Troponin and a Myopathy-Linked Mutation in TPM3 Inhibit Cofilin-2-Induced Thin Filament Depolymerization
Abstract
:1. Introduction
2. Results
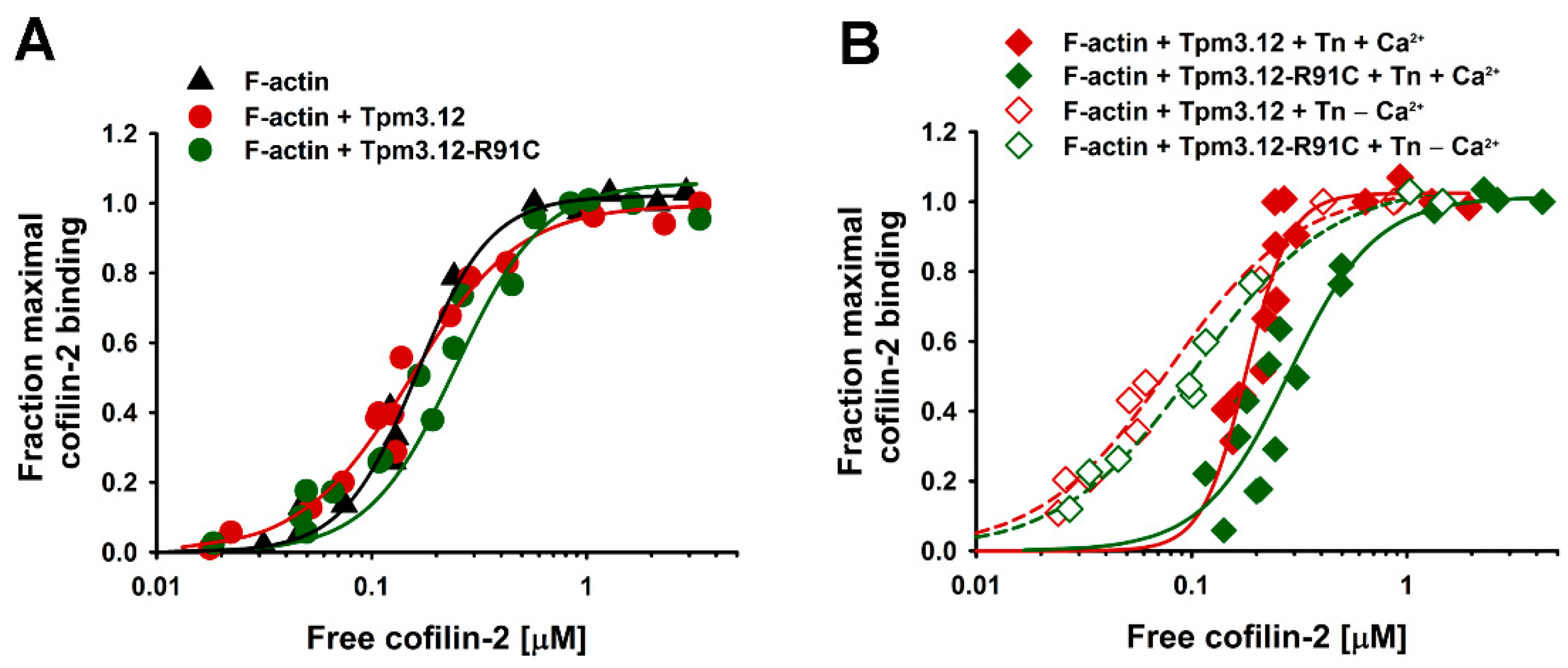
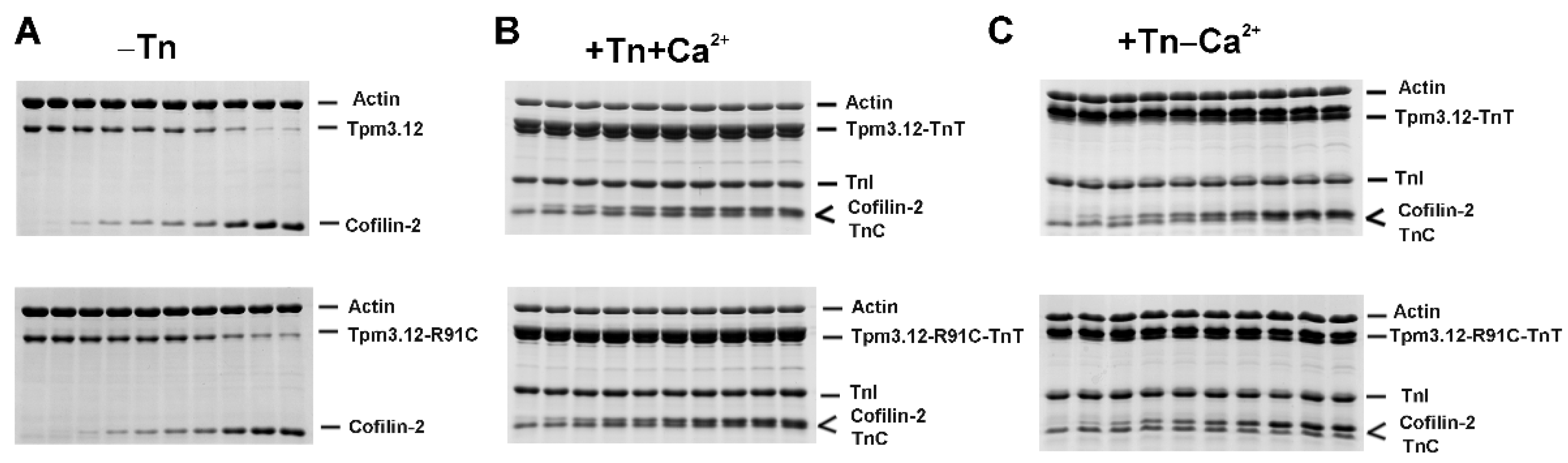
2.1. Effects of the p.R91C Mutation in Tpm3.12 and the Troponin Complex on Cofilin-2 Binding to F-Actin
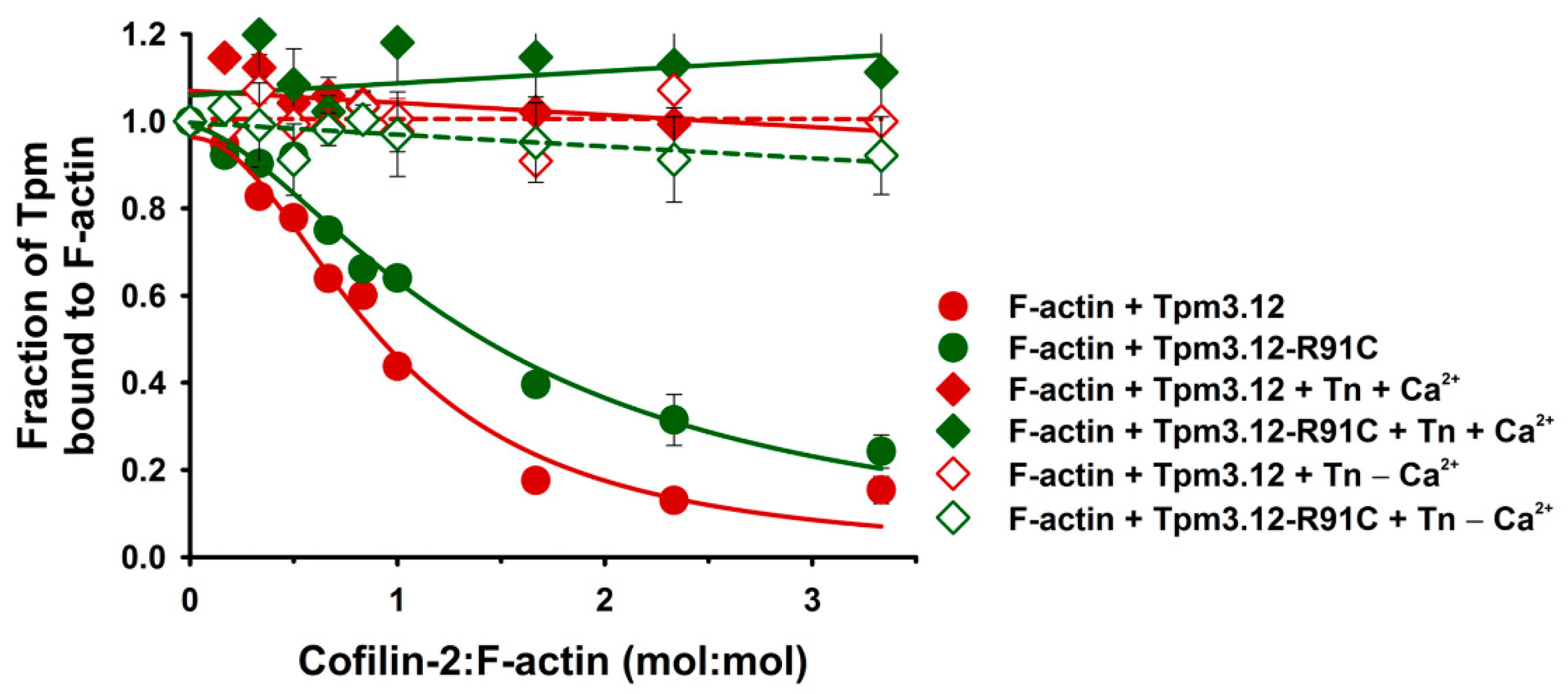
2.2. Effects of the Troponin Complex on Dissociation of Tpm3.12 and Tpm3.12-R91C from Actin Filaments
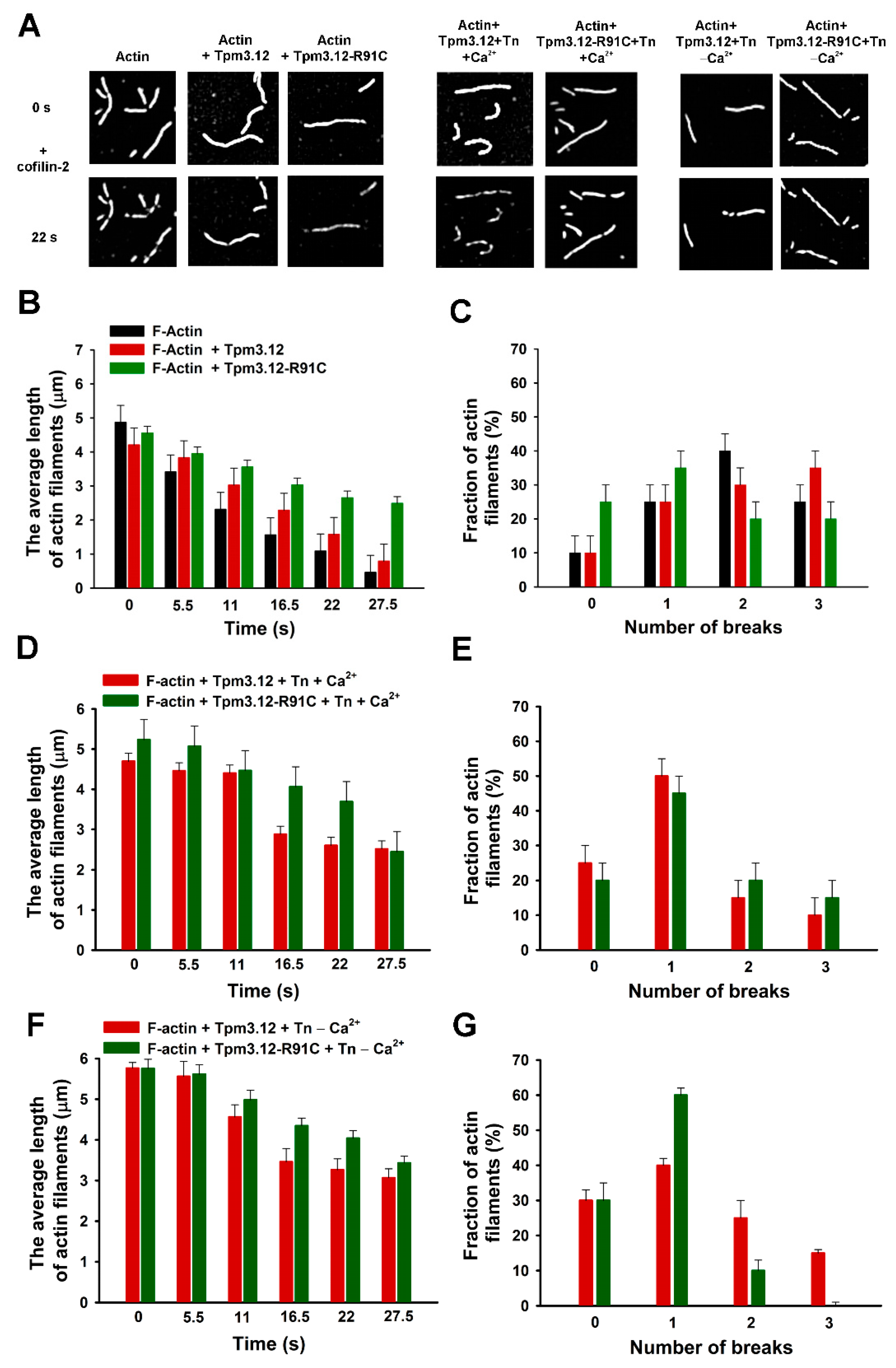
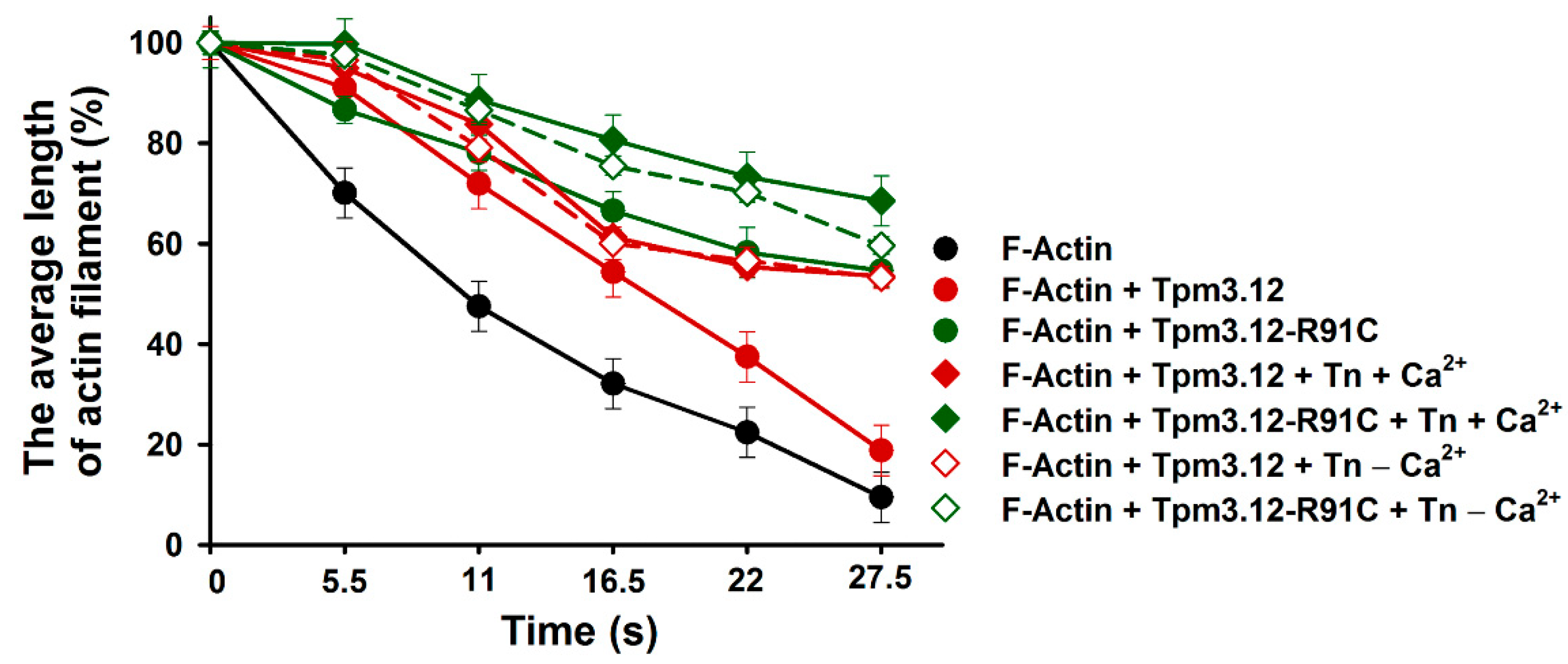
2.3. Effects of Tpm3.12 Variants and Troponin on Cofilin-2-Induced Fragmentation and Depolymerization of F-Actin
3. Discussion
3.1. Effects of Troponin and Tpm3.12-R91C on Actin Affinity of Cofilin-2
3.2. Effects of Troponin and Tpm3.12-R91C on Severing and Depolymerization of the Thin Filament by Cofilin-2
4. Materials and Methods
4.1. Muscle Protein Preparation
4.2. Preparation of Recombinant Tropomyosin Tpm3.12 Variants and Cofilin-2
4.3. Cosedimentation Assay
4.4. In Vitro Severing/Depolymerization Assay
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cohen, E.; Bonne, G.; Rivier, F.; Hamroun, D. The 2022 version of the gene table of neuromuscular disorders (nuclear genome). Neuromuscul. Disord. 2021, 31, 1313–1357. [Google Scholar] [CrossRef] [PubMed]
- Pelin, K.; Wallgren-Pettersson, C. Update on the Genetics of Congenital Myopathies. Semin. Pediatr. Neurol. 2019, 29, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Benarroch, L.; Bonne, G.; Rivier, F.; Hamroun, D. The 2023 version of the gene table of neuromuscular disorders (nuclear genome). Neuromuscul. Disord. 2023, 33, 76–117. [Google Scholar] [CrossRef] [PubMed]
