Role of CB1 Cannabinoid Receptors in Vascular Responses and Vascular Remodeling of the Aorta in Female Mice
Abstract
:1. Introduction
2. Results
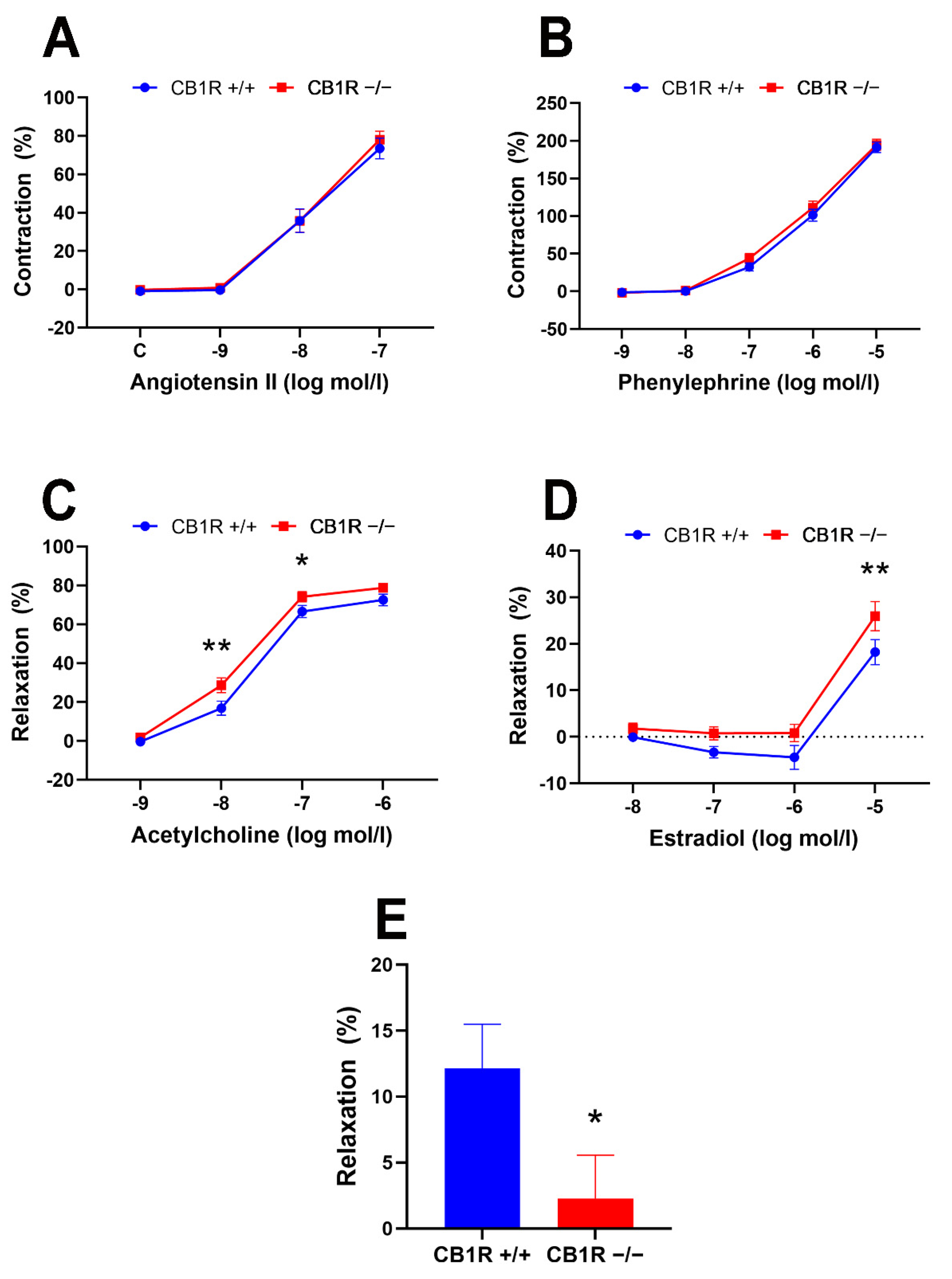
2.1. Effects of Presence of CB1 Receptors on Vascular Contraction and Relaxation Responses
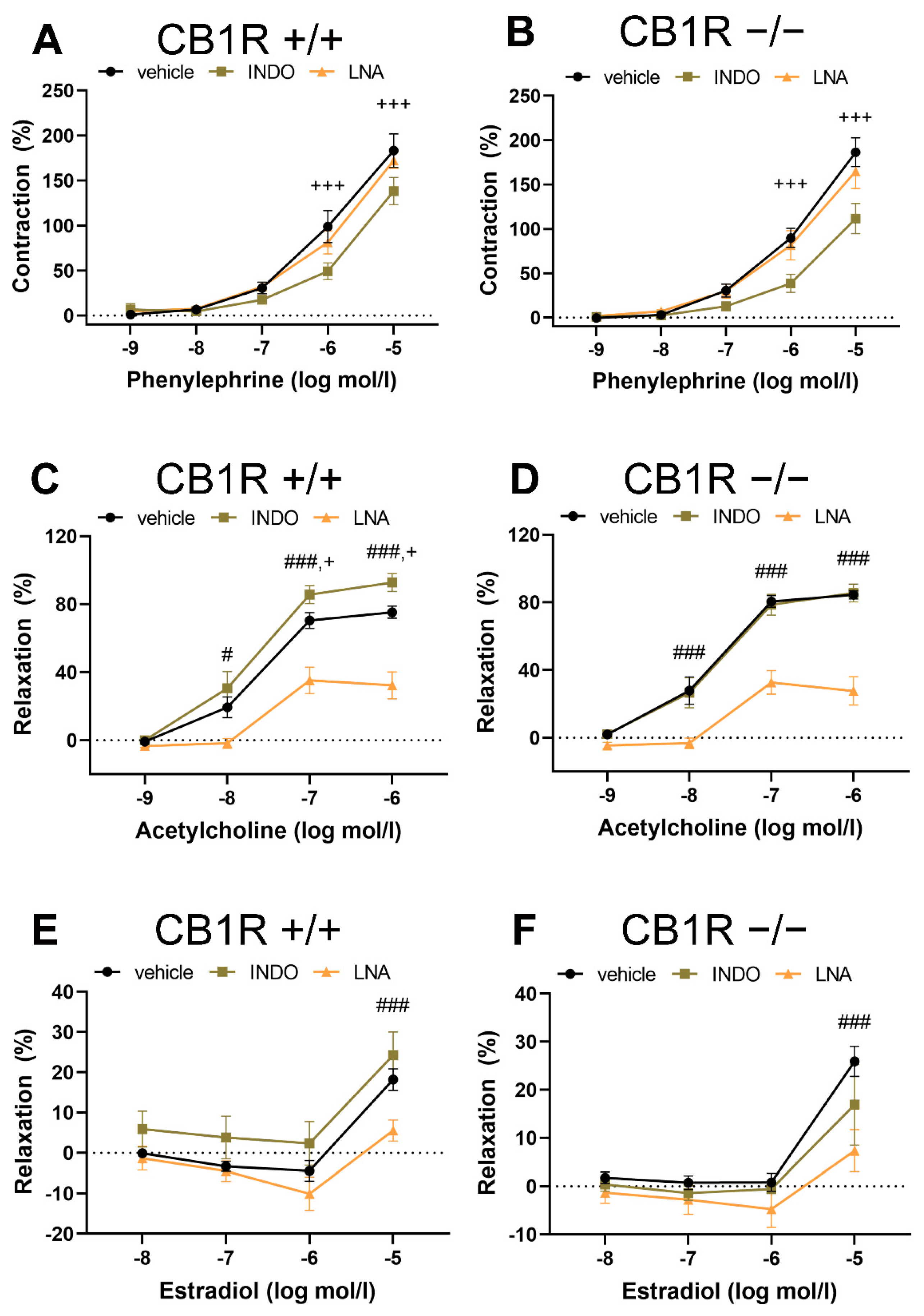
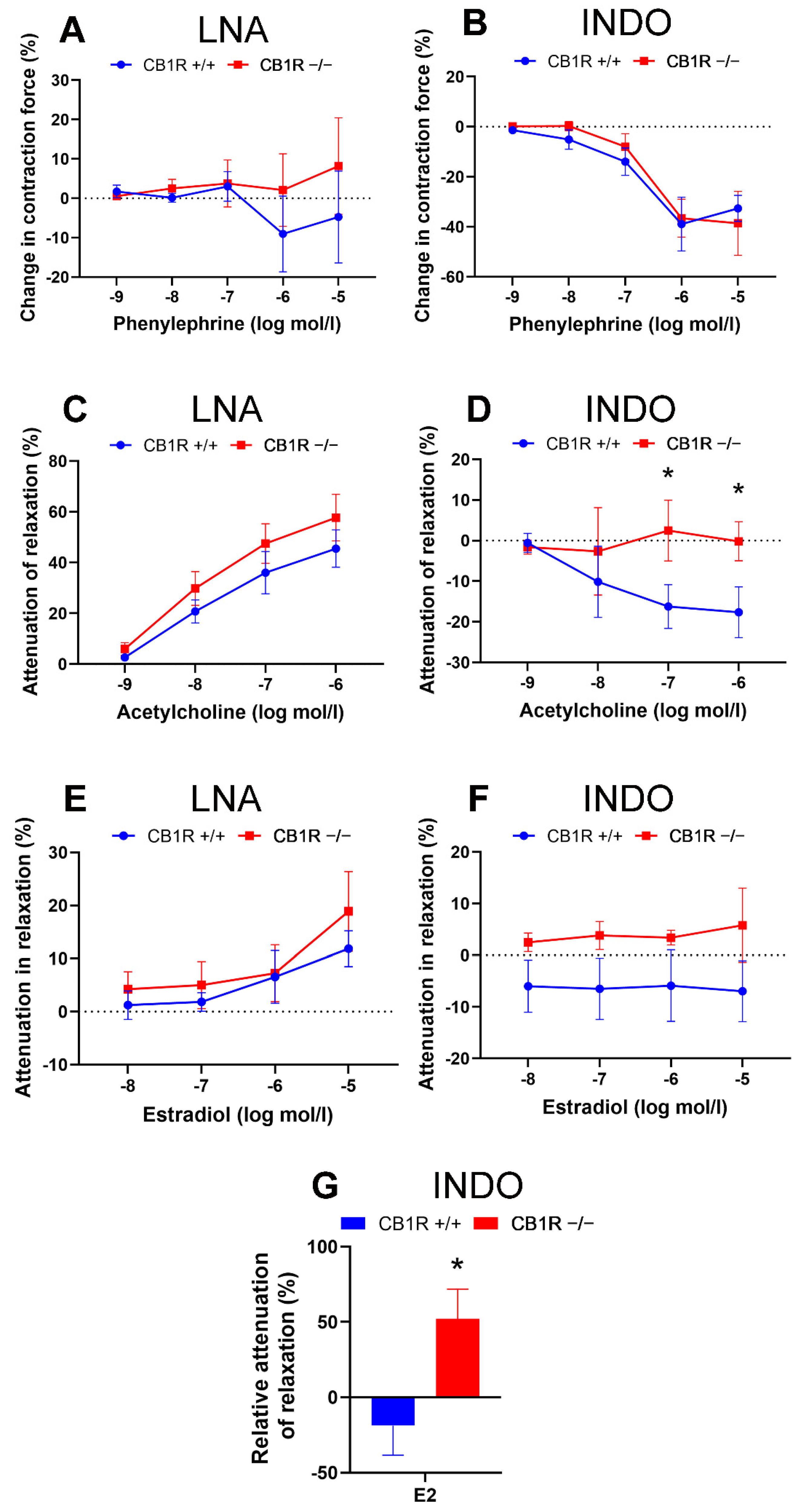
2.2. Effects of Specific Inhibitors on Contraction and Relaxation Vascular Responses in WT (CB1R+/+) and in CB1R KO (CB1R−/−) Female Mice
2.3. Estrogen Metabolite Levels
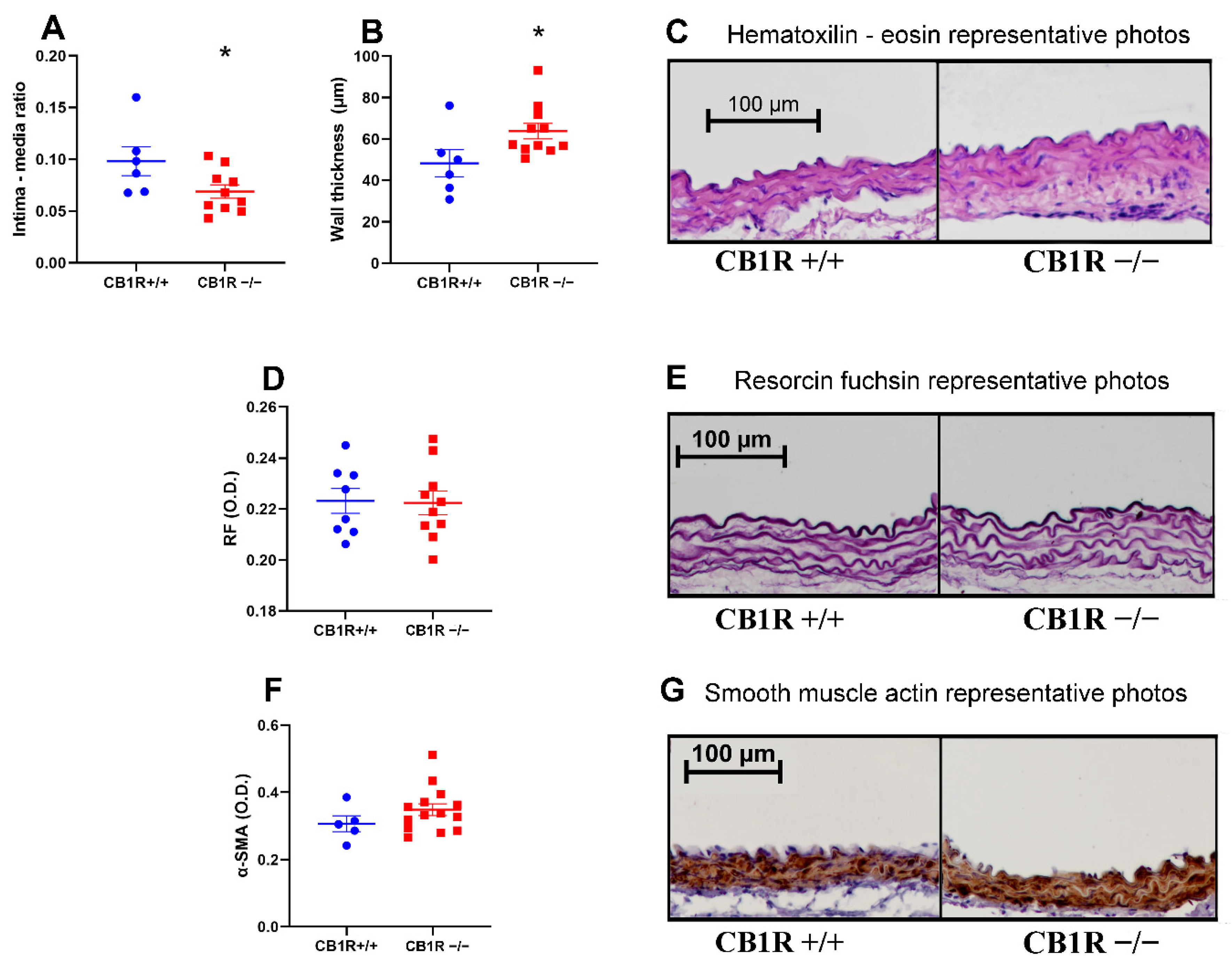
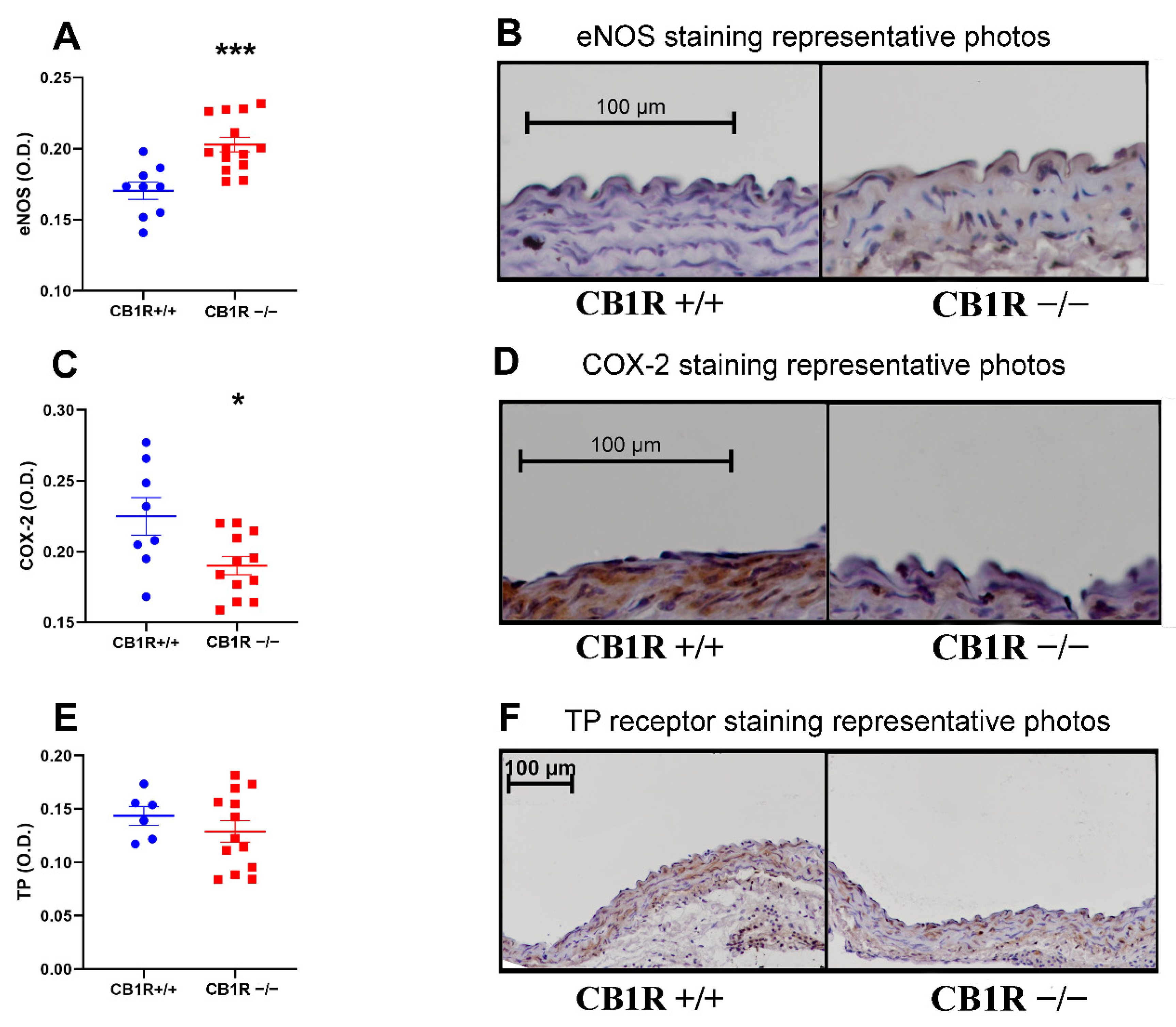
2.4. Histology and Immuno-Histochemistry
2.5. Vasoactive Markers/Molecular Contributors of Vascular Functions
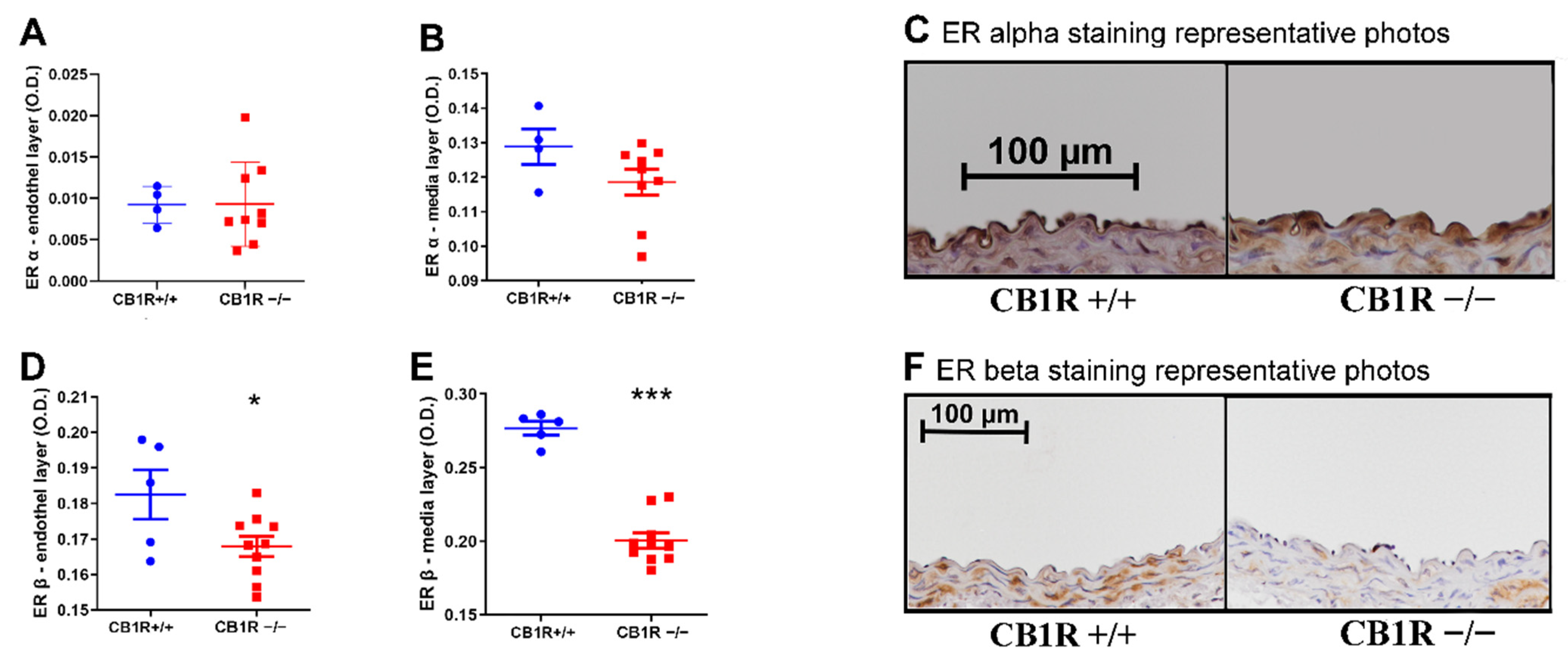
2.6. Estrogen Receptor Expression
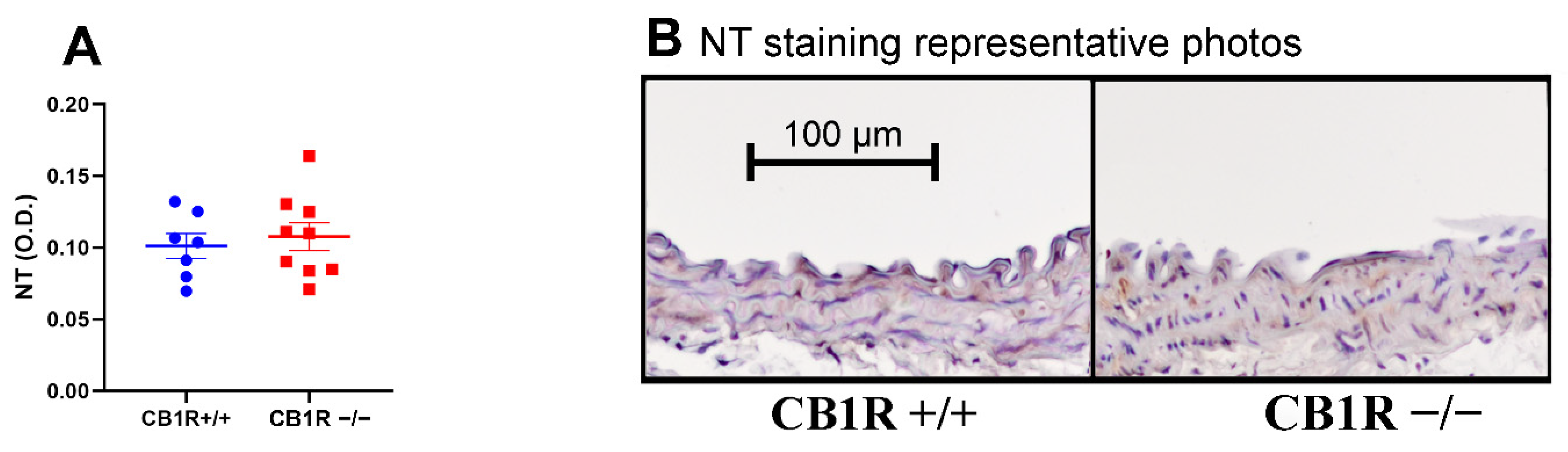
2.7. Nitrative Stress
3. Discussion
3.1. Main Functional Observations and Their Molecular Background
3.2. Vascular Effects of Cannabinoids and Endocannabinoid Signaling
3.3. Role of ECS in Cardiovascular Pathologies and Vascular Remodeling, Possible Therapeutic Effects
3.4. Estrogen-Induced Relaxation, Plasma Levels, Receptors
3.5. Nitrative Stress Levels
3.6. Gender Differences in Vascular Responses of Estrogens and Cannabinoids
4. Materials and Methods
4.1. Chemicals
4.2. Animals and Preparation
4.3. Myography
4.4. Hormone Determinations
4.5. Histological and Immuno-Histochemical Stainings
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Freund, T.F.; Katona, I.; Piomelli, D. Role of endogenous cannabinoids in synaptic signaling. Physiol. Rev. 2003, 83, 1017–1066. [Google Scholar] [CrossRef] [PubMed]
- Gyombolai, P.; Pap, D.; Turu, G.; Catt, K.J.; Bagdy, G.; Hunyady, L. Regulation of endocannabinoid release by G proteins: A paracrine mechanism of G protein-coupled receptor action. Mol. Cell. Endocrinol. 2012, 353, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Pacher, P.; Bátkai, S.; Kunos, G. Cardiovascular pharmacology of cannabinoids. In Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2005; pp. 599–625. [Google Scholar] [CrossRef]
- Pacher, P.; Bátkai, S.; Kunos, G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol. Rev. 2006, 58, 389–462. [Google Scholar] [CrossRef] [PubMed]
- Szekeres, M.; Nádasy, G.L.; Turu, G.; Soltész-Katona, E.; Benyó, Z.; Offermanns, S.; Ruisanchez, É.; Szabó, E.; Takáts, Z.; Bátkai, S.; et al. Endocannabinoid-mediated modulation of Gq/11 protein-coupled receptor signaling-induced vasoconstriction and hypertension. Mol. Cell. Endocrinol. 2015, 403, 46–56. [Google Scholar] [CrossRef]
- Turu, G.; Simon, A.; Gyombolai, P.; Szidonya, L.; Bagdy, G.; Lenkei, Z.; Hunyady, L. The role of diacylglycerol lipase in constitutive and angiotensin AT1 receptor-stimulated cannabinoid CB1 receptor activity. J. Biol. Chem. 2007, 282, 7753–7757. [Google Scholar] [CrossRef]
