Structural Insights into Protein–Aptamer Recognitions Emerged from Experimental and Computational Studies
Abstract
1. Introduction
2. Protein–Aptamer Complexes in the Protein Data Bank: Identification and Classification
2.1. Procedure Used to Select the Structures of Aptamers and Their Complexes with Proteins and Classification Tools
2.2. Chronological Evolution of Aptamer Structures Reported in the PDB: Impact of the Different Methodologies
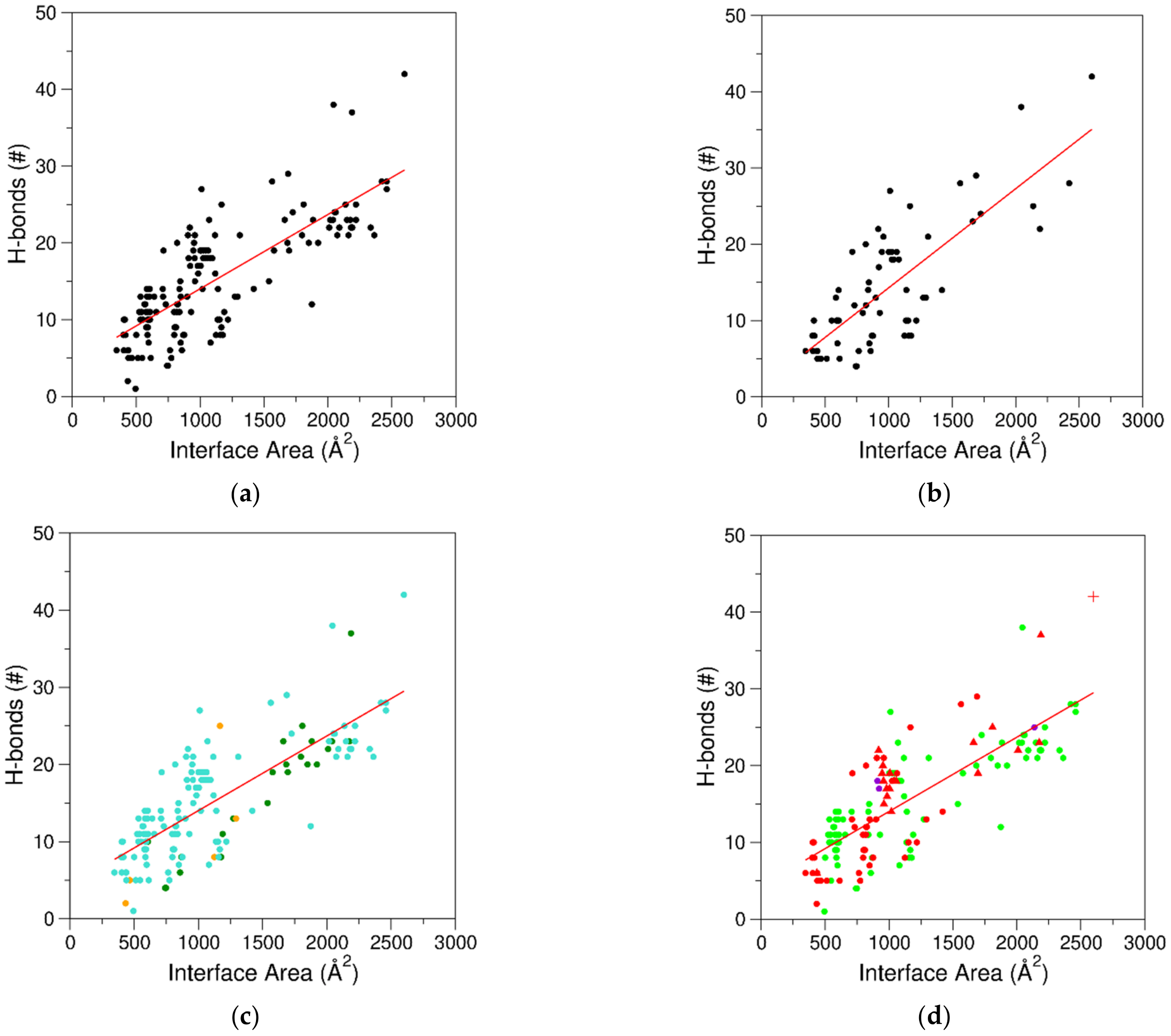
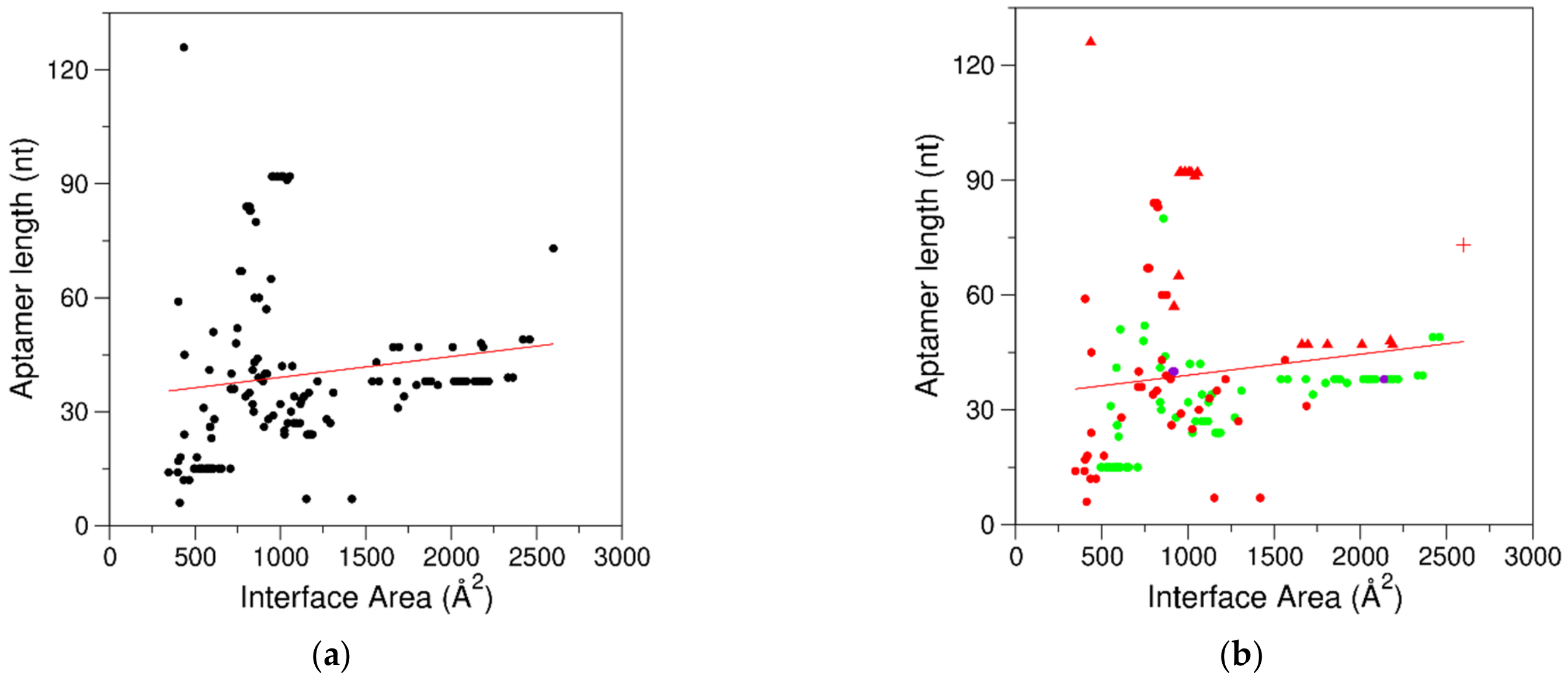
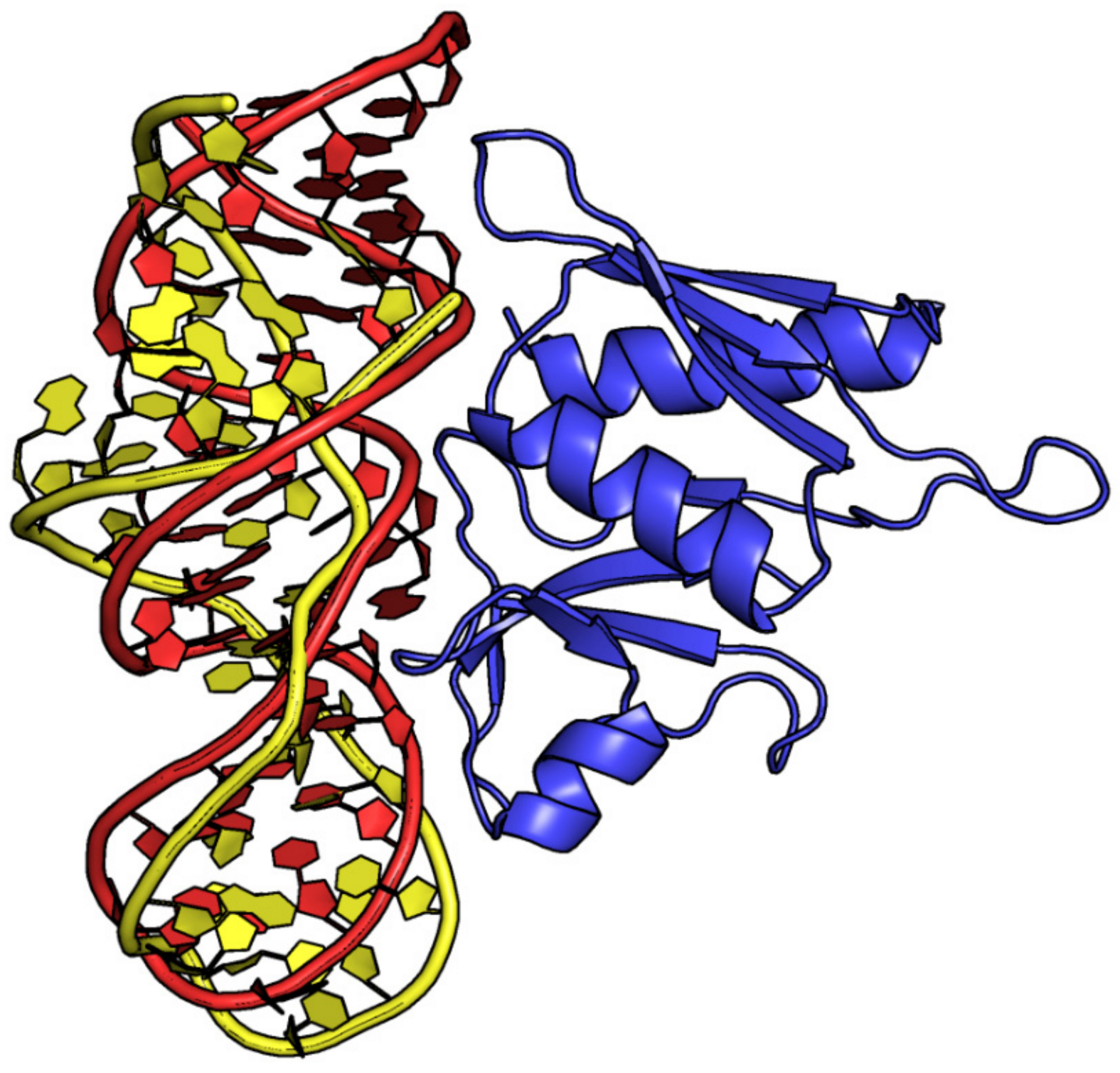
2.3. Characterization of the Interfaces That Stabilize Protein–Aptamer Complexes
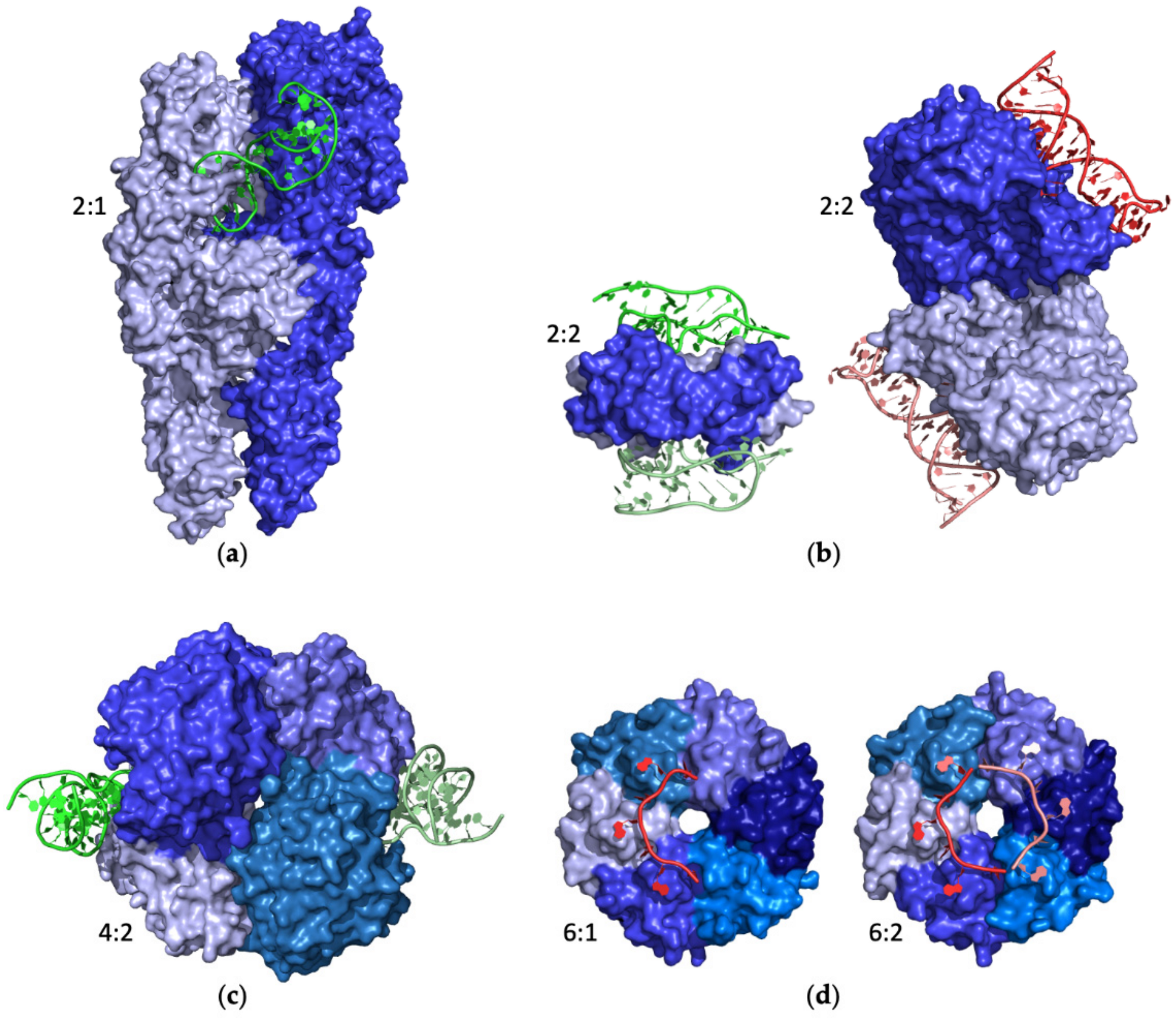
2.4. Stoichiometry of Protein–Aptamer Complexes
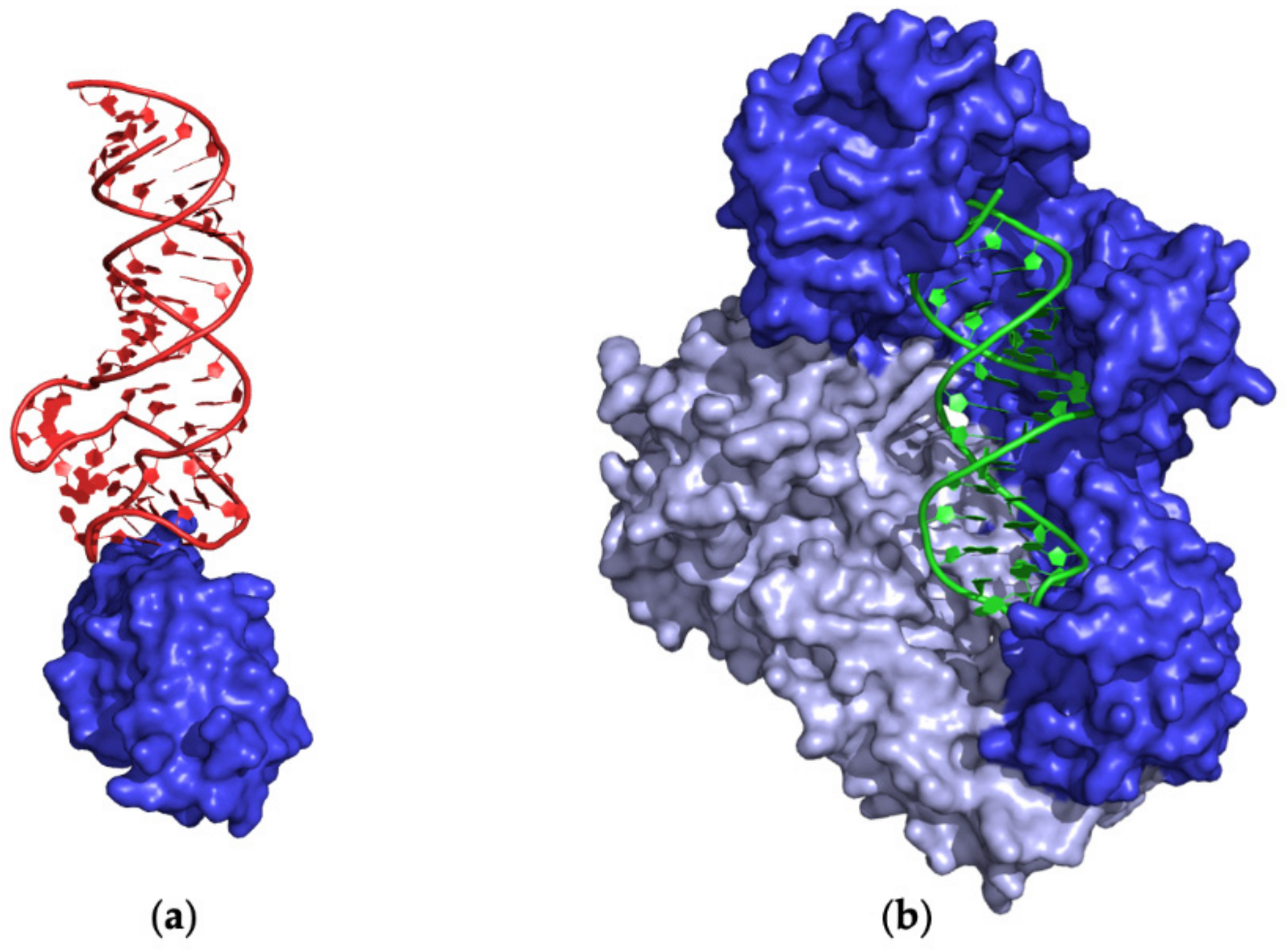
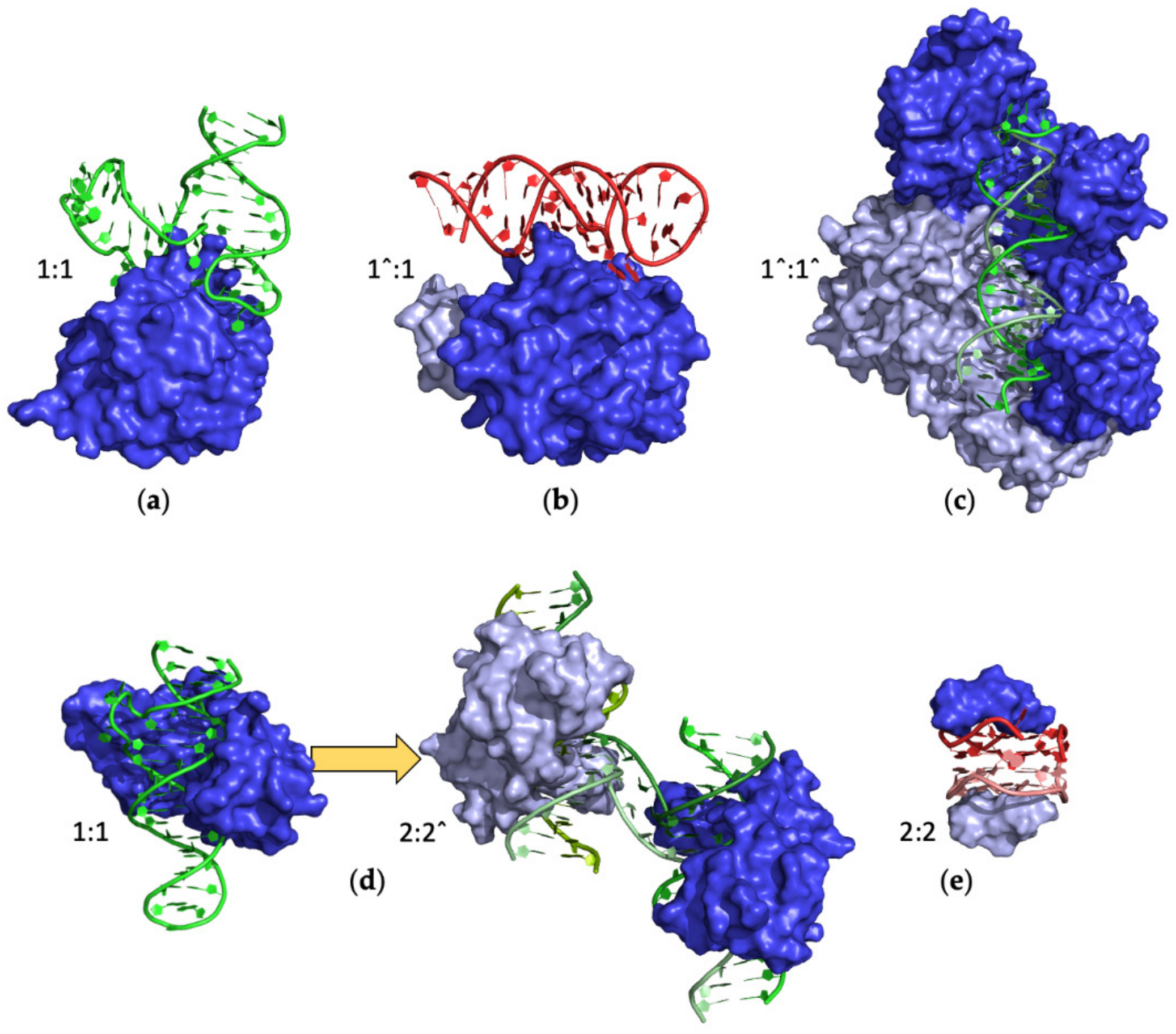
2.4.1. Monomeric Proteins
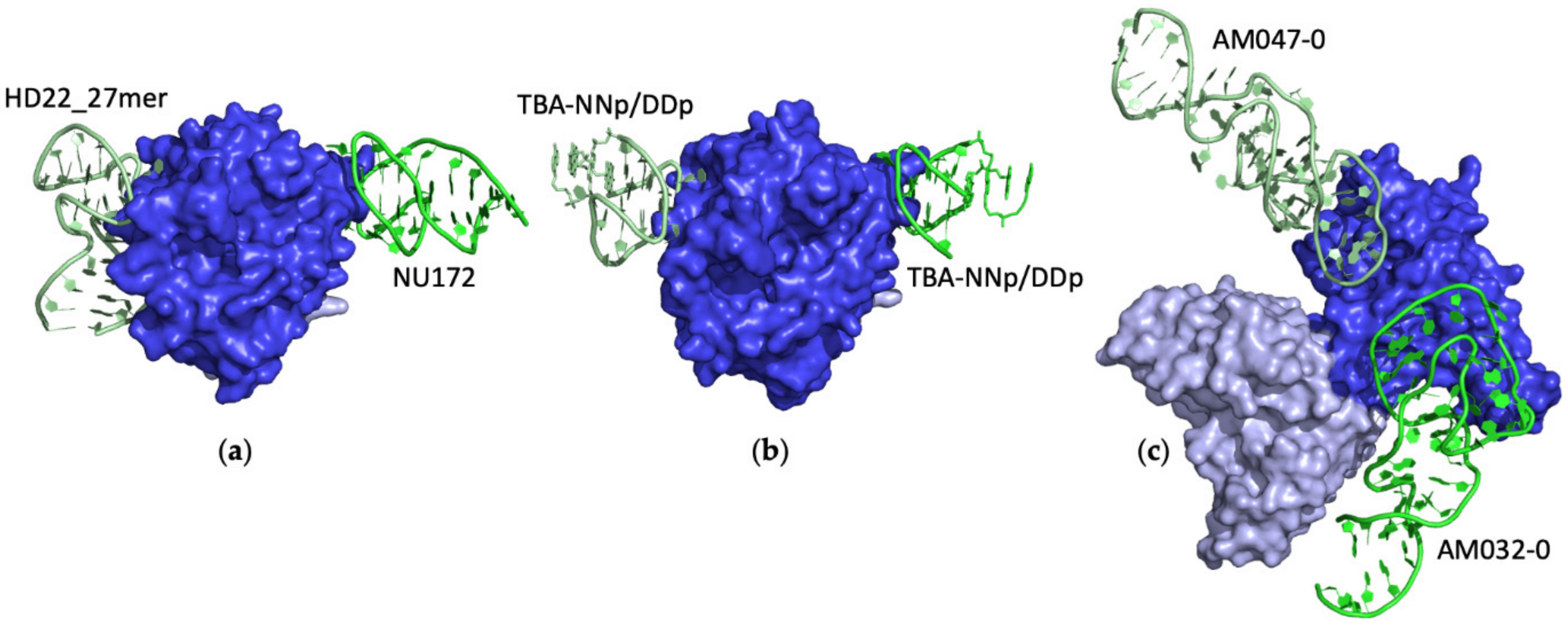
Ternary Complexes
2.4.2. Homomeric Proteins
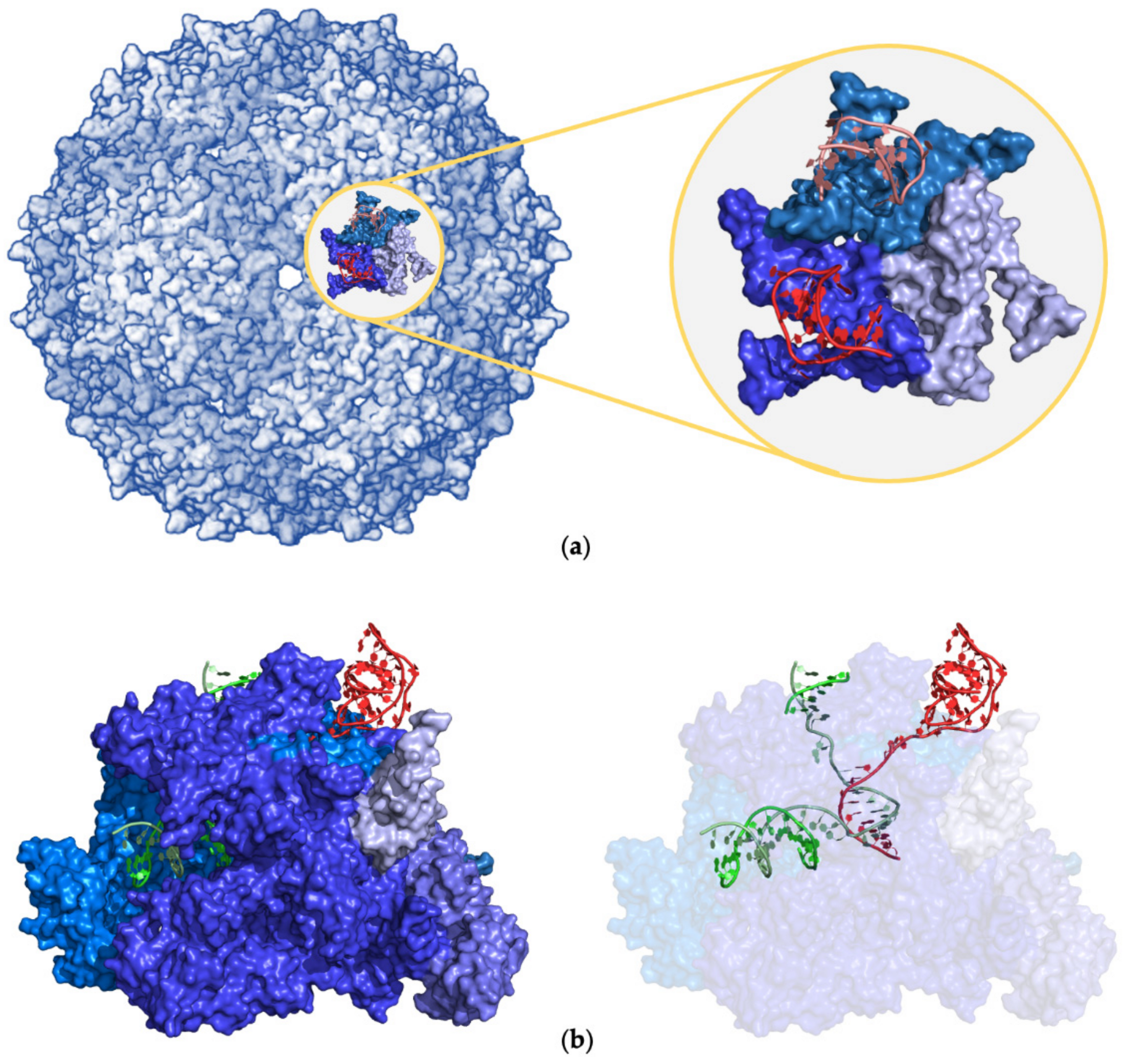
2.4.3. Large Protein Assemblies
3. Structural Versatility of Aptamers Targeting Proteins: Insights into the Recognition Mechanism
4. Conclusions and Perspectives
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Du, X.; Li, Y.; Xia, Y.-L.; Ai, S.-M.; Liang, J.; Sang, P.; Ji, X.-L.; Liu, S.-Q. Insights into Protein–Ligand Interactions: Mechanisms, Models, and Methods. Int. J. Mol. Sci. 2016, 17, 144. [Google Scholar] [CrossRef] [PubMed]
- Wells, J.A.; McClendon, C.L. Reaching for High-Hanging Fruit in Drug Discovery at Protein–Protein Interfaces. Nature 2007, 450, 1001–1009. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Zhou, Q.; He, J.; Jiang, Z.; Peng, C.; Tong, R.; Shi, J. Recent Advances in the Development of Protein–Protein Interactions Modulators: Mechanisms and Clinical Trials. Signal Transduct. Target. Ther. 2020, 5, 213. [Google Scholar] [CrossRef] [PubMed]
- Monti, A.; Vitagliano, L.; Caporale, A.; Ruvo, M.; Doti, N. Targeting Protein-Protein Interfaces with Peptides: The Contribution of Chemical Combinatorial Peptide Library Approaches. Int. J. Mol. Sci. 2023, 24, 7842. [Google Scholar] [CrossRef] [PubMed]
- Mabonga, L.; Kappo, A.P. Protein-Protein Interaction Modulators: Advances, Successes and Remaining Challenges. Biophys. Rev. 2019, 11, 559–581. [Google Scholar] [CrossRef]
- Chames, P.; Van Regenmortel, M.; Weiss, E.; Baty, D. Therapeutic Antibodies: Successes, Limitations and Hopes for the Future. Br. J. Pharmacol. 2009, 157, 220–233. [Google Scholar] [CrossRef] [PubMed]
- Keefe, A.D.; Pai, S.; Ellington, A. Aptamers as Therapeutics. Nat. Rev. Drug Discov. 2010, 9, 537–550. [Google Scholar] [CrossRef]
- Zhou, J.; Rossi, J. Aptamers as Targeted Therapeutics: Current Potential and Challenges. Nat. Rev. Drug Discov. 2017, 16, 181–202. [Google Scholar] [CrossRef] [PubMed]
- Basu, D.; Chakraborty, S.; Pal, R.; Sharma, T.K.; Sarkar, S. Identification and Engineering of Aptamers for Theranostic Application in Human Health and Disorders. Int. J. Mol. Sci. 2021, 22, 9661. [Google Scholar] [CrossRef]
- Byun, J. Recent Progress and Opportunities for Nucleic Acid Aptamers. Life 2021, 11, 193. [Google Scholar] [CrossRef]
- Troisi, R.; Sica, F. Aptamers: Functional-Structural Studies and Biomedical Applications. Int. J. Mol. Sci. 2022, 23, 4796. [Google Scholar] [CrossRef] [PubMed]
- Szeto, K.; Craighead, H.G. Devices and Approaches for Generating Specific High-Affinity Nucleic Acid Aptamers. Appl. Phys. Rev. 2014, 1, 031103. [Google Scholar] [CrossRef]
- Cai, S.; Yan, J.; Xiong, H.; Liu, Y.; Peng, D.; Liu, Z. Investigations on the Interface of Nucleic Acid Aptamers and Binding Targets. Analyst 2018, 143, 5317–5338. [Google Scholar] [CrossRef]
- Yan, J.; Xiong, H.; Cai, S.; Wen, N.; He, Q.; Liu, Y.; Peng, D.; Liu, Z. Advances in Aptamer Screening Technologies. Talanta 2019, 200, 124–144. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lai, B.S.; Juhas, M. Recent Advances in Aptamer Discovery and Applications. Molecules 2019, 24, 941. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Jiang, X.; Zhou, Y.; Ma, M.; Wang, M.; Ying, B. Systematic Evolution of Ligands by Exponential Enrichment Technologies and Aptamer-Based Applications: Recent Progress and Challenges in Precision Medicine of Infectious Diseases. Front. Bioeng. Biotechnol. 2021, 9, 704077. [Google Scholar] [CrossRef]
- Yang, L.F.; Ling, M.; Kacherovsky, N.; Pun, S.H. Aptamers 101: Aptamer Discovery and in Vitro Applications in Biosensors and Separations. Chem. Sci. 2023, 14, 4961–4978. [Google Scholar] [CrossRef]
- Tuerk, C.; Gold, L. Systematic Evolution of Ligands by Exponential Enrichment: RNA Ligands to Bacteriophage T4 DNA Polymerase. Science 1990, 249, 505–510. [Google Scholar] [CrossRef]
- Ellington, A.D.; Szostak, J.W. In Vitro Selection of RNA Molecules That Bind Specific Ligands. Nature 1990, 346, 818–822. [Google Scholar] [CrossRef]
- Sun, H.; Zu, Y. Aptamers and Their Applications in Nanomedicine. Small 2015, 11, 2352–2364. [Google Scholar] [CrossRef]
- Kumar Kulabhusan, P.; Hussain, B.; Yüce, M. Current Perspectives on Aptamers as Diagnostic Tools and Therapeutic Agents. Pharmaceutics 2020, 12, 646. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zaman, K.; Fortenberry, Y.M. Overview of the Therapeutic Potential of Aptamers Targeting Coagulation Factors. Int. J. Mol. Sci. 2021, 22, 3897. [Google Scholar] [CrossRef] [PubMed]
- Di Mauro, V.; Lauta, F.C.; Modica, J.; Appleton, S.L.; de Franciscis, V.; Catalucci, D. Diagnostic and Therapeutic Aptamers: A Promising Pathway to Improved Cardiovascular Disease Management. JACC Basic Transl. Sci. 2023, in press. [Google Scholar] [CrossRef]
- Nimjee, S.M.; White, R.R.; Becker, R.C.; Sullenger, B.A. Aptamers as Therapeutics. Annu. Rev. Pharmacol. Toxicol. 2017, 57, 61–79. [Google Scholar] [CrossRef]
- Binning, J.; Leung, D.; Amarasinghe, G. Aptamers in Virology: Recent Advances and Challenges. Front. Microbiol. 2012, 3, 29. [Google Scholar] [CrossRef] [PubMed]
- Wandtke, T.; Woźniak, J.; Kopiński, P. Aptamers in Diagnostics and Treatment of Viral Infections. Viruses 2015, 7, 751–780. [Google Scholar] [CrossRef]
