Improved Reversion of Calcifications in Porcine Aortic Heart Valves Using Elastin-Targeted Nanoparticles
Abstract
:1. Introduction
2. Results
2.1. Preparation and Characterization of Nanoparticles
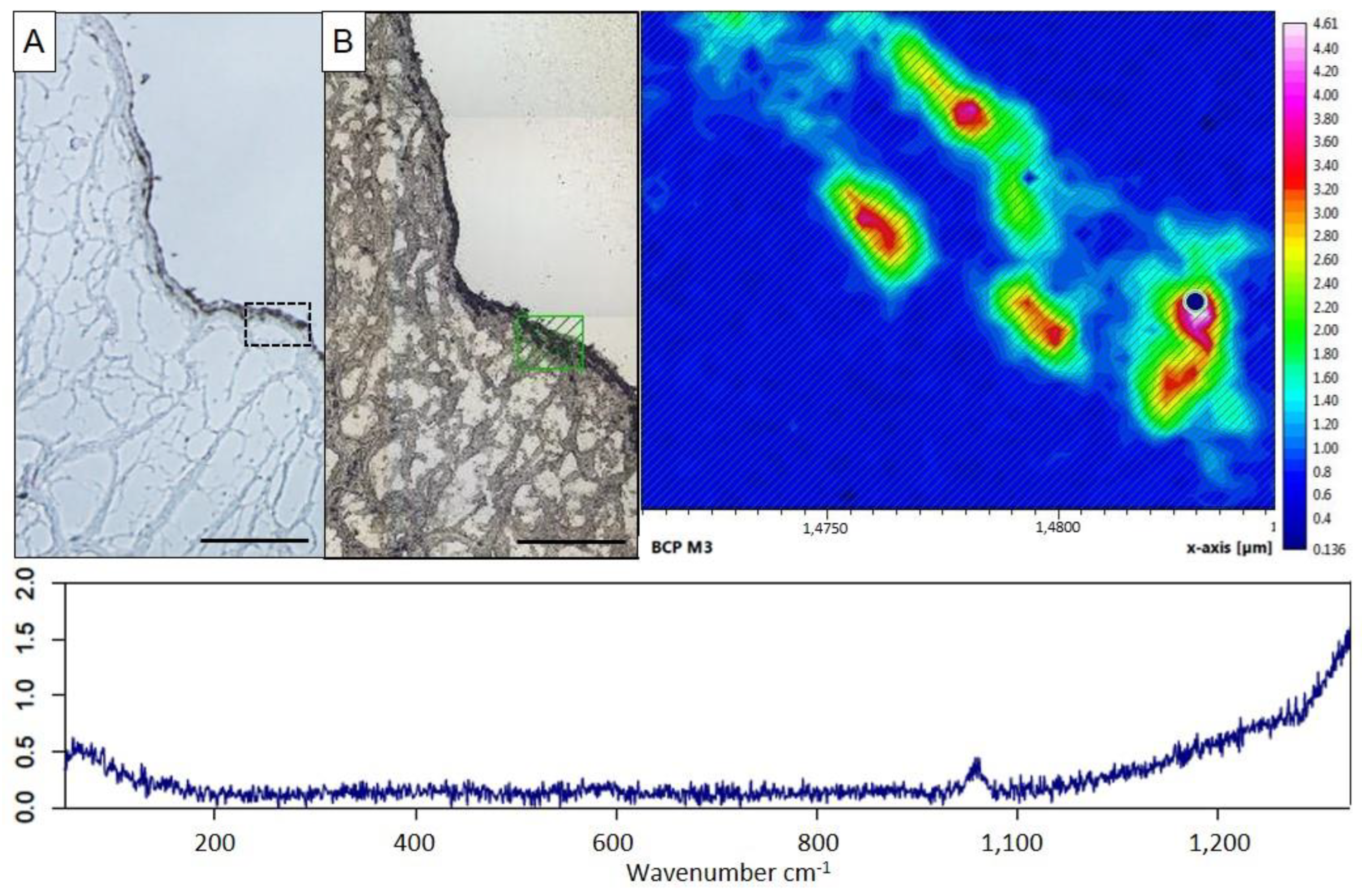
2.2. Characterization of Ex Vivo Calcification of Porcine Aortic Valve Leaflets
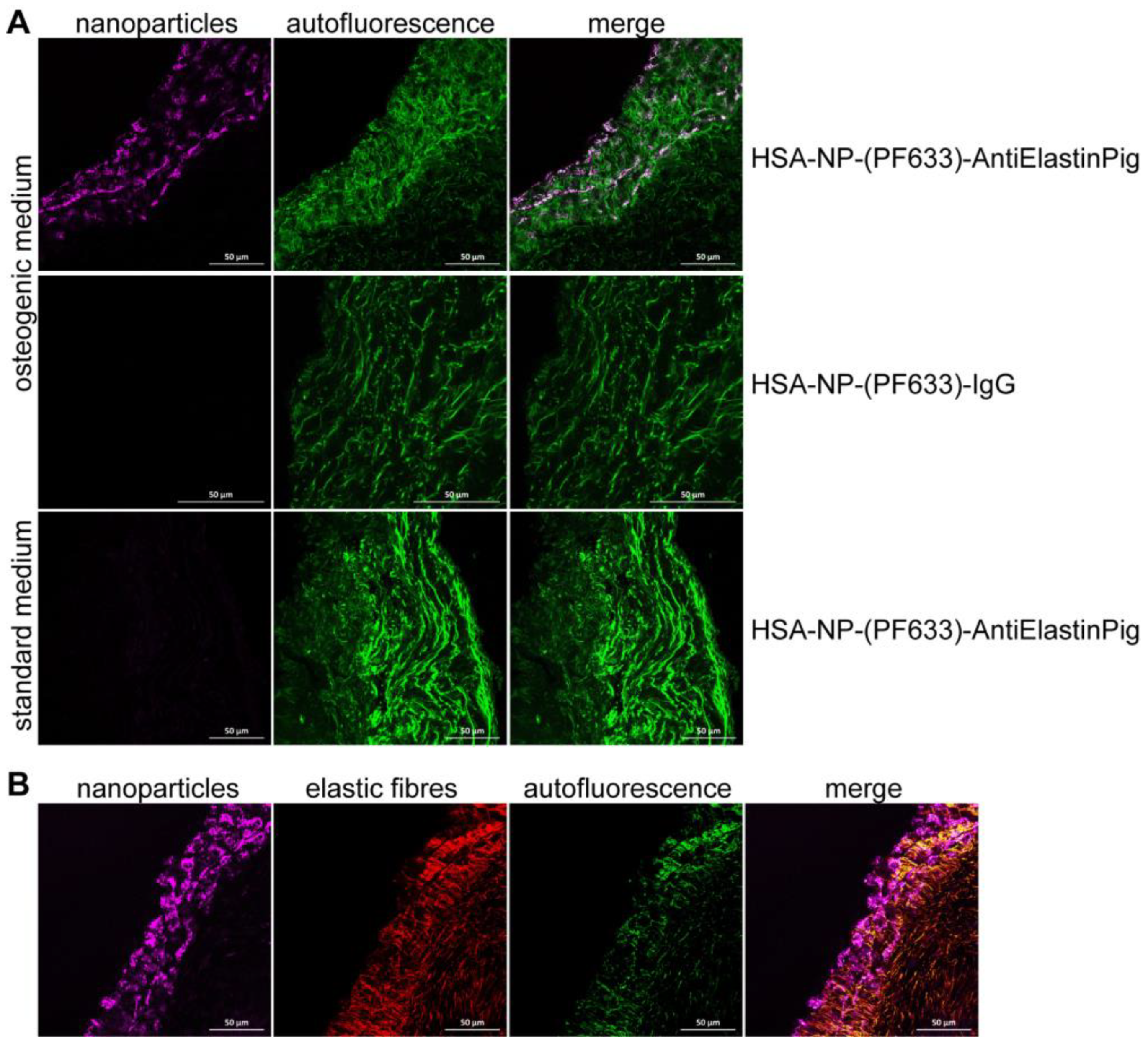
2.3. Ex Vivo Targeting of Elastin Antibody-Labeled NPs within Aortic Valve Leaflets
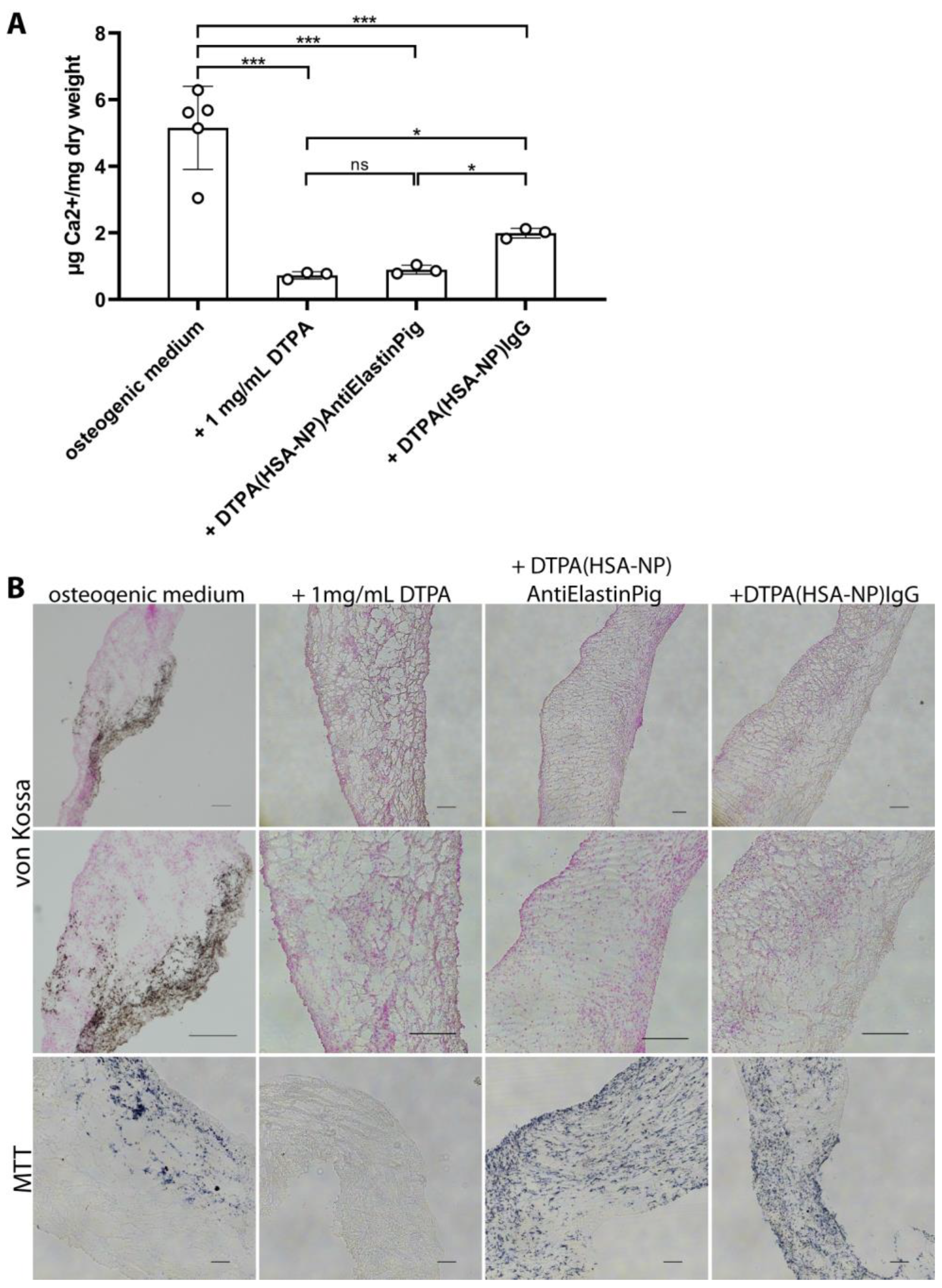
2.4. DTPA-Loaded NPs Reversed Induced Calcification in Aortic Valve Leaflets Ex Vivo
3. Discussion
4. Materials and Methods
4.1. Nanoparticle Preparation
4.2. Nanoparticle Characterization
4.3. Porcine Heart Preparation and Tissue Culture
4.4. Viability of the Porcine Aortic Valve Leaflet Cells
4.5. Calcium Quantification
4.6. Histology and Immunofluorescence Staining Methods
4.7. Raman Spectroscopy of Aortic Valves
4.8. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nitschke, Y.; Rutsch, F. Inherited Arterial Calcification Syndromes: Etiologies and Treatment Concepts. Curr. Osteoporos. Rep. 2017, 15, 255–270. [Google Scholar] [CrossRef]
- Rutsch, F.; Buers, I.; Nitschke, Y. Hereditary Disorders of Cardiovascular Calcification. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 35–47. [Google Scholar] [CrossRef]
