Moringa oleifera Leaves Protein Enhances Intestinal Permeability by Activating TLR4 Upstream Signaling and Disrupting Tight Junctions
Abstract
:1. Introduction
2. Results
2.1. Viability of Caco-2 Cells
2.2. Changes in the TEER of Monolayers of Cells
2.3. FITC-Dextran Cellular Transport
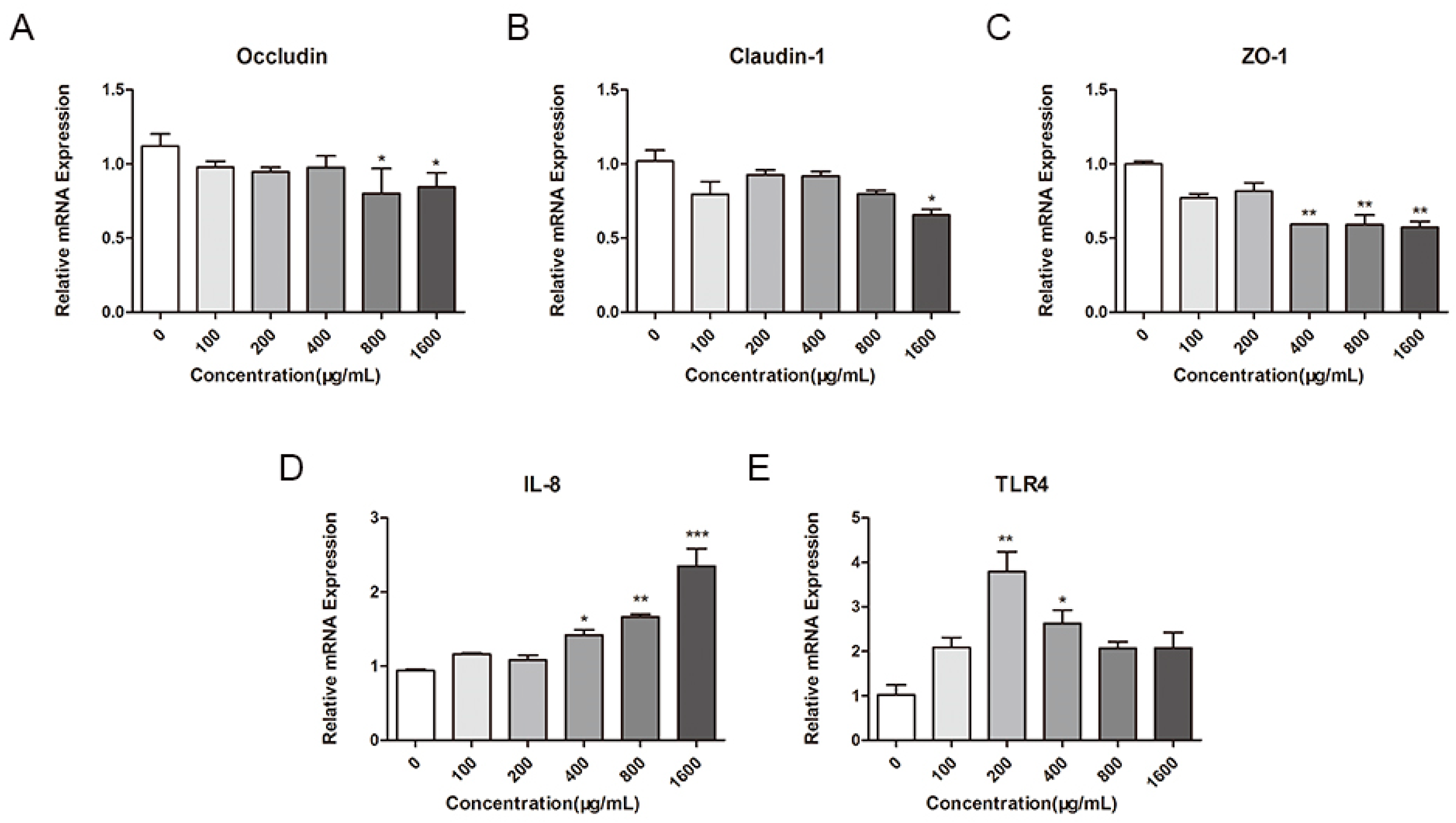
2.4. Effect of M. oleifera Leaves Protein on the mRNA Expression of Tight Junction Proteins in Caco-2 Cells
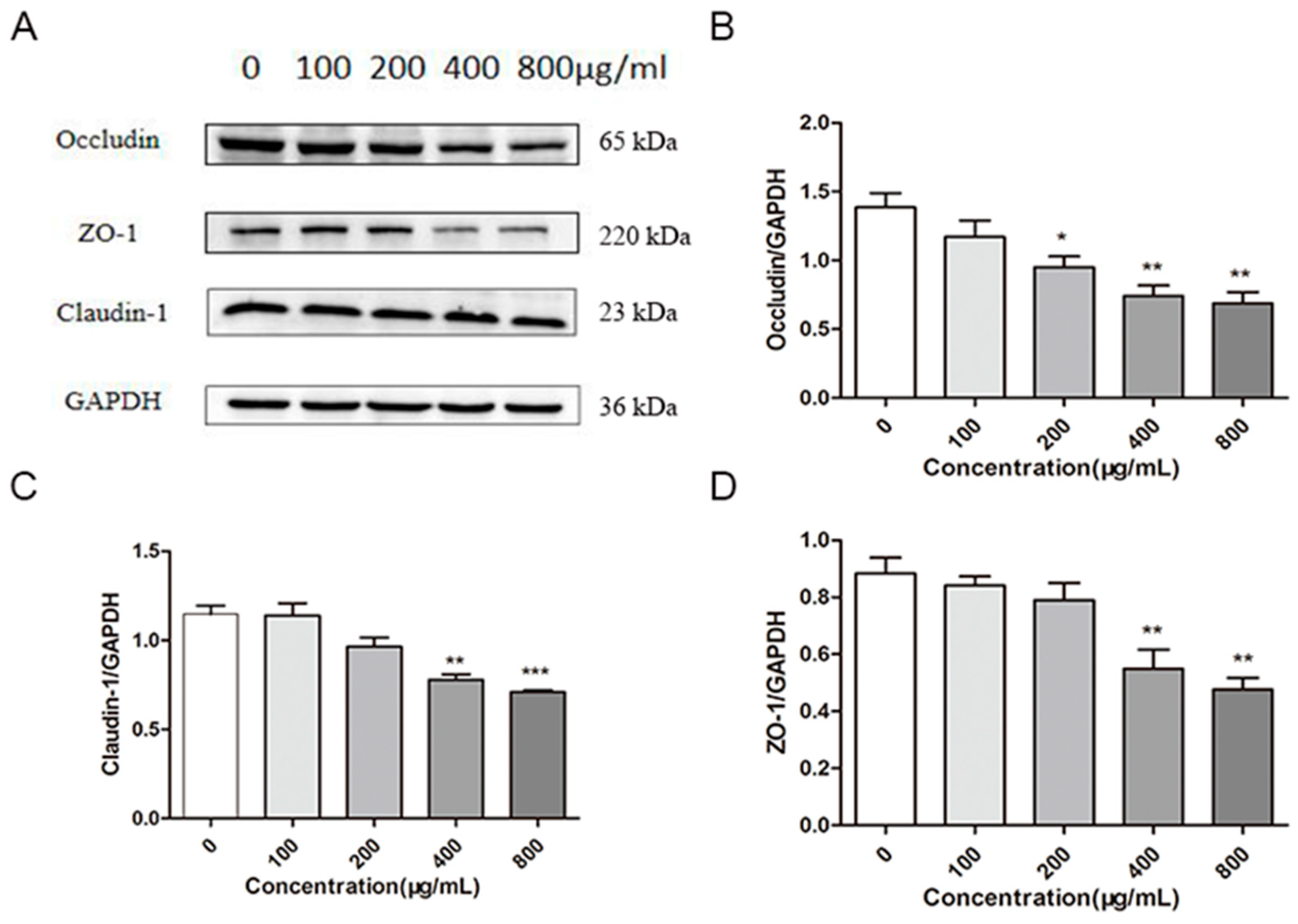
2.5. Effects of M. oleifera Leaves Protein on the Expression of Tight Junction Proteins in Caco-2 Cells
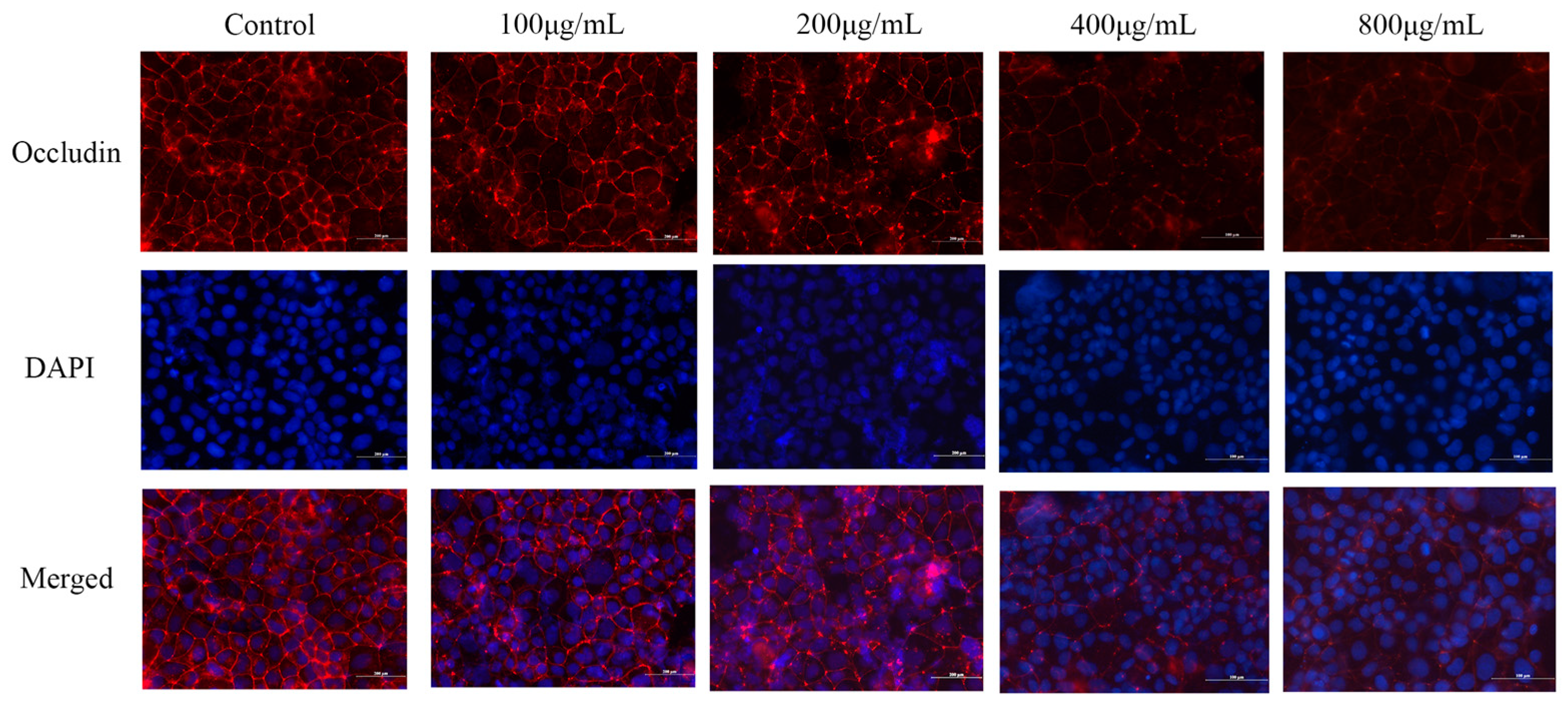
2.6. Effect of M. oleifera Leaves Protein Treatment on Occludin Protein Localization in Caco-2 Cells
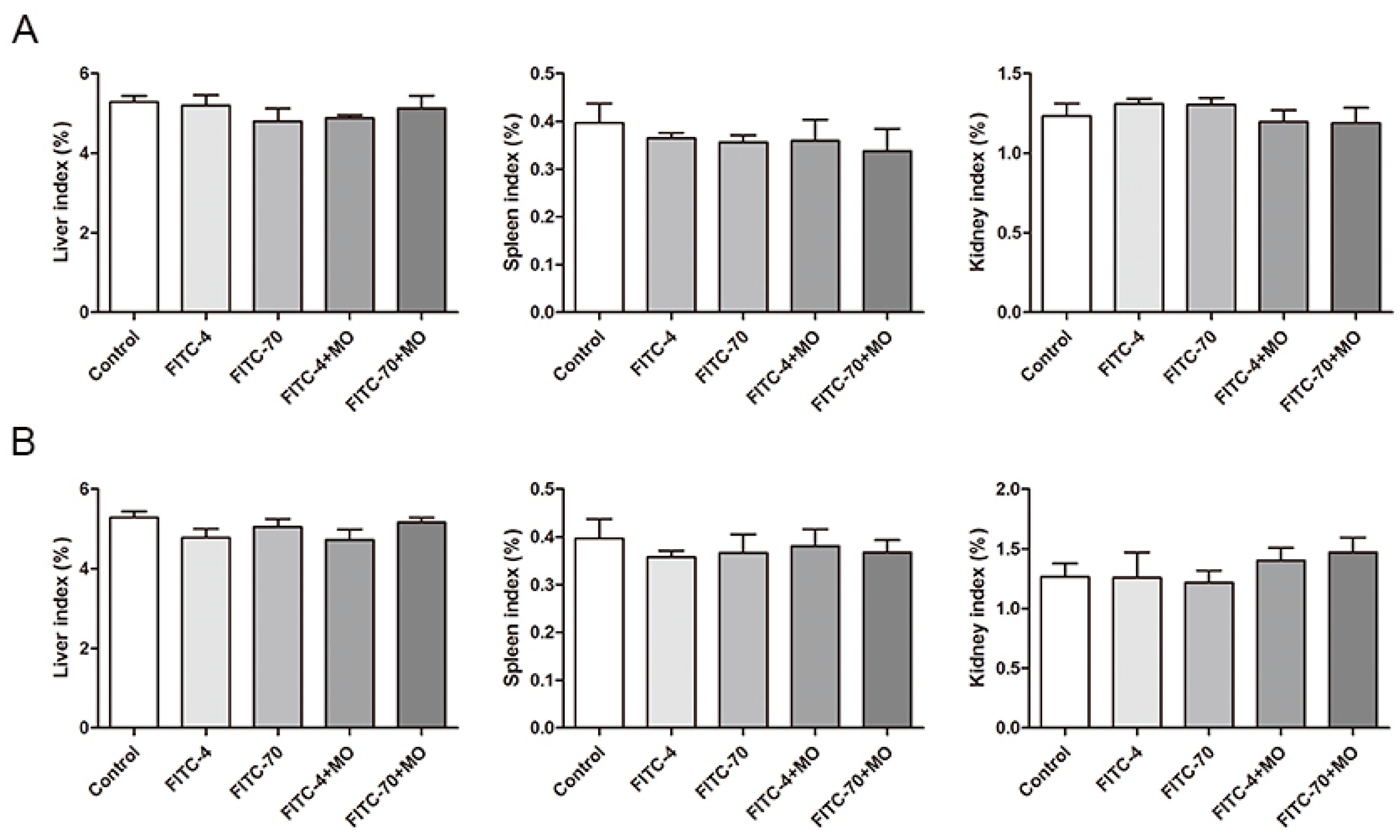
2.7. Effects of M. oleifera Leaves Protein on Organ Indices in Mice
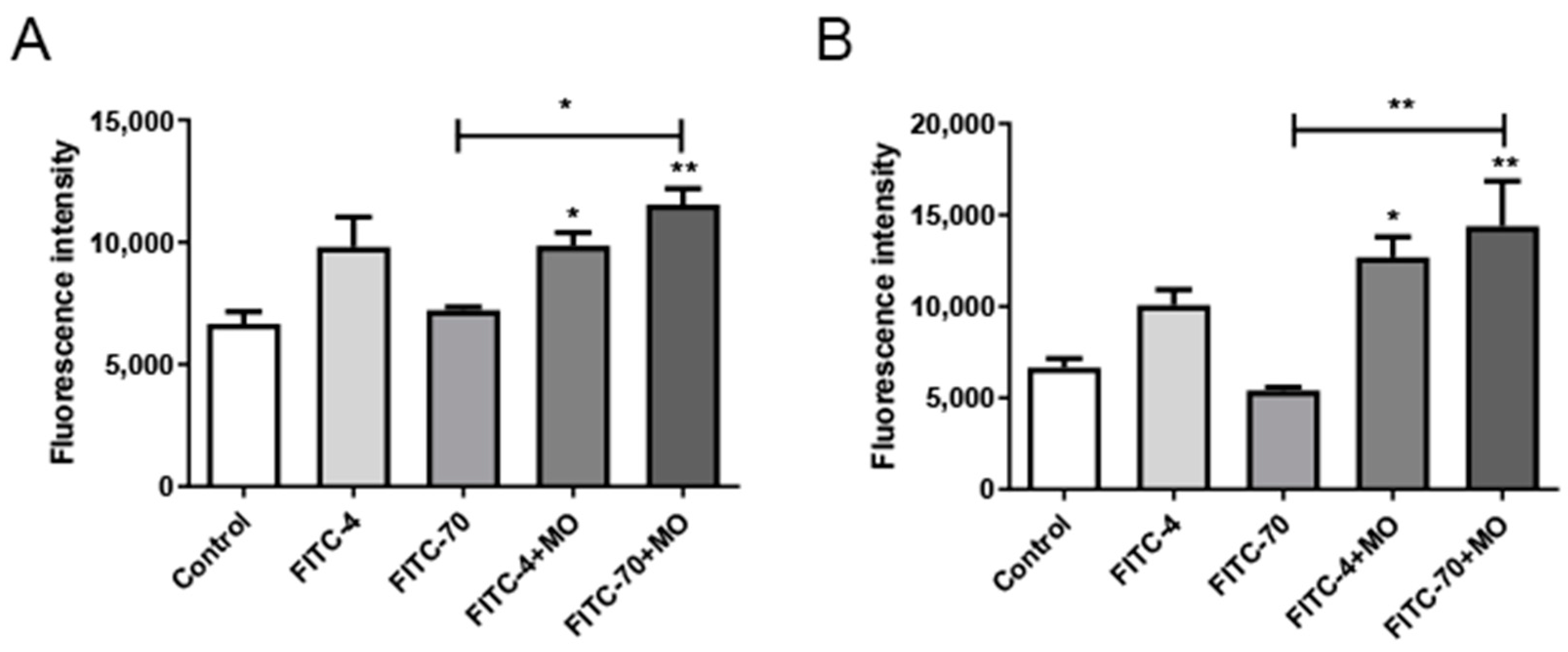
2.8. Serum Fluorescence Intensity in Mice
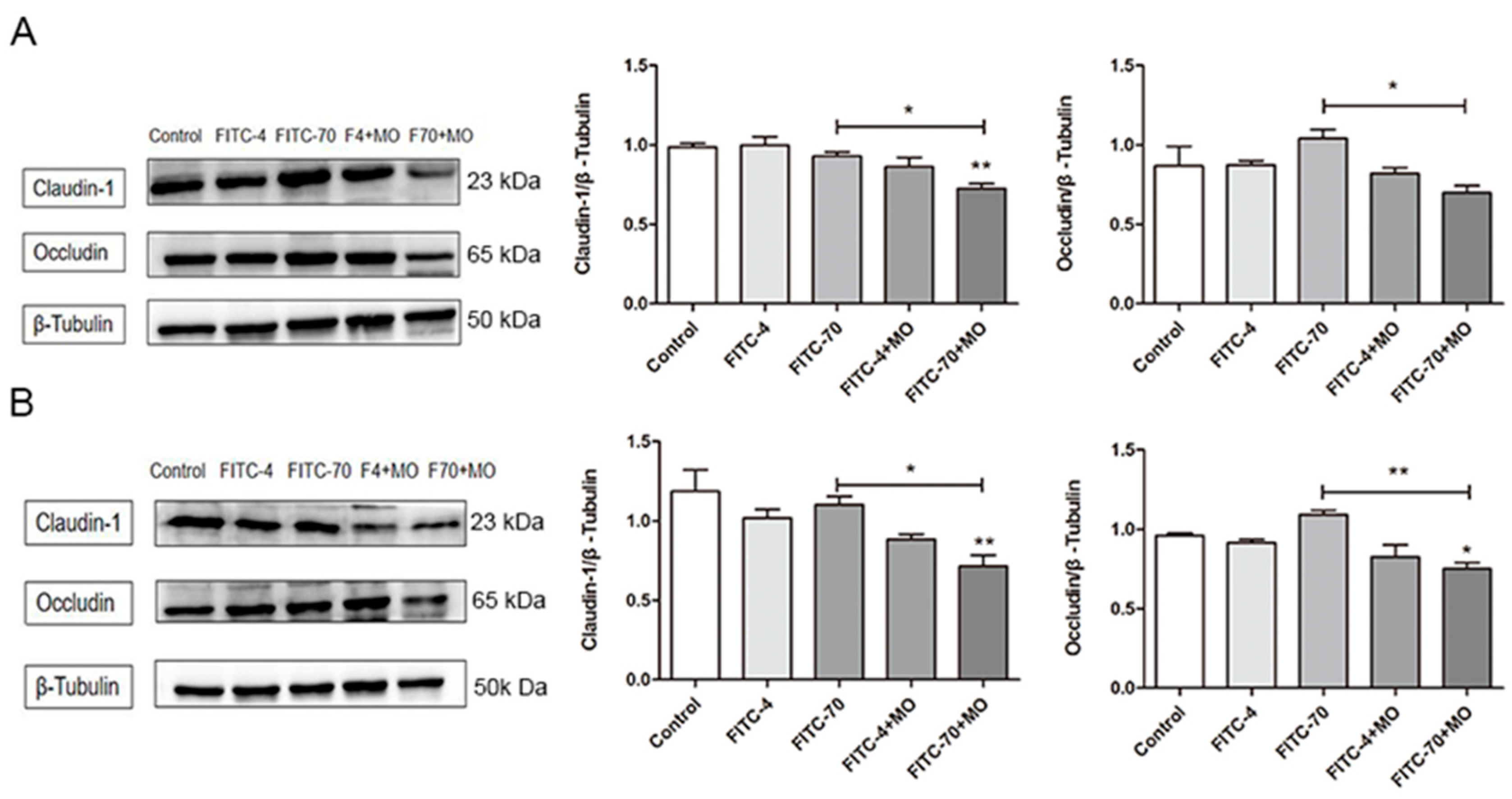
2.9. Effects of M. oleifera Leaves Protein on Tight Junction Protein Expression in the Small Intestinal Epithelium
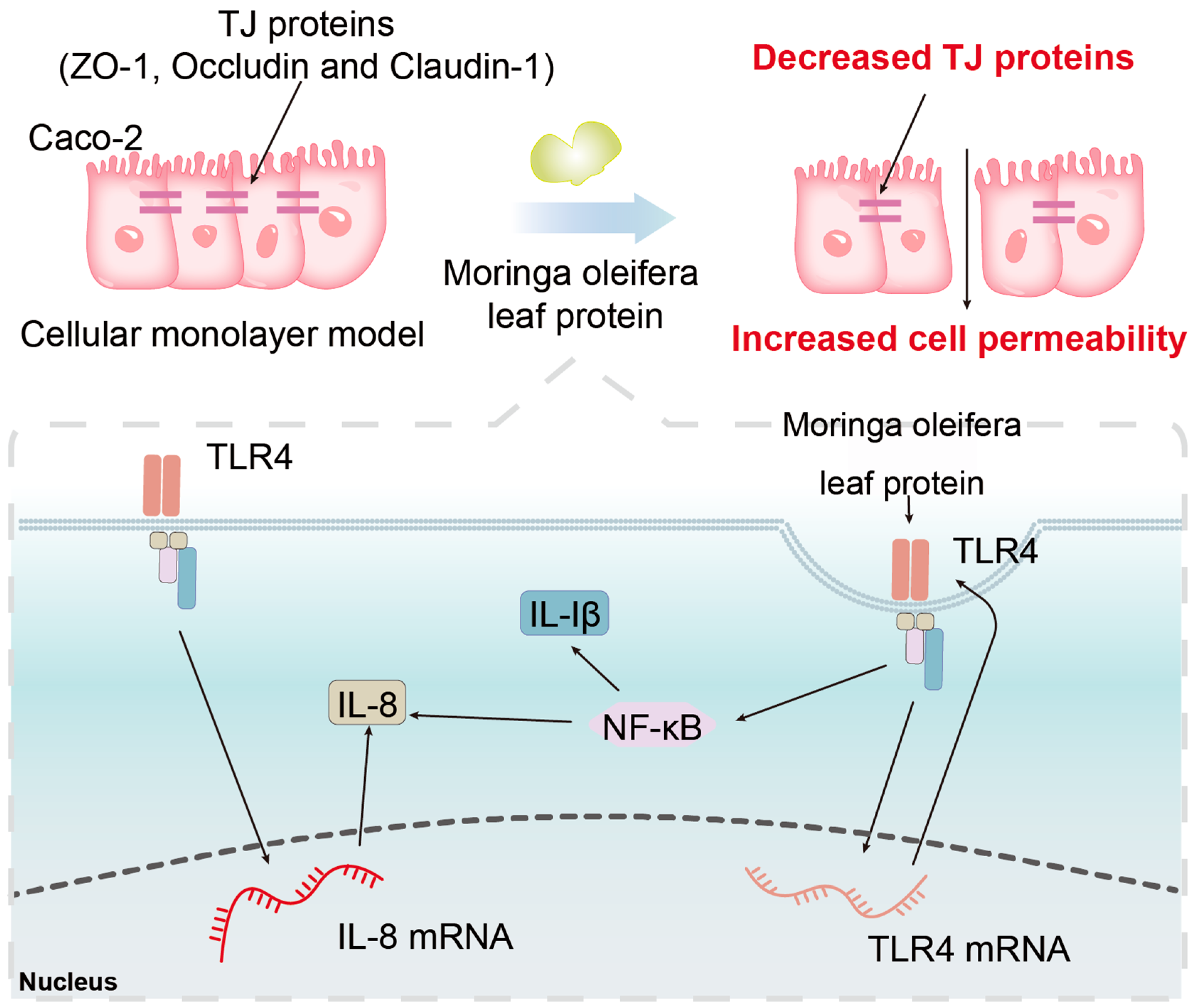
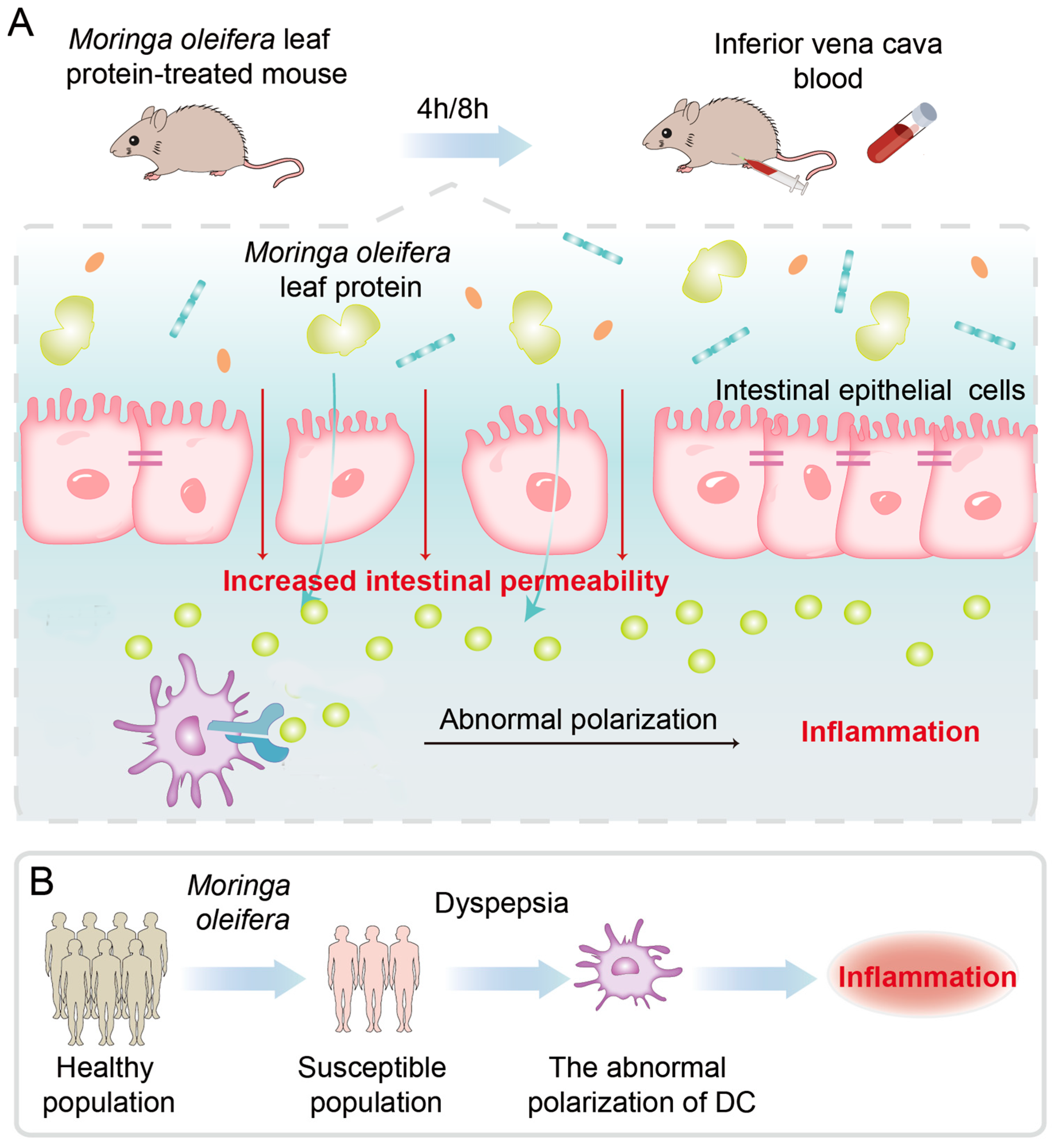
3. Discussion
4. Materials and Methods
4.1. Materials and Chemicals
4.2. Caco-2 Cell Assay
4.2.1. Cell Viability Assay
4.2.2. Establishment and Grouping of the Caco-2 Cell Permeability Model
4.2.3. Evaluation of Cell Transepithelial Electrical Resistance (TEER)
4.2.4. Permeability Assays
4.2.5. Determination of the mRNA Expression of Tight Junction Proteins by Real-Time PCR (RT–PCR)
4.2.6. Immunoblot Analysis
4.2.7. Effect of M. oleifera Leaves Protein Treatment on Occludin Protein Localization in Caco-2 Cells
4.3. Animal Experiments
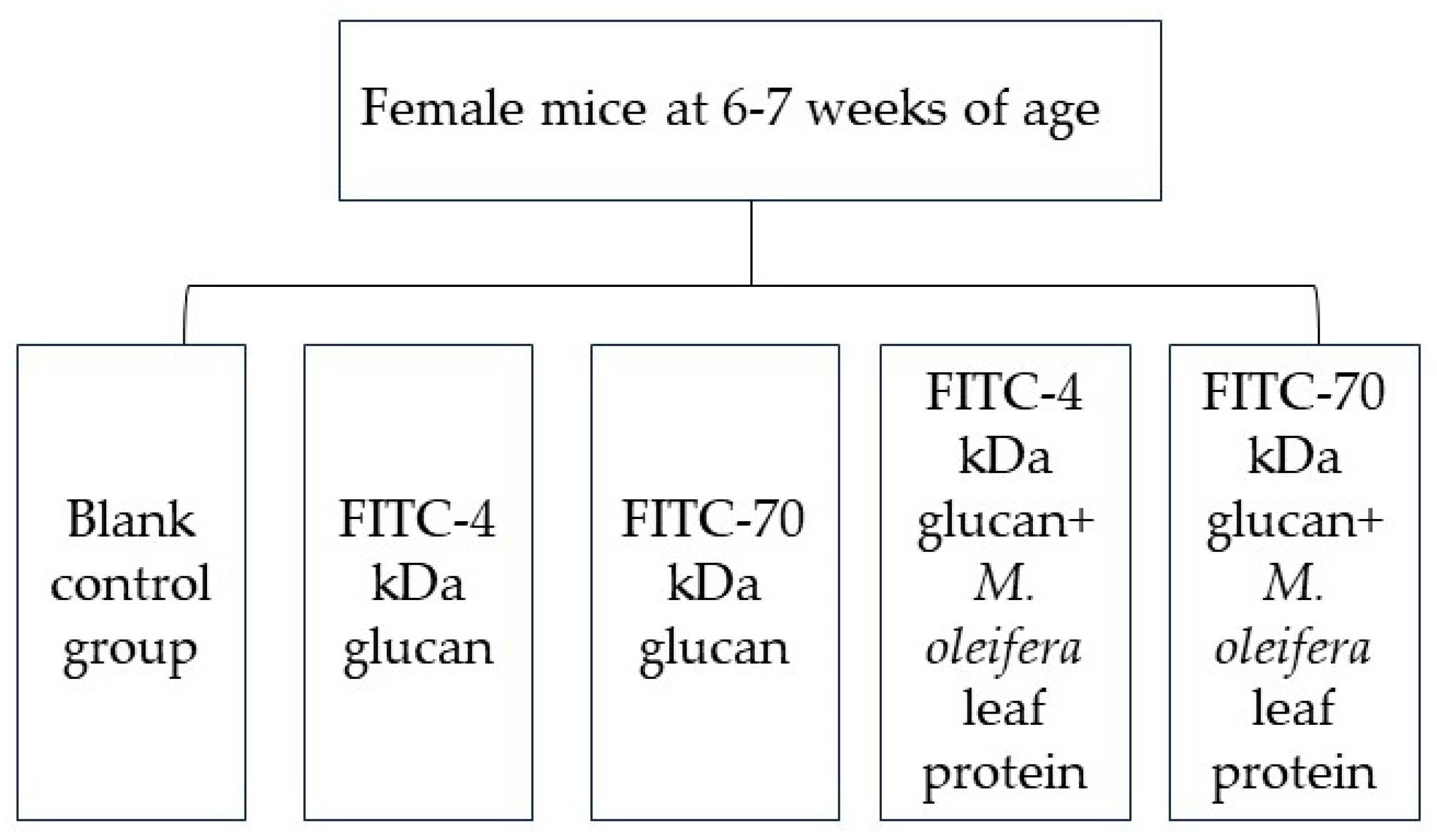
4.3.1. Study Subjects and Subgroups
4.3.2. Mouse Serum Fluorescence Intensity Assay
4.3.3. Assessment of Physiological Indicators in Mice
4.3.4. Western Blot Analysis
4.4. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Vaknin, Y.; Mishal, A. The potential of the tropical “miracle tree” Moringa oleifera and its desert relative Moringa peregrina as edible seed-oil and protein crops under Mediterranean conditions. Sci. Hortic. 2017, 225, 431–437. [Google Scholar] [CrossRef]
