Systemic Evidence for Mitochondrial Dysfunction in Age-Related Macular Degeneration as Revealed by mtDNA Copy Number Measurements in Peripheral Blood
Abstract
:1. Introduction
2. Results
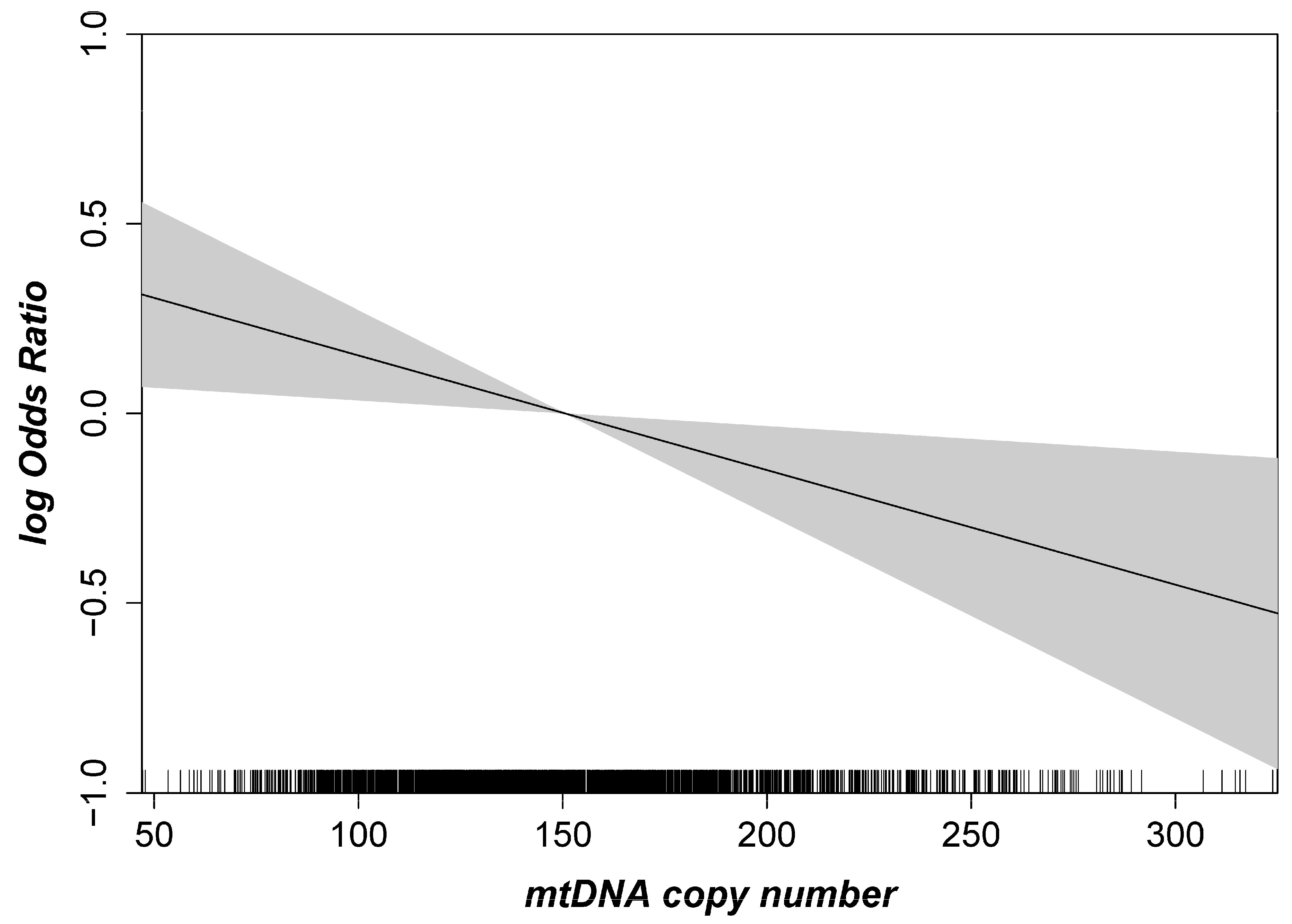
2.1. Mitochondrial DNA Copy Number and AMD
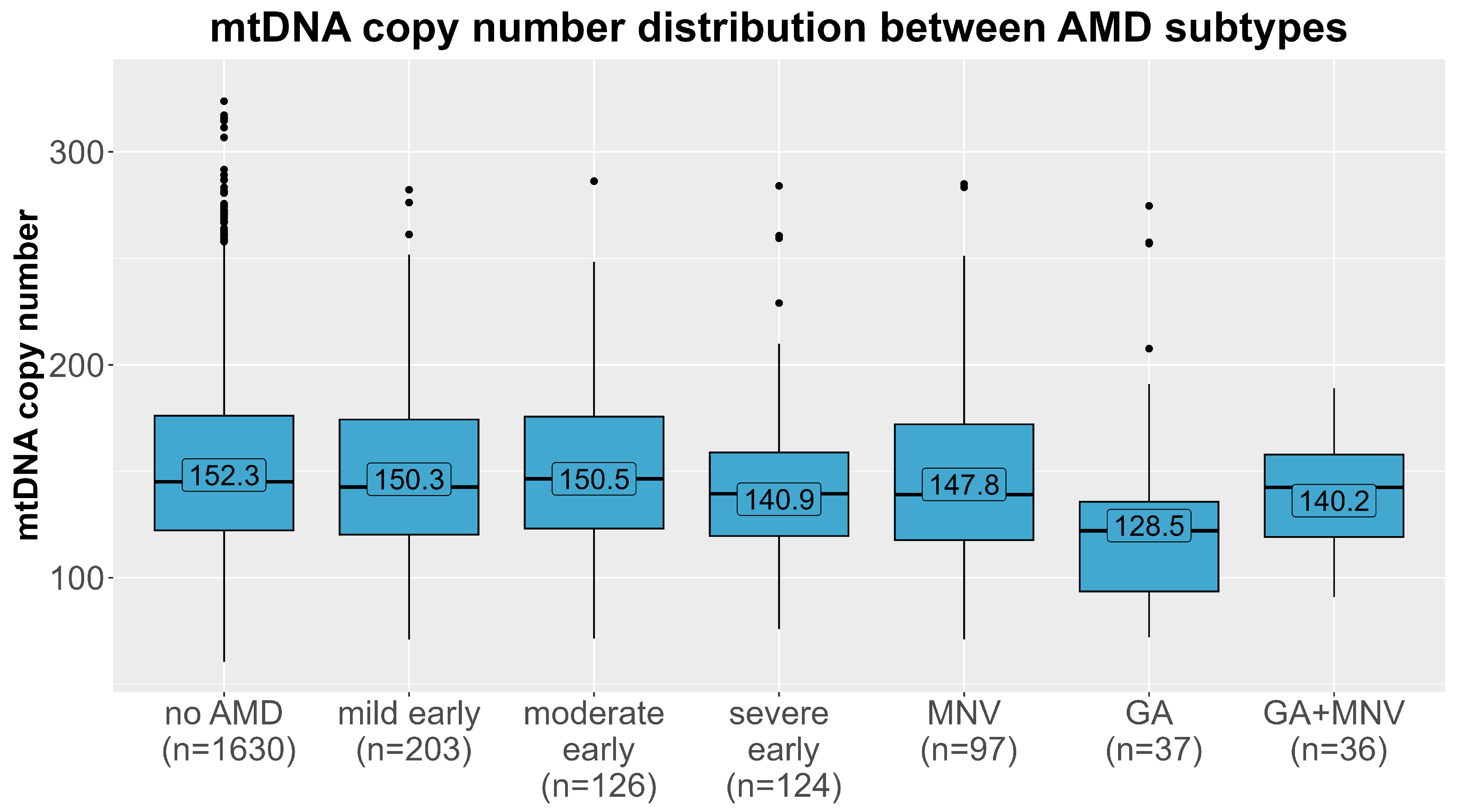
2.2. Effects Differ by AMD Subtypes
2.3. Mitochondrial DNA Haplogroup Analyses
3. Discussion
3.1. Biological Hypothesis
3.2. Strengths and Limitations
4. Materials and Methods
4.1. Study Design, Data Collection and AMD Grading
4.2. DNA Extraction
4.3. mtDNA Copy Number Measurements and Mitochondrial Haplogroup Determination
4.4. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Korb, C.A.; Kottler, U.B.; Wolfram, C.; Hoehn, R.; Schulz, A.; Zwiener, I.; Wild, P.S.; Pfeiffer, N.; Mirshahi, A. Prevalence of age-related macular degeneration in a large European cohort: Results from the population-based Gutenberg Health Study. Graefes Arch. Clin. Exp. Ophthalmol. 2014, 252, 1403–1411. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Q.; Welchowski, T.; Schmid, M.; Mauschitz, M.M.; Holz, F.G.; Finger, R.P. Prevalence and incidence of age-related macular degeneration in Europe: A systematic review and meta-analysis. Br. J. Ophthalmol. 2020, 104, 1077–1084. [Google Scholar] [CrossRef] [PubMed]
- Al-Zamil, W.M.; Yassin, S.A. Recent developments in age-related macular degeneration: A review. Clin. Interv. Aging 2017, 12, 1313–1330. [Google Scholar] [CrossRef]
- Brandl, C.; Zimmermann, M.E.; Günther, F.; Barth, T.; Olden, M.; Schelter, S.C.; Kronenberg, F.; Loss, J.; Küchenhoff, H.; Helbig, H.; et al. On the impact of different approaches to classify age-related macular degeneration: Results from the German AugUR study. Sci. Rep. 2018, 8, 8675. [Google Scholar] [CrossRef] [PubMed]
- Lim, L.S.; Mitchell, P.; Seddon, J.M.; Holz, F.G.; Wong, T.Y. Age-related macular degeneration. Lancet 2012, 379, 1728–1738. [Google Scholar] [CrossRef]
- Rosenfeld, P.J.; Brown, D.M.; Heier, J.S.; Boyer, D.S.; Kaiser, P.K.; Chung, C.Y.; Kim, R.Y.; Group, M.S. Ranibizumab for neovascular age-related macular degeneration. N. Engl. J. Med. 2006, 355, 1419–1431. [Google Scholar] [CrossRef]
- Rosenfeld, P.J. Bevacizumab versus ranibizumab for AMD. N. Engl. J. Med. 2011, 364, 1966–1967. [Google Scholar] [CrossRef]
- Wu, J.; Sun, X. Complement system and age-related macular degeneration: Drugs and challenges. Drug Des. Devel Ther. 2019, 13, 2413–2425. [Google Scholar] [CrossRef]
