Tff3 Deficiency Differentially Affects the Morphology of Male and Female Intestines in a Long-Term High-Fat-Diet-Fed Mouse Model
Abstract
:1. Introduction
2. Results
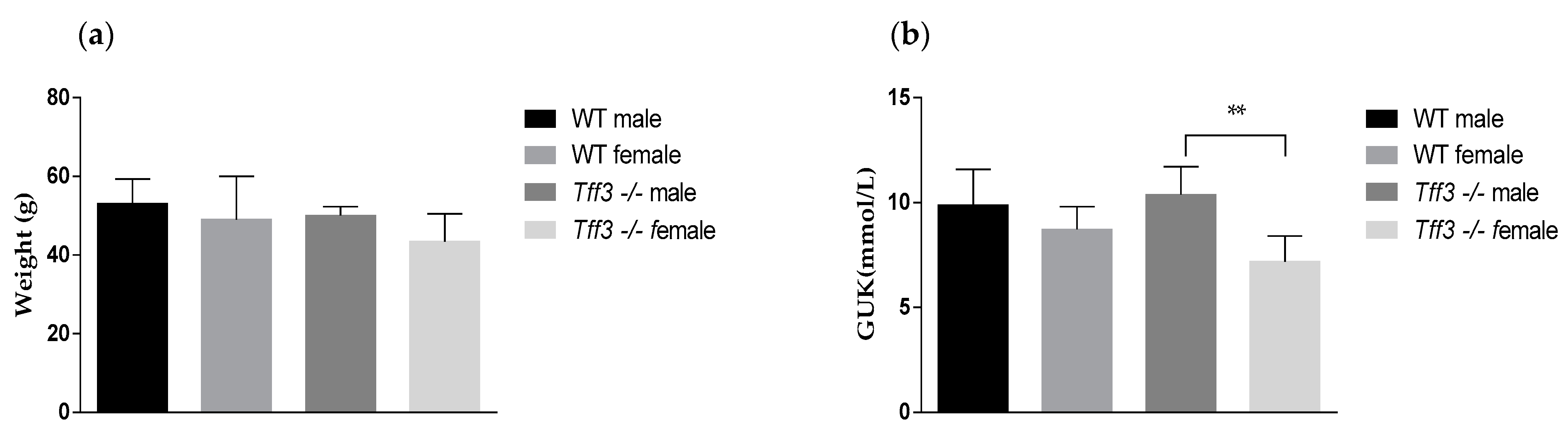
2.1. Body Weight and Glucose Level
2.2. Tff3 Deficiency Affects Intestinal Morphology in the HFD Model
2.3. SCFA Content as an Indicator of the Microbiome
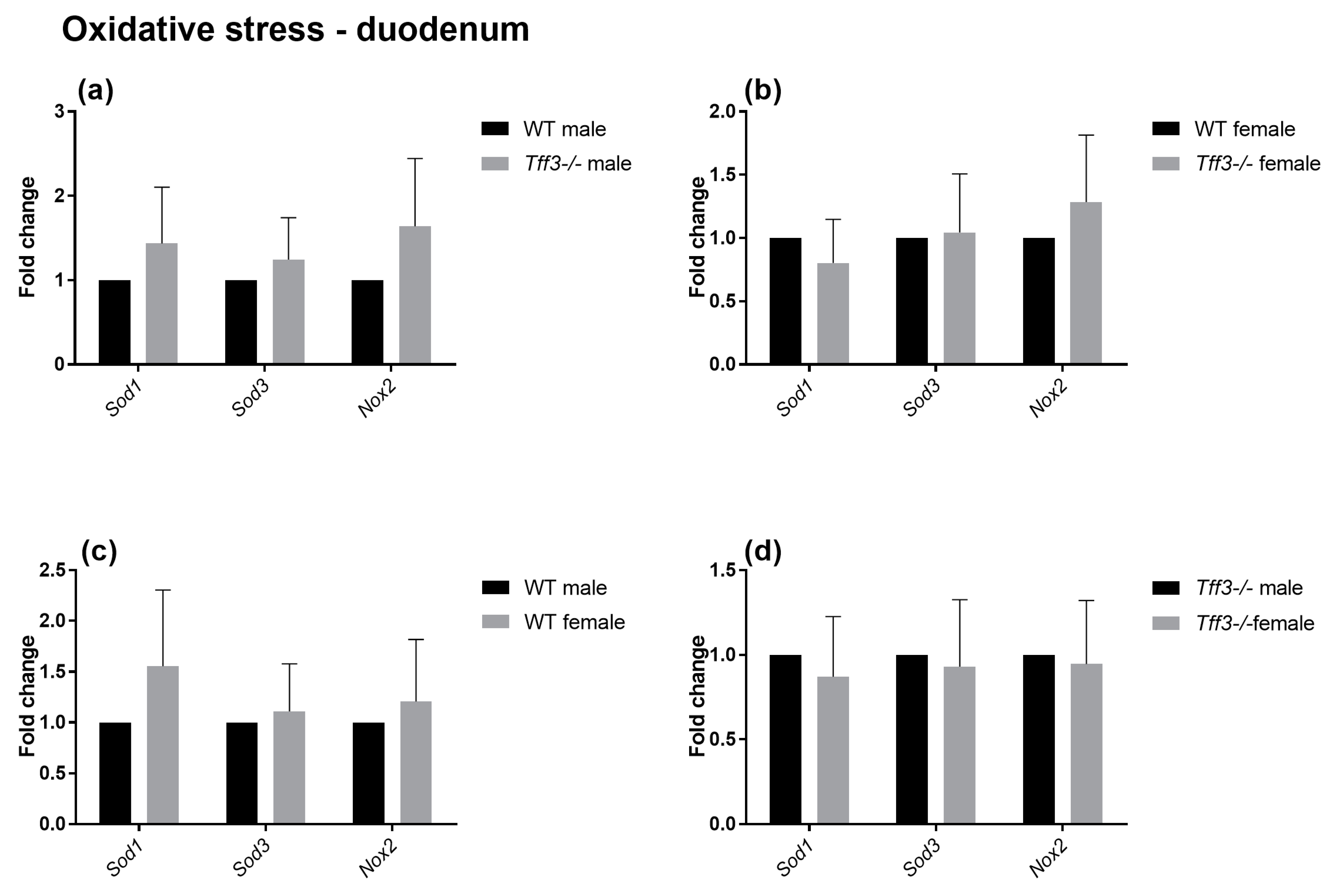
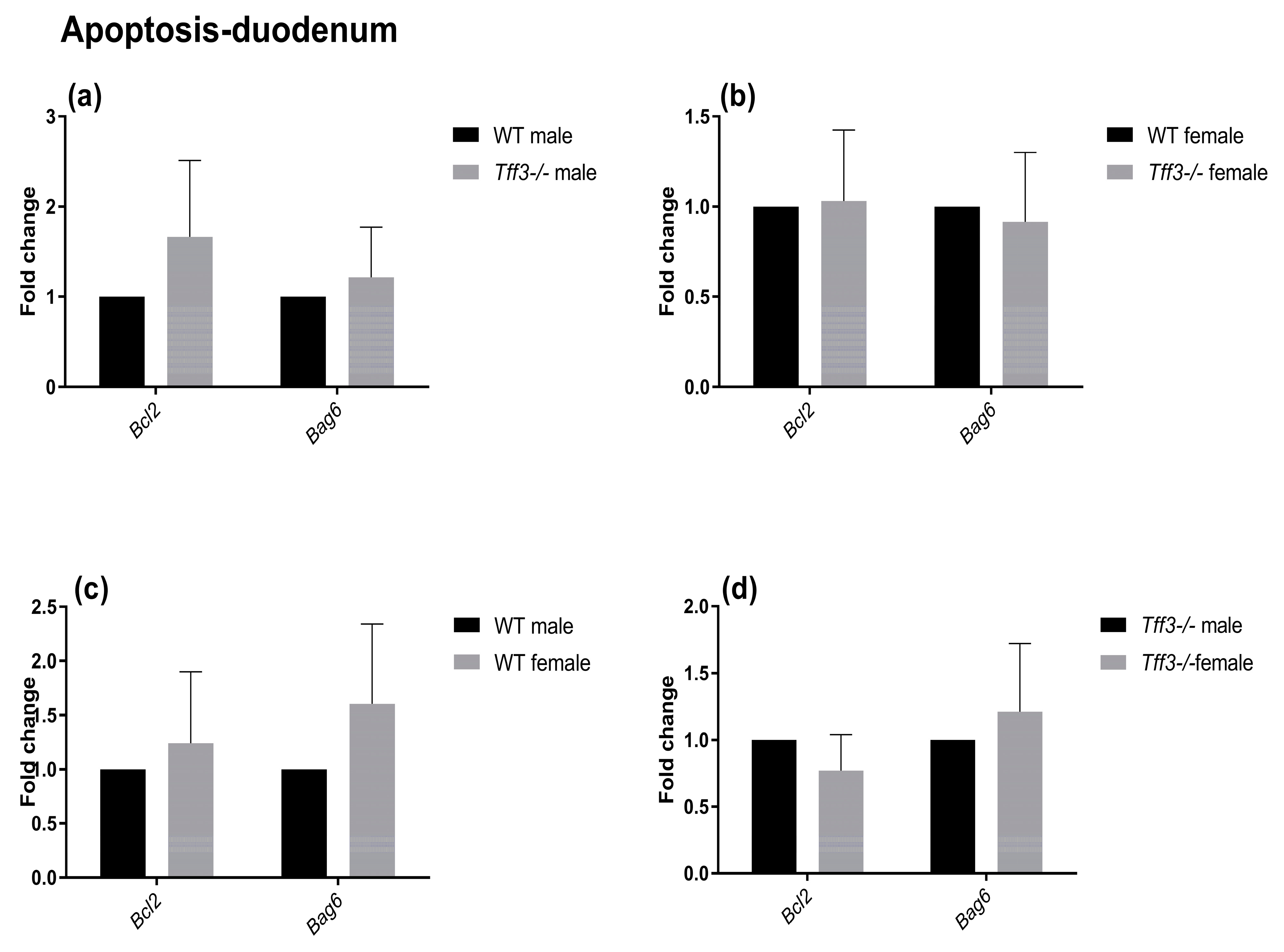
2.4. Expression of Genes Involved in Disease-Relevant Pathways
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Diet
4.3. Chemical Composition of the Diet
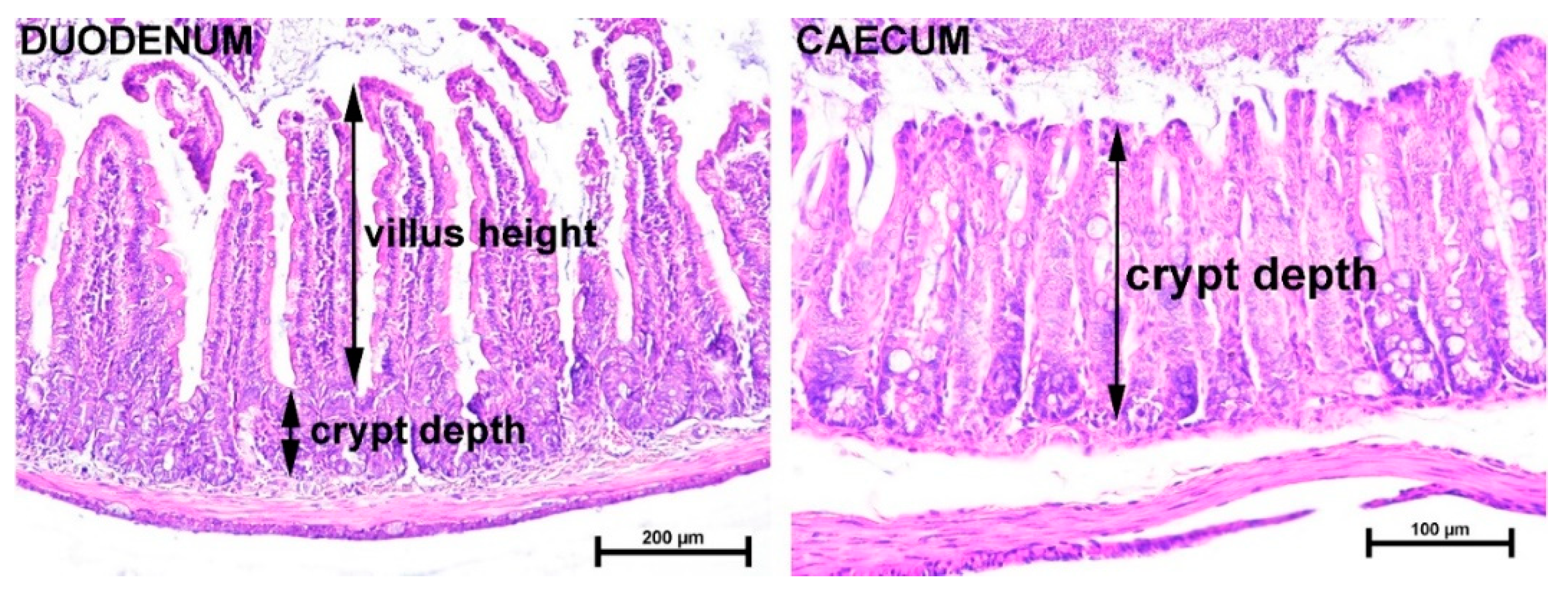
4.4. Tissue Sampling, Staining, and Histological Measurements of Intestinal Tissues
4.5. SCFA Analyses
4.6. Q-PCR Analysis
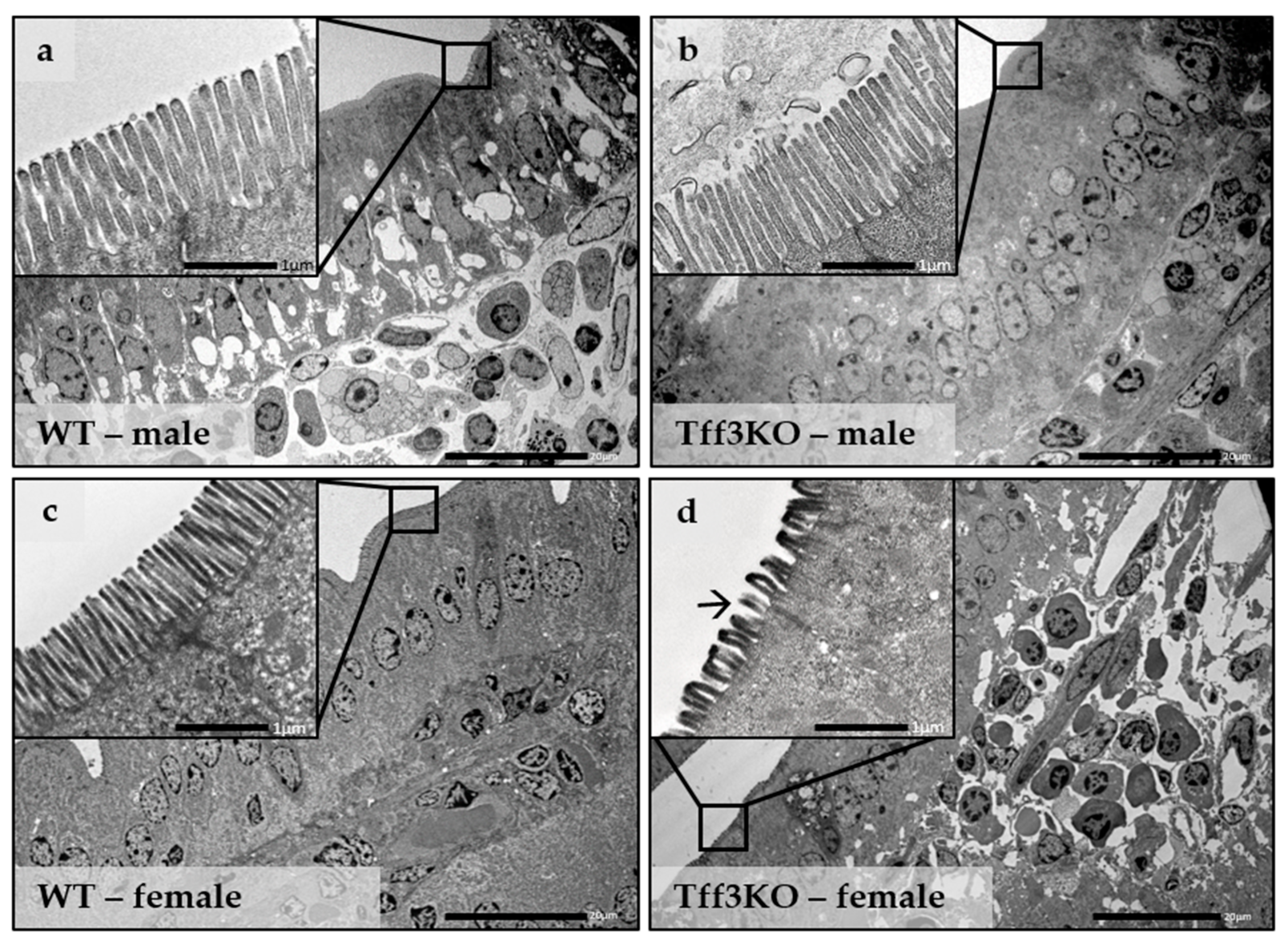
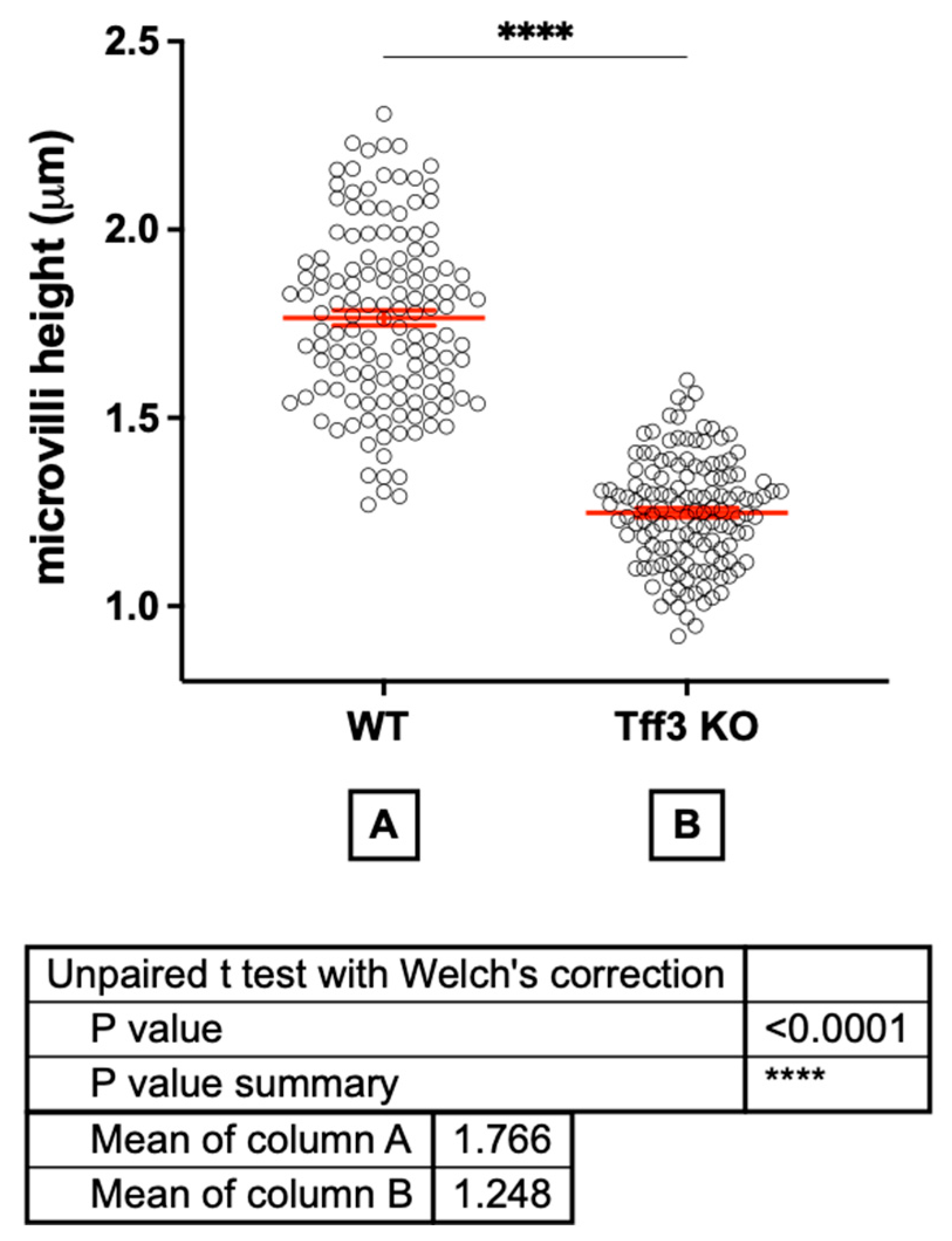
4.7. Ultrastructure
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ARHGAP17 | Rho GTPase Activating Protein 17 |
| ATF6 | Activating transcription factor 6 |
| ATF4 | Activating transcription factor 4 |
| BB | Brush border |
| BiP | HSP70 molecular chaperone located in the lumen of the ER |
| CHOP | DNA damage-inducible transcript 3 |
| CNS | Central nervous system |
| CRP-ductin | C reactive protein ductin |
| CXCR4 | C-X-C chemokine receptor type 4 |
| CXCR7 | Seven transmembrane-spanning receptor |
| DMBT1gp340 | gp-340/deleted in malignant brain tumors 1 |
| DSS | Dextran sulphate sodium |
| EDEM1 | ER degradation enhancing alpha-mannosidase like protein 1 |
| EIF2AK3/PERK | Eukaryotic translation initiation factor 2 alpha kinase 3 |
| ER | Endoplasmic reticulum |
| ERAD | ER-associated protein degradation |
| ERN1 | Serine/threonine-protein kinase and endoribonuclease |
| FCGBP | Fc Gamma Binding Protein |
| GLB axis | Gut-liver-brain axis |
| GRP94 | Heat shock protein 90 beta family member 1 |
| HE | Hematoxylin and eosin |
| HFD | High-fat diet |
| IBD | Inflammatory bowel diseases |
| IRE1 | Inositol-requiring enzyme 1 |
| ITO buffer | Indium tio oxide buffer |
| LINGO2 | Leucine Rich Repeat And Ig Domain Containing 2 |
| LPS | Lipopolysaccharide |
| LSM | Least square means |
| NADPH | Reduced nicotinamide adenine dinucleotide phosphate |
| Nnt gene | Nicotinamide nucleotide transhydrogenase gene |
| qPCR | Quantitative PCR |
| QTL | Quantitative trait locus |
| PAR2 | Proteinase-activated receptor 2 |
| PBS | Phosphate-buffered saline |
| PERK | Pancreatic EIF2-α kinase |
| PFA | Paraformaldehyde |
| Rho-family GTPases | Member of the Ras superfamily of small GTPases |
| SCFA | Short chain fatty acids |
| SD | Standard diet |
| TEM | Transmission electron microscopy |
| Tff3 | Trefoil factor family protein 3 |
| Tff3-FCGBP | Fc Fragment of IgG Binding Protein |
| sXBP1 | Spliced X-box binding protein 1 |
| TLR2 | Toll-like receptor 2 |
| UPR | Unfolded protein response |
| Wt | Wild type |
References
- Thim, L.; May, F.E.B. Structure of mammalian trefoil factors and functional insights. Cell. Mol. Life Sci. 2005, 62, 2956–2973. [Google Scholar] [CrossRef]