- Ogasawara, M.; Nishino, I. A review of major causative genes in congenital myopathies. J. Hum. Genet. 2023, 68, 215–225. [Google Scholar] [CrossRef]
- Marttila, M.; Lehtokari, V.L.; Marston, S.; Nyman, T.A.; Barnerias, C.; Beggs, A.H.; Bertini, E.; Ceyhan-Birsoy, O.; Cintas, P.; Gerard, M.; et al. Mutation update and genotype-phenotype correlations of novel and previously described mutations in TPM2 and TPM3 causing congenital myopathies. Hum. Mutat. 2014, 35, 779–790. [Google Scholar] [CrossRef]
- Moraczewska, J. Thin filament dysfunctions caused by mutations in tropomyosin Tpm3.12 and Tpm1.1. J. Muscle Res. Cell Motil. 2020, 41, 39–53. [Google Scholar] [CrossRef]
- Kee, A.J.; Hardeman, E.C. Tropomyosins in skeletal muscle diseases. Adv. Exp. Med. Biol. 2008, 644, 143–157. [Google Scholar]
- Robaszkiewicz, K.; Dudek, E.; Kasprzak, A.A.; Moraczewska, J. Functional effects of congenital myopathy-related mutations in gamma-tropomyosin gene. Biochim. Biophys. Acta 2012, 1822, 1562–1569. [Google Scholar] [CrossRef]
- Sliwinska, M.; Robaszkiewicz, K.; Czajkowska, M.; Zheng, W.J.; Moraczewska, J. Functional effects of substitutions I92T and V95A in actin-binding period 3 of tropomyosin. Biochim. Biophys. Acta 2018, 1866, 558–568. [Google Scholar] [CrossRef]
- Orzechowski, M.; Fischer, S.; Moore, J.R.; Lehman, W.; Farman, G.P. Energy landscapes reveal the myopathic effects of tropomyosin mutations. Arch. Biochem. Biophys. 2014, 564, 89–99. [Google Scholar] [CrossRef]
- Memo, M.; Marston, S. Skeletal muscle myopathy mutations at the actin tropomyosin interface that cause gain- or loss-of-function. J. Muscle Res. Cell Motil. 2013, 34, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Gokhin, D.S.; Kim, N.E.; Lewis, S.A.; Hoenecke, H.R.; D’Lima, D.D.; Fowler, V.M. Thin-filament length correlates with fiber type in human skeletal muscle. Am. J. Physiol. Cell Physiol. 2012, 302, C555–C565. [Google Scholar] [CrossRef] [PubMed]