- Turu, G.; Várnai, P.; Gyombolai, P.; Szidonya, L.; Offertaler, L.; Bagdy, G.; Kunos, G.; Hunyady, L. Paracrine transactivation of the CB1 cannabinoid receptor by AT1 angiotensin and other Gq/11 protein-coupled receptors. J. Biol. Chem. 2009, 284, 16914–16921. [Google Scholar] [CrossRef]
- Aizpurua-Olaizola, O.; Elezgarai, I.; Rico-Barrio, I.; Zarandona, I.; Etxebarria, N.; Usobiaga, A. Targeting the endocannabinoid system: Future therapeutic strategies. Drug Discov. Today 2017, 22, 105–110. [Google Scholar] [CrossRef]
- Dörnyei, G.; Vass, Z.; Juhász, C.B.; Nádasy, G.L.; Hunyady, L.; Szekeres, M. Role of the Endocannabinoid System in Metabolic Control Processes and in the Pathogenesis of Metabolic Syndrome: An Update. Biomedicines 2023, 11, 306. [Google Scholar] [CrossRef]
- Pacher, P.; Mukhopadhyay, P.; Mohanraj, R.; Godlewski, G.; Bátkai, S.; Kunos, G. Modulation of the endocannabinoid system in cardiovascular disease: Therapeutic potential and limitations. Hypertension 2008, 52, 601–607. [Google Scholar] [CrossRef]
- Bátkai, S.; Pacher, P.; Osei-Hyiaman, D.; Radaeva, S.; Liu, J.; Harvey-White, J.; Offertáler, L.; Mackie, K.; Rudd, M.A.; Bukoski, R.D.; et al. Endocannabinoids acting at cannabinoid-1 receptors regulate cardiovascular function in hypertension. Circulation 2004, 110, 1996–2002. [Google Scholar] [CrossRef]
- Ueda, N.; Tsuboi, K.; Uyama, T.; Ohnishi, T. Biosynthesis and degradation of the endocannabinoid 2-arachidonoylglycerol. Biofactors 2011, 37, 1–7. [Google Scholar] [CrossRef]
- Kunos, G.; Osei-Hyiaman, D.; Bátkai, S.; Sharkey, K.A.; Makriyannis, A. Should peripheral CB1 cannabinoid receptors be selectively targeted for therapeutic gain? Trends Pharmacol. Sci. 2009, 30, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Szekeres, M.; Nádasy, G.L.; Turu, G.; Soltész-Katona, E.; Tóth, Z.E.; Balla, A.; Catt, K.J.; Hunyady, L. Angiotensin II induces vascular endocannabinoid release, which attenuates its vasoconstrictor effect via CB1 cannabinoid receptors. J. Biol. Chem. 2012, 287, 31540–31550. [Google Scholar] [CrossRef] [PubMed]
- Karpińska, O.; Baranowska-Kuczko, M.; Kloza, M.; Kozłowska, H. Endocannabinoids modulate Gq/11 protein-coupled receptor agonist-induced vasoconstriction via a negative feedback mechanism. J. Pharm. Pharmacol. 2018, 70, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Szekeres, M.; Nádasy, G.L.; Soltész-Katona, E.; Hunyady, L. Control of myogenic tone and agonist induced contraction of intramural coronary resistance arterioles by cannabinoid type 1 receptors and endocannabinoids. Prostaglandins Other Lipid Mediat. 2018, 134, 77–83. [Google Scholar] [CrossRef]
- Mechoulam, R.; Fride, E.; Ben-Shabat, S.; Meiri, U.; Horowitz, M. Carbachol, an acetylcholine receptor agonist, enhances production in rat aorta of 2-arachidonoyl glycerol, a hypotensive endocannabinoid. Eur. J. Pharmacol. 1998, 362, R1–R3. [Google Scholar] [CrossRef] [PubMed]