- Nebbioso, M.; Lambiase, A.; Cerini, A.; Limoli, P.G.; La Cava, M.; Greco, A. Therapeutic Approaches with Intravitreal Injections in Geographic Atrophy Secondary to Age-Related Macular Degeneration: Current Drugs and Potential Molecules. Int. J. Mol. Sci. 2019, 20, 1693. [Google Scholar] [CrossRef]
- Qin, S.; Dong, N.; Yang, M.; Wang, J.; Feng, X.; Wang, Y. Complement Inhibitors in Age-Related Macular Degeneration: A Potential Therapeutic Option. J. Immunol. Res. 2021, 2021, 9945725. [Google Scholar] [CrossRef]
- Sharma, A.; Jaganathan, B.G. Stem Cell Therapy for Retinal Degeneration: The Evidence to Date. Biologics 2021, 15, 299–306. [Google Scholar] [CrossRef]
- Chakravarthy, U.; Wong, T.Y.; Fletcher, A.; Piault, E.; Evans, C.; Zlateva, G.; Buggage, R.; Pleil, A.; Mitchell, P. Clinical risk factors for age-related macular degeneration: A systematic review and meta-analysis. BMC Ophthalmol. 2010, 10, 31. [Google Scholar] [CrossRef] [PubMed]
- van Leeuwen, R.; Boekhoorn, S.; Vingerling, J.R.; Witteman, J.C.; Klaver, C.C.; Hofman, A.; de Jong, P.T. Dietary intake of antioxidants and risk of age-related macular degeneration. JAMA 2005, 294, 3101–3107. [Google Scholar] [CrossRef]
- Fritsche, L.G.; Igl, W.; Bailey, J.N.; Grassmann, F.; Sengupta, S.; Bragg-Gresham, J.L.; Burdon, K.P.; Hebbring, S.J.; Wen, C.; Gorski, M.; et al. A large genome-wide association study of age-related macular degeneration highlights contributions of rare and common variants. Nat. Genet. 2016, 48, 134–143. [Google Scholar] [CrossRef]
- Kaarniranta, K.; Uusitalo, H.; Blasiak, J.; Felszeghy, S.; Kannan, R.; Kauppinen, A.; Salminen, A.; Sinha, D.; Ferrington, D. Mechanisms of mitochondrial dysfunction and their impact on age-related macular degeneration. Prog. Retin. Eye Res. 2020, 79, 100858. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.; Cano, M.; Ebrahimi, K.; Wang, L.; Handa, J.T. The impact of oxidative stress and inflammation on RPE degeneration in non-neovascular AMD. Prog. Retin. Eye Res. 2017, 60, 201–218. [Google Scholar] [CrossRef]
- Liu, M.M.; Chan, C.C.; Tuo, J. Genetic mechanisms and age-related macular degeneration: Common variants, rare variants, copy number variations, epigenetics, and mitochondrial genetics. Hum. Genom. 2012, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Udar, N.; Atilano, S.R.; Memarzadeh, M.; Boyer, D.S.; Chwa, M.; Lu, S.; Maguen, B.; Langberg, J.; Coskun, P.; Wallace, D.C.; et al. Mitochondrial DNA haplogroups associated with age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2009, 50, 2966–2974. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.M.; Manwaring, N.; Wang, J.J.; Rochtchina, E.; Mitchell, P.; Sue, C.M. Mitochondrial DNA haplogroups and age-related maculopathy. Arch. Ophthalmol. 2007, 125, 1235–1240. [Google Scholar] [CrossRef]
