Total Flavonoids in Artemisia absinthium L. and Evaluation of Its Anticancer Activity
Abstract
:1. Introduction
2. Results
2.1. Standard Curve
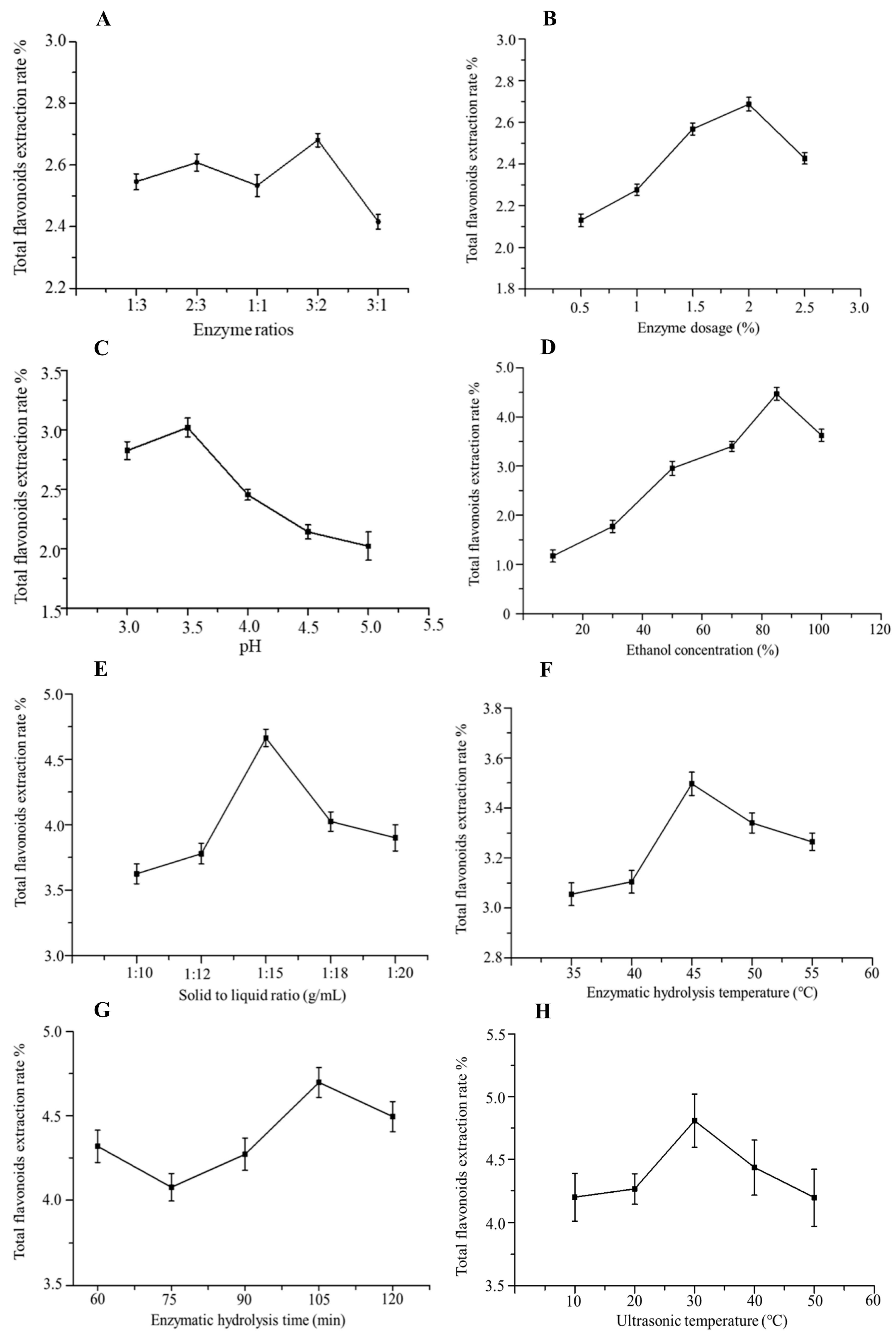
2.2. Single-Factor Experiments
2.2.1. Effect of Compound Enzyme Ratio on the Extraction Rate of Total Flavonoids
2.2.2. Effect of Enzyme Dosage on the Extraction Rate of Total Flavonoids
2.2.3. Effect of pH on the Extraction Rate of Total Flavonoids
2.2.4. Effect of Ethanol Concentration on the Extraction Rate of Total Flavonoids
2.2.5. Effect of Solid–Liquid Ratio on the Extraction Rate of Total Flavonoids
2.2.6. Effect of Enzymatic Hydrolysis Temperature on the Extraction Rate of Total Flavonoids
2.2.7. Effect of Enzymatic Hydrolysis Time on the Extraction Rate of Total Flavonoids
2.2.8. Effect of Ultrasonic Temperature on the Extraction Rate of Total Flavonoids
2.3. Analysis of Extraction Parameters Using Response Surface Method
2.3.1. Response Surface Experimental Design
2.3.2. Response Surface Analysis
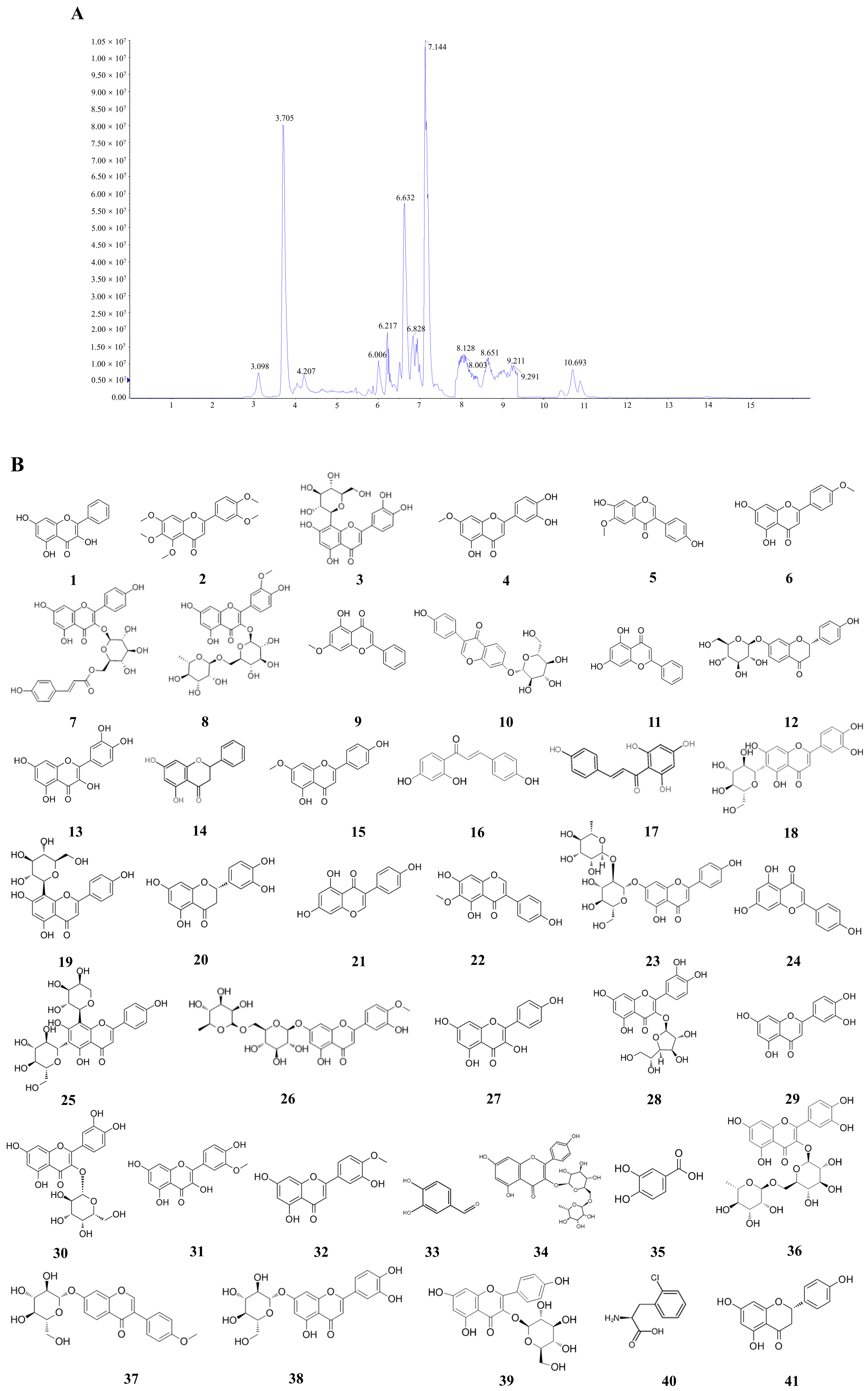
2.4. LC-MS
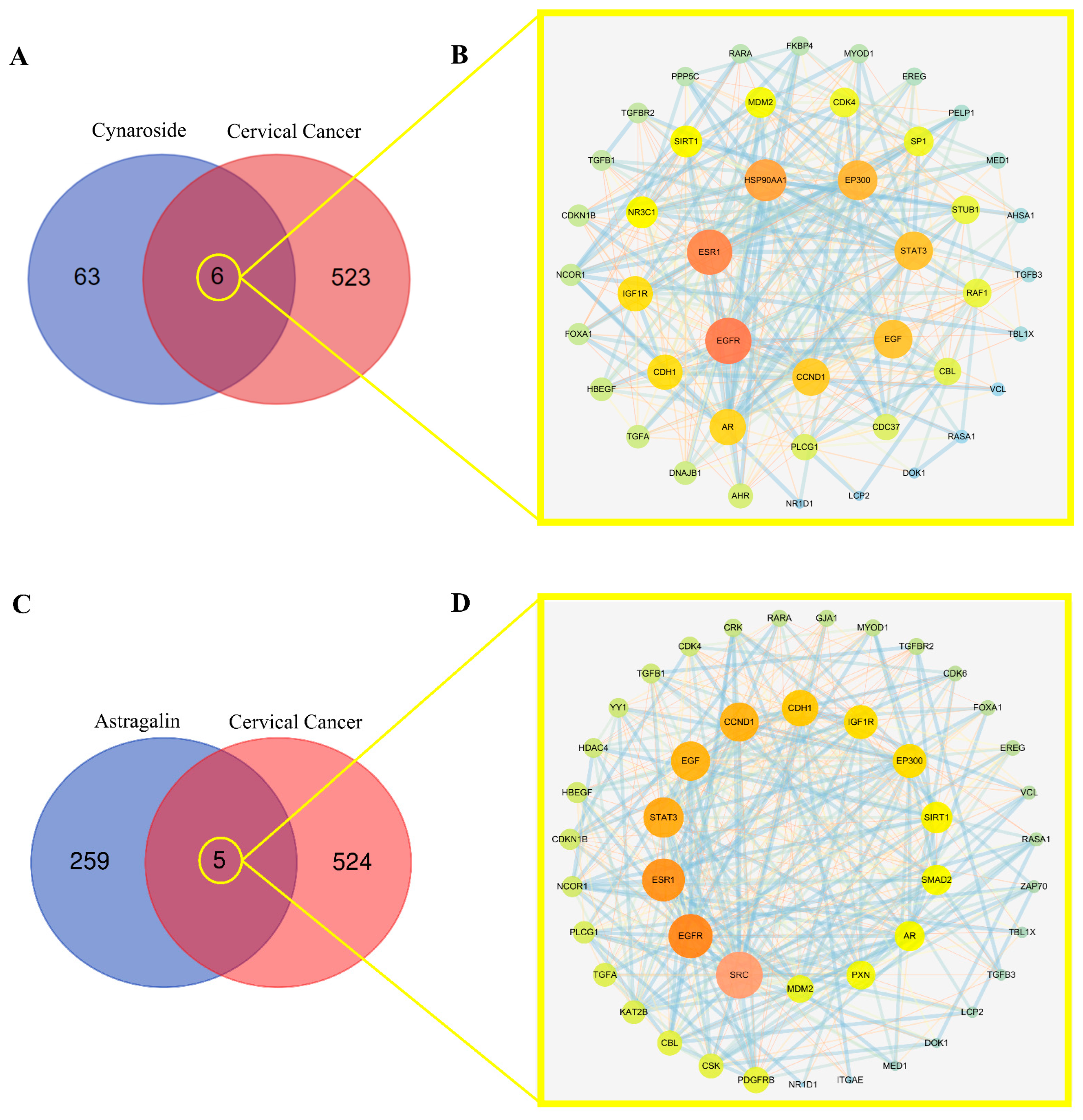
2.5. Screening Mutual Targets of Drugs and Diseases
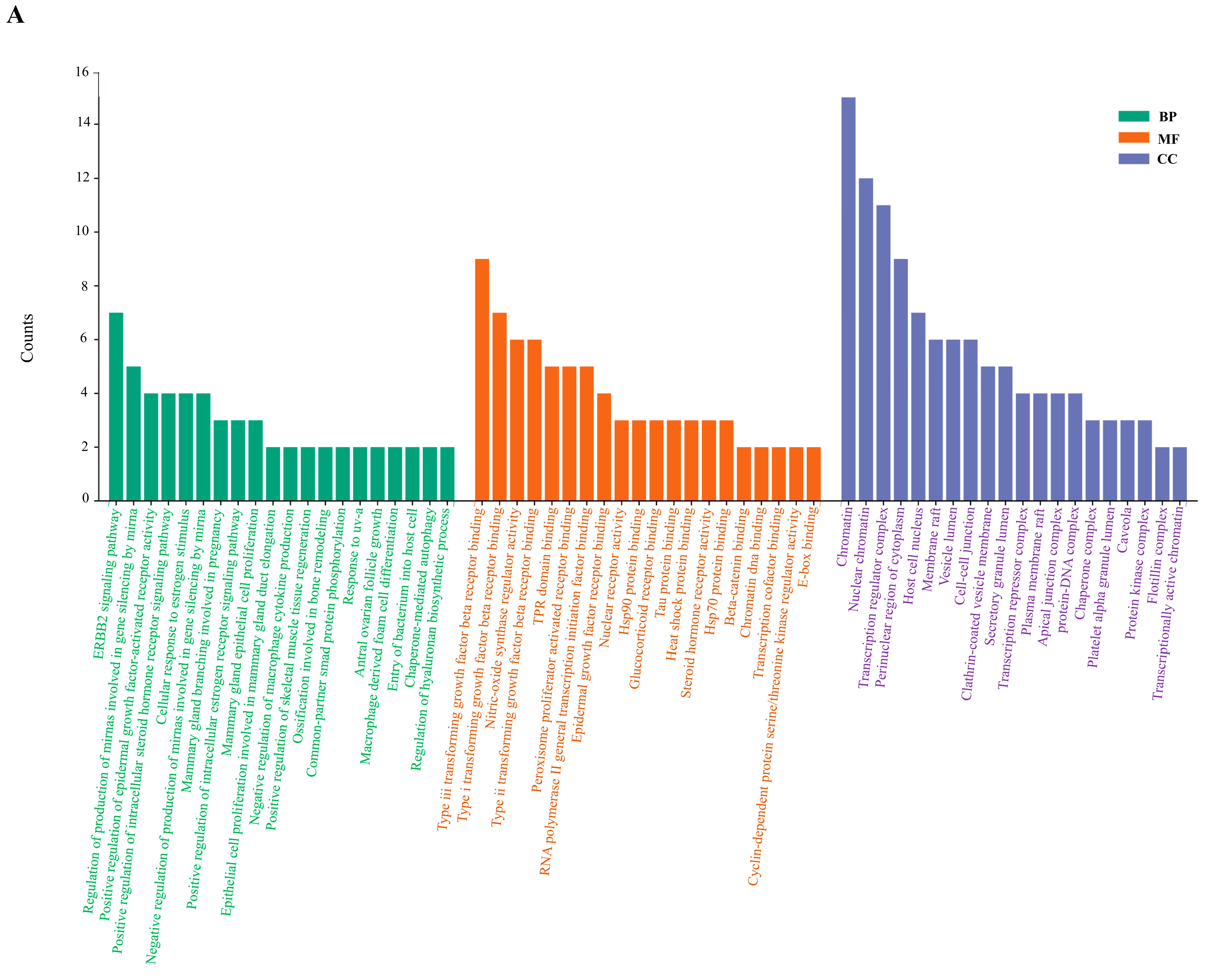
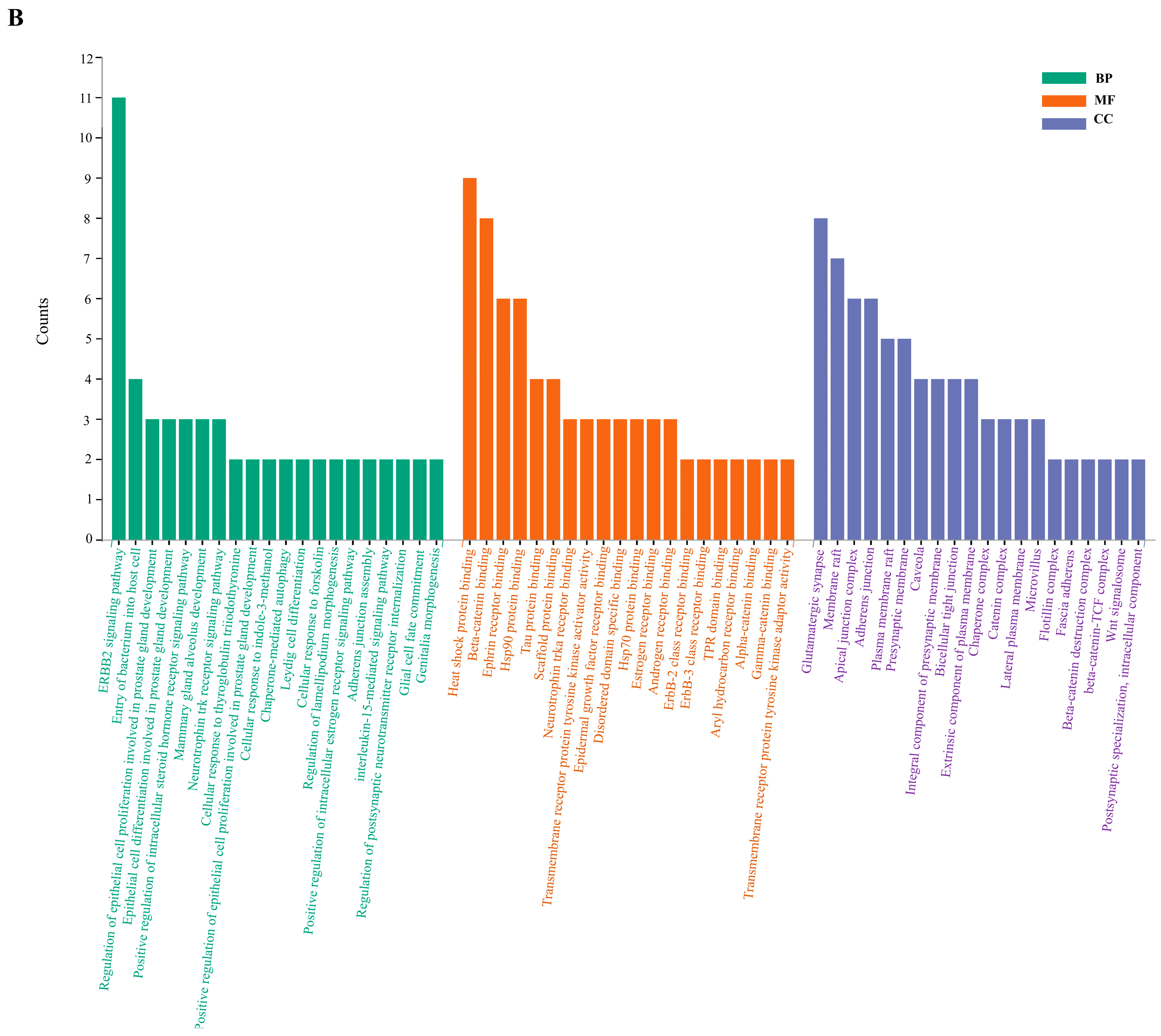
2.6. Enrichment Analysis of GO and KEGG Pathway
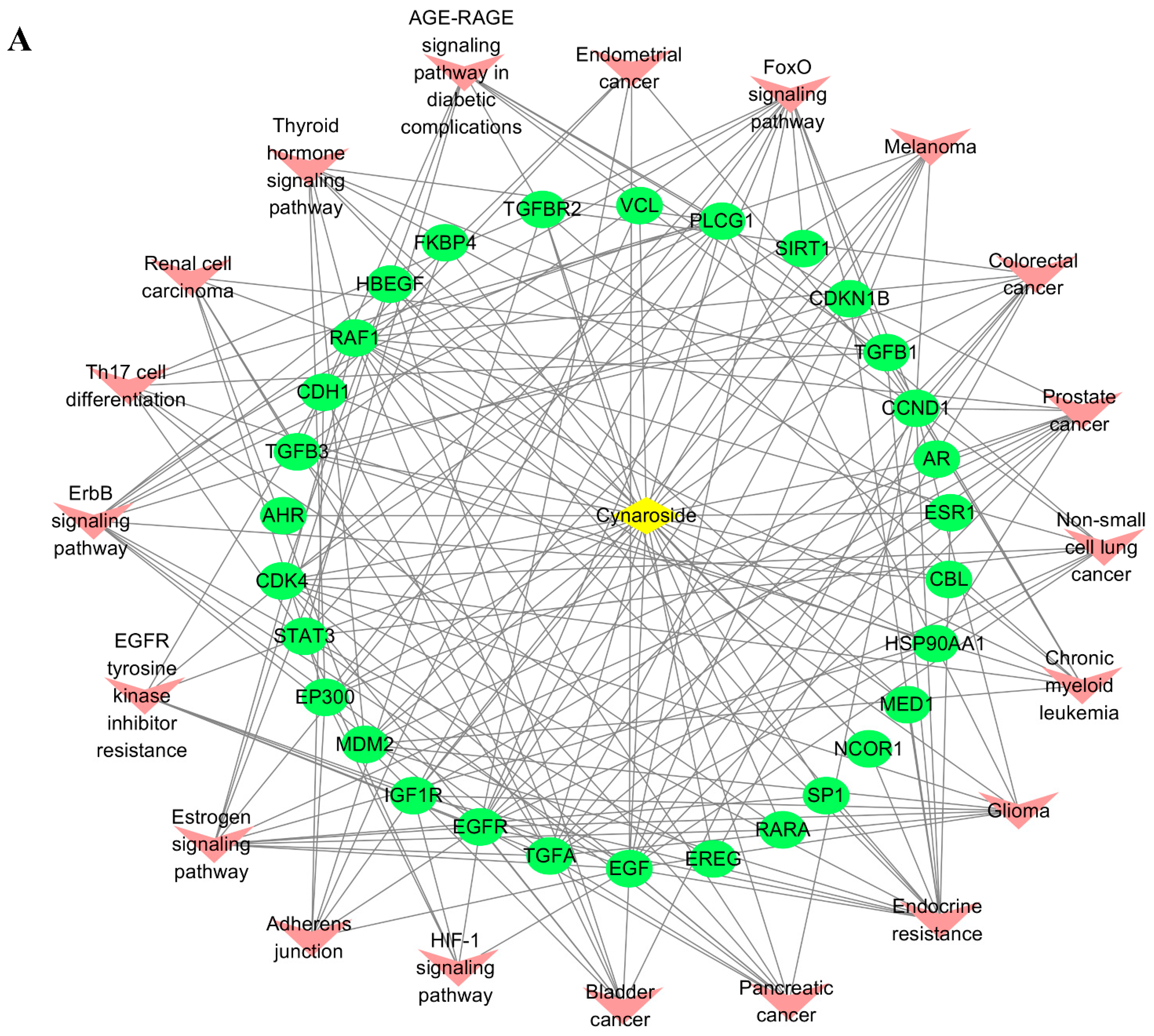
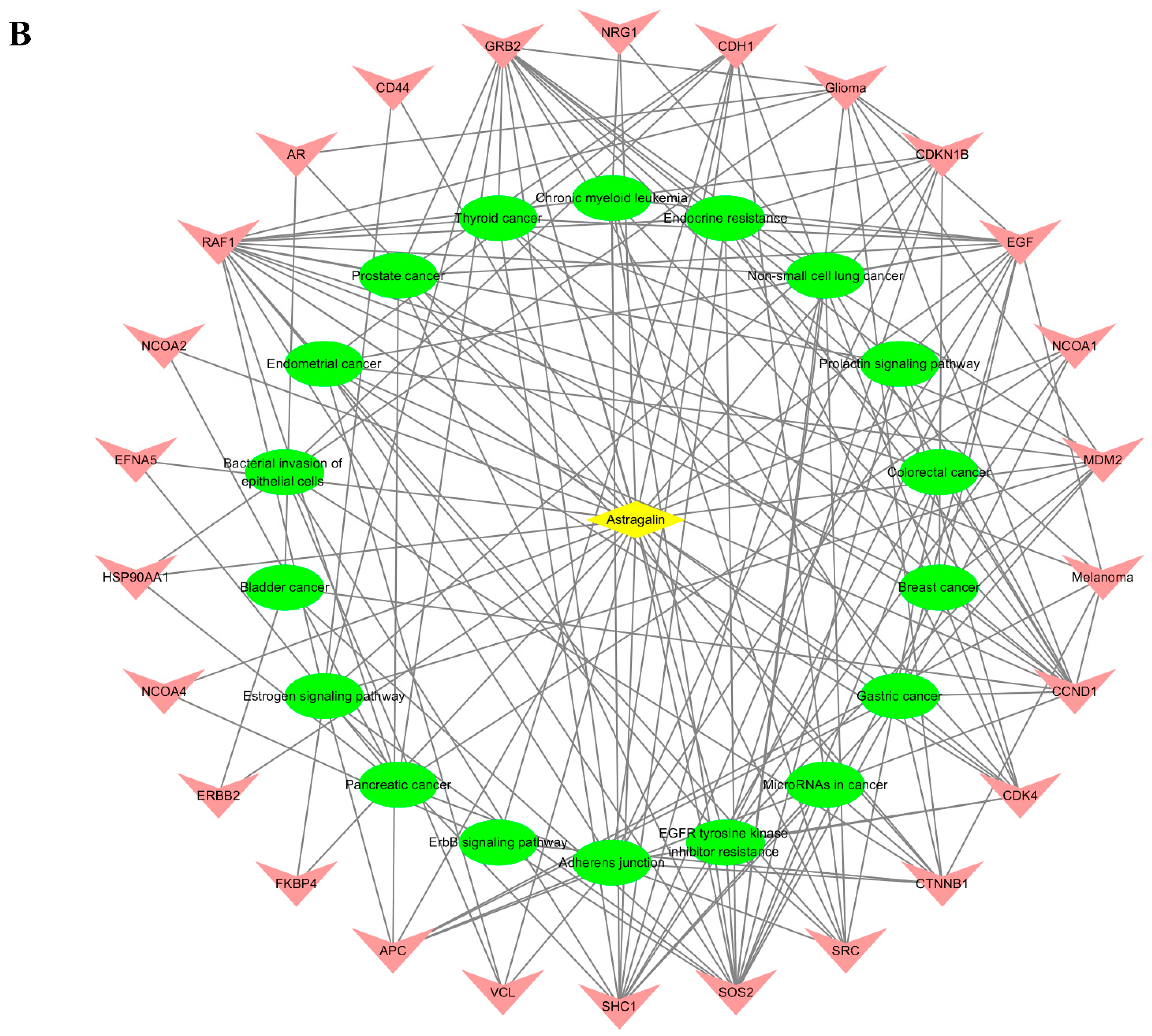
2.7. Active Ingredients–Shared Key Targets–Signal Pathway Network Diagram Construction
2.8. Key Active Ingredients and Core Target Molecular Docking Results
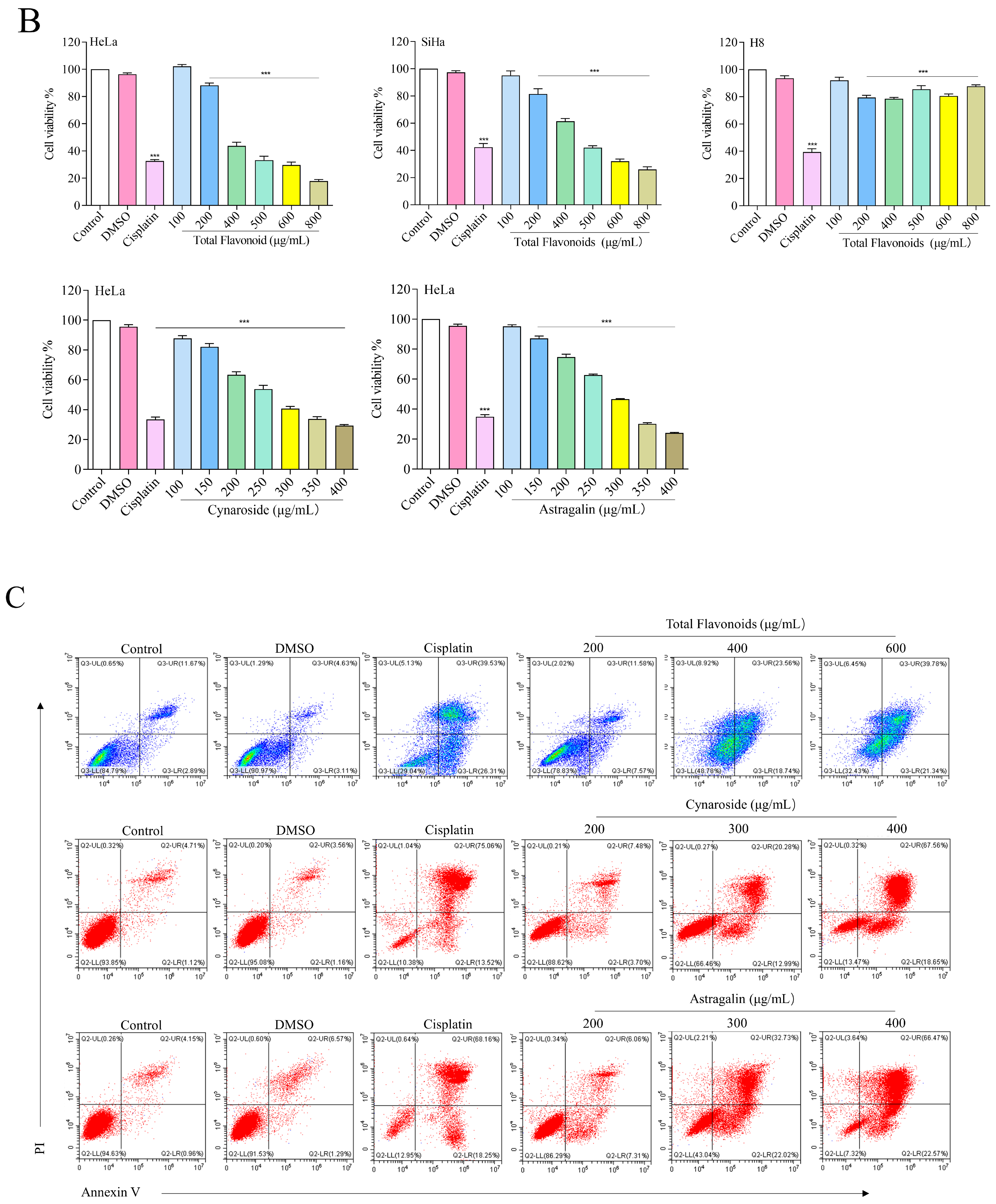
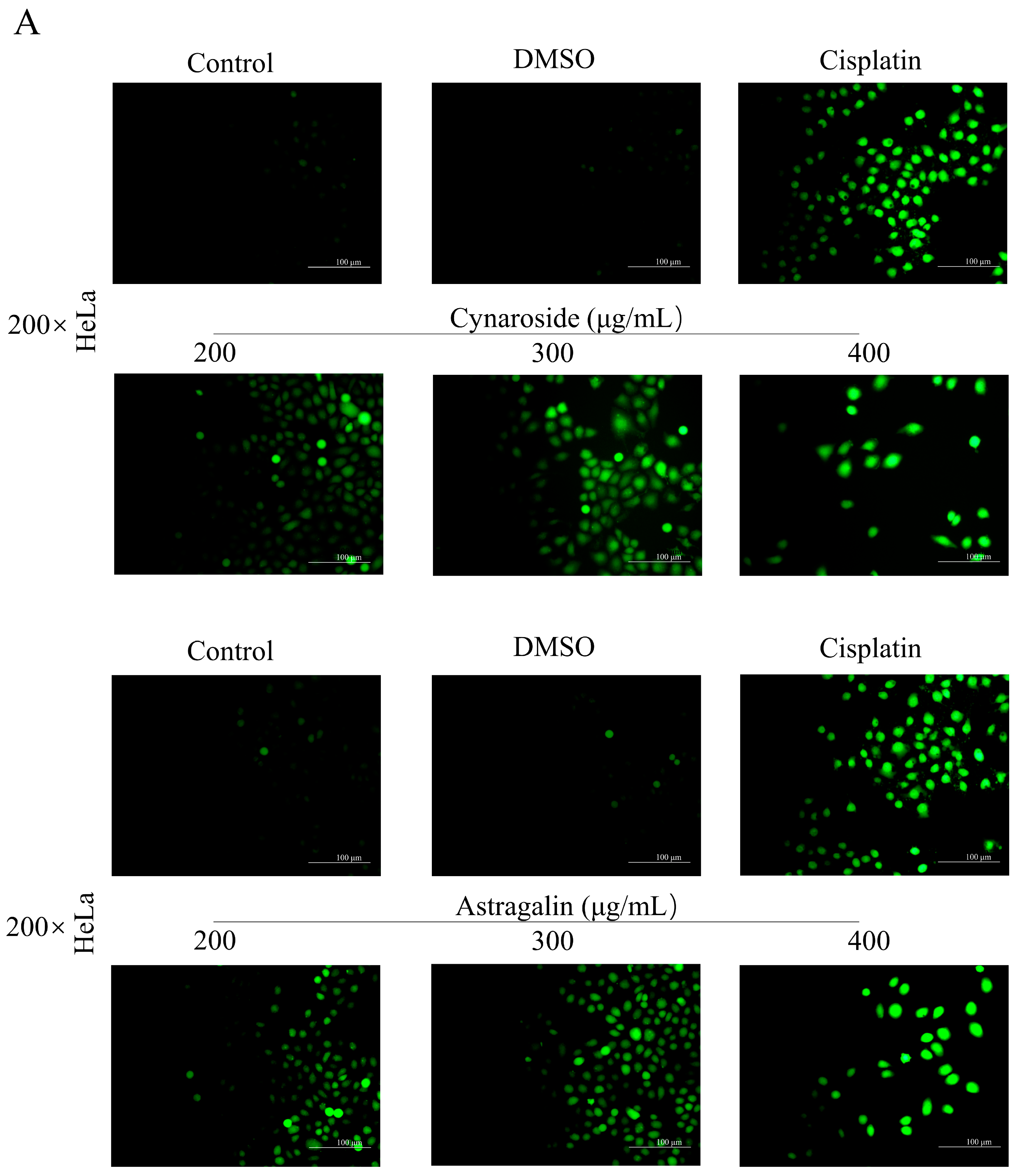
2.9. Total Flavonoids and Active Ingredients Can Inhibit Cell Proliferation and Induce Apoptosis
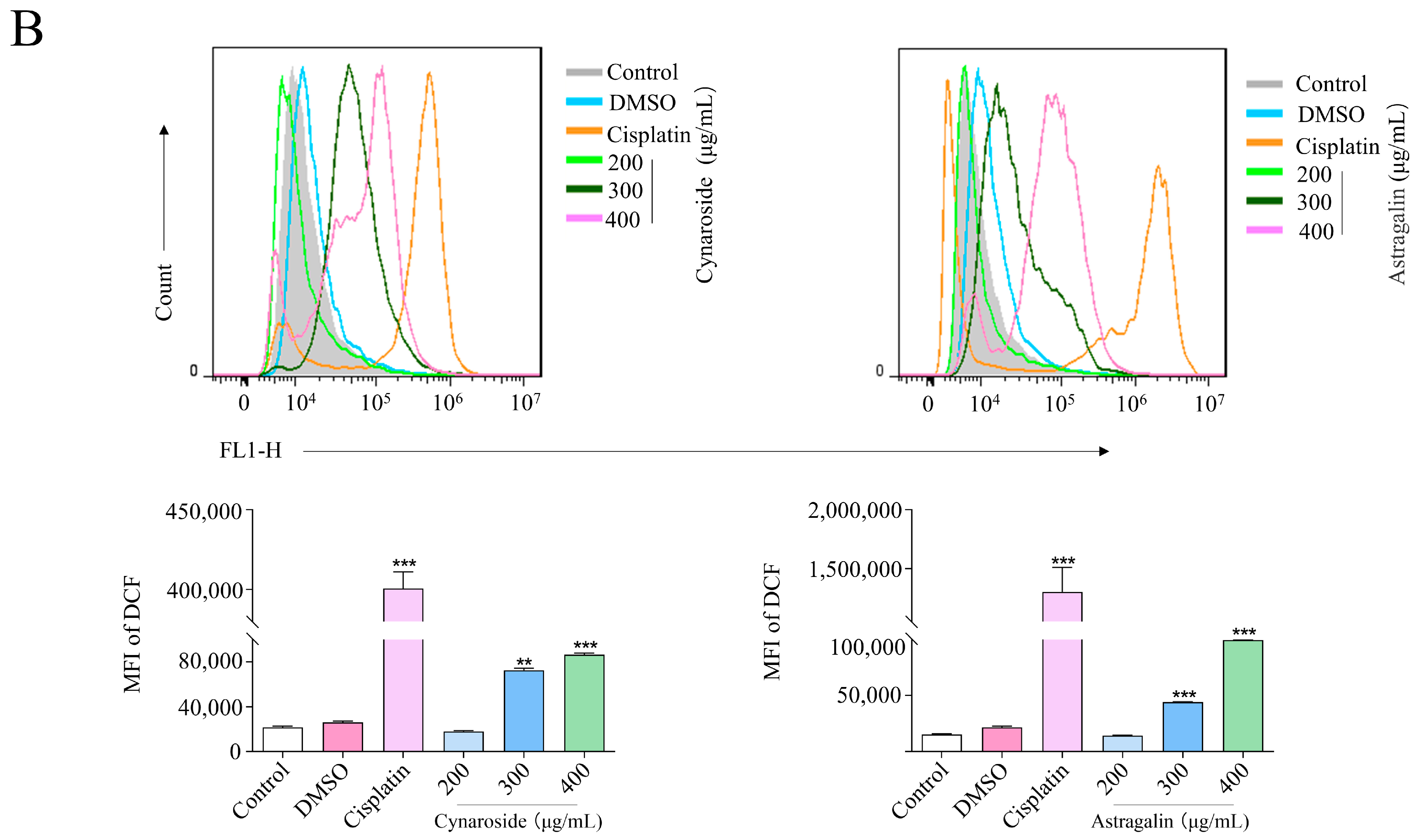
2.10. Induction of ROS Accumulation by Active Ingredients of Total Flavonoids
3. Discussion
4. Materials and Methods
4.1. Materials and Reagents
4.2. Methods
4.2.1. Quercetin Standard Curve Plotting
4.2.2. Extraction of Total Flavonoids
4.2.3. Determination of Total Flavonoid Content
4.2.4. Single-Factor Experimental Design
4.3. Optimization of Extraction Conditions of Total Flavonoids by Response Surface Methodology
4.4. LC-MS
4.4.1. Metabolites Extraction
4.4.2. MS Analysis
4.4.3. Screening of Core Active Ingredients and Targets
4.4.4. Disease-Related Gene Mining
4.4.5. Protein–Protein Interaction Network Construction and Analysis
4.4.6. Gene Ontology (GO) and the Kyoto Encyclopedia of Genes and Genomes (KEGG) Enrichment
4.4.7. Network Construction
4.4.8. Molecular Docking Ensures the Interaction between Targets and Key Compounds
4.5. Cell Culture
4.6. MTT
4.7. Apoptosis
4.8. Hochest
4.9. ROS
4.10. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marquina, G.; Manzano, A.; Casado, A. Targeted Agents in Cervical Cancer: Beyond Bevacizumab. Curr. Oncol. Rep. 2018, 20, 40. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Hu, Z.; Ma, D. The precision prevention and therapy of HPV-related cervical cancer: New concepts and clinical implications. Cancer Med. 2018, 7, 5217–5236. [Google Scholar] [CrossRef] [PubMed]
- Eifel, P.J. Chemoradiotherapy in the treatment of cervical cancer. Semin. Radiat. Oncol. 2006, 16, 177–185. [Google Scholar] [CrossRef]
- Kumar, L.; Harish, P.; Malik, P.S.; Khurana, S. Chemotherapy and targeted therapy in the management of cervical cancer. Curr. Probl. Cancer 2018, 42, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Mauricio, D.; Zeybek, B.; Tymon-Rosario, J.; Harold, J.; Santin, A.D. Immunotherapy in Cervical Cancer. Curr. Oncol. Rep. 2021, 23, 61. [Google Scholar] [CrossRef] [PubMed]
- Liontos, M.; Kyriazoglou, A.; Dimitriadis, I.; Dimopoulos, M.A.; Bamias, A. Systemic therapy in cervical cancer: 30 years in review. Crit. Rev. Oncol. Hematol. 2019, 137, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.; Jabeen, A.; Maharjan, R.; Nadeem-Ul-Haque, M.; Aamra, H.; Nazir, S.; Khan, S.; Olleik, H.; Maresca, M.; Shaheen, F. Furan-Conjugated Tripeptides as Potent Antitumor Drugs. Biomolecules 2020, 10, 1684. [Google Scholar] [CrossRef]
- Takahashi, M.; Kataoka, S.; Kobayashi, E.; Udagawa, Y.; Aoki, D.; Oie, S.; Kozu, A.; Nozawa, S. Differences in apoptosis induced by anticancer drugs in sublines (SKG-3a, SKG-3b) from a human uterine cervical epidermoid carcinoma. Oncol. Res. 1999, 11, 71–75. [Google Scholar]
- Bora, K.S.; Sharma, A. The genus Artemisia: A comprehensive review. Pharm. Biol. 2011, 49, 101–109. [Google Scholar] [CrossRef]
- Trendafilova, A.; Moujir, L.M.; Sousa, P.M.; Seca, A.M. Research advances on health effects of edible Artemisia species and some sesquiterpene lactones constituents. Foods 2020, 10, 65. [Google Scholar] [CrossRef] [PubMed]
- Szopa, A.; Pajor, J.; Klin, P.; Rzepiela, A.; Elansary, H.O.; Al-Mana, F.A.; Mattar, M.A.; Ekiert, H. Artemisia absinthium L.—Importance in the history of medicine, the latest advances in phytochemistry and therapeutical, cosmetological and culinary uses. Plants 2020, 9, 1063. [Google Scholar] [CrossRef]
- Parada, M.; Carrió, E.; Vallès, J. Ethnobotany of food plants in the Alt Emporda region (Catalonia, Iberian Peninsula). J. Appl. Bot. Food Qual. 2011, 84, 11–25. [Google Scholar]
- Allen, G. The Herbalist in the Kitchen; University of Illinois Press: Champaign, IL, USA, 2010. [Google Scholar]
- Wei, X.; Xia, L.; Ziyayiding, D.; Chen, Q.; Liu, R.; Xu, X.; Li, J. The Extracts of Artemisia absinthium L. Suppress the Growth of Hepatocellular Carcinoma Cells through Induction of Apoptosis via Endoplasmic Reticulum Stress and Mitochondrial-Dependent Pathway. Molecules 2019, 24, 913. [Google Scholar] [CrossRef] [PubMed]
- Nazeri, M.; Mirzaie-Asl, A.; Saidijam, M.; Moradi, M. Methanolic extract of Artemisia absinthium prompts apoptosis, enhancing expression of Bax/Bcl-2 ratio, cell cycle arrest, caspase-3 activation and mitochondrial membrane potential destruction in human colorectal cancer HCT-116 cells. Mol. Biol. Rep. 2020, 47, 8831–8840. [Google Scholar] [CrossRef]
- Shafi, G.; Hasan, T.N.; Syed, N.A.; Al-Hazzani, A.A.; Alshatwi, A.A.; Jyothi, A.; Munshi, A. Artemisia absinthium (AA): A novel potential complementary and alternative medicine for breast cancer. Mol. Biol. Rep. 2012, 39, 7373–7379. [Google Scholar] [CrossRef]
- Moacă, E.-A.; Pavel, I.Z.; Danciu, C.; Crăiniceanu, Z.; Minda, D.; Ardelean, F.; Antal, D.S.; Ghiulai, R.; Cioca, A.; Derban, M. Romanian wormwood (Artemisia absinthium L.): Physicochemical and nutraceutical screening. Molecules 2019, 24, 3087. [Google Scholar] [CrossRef]