- Afshar, S.; Abbasinazari, M.; Amin, G.; Farrokhian, A.; Sistanizad, M.; Afshar, F.; Khalili, S. Endocannabinoids and related compounds as modulators of angiogenesis: Concepts and clinical significance. Cell Biochem. Funct. 2022, 40, 826–837. [Google Scholar] [CrossRef]
- Matrai, M.; Mericli, M.; Nadasy, G.L.; Szekeres, M.; Varbiro, S.; Banhidy, F.; Acs, N.; Monos, E.; Szekacs, B. Gender differences in biomechanical properties of intramural coronary resistance arteries of rats, an in vitro microarteriographic study. J. Biomech. 2007, 40, 1024–1030. [Google Scholar] [CrossRef]
- Mericli, M.; Nádasy, G.L.; Szekeres, M.; Várbíró, S.; Vajo, Z.; Mátrai, M.; Acs, N.; Monos, E.; Székács, B. Estrogen replacement therapy reverses changes in intramural coronary resistance arteries caused by female sex hormone depletion. Cardiovasc. Res. 2004, 61, 317–324. [Google Scholar] [CrossRef]
- Huang, A.; Sun, D.; Koller, A.; Kaley, G. Gender difference in flow-induced dilation and regulation of shear stress: Role of estrogen and nitric oxide. Am. J. Physiol. 1998, 275, R1571–R1577. [Google Scholar] [CrossRef]
- Kaley, G.; Koller, A. Prostaglandin-nitric oxide interactions in the microcirculation. Adv. Prostaglandin Thromboxane Leukot. Res. 1995, 23, 485–490. [Google Scholar] [PubMed]
- Koller, A.; Dörnyei, G.; Kaley, G. Flow-induced responses in skeletal muscle venules: Modulation by nitric oxide and prostaglandins. Am. J. Physiol. 1998, 275, H831–H836. [Google Scholar] [CrossRef] [PubMed]
- Szekeres, M.; Nádasy, G.L.; Kaley, G.; Koller, A. Nitric oxide and prostaglandins modulate pressure-induced myogenic responses of intramural coronary arterioles. J. Cardiovasc. Pharmacol. 2004, 43, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Khan, H.; Xiao, J.; Cheang, W.S. Effects of Arachidonic Acid Metabolites on Cardiovascular Health and Disease. Int. J. Mol. Sci. 2021, 22, 12029. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.; Sun, D.; Kaley, G.; Koller, A. Estrogen maintains nitric oxide synthesis in arterioles of female hypertensive rats. Hypertension 1997, 29, 1351–1356. [Google Scholar] [CrossRef]
- Kakucs, R.; Várbíró, S.; Nádasy, G.L.; Monos, E.; Székács, B. Acute, nongenomic vasodilatory action of estradiol is attenuated by chronic estradiol treatment. Exp. Biol. Med. 2001, 226, 538–542. [Google Scholar] [CrossRef]
- Kakucs, R.; Várbíró, S.; Székács, B.; Nádasy, G.L.; Acs, N.; Monos, E. Direct relaxing effect of estradiol-17beta and progesterone on rat saphenous artery. Microvasc. Res. 1998, 56, 139–143. [Google Scholar] [CrossRef]
- Acs, N.; Székács, B.; Nádasy, G.L.; Várbíró, S.; Miklós, Z.; Szentiványi, M., Jr.; Monos, E. Effects of combined sex hormone replacement therapy on small artery biomechanics in pharmacologically ovariectomized rats. Maturitas 2000, 34, 83–92. [Google Scholar] [CrossRef]