- Braga Emidio, N.; Brierley, S.M.; Schroeder, C.I.; Muttenthaler, M. Structure, Function, and Therapeutic Potential of the Trefoil Factor Family in the Gastrointestinal Tract. ACS Pharmacol. Transl. Sci. 2020, 3, 583–597. [Google Scholar] [CrossRef]
- Hoffman, W.; Jagla, W.; Wiede, A. Molecular medicine of TFF-peptides: From gut to brain. Histol. Histopathol. 2001, 16, 319–334. [Google Scholar] [CrossRef]
- Madsen, J.; Nielsen, O.; Tornøe, I.; Thim, L.; Holmskov, U. Tissue localization of human trefoil factors 1, 2, and 3. J. Histochem. Cytochem. 2007, 55, 505–513. [Google Scholar] [CrossRef]
- Baus-Loncar, M.; Schmid, J.; Lalani, E.; Goodlad, R.A.; Stamp, G.W.H.; Blin, N. Trefoil Factor 2 (Tff2) Deficiency in Murine Digestive Tract Influences the Immune System. Cell Physiol. Biochem. 2005, 16, 31–42. [Google Scholar] [CrossRef]
- Hoffmann, W. Trefoil factor family (Tff) peptides and their links to inflammation: A re-evaluation and new medical perspectives. Int. J. Mol. Sci. 2021, 22, 4909. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Wang, L.; Song, H. The expression and role of trefoil factors in human tumors. Transl. Cancer Res. 2019, 8, 1609–1617. [Google Scholar] [CrossRef]
- Westley, B.R.; Griffin, S.M.; May, F.E.B. Interaction between TFF1, a gastric tumor suppressor trefoil protein, and TFIZ1, a brichos domain-containing protein with homology to SP-C. Biochemistry 2005, 44, 7967–7975. [Google Scholar] [CrossRef]
- Albert, T.K.; Laubinger, W.; Müller, S.; Hanisch, F.G.; Kalinski, T.; Meyer, F.; Hoffmann, W. Human intestinal TFF3 forms disulfide-linked heteromers with the mucus-associated FCGBP protein and is released by hydrogen sulfide. J. Proteome Res. 2010, 9, 3108–3117. [Google Scholar] [CrossRef]
- Houben, T.; Harder, S.; Schlüter, H.; Kalbacher, H.; Hoffmann, W. Different forms of TFF3 in the human saliva: Heterodimerization with IgG Fc binding protein (FCGBP). Int. J. Mol. Sci. 2019, 20, 5000. [Google Scholar] [CrossRef]
- Heuer, J.; Heuer, F.; Stürmer, R.; Harder, S.; Schlüter, H.; Emidio, N.B.; Muttenthaler, M.; Jechorek, D.; Meyer, F.; Hoffmann, W. The tumor suppressor TFF1 occurs in different forms and interacts with multiple partners in the human gastric mucus barrier: Indications for diverse protective Functions. Int. J. Mol. Sci. 2020, 21, 2508. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, W. Trefoil factor family (TFF) peptides and their diverse molecular functions in mucus barrier protection and more: Changing the paradigm. Int. J. Mol. Sci. 2020, 21, 4535. [Google Scholar] [CrossRef] [PubMed]
- Cook, G.A.; Familari, M.; Thim, L.; Giraud, A.S. The trefoil peptides TFF2 and TFF3 are expressed in rat lymphoid tissues and participate in the immune response. FEBS Lett. 1999, 456, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.C.; Olver, W.I.; Donnelly, C.J.; May, M.E.; Naggert, J.K.; Shaffer, D.J.; Roopenian, D.C. Searching QTL by gene expression: Analysis of diabesity. BMC Genet. 2005, 6, 12. [Google Scholar] [CrossRef] [PubMed]