- Mohammadian, A.; Moradkhani, S.; Ataei, S.; Shayesteh, T.H.; Sedaghat, M.; Kheiripour, N.; Ranjbar, A. Antioxidative and hepatoprotective effects of hydroalcoholic extract of Artemisia absinthium L. in rat. J. HerbMed Pharmacol. 2016, 5, 29–32. [Google Scholar]
- Ahmad, F.; Khan, R.A.; Rasheed, S. Study of analgesic and anti-inflammatory activity from plant extracts of Lactuca scariola and Artemisia absinthium. J. Islam. Acad. Sci. 1992, 5, 111–114. [Google Scholar]
- Mishra, K.; Sharma, N.; Diwaker, D.; Ganju, L.; Singh, S. Plant derived antivirals: A potential source of drug development. J. Virol. Antivir. Res. 2013, 2, 2. [Google Scholar]
- Juteau, F.; Jerkovic, I.; Masotti, V.; Milos, M.; Mastelic, J.; Bessière, J.-M.; Viano, J. Composition and antimicrobial activity of the essential oil of Artemisia absinthium from Croatia and France. Planta Med. 2003, 69, 158–161. [Google Scholar] [CrossRef] [PubMed]
- Koyuncu, I. Evaluation of anticancer, antioxidant activity and phenolic compounds of Artemisia absinthium L. extract. Cell. Mol. Biol. 2018, 64, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Anadanovic-Brunet, J.M.; Djilas, S.M.; Cetkovic, G.S.; Tumbas, V.T. Free-radical scavenging activity of wormwood (Artemisia absinthium L.) extracts. J. Sci. Food Agr. 2005, 85, 265–272. [Google Scholar] [CrossRef]
- Kshirsagar, S.G.; Rao, R.V. Antiviral and immunomodulation effects of Artemisia. Medicina 2021, 57, 217. [Google Scholar] [CrossRef] [PubMed]
- Mladenova, O.M. Grapes and Wine in the Balkans: An Ethno-Linguistic Study; Otto Harrassowitz Verlag: Wiesbaden, Germany, 1998; Volume 32. [Google Scholar]
- Craciunescu, O.; Constantin, D.; Gaspar, A.; Toma, L.; Utoiu, E.; Moldovan, L. Evaluation of antioxidant and cytoprotective activities of Arnica montana L. and Artemisia absinthium L. ethanolic extracts. Chem. Cent. J. 2012, 6, 97. [Google Scholar] [CrossRef]
- Ali, M.; Iqbal, R.; Safdar, M.; Murtaza, S.; Mustafa, G.; Sajjad, M.; Bukhari, S.A.; Huma, T. Antioxidant and antibacterial activities of Artemisia absinthium and Citrus paradisi extracts repress viability of aggressive liver cancer cell line. Mol. Biol. Rep. 2021, 48, 7703–7710. [Google Scholar] [CrossRef]
- Cheng, Y.; Xue, F.; Yu, S.; Du, S.; Yang, Y. Subcritical water extraction of natural products. Molecules 2021, 26, 4004. [Google Scholar] [CrossRef]
- Pietta, P.-G. Flavonoids as antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, W.; Liu, J.; Liu, H.; Lv, Z.; Zhang, C.; Chen, D.; Jiao, Z. Identification of Six Flavonoids as Novel Cellular Antioxidants and Their Structure-Activity Relationship. Oxid. Med. Cell. Longev. 2020, 2020, 4150897. [Google Scholar] [CrossRef]
- Choy, K.W.; Murugan, D.; Leong, X.-F.; Abas, R.; Alias, A.; Mustafa, M.R. Flavonoids as natural anti-inflammatory agents targeting nuclear factor-kappa B (NFκB) signaling in cardiovascular diseases: A mini review. Front. Pharmacol. 2019, 10, 1295. [Google Scholar] [CrossRef]
- Iwashina, T. The structure and distribution of the flavonoids in plants. J. Plant Res. 2000, 113, 287. [Google Scholar] [CrossRef]
- Maleki, S.J.; Crespo, J.F.; Cabanillas, B. Anti-inflammatory effects of flavonoids. Food Chem. 2019, 299, 125124. [Google Scholar] [CrossRef] [PubMed]
- Chanput, W.; Krueyos, N.; Ritthiruangdej, P. Anti-oxidative assays as markers for anti-inflammatory activity of flavonoids. Int. Immunopharmacol. 2016, 40, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Luan, F.; Peng, L.; Lei, Z.; Jia, X.; Zou, J.; Yang, Y.; He, X.; Zeng, N. Traditional Uses, Phytochemical Constituents and Pharmacological Properties of Averrhoa carambola L.: A Review. Front. Pharmacol. 2021, 1814, 699899. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Wu, L.; Yang, L.; Zhao, B.; Li, C. Extraction of Phenolics and Flavonoids from Four Hosta Species Using Reflux and Ultrasound-Assisted Methods with Antioxidant and α-Glucosidase Inhibitory Activities. BioMed Res. Int. 2020, 2020, 6124153. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Guo, Z.; Yu, G. Process intensification and kinetic studies of ultrasound-assisted extraction of flavonoids from peanut shells. Ultrason. Sonochem. 2021, 76, 105661. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhou, C.-L.; Zhao, J.; Chen, D.; Li, Q.-H. Optimized microwave-assisted extraction of 6-gingerol from Zingiber officinale roscoeand evaluation of antioxidant activity in vitro. Acta Sci. Pol. Technol. Aliment. 2014, 13, 155–168. [Google Scholar] [CrossRef]
- Liu, L.; Wen, W.; Zhang, R.; Wei, Z.; Deng, Y.; Xiao, J.; Zhang, M. Complex enzyme hydrolysis releases antioxidative phenolics from rice bran. Food Chem. 2017, 214, 1–8. [Google Scholar] [CrossRef]
- Yu, M.; Wang, B.; Qi, Z.; Xin, G.; Li, W. Response surface method was used to optimize the ultrasonic assisted extraction of flavonoids from Crinum asiaticum. Saudi J. Biol. Sci. 2019, 26, 2079–2084. [Google Scholar] [CrossRef]
- Lee, L.-S.; Lee, N.; Kim, Y.H.; Lee, C.-H.; Hong, S.P.; Jeon, Y.-W.; Kim, Y.-E. Optimization of ultrasonic extraction of phenolic antioxidants from green tea using response surface methodology. Molecules 2013, 18, 13530–13545. [Google Scholar] [CrossRef]
- Chávez-González, M.L.; Sepúlveda, L.; Verma, D.K.; Luna-García, H.A.; Rodríguez-Durán, L.V.; Ilina, A.; Aguilar, C.N. Conventional and emerging extraction processes of flavonoids. Processes 2020, 8, 434. [Google Scholar] [CrossRef]
- Chaves, J.O.; De Souza, M.C.; Da Silva, L.C.; Lachos-Perez, D.; Torres-Mayanga, P.C.; Machado, A.P.d.F.; Forster-Carneiro, T.; Vázquez-Espinosa, M.; González-de-Peredo, A.V.; Barbero, G.F. Extraction of flavonoids from natural sources using modern techniques. Front. Chem. 2020, 864, 507887. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sun, B.; Cao, Y.; Tian, Y.; Li, X. Optimisation of ultrasound-assisted extraction of phenolic compounds from wheat bran. Food Chem. 2008, 106, 804–810. [Google Scholar] [CrossRef]
- Daoying, Z.; Xiangtin, S.; Liang, Z.; Haowen, Z.; Hao, H. Ultrasound-assisted Enzymatic Extraction of Total Flavonoids from Ploygonum perfoliatum L. J. Gannan Med. Univ. 2019, 39, 552–557. [Google Scholar]
- Du, L.; Gu, L.; Wei, Z.; Gao, H.; Guangjie, Z. Studyon Extraction of Flavonoids from Peanut Shells by Enzymatic Hydrolysis Method and Their Antioxidant Activity and Antibacterial Activity. China Condiment 2022, 47, 195–199. [Google Scholar]
