The Neuroprotective Effects of Dendropanax morbifera Water Extract on Scopolamine-Induced Memory Impairment in Mice
Abstract
:1. Introduction
2. Results
2.1. Analysis of DMLS
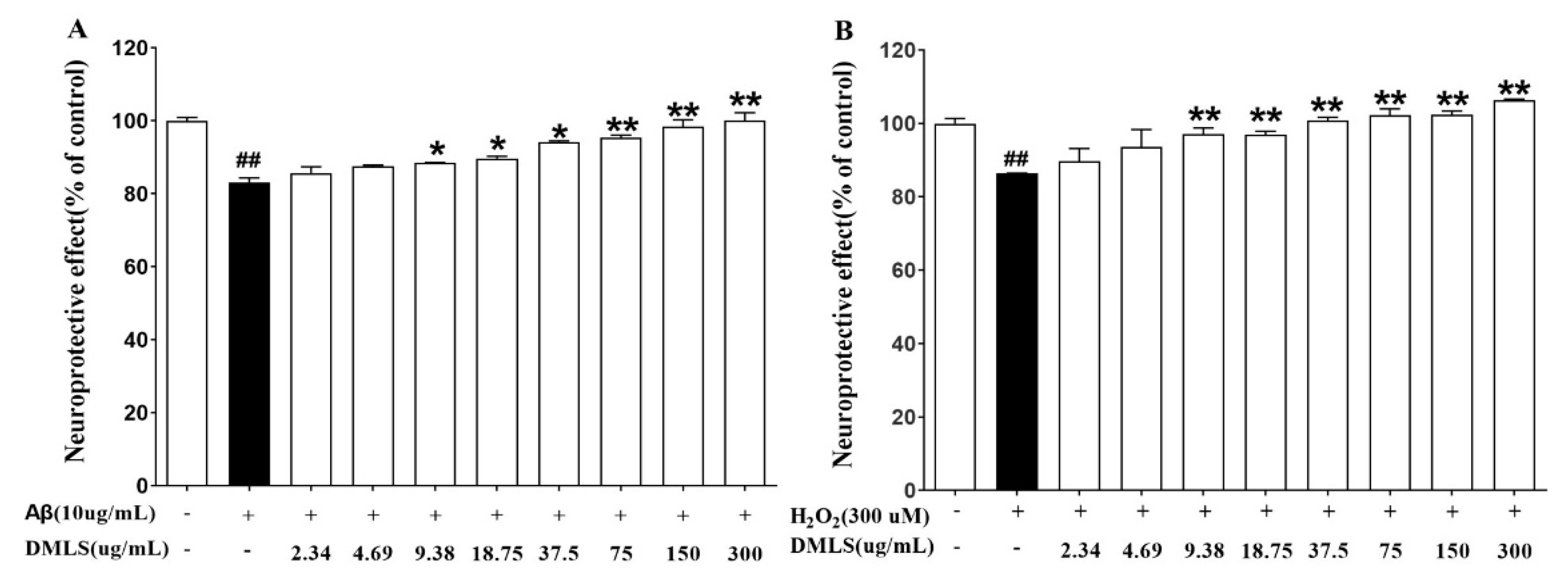
2.2. Neuroprotection Assay
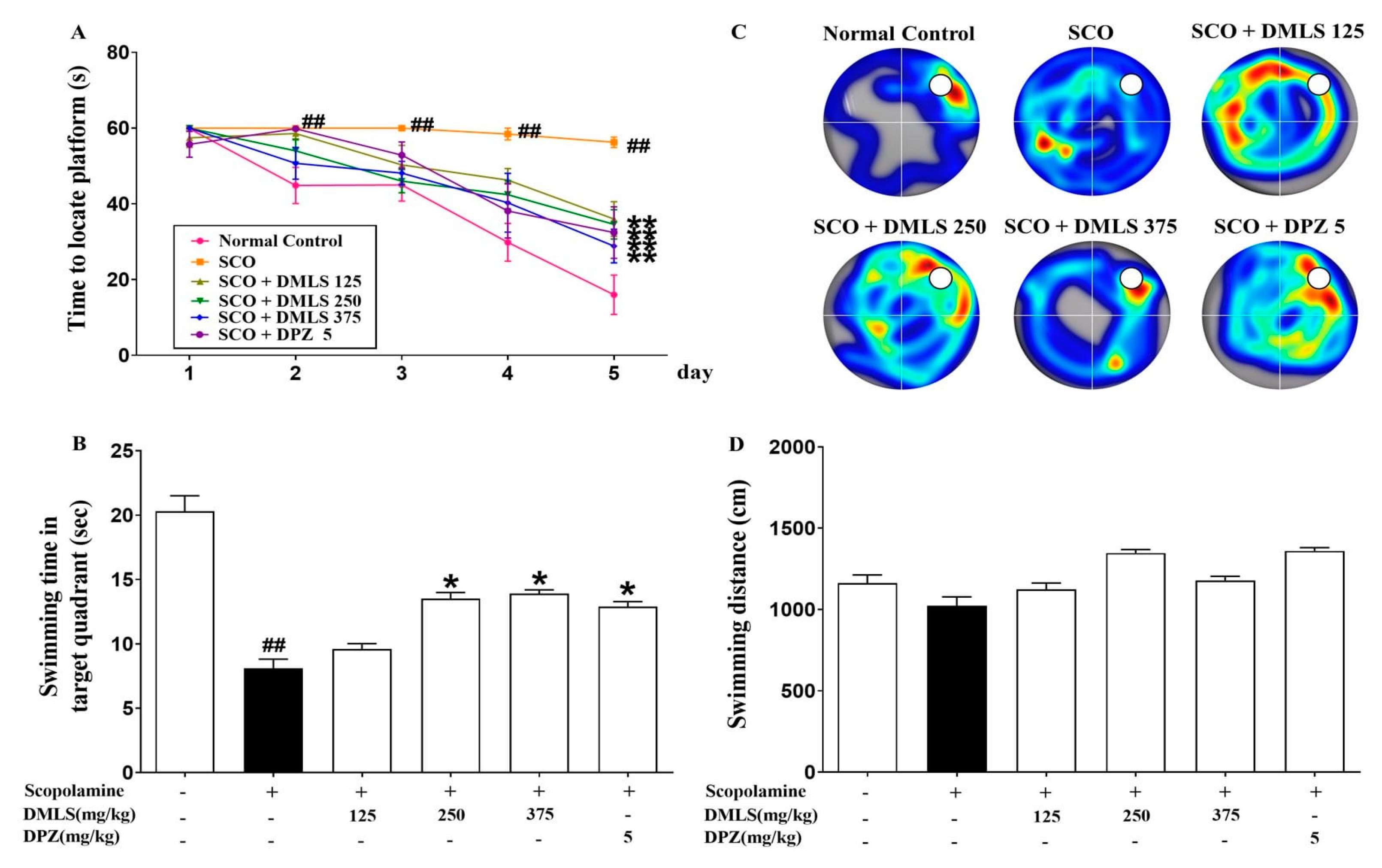
2.3. Effects DMLS on SCO-Induced Memory Impairment in the Morris Water-Maze Task
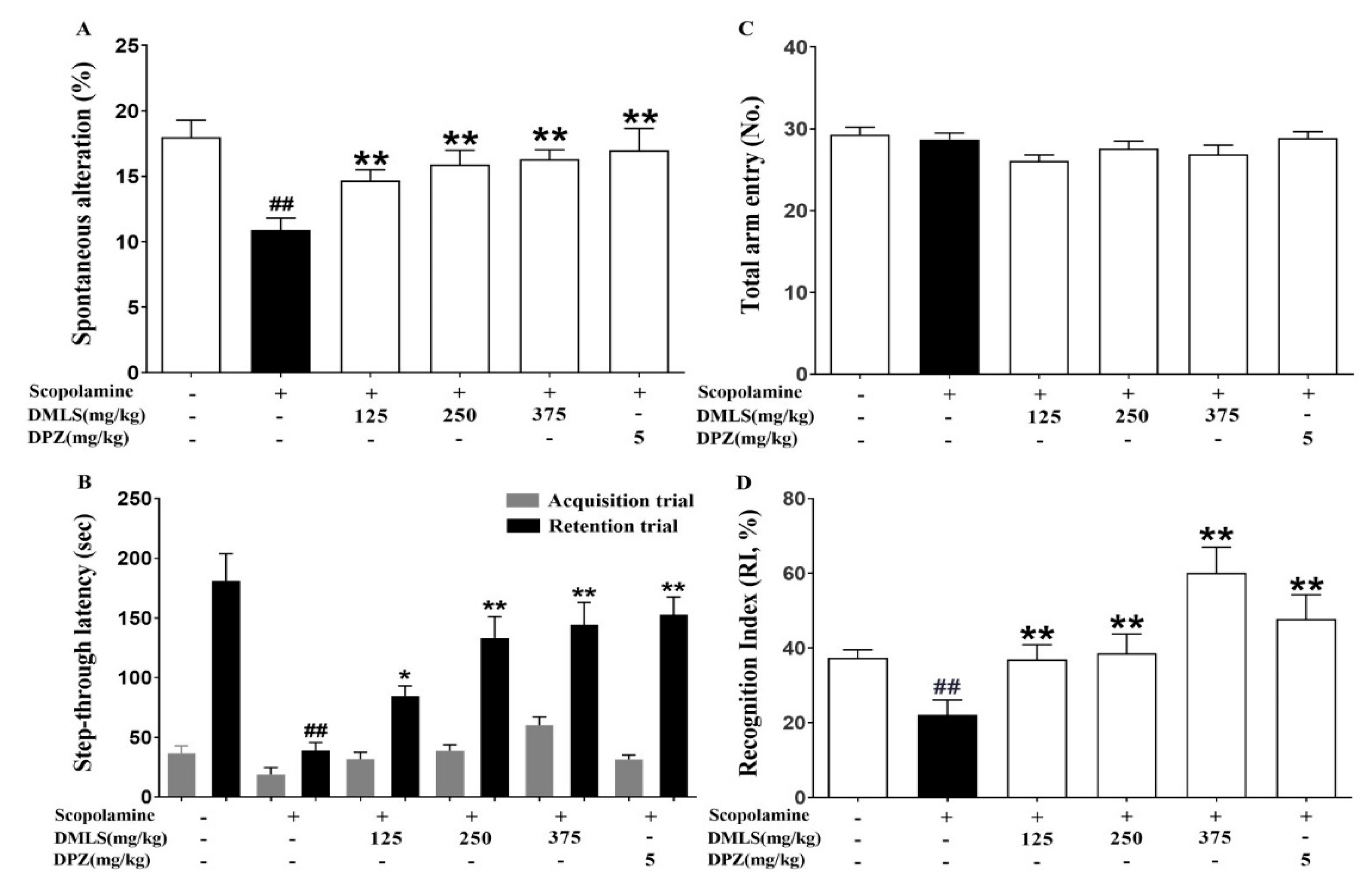
2.4. Effects DMLS on SCO-Induced Memory Impairment in the Y-Maze Task
2.5. Effects DMLS on SCO-Induced Memory Impairment in the Passive Avoidance Task
2.6. Effects DMLS on SCO-Induced Memory Impairment in the Novel Object Recognition (NOR) Test
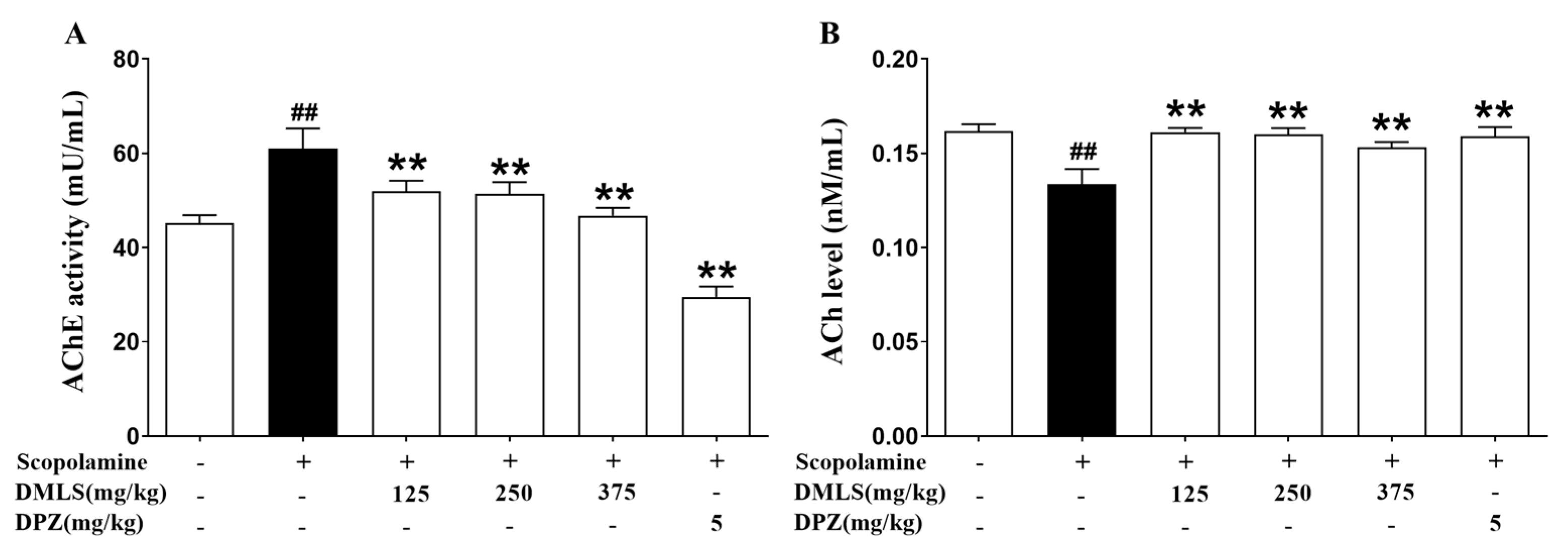
2.7. Effects DMLS on Parameters of Acetylcholinesterase (AChE) Activity and Acetylcholine (ACh) Contents
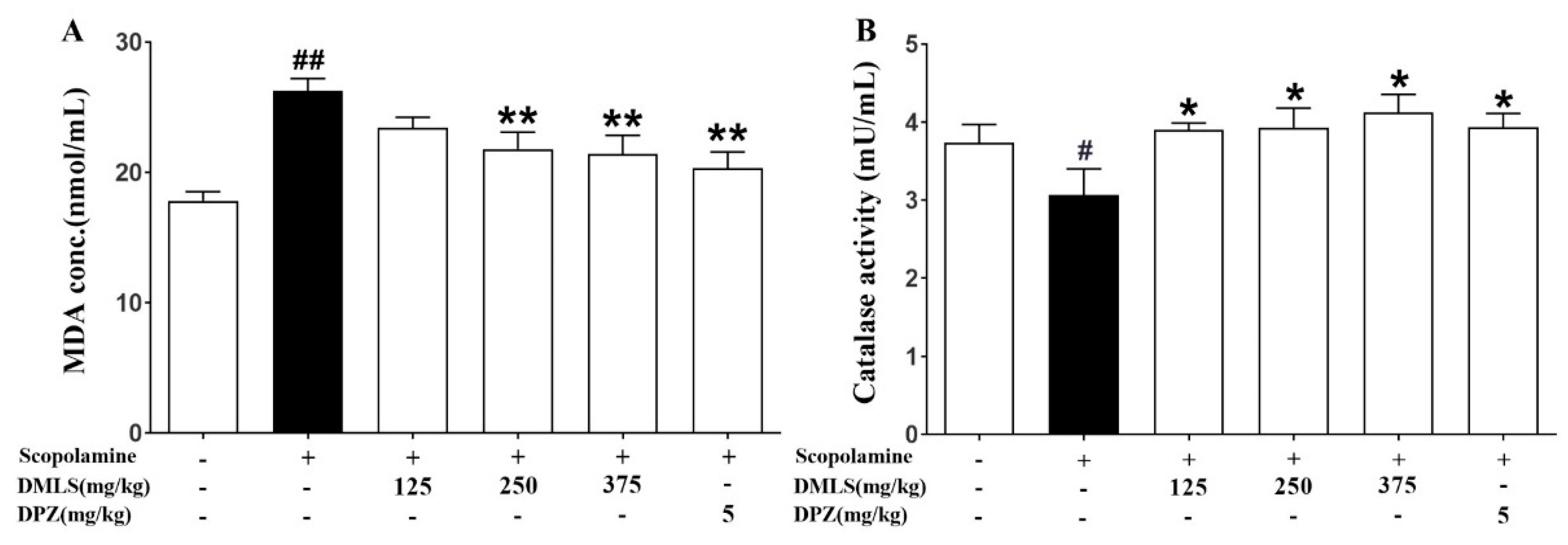
2.8. Effects DMLS on Parameters of Oxidative Stress
3. Discussion
4. Materials and Methods
4.1. Plant Preparation
4.1.1. Plant Material and Preparation of DMLS Extract
4.1.2. Manufacturing Process and Quantitative High-Performance Liquid Chromatography (HPLC) of DMLS
4.2. In Vitro Experiment
4.2.1. Cell Line and Cell Culture
4.2.2. Neuroprotection Assay
4.3. In Vivo Experiment
4.3.1. Experimental Animals
4.3.2. Mice Grouping and Treatment
4.4. Y-Maze Test
4.5. Morris Water-Maze Test
4.6. Passive Avoidance Task
4.7. Novel Object Recognition Test
4.8. AChE Activity and Contents of ACh in Brain Tissues
4.9. Oxidative Stress Parameters
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Spires-Jones, T.L.; Attems, J.; Thal, D.R. Interactions of pathological proteins in neurodegenerative diseases. Acta Neuropathol. 2017, 134, 187–205. [Google Scholar] [CrossRef] [PubMed]