- Ge, H.; Gardner, J.; Wu, X.; Rulifson, I.; Wang, J.; Xiong, Y.; Ye, J.; Belouski, E.; Cao, P.; Tang, J.; et al. Trefoil factor 3 (TFF3) is regulated by food intake, improves glucose tolerance and induces mucinous metaplasia. PLoS ONE 2015, 10, e0126924. [Google Scholar] [CrossRef]
- Mashimo, H.; Wu, D.C.; Podolsky, D.K.; Fishman, M.C. Impaired defense of intestinal mucosa in mice lacking intestinal trefoil factor. Science 1996, 274, 262–265. [Google Scholar] [CrossRef]
- Beck, P.L.; Wong, J.F.; Li, Y.; Swaminathan, S.; Xavier, R.J.; Devaney, K.L.; Podolsky, D.K. Chemotherapy- and Radiotherapy-Induced Intestinal Damage Is Regulated by Intestinal Trefoil Factor. Gastroenterology 2004, 126, 796–808. [Google Scholar] [CrossRef] [PubMed]
- Bujak, M.; Bujak, I.T.; Sobočanec, S.; Mihalj, M.; Novak, S.; Cosić, A.; Levak, M.T.; Kopačin, V.; Mihaljević, B.; Balog, T.; et al. Trefoil Factor 3 Deficiency Affects Liver Lipid Metabolism. Cell. Physiol. Biochem. 2018, 47, 827–841. [Google Scholar] [CrossRef]
- Montagutelli, X. Effect of the genetic background on the phenotype of mouse mutations. J. Am. Soc. Nephrol. 2000, 11, 101–105. [Google Scholar] [CrossRef]
- Freeman, H.C.; Hugill, A.; Dear, N.T.; Ashcroft, F.M.; Cox, R.D. Deletion of nicotinamide nucleotide transhydrogenase: A new quantitive trait locus accounting for glucose intolerance in C57BL/6J mice. Diabetes 2006, 55, 2153–2156. [Google Scholar] [CrossRef]
- Shimomura, K.; Galvanovskis, J.; Goldsworthy, M.; Hugill, A.; Teboul, L.; Ashcroft, F.; Cox, R.D. Europe PMC Funders Group Insulin secretion from beta cells is affected by deletion of Nicotinamide Nucleotide Transhydrogenase. Methods Enzymol. 2018, 6879, 451–480. [Google Scholar] [CrossRef]
- Ripoll, V.M.; Meadows, N.A.; Bangert, M.; Lee, A.W.; Kadioglu, A.; Cox, R.D. Nicotinamide nucleotide transhydrogenase (NNT) acts as a novel modulator of macrophage inflammatory responses. FASEB J. 2012, 26, 3550–3562. [Google Scholar] [CrossRef] [PubMed]
- Sigmund, C.D. Viewpoint: Are studies in genetically altered mice out of control? Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1425–1429. [Google Scholar] [CrossRef] [PubMed]
- Šešelja, K.; Bazina, I.; Welss, J.; Schicht, M.; Paulsen, F.; Bijelić, N.; Roðak, E.; Horvatić, A.; Gelemanović, A.; Mihalj, M.; et al. Effect of Tff3 deficiency and ER stress in the liver. Int. J. Mol. Sci. 2019, 20, 4389. [Google Scholar] [CrossRef] [PubMed]
- May, F.E.B.; Church, S.T.; Major, S.; Westley, B.R. The closely related estrogen-regulated trefoil proteins TFF1 and TFF3 have markedly different hydrodynamic properties, overall charge, and distribution of surface charge. Biochemistry 2003, 42, 8250–8259. [Google Scholar] [CrossRef]
- Wali, J.A.; Jarzebska, N.; Raubenheimer, D.; Simpson, S.J.; Rodionov, R.N.; O’Sullivan, J.F. (Cardio-Metabolic Effects of High-Fat Diets and Their Underlying Mechanisms—A Narrative Review. Nutrients 2020, 12, 1505. [Google Scholar] [CrossRef]
- Tan, B.L.; Norhaizan, M.E. Effect of high-fat diets on oxidative stress, cellular inflammatory response and cognitive function. Nutrients 2019, 11, 2579. [Google Scholar] [CrossRef]