- Zou, T.-B.; Wang, M.; Gan, R.-Y.; Ling, W.-H. Optimization of ultrasound-assisted extraction of anthocyanins from mulberry, using response surface methodology. Int. J. Mol. Sci. 2011, 12, 3006–3017. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, Y.; Pang, X.; Hua, P.; Gao, X.; Li, Q.; Li, Z. Simultaneous optimization of ultrasound-assisted extraction for flavonoids and antioxidant activity of Angelica keiskei using response surface methodology (RSM). Molecules 2019, 24, 3461. [Google Scholar] [CrossRef]
- Bezerra, M.A.; Santelli, R.E.; Oliveira, E.P.; Villar, L.S.; Escaleira, L.A. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 2008, 76, 965–977. [Google Scholar] [CrossRef]
- Arya, A.; Chahal, R.; Nanda, A.; Kaushik, D.; Bin-Jumah, M.; Rahman, M.H.; Abdel-Daim, M.M.; Mittal, V. Statistically Designed Extraction of Herbs Using Ultrasound Waves: A Review. Curr. Pharm. Des. 2021, 27, 3638–3655. [Google Scholar] [CrossRef]
- Jing, C.L.; Dong, X.F.; Tong, J.M. Optimization of Ultrasonic-Assisted Extraction of Flavonoid Compounds and Antioxidants from Alfalfa Using Response Surface Method. Molecules 2015, 20, 15550–15571. [Google Scholar] [CrossRef]
- Chen, L.; Cao, Y.; Zhang, H.; Lv, D.; Zhao, Y.; Liu, Y.; Ye, G.; Chai, Y. Network pharmacology-based strategy for predicting active ingredients and potential targets of Yangxinshi tablet for treating heart failure. J. Ethnopharmacol. 2018, 219, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Wang, M.H.; Wang, F.; Chen, P.Y.; Ke, X.G.; Yu, B.; Yang, Y.F.; You, P.T.; Wu, H.Z. Network pharmacology and molecular docking reveal the mechanism of Scopoletin against non-small cell lung cancer. Life Sci. 2021, 270, 119105. [Google Scholar] [CrossRef] [PubMed]
- Saikia, S.; Bordoloi, M. Molecular Docking: Challenges, Advances and its Use in Drug Discovery Perspective. Curr. Drug Targets 2019, 20, 501–521. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Chen, A.Y.; Rojanasakul, Y.; Ye, X.; Rankin, G.O.; Chen, Y.C. Dietary compounds galangin and myricetin suppress ovarian cancer cell angiogenesis. J. Funct. Foods 2015, 15, 464–475. [Google Scholar] [CrossRef] [PubMed]
- Ha, T.K.; Kim, M.E.; Yoon, J.H.; Bae, S.J.; Yeom, J.; Lee, J.S. Galangin induces human colon cancer cell death via the mitochondrial dysfunction and caspase-dependent pathway. Exp. Biol. Med. 2013, 238, 1047–1054. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.T.; Luo, H.; Wu, J.; Lan, L.B.; Fan, D.H.; Zhu, K.D.; Chen, X.Y.; Wen, M.; Liu, H.M. Galangin induces apoptosis of hepatocellular carcinoma cells via the mitochondrial pathway. World J. Gastroenterol. 2010, 16, 3377–3384. [Google Scholar] [CrossRef]
- Liang, X.; Wang, P.; Yang, C.; Huang, F.; Wu, H.; Shi, H.; Wu, X. Galangin Inhibits Gastric Cancer Growth Through Enhancing STAT3 Mediated ROS Production. Front. Pharmacol. 2021, 12, 646628. [Google Scholar] [CrossRef]
- Liu, D.; You, P.; Luo, Y.; Yang, M.; Liu, Y. Galangin Induces Apoptosis in MCF-7 Human Breast Cancer Cells Through Mitochondrial Pathway and Phosphatidylinositol 3-Kinase/Akt Inhibition. Pharmacology 2018, 102, 58–66. [Google Scholar] [CrossRef]
- Dong, Y.; Ji, G.; Cao, A.; Shi, J.; Shi, H.; Xie, J.; Wu, D. Effects of sinensetin on proliferation and apoptosis of human gastric cancer AGS cells. Zhongguo Zhong Yao Za Zhi = Zhongguo Zhongyao Zazhi = China J. Chin. Mater. Medica 2011, 36, 790–794. [Google Scholar]
- Kim, S.M.; Ha, S.E.; Lee, H.J.; Rampogu, S.; Vetrivel, P.; Kim, H.H.; Venkatarame Gowda Saralamma, V.; Lee, K.W.; Kim, G.S. Sinensetin Induces Autophagic Cell Death through p53-Related AMPK/mTOR Signaling in Hepatocellular Carcinoma HepG2 Cells. Nutrients 2020, 12, 2462. [Google Scholar] [CrossRef]
- Rezakhani, N.; Goliaei, B.; Parivar, K.; Nikoofar, A. Effects of X-irradiation and sinensetin on apoptosis induction in MDA-MB-231 human breast cancer cells. Int. J. Radiat. Res. 2020, 18, 75–82. [Google Scholar]
- Kim, S.J.; Pham, T.H.; Bak, Y.; Ryu, H.W.; Oh, S.R.; Yoon, D.Y. Orientin inhibits invasion by suppressing MMP-9 and IL-8 expression via the PKCα/ ERK/AP-1/STAT3-mediated signaling pathways in TPA-treated MCF-7 breast cancer cells. Phytomed. Int. J. Phytother. Phytopharm. 2018, 50, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Thangaraj, K.; Balasubramanian, B.; Park, S.; Natesan, K.; Liu, W.; Manju, V. Orientin Induces G0/G1 Cell Cycle Arrest and Mitochondria Mediated Intrinsic Apoptosis in Human Colorectal Carcinoma HT29 Cells. Biomolecules 2019, 9, 418. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Tong, M.; Li, Z.; Huang, W.; Jin, Y.; Cao, Q.; Zhou, X.; Tong, G. The Effects of Orientin on Proliferation and Apoptosis of T24 Human Bladder Carcinoma Cells Occurs Through the Inhibition of Nuclear Factor-kappaB and the Hedgehog Signaling Pathway. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2019, 25, 9547–9554. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Tian, X.; Yang, A.; Zhou, Y.; Wu, D.; Wang, Z. Orientin in Trollius chinensis Bunge inhibits proliferation of HeLa human cervical carcinoma cells by induction of apoptosis. Monatshefte Chem. Chem. Mon. 2014, 145, 229–233. [Google Scholar] [CrossRef]
- Chou, L.F.; Chen, C.Y.; Yang, W.H.; Chen, C.C.; Chang, J.L.; Leu, Y.L.; Liou, M.J.; Wang, T.H. Suppression of Hepatocellular Carcinoma Progression through FOXM1 and EMT Inhibition via Hydroxygenkwanin-Induced miR-320a Expression. Biomolecules 2019, 10, 20. [Google Scholar] [CrossRef]
- Ao, H.; Li, Y.; Li, H.; Wang, Y.; Han, M.; Guo, Y.; Shi, R.; Yue, F.; Wang, X. Preparation of hydroxy genkwanin nanosuspensions and their enhanced antitumor efficacy against breast cancer. Drug Deliv. 2020, 27, 816–824. [Google Scholar] [CrossRef]
- Leu, Y.L.; Wang, T.H.; Wu, C.C.; Huang, K.Y.; Jiang, Y.W.; Hsu, Y.C.; Chen, C.Y. Hydroxygenkwanin Suppresses Non-Small Cell Lung Cancer Progression by Enhancing EGFR Degradation. Molecules 2020, 25, 941. [Google Scholar] [CrossRef]
- Zang, Y.Q.; Feng, Y.Y.; Luo, Y.H.; Zhai, Y.Q.; Ju, X.Y.; Feng, Y.C.; Wang, J.R.; Yu, C.Q.; Jin, C.H. Glycitein induces reactive oxygen species-dependent apoptosis and G0/G1 cell cycle arrest through the MAPK/STAT3/NF-κB pathway in human gastric cancer cells. Drug Dev. Res. 2019, 80, 573–584. [Google Scholar] [CrossRef]
- Lee, E.J.; Kim, S.Y.; Hyun, J.W.; Min, S.W.; Kim, D.H.; Kim, H.S. Glycitein inhibits glioma cell invasion through down-regulation of MMP-3 and MMP-9 gene expression. Chem. Biol. Interact. 2010, 185, 18–24. [Google Scholar] [CrossRef]