- Deschamps, R.; Moulignier, A. Memory and related disorders. EMC-Neurol. 2005, 2, 505–525. [Google Scholar] [CrossRef]
- Bubser, M.; Byun, N.; Wood, M.R.; Jones, C.K. Muscarinic receptor pharmacology and circuitry for the modulation of cognition. Muscarinic Recept. 2012, 208, 121–166. [Google Scholar]
- Vandesquille, M. Implication du Système Cholinergique dans L’altération de la Mémoire de Travail au Cours du Vieillissement Chez la Souris. Approche Comportementale, Pharmacologique et Neurofonctionnelle; Bordeaux University: Bordeaux, France, 2011. [Google Scholar]
- Kim, S.-B.; Lee, A.Y.; Chun, J.M.; Lee, A.R.; Kim, H.S.; Seo, Y.S.; Moon, B.C.; Kwon, B.-I. Anthriscus sylvestris root extract reduces allergic lung inflammation by regulating interferon regulatory factor 4-mediated Th2 cell activation. J. Ethnopharmacol. 2019, 232, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Hafez, H.S.; Ghareeb, D.A.; Saleh, S.R.; Abady, M.M.; El Demellawy, M.A.; Hussien, H.; Abdel-Monem, N. Neuroprotective effect of ipriflavone against scopolamine-induced memory impairment in rats. Psychopharmacology 2017, 234, 3037–3053. [Google Scholar] [CrossRef] [PubMed]
- Mendiola-Precoma, J.; Berumen, L.; Padilla, K.; Garcia-Alcocer, G. Therapies for prevention and treatment of Alzheimer’s disease. BioMed Res. Int. 2016, 2016, 2589276. [Google Scholar] [CrossRef]
- Slader, C.A.; Reddel, H.K.; Jenkins, C.R.; Armour, C.L.; Bosnic-Anticevich, S.Z. Complementary and alternative medicine use in asthma: Who is using what? Respirology 2006, 11, 373–387. [Google Scholar] [CrossRef]
- Zhang, T.; Srivastava, K.; Wen, M.C.; Yang, N.; Cao, J.; Busse, P.; Birmingham, N.; Goldfarb, J.; Li, X.M. Pharmacology and immunological actions of a herbal medicine ASHMITM on allergic asthma. Phytother. Res. 2010, 24, 1047–1055. [Google Scholar] [CrossRef]