- Kim, D.G.; Krenz, A.; Toussaint, L.E.; Maurer, K.J.; Robinson, S.A.; Yan, A.; Torres, L.; Bynoe, M.S. Non-alcoholic fatty liver disease induces signs of Alzheimer’s disease (AD) in wild-type mice and accelerates pathological signs of AD in an AD model. J. Neuroinflamm. 2016, 13, 1. [Google Scholar] [CrossRef]
- Bojková, B.; Winklewski, P.J.; Wszedybyl-Winklewska, M. Dietary fat and cancer—Which is good, which is bad, and the body of evidence. Int. J. Mol. Sci. 2020, 21, 4114. [Google Scholar] [CrossRef]
- Hoffmann, W. TFF2, a MUC6-binding lectin stabilizing the gastric mucus barrier and more (Review). Int. J. Oncol. 2015, 47, 806–816. [Google Scholar] [CrossRef]
- Thim, L.; Mørtz, E. Isolation and characterization of putative trefoil peptide receptors. Regul. Pept. 2000, 90, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Madsen, J.; Sorensen, G.L.; Nielsen, O.; Tornøe, I.; Thim, L.; Fenger, C.; Mollenhauer, J.; Holmskov, U. A Variant Form of the Human Deleted in Malignant Brain Tumor 1 (DMBT1) Gene Shows Increased Expression in Inflammatory Bowel Diseases and Interacts with Dimeric Trefoil Factor 3 (TFF3). PLoS ONE 2013, 8, e64441. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, W. Trefoil factor family (TFF) peptides and chemokine receptors: A promising relationship. J. Med. Chem. 2009, 52, 6505–6510. [Google Scholar] [CrossRef] [PubMed]
- Dieckow, J.; Brandt, W.; Hattermann, K.; Schob, S.; Schulze, U.; Mentlein, R.; Ackermann, P.; Sel, S.; Paulsen, F.P. CXCR4 and CXCR7 mediate TFF3-induced cell migration independently from the ERK1/2 signaling pathway. Investig. Ophthalmol. Vis. Sci. 2016, 57, 56–65. [Google Scholar] [CrossRef]
- Barrera Roa, G.J.; Sanchez Tortolero, G. Trefoil factor 3 (TFF3) from human breast milk activates PAR-2 receptors, of the intestinal epithelial cells HT-29, regulating cytokines and defensins. Bratislava Med. J. 2016, 117, 332–339. [Google Scholar] [CrossRef]
- Belle, N.M.; Ji, Y.; Herbine, K.; Wei, Y.; Park, J.; Zullo, K.; Hung, L.Y.; Srivatsa, S.; Young, T.; Oniskey, T.; et al. TFF3 interacts with LINGO2 to regulate EGFR activation for protection against colitis and gastrointestinal helminths. Nat. Commun. 2019, 10, 4408. [Google Scholar] [CrossRef]
- Loncar, M.B.; Al-Azzeh, E.D.; Sommer, P.S.M.; Marinovic, M.; Schmehl, K.; Kruschewski, M.; Blin, N.; Stohwasser, R.; Gött, P.; Kayademir, T. Tumour necrosis factor α and nuclear factor κB inhibit transcription of human TFF3 encoding a gastrointestinal healing peptide. Gut 2003, 52, 1297–1303. [Google Scholar] [CrossRef]
- Baus-Loncar, M.; Al-azzeh, E.D.; Romanska, H.; Lalani, E.N.; Stamp, G.W.H.; Blin, N.; Kayademir, T. Transcriptional control of TFF3 (intestinal trefoil factor) via promoter binding sites for the nuclear factor κB and C/EBPβ. Peptides 2004, 25, 849–854. [Google Scholar] [CrossRef]
- Xue, Y.; Shen, L.; Cui, Y.; Zhang, H.; Chen, Q.; Cui, A.; Fang, F.; Chang, Y. Tff3, as a Novel Peptide, Regulates Hepatic Glucose Metabolism. PLoS ONE 2013, 8, e0075240. [Google Scholar] [CrossRef]