- Zhang, B.; Su, J.P.; Bai, Y.; Li, J.; Liu, Y.H. Inhibitory effects of O-methylated isoflavone glycitein on human breast cancer SKBR-3 cells. Int. J. Clin. Exp. Pathol. 2015, 8, 7809–7817. [Google Scholar] [PubMed]
- Shim, H.Y.; Park, J.H.; Paik, H.D.; Nah, S.Y.; Kim, D.S.; Han, Y.S. Acacetin-induced apoptosis of human breast cancer MCF-7 cells involves caspase cascade, mitochondria-mediated death signaling and SAPK/JNK1/2-c-Jun activation. Mol. Cells 2007, 24, 95–104. [Google Scholar] [PubMed]
- Hsu, Y.L.; Kuo, P.L.; Lin, C.C. Acacetin inhibits the proliferation of Hep G2 by blocking cell cycle progression and inducing apoptosis. Biochem. Pharmacol. 2004, 67, 823–829. [Google Scholar] [CrossRef] [PubMed]
- Shen, K.H.; Hung, S.H.; Yin, L.T.; Huang, C.S.; Chao, C.H.; Liu, C.L.; Shih, Y.W. Acacetin, a flavonoid, inhibits the invasion and migration of human prostate cancer DU145 cells via inactivation of the p38 MAPK signaling pathway. Mol. Cell. Biochem. 2010, 333, 279–291. [Google Scholar] [CrossRef]
- Hsu, Y.L.; Kuo, P.L.; Liu, C.F.; Lin, C.C. Acacetin-induced cell cycle arrest and apoptosis in human non-small cell lung cancer A549 cells. Cancer Lett. 2004, 212, 53–60. [Google Scholar] [CrossRef]
- Han, R.; Yang, H.; Lu, L.; Lin, L. Tiliroside as a CAXII inhibitor suppresses liver cancer development and modulates E2Fs/Caspase-3 axis. Sci. Rep. 2021, 11, 8626. [Google Scholar] [CrossRef]
- Da’i, M.; Wikantyasning, E.R.; Wahyuni, A.S.; Kusumawati, I.T.D.; Saifudin, A.; Suhendi, A. Antiproliferative properties of tiliroside from Guazuma ulmifolia lamk on T47D and MCF7 cancer cell lines. Natl. J. Physiol. Pharm. Pharmacol. 2016, 6, 627. [Google Scholar] [CrossRef]
- Liu, T.; Cao, L.; Zhang, T.; Fu, H. Molecular docking studies, anti-Alzheimer’s disease, antidiabetic, and anti-acute myeloid leukemia potentials of narcissoside. Arch. Physiol. Biochem. 2020, 129, 405–415. [Google Scholar] [CrossRef]
- Park, M.H.; Hong, J.E.; Park, E.S.; Yoon, H.S.; Seo, D.W.; Hyun, B.K.; Han, S.-B.; Ham, Y.W.; Hwang, B.Y.; Hong, J.T. Anticancer effect of tectochrysin in colon cancer cell via suppression of NF-kappaB activity and enhancement of death receptor expression. Mol. Cancer 2015, 14, 124. [Google Scholar] [CrossRef]
- Oh, S.-B.; Hwang, C.J.; Song, S.-Y.; Jung, Y.Y.; Yun, H.-M.; Sok, C.H.; Sung, H.C.; Yi, J.-M.; Park, D.H.; Ham, Y.W. Anti-cancer effect of tectochrysin in NSCLC cells through overexpression of death receptor and inactivation of STAT3. Cancer Lett. 2014, 353, 95–103. [Google Scholar] [CrossRef]
- Wang, Y.; Ke, R.-J.; Jiang, P.-R.; Ying, J.-H.; Lou, E.-Z.; Chen, J.-Y. The effects of tectochrysin on prostate cancer cells apoptosis and its mechanism. Zhongguo Ying Yong Sheng Li Xue Za Zhi = Zhongguo Yingyong Shenglixue Zazhi = Chin. J. Appl. Physiol. 2019, 35, 283–288. [Google Scholar]
- Yao, Z.; Xu, X.; Huang, Y. Daidzin inhibits growth and induces apoptosis through the JAK2/STAT3 in human cervical cancer HeLa cells. Saudi J. Biol. Sci. 2021, 28, 7077–7081. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.; Lim, W.; Bazer, F.W.; Song, G. Chrysin induces death of prostate cancer cells by inducing ROS and ER stress. J. Cell. Physiol. 2017, 232, 3786–3797. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Liu, D.; Jiang, Z.; Li, C.; Chen, L.; Xia, Y.; Liu, D.; Yao, Q.; Wang, D. Chrysin induced cell apoptosis and inhibited invasion through regulation of TET1 expression in gastric cancer cells. OncoTargets Ther. 2020, 13, 3277. [Google Scholar] [CrossRef]
- Lima, A.P.B.; Almeida, T.C.; Barros, T.M.B.; Rocha, L.C.M.; Garcia, C.C.M.; da Silva, G.N. Toxicogenetic and antiproliferative effects of chrysin in urinary bladder cancer cells. Mutagenesis 2020, 35, 361–371. [Google Scholar] [CrossRef]
- Ronnekleiv-Kelly, S.M.; Nukaya, M.; Díaz-Díaz, C.J.; Megna, B.W.; Carney, P.R.; Geiger, P.G.; Kennedy, G.D. Aryl hydrocarbon receptor-dependent apoptotic cell death induced by the flavonoid chrysin in human colorectal cancer cells. Cancer Lett. 2016, 370, 91–99. [Google Scholar] [CrossRef]
- Zhou, Y.; Ho, W.S. Combination of liquiritin, isoliquiritin and isoliquirigenin induce apoptotic cell death through upregulating p53 and p21 in the A549 non-small cell lung cancer cells. Oncol. Rep. 2014, 31, 298–304. [Google Scholar] [CrossRef]
- He, S.-H.; Liu, H.-G.; Zhou, Y.-F.; Yue, Q.-F. Liquiritin (LT) exhibits suppressive effects against the growth of human cervical cancer cells through activating Caspase-3 in vitro and xenograft mice in vivo. Biomed. Pharmacother. 2017, 92, 215–228. [Google Scholar] [CrossRef]
- Xie, R.; Gao, C.-C.; Yang, X.-Z.; Wu, S.-N.; Wang, H.-G.; Zhang, J.-L.; Yan, W.; Ma, T.-H. Combining TRAIL and liquiritin exerts synergistic effects against human gastric cancer cells and xenograft in nude mice through potentiating apoptosis and ROS generation. Biomed. Pharmacother. 2017, 93, 948–960. [Google Scholar] [CrossRef]
- Wang, J.-R.; Li, T.-Z.; Wang, C.; Li, S.-M.; Luo, Y.-H.; Piao, X.-J.; Feng, Y.-C.; Zhang, Y.; Xu, W.-T.; Zhang, Y. Liquiritin inhibits proliferation and induces apoptosis in HepG2 hepatocellular carcinoma cells via the ROS-mediated MAPK/AKT/NF-κB signaling pathway. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2020, 393, 1987–1999. [Google Scholar] [CrossRef]
- Yoshida, M.; Sakai, T.; Hosokawa, N.; Marui, N.; Matsumoto, K.; Fujioka, A.; Nishino, H.; Aoike, A. The effect of quercetin on cell cycle progression and growth of human gastric cancer cells. FEBS Lett. 1990, 260, 10–13. [Google Scholar] [CrossRef]
- Choi, J.-A.; Kim, J.-Y.; Lee, J.-Y.; Kang, C.-M.; Kwon, H.-J.; Yoo, Y.-D.; Kim, T.-W.; Lee, Y.-S.; Lee, S.-J. Induction of cell cycle arrest and apoptosis in human breast cancer cells by quercetin. Int. J. Oncol. 2001, 19, 837–844. [Google Scholar] [CrossRef] [PubMed]
- Shafabakhsh, R.; Asemi, Z. Quercetin: A natural compound for ovarian cancer treatment. J. Ovarian Res. 2019, 12, 55. [Google Scholar] [CrossRef] [PubMed]