- Matrai, M.; Hetthéssy, J.R.; Nadasy, G.L.; Szekacs, B.; Mericli, M.; Acs, N.; Monos, E.; Arbib, N.; Varbiro, S. Estrogen therapy may counterbalance eutrophic remodeling of coronary arteries and increase bradykinin relaxation in a rat model of menopausal hypertension. Menopause 2016, 23, 778–783. [Google Scholar] [CrossRef]
- Vallée, A. Association between lifetime cannabis use and arterial stiffness in a middle-aged general population. J. Hypertens. 2023, 41, 658–669. [Google Scholar] [CrossRef]
- Bari, M.; Battista, N.; Pirazzi, V.; Maccarrone, M. The manifold actions of endocannabinoids on female and male reproductive events. Front. Biosci. Landmark Ed. 2011, 16, 498–516. [Google Scholar] [CrossRef] [PubMed]
- Brents, L.K. Marijuana, the Endocannabinoid System and the Female Reproductive System. Yale J. Biol. Med. 2016, 89, 175–191. [Google Scholar] [PubMed]
- Alvarez, S. Do some addictions interfere with fertility? Fertil. Steril. 2015, 103, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Park, B.; McPartland, J.M.; Glass, M. Cannabis, cannabinoids and reproduction. Prostaglandins Leukot. Essent. Fatty Acids 2004, 70, 189–197. [Google Scholar] [CrossRef]
- Wang, H.; Dey, S.K.; Maccarrone, M. Jekyll and hyde: Two faces of cannabinoid signaling in male and female fertility. Endocr. Rev. 2006, 27, 427–448. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.H.; Abbas, M.S.; Habiba, M.A.; Konje, J.C. Histomorphometric evaluation of cannabinoid receptor and anandamide modulating enzyme expression in the human endometrium through the menstrual cycle. Histochem. Cell Biol. 2010, 133, 557–565. [Google Scholar] [CrossRef]
- Amantea, D.; Spagnuolo, P.; Bari, M.; Fezza, F.; Mazzei, C.; Tassorelli, C.; Morrone, L.A.; Corasaniti, M.T.; Maccarrone, M.; Bagetta, G. Modulation of the endocannabinoid system by focal brain ischemia in the rat is involved in neuroprotection afforded by 17beta-estradiol. FEBS J. 2007, 274, 4464–4775. [Google Scholar] [CrossRef]
- El-Talatini, M.R.; Taylor, A.H.; Elson, J.C.; Brown, L.; Davidson, A.C.; Konje, J.C. Localisation and function of the endocannabinoid system in the human ovary. PLoS ONE 2009, 4, e4579. [Google Scholar] [CrossRef]
- Darblade, B.; Pendaries, C.; Krust, A.; Dupont, S.; Fouque, M.J.; Rami, J.; Chambon, P.; Bayard, F.; Arnal, J.F. Estradiol alters nitric oxide production in the mouse aorta through the alpha-, but not beta-, estrogen receptor. Circ. Res. 2002, 90, 413–419. [Google Scholar] [CrossRef]
- Savva, C.; Korach-André, M. Estrogen Receptor beta (ERβ) Regulation of Lipid Homeostasis-Does Sex Matter? Metabolites 2020, 10, 116. [Google Scholar] [CrossRef]
- Iorga, A.; Umar, S.; Ruffenach, G.; Aryan, L.; Li, J.; Sharma, S.; Motayagheni, N.; Nadadur, R.D.; Bopassa, J.C.; Eghbali, M. Estrogen rescues heart failure through estrogen receptor Beta activation. Biol. Sex. Differ. 2018, 9, 48. [Google Scholar] [CrossRef] [PubMed]