- Joseph, J.; Naqvi, S.Y.; Giri, J.; Goldberg, S. Aortic Stenosis: Pathophysiology, Diagnosis, and Therapy. Am. J. Med. 2017, 130, 253–263. [Google Scholar] [CrossRef]
- Zheng, K.H.; Tzolos, E.; Dweck, M.R. Pathophysiology of Aortic Stenosis and Future Perspectives for Medical Therapy. Cardiol. Clin. 2020, 38, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hai, J.J.; Lachman, N.; Syed, F.F.; Desimone, C.V.; Asirvatham, S.J. The anatomic basis for ventricular arrhythmia in the normal heart: What the student of anatomy needs to know. Clin. Anat. 2014, 27, 885–893. [Google Scholar] [CrossRef]
- Hinton, R.B.; Yutzey, K.E. Heart valve structure and function in development and disease. Annu. Rev. Physiol. 2011, 73, 29–46. [Google Scholar] [CrossRef] [PubMed]
- Vesely, I. The role of elastin in aortic valve mechanics. J. Biomech. 1998, 31, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Cocciolone, A.J.; Hawes, J.Z.; Staiculescu, M.C.; Johnson, E.O.; Murshed, M.; Wagenseil, J.E. Elastin, arterial mechanics, and cardiovascular disease. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H189–H205. [Google Scholar] [CrossRef] [PubMed]