- Binayi, F.; Moslemi, M.; Khodagholi, F.; Hedayati, M.; Zardooz, H. Long-term high-fat diet disrupts lipid metabolism and causes inflammation in adult male rats: Possible intervention of endoplasmic reticulum stress. Arch. Physiol. Biochem. 2020, 129, 204–212. [Google Scholar] [CrossRef]
- Gulhane, M.; Murray, L.; Lourie, R.; Tong, H.; Sheng, Y.H.; Wang, R.; Kang, A.; Schreiber, V.; Wong, K.Y.; Magor, G.; et al. High Fat Diets Induce Colonic Epithelial Cell Stress and Inflammation that is Reversed by IL-22. Sci. Rep. 2016, 6, 28990. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kim, H.; Kim, K.; Lee, H.; Lee, S.; Lee, D. Arhgap17, a RhoGTPase activating protein, regulates mucosal and epithelial barrier function in the mouse colon. Sci. Rep. 2016, 6, 26923. [Google Scholar] [CrossRef]
- Podolsky, D.K.; Gerken, G.; Eyking, A.; Cario, E. Colitis-Associated Variant of TLR2 Causes Impaired Mucosal Repair Because of TFF3 Deficiency. Gastroenterology 2009, 137, 209–220. [Google Scholar] [CrossRef]
- Marchbank, T.; Cox, H.M.; Goodlad, R.A.; Giraud, A.S.; Moss, S.F.; Poulsom, R.; Wright, N.A.; Jankowski, J.; Playford, R.J. Effect of Ectopic Expression of Rat Trefoil Factor Family 3 (Intestinal Trefoil Factor) in the Jejunum of Transgenic Mice. J. Biol. Chem. 2001, 276, 24088–24096. [Google Scholar] [CrossRef] [PubMed]
- Clara, R.; Schumacher, M.; Ramachandran, D.; Fedele, S.; Krieger, J.P.; Langhans, W.; Mansouri, A. Metabolic Adaptation of the Small Intestine to Short- and Medium-Term High-Fat Diet Exposure. J. Cell. Physiol. 2017, 232, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Šešelja, K.; Bazina, I.; Vrecl, M.; Welss, J.; Schischt, M.; Mihalj, M.; Kopačin, V.; Paulsen, F.; Pirman, T.; Baus Lončar, M. Tff3 -/-/C57B16N mice are protected from hepatic fat accumulation after a prolonged high-fat diet. Life 2022, 12, 1288. [Google Scholar] [CrossRef] [PubMed]
- Geyra, A.; Uni, Z.; Sklan, D. Enterocyte dynamics and mucosal development in the posthatch chick. Poult. Sci. 2001, 80, 776–782. [Google Scholar] [CrossRef]
- Willing, B.P.; Van Kessel, A.G. Enterocyte proliferation and apoptosis in the caudal small intestine is influenced by the composition of colonizing commensal bacteria in the neonatal gnotobiotic pig. J. Anim. Sci. 2007, 85, 3256–3266. [Google Scholar] [CrossRef]
- Revenu, C.; Athman, R.; Robine, S.; Louvard, D. The co-workers of actin filaments: From cell structures to signals. Nat. Rev. Mol. Cell Biol. 2004, 5, 635–646. [Google Scholar] [CrossRef]
- Crawley, S.W.; Mooseker, M.S.; Tyska, M.J. Shaping the intestinal brush border. J. Cell Biol. 2014, 207, 441–451. [Google Scholar] [CrossRef]
- Delacour, D.; Salomon, J.; Robine, S.; Louvard, D. Plasticity of the brush border-the yin and yang of intestinal homeostasis. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 161–174. [Google Scholar] [CrossRef] [PubMed]