- Aryan, L.; Younessi, D.; Zargari, M.; Banerjee, S.; Agopian, J.; Rahman, S.; Borna, R.; Ruffenach, G.; Umar, S.; Eghbali, M. The Role of Estrogen Receptors in Cardiovascular Disease. Int. J. Mol. Sci. 2020, 21, 4314. [Google Scholar] [CrossRef] [PubMed]
- Paterni, I.; Granchi, C.; Katzenellenbogen, J.A.; Minutolo, F. Estrogen receptors alpha (ERα) and beta (ERβ): Subtype-selective ligands and clinical potential. Steroids 2014, 90, 13–29. [Google Scholar] [CrossRef] [PubMed]
- Karpińska, O.; Baranowska-Kuczko, M.; Kloza, M.; Ambroz Ewicz, E.; Kozłowski, T.; Kasacka, I.; Malinowska, B.; Kozłowska, H. Activation of CB1 receptors by 2-arachidonoylglycerol attenuates vasoconstriction induced by U46619 and angiotensin II in human and rat pulmonary arteries. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2017, 312, R883–R893. [Google Scholar] [CrossRef]
- Dannert, M.T.; Alsasua, A.; Herradon, E.; Martín, M.I.; López-Miranda, V. Vasorelaxant effect of Win 55,212-2 in rat aorta: New mechanisms involved. Vascul. Pharmacol. 2007, 46, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Hillard, C.J. Endocannabinoids and vascular function. J. Pharmacol. Exp. Ther. 2000, 294, 27–32. [Google Scholar]
- Randall, M.D.; Kendall, D.A.; O’Sullivan, S. The complexities of the cardiovascular actions of cannabinoids. Br. J. Pharmacol. 2004, 142, 20–26. [Google Scholar] [CrossRef]
- Wagner, J.A.; Járai, Z.; Bátkai, S.; Kunos, G. Hemodynamic effects of cannabinoids: Coronary and cerebral vasodilation mediated by cannabinoid CB1 receptors. Eur. J. Pharmacol. 2001, 423, 203–210. [Google Scholar] [CrossRef]
- O’Sullivan, S.E.; Randall, M.D.; Gardiner, S.M. The in vitro and in vivo cardiovascular effects of Delta9-tetrahydrocannabinol in rats made hypertensive by chronic inhibition of nitric-oxide synthase. J. Pharmacol. Exp. Ther. 2007, 321, 663–672. [Google Scholar] [CrossRef]
- White, R.; Hiley, C.R. The actions of some cannabinoid receptor ligands in the rat isolated mesenteric artery. Br. J. Pharmacol. 1998, 125, 533–541. [Google Scholar] [CrossRef]
- Járai, Z.; Wagner, J.A.; Goparaju, S.K.; Wang, L.; Razdan, R.K.; Sugiura, T.; Zimmer, A.M.; Bonner, T.I.; Zimmer, A.; Kunos, G. Cardiovascular effects of 2-arachidonoyl glycerol in anesthetized mice. Hypertension 2000, 35, 679–684. [Google Scholar] [CrossRef] [PubMed]
- Turu, G.; Hunyady, L. Signal transduction of the CB1 cannabinoid receptor. J. Mol. Endocrinol. 2010, 44, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Rademacher, D.J.; Patel, S.; Ho, W.S.; Savoie, A.M.; Rusch, N.J.; Gauthier, K.M.; Hillard, C.J. U-46619 but not serotonin increases endocannabinoid content in middle cerebral artery: Evidence for functional relevance. Am. J. Physiol. Heart Circ. Physiol. 2005, 288, H2694–H2701. [Google Scholar] [CrossRef]
- Baranowska-Kuczko, M.; Kozłowska, H.; Kloza, M.; Harasim-Symbor, E.; Biernacki, M.; Kasacka, I.; Malinowska, B. Beneficial Changes in Rat Vascular Endocannabinoid System in Primary Hypertension and under Treatment with Chronic Inhibition of Fatty Acid Amide Hydrolase by URB597. Int. J. Mol. Sci. 2021, 22, 4833. [Google Scholar] [CrossRef] [PubMed]