- Lerman, D.A.; Prasad, S.; Alotti, N. Calcific Aortic Valve Disease: Molecular Mechanisms and Therapeutic Approaches. Eur. Cardiol. 2015, 10, 108–112. [Google Scholar] [CrossRef]
- Peeters, F.; Meex, S.J.R.; Dweck, M.R.; Aikawa, E.; Crijns, H.; Schurgers, L.J.; Kietselaer, B. Calcific aortic valve stenosis: Hard disease in the heart: A biomolecular approach towards diagnosis and treatment. Eur. Heart J. 2018, 39, 2618–2624. [Google Scholar] [CrossRef]
- Tseng, H.; Grande-Allen, K.J. Elastic fibers in the aortic valve spongiosa: A fresh perspective on its structure and role in overall tissue function. Acta Biomater. 2011, 7, 2101–2108. [Google Scholar] [CrossRef] [PubMed]
- Daamen, W.F.; Quaglino, D. Signaling pathways in elastic tissues. Cell Signal 2019, 63, 109364. [Google Scholar] [CrossRef]
- Bashey, R.I.; Torii, S.; Angrist, A. Age-related collagen and elastin content of human heart valves. J. Gerontol. 1967, 22, 203–208. [Google Scholar] [CrossRef]
- Ronchetti, I.; Boraldi, F.; Annovi, G.; Cianciulli, P.; Quaglino, D. Fibroblast involvement in soft connective tissue calcification. Front. Genet. 2013, 4, 22. [Google Scholar] [CrossRef] [PubMed]