- Leija-Montoya, A.G.; Benítez-Hess, M.L.; Alvarez-Salas, L.M.; Leija-Montoya, A.G.; Benítez-Hess, M.L.; Alvarez-Salas, L.M. Application of Nucleic Acid Aptamers to Viral Detection and Inhibition. In Nucleic Acids—From Basic Aspects to Laboratory Tools; IntechOpen: London, UK, 2016; ISBN 978-953-51-2264-7. [Google Scholar]
- Niederlender, S.; Fontaine, J.-J.; Karadjian, G. Potential Applications of Aptamers in Veterinary Science. Vet. Res. 2021, 52, 79. [Google Scholar] [CrossRef]
- Otte, D.-M.; Choukeife, M.; Patwari, T.; Mayer, G. Nucleic Acid Aptamers: From Basic Research to Clinical Applications. In Handbook of Chemical Biology of Nucleic Acids; Sugimoto, N., Ed.; Springer Nature: Singapore, 2022; pp. 1–25. ISBN 9789811613135. [Google Scholar]
- Esposito, C.L.; Autiero, I.; Sandomenico, A.; Li, H.; Bassal, M.A.; Ibba, M.L.; Wang, D.; Rinaldi, L.; Ummarino, S.; Gaggi, G.; et al. Targeted Systematic Evolution of an RNA Platform Neutralizing DNMT1 Function and Controlling DNA Methylation. Nat. Commun. 2023, 14, 99. [Google Scholar] [CrossRef]
- Karadeniz Saygılı, S.; Szymanowska, A.; Lopez-Berestein, G.; Rodriguez-Aguayo, C.; Amero, P. Aptamers as Insights for Targeting SARS-CoV-2. Biologics 2023, 3, 116–137. [Google Scholar] [CrossRef]
- Ng, E.W.M.; Shima, D.T.; Calias, P.; Cunningham, E.T.; Guyer, D.R.; Adamis, A.P. Pegaptanib, a Targeted Anti-VEGF Aptamer for Ocular Vascular Disease. Nat. Rev. Drug Discov. 2006, 5, 123–132. [Google Scholar] [CrossRef]
- Mullard, A. FDA Approves Second RNA Aptamer. Nat. Rev. Drug Discov. 2023, 22, 774. [Google Scholar] [CrossRef]
- Haßel, S.K.; Mayer, G. Aptamers as Therapeutic Agents: Has the Initial Euphoria Subsided? Mol. Diagn. Ther. 2019, 23, 301–309. [Google Scholar] [CrossRef]
- Di Ruscio, A.; de Franciscis, V. Minding the Gap: Unlocking the Therapeutic Potential of Aptamers and Making up for Lost Time. Mol. Ther. Nucleic Acids 2022, 29, 384–386. [Google Scholar] [CrossRef]
- Padmanabhan, K.; Padmanabhan, K.P.; Ferrara, J.D.; Sadler, J.E.; Tulinsky, A. The Structure of Alpha-Thrombin Inhibited by a 15-Mer Single-Stranded DNA Aptamer. J. Biol. Chem. 1993, 268, 17651–17654. [Google Scholar] [CrossRef] [PubMed]
- Ruigrok, V.J.B.; Levisson, M.; Hekelaar, J.; Smidt, H.; Dijkstra, B.W.; Van der Oost, J. Characterization of Aptamer-Protein Complexes by X-Ray Crystallography and Alternative Approaches. Int. J. Mol. Sci. 2012, 13, 10537–10552. [Google Scholar] [CrossRef]
- Gelinas, A.D.; Davies, D.R.; Janjic, N. Embracing Proteins: Structural Themes in Aptamer-Protein Complexes. Curr. Opin. Struct. Biol. 2016, 36, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Novoseltseva, A.A.; Zavyalova, E.G.; Golovin, A.V.; Kopylov, A.M. An Insight into Aptamer–Protein Complexes. Aptamers 2018, 2, 55–63. [Google Scholar]
- Zhang, N.; Chen, Z.; Liu, D.; Jiang, H.; Zhang, Z.-K.; Lu, A.; Zhang, B.-T.; Yu, Y.; Zhang, G. Structural Biology for the Molecular Insight between Aptamers and Target Proteins. Int. J. Mol. Sci. 2021, 22, 4093. [Google Scholar] [CrossRef]
- Reverdatto, S.; Burz, D.S.; Shekhtman, A. Peptide Aptamers: Development and Applications. Curr. Top. Med. Chem. 2015, 15, 1082–1101. [Google Scholar] [CrossRef] [PubMed]
- Krissinel, E.; Henrick, K. Inference of Macromolecular Assemblies from Crystalline State. J. Mol. Biol. 2007, 372, 774–797. [Google Scholar] [CrossRef]
- Padmanabhan, K.; Tulinsky, A. An Ambiguous Structure of a DNA 15-Mer Thrombin Complex. Acta Crystallogr. D Biol. Crystallogr. 1996, 52, 272–282. [Google Scholar] [CrossRef]
- Ye, X.; Gorin, A.; Ellington, A.D.; Patel, D.J. Deep Penetration of an Alpha-Helix into a Widened RNA Major Groove in the HIV-1 Rev Peptide-RNA Aptamer Complex. Nat. Struct. Biol. 1996, 3, 1026–1033. [Google Scholar] [CrossRef] [PubMed]
- Convery, M.A.; Rowsell, S.; Stonehouse, N.J.; Ellington, A.D.; Hirao, I.; Murray, J.B.; Peabody, D.S.; Phillips, S.E.; Stockley, P.G. Crystal Structure of an RNA Aptamer-Protein Complex at 2.8 A Resolution. Nat. Struct. Biol. 1998, 5, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Rowsell, S.; Stonehouse, N.J.; Convery, M.A.; Adams, C.J.; Ellington, A.D.; Hirao, I.; Peabody, D.S.; Stockley, P.G.; Phillips, S.E. Crystal Structures of a Series of RNA Aptamers Complexed to the Same Protein Target. Nat. Struct. Biol. 1998, 5, 970–975. [Google Scholar] [CrossRef]
- Ye, X.; Gorin, A.; Frederick, R.; Hu, W.; Majumdar, A.; Xu, W.; McLendon, G.; Ellington, A.; Patel, D.J. RNA Architecture Dictates the Conformations of a Bound Peptide. Chem. Biol. 1999, 6, 657–669. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bullock, T.L.; Sherlin, L.D.; Perona, J.J. Tertiary Core Rearrangements in a Tight Binding Transfer RNA Aptamer. Nat. Struct. Biol. 2000, 7, 497–504. [Google Scholar] [CrossRef]
- Jiang, F.; Gorin, A.; Hu, W.; Majumdar, A.; Baskerville, S.; Xu, W.; Ellington, A.; Patel, D.J. Anchoring an Extended HTLV-1 Rex Peptide within an RNA Major Groove Containing Junctional Base Triples. Structure 1999, 7, 1461–1472. [Google Scholar] [CrossRef]
- Huang, D.-B.; Vu, D.; Cassiday, L.A.; Zimmerman, J.M.; Maher, L.J.; Ghosh, G. Crystal Structure of NF-kappaB (P50)2 Complexed to a High-Affinity RNA Aptamer. Proc. Natl. Acad. Sci. USA 2003, 100, 9268–9273. [Google Scholar] [CrossRef]
- Horn, W.T.; Convery, M.A.; Stonehouse, N.J.; Adams, C.J.; Liljas, L.; Phillips, S.E.V.; Stockley, P.G. The Crystal Structure of a High Affinity RNA Stem-Loop Complexed with the Bacteriophage MS2 Capsid: Further Challenges in the Modeling of Ligand-RNA Interactions. RNA 2004, 10, 1776–1782. [Google Scholar] [CrossRef]
- Kettenberger, H.; Eisenführ, A.; Brueckner, F.; Theis, M.; Famulok, M.; Cramer, P. Structure of an RNA Polymerase II-RNA Inhibitor Complex Elucidates Transcription Regulation by Noncoding RNAs. Nat. Struct. Mol. Biol. 2006, 13, 44–48. [Google Scholar] [CrossRef]
- Long, S.B.; Long, M.B.; White, R.R.; Sullenger, B.A. Crystal Structure of an RNA Aptamer Bound to Thrombin. RNA 2008, 14, 2504–2512. [Google Scholar] [CrossRef]
- Xiao, H.; Edwards, T.E.; Ferré-D’Amaré, A.R. Structural Basis for Specific, High-Affinity Tetracycline Binding by an in Vitro Evolved Aptamer and Artificial Riboswitch. Chem. Biol. 2008, 15, 1125–1137. [Google Scholar] [CrossRef]