- Mueller, E.E.; Schaier, E.; Brunner, S.M.; Eder, W.; Mayr, J.A.; Egger, S.F.; Nischler, C.; Oberkofler, H.; Reitsamer, H.A.; Patsch, W.; et al. Mitochondrial haplogroups and control region polymorphisms in age-related macular degeneration: A case-control study. PLoS ONE 2012, 7, e30874. [Google Scholar] [CrossRef]
- Kenney, M.C.; Chwa, M.; Atilano, S.R.; Pavlis, J.M.; Falatoonzadeh, P.; Ramirez, C.; Malik, D.; Hsu, T.; Woo, G.; Soe, K.; et al. Mitochondrial DNA variants mediate energy production and expression levels for CFH, C3 and EFEMP1 genes: Implications for age-related macular degeneration. PLoS ONE 2013, 8, e54339. [Google Scholar] [CrossRef] [PubMed]
- Robin, E.D.; Wong, R. Mitochondrial DNA molecules and virtual number of mitochondria per cell in mammalian cells. J. Cell Physiol. 1988, 136, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Silva, P.; Enriquez, J.A.; Montoya, J. Replication and transcription of mammalian mitochondrial DNA. Exp. Physiol. 2003, 88, 41–56. [Google Scholar] [CrossRef]
- Karunadharma, P.P.; Nordgaard, C.L.; Olsen, T.W.; Ferrington, D.A. Mitochondrial DNA damage as a potential mechanism for age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2010, 51, 5470–5479. [Google Scholar] [CrossRef]
- Bilbao-Malavé, V.; González-Zamora, J.; de la Puente, M.; Recalde, S.; Fernandez-Robredo, P.; Hernandez, M.; Layana, A.G.; Saenz de Viteri, M. Mitochondrial Dysfunction and Endoplasmic Reticulum Stress in Age Related Macular Degeneration, Role in Pathophysiology, and Possible New Therapeutic Strategies. Antioxidants 2021, 10, 1170. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.N.; Czajka, A. Is mitochondrial DNA content a potential biomarker of mitochondrial dysfunction? Mitochondrion 2013, 13, 481–492. [Google Scholar] [CrossRef]
- Clay Montier, L.L.; Deng, J.J.; Bai, Y. Number matters: Control of mammalian mitochondrial DNA copy number. J. Genet. Genom. 2009, 36, 125–131. [Google Scholar] [CrossRef]
- Pyle, A.; Anugrha, H.; Kurzawa-Akanbi, M.; Yarnall, A.; Burn, D.; Hudson, G. Reduced mitochondrial DNA copy number is a biomarker of Parkinson’s disease. Neurobiol. Aging 2016, 38, 216.e7–216.e10. [Google Scholar] [CrossRef]