- Ghafouri-Fard, S.; Shabestari, F.A.; Vaezi, S.; Abak, A.; Shoorei, H.; Karimi, A.; Taheri, M.; Basiri, A. Emerging impact of quercetin in the treatment of prostate cancer. Biomed. Pharmacother. 2021, 138, 111548. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Fang, L.; Liao, J.; Li, L.; Yao, W.; Xiong, Z.; Zhou, X. Investigation of the anti-cancer effect of quercetin on HepG2 cells in vivo. PLoS ONE 2017, 12, e0172838. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.S.; Nair, M.; Hema, P.; Mohan, J.; Santhoshkumar, T. Pinocembrin triggers Bax-dependent mitochondrial apoptosis in colon cancer cells. Mol. Carcinog. 2007, 46, 231–241. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, K.; Wu, Y.; Chen, Y.; Chen, X.; Hu, C.W.; Hu, F. Pinocembrin induces ER stress mediated apoptosis and suppresses autophagy in melanoma cells. Cancer Lett. 2018, 431, 31–42. [Google Scholar] [CrossRef]
- Gao, J.; Lin, S.; Gao, Y.; Zou, X.; Zhu, J.; Chen, M.; Wan, H.; Zhu, H. Pinocembrin inhibits the proliferation and migration and promotes the apoptosis of ovarian cancer cells through down-regulating the mRNA levels of N-cadherin and GABAB receptor. Biomed. Pharmacother. 2019, 120, 109505. [Google Scholar] [CrossRef]
- Gong, H. Pinocembrin suppresses proliferation and enhances apoptosis in lung cancer cells in vitro by restraining autophagy. Bioengineered 2021, 12, 6035–6044. [Google Scholar] [CrossRef]
- Wei, C.; Lu, J.; Zhang, Z.; Hua, F.; Chen, Y.; Shen, Z. Genkwanin attenuates lung cancer development by repressing proliferation and invasion via phosphatidylinositol 3-kinase/protein kinase B pathway. Mater. Express 2021, 11, 319–325. [Google Scholar]
- Kanazawa, M.; Satomi, Y.; Mizutani, Y.; Ukimura, O.; Kawauchi, A.; Sakai, T.; Baba, M.; Okuyama, T.; Nishino, H.; Miki, T. Isoliquiritigenin inhibits the growth of prostate cancer. Eur. Urol. 2003, 43, 580–586. [Google Scholar] [CrossRef] [PubMed]
- Maggiolini, M.; Statti, G.; Vivacqua, A.; Gabriele, S.; Rago, V.; Loizzo, M.; Menichini, F.; Amdò, S. Estrogenic and antiproliferative activities of isoliquiritigenin in MCF7 breast cancer cells. J. Steroid Biochem. Mol. Biol. 2002, 82, 315–322. [Google Scholar] [CrossRef]
- Ii, T.; Satomi, Y.; Katoh, D.; Shimada, J.; Baba, M.; Okuyama, T.; Nishino, H.; Kitamura, N. Induction of cell cycle arrest and p21CIP1/WAF1 expression in human lung cancer cells by isoliquiritigenin. Cancer Lett. 2004, 207, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Hirchaud, F.; Hermetet, F.; Ablise, M.; Fauconnet, S.; Vuitton, D.A.; Prétet, J.-L.; Mougin, C. Isoliquiritigenin induces caspase-dependent apoptosis via downregulation of HPV16 E6 expression in cervical cancer Ca Ski cells. Planta Medica 2013, 79, 1628–1635. [Google Scholar] [CrossRef]
- Fernandes, I.; Leça, J.M.; Aguiar, R.; Fernandes, T.; Marques, J.C.; Cordeiro, N. Influence of crop system fruit quality, carotenoids, fatty acids and phenolic compounds in cherry tomatoes. Agric. Res. 2021, 10, 56–65. [Google Scholar] [CrossRef]
- Vadde, R.; Radhakrishnan, S.; Reddivari, L.; Vanamala, J.K. Triphala extract suppresses proliferation and induces apoptosis in human colon cancer stem cells via suppressing c-Myc/Cyclin D1 and elevation of Bax/Bcl-2 ratio. BioMed Res. Int. 2015, 2015, 649263. [Google Scholar] [CrossRef]
- Liu, X.; Jiang, Q.; Liu, H.; Luo, S. Vitexin induces apoptosis through mitochondrial pathway and PI3K/Akt/mTOR signaling in human non-small cell lung cancer A549 cells. Biol. Res. 2019, 52, 7. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Guan, X.; Hou, R.; Zhang, X.; Guo, F.; Zhang, Z.; Hua, C. Vitexin attenuates epithelial ovarian cancer cell viability and motility in vitro and carcinogenesis in vivo via p38 and ERK1/2 pathways related VEGFA. Ann. Transl. Med. 2020, 8, 1139. [Google Scholar] [CrossRef]
- Zhou, P.; Zheng, Z.-H.; Wan, T.; Wu, J.; Liao, C.-W.; Sun, X.-J. Vitexin Inhibits Gastric Cancer Growth and Metastasis through HMGB1-mediated Inactivation of the PI3K/AKT/mTOR/HIF-1α Signaling Pathway. J. Gastric Cancer 2021, 21, 439. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, R.; Ni, H. Eriodictyol exerts potent anticancer activity against A549 human lung cancer cell line by inducing mitochondrial-mediated apoptosis, G2/M cell cycle arrest and inhibition of m-TOR/PI3K/Akt signalling pathway. Arch. Med. Sci. AMS 2020, 16, 446. [Google Scholar] [CrossRef]
- Yu, L.; Liu, X. Eriodictyol Suppresses Survival of Cervical Cancer Cells Through Mediation of PTEN/Akt Signaling Pathway. Curr. Top. Nutraceutical Res. 2020, 18, 196–201. [Google Scholar]
- Gossner, G.; Choi, M.; Tan, L.; Fogoros, S.; Griffith, K.A.; Kuenker, M.; Liu, J.R. Genistein-induced apoptosis and autophagocytosis in ovarian cancer cells. Gynecol. Oncol. 2007, 105, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Pagliacci, M.; Smacchia, M.; Migliorati, G.; Grignani, F.; Riccardi, C.; Nicoletti, I. Growth-inhibitory effects of the natural phyto-oestrogen genistein in MCF-7 human breast cancer cells. Eur. J. Cancer 1994, 30, 1675–1682. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Kim, S.H.; Kim, Y.B.; Jeon, Y.T.; Lee, S.C.; Song, Y.S. Genistein inhibits cell growth by modulating various mitogen-activated protein kinases and AKT in cervical cancer cells. Ann. N. Y. Acad. Sci. 2009, 1171, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-I.; Lee, K.-T.; Park, H.-J.; Kim, T.J.; Choi, Y.S.; Shih, I.-M.; Choi, J.-H. Tectorigenin sensitizes paclitaxel-resistant human ovarian cancer cells through downregulation of the Akt and NFκB pathway. Carcinogenesis 2012, 33, 2488–2498. [Google Scholar] [CrossRef]
- Zeng, L.; Yuan, S.; Shen, J.; Wu, M.; Pan, L.; Kong, X. Suppression of human breast cancer cells by tectorigenin through downregulation of matrix metalloproteinases and MAPK signaling in vitro. Mol. Med. Rep. 2018, 17, 3935–3943. [Google Scholar] [CrossRef]
- Jiang, C.-P.; Ding, H.; Shi, D.-H.; Wang, Y.-R.; Li, E.-G.; Wu, J.-H. Pro-apoptotic effects of tectorigenin on human hepatocellular carcinoma HepG2 cells. World J. Gastroenterol. WJG 2012, 18, 1753. [Google Scholar] [CrossRef]
- Xiong, L.; Lu, H.; Hu, Y.; Wang, W.; Liu, R.; Wan, X.; Fu, J. In vitro anti-motile effects of Rhoifolin, a flavonoid extracted from Callicarpa nudiflora on breast cancer cells via downregulating Podocalyxin-Ezrin interaction during Epithelial Mesenchymal Transition. Phytomed. Int. J. Phytother. Phytopharm. 2021, 93, 153486. [Google Scholar] [CrossRef]
- Ujiki, M.B.; Ding, X.-Z.; Salabat, M.R.; Bentrem, D.J.; Golkar, L.; Milam, B.; Talamonti, M.S.; Bell, R.H.; Iwamura, T.; Adrian, T.E. Apigenin inhibits pancreatic cancer cell proliferation through G2/M cell cycle arrest. Mol. Cancer 2006, 5, 76. [Google Scholar] [CrossRef]