- Li, X.M. Treatment of asthma and food allergy with herbal interventions from traditional chinese medicine. Mt. Sinai J. Med. J. Transl. Pers. Med. 2011, 78, 697–716. [Google Scholar] [CrossRef]
- Park, B.-Y.; Min, B.-S.; Oh, S.-R.; Kim, J.-H.; Kim, T.-J.; Kim, D.-H.; Bae, K.-H.; Lee, H.-K. Isolation and anticomplement activity of compounds from Dendropanax morbifera. J. Ethnopharmacol. 2004, 90, 403–408. [Google Scholar] [CrossRef]
- Yang, H.Y.; Kim, K.S.; Lee, Y.H.; Park, J.H.; Kim, J.H.; Lee, S.-Y.; Kim, Y.-M.; Kim, I.S.; Kacew, S.; Lee, B.M.; et al. Dendropanax morbifera ameliorates thioacetamide-induced hepatic fibrosis via TGF-β1/Smads pathways. Int. J. Biol. Sci. 2019, 15, 800–811. [Google Scholar] [CrossRef] [PubMed]
- Youn, J.S.; Kim, Y.J.; Na, H.J.; Jung, H.R.; Song, C.K.; Kang, S.Y.; Kim, J.Y. Antioxidant activity and contents of leaf extracts obtained from Dendropanax morbifera LEV are dependent on the collecting season and extraction conditions. Food Sci. Biotechnol. 2019, 28, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.Y.; Lee, J.M.; Lee, D.G.; Cho, S.; Yoon, Y.H.; Cho, E.J.; Lee, S. The n-butanol fraction and rutin from tartary buckwheat improve cognition and memory in an in vivo model of amyloid-β-induced Alzheimer’s disease. J. Med. Food 2015, 18, 631–641. [Google Scholar] [CrossRef] [PubMed]
- Bellavite, P. Neuroprotective potentials of flavonoids: Experimental studies and mechanisms of action. Antioxidants 2023, 12, 280. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-Y.; Karthivashan, G.; Ko, H.M.; Cho, D.-Y.; Kim, J.; Cho, D.J.; Ganesan, P.; Su-Kim, I.; Choi, D.-K. Aqueous extract of Dendropanax morbiferus leaves effectively alleviated neuroinflammation and behavioral impediments in MPTP-induced Parkinson’s mouse model. Oxid. Med. Cell. Longev. 2018, 2018, 3175214. [Google Scholar] [CrossRef]