- Antonicelli, F.; Bellon, G.; Debelle, L.; Hornebeck, W. Elastin-elastases and inflamm-aging. Curr. Top. Dev. Biol. 2007, 79, 99–155. [Google Scholar] [CrossRef]
- de Oliveira Sa, M.P.B.; Cavalcanti, L.R.P.; Perazzo, A.M.; Gomes, R.A.F.; Clavel, M.A.; Pibarot, P.; Biondi-Zoccai, G.; Zhigalov, K.; Weymann, A.; Ruhparwar, A.; et al. Calcific Aortic Valve Stenosis and Atherosclerotic Calcification. Curr. Atheroscler. Rep. 2020, 22, 2. [Google Scholar] [CrossRef] [PubMed]
- Otto, C.M.; Kuusisto, J.; Reichenbach, D.D.; Gown, A.M.; O’Brien, K.D. Characterization of the early lesion of ‘degenerative’ valvular aortic stenosis. Histological and immunohistochemical studies. Circulation 1994, 90, 844–853. [Google Scholar] [CrossRef]
- Hinton, R.B., Jr.; Lincoln, J.; Deutsch, G.H.; Osinska, H.; Manning, P.B.; Benson, D.W.; Yutzey, K.E. Extracellular matrix remodeling and organization in developing and diseased aortic valves. Circ. Res. 2006, 98, 1431–1438. [Google Scholar] [CrossRef]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. J. Cardiothorac. Surg. 2021, 60, 727–800. [Google Scholar] [CrossRef]
- Kraler, S.; Blaser, M.C.; Aikawa, E.; Camici, G.G.; Luscher, T.F. Calcific aortic valve disease: From molecular and cellular mechanisms to medical therapy. Eur. Heart J. 2022, 43, 683–697. [Google Scholar] [CrossRef]