- Szekeres, M.; Nádasy, G.L.; Dörnyei, G.; Szénási, A.; Koller, A. Remodeling of Wall Mechanics and the Myogenic Mechanism of Rat Intramural Coronary Arterioles in Response to a Short-Term Daily Exercise Program: Role of Endothelial Factors. J. Vasc. Res. 2018, 55, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Stanley, C.P.; Hind, W.H.; Tufarelli, C.; O’Sullivan, S.E. Cannabidiol causes endothelium-dependent vasorelaxation of human mesenteric arteries via CB1 activation. Cardiovasc. Res. 2015, 107, 568–578. [Google Scholar] [CrossRef]
- Morales, P.; Hurst, D.P.; Reggio, P.H. Molecular Targets of the Phytocannabinoids: A Complex Picture. Prog. Chem. Org. Nat. Prod. 2017, 103, 103–131. [Google Scholar] [CrossRef]
- Eckenstaler, R.; Ripperger, A.; Hauke, M.; Petermann, M.; Hemkemeyer, S.A.; Schwedhelm, E.; Ergün, S.; Frye, M.; Werz, O.; Koeberle, A.; et al. A Thromboxane A2 Receptor-Driven COX-2-Dependent Feedback Loop That Affects Endothelial Homeostasis and Angiogenesis. Arterioscler. Thromb. Vasc. Biol. 2022, 42, 444–461. [Google Scholar] [CrossRef]
- Wang, L.N.; Xing, M.D.; Qu, W.T.; Wang, C.B.; Liu, Z.Q.; Han, J.; Ren, W.; Qiao, Y.N. Impaired vessel relaxation response and increased infarct size in smooth muscle cannabinoid receptor 1 knockout mice. Microvasc. Res. 2022, 139, 104263. [Google Scholar] [CrossRef]
- Molica, F.; Burger, F.; Thomas, A.; Staub, C.; Tailleux, A.; Staels, B.; Pelli, G.; Zimmer, A.; Cravatt, B.; Matter, C.M.; et al. Endogenous cannabinoid receptor CB1 activation promotes vascular smooth-muscle cell proliferation and neointima formation. J. Lipid Res. 2013, 54, 1360–1368. [Google Scholar] [CrossRef]
- Bondarenko, A.I. Cannabinoids and Cardiovascular System. Adv. Exp. Med. Biol. 2019, 1162, 63–87. [Google Scholar] [CrossRef] [PubMed]
- Ho, W.S.; Gardiner, S.M. Acute hypertension reveals depressor and vasodilator effects of cannabinoids in conscious rats. Br. J. Pharmacol. 2009, 156, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Malinowska, B.; Baranowska-Kuczko, M.; Schlicker, E. Triphasic blood pressure responses to cannabinoids: Do we understand the mechanism? Br. J. Pharmacol. 2012, 165, 2073–2088. [Google Scholar] [CrossRef]
- Dobovišek, L.; Hojnik, M.; Ferk, P. Overlapping molecular pathways between cannabinoid receptors type 1 and 2 and estrogens/androgens on the periphery and their involvement in the pathogenesis of common diseases (Review). Int. J. Mol. Med. 2016, 38, 1642–1651. [Google Scholar] [CrossRef] [PubMed]
- Dol-Gleizes, F.; Paumelle, R.; Visentin, V.; Marés, A.M.; Desitter, P.; Hennuyer, N.; Gilde, A.; Staels, B.; Schaeffer, P.; Bono, F. Rimonabant, a selective cannabinoid CB1 receptor antagonist, inhibits atherosclerosis in LDL receptor-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 12–18. [Google Scholar] [CrossRef]