- Lee, K.Y.; Jung, H.Y.; Yoo, D.Y.; Kim, W.; Kim, J.W.; Kwon, H.J.; Kim, D.W.; Yoon, Y.S.; Hwang, I.K.; Choi, J.H. Dendropanax morbifera Léveille extract ameliorates D-galactose-induced memory deficits by decreasing inflammatory responses in the hippocampus. Lab. Anim. Res. 2017, 33, 283–290. [Google Scholar] [CrossRef]
- Kim, W.; Kim, D.W.; Yoo, D.Y.; Jung, H.Y.; Kim, J.W.; Kim, D.-W.; Choi, J.H.; Moon, S.M.; Yoon, Y.S.; Hwang, I.K. Antioxidant effects of Dendropanax morbifera Léveille extract in the hippocampus of mercury-exposed rats. BMC Complement. Altern. Med. 2015, 15, 247. [Google Scholar] [CrossRef]
- Kim, W.; Yim, H.S.; Yoo, D.Y.; Jung, H.Y.; Kim, J.W.; Choi, J.H.; Yoon, Y.S.; Kim, D.W.; Hwang, I.K. Dendropanax morbifera Léveille extract ameliorates cadmium-induced impairment in memory and hippocampal neurogenesis in rats. BMC Complement. Altern. Med. 2016, 16, 452. [Google Scholar] [CrossRef]
- Kim, W.; Kim, D.W.; Yoo, D.Y.; Jung, H.Y.; Nam, S.M.; Kim, J.W.; Hong, S.-M.; Kim, D.-W.; Choi, J.H.; Moon, S.M. Dendropanax morbifera Léveille extract facilitates cadmium excretion and prevents oxidative damage in the hippocampus by increasing antioxidant levels in cadmium-exposed rats. BMC Complement. Altern. Med. 2014, 14, 428. [Google Scholar] [CrossRef]
- Dugger, B.N.; Dickson, D.W. Pathology of neurodegenerative diseases. Cold Spring Harb. Perspect. Biol. 2017, 9, a028035. [Google Scholar] [CrossRef]
- Vorhees, C.V.; Williams, M.T. Morris water maze: Procedures for assessing spatial and related forms of learning and memory. Nat. Protoc. 2006, 1, 848–858. [Google Scholar] [CrossRef] [PubMed]