- Moraczewska, J.; Robaszkiewicz, K.; Sliwinska, M.; Czajkowska, M.; Ly, T.; Kostyukova, A.; Wen, H.; Zheng, W. Congenital myopathy-related mutations in tropomyosin disrupt regulatory function through altered actin affinity and tropomodulin binding. FEBS J. 2019, 286, 1877–1893. [Google Scholar] [CrossRef] [PubMed]
- Pieples, K.; Wieczorek, D.F. Tropomyosin 3 increases striated muscle isoform diversity. Biochemistry 2000, 39, 8291–8297. [Google Scholar] [CrossRef] [PubMed]
- Corbett, M.A.; Akkari, P.A.; Domazetovska, A.; Cooper, S.T.; North, K.N.; Laing, N.G.; Gunning, P.W.; Hardeman, E.C. An alphaTropomyosin mutation alters dimer preference in nemaline myopathy. Ann. Neurol. 2005, 57, 42–49. [Google Scholar] [CrossRef]
- Gordon, A.M.; Homsher, E.; Regnier, M. Regulation of contraction in striated muscle. Physiol. Rev. 2000, 80, 853–924. [Google Scholar] [CrossRef]
- Lehman, W. Thin Filament Structure and the Steric Blocking Model. Comp. Phys. 2016, 6, 1043–1069. [Google Scholar] [CrossRef]
- Poole, K.J.; Lorenz, M.; Evans, G.; Rosenbaum, G.; Pirani, A.; Craig, R.; Tobacman, L.S.; Lehman, W.; Holmes, K.C. A comparison of muscle thin filament models obtained from electron microscopy reconstructions and low-angle X-ray fibre diagrams from non-overlap muscle. J. Struct. Biol. 2006, 155, 273–284. [Google Scholar] [CrossRef]
- Robaszkiewicz, K.; Sliwinska, M.; Moraczewska, J. Regulation of Actin Filament Length by Muscle Isoforms of Tropomyosin and Cofilin. Int. J. Mol. Sci. 2020, 21, 4285. [Google Scholar] [CrossRef]
