22, 8843. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, Y.; Zhang, Y.; Li, C.; Gong, S.; Yan, S.; Li, G.; Hu, G.; Ren, H.; Yang, J.; et al. comparative transcriptome analysis of salt-sensitive and salt-tolerant maize reveals potential mechanisms to enhance salt resistance. Genes Genom. 2019, 41, 781–801. [Google Scholar] [CrossRef]
- Chen, J.; Wang, L.; Liang, H.; Jin, X.; Wan, J.; Liu, F.; Zhao, K.; Huang, J.; Tian, M. Overexpression of DoUGP enhanced biomass and stress tolerance by promoting polysaccharide accumulation in dendrobium officinale. Front. Plant Sci. 2020, 11. [Google Scholar] [CrossRef]
- Valledor, L.; Furuhashi, T.; Hanak, A.-M.; Weckwerth, W. Systemic cold stress adaptation of chlamydomonas reinhardtii. Mol. Cell. Proteom. 2013, 12, 2032–2047. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Song, X.; Li, Y.; Yu, C.; Zhao, Y.; Gong, M.; Shen, X.; Chen, M. Gene expression related to trehalose metabolism and its effect on Volvariella volvacea under low temperature stress. Sci. Rep. 2018, 8, 11011. [Google Scholar] [CrossRef] [PubMed]
- Barati, B.; Gan, S.Y.; Lim, P.E.; Beardall, J.; Phang, S.M. Green algal molecular responses to temperature stress. Acta Physiol. Plant. 2019, 41, 26. [Google Scholar] [CrossRef]
- Nagao, M.; Matsui, K.; Uemura, M. Klebsormidium flaccidum, a charophycean green alga, exhibits cold acclimation that is closely associated with compatible solute accumulation and ultrastructural changes. Plant Cell Environ. 2008, 31, 872–885. [Google Scholar] [CrossRef] [PubMed]
- Admiraal, W. Influence of light and temperature on the growth rate of estuarine benthic diatoms in culture. Mar. Biollogy 1977, 39, 1–9. [Google Scholar] [CrossRef]
- Shin, H.S.; Hong, S.J.; Yoo, C.; Han, M.A.; Lee, H.K.; Choi, H.K.; Cho, S.; Lee, C.G. Genome-wide transcriptome analysis revealed organelle specific responses to temperature variations in algae. Sci. Rep. 2016, 6, 37770. [Google Scholar] [CrossRef]
- Chong, G.L.; Chu, W.L.; Othman, R.Y.; Phang, S.M. Differential gene expression of an Antarctic Chlorella in response to temperature stress. Polar Biol. 2011, 34, 637–645. [Google Scholar] [CrossRef]
- Mock, T.; Hoch, N. Long-term temperature acclimation of photosynthesis in steady-state cultures of the Polar Diatom Fragilariopsis cylindrus. Photosynth. Res. 2005, 85, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Zhou, Y.; Ye, M.; Ling, T.; Chen, K.; Xu, J.; Zhou, C.; Liao, K.; Zhang, L.; Yan, X. Comprehensive comparable study of metabolomic and transcriptomic profiling of Isochrysis galbana exposed to high temperature, an important diet microalgae species. Aquaculture 2020, 521, 735034. [Google Scholar] [CrossRef]
- Vona, V.; Rigano, V.D.M.; Lobosco, O.; Carfagna, S.; Esposito, S.; Rigano, C. Temperature responses of growth, photosynthesis, respiration and NADH: Nitrate reductase in cryophilic and mesophilic algae. New Phytol. 2004, 163, 325–331. [Google Scholar] [CrossRef]
- Rigano, V.D.M.; Vona, V.; Lobosco, O.; Carillo, P.; Lunn, J.E.; Carfagna, S.; Esposito, S.; Caiazzo, M.; Rigano, C. Temperature dependence of nitrate reductase in the psychrophilic unicellular alga Koliella antarctica and the mesophilic alga Chlorella sorokiniana. Plant Cell Environ. 2006, 29, 1400–1409. [Google Scholar] [CrossRef] [PubMed]
- Thangaraj, B.; Rajasekar, D.P.; Vijayaraghavan, R.; Garlapati, D.; Devanesan, A.A.; Lakshmanan, U.; Dharmar, P. Cytomorphological and nitrogen metabolic enzyme analysis of psychrophilic and mesophilic Nostoc sp.: A comparative outlook. 3 Biotech 2017, 7, 107. [Google Scholar] [CrossRef]