- Hoogland, I.; Houbolt, C.; van Westerloo, D.J.; van Gool, W.A.; van de Beek, D. Systemic inflammation and microglial activation: Systematic review of animal experiments. J. Neuroinflammation 2015, 12, 114. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Lee, J.Y.; Kim, J.W.; An, Y.J. Effect of leaf extracts of Dendropanax morbifera on selected probiotics and pathogenic bacteria. FASEB J. 2017, 31, lb407. [Google Scholar]
- Park, Y.M.; Han, J.S.; Park, Y.M.; Han, J.S. A study on the utilization of Dendropanax morbifera Lev. leaf extract for material of functional cosmetics and hair growth products. Asian J. Beauty Cosmetol. 2016, 14, 277–288. [Google Scholar] [CrossRef]
- Yang, E.-J.; Kim, S.-I.; Ku, H.-Y.; Lee, D.-S.; Lee, J.-W.; Kim, Y.-S.; Seong, Y.-H.; Song, K.-S. Syringin from stem bark of Fraxinus rhynchophylla protects Aβ (25–35)-induced toxicity in neuronal cells. Arch. Pharmacal Res. 2010, 33, 531–538. [Google Scholar] [CrossRef]
- Moshahid Khan, M.; Raza, S.S.; Javed, H.; Ahmad, A.; Khan, A.; Islam, F.; Safhi, M.M.; Islam, F. Rutin protects dopaminergic neurons from oxidative stress in an animal model of Parkinson’s disease. Neurotoxic. Res. 2012, 22, 1–15. [Google Scholar] [CrossRef]
- Gao, L.-J.; Dai, Y.; Li, X.-Q.; Meng, S.; Zhong, Z.-Q.; Xu, S.-J. Chlorogenic acid enhances autophagy by upregulating lysosomal function to protect against SH-SY5Y cell injury induced by H2O2. Exp. Ther. Med. 2021, 21, 426. [Google Scholar] [CrossRef]