- Ono, S.; Minami, N.; Abe, H.; Obinata, T. Characterization of a novel cofilin isoform that is predominantly expressed in mammalian skeletal muscle. J. Biol. Chem. 1994, 269, 15280–15286. [Google Scholar] [CrossRef]
- Vartiainen, M.K.; Mustonen, T.; Mattila, P.K.; Ojala, P.J.; Thesleff, I.; Partanen, J.; Lappalainen, P. The three mouse actin-depolymerizing factor/cofilins evolved to fulfill cell-type-specific requirements for actin dynamics. Mol. Biol. Cell 2002, 13, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Kremneva, E.; Makkonen, M.H.; Skwarek-Maruszewska, A.; Gateva, G.; Michelot, A.; Dominguez, R.; Lappalainen, P. Cofilin-2 controls actin filament length in muscle sarcomeres. Dev. Cell 2014, 31, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, P.B.; Joshi, M.; Savic, T.; Chen, Z.; Beggs, A.H. Normal myofibrillar development followed by progressive sarcomeric disruption with actin accumulations in a mouse Cfl2 knockout demonstrates requirement of cofilin-2 for muscle maintenance. Hum. Mol. Genet. 2012, 21, 2341–2356. [Google Scholar] [CrossRef] [PubMed]
- Miyauchi-Nomura, S.; Obinata, T.; Sato, N. Cofilin is required for organization of sarcomeric actin filaments in chicken skeletal muscle cells. Cytoskeleton 2012, 69, 290–302. [Google Scholar] [CrossRef] [PubMed]
- Gurniak, C.B.; Chevessier, F.; Jokwitz, M.; Jonsson, F.; Perlas, E.; Richter, H.; Matern, G.; Boyl, P.P.; Chaponnier, C.; Furst, D.; et al. Severe protein aggregate myopathy in a knockout mouse model points to an essential role of cofilin2 in sarcomeric actin exchange and muscle maintenance. Eur. J. Cell Biol. 2014, 93, 252–266. [Google Scholar] [CrossRef] [PubMed]