- Hernández, M.L.; María, N.P.; Mancha, M.; José, M.; Rivas, M. Expression analysis identifies FAD2-2 as the olive oleate desaturase gene mainly responsible for the linoleic acid content in virgin olive oil. J. Agric. Food Chem. 2009, 57, 6199–6206. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Li, H.; Xiao, Y.; Liu, H. A state-of-the-art review on the synthetic mechanisms, production technologies, and practical application of polyunsaturated fatty acids from microalgae. Algal Res. 2021, 55, 102281. [Google Scholar] [CrossRef]
- Hoffmann, M.; Marxen, K.; Schulz, R.; Vanselow, K.H. TFA and EPA productivities of Nannochloropsis salina influenced by temperature and nitrate stimuli in turbidostatic controlled experiments. Mar. Drugs 2010, 8, 2526–2545. [Google Scholar] [CrossRef] [PubMed]
- An, M.; Mou, S.; Zhang, X.; Ye, N.; Zheng, Z.; Cao, S.; Xu, D.; Fan, X.; Wang, Y.; Miao, J. Temperature regulates fatty acid desaturases at a transcriptional level and modulates the fatty acid profile in the antarctic microalga Chlamydomonas Sp. ICE-L. Bioresour. Technol. 2013, 134, 151–157. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.; Hamilton, J.K.; Rebers, P.A.; Smith, F. A colorimetric method for the determination of sugars. Nature 1951, 168, 167. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Yoon, B.-D.; Oh, H.-M. Rapid method for the determination of lipid from the green alga Botryococcus Braunii. Biotechnol. Tech. 1998, 12, 553–556. [Google Scholar] [CrossRef]
- Zetsche, E.M.; Meysman, F.J.R. Dead or alive? Viability assessment of micro- and mesoplankton. J. Plankton Res. 2012, 34, 493–509. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef] [PubMed]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Chen, Z.H.; Rhind, N.; Birren, B.W.; Zwng, Q.; et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Simão, F.A.; Robert, M.; Waterhouse, R.M.; Ioannidis, P.; Kriventseva, E.V.; Zdobnov, E.M. BUSCO: Assessing genome assembly and annotation completeness with single copy orthologs. Bioinformatics 2015, 31, 3210–3212. [Google Scholar] [CrossRef]
- Kanehisa, M. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W. Simon Anders Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, N.; Deng, C.; Hong, T.; Ren, J.; Zhang, Y.; Li, F.; Dong, Z.; Hu, Z.; Huang, X.; Li, C. Transcriptome Analysis Revealed the Advantages of Room Temperature Preservation of Concentrated Oocystis borgei Cultures for Use in Aquaculture. Int. J. Mol. Sci. 2023, 24, 16225. https://doi.org/10.3390/ijms242216225
Zhang N, Deng C, Hong T, Ren J, Zhang Y, Li F, Dong Z, Hu Z, Huang X, Li C. Transcriptome Analysis Revealed the Advantages of Room Temperature Preservation of Concentrated Oocystis borgei Cultures for Use in Aquaculture. International Journal of Molecular Sciences. 2023; 24(22):16225. https://doi.org/10.3390/ijms242216225
Chicago/Turabian StyleZhang, Ning, Chengcheng Deng, Ting Hong, Jiajia Ren, Yulei Zhang, Feng Li, Zhongdian Dong, Zhangxi Hu, Xianghu Huang, and Changling Li. 2023. "Transcriptome Analysis Revealed the Advantages of Room Temperature Preservation of Concentrated Oocystis borgei Cultures for Use in Aquaculture" International Journal of Molecular Sciences 24, no. 22: 16225. https://doi.org/10.3390/ijms242216225
APA StyleZhang, N., Deng, C., Hong, T., Ren, J., Zhang, Y., Li, F., Dong, Z., Hu, Z., Huang, X., & Li, C. (2023). Transcriptome Analysis Revealed the Advantages of Room Temperature Preservation of Concentrated Oocystis borgei Cultures for Use in Aquaculture. International Journal of Molecular Sciences, 24(22), 16225. https://doi.org/10.3390/ijms242216225