- Limón, I.D.; Díaz, A.; Mendieta, L.; Chamorro, G.; Espinosa, B.; Zenteno, E.; Guevara, J. Amyloid-β25–35 impairs memory and increases NO in the temporal cortex of rats. Neurosci. Res. 2009, 63, 129–137. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Boyd-Kimball, D. The critical role of methionine 35 in Alzheimer’s amyloid β-peptide (1–42)-induced oxidative stress and neurotoxicity. Biochim. Biophys. Acta. Proteins Proteom. 2005, 1703, 149–156. [Google Scholar] [CrossRef]
- Behl, C.; Davis, J.; Lesley, R.; Schubert, D. Hydrogen peroxide mediates amyloid β protein toxicity. Cell 1994, 77, 817–827. [Google Scholar] [CrossRef]
- Hyslop, P.A.; Zhang, Z.; Pearson, D.V.; Phebus, L.A. Measurement of striatal H2O2 by microdialysis following global forebrain ischemia and reperfusion in the rat: Correlation with the cytotoxic potential of H2O2 in vitro. Brain Res. 1995, 671, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Simonian, N.; Coyle, J. Oxidative stress in neurodegenerative diseases. Annu. Rev. Pharmacol. Toxicol. 1996, 36, 83–106. [Google Scholar] [CrossRef]
- Ishikawa, Y.; Satoh, T.; Enokido, Y.; Nishio, C.; Ikeuchi, T.; Hatanaka, H. Generation of reactive oxygen species, release of L-glutamate and activation of caspases are required for oxygen-induced apoptosis of embryonic hippocampal neurons in culture. Brain Res. 1999, 824, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Stoy, N.; Mackay, G.; Forrest, C.; Christofides, J.; Egerton, M.; Stone, T.; Darlington, L. Tryptophan metabolism and oxidative stress in patients with Huntington’s disease. J. Neurochem. 2005, 93, 611–623. [Google Scholar] [CrossRef]
- Choi, J.; Sullards, M.C.; Olzmann, J.A.; Rees, H.D.; Weintraub, S.T.; Bostwick, D.E.; Gearing, M.; Levey, A.I.; Chin, L.-S.; Li, L. Oxidative damage of DJ-1 is linked to sporadic Parkinson and Alzheimer diseases. J. Biol. Chem. 2006, 281, 10816–10824. [Google Scholar] [CrossRef] [PubMed]