- Huang, R.-H.; Fremont, D.H.; Diener, J.L.; Schaub, R.G.; Sadler, J.E. A Structural Explanation for the Antithrombotic Activity of ARC1172, a DNA Aptamer That Binds von Willebrand Factor Domain A1. Structure 2009, 17, 1476–1484. [Google Scholar] [CrossRef]
- Smith, K.D.; Lipchock, S.V.; Ames, T.D.; Wang, J.; Breaker, R.R.; Strobel, S.A. Structural Basis of Ligand Binding by a C-Di-GMP Riboswitch. Nat. Struct. Mol. Biol. 2009, 16, 1218–1223. [Google Scholar] [CrossRef] [PubMed]
- Nomura, Y.; Sugiyama, S.; Sakamoto, T.; Miyakawa, S.; Adachi, H.; Takano, K.; Murakami, S.; Inoue, T.; Mori, Y.; Nakamura, Y.; et al. Conformational Plasticity of RNA for Target Recognition as Revealed by the 2.15 A Crystal Structure of a Human IgG-Aptamer Complex. Nucleic Acids Res. 2010, 38, 7822–7829. [Google Scholar] [CrossRef]
- Someya, T.; Baba, S.; Fujimoto, M.; Kawai, G.; Kumasaka, T.; Nakamura, K. Crystal Structure of Hfq from Bacillus Subtilis in Complex with SELEX-Derived RNA Aptamer: Insight into RNA-Binding Properties of Bacterial Hfq. Nucleic Acids Res. 2012, 40, 1856–1867. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.D.; Lipchock, S.V.; Livingston, A.L.; Shanahan, C.A.; Strobel, S.A. Structural and Biochemical Determinants of Ligand Binding by the C-Di-GMP Riboswitch. Biochemistry 2010, 49, 7351–7359. [Google Scholar] [CrossRef]
- Russo Krauss, I.; Merlino, A.; Giancola, C.; Randazzo, A.; Mazzarella, L.; Sica, F. Thrombin-Aptamer Recognition: A Revealed Ambiguity. Nucleic Acids Res. 2011, 39, 7858–7867. [Google Scholar] [CrossRef]
- Smith, K.D.; Lipchock, S.V.; Strobel, S.A. Structural and Biochemical Characterization of Linear Dinucleotide Analogues Bound to the C-Di-GMP-I Aptamer. Biochemistry 2012, 51, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Tesmer, V.M.; Lennarz, S.; Mayer, G.; Tesmer, J.J.G. Molecular Mechanism for Inhibition of g Protein-Coupled Receptor Kinase 2 by a Selective RNA Aptamer. Structure 2012, 20, 1300–1309. [Google Scholar] [CrossRef]
- Baird, N.J.; Zhang, J.; Hamma, T.; Ferré-D’Amaré, A.R. YbxF and YlxQ Are Bacterial Homologs of L7Ae and Bind K-Turns but Not K-Loops. RNA 2012, 18, 759–770. [Google Scholar] [CrossRef][Green Version]
- Russo Krauss, I.; Merlino, A.; Randazzo, A.; Novellino, E.; Mazzarella, L.; Sica, F. High-Resolution Structures of Two Complexes between Thrombin and Thrombin-Binding Aptamer Shed Light on the Role of Cations in the Aptamer Inhibitory Activity. Nucleic Acids Res. 2012, 40, 8119–8128. [Google Scholar] [CrossRef]
- Davies, D.R.; Gelinas, A.D.; Zhang, C.; Rohloff, J.C.; Carter, J.D.; O’Connell, D.; Waugh, S.M.; Wolk, S.K.; Mayfield, W.S.; Burgin, A.B.; et al. Unique Motifs and Hydrophobic Interactions Shape the Binding of Modified DNA Ligands to Protein Targets. Proc. Natl. Acad. Sci. USA 2012, 109, 19971–19976. [Google Scholar] [CrossRef]
- Mashima, T.; Nishikawa, F.; Kamatari, Y.O.; Fujiwara, H.; Saimura, M.; Nagata, T.; Kodaki, T.; Nishikawa, S.; Kuwata, K.; Katahira, M. Anti-Prion Activity of an RNA Aptamer and Its Structural Basis. Nucleic Acids Res. 2013, 41, 1355–1362. [Google Scholar] [CrossRef]
- Hayashi, T.; Oshima, H.; Mashima, T.; Nagata, T.; Katahira, M.; Kinoshita, M. Binding of an RNA Aptamer and a Partial Peptide of a Prion Protein: Crucial Importance of Water Entropy in Molecular Recognition. Nucleic Acids Res. 2014, 42, 6861–6875. [Google Scholar] [CrossRef] [PubMed]
- Cheung, Y.-W.; Kwok, J.; Law, A.W.L.; Watt, R.M.; Kotaka, M.; Tanner, J.A. Structural Basis for Discriminatory Recognition of Plasmodium Lactate Dehydrogenase by a DNA Aptamer. Proc. Natl. Acad. Sci. USA 2013, 110, 15967–15972. [Google Scholar] [CrossRef]
- Russo Krauss, I.; Pica, A.; Merlino, A.; Mazzarella, L.; Sica, F. Duplex-Quadruplex Motifs in a Peculiar Structural Organization Cooperatively Contribute to Thrombin Binding of a DNA Aptamer. Acta Crystallogr. D Biol. Crystallogr. 2013, 69, 2403–2411. [Google Scholar] [CrossRef]
- Padlan, C.S.; Malashkevich, V.N.; Almo, S.C.; Levy, M.; Brenowitz, M.; Girvin, M.E. An RNA Aptamer Possessing a Novel Monovalent Cation-Mediated Fold Inhibits Lysozyme Catalysis by Inhibiting the Binding of Long Natural Substrates. RNA 2014, 20, 447–461. [Google Scholar] [CrossRef]
- Huang, H.; Suslov, N.B.; Li, N.-S.; Shelke, S.A.; Evans, M.E.; Koldobskaya, Y.; Rice, P.A.; Piccirilli, J.A. A G-Quadruplex-Containing RNA Activates Fluorescence in a GFP-like Fluorophore. Nat. Chem. Biol. 2014, 10, 686–691. [Google Scholar] [CrossRef] [PubMed]
- Pica, A.; Russo Krauss, I.; Merlino, A.; Nagatoishi, S.; Sugimoto, N.; Sica, F. Dissecting the Contribution of Thrombin Exosite I in the Recognition of Thrombin Binding Aptamer. FEBS J. 2013, 280, 6581–6588. [Google Scholar] [CrossRef] [PubMed]
- Gelinas, A.D.; Davies, D.R.; Edwards, T.E.; Rohloff, J.C.; Carter, J.D.; Zhang, C.; Gupta, S.; Ishikawa, Y.; Hirota, M.; Nakaishi, Y.; et al. Crystal Structure of Interleukin-6 in Complex with a Modified Nucleic Acid Ligand. J. Biol. Chem. 2014, 289, 8720–8734. [Google Scholar] [CrossRef] [PubMed]
- Davlieva, M.; Donarski, J.; Wang, J.; Shamoo, Y.; Nikonowicz, E.P. Structure Analysis of Free and Bound States of an RNA Aptamer against Ribosomal Protein S8 from Bacillus Anthracis. Nucleic Acids Res. 2014, 42, 10795–10808. [Google Scholar] [CrossRef]
- Oberthür, D.; Achenbach, J.; Gabdulkhakov, A.; Buchner, K.; Maasch, C.; Falke, S.; Rehders, D.; Klussmann, S.; Betzel, C. Crystal Structure of a Mirror-Image L-RNA Aptamer (Spiegelmer) in Complex with the Natural L-Protein Target CCL2. Nat. Commun. 2015, 6, 6923. [Google Scholar] [CrossRef]
- Yatime, L.; Maasch, C.; Hoehlig, K.; Klussmann, S.; Andersen, G.R.; Vater, A. Structural Basis for the Targeting of Complement Anaphylatoxin C5a Using a Mixed L-RNA/L-DNA Aptamer. Nat. Commun. 2015, 6, 6481. [Google Scholar] [CrossRef]
- Ren, A.; Wang, X.C.; Kellenberger, C.A.; Rajashankar, K.R.; Jones, R.A.; Hammond, M.C.; Patel, D.J. Structural Basis for Molecular Discrimination by a 3′,3′-cGAMP Sensing Riboswitch. Cell Rep. 2015, 11, 1–12. [Google Scholar] [CrossRef]
- Jarvis, T.C.; Davies, D.R.; Hisaminato, A.; Resnicow, D.I.; Gupta, S.; Waugh, S.M.; Nagabukuro, A.; Wadatsu, T.; Hishigaki, H.; Gawande, B.; et al. Non-Helical DNA Triplex Forms a Unique Aptamer Scaffold for High Affinity Recognition of Nerve Growth Factor. Structure 2015, 23, 1293–1304. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.T.; Tuske, S.; Das, K.; DeStefano, J.J.; Arnold, E. Structure of HIV-1 Reverse Transcriptase Bound to a Novel 38-Mer Hairpin Template-Primer DNA Aptamer. Protein Sci. 2016, 25, 46–55. [Google Scholar] [CrossRef]