- Pacher, P. Cannabinoid CB1 receptor antagonists for atherosclerosis and cardiometabolic disorders: New hopes, old concerns? Arterioscler. Thromb. Vasc. Biol. 2009, 29, 7–9. [Google Scholar] [CrossRef]
- Kovács, K.; Vásárhelyi, B.; Mészáros, K.; Patócs, A.; Karvaly, G. The biological and clinical relevance of estrogen metabolome. Orv. Hetil. 2017, 158, 929–937. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Morton, J.S.; Davidge, S.T. Mechanisms of estrogen effects on the endothelium: An overview. Can. J. Cardiol. 2014, 30, 705–712. [Google Scholar] [CrossRef]
- Ing, N.H.; Tornesi, M.B. Estradiol up-regulates estrogen receptor and progesterone receptor gene expression in specific ovine uterine cells. Biol. Reprod. 1997, 56, 1205–1215. [Google Scholar] [CrossRef]
- Ropero, A.B.; Alonso-Magdalena, P.; Quesada, I.; Nadal, A. The role of estrogen receptors in the control of energy and glucose homeostasis. Steroids 2008, 73, 874–879. [Google Scholar] [CrossRef]
- Nogueiras, R.; Diaz-Arteaga, A.; Lockie, S.H.; Velásquez, D.A.; Tschop, J.; López, M.; Cadwell, C.C.; Diéguez, C.; Tschöp, M.H. The endocannabinoid system: Role in glucose and energy metabolism. Pharmacol. Res. 2009, 60, 93–98. [Google Scholar] [CrossRef]
- Pabbidi, M.R.; Kuppusamy, M.; Didion, S.P.; Sanapureddy, P.; Reed, J.T.; Sontakke, S.P. Sex differences in the vascular function and related mechanisms: Role of 17β-estradiol. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H1499–H1518. [Google Scholar] [CrossRef]
- Santoro, A.; Mele, E.; Marino, M.; Viggiano, A.; Nori, S.L.; Meccariello, R. The Complex Interplay between Endocannabinoid System and the Estrogen System in Central Nervous System and Periphery. Int. J. Mol. Sci. 2021, 22, 972. [Google Scholar] [CrossRef] [PubMed]
- Takeda, S.; Yoshida, K.; Nishimura, H.; Harada, M.; Okajima, S.; Miyoshi, H.; Okamoto, Y.; Amamoto, T.; Watanabe, K.; Omiecinski, C.J.; et al. Δ9-Tetrahydrocannabinol disrupts estrogen-signaling through up-regulation of estrogen receptor β (ERβ). Chem. Res. Toxicol. 2013, 26, 1073–1079. [Google Scholar] [CrossRef] [PubMed]
- Szabó, R.; Börzsei, D.; Szabó, Z.; Hoffmann, A.; Zupkó, I.; Priksz, D.; Kupai, K.; Varga, C.; Pósa, A. A Potential Involvement of Anandamide in the Modulation of HO/NOS Systems: Women, Menopause, and “Medical Cannabinoids”. Int. J. Mol. Sci. 2020, 21, 8801. [Google Scholar] [CrossRef]
- Mukhopadhyay, P.; Rajesh, M.; Bátkai, S.; Patel, V.; Kashiwaya, Y.; Liaudet, L.; Evgenov, O.V.; Mackie, K.; Haskó, G.; Pacher, P. CB1 cannabinoid receptors promote oxidative stress and cell death in murine models of doxorubicin-induced cardiomyopathy and in human cardiomyocytes. Cardiovasc. Res. 2010, 85, 773–784. [Google Scholar] [CrossRef]