- Ockeloen, C.W.; Gilhuis, H.J.; Pfundt, R.; Kamsteeg, E.J.; Agrawal, P.B.; Beggs, A.H.; Dara Hama-Amin, A.; Diekstra, A.; Knoers, N.V.; Lammens, M.; et al. Congenital myopathy caused by a novel missense mutation in the CFL2 gene. Neuromuscul. Disord. 2012, 22, 632–639. [Google Scholar] [CrossRef]
- Agrawal, P.B.; Greenleaf, R.S.; Tomczak, K.K.; Lehtokari, V.L.; Wallgren-Pettersson, C.; Wallefeld, W.; Laing, N.G.; Darras, B.T.; Maciver, S.K.; Dormitzer, P.R.; et al. Nemaline myopathy with minicores caused by mutation of the CFL2 gene encoding the skeletal muscle actin-binding protein, cofilin-2. Am. J. Hum. Genet. 2007, 80, 162–167. [Google Scholar] [CrossRef]
- Clarke, N.F. Skeletal muscle disease due to mutations in tropomyosin, troponin and cofilin. Adv. Exp. Med. Biol. 2008, 642, 40–54. [Google Scholar]
- Marston, S.B. Why Is there a Limit to the Changes in Myofilament Ca(2+)-Sensitivity Associated with Myopathy Causing Mutations? Front. Physiol. 2016, 7, 415. [Google Scholar] [CrossRef]
- Ochala, J. Thin filament proteins mutations associated with skeletal myopathies: Defective regulation of muscle contraction. J. Mol. Med. 2008, 86, 1197–1204. [Google Scholar] [CrossRef]
- Ochala, J.; Gokhin, D.S.; Penisson-Besnier, I.; Quijano-Roy, S.; Monnier, N.; Lunardi, J.; Romero, N.B.; Fowler, V.M. Congenital myopathy-causing tropomyosin mutations induce thin filament dysfunction via distinct physiological mechanisms. Hum. Mol. Genet. 2012, 21, 4473–4485. [Google Scholar] [CrossRef]
- Robaszkiewicz, K.; Ostrowska, Z.; Marchlewicz, K.; Moraczewska, J. Tropomyosin isoforms differentially modulate the regulation of actin filament polymerization and depolymerization by cofilins. FEBS J. 2016, 283, 723–737. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, T.B.; Bamburg, J.R. Tropomyosin and ADF/cofilin as collaborators and competitors. Adv. Exp. Med. Biol. 2008, 644, 232–249. [Google Scholar] [PubMed]