- Liu, L.-Z.; Fang, J.; Zhou, Q.; Hu, X.; Shi, X.; Jiang, B.-H. Apigenin inhibits expression of vascular endothelial growth factor and angiogenesis in human lung cancer cells: Implication of chemoprevention of lung cancer. Mol. Pharmacol. 2005, 68, 635–643. [Google Scholar] [CrossRef]
- Oh, E.-K.; Kim, H.-J.; Bae, S.-M.; Park, M.-Y.; Kim, Y.-W.; Kim, T.-E.; Ahn, W.-S. Apigenin-induced apoptosis in cervical cancer cell lines. Korean J. Obstet. Gynecol. 2008, 874–881. [Google Scholar] [CrossRef]
- Kim, P.S.; Shin, J.H.; Jo, D.S.; Shin, D.W.; Choi, D.-H.; Kim, W.J.; Park, K.; Kim, J.K.; Joo, C.G.; Lee, J.S. Anti-melanogenic activity of schaftoside in Rhizoma Arisaematis by increasing autophagy in B16F1 cells. Biochem. Biophys. Res. Commun. 2018, 503, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Lewinska, A.; Adamczyk-Grochala, J.; Kwasniewicz, E.; Deregowska, A.; Wnuk, M. Diosmin-induced senescence, apoptosis and autophagy in breast cancer cells of different p53 status and ERK activity. Toxicol. Lett. 2017, 265, 117–130. [Google Scholar] [CrossRef] [PubMed]
- Helmy, M.; Ghoneim, A.I.; Katary, M.A.; Elmahdy, R.K. The synergistic anti-proliferative effect of the combination of diosmin and BEZ-235 (dactolisib) on the HCT-116 colorectal cancer cell line occurs through inhibition of the PI3K/Akt/mTOR/NF-κB axis. Mol. Biol. Rep. 2020, 47, 2217–2230. [Google Scholar] [CrossRef]
- Luo, H.; Rankin, G.O.; Li, Z.; DePriest, L.; Chen, Y.C. Kaempferol induces apoptosis in ovarian cancer cells through activating p53 in the intrinsic pathway. Food Chem. 2011, 128, 513–519. [Google Scholar] [CrossRef]
- Yoshida, T.; Konishi, M.; Horinaka, M.; Yasuda, T.; Goda, A.E.; Taniguchi, H.; Yano, K.; Wakada, M.; Sakai, T. Kaempferol sensitizes colon cancer cells to TRAIL-induced apoptosis. Biochem. Biophys. Res. Commun. 2008, 375, 129–133. [Google Scholar] [CrossRef]
- Wang, X.; Yang, Y.; An, Y.; Fang, G. The mechanism of anticancer action and potential clinical use of kaempferol in the treatment of breast cancer. Biomed. Pharmacother. 2019, 117, 109086. [Google Scholar] [CrossRef]
- Amado, N.G.; Predes, D.; Fonseca, B.F.; Cerqueira, D.M.; Reis, A.H.; Dudenhoeffer, A.C.; Borges, H.L.; Mendes, F.A.; Abreu, J.G. Isoquercitrin suppresses colon cancer cell growth in vitro by targeting the Wnt/β-catenin signaling pathway. J. Biol. Chem. 2014, 289, 35456–35467. [Google Scholar] [CrossRef]
- Huang, G.; Tang, B.; Tang, K.; Dong, X.; Deng, J.; Liao, L.; Liao, Z.; Yang, H.; He, S. Isoquercitrin inhibits the progression of liver cancer in vivo and in vitro via the MAPK signalling pathway. Oncol. Rep. 2014, 31, 2377–2384. [Google Scholar] [CrossRef]
- Horinaka, M.; Yoshida, T.; Shiraishi, T.; Nakata, S.; Wakada, M.; Nakanishi, R.; Nishino, H.; Sakai, T. The combination of TRAIL and luteolin enhances apoptosis in human cervical cancer HeLa cells. Biochem. Biophys. Res. Commun. 2005, 333, 833–838. [Google Scholar] [CrossRef]
- Cook, M.T. Mechanism of metastasis suppression by luteolin in breast cancer. Breast Cancer Targets Ther. 2018, 10, 89. [Google Scholar] [CrossRef] [PubMed]
- Pandurangan, A.K.; Esa, N.M. Luteolin, a bioflavonoid inhibits colorectal cancer through modulation of multiple signaling pathways: A review. Asian Pac. J. Cancer Prev. 2014, 15, 5501–5508. [Google Scholar] [CrossRef] [PubMed]
- Fu, T.; Wang, L.; Jin, X.-N.; Sui, H.-J.; Liu, Z.; Jin, Y. Hyperoside induces both autophagy and apoptosis in non-small cell lung cancer cells in vitro. Acta Pharmacol. Sin. 2016, 37, 505–518. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Zhang, T.; Zhu, X.; Yang, C.; Wang, Y.; Zhou, N.; Ju, B.; Zhou, T.; Deng, G.; Qiu, C. Hyperoside Induces Breast Cancer Cells Apoptosis via ROS-Mediated NF-κB Signaling Pathway. Int. J. Mol. Sci. 2019, 21, 131. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Ren, F.-Q.; Yang, C.-L.; Zhou, L.-M.; Liu, Y.-Y.; Xiao, J.; Zhu, L.; Wang, Z.-G. Anti-proliferation effects of isorhamnetin on lung cancer cells in vitro and in vivo. Asian Pac. J. Cancer Prev. 2015, 16, 3035–3042. [Google Scholar] [CrossRef] [PubMed]
- Saud, S.M.; Young, M.R.; Jones-Hall, Y.L.; Ileva, L.; Evbuomwan, M.O.; Wise, J.; Colburn, N.H.; Kim, Y.S.; Bobe, G. Chemopreventive activity of plant flavonoid isorhamnetin in colorectal cancer is mediated by oncogenic Src and β-catenin. Cancer Res. 2013, 73, 5473–5484. [Google Scholar] [CrossRef]
- Ma, A.; Zhang, R. Diosmetin inhibits cell proliferation, induces cell apoptosis and cell cycle arrest in liver cancer. Cancer Manag. Res. 2020, 12, 3537. [Google Scholar] [CrossRef]
- Choi, J.; Jiang, X.; Jeong, J.B.; Lee, S.-H. Anticancer activity of protocatechualdehyde in human breast cancer cells. J. Med. Food 2014, 17, 842–848. [Google Scholar] [CrossRef]
- Li, Y.; Yu, X.; Wang, Y.; Zheng, X.; Chu, Q. Kaempferol-3-O-rutinoside, a flavone derived from Tetrastigma hemsleyanum, suppresses lung adenocarcinoma via the calcium signaling pathway. Food Funct. 2021, 12, 8351–8365. [Google Scholar] [CrossRef]
- Yee Kuen, C.; Galen, T.; Fakurazi, S.; Othman, S.S.; Masarudin, M.J. Increased cytotoxic efficacy of protocatechuic acid in A549 human lung cancer delivered via hydrophobically modified-chitosan nanoparticles as an anticancer modality. Polymers 2020, 12, 1951. [Google Scholar] [CrossRef]
- Nafees, S.; Mehdi, S.H.; Zafaryab, M.; Zeya, B.; Sarwar, T.; Rizvi, M.A. Synergistic Interaction of Rutin and Silibinin on Human Colon Cancer Cell Line. Arch. Med. Res. 2018, 49, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Paudel, K.R.; Wadhwa, R.; Tew, X.N.; Lau, N.J.X.; Madheswaran, T.; Panneerselvam, J.; Zeeshan, F.; Kumar, P.; Gupta, G.; Anand, K. Rutin loaded liquid crystalline nanoparticles inhibit non-small cell lung cancer proliferation and migration in vitro. Life Sci. 2021, 276, 119436. [Google Scholar] [CrossRef] [PubMed]
- Zuo, F. Anti-breast Cancer Effect of Ononin and Its Mechanism in Vitro. Chin. Pharm. J. 2020, 194–198. [Google Scholar] [CrossRef]
- Ji, J.; Wang, Z.; Sun, W.; Li, Z.; Cai, H.; Zhao, E.; Cui, H. Effects of Cynaroside on Cell Proliferation, Apoptosis, Migration and Invasion though the MET/AKT/mTOR Axis in Gastric Cancer. Int. J. Mol. Sci. 2021, 22, 12125. [Google Scholar] [CrossRef]
- Chen, M.; Cai, F.; Zha, D.; Wang, X.; Zhang, W.; He, Y.; Huang, Q.; Zhuang, H.; Hua, Z.-C. Astragalin-induced cell death is caspase-dependent and enhances the susceptibility of lung cancer cells to tumor necrosis factor by inhibiting the NF-κB pathway. Oncotarget 2017, 8, 26941. [Google Scholar] [CrossRef]