- Fazzini, F.; Lamina, C.; Raftopoulou, A.; Koller, A.; Fuchsberger, C.; Pattaro, C.; Del Greco, F.M.; Döttelmayer, P.; Fendt, L.; Fritz, J.; et al. Association of mitochondrial DNA copy number with metabolic syndrome and type 2 diabetes in 14 176 individuals. J. Intern. Med. 2021, 290, 190–202. [Google Scholar] [CrossRef]
- Koller, A.; Fazzini, F.; Lamina, C.; Rantner, B.; Kollerits, B.; Stadler, M.; Klein-Weigel, P.; Fraedrich, G.; Kronenberg, F. Mitochondrial DNA copy number is associated with all-cause mortality and cardiovascular events in patients with peripheral arterial disease. J. Intern. Med. 2020, 287, 569–579. [Google Scholar] [CrossRef]
- Tin, A.; Grams, M.E.; Ashar, F.N.; Lane, J.A.; Rosenberg, A.Z.; Grove, M.L.; Boerwinkle, E.; Selvin, E.; Coresh, J.; Pankratz, N.; et al. Association between Mitochondrial DNA Copy Number in Peripheral Blood and Incident CKD in the Atherosclerosis Risk in Communities Study. J. Am. Soc. Nephrol. 2016, 27, 2467–2473. [Google Scholar] [CrossRef] [PubMed]
- Ashar, F.N.; Moes, A.; Moore, A.Z.; Grove, M.L.; Chaves, P.H.M.; Coresh, J.; Newman, A.B.; Matteini, A.M.; Bandeen-Roche, K.; Boerwinkle, E.; et al. Association of mitochondrial DNA levels with frailty and all-cause mortality. J. Mol. Med. 2015, 93, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Monzel, A.S.; Enríquez, J.A.; Picard, M. Multifaceted mitochondria: Moving mitochondrial science beyond function and dysfunction. Nat. Metab. 2023, 5, 546–562. [Google Scholar] [CrossRef]
- Riley, J.S.; Tait, S.W. Mitochondrial DNA in inflammation and immunity. EMBO Rep. 2020, 21, e49799. [Google Scholar] [CrossRef] [PubMed]
- Saki, M.; Prakash, A. DNA damage related crosstalk between the nucleus and mitochondria. Free Radic. Biol. Med. 2017, 107, 216–227. [Google Scholar] [CrossRef]
- Sreekumar, P.G.; Ishikawa, K.; Spee, C.; Mehta, H.H.; Wan, J.; Yen, K.; Cohen, P.; Kannan, R.; Hinton, D.R. The Mitochondrial-Derived Peptide Humanin Protects RPE Cells From Oxidative Stress, Senescence, and Mitochondrial Dysfunction. Investig. Ophthalmol. Vis. Sci. 2016, 57, 1238–1253. [Google Scholar] [CrossRef]
- Nashine, S.; Cohen, P.; Chwa, M.; Lu, S.; Nesburn, A.B.; Kuppermann, B.D.; Kenney, M.C. Humanin G (HNG) protects age-related macular degeneration (AMD) transmitochondrial ARPE-19 cybrids from mitochondrial and cellular damage. Cell Death Dis. 2017, 8, e2951. [Google Scholar] [CrossRef]
- Kenney, M.C.; Atilano, S.R.; Boyer, D.; Chwa, M.; Chak, G.; Chinichian, S.; Coskun, P.; Wallace, D.C.; Nesburn, A.B.; Udar, N.S. Characterization of retinal and blood mitochondrial DNA from age-related macular degeneration patients. Investig. Ophthalmol. Vis. Sci. 2010, 51, 4289–4297. [Google Scholar] [CrossRef]