- Bellingham, M.C. A review of the neural mechanisms of action and clinical efficiency of riluzole in treating amyotrophic lateral sclerosis: What have we learned in the last decade? CNS Neurosci. Ther. 2011, 17, 4–31. [Google Scholar] [CrossRef] [PubMed]
- Caviness, J.N.; Lue, L.; Adler, C.H.; Walker, D.G. Parkinson’s disease dementia and potential therapeutic strategies. CNS Neurosci. Ther. 2011, 17, 32–44. [Google Scholar] [CrossRef]
- Kelsey, N.A.; Wilkins, H.M.; Linseman, D.A. Nutraceutical antioxidants as novel neuroprotective agents. Molecules 2010, 15, 7792–7814. [Google Scholar] [CrossRef]
- Shibata, S.; Tanaka, O.; Soma, K.; Iida, Y.; Ando, T.; Nakamura, H. Studies on saponins and sapogenins of ginseng the structure of panaxatriol. Tetrahedron Lett. 1965, 6, 207–213. [Google Scholar] [CrossRef]
- Lorenzini, C.A.; Baldi, E.; Bucherelli, C.; Sacchetti, B.; Tassoni, G. Role of dorsal hippocampus in acquisition, consolidation and retrieval of rat’s passive avoidance response: A tetrodotoxin functional inactivation study. Brain Res. 1996, 730, 32–39. [Google Scholar] [CrossRef]
- Lueptow, L.M. Novel object recognition test for the investigation of learning and memory in mice. J. Vis. Exp. 2017, 126, e55718. [Google Scholar]
- Kim, J.; Shim, J.; Lee, S.; Cho, W.-H.; Hong, E.; Lee, J.H.; Han, J.-S.; Lee, H.J.; Lee, K.W. Rg3-enriched ginseng extract ameliorates scopolamine-induced learning deficits in mice. BMC Complement. Altern. Med. 2016, 16, 66. [Google Scholar] [CrossRef] [PubMed]
- Chance, B.; Sies, H.; Boveris, A. Hydroperoxide metabolism in mammalian organs. Physiol. Rev. 1979, 59, 527–605. [Google Scholar] [CrossRef] [PubMed]