- Wang, Z.; Lv, J.; Li, X.; Lin, Q. The flavonoid Astragalin shows anti-tumor activity and inhibits PI3K/AKT signaling in gastric cancer. Chem. Biol. Drug Des. 2021, 98, 779–786. [Google Scholar] [CrossRef]
- Wang, C.; Lyu, H.; Guo, Z. Metabolomic and Pathway Changes in Large-Leaf, Middle-Leaf and Small-Leaf Cultivars of Camellia sinensis (L.) Kuntze var. niaowangensis. Chem. Biodivers. 2021, 18, e2100132. [Google Scholar] [CrossRef]
- Shi, X.; Luo, X.; Chen, T.; Guo, W.; Liang, C.; Tang, S.; Mo, J. Naringenin inhibits migration, invasion, induces apoptosis in human lung cancer cells and arrests tumour progression in vitro. J. Cell. Mol. Med. 2021, 25, 2563–2571. [Google Scholar] [CrossRef]
- Lim, W.; Park, S.; Bazer, F.W.; Song, G. Naringenin-induced apoptotic cell death in prostate cancer cells is mediated via the PI3K/AKT and MAPK signaling pathways. J. Cell. Biochem. 2017, 118, 1118–1131. [Google Scholar] [CrossRef]
- Zhang, F.; Dong, W.; Zeng, W.; Zhang, L.; Zhang, C.; Qiu, Y.; Wang, L.; Yin, X.; Zhang, C.; Liang, W. Naringenin prevents TGF-β1 secretion from breast cancer and suppresses pulmonary metastasis by inhibiting PKC activation. Breast Cancer Res. 2016, 18, 38. [Google Scholar] [CrossRef]
- Bodet, C.; La, V.; Epifano, F.; Grenier, D. Naringenin has anti-inflammatory properties in macrophage and ex vivo human whole-blood models. J. Periodontal Res. 2008, 43, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Sahin, S.; Aybastıer, O.; Işık, E. Optimisation of ultrasonic-assisted extraction of antioxidant compounds from Artemisia absinthium using response surface methodology. Food Chem. 2013, 141, 1361–1368. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.Y.; Liao, P.R.; Zhao, M.Z.; Gong, C.; Dang, Y.; Qu, Y.; Qiu, L.S. Optimization of Ultrasonic Flavonoid Extraction from Saussurea involucrate, and the Ability of Flavonoids to Block Melanin Deposition in Human Melanocytes. Molecules 2020, 25, 313. [Google Scholar] [CrossRef]
- Yuxia, X.; Huabin, W. Effects of extracting technology of total flavonoids in Malus micromalus Makino by enzymic treatment and its flavonoid crude extract on proliferation of Hela cells in vitro. J. China Agric. Univ. 2013, 18, 119–127. [Google Scholar]
- Yun, C.; Wang, S.; Gao, Y.; Zhao, Z.; Miao, N.; Shi, Y.; Ri, I.; Wang, W.; Wang, H. Optimization of ultrasound-assisted enzymatic pretreatment for enhanced extraction of baicalein and wogonin from Scutellaria baicalensis roots. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2022, 1188, 123077. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Zhao, H.; Cui, L.; Hussain, H.; Nadolnik, L.; Zhang, Z.; Zhao, Y.; Qin, X.; Li, J.; Park, J.H.; et al. Ultrasonic-assisted extraction of flavonoids from peanut leave and stem using deep eutectic solvents and its molecular mechanism. Food Chem. 2024, 434, 137497. [Google Scholar] [CrossRef]
- Ou, H.; Zuo, J.; Gregersen, H.; Liu, X.-Y. Combination of supercritical CO2 and ultrasound for flavonoids extraction from Cosmos sulphureus: Optimization, kinetics, characterization and antioxidant capacity. Food Chem. 2024, 435, 137598. [Google Scholar] [CrossRef]
- Gerl, R.; Vaux, D.L. Apoptosis in the development and treatment of cancer. Carcinogenesis 2005, 26, 263–270. [Google Scholar] [CrossRef]
- Yuan, X.; Zhang, B.; Chen, N.; Chen, X.Y.; Liu, L.L.; Zheng, Q.S.; Wang, Z.P. Isoliquiritigenin treatment induces apoptosis by increasing intracellular ROS levels in HeLa cells. J. Asian Nat. Prod. Res. 2012, 14, 789–798. [Google Scholar] [CrossRef]
- Lim, H.M.; Park, S.H.; Nam, M.J. Induction of apoptosis in indole-3-carbinol-treated lung cancer H1299 cells via ROS level elevation. Hum. Exp. Toxicol. 2021, 40, 812–825. [Google Scholar] [CrossRef]






| A | B | C | |
|---|---|---|---|
| Level | Solid-to-Liquid Ratio (g/mL) | Enzymatic Hydrolysis Temperature (°C) | Ethanol Concentration (%) |
| −1 | 12 | 40 | 70 |
| 0 | 15 | 45 | 85 |
| 1 | 18 | 50 | 100 |
| Tests | A-Solid-to-Liquid Ratio (g/mL) | B-Enzymatic Hydrolysis Temperature (°C) | C-Ethanol Concentration (%) | Extraction Yield (%) |
|---|---|---|---|---|
| 1 | −1 | −1 | 0 | 2.4968 |
| 2 | 1 | 1 | 0 | 2.29127 |
| 3 | −1 | 0 | −1 | 2.72895 |
| 4 | 1 | 0 | 1 | 2.84924 |
| 5 | 0 | 0 | 0 | 3.62145 |
| 6 | 1 | −1 | 0 | 2.51907 |
| 7 | −1 | 0 | 1 | 2.85513 |
| 8 | 0 | 1 | 1 | 2.86716 |
| 9 | 0 | −1 | −1 | 2.8646 |
| 10 | 0 | 0 | 0 | 3.99514 |
| 11 | 0 | 0 | 0 | 3.69056 |
| 12 | 0 | 0 | 0 | 3.91323 |
| 13 | 1 | 0 | −1 | 2.27592 |
| 14 | −1 | 1 | 0 | 3.36115 |
| 15 | 0 | 0 | 0 | 3.83645 |
| 16 | 0 | 1 | −1 | 2.61121 |
| 17 | 0 | −1 | 1 | 3.22293 |
| Sources of Variation | Sum of Squares | Degree of Freedom (DOF) | Mean Square | F Value | p Value | Significance |
|---|---|---|---|---|---|---|
| Model | 4.99 | 9 | 0.55 | 11.63 | 0.0019 | significant |
| A | 0.28 | 1 | 0.28 | 5.95 | 0.0448 | |
| B | 9.38 × 10−5 | 1 | 9.38 × 10−5 | 1.97 × 10−3 | 0.9659 | |
| C | 0.22 | 1 | 0.22 | 4.53 | 0.0709 | |
| AB | 0.3 | 1 | 0.3 | 6.26 | 0.0409 | |
| AC | 0.05 | 1 | 0.05 | 1.05 | 0.3399 | |
| BC | 2.62 × 10−3 | 1 | 2.62 × 10−3 | 0.055 | 0.8213 | |
| A2 | 1.94 | 1 | 1.94 | 40.76 | 0.0004 | |
| B2 | 0.91 | 1 | 0.91 | 19.11 | 0.0033 | |
| C2 | 0.87 | 1 | 0.87 | 18.27 | 0.0037 | |
| Residual | 0.33 | 7 | 0.048 | |||
| Lack of Fit | 0.24 | 3 | 0.079 | 3.33 | 0.1379 | not significant |
| Pure Error | 0.095 | 4 | 0.024 | |||
| Cor Total | 5.32 | 16 | ||||
| R2 | 0.9373 | |||||
| CV% | 7.14 |
| Number | Compound Name | Structured | Compound Class | Active | Tumor Type | Mechanism | References |
|---|---|---|---|---|---|---|---|
| 1 | Galangin | C15H10O5 | Flavonoids | Anticancer | Ovarian cancer, colon cancer, liver cancer, gastric cancer, breast cancer, | Mitochondrial pathway induces apoptosis and cell cycle arrest and inhibits angiogenesis | [56,57,58,59,60] |
| 2 | Sinensetin | C20H20O7 | Flavonoids | Anticancer | Gastric cancer, liver cancer, breast cancer | Regulation of AMPK/mTOR pathway | [61,62,63] |
| 3 | Orientin | C21H20O11 | Flavonoids | Anticancer | Breast cancer, colon cancer, bladder cancer, cervical cancer | Inhibition of cell cycle arrest, inhibition of migration, and invasion | [64,65,66,67] |
| 4 | Hydroxygenkwanin | C16H12O6 | Flavonoids | Anticancer | Liver cancer, breast cancer, lung cancer | Induces apoptosis and inhibits cell proliferation | [68,69,70] |