- Sagona, W.C.J.; Chirwa, P.W.; Sajidu, S.M. The miracle mix of Moringa: Status of Moringa research and development in Malawi. S. Afr. J. Bot. 2020, 129, 138–145. [Google Scholar] [CrossRef]
- Pavan, A.R.; Ahad, H.A.; Babu, G.N.; Keerthi, P.S. Moringa oleifera, Phyto Contituents and its Therapeutic Actions: A Review. Food Sci. Hum. Wellness 2016, 5, 49–56. [Google Scholar] [CrossRef]
- Ufele, A.N.; Ebenebe, C.I.; Igwe, I.I.; Mogbo, T.C.; Akunne, E.C.; Aziagba, B.O. The Effects of Drumstick Tree (Moringa oleifera) Leaves Meal on the Average Weight Gain of Domestic Rabbits (Oryctolagus cuniculus). Biosci. J. 2013, 1, 106–108. Available online: http://www.bioscientistjournal.com (accessed on 27 October 2023).
- Bassey, K.; Mabowe, M.; Mothibe, M.; Witika, B.A. Chemical Characterization and Nutritional Markers of South African Moringa oleifera Seed Oils. Molecules 2022, 27, 5749. [Google Scholar] [CrossRef] [PubMed]
- Hany, M.R.A.-L.; Mohamed, M.A.-D.; Mustafa, S.; Joanna, N.; Dariusz, K. Benefits and applications of Moringa oleifera as a plant protein source in Aquafeed: A review. Aquaculture 2021, 547, 737369. [Google Scholar] [CrossRef]
- Paul, C.W.; Didia, B.C. The effects of methanolic extract of Moringa oleifera lam roots on the histology of ovary and female reproductive tract of guinea pigs. Transp. Res. Rec. 2012, 4, 55–60. [Google Scholar]
- You, X.-Y.; Zhang, H.-Y.; Han, X.; Wang, F.; Zhuang, P.-W.; Zhang, Y.-J. Intestinal Mucosal Barrier is regulated by Intestinal tract Neuro-Immune interplay. Front. Pharmacol. 2021, 12, 659716. [Google Scholar] [CrossRef] [PubMed]
- Tranah, T.H.; Edwards, L.A.; Schnabl, B.; Shawcross, D.L. Targeting the gut-liver-immune axis to treat cirrhosis. Gut 2020, 70, 982–994. [Google Scholar] [CrossRef]
- Hensley-McBain, T.; Berard, A.R.; Manuzak, J.A.; Miller, C.J.; Zevin, A.S.; Polacino, P.; Gile, J.; Agricola, B.; Cameron, M.; Hu, S.-L.; et al. Intestinal damage precedes mucosal immune dysfunction in SIV infection. Mucosal Immunol. 2018, 11, 1429–1440. [Google Scholar] [CrossRef]