- Klein, R.; Meuer, S.M.; Myers, C.E.; Buitendijk, G.H.; Rochtchina, E.; Choudhury, F.; de Jong, P.T.; McKean-Cowdin, R.; Iyengar, S.K.; Gao, X.; et al. Harmonizing the classification of age-related macular degeneration in the three-continent AMD consortium. Ophthalmic Epidemiol. 2014, 21, 14–23. [Google Scholar] [CrossRef]
- Hurtado-Roca, Y.; Ledesma, M.; Gonzalez-Lazaro, M.; Moreno-Loshuertos, R.; Fernandez-Silva, P.; Enriquez, J.A.; Laclaustra, M. Adjusting MtDNA Quantification in Whole Blood for Peripheral Blood Platelet and Leukocyte Counts. PLoS ONE 2016, 11, e0163770. [Google Scholar] [CrossRef]
- López, S.; Buil, A.; Souto, J.C.; Casademont, J.; Blangero, J.; Martinez-Perez, A.; Fontcuberta, J.; Lathrop, M.; Almasy, L.; Soria, J.M. Sex-specific regulation of mitochondrial DNA levels: Genome-wide linkage analysis to identify quantitative trait loci. PLoS ONE 2012, 7, e42711. [Google Scholar] [CrossRef] [PubMed]
- Ventura-Clapier, R.; Dworatzek, E.; Seeland, U.; Kararigas, G.; Arnal, J.F.; Brunelleschi, S.; Carpenter, T.C.; Erdmann, J.; Franconi, F.; Giannetta, E.; et al. Sex in basic research: Concepts in the cardiovascular field. Cardiovasc. Res. 2017, 113, 711–724. [Google Scholar] [CrossRef] [PubMed]
- Mengel-From, J.; Svane, A.M.; Pertoldi, C.; Nygaard Kristensen, T.; Loeschcke, V.; Skytthe, A.; Christensen, K.; Lindahl-Jacobsen, R.; Hjelmborg, J.; Christiansen, L. Advanced Parental Age at Conception and Sex Affects Mitochondrial DNA Copy Number in Human and Fruit Flies. J. Gerontol. A Biol. Sci. Med. Sci. 2019, 74, 1853–1860. [Google Scholar] [CrossRef]
- Country, M.W. Retinal metabolism: A comparative look at energetics in the retina. Brain Res. 2017, 1672, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Canter, J.A.; Olson, L.M.; Spencer, K.; Schnetz-Boutaud, N.; Anderson, B.; Hauser, M.A.; Schmidt, S.; Postel, E.A.; Agarwal, A.; Pericak-Vance, M.A.; et al. Mitochondrial DNA polymorphism A4917G is independently associated with age-related macular degeneration. PLoS ONE 2008, 3, e2091. [Google Scholar] [CrossRef]
- SanGiovanni, J.P.; Arking, D.E.; Iyengar, S.K.; Elashoff, M.; Clemons, T.E.; Reed, G.F.; Henning, A.K.; Sivakumaran, T.A.; Xu, X.; DeWan, A.; et al. Mitochondrial DNA variants of respiratory complex I that uniquely characterize haplogroup T2 are associated with increased risk of age-related macular degeneration. PLoS ONE 2009, 4, e5508. [Google Scholar] [CrossRef] [PubMed]