| 5 | Glycitein | C16H12O5 | Flavonoids | Anticancer | Gastric cancer, glioma, breast cancer | NF-κB/AP-1-dependent and non-dependent pathways inhibit cell invasion and cell cycle arrest | [71,72,73] |
| 6 | Acacetin | C16H12O5 | Flavonoids | Anticancer | Breast cancer, liver cancer, prostate cancer, lung cancer | Inhibition of cell cycle arrest and cell migration | [74,75,76,77] |
| 7 | Tiliroside | C30H26O13 | Flavonoids | Anticancer | Liver cancer, breast cancer | Inhibits cell migration and invasion | [78,79] |
| 8 | Narcissoside | C28H32O16 | Flavonoids | Antidiabetic | [80] | ||
| 9 | Tectochrysin | C16H12O4 | Flavonoids | Anticancer | Colon cancer, lung cancer, prostate cancer | The death receptor pathway induces apoptosis | [81,82,83] |
| 10 | Daidzin | C21H20O9 | Flavonoids | Anticancer | Cervical cancer | JAK2/STAT3 inhibits cell growth and induces apoptosis | [84] |
| 11 | Chrysin | C15H10O4 | Flavonoids | Anticancer | Prostate cancer, gastric cancer, bladder cancer, colorectal cancer | Downregulation of PI3K-AKT and ERK inhibits cell proliferation, cell | [85,86,87,88] |
| 12 | Liquiritin | C21H22O9 | Flavonoids | Anti-inflammatory, anticancer | cervical cancer, gastric cancer, liver cancer | Upregulation of p53 and p21 induces apoptosis | [88,89,90,91,92] |
| 13 | Quercetin | C15H10O7 | Flavonoids | Anticancer | Gastric cancer, breast cancer, ovarian cancer, prostate cancer, liver cancer | Inhibition of cell cycle arrest, induction of apoptosis | [93,94,95,96,97] |
| 14 | Pinocembrin | C15H12O4 | Flavonoids | Anticancer | Colon cancer, melanoma, ovarian cancer, lung cancer | Inhibition of cell autophagy, proliferation, and migration | [98,99,100,101] |
| 15 | Genkwanin | C16H12O5 | Flavonoids | Anticancer | Lung cancer | Inhibition of cell cycle blockade | [102] |
| 16 | Isoliquiritigenin | C15H12O4 | Flavonoids | Anticancer | Prostate cancer, breast cancer, lung cancer, cervical cancer | Inhibition of cell cycle blockade | [103,104,105,106] |
| 17 | Chalconaringenin | C20H14O5 | Terpenoids | Antioxidant | [107] | ||
| 18 | Homoorientin | C21H20O11 | Flavonoids | Anticancer | Colon cancer | Inhibition of cell proliferation and induction of apoptosis | [108] |
| 19 | Vitexin | C21H20O10 | Flavonoids | Anticancer | Lung cancer, ovarian cancer, gastric cancer | Downregulation of p-p38/p38 and p-ERK1/2/ERK1/2; Bcl-2/Bax ratios | [109,110,111] |
| 20 | Eriodictyol | C15H12O6 | Flavonoids | Anticancer | Lung cancer, cervical cancer | Inhibition of mTOR/PI3K/AKT signaling pathway and cell cycle blockade | [112,113] |
| 21 | Genistein | C15H10O5 | Flavonoids | Anticancer | Ovarian cancer, breast cancer, cervical cancer | Inhibition of cell cycle arrest, induction of apoptosis | [114,115,116] |
| 22 | Tectorigenin | C16H12O6 | Flavonoids | Anticancer | Ovarian cancer, breast cancer, liver cancer | Inactivation of AKT/IKK/IκB/NF-κB signaling pathway induces apoptosis | [117,118,119] |
| 23 | Rhoifolin | C27H30O14 | Flavonoids | Anticancer | Breast cancer | Suppression of EMT | [120] |
| 24 | Apigenin | C15H10O5 | Flavonoids | Anticancer | Pancreatic cancer, lung cancer, cervical cancer | Induction of cell cycle arrest and apoptosis | [121,122,123] |
| 25 | Schaftoside | C26H28O14 | Flavonoids | Anticancer | Melanoma | Increased cellular autophagy | [124] |
| 26 | Diosmin | C28H32O15 | Flavonoids | Anticancer | Breast cancer, colorectal cancer | Inhibition of cell cycle arrest, induction of apoptosis | [125,126] |
| 27 | Kaempferol | C15H10O6 | Flavonoids | Anticancer | Ovarian cancer, colon cancer, breast cancer | Downregulation of PI3K/AKT and hTERT induces apoptosis and senescence | [127,128,129] |
| 28 | Isoquercitrin | C21H20O12 | Flavonoids | Anticancer | Colon cancer, liver cancer | Downregulation of the PI3K-AKT/mTOR signaling pathway inhibits cell proliferation | [130,131] |
| 29 | Luteolin | C15H10O6 | Flavonoids | Anticancer | Cervical cancer, breast cancer, colorectal cancer | Cell cycle arrest, inhibition of cell growth, and invasion | [132,133,134] |
| 30 | Hyperoside | C21H20O12 | Flavonoids | Anticancer | breast cancer, Lung cancer | AKT/mTOR-p70S6K signaling pathway induces autophagy | [135,136] |
| 31 | Isorhamnetin | C16H12O7 | Flavonoids | Anticancer | Lung cancer, colorectal cancer | Inhibition of PI3K AKT/mTOR signaling pathway to sup-press cell prolifera-tion | [137,138] |
| 32 | Diosmetin | C16H12O6 | Flavonoids | Anticancer | Liver cancer | Inhibits cell cycle arrest and induces apoptosis | [139] |
| 33 | Protocatechualdeh-yde | C7H6O3 | Phenols | Anticancer | Breast cancer | Cell cycle arrest | [140] |
| 34 | Kaempferol-3-O-rutinoside | C27H30O15 | Flavonoids | Anti-inflammatory | Lung cancer | Inhibition of cancer cell growth through calcium signaling pathway calcium signaling pathway | [141] |
| 35 | protocatechuic acid | C7H6O4 | Phenols | Anticancer | Lung cancer | Induction of apoptosis | [142] |
| 36 | Rutin | C27H30O16 | Flavonoids | Anticancer | Colon cancer, lung cancer | Induction of apoptosis | [143,144] |
| 37 | Ononin | C22H22O9 | Flavonoids | Anticancer | Breast cancer | Promotes cell apoptosis; decreases cell invasion and migration | [145] |
| 38 | Cynaroside | C21H20O11 | Flavonoids | Anticancer | Gastric cancer. | Cell cycle arrest | [146] |
| 39 | Astragalin | C21H20O11 | Flavonoids | Anticancer | Lung cancer, gastric cancer | Inhibits cell proliferation, cell migration, and invasion | [147,148] |
| 40 | 2-Chloro-L-phenylalanine | C9H10ClNO2 | Amino acids and their derivatives | Antioxidant | [149] | ||
| 41 | Naringenin | C15H12O5 | Flavonoids | Anti-inflammatory, anticancer | Lung cancer, breast cancer | Inhibition of cell proliferation, cell cycle arrest | [150,151,152,153] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, M.; Yasin, K.; Yu, S.; Li, J.; Xia, L. Total Flavonoids in Artemisia absinthium L. and Evaluation of Its Anticancer Activity. Int. J. Mol. Sci. 2023, 24, 16348. https://doi.org/10.3390/ijms242216348
He M, Yasin K, Yu S, Li J, Xia L. Total Flavonoids in Artemisia absinthium L. and Evaluation of Its Anticancer Activity. International Journal of Molecular Sciences. 2023; 24(22):16348. https://doi.org/10.3390/ijms242216348
Chicago/Turabian StyleHe, Meizhu, Kamarya Yasin, Shaoqi Yu, Jinyao Li, and Lijie Xia. 2023. "Total Flavonoids in Artemisia absinthium L. and Evaluation of Its Anticancer Activity" International Journal of Molecular Sciences 24, no. 22: 16348. https://doi.org/10.3390/ijms242216348