- Yin, J.; Sheng, B.; Yang, K.; Sun, L.; Xiao, W.; Yang, H. The protective roles of NLRP6 in intestinal epithelial cells. Cell Prolif. 2018, 52, e12555. [Google Scholar] [CrossRef] [PubMed]
- Okumura, R.; Takeda, K. Roles of intestinal epithelial cells in the maintenance of gut homeostasis. Exp. Mol. Med. 2017, 49, e338. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Liu, Y.; Wang, Y.; Fan, R.; Hu, X.; Zhang, F.; Yang, J.; Chen, J. The Role of Intestinal Mucosal Barrier in Autoimmune Disease: A Potential Target. Front. Immunol. 2022, 13, 871713. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Feng, G.; Zhang, X.; Hu, Q.; Sun, S.; Sun, J.; Sun, Y.; Wang, R.; Zhang, Y.; Wang, P.; et al. The Functional Role of Lactoferrin in Intestine Mucosal Immune System and Inflammatory Bowel Disease. Front. Nutr. 2021, 8, 759507. [Google Scholar] [CrossRef]
- Ronaghan, N.J.; Shang, J.; Iablokov, V.; Zaheer, R.; Colarusso, P.; Dion, S.; Désilets, A.; Leduc, R.; Turner, J.R.; MacNaughton, W.K. The serine protease-mediated increase in intestinal epithelial barrier function is dependent on occludin and requires an intact tight junction. Am. J. Physiol.-Gastrointest. Liver Physiol. 2016, 311, G466–G479. [Google Scholar] [CrossRef]
- Pan, L.; Qin, G.; Zhao, Y.; Wang, J.; Liu, F.; Che, D. Effects of Soybean Agglutinin on Mechanical Barrier Function and Tight Junction Protein Expression in Intestinal Epithelial Cells from Piglets. Int. J. Mol. Sci. 2013, 14, 21689–21704. [Google Scholar] [CrossRef] [PubMed]
- Marchelletta, R.R.; Krishnan, M.; Spalinger, M.R.; Placone, T.W.; Alvarez, R.; Sayoc-Becerra, A.; Canale, V.; Shawki, A.; Park, Y.S.; Bernts, L.H.; et al. T cell protein tyrosine phosphatase protects intestinal barrier function by restricting epithelial tight junction remodeling. J. Clin. Investig. 2016, 131, e138230. [Google Scholar] [CrossRef]
- Haq, S.; Grondin, J.; Banskota, S.; Khan, W.I. Autophagy: Roles in intestinal mucosal homeostasis and inflammation. J. Biomed. Sci. 2019, 26, 19. [Google Scholar] [CrossRef]