- Russo Krauss, I.; Spiridonova, V.; Pica, A.; Napolitano, V.; Sica, F. Different Duplex/Quadruplex Junctions Determine the Properties of Anti-Thrombin Aptamers with Mixed Folding. Nucleic Acids Res. 2016, 44, 983–991. [Google Scholar] [CrossRef]
- Abeydeera, N.D.; Egli, M.; Cox, N.; Mercier, K.; Conde, J.N.; Pallan, P.S.; Mizurini, D.M.; Sierant, M.; Hibti, F.-E.; Hassell, T.; et al. Evoking Picomolar Binding in RNA by a Single Phosphorodithioate Linkage. Nucleic Acids Res. 2016, 44, 8052–8064. [Google Scholar] [CrossRef] [PubMed]
- Pica, A.; Russo Krauss, I.; Parente, V.; Tateishi-Karimata, H.; Nagatoishi, S.; Tsumoto, K.; Sugimoto, N.; Sica, F. Through-Bond Effects in the Ternary Complexes of Thrombin Sandwiched by Two DNA Aptamers. Nucleic Acids Res. 2017, 45, 461–469. [Google Scholar] [CrossRef]
- Mullins, N.D.; Maguire, N.M.; Ford, A.; Das, K.; Arnold, E.; Balzarini, J.; Maguire, A.R. Exploring the Role of the α-Carboxyphosphonate Moiety in the HIV-RT Activity of α-Carboxy Nucleoside Phosphonates. Org. Biomol. Chem. 2016, 14, 2454–2465. [Google Scholar] [CrossRef]
- Das, K.; Balzarini, J.; Miller, M.T.; Maguire, A.R.; DeStefano, J.J.; Arnold, E. Conformational States of HIV-1 Reverse Transcriptase for Nucleotide Incorporation vs Pyrophosphorolysis-Binding of Foscarnet. ACS Chem. Biol. 2016, 11, 2158–2164. [Google Scholar] [CrossRef]
- Kato, K.; Ikeda, H.; Miyakawa, S.; Futakawa, S.; Nonaka, Y.; Fujiwara, M.; Okudaira, S.; Kano, K.; Aoki, J.; Morita, J.; et al. Structural Basis for Specific Inhibition of Autotaxin by a DNA Aptamer. Nat. Struct. Mol. Biol. 2016, 23, 395–401. [Google Scholar] [CrossRef]
- Choi, S.-J.; Ban, C. Crystal Structure of a DNA Aptamer Bound to PvLDH Elucidates Novel Single-Stranded DNA Structural Elements for Folding and Recognition. Sci. Rep. 2016, 6, 34998. [Google Scholar] [CrossRef]
- Shakeel, S.; Dykeman, E.C.; White, S.J.; Ora, A.; Cockburn, J.J.B.; Butcher, S.J.; Stockley, P.G.; Twarock, R. Genomic RNA Folding Mediates Assembly of Human Parechovirus. Nat. Commun. 2017, 8, 5. [Google Scholar] [CrossRef]
- Ren, X.; Gelinas, A.D.; von Carlowitz, I.; Janjic, N.; Pyle, A.M. Structural Basis for IL-1α Recognition by a Modified DNA Aptamer That Specifically Inhibits IL-1α Signaling. Nat. Commun. 2017, 8, 810. [Google Scholar] [CrossRef]
- Koirala, D.; Shelke, S.A.; Dupont, M.; Ruiz, S.; DasGupta, S.; Bailey, L.J.; Benner, S.A.; Piccirilli, J.A. Affinity Maturation of a Portable Fab-RNA Module for Chaperone-Assisted RNA Crystallography. Nucleic Acids Res. 2018, 46, 2624–2635. [Google Scholar] [CrossRef] [PubMed]
- Dolot, R.; Lam, C.H.; Sierant, M.; Zhao, Q.; Liu, F.-W.; Nawrot, B.; Egli, M.; Yang, X. Crystal Structures of Thrombin in Complex with Chemically Modified Thrombin DNA Aptamers Reveal the Origins of Enhanced Affinity. Nucleic Acids Res. 2018, 46, 4819–4830. [Google Scholar] [CrossRef] [PubMed]
- Gunaratne, R.; Kumar, S.; Frederiksen, J.W.; Stayrook, S.; Lohrmann, J.L.; Perry, K.; Bompiani, K.M.; Chabata, C.V.; Thalji, N.K.; Ho, M.D.; et al. Combination of Aptamer and Drug for Reversible Anticoagulation in Cardiopulmonary Bypass. Nat. Biotechnol. 2018, 36, 606–613. [Google Scholar] [CrossRef]
- Yasutake, Y.; Hattori, S.-I.; Hayashi, H.; Matsuda, K.; Tamura, N.; Kohgo, S.; Maeda, K.; Mitsuya, H. HIV-1 with HBV-Associated Q151M Substitution in RT Becomes Highly Susceptible to Entecavir: Structural Insights into HBV-RT Inhibition by Entecavir. Sci. Rep. 2018, 8, 1624. [Google Scholar] [CrossRef] [PubMed]
- Tuske, S.; Zheng, J.; Olson, E.D.; Ruiz, F.X.; Pascal, B.D.; Hoang, A.; Bauman, J.D.; Das, K.; DeStefano, J.J.; Musier-Forsyth, K.; et al. Integrative Structural Biology Studies of HIV-1 Reverse Transcriptase Binding to a High-Affinity DNA Aptamer. Curr. Res. Struct. Biol. 2020, 2, 116–129. [Google Scholar] [CrossRef] [PubMed]
- Dearborn, A.D.; Eren, E.; Watts, N.R.; Palmer, I.W.; Kaufman, J.D.; Steven, A.C.; Wingfield, P.T. Structure of an RNA Aptamer That Can Inhibit HIV-1 by Blocking Rev-Cognate RNA (RRE) Binding and Rev-Rev Association. Structure 2018, 26, 1187–1195.e4. [Google Scholar] [CrossRef] [PubMed]
- Shelke, S.A.; Shao, Y.; Laski, A.; Koirala, D.; Weissman, B.P.; Fuller, J.R.; Tan, X.; Constantin, T.P.; Waggoner, A.S.; Bruchez, M.P.; et al. Structural Basis for Activation of Fluorogenic Dyes by an RNA Aptamer Lacking a G-Quadruplex Motif. Nat. Commun. 2018, 9, 4542. [Google Scholar] [CrossRef] [PubMed]
- Troisi, R.; Napolitano, V.; Spiridonova, V.; Russo Krauss, I.; Sica, F. Several Structural Motifs Cooperate in Determining the Highly Effective Anti-Thrombin Activity of NU172 Aptamer. Nucleic Acids Res. 2018, 46, 12177–12185. [Google Scholar] [CrossRef] [PubMed]
- Ptacek, J.; Zhang, D.; Qiu, L.; Kruspe, S.; Motlova, L.; Kolenko, P.; Novakova, Z.; Shubham, S.; Havlinova, B.; Baranova, P.; et al. Structural Basis of Prostate-Specific Membrane Antigen Recognition by the A9g RNA Aptamer. Nucleic Acids Res. 2020, 48, 11130–11145. [Google Scholar] [CrossRef]
- Grau, F.C.; Jaeger, J.; Groher, F.; Suess, B.; Muller, Y.A. The Complex Formed between a Synthetic RNA Aptamer and the Transcription Repressor TetR Is a Structural and Functional Twin of the Operator DNA-TetR Regulator Complex. Nucleic Acids Res. 2020, 48, 3366–3378. [Google Scholar] [CrossRef]
- Cheung, Y.-W.; Röthlisberger, P.; Mechaly, A.E.; Weber, P.; Levi-Acobas, F.; Lo, Y.; Wong, A.W.C.; Kinghorn, A.B.; Haouz, A.; Savage, G.P.; et al. Evolution of Abiotic Cubane Chemistries in a Nucleic Acid Aptamer Allows Selective Recognition of a Malaria Biomarker. Proc. Natl. Acad. Sci. USA 2020, 117, 16790–16798. [Google Scholar] [CrossRef]
- Klingler, C.; Ashley, J.; Shi, K.; Stiefvater, A.; Kyba, M.; Sinnreich, M.; Aihara, H.; Kinter, J. DNA Aptamers against the DUX4 Protein Reveal Novel Therapeutic Implications for FSHD. FASEB J. 2020, 34, 4573–4590. [Google Scholar] [CrossRef]