- Ngo, K.X.; Umeki, N.; Kijima, S.T.; Kodera, N.; Ueno, H.; Furutani-Umezu, N.; Nakajima, J.; Noguchi, T.Q.; Nagasaki, A.; Tokuraku, K.; et al. Allosteric regulation by cooperative conformational changes of actin filaments drives mutually exclusive binding with cofilin and myosin. Sci. Rep. 2016, 6, 35449. [Google Scholar] [CrossRef] [PubMed]
- Colpan, M.; Moroz, N.A.; Kostyukova, A.S. Tropomodulins and tropomyosins: Working as a team. J. Muscle Res. Cell Motil. 2013, 34, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Chereau, D.; Boczkowska, M.; Skwarek-Maruszewska, A.; Fujiwara, I.; Hayes, D.B.; Rebowski, G.; Lappalainen, P.; Pollard, T.D.; Dominguez, R. Leiomodin is an actin filament nucleator in muscle cells. Science 2008, 320, 239–243. [Google Scholar] [CrossRef]
- Mannherz, H.G.; Ballweber, E.; Galla, M.; Villard, S.; Granier, C.; Steegborn, C.; Schmidtmann, A.; Jaquet, K.; Pope, B.; Weeds, A.G. Mapping the ADF/cofilin binding site on monomeric actin by competitive cross-linking and peptide array: Evidence for a second binding site on monomeric actin. J. Mol. Biol. 2007, 366, 745–755. [Google Scholar] [CrossRef]
- Tanaka, K.; Takeda, S.; Mitsuoka, K.; Oda, T.; Kimura-Sakiyama, C.; Maeda, Y.; Narita, A. Structural basis for cofilin binding and actin filament disassembly. Nat. Commun. 2018, 9, 1860. [Google Scholar] [CrossRef]
- Kraus, J.; Russell, R.W.; Kudryashova, E.; Xu, C.; Katyal, N.; Perilla, J.R.; Kudryashov, D.S.; Polenova, T. Magic angle spinning NMR structure of human cofilin-2 assembled on actin filaments reveals isoform-specific conformation and binding mode. Nat. Commun. 2022, 13, 2114. [Google Scholar] [CrossRef]