- González-Castro, A.M.; Martínez, C.; Salvo-Romero, E.; Fortea, M.; Pardo-Camacho, C.; Pérez-Berezo, T.; Alonso-Cotoner, C.; Santos, J.; Vicario, M. Mucosal pathobiology and molecular signature of epithelial barrier dysfunction in the small intestine in irritable bowel syndrome. J. Gastroenterol. Hepatol. 2016, 32, 53–63. [Google Scholar] [CrossRef]
- Tanaka, H.; Yamamoto, Y.; Kashihara, H.; Yamazaki, Y.; Tani, K.; Fujiyoshi, Y.; Mineta, K.; Takeuchi, K.; Tamura, A.; Tsukita, S. Claudin-21 Has a Paracellular Channel Role at Tight Junctions. Mol. Cell. Biol. 2016, 36, 954–964. [Google Scholar] [CrossRef]
- Fromm, M.; Piontek, J.; Rosenthal, R.; Günzel, D.; Krug, S.M. Tight junctions of the proximal tubule and their channel proteins. Pflügers Arch.-Eur. J. Physiol. 2017, 469, 877–887. [Google Scholar] [CrossRef] [PubMed]
- Dyah Ika, K.; Moh, A.; Didik Susetiyanto, A.; Elfi Quyumi, R.; Dwi, R.; Erna, S.; Tsung-Rong, K. Food Allergies: Immunosensors and Management. Appl. Sci. 2022, 12, 2393. [Google Scholar] [CrossRef]
- Jaklevic, M.C. Sesame Should Be on Food Labels to Warn Consumers with Allergy. JAMA 2020, 324, 2357. [Google Scholar] [CrossRef] [PubMed]
- Seth, D.; Poowutikul, P.; Pansare, M.; Kamat, D. Food Allergy: A Review. Pediatr. Ann. 2020, 49, e50–e58. [Google Scholar] [CrossRef] [PubMed]
- Liao, E.C.; Tsai, J.J. The prevalence of specific IgE to common foods and dust mite in the sera of allergic patients in Taiwan. J. Food Drug Anal. 2020, 20, 125–132+170. [Google Scholar] [CrossRef]
- Yang, H.; Liu, X.; Su, H.; Li, L.; Song, S. Optimization of extraction process and agglutination activity of Moringa oleifera leaves protein by response surface method. Sci. Technol. Food Ind. 2022, 43, 150–157. [Google Scholar] [CrossRef]
- Edelblum, K.L.; Sharon, G.; Singh, G.; Odenwald, M.A.; Sailer, A.; Cao, S.; Ravens, S.; Thomsen, I.; El Bissati, K.; McLeod, R.; et al. The Microbiome Activates CD4 T-cell-mediated Immunity to Compensate for Increased Intestinal Permeability. Cell. Mol. Gastroenterol. Hepatol. 2017, 10, 285–297. [Google Scholar] [CrossRef]