- Bányai, B.; Répás, C.; Miklós, Z.; Johnsen, J.; Horváth, E.M.; Benkő, R. Delta 9-tetrahydrocannabinol conserves cardiovascular functions in a rat model of endotoxemia: Involvement of endothelial molecular mechanisms and oxidative-nitrative stress. PLoS ONE 2023, 18, e0287168. [Google Scholar] [CrossRef] [PubMed]
- Varbiro, S.; Matrai, M.; Szekeres, M.; Nadasy, G.L.; Szaky, E.; Mericli, M.; Banhidy, F.; Monos, E.; Szekacs, B. Intramural coronary artery constrictor reactivity to thromboxane is higher in male than in female rats. Gynecol. Endocrinol. 2006, 22, 44–47. [Google Scholar] [CrossRef]
- Ho, W.S. Modulation by 17β-estradiol of anandamide vasorelaxation in normotensive and hypertensive rats: A role for TRPV1 but not fatty acid amide hydrolase. Eur. J. Pharmacol. 2013, 701, 49–56. [Google Scholar] [CrossRef]
- Zimmer, A.; Zimmer, A.M.; Hohmann, A.G.; Herkenham, M.; Bonner, T.I. Increased mortality, hypoactivity, and hypoalgesia in cannabinoid CB1 receptor knockout mice. Proc. Natl. Acad. Sci. USA 1999, 96, 5780–5785. [Google Scholar] [CrossRef]
- Horváth, B.; Orsy, P.; Benyó, Z. Endothelial NOS-mediated relaxations of isolated thoracic aorta of the C57BL/6J mouse: A methodological study. J. Cardiovasc. Pharmacol. 2005, 45, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Szenasi, A.; Amrein, K.; Czeiter, E.; Szarka, N.; Toth, P.; Koller, A. Molecular Pathomechanisms of Impaired Flow-Induced Constriction of Cerebral Arteries Following Traumatic Brain Injury: A Potential Impact on Cerebral Autoregulation. Int. J. Mol. Sci. 2021, 22, 6624. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bányai, B.; Vass, Z.; Kiss, S.; Balogh, A.; Brandhuber, D.; Karvaly, G.; Kovács, K.; Nádasy, G.L.; Hunyady, L.; Dörnyei, G.; et al. Role of CB1 Cannabinoid Receptors in Vascular Responses and Vascular Remodeling of the Aorta in Female Mice. Int. J. Mol. Sci. 2023, 24, 16429. https://doi.org/10.3390/ijms242216429
Bányai B, Vass Z, Kiss S, Balogh A, Brandhuber D, Karvaly G, Kovács K, Nádasy GL, Hunyady L, Dörnyei G, et al. Role of CB1 Cannabinoid Receptors in Vascular Responses and Vascular Remodeling of the Aorta in Female Mice. International Journal of Molecular Sciences. 2023; 24(22):16429. https://doi.org/10.3390/ijms242216429
Chicago/Turabian StyleBányai, Bálint, Zsolt Vass, Stella Kiss, Anikó Balogh, Dóra Brandhuber, Gellért Karvaly, Krisztián Kovács, György L. Nádasy, László Hunyady, Gabriella Dörnyei, and et al. 2023. "Role of CB1 Cannabinoid Receptors in Vascular Responses and Vascular Remodeling of the Aorta in Female Mice" International Journal of Molecular Sciences 24, no. 22: 16429. https://doi.org/10.3390/ijms242216429
APA StyleBányai, B., Vass, Z., Kiss, S., Balogh, A., Brandhuber, D., Karvaly, G., Kovács, K., Nádasy, G. L., Hunyady, L., Dörnyei, G., Horváth, E. M., & Szekeres, M. (2023). Role of CB1 Cannabinoid Receptors in Vascular Responses and Vascular Remodeling of the Aorta in Female Mice. International Journal of Molecular Sciences, 24(22), 16429. https://doi.org/10.3390/ijms242216429