- Chen, H.; Egger, M.; Xu, X.; Flemmich, L.; Krasheninina, O.; Sun, A.; Micura, R.; Ren, A. Structural Distinctions between NAD+ Riboswitch Domains 1 and 2 Determine Differential Folding and Ligand Binding. Nucleic Acids Res. 2020, 48, 12394–12406. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, C.; Denton, D.T.; O’Connell, D.; Drolet, D.W.; Geisbrecht, B.V. Inhibition of the Complement Alternative Pathway by Chemically Modified DNA Aptamers That Bind with Picomolar Affinity to Factor B. J. Immunol. 2021, 206, 861–873. [Google Scholar] [CrossRef]
- Chesterman, C.; Arnold, E. Co-Crystallization with Diabodies: A Case Study for the Introduction of Synthetic Symmetry. Structure 2021, 29, 598–605.e3. [Google Scholar] [CrossRef]
- Smirnov, I.; Kolganova, N.; Troisi, R.; Sica, F.; Timofeev, E. Expanding the Recognition Interface of the Thrombin-Binding Aptamer HD1 through Modification of Residues T3 and T12. Mol. Ther. Nucleic Acids 2021, 23, 863–871. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Gilbert, J.C.; Hatala, P.; Harvey, W.; Liang, Z.; Gao, S.; Kang, D.; Jilma, B. The Development and Characterization of a Long Acting Anti-Thrombotic von Willebrand Factor (VWF) Aptamer. J. Thromb. Haemost. 2020, 18, 1113–1123. [Google Scholar] [CrossRef]
- Ren, X.; Gelinas, A.D.; Linehan, M.; Iwasaki, A.; Wang, W.; Janjic, N.; Pyle, A.M. Evolving A RIG-I Antagonist: A Modified DNA Aptamer Mimics Viral RNA. J. Mol. Biol. 2021, 433, 167227. [Google Scholar] [CrossRef]
- Troisi, R.; Balasco, N.; Santamaria, A.; Vitagliano, L.; Sica, F. Structural and Functional Analysis of the Simultaneous Binding of Two Duplex/Quadruplex Aptamers to Human α-Thrombin. Int. J. Biol. Macromol. 2021, 181, 858–867. [Google Scholar] [CrossRef]
- Singh, A.K.; Martinez, S.E.; Gu, W.; Nguyen, H.; Schols, D.; Herdewijn, P.; De Jonghe, S.; Das, K. Sliding of HIV-1 Reverse Transcriptase over DNA Creates a Transient P Pocket–Targeting P-Pocket by Fragment Screening. Nat. Commun. 2021, 12, 7127. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, F.X.; Hoang, A.; Dilmore, C.R.; DeStefano, J.J.; Arnold, E. Structural Basis of HIV Inhibition by L-Nucleosides: Opportunities for Drug Development and Repurposing. Drug Discov. Today 2022, 27, 1832–1846. [Google Scholar] [CrossRef]
- Rees, H.C.; Gogacz, W.; Li, N.-S.; Koirala, D.; Piccirilli, J.A. Structural Basis for Fluorescence Activation by Pepper RNA. ACS Chem. Biol. 2022, 17, 1866–1875. [Google Scholar] [CrossRef]
- Saito, T.; Shimizu, Y.; Tsukakoshi, K.; Abe, K.; Lee, J.; Ueno, K.; Asano, R.; Jones, B.V.; Yamada, T.; Nakano, T.; et al. Development of a DNA Aptamer That Binds to the Complementarity-Determining Region of Therapeutic Monoclonal Antibody and Affinity Improvement Induced by pH-Change for Sensitive Detection. Biosens. Bioelectron. 2022, 203, 114027. [Google Scholar] [CrossRef]
- Kim, J.; Yunn, N.-O.; Park, M.; Kim, J.; Park, S.; Kim, Y.; Noh, J.; Ryu, S.H.; Cho, Y. Functional Selectivity of Insulin Receptor Revealed by Aptamer-Trapped Receptor Structures. Nat. Commun. 2022, 13, 6500. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; De Wijngaert, B.; Bijnens, M.; Uyttersprot, K.; Nguyen, H.; Martinez, S.E.; Schols, D.; Herdewijn, P.; Pannecouque, C.; Arnold, E.; et al. Cryo-EM Structures of Wild-Type and E138K/M184I Mutant HIV-1 RT/DNA Complexed with Inhibitors Doravirine and Rilpivirine. Proc. Natl. Acad. Sci. USA 2022, 119, e2203660119. [Google Scholar] [CrossRef] [PubMed]
- Troisi, R.; Riccardi, C.; Pérez de Carvasal, K.; Smietana, M.; Morvan, F.; Del Vecchio, P.; Montesarchio, D.; Sica, F. A Terminal Functionalization Strategy Reveals Unusual Binding Abilities of Anti-Thrombin Anticoagulant Aptamers. Mol. Ther. Nucleic Acids 2022, 30, 585–594. [Google Scholar] [CrossRef]
- Cheng, E.L.; Cardle, I.I.; Kacherovsky, N.; Bansia, H.; Wang, T.; Zhou, Y.; Raman, J.; Yen, A.; Gutierrez, D.; Salipante, S.J.; et al. Discovery of a Transferrin Receptor 1-Binding Aptamer and Its Application in Cancer Cell Depletion for Adoptive T-Cell Therapy Manufacturing. J. Am. Chem. Soc. 2022, 144, 13851–13864. [Google Scholar] [CrossRef]
- Menichelli, E.; Lam, B.J.; Wang, Y.; Wang, V.S.; Shaffer, J.; Tjhung, K.F.; Bursulaya, B.; Nguyen, T.N.; Vo, T.; Alper, P.B.; et al. Discovery of Small Molecules That Target a Tertiary-Structured RNA. Proc. Natl. Acad. Sci. USA 2022, 119, e2213117119. [Google Scholar] [CrossRef] [PubMed]
- Troisi, R.; Napolitano, V.; Rossitto, E.; Osman, W.; Nagano, M.; Wakui, K.; Popowicz, G.M.; Yoshimoto, K.; Sica, F. Steric Hindrance and Structural Flexibility Shape the Functional Properties of a Guanine-Rich Oligonucleotide. Nucleic Acids Res. 2023, 51, 8880–8890. [Google Scholar] [CrossRef] [PubMed]
- Chauvier, A.; Porta, J.C.; Deb, I.; Ellinger, E.; Meze, K.; Frank, A.T.; Ohi, M.D.; Walter, N.G. Structural Basis for Control of Bacterial RNA Polymerase Pausing by a Riboswitch and Its Ligand. Nat. Struct. Mol. Biol. 2023, 30, 902–913. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.S.; Han, M.J.; Kim, S.W.; Kang, S.M.; Kim, B.R.; Kim, H.; Lee, C.J.; Noh, J.E.; Kim, H.; Lee, J.-O.; et al. Structure-Guided Development of Bivalent Aptamers Blocking SARS-CoV-2 Infection. Molecules 2023, 28, 4645. [Google Scholar] [CrossRef]
- Troisi, R.; Balasco, N.; Autiero, I.; Sica, F.; Vitagliano, L. New Insight into the Traditional Model of the Coagulation Cascade and Its Regulation: Illustrated Review of a Three-Dimensional View. Res. Pract. Thromb. Haemost. 2023, 7, 102160. [Google Scholar] [CrossRef]
- Troisi, R.; Balasco, N.; Autiero, I.; Vitagliano, L.; Sica, F. Exosite Binding in Thrombin: A Global Structural/Dynamic Overview of Complexes with Aptamers and Other Ligands. Int. J. Mol. Sci. 2021, 22, 10803. [Google Scholar] [CrossRef]
- Riccardi, C.; Napolitano, E.; Platella, C.; Musumeci, D.; Montesarchio, D. G-Quadruplex-Based Aptamers Targeting Human Thrombin: Discovery, Chemical Modifications and Antithrombotic Effects. Pharmacol. Ther. 2021, 217, 107649. [Google Scholar] [CrossRef]
- Callaway, E. Revolutionary Cryo-EM Is Taking over Structural Biology. Nature 2020, 578, 201. [Google Scholar] [CrossRef]
- Chari, A.; Stark, H. Prospects and Limitations of High-Resolution Single-Particle Cryo-Electron Microscopy. Annu. Rev. Biophys. 2023, 52, 391–411. [Google Scholar] [CrossRef]