- Pollard, T.D.; Mooseker, M.S. Direct measurement of actin polymerization rate constants by electron microscopy of actin filaments nucleated by isolated microvillus cores. J. Cell Biol. 1981, 88, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Mooseker, M.S.; Tilney, L.G. Organization of an actin filament-membrane complex: Filament polarity and membrane attachment in the microvilli of intestinal epithelial cells. J. Cell Biol. 1975, 67, 725–743. [Google Scholar] [CrossRef] [PubMed]
- Pollard, T.D.; Borisy, G.G. Cellular motility driven by assembly and disassembly of actin filaments. Cell 2003, 112, 453–465. [Google Scholar] [CrossRef]
- Nobes, C.D.; Hall, A. Rho, Rac, and Cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell 1995, 81, 53–62. [Google Scholar] [CrossRef]
- Machesky, L.M.; Hall, A. Role of actin polymerization and adhesion to extracellular matrix in Rac- and Rho-induced cytoskeletal reorganization. J. Cell Biol. 1997, 138, 913–926. [Google Scholar] [CrossRef]
- Timpson, P.; Jones, G.E.; Frame, M.C.; Brunton, V.G. Coordination of cell polarization and migration by the Rho family GTPases requires Src tyrosine kinase activity. Curr. Biol. 2001, 11, 1836–1846. [Google Scholar] [CrossRef]
- Emami, S.; Floch, N.L.; Bruyneel, E.; Thim, L.; May, F.; Westley, B.; Rio, M.; Mareel, M.; Gespach, C. Induction of scattering and cellular invasion by trefoil peptides in src- and RhoA-transformed kidney and colonic epithelial cells. FASEB J. 2001, 15, 351–361. [Google Scholar] [CrossRef]
- Emami, S.; Rodrigues, S.; Rodrigue, C.M.; Le Floch, N.; Rivat, C.; Attoub, S.; Bruyneel, E.; Gespach, C. Trefoil factor family (TFF) peptides and cancer progression. Peptides 2004, 25, 885–898. [Google Scholar] [CrossRef]
- Xu, L.F.; Xu, C.; Mao, Z.Q.; Teng, X.; Ma, L.; Sun, M. Disruption of the F-actin cytoskeleton and monolayer barrier integrity induced by PAF and the protective effect of ITF on intestinal epithelium. Arch. Pharm. Res. 2011, 34, 245–251. [Google Scholar] [CrossRef]
- Wiśniewski, J.R.; Friedrich, A.; Keller, T.; Mann, M.; Koepsell, H. The impact of high-fat diet on metabolism and immune defense in small intestine mucosa. J. Proteome Res. 2015, 14, 353–365. [Google Scholar] [CrossRef] [PubMed]
- Murphy, E.A.; Velazquez, K.T.; Herbert, K.M. Influence of high-fat diet on gut microbiota: A driving force for chronic disease risk. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Donohoe, D.R.; Garge, N.; Zhang, X.; Sun, W.; O’Connell, T.M.; Bunger, M.K.; Bultman, S.J. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab. 2011, 13, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Corrêa-Oliveira, R.; Fachi, J.L.; Vieira, A.; Sato, F.T.; Vinolo, M.A.R. Regulation of immune cell function by short-chain fatty acids. Clin. Transl. Immunol. 2016, 5, 73. [Google Scholar] [CrossRef]
- Venegas, D.P.; De La Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short chain fatty acids (SCFAs)mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef]
- Li, C.; Wang, R.; Su, B.; Luo, Y.; Terhune, J.; Beck, B.; Peatman, E. Evasion of mucosal defenses during Aeromonas hydrophila infection of channel catfish (Ictalurus punctatus) skin. Dev. Comp. Immunol. 2013, 39, 447–455. [Google Scholar] [CrossRef]