- Li, X.-J.; You, X.-Y.; Wang, C.-Y.; Li, X.-L.; Sheng, Y.-Y.; Zhuang, P.-W.; Zhang, Y.-J. Bidirectional Brain-gut-microbiota Axis in increased intestinal permeability induced by central nervous system injury. CNS Neurosci. Ther. 2020, 26, 783–790. [Google Scholar] [CrossRef]
- Li, C.; Duan, S.; Li, Y.; Pan, X.; Han, L. Polysaccharides in natural products that repair the damage to intestinal mucosa caused by cyclophosphamide and their mechanisms: A review. Carbohydr. Polym. 2021, 261, 117876. [Google Scholar] [CrossRef]
- Schwarzmeier, L.Â.T.; da Cruz, B.S.; Ferreira, C.C.P.; Carvalho, B.F.d.C.; Alves, M.G.O.; Lima Carta, C.F.; Scholz, J.R.; Almeida, J.D. E-cig might cause cell damage of oral mucosa. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2020, 131, 435–443. [Google Scholar] [CrossRef]
- Zhu, L.; Lu, X.; Liu, L.; Voglmeir, J.; Zhong, X.; Yu, Q. Akkermansia muciniphila protects intestinal mucosa from damage caused by S. pullorum by initiating proliferation of intestinal epithelium. Vet. Res. 2020, 51, 34. [Google Scholar] [CrossRef] [PubMed]
- Weiss, G.A.; Hennet, T. Mechanisms and consequences of intestinal dysbiosis. Cell. Mol. Life Sci. 2017, 74, 2959–2977. [Google Scholar] [CrossRef] [PubMed]
- Underwood, M.A.; Mukhopadhyay, S.; Lakshminrusimha, S.; Bevins, C.L. Neonatal intestinal dysbiosis. J. Perinatol. 2020, 40, 1597–1608. [Google Scholar] [CrossRef]
- Ciccia, F.; Ferrante, A.; Triolo, G. Intestinal dysbiosis and innate immune responses in axial spondyloarthritis. Curr. Opin. Rheumatol. 2016, 28, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Yuan, Y.; Zhang, S.; Guo, C.; Li, X.; Li, G.; Xiong, W.; Zeng, Z. Intestinal Flora and Disease Mutually Shape the Regional Immune System in the Intestinal Tract. Front. Immunol. 2020, 11, 575. [Google Scholar] [CrossRef]
- Wang, T.; Gao, L.; Yang, Z.; Wang, F.; Guo, Y.; Wang, B.; Hua, R.; Shang, H.; Xu, J. Restraint Stress in Hypertensive Rats Activates the Intestinal Macrophages and Reduces Intestinal Barrier Accompanied by Intestinal Flora Dysbiosis. J. Inflamm. Res. 2021, 14, 1085–1110. [Google Scholar] [CrossRef]