- Ji, C.; Wei, J.; Zhang, L.; Hou, X.; Tan, J.; Yuan, Q.; Tan, W. Aptamer-Protein Interactions: From Regulation to Biomolecular Detection. Chem. Rev. 2023, in press. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.; Daley, D.T.; Luscombe, N.M.; Berman, H.M.; Thornton, J.M. Protein-RNA Interactions: A Structural Analysis. Nucleic Acids Res. 2001, 29, 943–954. [Google Scholar] [CrossRef]
- Stone, S.R.; Hofsteenge, J. Effect of Heparin on the Interaction between Thrombin and Hirudin. Eur. J. Biochem. 1987, 169, 373–376. [Google Scholar] [CrossRef]
- Fredenburgh, J.C.; Stafford, A.R.; Weitz, J.I. Evidence for Allosteric Linkage between Exosites 1 and 2 of Thrombin. J. Biol. Chem. 1997, 272, 25493–25499. [Google Scholar] [CrossRef] [PubMed]
- Koeppe, J.R.; Seitova, A.; Mather, T.; Komives, E.A. Thrombomodulin Tightens the Thrombin Active Site Loops to Promote Protein C Activation. Biochemistry 2005, 44, 14784–14791. [Google Scholar] [CrossRef] [PubMed]
- Sabo, T.M.; Farrell, D.H.; Maurer, M.C. Conformational Analysis of γ‘ Peptide (410−427) Interactions with Thrombin Anion Binding Exosite II. Biochemistry 2006, 45, 7434–7445. [Google Scholar] [CrossRef]
- Sabo, T.M.; Maurer, M.C. Biophysical Investigation of GpIbalpha Binding to Thrombin Anion Binding Exosite II. Biochemistry 2009, 48, 7110–7122. [Google Scholar] [CrossRef]
- Petrera, N.S.; Stafford, A.R.; Leslie, B.A.; Kretz, C.A.; Fredenburgh, J.C.; Weitz, J.I. Long Range Communication between Exosites 1 and 2 Modulates Thrombin Function. J. Biol. Chem. 2009, 284, 25620–25629. [Google Scholar] [CrossRef]
- Olmsted, I.R.; Xiao, Y.; Cho, M.; Csordas, A.T.; Sheehan, J.H.; Meiler, J.; Soh, H.T.; Bornhop, D.J. Measurement of Aptamer-Protein Interactions with Back-Scattering Interferometry. Anal. Chem. 2011, 83, 8867–8870. [Google Scholar] [CrossRef]
- Malovichko, M.V.; Sabo, T.M.; Maurer, M.C. Ligand Binding to Anion-Binding Exosites Regulates Conformational Properties of Thrombin. J. Biol. Chem. 2013, 288, 8667–8678. [Google Scholar] [CrossRef]
- Chen, K.; Stafford, A.R.; Wu, C.; Yeh, C.H.; Kim, P.Y.; Fredenburgh, J.C.; Weitz, J.I. Exosite 2-Directed Ligands Attenuate Protein C Activation by the Thrombin-Thrombomodulin Complex. Biochemistry 2017, 56, 3119–3128. [Google Scholar] [CrossRef] [PubMed]
- Billur, R.; Sabo, T.M.; Maurer, M.C. Thrombin Exosite Maturation and Ligand Binding at ABE II Help Stabilize PAR-Binding Competent Conformation at ABE I. Biochemistry 2019, 58, 1048–1060. [Google Scholar] [CrossRef]
- Feng, X.; Yu, C.; Feng, F.; Lu, P.; Chai, Y.; Li, Q.; Zhang, D.; Wang, X.; Yao, L. Direct Measurement of Through-Bond Effects in Molecular Multivalent Interactions. Chem. Eur. J. 2019, 25, 2978–2982. [Google Scholar] [CrossRef] [PubMed]
- Troisi, R.; Balasco, N.; Vitagliano, L.; Sica, F. Molecular Dynamics Simulations of Human α-Thrombin in Different Structural Contexts: Evidence for an Aptamer-Guided Cooperation between the Two Exosites. J. Biomol. Struct. Dyn. 2021, 39, 2199–2209. [Google Scholar] [CrossRef] [PubMed]
- Jo, H.; Ban, C. Aptamer–Nanoparticle Complexes as Powerful Diagnostic and Therapeutic Tools. Exp. Mol. Med. 2016, 48, e230. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M. Aptamers Targeting Cell Surface Proteins. Biochimie 2018, 145, 63–72. [Google Scholar] [CrossRef]
- Reiter, N.J.; Maher, L.J., III; Butcher, S.E. DNA Mimicry by a High-Affinity Anti-NF-κB RNA Aptamer. Nucleic Acids Res. 2008, 36, 1227–1236. [Google Scholar] [CrossRef]
- Mashima, T.; Lee, J.-H.; Kamatari, Y.O.; Hayashi, T.; Nagata, T.; Nishikawa, F.; Nishikawa, S.; Kinoshita, M.; Kuwata, K.; Katahira, M. Development and Structural Determination of an Anti-PrPC Aptamer That Blocks Pathological Conformational Conversion of Prion Protein. Sci. Rep. 2020, 10, 4934. [Google Scholar] [CrossRef] [PubMed]
- Autiero, I.; Ruvo, M.; Improta, R.; Vitagliano, L. The Intrinsic Flexibility of the Aptamer Targeting the Ribosomal Protein S8 Is a Key Factor for the Molecular Recognition. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 1006–1016. [Google Scholar] [CrossRef]
- Autiero, I.; Vitagliano, L. Enhanced Molecular Dynamic Simulation Studies Unravel Long-Range Effects Caused by Sequence Variations and Partner Binding in RNA Aptamers. Mol. Ther. Nucleic Acids 2023, 34, 102039. [Google Scholar] [CrossRef]
- Koldobskaya, Y.; Duguid, E.M.; Shechner, D.M.; Suslov, N.B.; Ye, J.; Sidhu, S.S.; Bartel, D.P.; Koide, S.; Kossiakoff, A.A.; Piccirilli, J.A. A Portable RNA Sequence Whose Recognition by a Synthetic Antibody Facilitates Structural Determination. Nat. Struct. Mol. Biol. 2011, 18, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.; Thornton, J.M. Principles of Protein-Protein Interactions. Proc. Natl. Acad. Sci. USA 1996, 93, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Emami, N.; Pakchin, P.S.; Ferdousi, R. Computational Predictive Approaches for Interaction and Structure of Aptamers. J. Theor. Biol. 2020, 497, 110268. [Google Scholar] [CrossRef]
- Ropii, B.; Bethasari, M.; Anshori, I.; Koesoema, A.P.; Shalannanda, W.; Satriawan, A.; Setianingsih, C.; Akbar, M.R.; Aditama, R. The Assessment of Molecular Dynamics Results of Three-Dimensional RNA Aptamer Structure Prediction. PLoS ONE 2023, 18, e0288684. [Google Scholar] [CrossRef]
- Schneider, B.; Sweeney, B.A.; Bateman, A.; Cerny, J.; Zok, T.; Szachniuk, M. When Will RNA Get Its AlphaFold Moment? Nucleic Acids Res. 2023, 51, 9522–9532. [Google Scholar] [CrossRef] [PubMed]
- Esmaeeli, R.; Bauzá, A.; Perez, A. Structural Predictions of Protein–DNA Binding: MELD-DNA. Nucleic Acids Res. 2023, 51, 1625–1636. [Google Scholar] [CrossRef]
- Wei, J.; Chen, S.; Zong, L.; Gao, X.; Li, Y. Protein–RNA Interaction Prediction with Deep Learning: Structure Matters. Brief. Bioinform. 2022, 23, bbab540. [Google Scholar] [CrossRef]
- Chen, Z.; Hu, L.; Zhang, B.-T.; Lu, A.; Wang, Y.; Yu, Y.; Zhang, G. Artificial Intelligence in Aptamer–Target Binding Prediction. Int. J. Mol. Sci. 2021, 22, 3605. [Google Scholar] [CrossRef]
- Varadi, M.; Anyango, S.; Deshpande, M.; Nair, S.; Natassia, C.; Yordanova, G.; Yuan, D.; Stroe, O.; Wood, G.; Laydon, A.; et al. AlphaFold Protein Structure Database: Massively Expanding the Structural Coverage of Protein-Sequence Space with High-Accuracy Models. Nucleic Acids Res. 2022, 50, D439–D444. [Google Scholar] [CrossRef] [PubMed]