- Ron, D.; Walter, P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell Biol. 2007, 8, 519–529. [Google Scholar] [CrossRef]
- Sidrauski, C.; Walter, P. The transmembrane kinase Ire1p is a site-specific endonuclease that initiates mRNA splicing in the unfolded protein response. Cell 1997, 90, 1031–1039. [Google Scholar] [CrossRef]
- Tanaka, N.; Meineke, B.; Shuman, S. RtcB, a novel RNA ligase, can catalyze tRNA splicing and HAC1 mRNA splicing in vivo. J. Biol. Chem. 2011, 286, 30253–30257. [Google Scholar] [CrossRef]
- Oslowski, C.M.; Urano, F. Measuring ER stress and the unfolded protein response using mammalian tissue culture system. In Methods in Enzymology; Academic Press Inc.: Cambridge, MA, USA, 2011; Volume 490, pp. 71–92. [Google Scholar]
- Luhr, M.; Torgersen, M.L.; Szalai, P.; Hashim, A.; Brech, A.; Staerk, J.; Engedal, N. The kinase PERK and the transcription factor ATF4 play distinct and essential roles in autophagy resulting from tunicamycin-induced ER stress. J. Biol. Chem. 2019, 294, 8197–8217. [Google Scholar] [CrossRef]
- Li, Y.; Guo, Y.; Tang, J.; Jiang, J.; Chen, Z. New insights into the roles of CHOP-induced apoptosis in ER stress. Acta Biochim. Biophys. Sin. 2014, 46, 629–640. [Google Scholar] [CrossRef]
- Wang, Z.V.; Deng, Y.; Gao, N.; Pedrozo, Z.; Li, D.L.; Morales, C.R.; Criollo, A.; Luo, X.; Tan, W.; Jiang, N.; et al. Spliced X-box binding protein 1 couples the unfolded protein response to hexosamine biosynthetic pathway. Cell 2014, 156, 1179–1192. [Google Scholar] [CrossRef] [PubMed]
- Cormier, J.H.; Tamura, T.; Sunryd, J.C.; Hebert, D.N. EDEM1 Recognition and Delivery of Misfolded Proteins to the SEL1L-Containing ERAD Complex. Mol. Cell 2009, 34, 627–633. [Google Scholar] [CrossRef] [PubMed]
- Eletto, D.; Dersh, D.; Argon, Y. GRP94 in ER quality control and stress responses. Semin. Cell Dev. Biol. 2010, 21, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.H.; Scapa, E.F.; Cohen, D.E.; Glimcher, L.H. Regulation of hepatic lipogenesis by the transcription factor XBP1. Science 2008, 320, 1492–1496. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Lee, J.; Reno, C.M.; Sun, C.; Park, S.W.; Chung, J.; Lee, J.; Fisher, S.J.; White, M.F.; Biddinger, S.B.; et al. Regulation of glucose homeostasis through a XBP-1-FoxO1 interaction. Nat. Med. 2011, 17, 356–365. [Google Scholar] [CrossRef]
- Eugene, S.P.; Reddy, V.S.; Trinath, J. Endoplasmic Reticulum Stress and Intestinal Inflammation: A Perilous Union. Front. Immunol. 2020, 11, 3061. [Google Scholar] [CrossRef]
- AOAC International. Official Methods of Analysis, 21st ed.; Horwith, W., Ed.; AOAC: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Trebušak, T.; Vrecl Fazarinc, M.; Salobir, J.; Pirman, T. The Effect of Substitution of Palm Fat with Linseed Oil on the Lipid Peroxidation, Antioxidative Capacity and Intestinal Morphology in Rabbits (Oryctolagus cuniculus). Animals 2019, 9, 830. [Google Scholar] [CrossRef]
- Holdeman, L.V.; Moore, W.E.C. Anaerobe Laboratory Manual; Virginia Polytechnic Institute and State University, University of Michigan: Ann Arbor, MI, USA, 1975. [Google Scholar]