- Miles, B.T.; Gabrielli, S.; Clarke, A.; Eisman, H.; Shand, G.; Ben-Shoshan, M. Rates of anaphylaxis for the most common food allergies. J. Allergy Clin. Immunol. Pract. 2020, 8, 2402–2405. [Google Scholar] [CrossRef]
- Santos, A.F.; Brough, H.A. Making the Most of In Vitro Tests to Diagnose Food Allergy. J. Allergy Clin. Immunol. Pract. 2017, 5, 237–248. [Google Scholar] [CrossRef]
- Bryce, P.J. Balancing Tolerance or Allergy to Food Proteins. Trends Immunol. 2016, 37, 659–667. [Google Scholar] [CrossRef]
- Herath, H.M.L.P.B.; Elvitigala, D.A.S.; Godahewa, G.I.; Umasuthan, N.; Whang, I.; Noh, J.K.; Lee, J. Molecular characterization and comparative expression analysis of two teleostean pro-inflammatory cytokines, IL-1β and IL-8, from Sebastes schlegeli. Gene 2016, 575 Pt 3, 732–742. [Google Scholar] [CrossRef]
- Turner, J.R. Molecular basis of epithelial barrier regulation: From basic mechanisms to clinical application. Am. J. Pathol. 2006, 169, 1901–1909. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.; Fogg, V.C.; Margolis, B. Tight junctions and cell polarity. Annu. Rev. Cell Dev. Biol. 2006, 22, 207–235. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, R.; Günzel, D.; Krug, S.M.; Schulzke, J.D.; Fromm, M.; Yu, A.S. Claudin-2-mediated cation and water transport share a common pore. Acta Physiol. 2017, 219, 521–536. [Google Scholar] [CrossRef] [PubMed]
- Nighot, P.K.; Hu, C.A.; Ma, T.Y. Autophagy enhances intestinal epithelial tight junction barrier function by targeting claudin-2 protein degradation. J. Biol. Chem. 2015, 290, 7234–7246. [Google Scholar] [CrossRef] [PubMed]
- Bao, J.; Yura, R.E.; Matters, G.L.; Bradley, S.G.; Shi, P.; Tian, F.; Bond, J.S. Meprin A impairs epithelial barrier function, enhances monocyte migration, and cleaves the tight junction protein occludin. Am. J. Physiol. Ren. Physiol. 2013, 305, F714–F726. [Google Scholar] [CrossRef]
- Nonaka, M.; Ma, B.Y.; Ohtani, M.; Yamamoto, A.; Murata, M.; Totani, K.; Ito, Y.; Miwa, K.; Nogami, W.; Kawasaki, N.; et al. Subcellular localization and physiological significance of intracellular mannan-binding protein. J. Biol. Chem. 2007, 282, 17908–17920. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, A.D.; Lei, Y.M.; Shan, G.Q.; Zhang, L.Y.; Lu, X.; Chen, Z.L. Mannose-binding lectin inhibits monocyte proliferation through transforming growth factor-beta1 and p38 signaling pathways. PLoS ONE 2013, 8, e72505. [Google Scholar] [CrossRef]