- Varadi, M.; Bertoni, D.; Magana, P.; Paramval, U.; Pidruchna, I.; Radhakrishnan, M.; Tsenkov, M.; Nair, S.; Mirdita, M.; Yeo, J.; et al. AlphaFold Protein Structure Database in 2024: Providing Structure Coverage for over 214 Million Protein Sequences. Nucleic Acids Res. 2023, in press. [Google Scholar] [CrossRef] [PubMed]

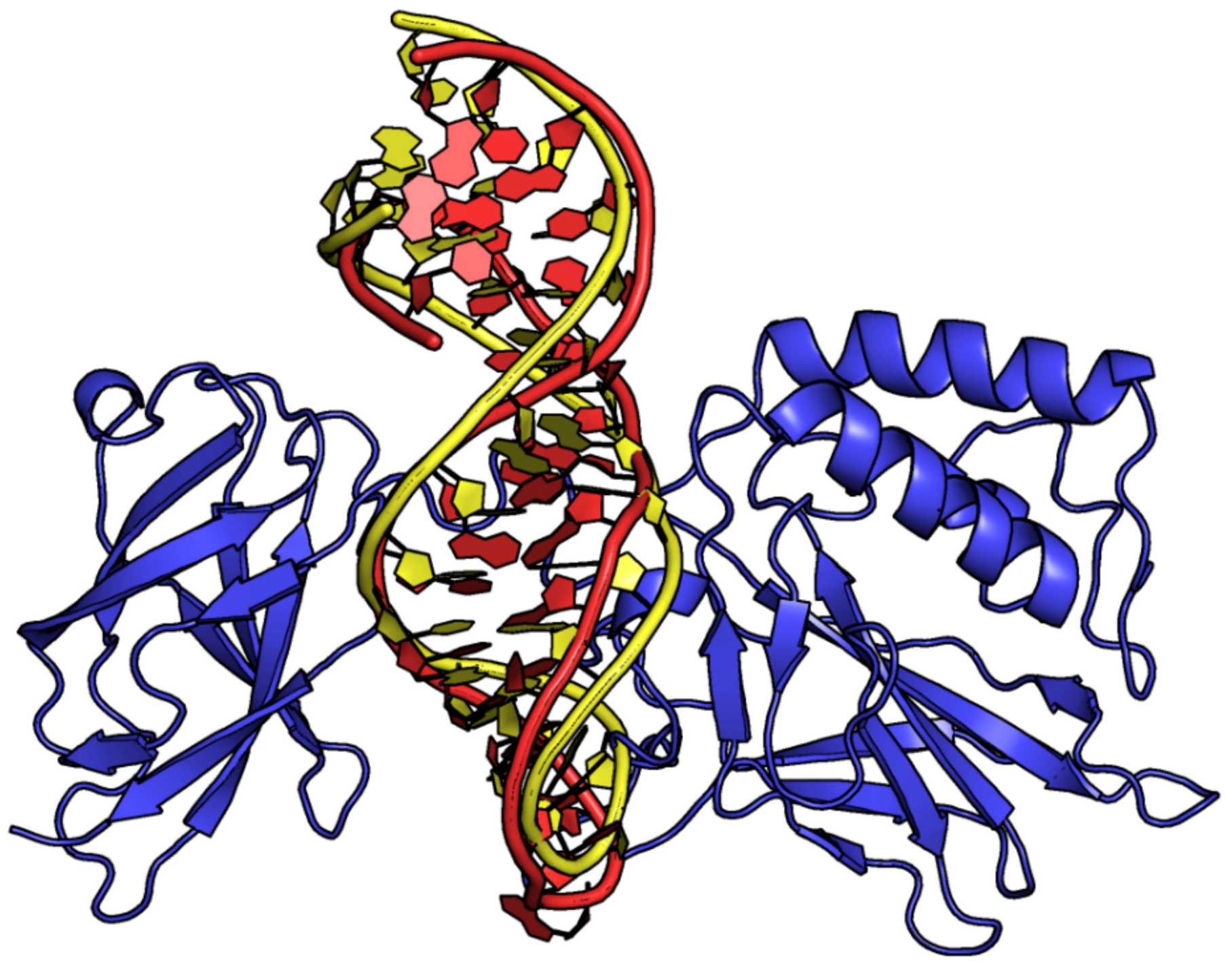
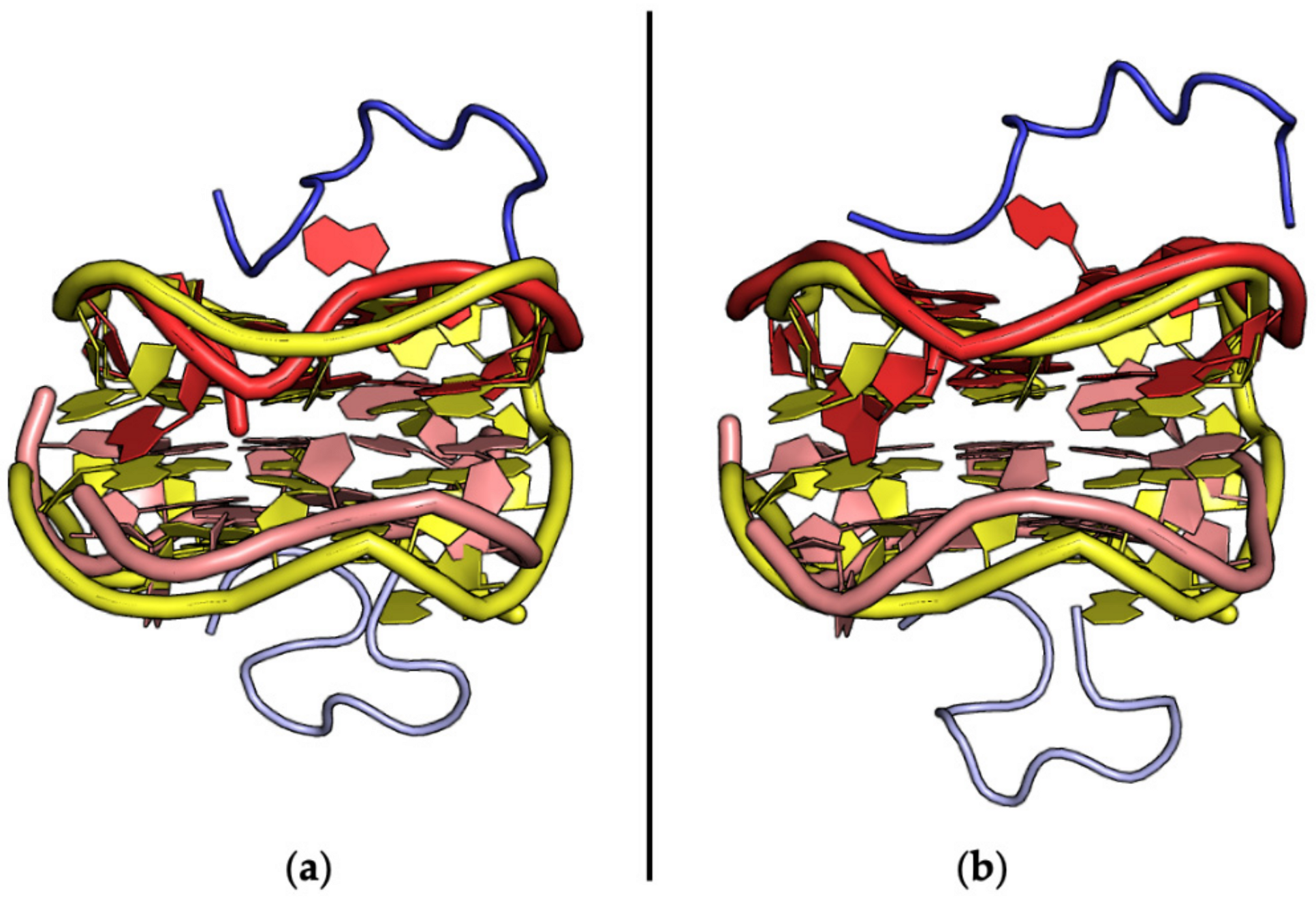
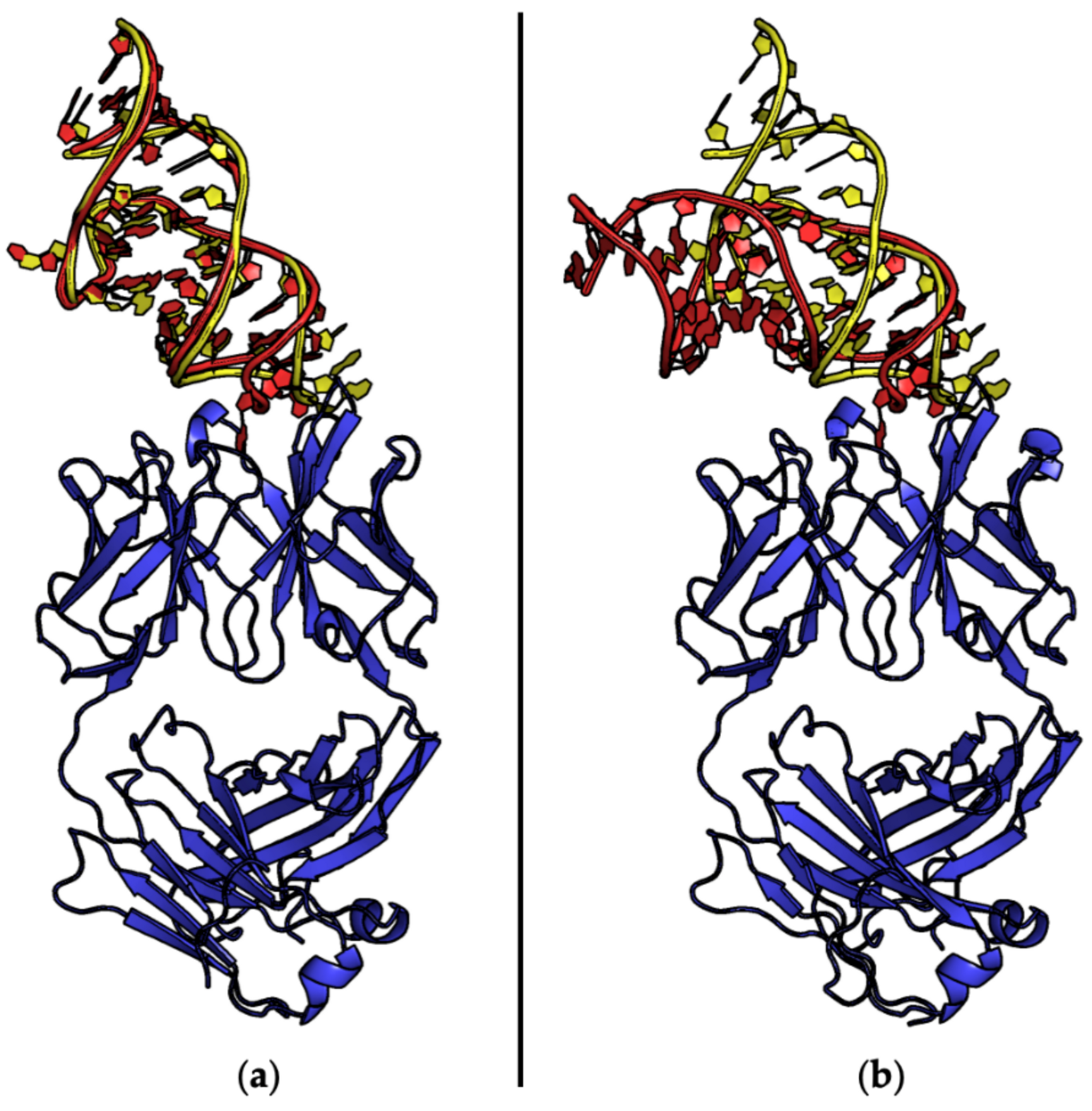
| Entry | PDB ID | Aptamer | Interacting Protein or Peptide | Protein–Aptamer Stoichiometry | Interface Area (Å2)/H-Bonds | Ref. | |
|---|---|---|---|---|---|---|---|
| Name | Type/ Length (nt) | ||||||
| 1 | 1HUT | TBA | DNA/15 | Thrombin (exosite I) | 1ˆ:1 | 500.6/8 | [36] |
| 2 | 1HAO | 1ˆ:1 | 546.6/5 | [43] | |||
| 3 | 1HAP | 1ˆ:1 | 494.6/1 | ||||
| 4 | 1ULL | 35-mer RRE RNA aptamer I | RNA/35 | HIV-1 Rev peptide | 1:1 | 1167.3/25 | [44] |
| 5 | 6MSF | F6 | RNA/14 | MS2 coat protein | Large assembly | 346.8/6 | [45] |
| 6 | 5MSF | F5 | RNA/18 | Large assembly | 416.2/8 | [46] | |
| 7 | 7MSF | F7 | RNA/14 | Large assembly | 400.0/8 | ||
| 8 | 484D | 27-mer RNA aptamer II | RNA/27 | HIV-1 Rev peptide | 1:1 | 1293.1/13 | [47] |
| 9 | 1EXD | tRNAGln var-AGGU | RNA/73 | Glutaminyl-tRNA synthetase | 1:1 | 2599.3/42 | [48] |
| 10 | 1EXY | 33-mer RNA aptamer | RNA/33 | HTLV-1 Rex peptide | 1:1 | 1124.7/8 | [49] |
| 11 | 1OOA | 29-nt RNA aptamer | RNA/29 | Nuclear factor NF-κB | 2:2 | 958.9/21 | [50] |
| 12 | 1U1Y | F5/2AP10 | RNA §/17 | MS2 coat protein | Large assembly | 403.2/10 | [51] |
| 13 | 2B63 | FC* | RNA §/31 | RNA polymerase II | Large assembly | 1688.1/29 | [52] |
| 14 | 3DD2 | Toggle-25t | RNA §/26 | Thrombin (exosite II) | 1ˆ:1 | 904.6/21 | [53] |
| 15 | 3EGZ | Tetracycline aptamer (riboswitch) | RNA/65 | U1 small nuclear ribonucleoprotein A | 1:1 | 946.3/19 | [54] |
| 16 | 3HXO | ARC1172 | DNA/42 | Von Willebrand factor | 1:1 | 1011.1/27 | [55] |
| 17 | 3HXQ | 1:1 | 1070.8/23 | ||||
| 18 | 3IRW | Vc2 (riboswitch) | RNA §/92 | U1 small nuclear ribonucleoprotein A | 1:1 | 952.4/20 | [56] |
| 19 | 3AGV | anti-Fc | RNA §/24 | Fc fragment of IgG1 | 2:2 | 438.5/6 | [57] |
| 20 | 3HSB | AGr | RNA/7 | RNA-binding protein Hfq | 6:1 | 1419.3/14 | [58] |
| 21 | 3AHU | 6:2 | 1152.8/10 | ||||
| 22 | 3MUM | G20A mutant Vc2 (riboswitch) | RNA §/92 | U1 small nuclear ribonucleoprotein A | 1:1 | 957.0/18 | [59] |
| 23 | 3MUR | C92U mutant Vc2 (riboswitch) | RNA §/92 | 1:1 | 1006.7/19 | ||
| 24 | 3MUT | G20A/C92U mutant Vc2 (riboswitch) | RNA §/92 | 1:1 | 981.2/17 | ||
| 25 | 3MUV | 1:1 | 1007.7/19 | ||||
| 26 | 3MXH | Vc2 (riboswitch) | RNA §/92 | 1:1 | 984.8/16 | ||
| 27 | 3QLP | mTBA | DNA/15 | Thrombin (exosite I) | 1ˆ:1 | 656.6/11 | [60] |
| 28 | 3UCU | Vc2 (riboswitch) | RNA §/92 | U1 small nuclear ribonucleoprotein A | 1:1 | 1055.9/18 | [61] |
| 29 | 3UCZ | 1:1 | 1004.6/17 | ||||
| 30 | 3UD3 | C92U mutant Vc2 (riboswitch) | RNA §/92 | 1:1 | 960.0/15 | ||
| 31 | 3UD4 | 1:1 | 1018.6/14 | ||||
| 32 | 3UZS | C13.28 | RNA/28 | G-protein coupled receptor kinase 2 | 1:1 | 613.5/5 | [62] |
| 33 | 3UZT | C13.18 | RNA/18 | 1:1 | 512.3/5 | ||
| 34 | 3V7E | SAM-I (riboswitch) | RNA/126 | RNA-binding protein YbxF | 1:1 | 435.3/6 | [63] |
| 35 | 4DIH | TBA | DNA/15 | Thrombin (exosite I) | 1ˆ:1 | 565.0/12 | [64] |
| 36 | 4DII | 1ˆ:1 | 542.7/11 | ||||
| 37 | 4HQU | SL5 | DNA §/24 | Platelet-derived growth factor B | 2:2 | 1158.3/8 | [65] |
| 38 | 4HQX | SL4 | DNA §/24 | 2:2 | 1166.5/9 | ||
| 39 | 2RSK | R12 | RNA/12 | P16 peptide from major prion protein | 2:2 | 434.2/2 | [66] |
| 40 | 2RU7 | 2:2 | 466.7/5 | [67] | |||
| 41 | 3ZH2 | 2008s | DNA/35 | Lactate dehydrogenase | 4:2 | 1310.3/21 | [68] |
| 42 | 4I7Y | HD22_27mer | DNA/27 | Thrombin (exosite II) | 1ˆ:1 | 1079.5/18 | [69] |
| 43 | 4M4O | minE | RNA/59 | Lysozyme C | 1:1 | 403.1/6 | [70] |
| 44 | 4M6D | minF | RNA/45 | 1:1 | 439.2/5 | ||
| 45 | 4KZD | Spinach | RNA §/84 | Fab BL3-6 | 1ˆ:1 | 820.5/11 | [71] |
| 46 | 4KZE | 1ˆ:1 | 815.3/11 | ||||
| 47 | 4Q9Q | 1ˆ:1 | 801.3/9 | ||||
| 48 | 4Q9R | 1ˆ:1 | 811.2/9 | ||||
| 49 | 4LZ1 | TBA∆T12 | DNA §/15 | Thrombin (exosite I) | 1ˆ:1 | 533.8/13 | [72] |
| 50 | 4LZ4 | TBA∆T3 | DNA §/15 | 1ˆ:1 | 540.6/11 | ||
| 51 | 4NI7 | SL1025 | DNA §/32 | Interleukin-6 | 1:1 | 1000.8/19 | [73] |
| 52 | 4NI9 | 1:1 | 1118.2/16 | ||||
| 53 | 4PDB | RNA-2 | RNA/38 | 30s ribosomal protein S8 | 1:1 | 898.6/13 | [74] |
| 54 | 4R8I | NOX-E36 | RNA §/40 | Chemokine CCL2 | 1:1 | 714.2/19 | [75] |
| 55 | 4WB2 | NOX-D20 | NA-hybrid §/40 | Complement anaphylatoxin C5a | 1:1 | 922.3/17 | [76] |
| 56 | 4WB3 | 1:1 | 910.0/18 | ||||
| 57 | 4YB1 | G20A mutant Vc2 (riboswitch) | RNA/91 | U1 small nuclear ribonucleoprotein A | 1:1 | 1039.9/18 | [77] |
| 58 | 4ZBN | SL1049 | DNA §/28 | Nerve growth factor | 2:2 | 930.2/11 | [78] |
| 59 | 5D3G | 38NT2,4-methyl | DNA §/38 | HIV-1 reverse transcriptase | 1ˆ:1 | 2189.9/22 | [79] |
| 60 | 5CMX | RE31 | DNA/31 | Thrombin (exosite I) | 1ˆ:1 | 551.7/10 | [80] |
| 61 | 5DO4 | Toggle-25t/AF113-18 | RNA §/25 | Thrombin (exosite II) | 1ˆ:1 | 1024.6/18 | [81] |
| 62 | 5EW1 | TBA∆T3 | DNA §/15 | Thrombin (exosite I) | 1ˆ:2 | 523.7/11 | [82] |
| HD22_27mer | DNA/27 | Thrombin (exosite II) | 1116.2/21 | ||||
| 63 | 5EW2 | TBA∆T12 | DNA §/15 | Thrombin (exosite I) | 1ˆ:2 | 533.6/10 | |
| HD22_27mer | DNA/27 | Thrombin (exosite II) | 1044.2/19 | ||||
| 64 | 5HLF | 38NT2,4-methyl | DNA §/38 | HIV-1 reverse transcriptase | 1ˆ:1 | 2218.7/23 | [83] |
| 65 | 5HP1 | DNA §/39 | 1ˆ:1 | 2333.4/22 | [84] | ||
| 66 | 5HRO | DNA §/38 | 1ˆ:1 | 2149.5/23 | |||
| 67 | 5I3U | 38NT2,4-methyl (variant) | DNA §/39 | 1ˆ:1 | 2362.5/21 | ||
| 68 | 5I42 | 38NT2,4-methyl | DNA §/38 | 1ˆ:1 | 2051.9/24 | ||
| 69 | 5HRT | RB011 | DNA §/34 | Autotaxin ENPP2 | 1:1 | 1139.7/14 | [85] |
| 70 | 5HRU | pL1 | DNA/34 | Lactate dehydrogenase | 4:2 | 1136.8/10 | [86] |
| 71 | 5HTO | 4:2 | 1081.5/7 | ||||
| 72 | 5MJV | - | RNA/6 | Genome polyprotein HPeV-1 | Large assembly | 412.0/10 | [87] |
| 73 | 5UC6 | SL1067 | DNA §/23 | Interleukin-1α | 1:1 | 597.1/7 | [88] |
| 74 | 6B14 | Spinach | RNA/83 | Fab BL3-6 | 1ˆ:1 | 821.2/12 | [89] |