- Pirman, T.; Ribeyre, M.C.; Mosoni, L.; Rémond, D.; Vrecl, M.; Salobir, J.; Patureau Mirand, P. Dietary pectin stimulates protein metabolism in the digestive tract. Nutrition 2007, 23, 69–75. [Google Scholar] [CrossRef]


| Intestinal Histology | Wt Male (10) | Tff3-/- Male (10) | Wt Female (10) | Tff3-/- Female (10) |
|---|---|---|---|---|
| Duodenum | ||||
| Villi height (µm) | 420.6 ± 83.2 * | 501.6 ± 31.2 † | 531.7 ± 60.5 | 416.8 ± 33.6 § |
| Villi average diameter (µm) | 255 ± 31 | 278 ± 29 | 292 ± 26 | 249 ± 19 § |
| Villi area (µm2) | 57,321 ± 19,416 | 62,933 ± 1270 | 68,475 ± 1224 | 49,855 ± 7558 § |
| Ratio villi height:diameter | 1.64:1 | 1.81:1 | 1.82:1 | 1.68:1 |
| Crypt depth | 70.5 ± 5.0 | 77.1 ± 1.5 ‡ | 73.3 ± 6.0 | 71.7 ± 7.5 |
| Ratio villi:crypt | 5.9:1 * | 6.5:1 | 7.3:1 | 5.9:1 § |
| Cecum | ||||
| Crypt depth | 166.2 ± 20.2 * | 140.8 ± 12.0 †,‡ | 213.9 ± 26.1 | 206.9 ± 17.1 |
| Short-Chain Fatty Acids | Wt Male (5) | Tff3-/- Male (5) | Wt Female (5) | Tff3-/- Female (5) |
|---|---|---|---|---|
| Cecum | ||||
| Acetic acid | 1.80 ± 0.85 | 1.66 ± 0.46 | 1.99 ± 0.47 | 1.81 ± 0.41 |
| Propionic acid | 0.34 ± 0.12 | 0.38 ± 0.11 | 0.47 ± 0.10 | 0.42 ± 0.09 |
| i-Butyric acid | 0.04 ± 0.03 | 0.04 ± 0.02 | 0.04 ± 0.02 | 0.05 ± 0.01 |
| n-Butyric acid | 0.24 ± 0.15 | 0.29 ± 0.11 | 0.28 ± 0.09 | 0.30 ± 0.12 |
| i-Valerianic acid | 0.05 ± 0.03 | 0.06 ± 0.03 | 0.06 ± 0.02 | 0.06 ± 0.01 |
| n-Valerianic acid | 0.05 ± 0.03 | 0.05 ± 0.02 | 0.06 ± 0.02 | 0.06 ± 0.02 |
| Sum | 2.52 ± 1.13 | 2.49 ± 0.68 | 2.90 ± 0.57 | 2.70 ± 0.61 |
| Ratio AA:PA:BA | 5.33:1:0.80 | 4.77:1:0.90 | 4.31:1:0.68 | 4.33:1:082 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šešelja, K.; Bazina, I.; Vrecl, M.; Farger, J.; Schicht, M.; Paulsen, F.; Baus Lončar, M.; Pirman, T. Tff3 Deficiency Differentially Affects the Morphology of Male and Female Intestines in a Long-Term High-Fat-Diet-Fed Mouse Model. Int. J. Mol. Sci. 2023, 24, 16342. https://doi.org/10.3390/ijms242216342
Šešelja K, Bazina I, Vrecl M, Farger J, Schicht M, Paulsen F, Baus Lončar M, Pirman T. Tff3 Deficiency Differentially Affects the Morphology of Male and Female Intestines in a Long-Term High-Fat-Diet-Fed Mouse Model. International Journal of Molecular Sciences. 2023; 24(22):16342. https://doi.org/10.3390/ijms242216342
Chicago/Turabian StyleŠešelja, Kate, Iva Bazina, Milka Vrecl, Jessica Farger, Martin Schicht, Friedrich Paulsen, Mirela Baus Lončar, and Tatjana Pirman. 2023. "Tff3 Deficiency Differentially Affects the Morphology of Male and Female Intestines in a Long-Term High-Fat-Diet-Fed Mouse Model" International Journal of Molecular Sciences 24, no. 22: 16342. https://doi.org/10.3390/ijms242216342
APA StyleŠešelja, K., Bazina, I., Vrecl, M., Farger, J., Schicht, M., Paulsen, F., Baus Lončar, M., & Pirman, T. (2023). Tff3 Deficiency Differentially Affects the Morphology of Male and Female Intestines in a Long-Term High-Fat-Diet-Fed Mouse Model. International Journal of Molecular Sciences, 24(22), 16342. https://doi.org/10.3390/ijms242216342