- Zhang, G.H.; Wu, L.L.; Yu, G.Y. Tight junctions and paracellular fluid and ion transport in salivary glands. Chin. J. Dent. Res. Off. J. Sci. 2013, 16, 13–46. [Google Scholar]
- Suzuki, H.; Tani, K.; Tamura, A.; Tsukita, S.; Fujiyoshi, Y. Model for the Architecture of Claudin-Based Paracellular Ion Channels through Tight Junctions. J. Mol. Biol. 2015, 427, 291–297. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, L.; Tian, Y.; Ye, J.; Liu, Y.; Song, L.; Pan, Q.; He, Y.; Chen, W.; Peng, Z.; et al. Numb modulates the paracellular permeability of intestinal epithelial cells through regulating apical junctional complex assembly and myosin light chain phosphorylation. Exp. Cell Res. 2013, 319, 3214–3225. [Google Scholar] [CrossRef]
- Spalinger, M.R.; Sayoc-Becerra, A.; Santos, A.N.; Shawki, A.; Canale, V.; Krishnan, M.; Niechcial, A.; Obialo, N.; Scharl, M.; Li, J.; et al. PTPN2 Regulates Interactions between Macrophages and Intestinal Epithelial Cells to Promote Intestinal Barrier Function. Gastroenterology 2020, 159, 1763–1777.e14. [Google Scholar] [CrossRef] [PubMed]
- Hausmann, M. How Bacteria-Induced Apoptosis of Intestinal Epithelial Cells Contributes to Mucosal Inflammation. Int. J. Inflamm. 2010, 2010, 574568. [Google Scholar] [CrossRef] [PubMed]
- Chinthrajah, R.S.; Hernandez, J.D.; Boyd, S.D.; Galli, S.J.; Nadeau, K.C. Molecular and cellular mechanisms of food allergy and food tolerance. J. Allergy Clin. Immunol. 2016, 137, 984–997. [Google Scholar] [CrossRef] [PubMed]
- Burris, A.D.; Pizzarello, C.; Järvinen, K.M. Immunologic components in human milk and allergic diseases with focus on food allergy. Semin. Perinatol. 2021, 45, 151386. [Google Scholar] [CrossRef]
- Mohamed Husien, H.; Peng, W.; Su, H.; Zhou, R.; Tao, Y.; Huang, J.; Liu, M.; Bo, R.; Li, J. Moringa oleifera leaves polysaccharide alleviates experimental colitis by inhibiting inflammation and maintaining intestinal barrier. Front. Nutr. 2022, 9, 1055791. [Google Scholar] [CrossRef]