- Torun, A.N.; Kulaksizoglu, S.; Kulaksizoglu, M.; Pamuk, B.O.; Isbilen, E.; Tutuncu, N.B. Serum total antioxidant status and lipid peroxidation marker malondialdehyde levels in overt and subclinical hypothyroidism. Clin. Endocrinol. 2009, 70, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Beutler, E.; Blaisdell, R.K. Iron enzymes in iron deficiency. II. Catalase Hum. Erythrocytes. J. Clin. Investig. 1958, 37, 833–835. [Google Scholar] [CrossRef] [PubMed]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.B.; Ryu, H.Y.; Nam, W.; Lee, S.M.; Jang, M.R.; Kwak, Y.G.; Kang, G.I.; Song, K.S.; Lee, J.W. The Neuroprotective Effects of Dendropanax morbifera Water Extract on Scopolamine-Induced Memory Impairment in Mice. Int. J. Mol. Sci. 2023, 24, 16444. https://doi.org/10.3390/ijms242216444
Kim SB, Ryu HY, Nam W, Lee SM, Jang MR, Kwak YG, Kang GI, Song KS, Lee JW. The Neuroprotective Effects of Dendropanax morbifera Water Extract on Scopolamine-Induced Memory Impairment in Mice. International Journal of Molecular Sciences. 2023; 24(22):16444. https://doi.org/10.3390/ijms242216444
Chicago/Turabian StyleKim, Sung Bae, Hyun Yeoul Ryu, Woo Nam, So Min Lee, Mi Ran Jang, Youn Gil Kwak, Gyoo Il Kang, Kyung Seok Song, and Jae Won Lee. 2023. "The Neuroprotective Effects of Dendropanax morbifera Water Extract on Scopolamine-Induced Memory Impairment in Mice" International Journal of Molecular Sciences 24, no. 22: 16444. https://doi.org/10.3390/ijms242216444
APA StyleKim, S. B., Ryu, H. Y., Nam, W., Lee, S. M., Jang, M. R., Kwak, Y. G., Kang, G. I., Song, K. S., & Lee, J. W. (2023). The Neuroprotective Effects of Dendropanax morbifera Water Extract on Scopolamine-Induced Memory Impairment in Mice. International Journal of Molecular Sciences, 24(22), 16444. https://doi.org/10.3390/ijms242216444