- McGough, A.; Pope, B.; Chiu, W.; Weeds, A. Cofilin changes the twist of F-actin: Implications for actin filament dynamics and cellular function. J. Cell Biol. 1997, 138, 771–781. [Google Scholar] [CrossRef]
- Jansen, S.; Goode, B.L. Tropomyosin isoforms differentially tune actin filament length and disassembly. Mol. Biol. Cell 2019, 30, 671–679. [Google Scholar] [CrossRef]
- Ostrowska, Z.; Robaszkiewicz, K.; Moraczewska, J. Regulation of actin filament turnover by cofilin-1 and cytoplasmic tropomyosin isoforms. Bioch. Biophys. Acta 2017, 1865, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Vibert, P.; Craig, R.; Lehman, W. Steric-model for activation of muscle thin filaments. J. Mol. Biol. 1997, 266, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, M.; Pope, B.; Maciver, S.K.; Weeds, A.G. Human actin depolymerizing factor mediates a pH-sensitive destruction of actin filaments. Biochemistry 1993, 32, 9985–9993. [Google Scholar] [CrossRef] [PubMed]
- Yonezawa, N.; Nishida, E.; Sakai, H. pH control of actin polymerization by cofilin. J. Biol. Chem. 1985, 260, 14410–14412. [Google Scholar] [CrossRef]
- Chin, E.R.; Allen, D.G. The contribution of pH-dependent mechanisms to fatigue at different intensities in mammalian single muscle fibres. J. Physiol. 1998, 512 Pt 3, 831–840. [Google Scholar] [CrossRef]
- Pedersen, T.H.; Nielsen, O.B.; Lamb, G.D.; Stephenson, D.G. Intracellular acidosis enhances the excitability of working muscle. Science 2004, 305, 1144–1147. [Google Scholar] [CrossRef]
- Spudich, J.A.; Watt, S. The regulation of rabbit skeletal muscle contraction. I. Biochemical studies of the interaction of the tropomyosin-troponin complex with actin and the proteolytic fragments of myosin. J. Biol. Chem. 1971, 246, 4866–4871. [Google Scholar] [CrossRef]