- Lim, D.; Levesque, C.; Wales, P.; Vine, D.; Borthwick, F.; Nation, P. Alterations in intestinal morphology, histology and permeability with exogenous glucagon-like peptide 2 and epidermal growth factor treatment promote intestinal adaptation in neonatal intestinal failure (137.2). In Proceedings of the Experimental Biology Meeting, San Diego, CA, USA, 26–30 April 2014. [Google Scholar] [CrossRef]
- Xing, F.; Guo, B.; Li, Y.; Sun, T.; Yang, Y.; Chen, W.; Fu, X. Effect of epidermal growth factor on gut function and mucosal permeability in rats after gut ischemia-reperfusion injury. Chin. Crit. Care Med. 2002, 14, 650–653. [Google Scholar]

| Genes | Forward | Reverse |
|---|---|---|
| Occludin | CTTCCAATGGCAAAGTGAATG | TACCACCGCTGCTGTAACGAG |
| Claudin-1 | CCAGGTACGAATTTGGTCAGG | TGGTGTTGGGTAAGAGGTTGT |
| ZO-1 | GAGCCTAATCTGACCTATGAACC | TGAGGACTCGTATCTGTATGTGG |
| IL-8 | CCTGAACCTTCCAAAGATGGC | TTCACCAGGCAAGTCTCCTCA |
| TLR4 | GTACCTGGGGAACAACCTCTT | GCAGCTTGACTAGACTCTCCA |
| 1RPL-19 | GAAGGTCAAAGGGAATGTGTTCA | CCTTGTCTGCCTTCAGCTTGT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Xi, C.; Li, W.; Su, H.; Yang, H.; Bai, Z.; Tian, Y.; Song, S. Moringa oleifera Leaves Protein Enhances Intestinal Permeability by Activating TLR4 Upstream Signaling and Disrupting Tight Junctions. Int. J. Mol. Sci. 2023, 24, 16425. https://doi.org/10.3390/ijms242216425
Liu X, Xi C, Li W, Su H, Yang H, Bai Z, Tian Y, Song S. Moringa oleifera Leaves Protein Enhances Intestinal Permeability by Activating TLR4 Upstream Signaling and Disrupting Tight Junctions. International Journal of Molecular Sciences. 2023; 24(22):16425. https://doi.org/10.3390/ijms242216425
Chicago/Turabian StyleLiu, Xiaoxue, Chuyu Xi, Wenjie Li, Hairan Su, Hao Yang, Zhongbin Bai, Yang Tian, and Shuang Song. 2023. "Moringa oleifera Leaves Protein Enhances Intestinal Permeability by Activating TLR4 Upstream Signaling and Disrupting Tight Junctions" International Journal of Molecular Sciences 24, no. 22: 16425. https://doi.org/10.3390/ijms242216425