- Primiano, G.; Abed, E.; Corbo, G.; Minnella, A.M.; Servidei, S.; Vollono, C.; Savastano, M.C.; Falsini, B. Macular impairment in mitochondrial diseases: A potential biomarker of disease severity. Sci. Rep. 2020, 10, 8554. [Google Scholar] [CrossRef]
- Fazzini, F.; Schöpf, B.; Blatzer, M.; Coassin, S.; Hicks, A.A.; Kronenberg, F.; Fendt, L. Plasmid-normalized quantification of relative mitochondrial DNA copy number. Sci. Rep. 2018, 8, 15347. [Google Scholar] [CrossRef]
- Stark, K.; Olden, M.; Brandl, C.; Dietl, A.; Zimmermann, M.E.; Schelter, S.C.; Loss, J.; Leitzmann, M.F.; Böger, C.A.; Luchner, A.; et al. The German AugUR study: Study protocol of a prospective study to investigate chronic diseases in the elderly. BMC Geriatr. 2015, 15, 130. [Google Scholar] [CrossRef]
- Stanzick, K.J.; Simon, J.; Zimmermann, M.E.; Schachtner, M.; Peterhoff, D.; Niller, H.H.; Überla, K.; Wagner, R.; Heid, I.M.; Stark, K.J. DNA extraction from clotted blood in genotyping quality. Biotechniques 2023, 74, 23–29. [Google Scholar] [CrossRef]
- Weissensteiner, H.; Pacher, D.; Kloss-Brandstätter, A.; Forer, L.; Specht, G.; Bandelt, H.J.; Kronenberg, F.; Salas, A.; Schönherr, S. HaploGrep 2: Mitochondrial haplogroup classification in the era of high-throughput sequencing. Nucleic Acids Res. 2016, 44, W58–W63. [Google Scholar] [CrossRef] [PubMed]
- van Oven, M. PhyloTree Build 17: Growing the human mitochondrial DNA tree. Forensic Sci. Int. Genet. Suppl. Ser. 2015, 5, e392–e394. [Google Scholar] [CrossRef]
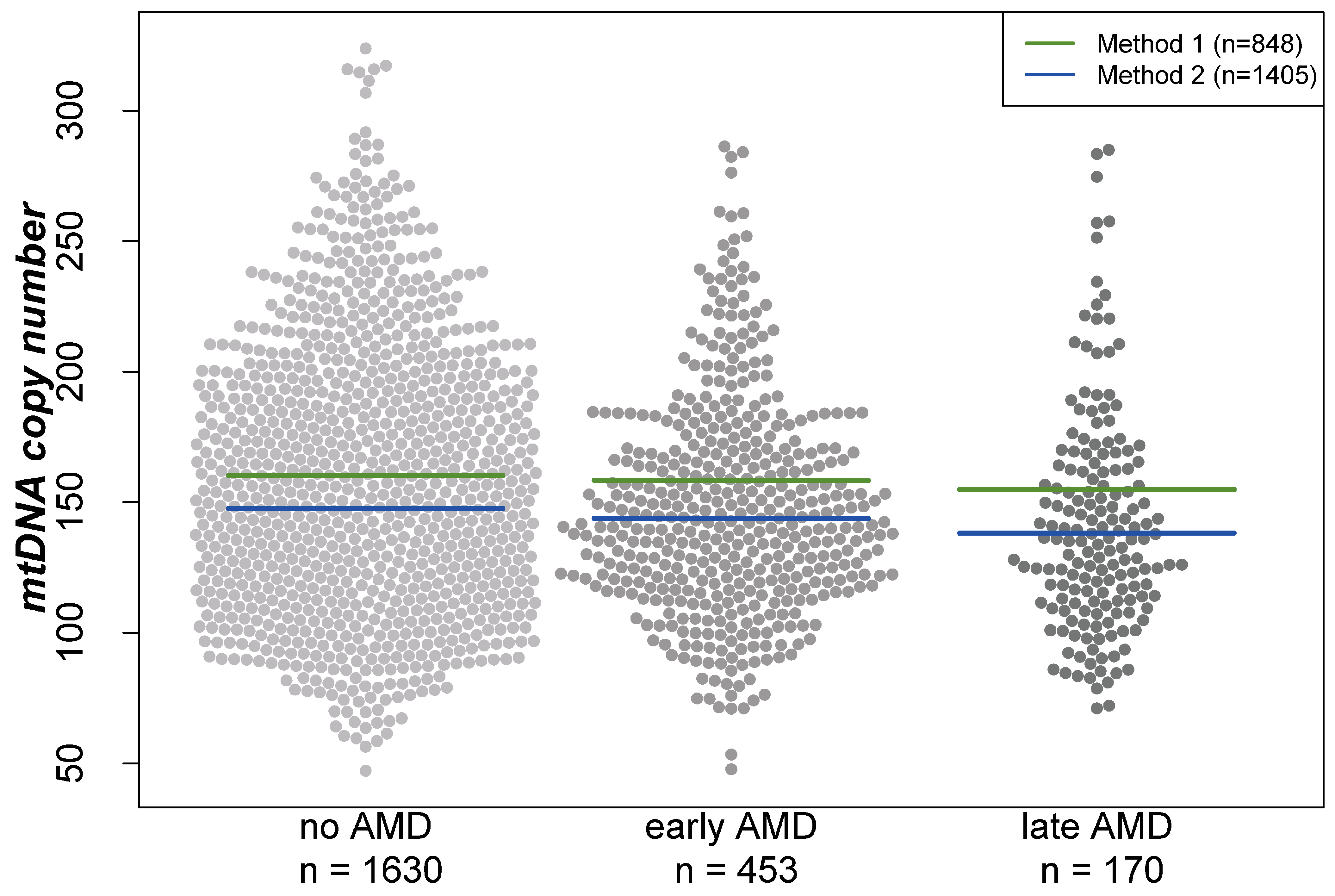
| No AMD (n = 1630) | Early AMD (n = 453) | Late AMD (n = 170) | |
|---|---|---|---|
| Sex, n (% male) | 794 (48.7%) | 198 (43.7%) | 81 (47.6%) |
| Age (years) | 77.6 ± 4.71 [74.0; 76.8; 80.6] | 79.1 ± 5.1 [75.2; 78.5; 82.6] | 81.9 ± 5.7 [77.7; 81.6; 86.4] |
| Smoking (current/ former/never), n (%) | 90 (5.5%)/644 (39.6)/892 (54.9%) | 20 (4.4%)/172 (38.1)/259 (57.4%) | 16 (9.5%)/57 (33.9)/95 (56.5%) |
| Body Mass Index (kg/m2) | 27.7 ± 4.5 [24.7; 27.2; 30.3] | 27.6 ± 4.7 [24.6; 27.0; 29.8] | 28.1 ± 4.4 [25.4; 27.7; 30.3] |
| Diabetes Mellitus, n (%) | 333 (20.4%) | 82 (18.2%) | 43 (25.3%) |
| Total cholesterol (mg/dL) | 218 ± 46 [185; 218; 248] | 218 ± 48 [187; 217; 250] | 215 ± 48 [184; 216; 243] |
| LDL cholesterol (mg/dL) | 142 ± 35 [116; 141; 164] | 140 ± 34 [116; 139; 163] | 139 ± 35 [115; 140; 161] |
| HDL cholesterol (mg/dL) | 60.7 ± 15.1 [49.6; 59.1; 69.8] | 63.6 ± 16.6 [52.1; 61.7; 72.8] | 59.4 ± 15.2 [47.6; 58.5; 68.9] |