| 75 | 6B3K | 1ˆ:1 | 826.1/12 | ||||
| 76 | 6EO6 | TBA-T4W | DNA §/15 | Thrombin (exosite I) | 1ˆ:1 | 707.6/14 | [90] |
| 77 | 6EO7 | TBA-T4K | DNA §/15 | 1ˆ:1 | 640.6/13 | ||
| 78 | 5VOE | 11F7t | RNA §/36 | Coagulation factor Xa | 1ˆ:1 | 730.9/12 | [91] |
| 79 | 5VOF | 1ˆ:1 | 710.2/13 | ||||
| 80 | 5XN0 | 38NT2,4-methyl (variant) | DNA §/38 | HIV-1 reverse transcriptase | 1ˆ:1 | 2073.1/21 | [92] |
| 81 | 5XN1 | 1ˆ:1 | 2061.2/24 | ||||
| 82 | 5XN2 | 1ˆ:1 | 2017.1/23 | ||||
| 83 | 6BHJ | 38NT2,4-methyl (variant) | NA-hybrid/ 38 | 1ˆ:1 | 2137.1/25 | [93] | |
| 84 | 6CF2 | 35-mer RRE RNA aptamer I | RNA/35 | HIV-1 Rev protein | 1:1 | 819.4/20 | [94] |
| 85 | 6DB8 | DIR2s | RNA/60 | Fab BL3-6 | 1ˆ:1 | 876.4/8 | [95] |
| 86 | 6DB9 | 1ˆ:1 | 849.1/13 | ||||
| 87 | 6EVV | NU172 | DNA/26 | Thrombin (exosite I) | 1ˆ:1 | 589.8/10 | [96] |
| 88 | 6GN7 | 1ˆ:1 | 588.6/8 | ||||
| 89 | 6RTI | A9g | RNA §/43 | Glutamate carboxypeptidase 2 | 2:2 | 1563.0/28 | [97] |
| 90 | 6SY4 | K1 | RNA/43 | Tet repressor protein | 2:1 | 848.3/7 | [98] |
| 91 | 6SY6 | K2 | RNA/39 | 2:1 | 873.0/8 | ||
| 92 | 6TXR | Cubamer | RNA §/38 | Lactate dehydrogenase | 4:2 | 1218.1/10 | [99] |
| 93 | 6U81 ‡ | - | DNA/ (17 + 20)2 | Double homeobox protein 4 DUX4 | 2:2ˆ | 1725.2/24 | [100] |
| 94 | 6U82 | - | DNA/38 | 1:1 | 2043.6/38 | ||
| 95 | 7D7V | 17delU1A (riboswitch) | RNA/57 | U1 small nuclear ribonucleoprotein A | 1:1 | 918.8/22 | [101] |
| 96 | 7JTN | SL1103 | DNA §/30 | Complement factor B | 1:1 | 844.7/15 | [102] |
| 97 | 7JTQ | SL1102 | DNA §/32 | 1:1 | 838.2/11 | ||
| 98 | 6VUG | 38NT2,4-methyl | DNA §/38 | HIV-1 reverse transcriptase | 1ˆ:1 | 1874.2/12 | [103] |
| 99 | 6Z8V | TBA-3L | DNA §/15 | Thrombin (exosite I) | 1ˆ:1 | 605.8/14 | [104] |
| 100 | 6Z8W | TBA-3G | DNA §/15 | 1ˆ:1 | 602.4/13 | ||
| 101 | 6Z8X | TBA-3Leu | DNA §/15 | 1ˆ:1 | 592.2/9 | ||
| 102 | 7F49 | BT-100 | RNA §/30 | Von Willebrand factor | 1:1 | 1063.3/19 | [105] |
| 103 | 7MK1 | SL1090 | DNA §/41 | Antiviral innate immune response receptor RIG-I | 1:1 | 838.8/14 | [106] |
| 104 | 7NTU | NU172 | DNA/26 | Thrombin (exosite I) | 1ˆ:2 | 591.1/11 | [107] |
| HD22_27mer | DNA/27 | Thrombin (exosite II) | 1095.1/18 | ||||
| 105 | 7OXQ | - | DNA/28 + 21 | HIV-1 reverse transcriptase | 1ˆ:1ˆ | 2459.7/28 | [108] |
| 106 | 7OZ2 | 1ˆ:1ˆ | 2421.2/28 | ||||
| 107 | 7OZ5 | 1ˆ:1ˆ | 2460.2/27 | ||||
| 108 | 7OZW | 38NT2,4-methyl (variant) | DNA §/37 | 1ˆ:1 | 1797.8/21 | ||
| 109 | 7P15 | 1ˆ:1 | 1922.7/20 | ||||
| 110 | 7LRI | 38NT2,4-methyl | DNA §/38 | 1ˆ:1 | 2160.9/21 | [109] | |
| 111 | 7LRM | 38NT2,4-methyl (variant) | DNA §/38 | 1ˆ:1 | 2179.3/22 | ||
| 112 | 7LRX | 1ˆ:1 | 2219.2/25 | ||||
| 113 | 7LRY | 1ˆ:1 | 2189.9/22 | ||||
| 114 | 7LSK | 38NT2,4-methyl | DNA §/38 | 1ˆ:1 | 2089.1/22 | ||
| 115 | 7SZU | Pepper | RNA §/67 | Fab BL3-6 | 1ˆ:1 | 764.7/6 | [110] |
| 116 | 7U0Y | 1ˆ:1 | 774.4/5 | ||||
| 117 | 7V5N | A14#1 | DNA/24 | Fab fragment of bevacizumab | 1ˆ:1 | 1025.1/19 | [111] |
| 118 | 7YQ3 | A43 | DNA §/28 | Insulin receptor | 2:1 | 1272.1/13 | [112] |
| 119 | 7YQ4 | A62 | DNA §/24 | 2:1 | 1177.4/8 | ||
| 120 | 7YQ5 | 2:1 | 1177.4/8 | ||||
| 121 | 7YQ6 | 2:2 | 1188.3/11 | ||||
| 122 | 7Z24 | 38NT2,4-methyl (variant) | DNA §/38 | HIV-1 reverse transcriptase | 1ˆ:1 | 1683.9/20 | [113] |
| 123 | 7Z29 | 38NT2,4-methyl | DNA §/38 | 1ˆ:1 | 1849.2/20 | ||
| 124 | 7Z2D | 38NT2,4-methyl (variant) | DNA §/38 | 1ˆ:1 | 1538.7/15 | ||
| 125 | 7Z2E | 38NT2,4-methyl | DNA §/38 | 1ˆ:1 | 1578.2/19 | ||
| 126 | 7Z2G | 38NT2,4-methyl (variant) | DNA §/38 | 1ˆ:1 | 2040.0/23 | ||
| 127 | 7Z2H | 38NT2,4-methyl | DNA §/38 | 1ˆ:1 | 1882.7/23 | ||
| 128 | 7ZKL | TBA-NNp/DDp | DNA/15 | Thrombin (exosite I) | 1ˆ:1 | 576.0/11 | [114] |
| 129 | 7ZKM | Thrombin (exosite I) | 1ˆ:2 | 583.1/14 | |||
| Thrombin (exosite II) | 595.1/10 | ||||||
| 130 | 7ZKN | Thrombin (exosite I) | 1ˆ:2 | 571.3/12 | |||
| Thrombin (exosite II) | 608.5/11 | ||||||
| 131 | 7ZKO | Thrombin (exosite I) | 1ˆ:1/1ˆ:2 | 579.1/9 | |||
| Thrombin (exosite II) | 597.0/14 | ||||||
| 132 | 7ZQS | tJBA8.1 | DNA/51 | Transferrin receptor protein 1 | 2:2 | 607.6/10 | [115] |
| 133 | 8D29 | Theophylline aptamer | RNA/34 | Fab BL3-6 | 1ˆ:1 | 796.9/11 | [116] |
| 134 | 8DK7 | 1ˆ:1 | 797.6/8 | ||||
| 135 | 8BW5 | M08s-1_41mer | DNA/41 | Thrombin (exosite I) | 1ˆ:1 | 585.1/13 | [117] |
| 136 | 8F3C | (riboswitch) | RNA/47 | DNA-directed RNA polymerase | Large assembly | 1661.1/23 | [118] |
| 137 | 8G00 | Large assembly | 1661.1/23 | ||||
| 138 | 8G1S | Large assembly | 1809.6/25 | ||||
| 139 | 8G2W | Large assembly | 1695.8/19 | ||||
| 140 | 8G4W | Large assembly | 2187.8/37 | ||||
| 141 | 8G7E | Large assembly | 2009.4/22 | ||||
| 142 | 8G8Z | (riboswitch) | RNA/48 | Large assembly | 2175.2/23 | ||
| 143 | 8J1Q | AM032-0 | DNA §/80 | Spike protein S1 (ACE2-binding site) | 1:2 | 857.5/6 | [119] |
| AM047-0 | DNA §/52 | Spike protein S1 (distal site) | 748.6/4 | ||||
| 144 | 8J26 | AM032-4 | DNA §/44 | Spike protein S1 (ACE2-binding site) | 1:2 | 867.5/8 | |
| AM047-6 | DNA §/48 | Spike protein S1 (distal site) | 741.0/4 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Troisi, R.; Balasco, N.; Autiero, I.; Vitagliano, L.; Sica, F. Structural Insights into Protein–Aptamer Recognitions Emerged from Experimental and Computational Studies. Int. J. Mol. Sci. 2023, 24, 16318. https://doi.org/10.3390/ijms242216318
Troisi R, Balasco N, Autiero I, Vitagliano L, Sica F. Structural Insights into Protein–Aptamer Recognitions Emerged from Experimental and Computational Studies. International Journal of Molecular Sciences. 2023; 24(22):16318. https://doi.org/10.3390/ijms242216318
Chicago/Turabian StyleTroisi, Romualdo, Nicole Balasco, Ida Autiero, Luigi Vitagliano, and Filomena Sica. 2023. "Structural Insights into Protein–Aptamer Recognitions Emerged from Experimental and Computational Studies" International Journal of Molecular Sciences 24, no. 22: 16318. https://doi.org/10.3390/ijms242216318
APA StyleTroisi, R., Balasco, N., Autiero, I., Vitagliano, L., & Sica, F. (2023). Structural Insights into Protein–Aptamer Recognitions Emerged from Experimental and Computational Studies. International Journal of Molecular Sciences, 24(22), 16318. https://doi.org/10.3390/ijms242216318