- Potter, J.D. Preparation of troponin and its subunits. Methods Enzymol. 1982, 85 Pt B, 241–263. [Google Scholar]
- Monteiro, P.B.; Lataro, R.C.; Ferro, J.A.; Reinach Fde, C. Functional alpha-tropomyosin produced in Escherichia coli. A dipeptide extension can substitute the amino-terminal acetyl group. J. Biol. Chem. 1994, 269, 10461–10466. [Google Scholar] [CrossRef]
| −Tn | +Tn (+Ca2+) | +Tn (−Ca2+) | ||||
|---|---|---|---|---|---|---|
| Kapp [μM−1] | αH | Kapp [μM−1] | αH | Kapp [μM−1] | αH | |
| F-actin | 6.0 ± 0.1 | 2.5 ± 0.1 | n.a. | n.a. | n.a. | n.a. |
| F-actin-Tpm3.12 | 6.6 ± 0.2 | 1.2 ± 0.4 | 5.5 ± 0.3 * | 3.2 ± 0.5 | 12.5 ± 1.8 * | 1.5 ± 0.3 |
| F-actin-Tpm3.12-R91C | 5.2 ± 0.2 | 1.7 ± 0.2 | 3.5 ± 0.4 * | 2.1 ± 0.3 | 10.5 ± 0.8 * | 1.4 ± 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Robaszkiewicz, K.; Wróbel, J.; Moraczewska, J. Troponin and a Myopathy-Linked Mutation in TPM3 Inhibit Cofilin-2-Induced Thin Filament Depolymerization. Int. J. Mol. Sci. 2023, 24, 16457. https://doi.org/10.3390/ijms242216457
Robaszkiewicz K, Wróbel J, Moraczewska J. Troponin and a Myopathy-Linked Mutation in TPM3 Inhibit Cofilin-2-Induced Thin Filament Depolymerization. International Journal of Molecular Sciences. 2023; 24(22):16457. https://doi.org/10.3390/ijms242216457
Chicago/Turabian StyleRobaszkiewicz, Katarzyna, Julia Wróbel, and Joanna Moraczewska. 2023. "Troponin and a Myopathy-Linked Mutation in TPM3 Inhibit Cofilin-2-Induced Thin Filament Depolymerization" International Journal of Molecular Sciences 24, no. 22: 16457. https://doi.org/10.3390/ijms242216457