| Triglycerides (mg/dL) | 160 ± 84 [102; 140; 195] | 149 ± 85 [92; 129; 183] | 159 ± 93 [96; 141; 188] |
| Leukocytes (G/L) | 6.5 ± 2.0 [5.3; 6.3; 7.4] | 6.4 ± 1.6 [5.4; 6.2; 7.2] | 6.8 ± 1.9 [5.6; 6.5; 7.5] |
| Thrombocytes (G/L) | 241 ± 63 [199; 235; 275] | 241 ± 64 [199; 237; 275] | 240 ± 61 [197; 242; 277] |
| HbA1c (mmol/mol) | 39.9 ± 7.3 [35.5; 38.4; 42.1] | 38.9 ± 6.7 [34.5; 37.7; 41.0] | 41.2 ± 9.4 [35.5; 38.8; 45.3] |
| Hypertension, n (%) * | 1174 (72.1%) | 325 (71.9%) | 131 (77.1%) |
| Cardiovascular disease, n (%) † | 343 (21.3%) | 87 (19.4%) | 53 (31.4%) |
| Mitochondrial haplogroups, n (%) ‡ | |||
| 792 (48.6%) | 247 (54.5%) | 86 (50.6%) |
| 354 (21.7%) | 77 (17.0%) | 38 (22.4%) |
| 347 (21.3%) | 90 (19.9%) | 36 (21.2%) |
| 112 (6.9%) | 34 (7.5%) | 10 (5.9%) |
| 25 (1.5%) | 5 (1.1%) | 0 (0%) |
| Mitochondrial DNA copy number | 152.3 ± 43.3 [122.0; 145.0; 176.0] | 147.8 ± 40.8 [120.4; 141.7; 169.6] | 142.0 ± 42.6 [112.2; 135.8; 162.7] |
| mtDNA Copy Number | All (n = 2253) | Women (n = 1073) | Men (n = 1180) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | |
| Early AMD vs. No AMD | Late AMD vs. No AMD | Early AMD vs. No AMD | Late AMD vs. No AMD | Early AMD vs. No AMD | Late AMD vs. No AMD | |||||||
| Model 1 adjusted for age | 1.05 (0.95–1.16) | 0.32 | 1.25 (1.06–1.47) | 0.008 | 1.02 (0.90–1.17) | 0.78 | 1.12 (0.90–1.41) | 0.29 | 1.10 (0.95–1.28) | 0.18 | 1.37 (1.07–1.76) | 0.01 |
| Model 2 adjusted for age, sex, smoking | 1.07 (0.97–1.19) | 0.20 | 1.22 (1.03–1.43) | 0.02 | 1.02 (0.89–1.17) | 0.76 | 1.09 (0.87–1.36) | 0.47 | 1.10 (0.95–1.28) | 0.20 | 1.35 (1.06–1.72) | 0.02 |
| Model 3 adjusted for age, sex, smoking, leukocyte, thrombocyte counts * | 1.10 (0.98–1.23) | 0.09 | 1.24 (1.04–1.47) | 0.02 | 1.04 (0.89–1.21) | 0.63 | 1.07 (0.84–1.36) | 0.59 | 1.16 (0.99–1.36) | 0.07 | 1.45 (1.11–1.90) | 0.006 |
| Model 4 adjusted for age, sex, smoking, CVD, HDL-C, hypertension | 1.10 (0.99–1.23) | 0.07 | 1.20 (1.01–1.42) | 0.04 | 1.02 (0.89–1.19) | 0.74 | 1.03 (0.82–1.30) | 0.79 | 1.19 (1.02–1.40) | 0.03 | 1.41 (1.08–1.82) | 0.01 |
| Model 5 adjusted for age, sex, smoking, diabetes, HbA1c | 1.08 (0.97–1.21) | 0.15 | 1.22 (1.02–1.44) | 0.03 | 1.02 (0.89–1.18) | 0.75 | 1.04 (0.83–1.32) | 0.71 | 1.14 (0.98–1.32) | 0.09 | 1.41 (1.08–1.84) | 0.01 |
| Model 6 fully adjusted † | 1.11 (1.00–1.25) | 0.06 | 1.23 (1.03–1.46) | 0.02 | 1.05 (0.90–1.22) | 0.56 | 1.05 (0.83–1.34) | 0.68 | 1.18 (0.01–1.39) | 0.04 | 1.45 (1.11–1.91) | 0.007 |
| mtDNA Copy Number | n | All | Women | Men | |||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | OR (95% CI) | p-Value | ||
| AMD vs. No AMD | AMD vs. No AMD | AMD vs. No AMD | |||||
| Mild early (early AMD) | 203 | 1.02 (0.88–1.18) | 0.83 | 0.96 (0.78–1.18) | 0.68 | 1.07 (0.86–1.32) | 0.54 |
| Moderate early (early AMD) | 126 | 1.10 (0.91–1.32) | 0.34 | 1.06 (0.84–1.34) | 0.63 | 1.13 (0.83–1.52) | 0.44 |
| Severe early (early AMD) | 124 | 1.32 (1.07–1.62) | 0.009 | 1.19 (0.90–1.58) | 0.21 | 1.25 (0.95–1.65) | 0.11 |
| Geographic atrophy (late AMD) | 37 | 1.76 (1.19–2.60) | 0.004 | 1.88 (1.07–3.32) | 0.03 | 1.54 (0.93–2.54) | 0.10 |
| Macular neovascular AMD (late AMD) | 97 | 1.08 (0.87–1.33) | 0.49 | 0.84 (0.63–1.11) | 0.22 | 1.32 (0.98–1.79) | 0.07 |
| Combination of Geographic atrophy and Macular neovascularization (late AMD) | 36 | 1.34 (0.95–1.91) | 0.10 | 1.45 (0.85–2.47) | 0.17 | 1.13 (0.73–1.75) | 0.57 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koller, A.; Lamina, C.; Brandl, C.; Zimmermann, M.E.; Stark, K.J.; Weissensteiner, H.; Würzner, R.; Heid, I.M.; Kronenberg, F. Systemic Evidence for Mitochondrial Dysfunction in Age-Related Macular Degeneration as Revealed by mtDNA Copy Number Measurements in Peripheral Blood. Int. J. Mol. Sci. 2023, 24, 16406. https://doi.org/10.3390/ijms242216406
Koller A, Lamina C, Brandl C, Zimmermann ME, Stark KJ, Weissensteiner H, Würzner R, Heid IM, Kronenberg F. Systemic Evidence for Mitochondrial Dysfunction in Age-Related Macular Degeneration as Revealed by mtDNA Copy Number Measurements in Peripheral Blood. International Journal of Molecular Sciences. 2023; 24(22):16406. https://doi.org/10.3390/ijms242216406
Chicago/Turabian StyleKoller, Adriana, Claudia Lamina, Caroline Brandl, Martina E. Zimmermann, Klaus J. Stark, Hansi Weissensteiner, Reinhard Würzner, Iris M. Heid, and Florian Kronenberg. 2023. "Systemic Evidence for Mitochondrial Dysfunction in Age-Related Macular Degeneration as Revealed by mtDNA Copy Number Measurements in Peripheral Blood" International Journal of Molecular Sciences 24, no. 22: 16406. https://doi.org/10.3390/ijms242216406
APA StyleKoller, A., Lamina, C., Brandl, C., Zimmermann, M. E., Stark, K. J., Weissensteiner, H., Würzner, R., Heid, I. M., & Kronenberg, F. (2023). Systemic Evidence for Mitochondrial Dysfunction in Age-Related Macular Degeneration as Revealed by mtDNA Copy Number Measurements in Peripheral Blood. International Journal of Molecular Sciences, 24(22), 16406. https://doi.org/10.3390/ijms242216406