- Keuth, J.; Nitschke, Y.; Mulac, D.; Riehemann, K.; Rutsch, F.; Langer, K. Reversion of arterial calcification by elastin-targeted DTPA-HSA nanoparticles. Eur. J. Pharm. Biopharm. Off. J. Arbeitsgemeinschaft Fur Pharm. Verfahrenstechnik e.V 2020, 150, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Grover, A.; Sinha, A.; Vyavahare, N. Efficacy of reversal of aortic calcification by chelating agents. Calcif. Tissue Int. 2013, 93, 426–435. [Google Scholar] [CrossRef]
- Lei, Y.; Nosoudi, N.; Vyavahare, N. Targeted chelation therapy with EDTA-loaded albumin nanoparticles regresses arterial calcification without causing systemic side effects. J. Control. Release Off. J. Control. Release Soc. 2014, 196, 79–86. [Google Scholar] [CrossRef]
- Glicklich, D.; Shin, C.T.; Frishman, W.H. Heavy Metal Toxicity in Chronic Renal Failure and Cardiovascular Disease: Possible Role for Chelation Therapy. Cardiol. Rev. 2020, 28, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Lamas, G.A.; Goertz, C.; Boineau, R.; Mark, D.B.; Rozema, T.; Nahin, R.L.; Drisko, J.A.; Lee, K.L. Design of the Trial to Assess Chelation Therapy (TACT). Am. Heart J. 2012, 163, 7–12. [Google Scholar] [CrossRef]
- Lamas, G.A.; Anstrom, K.J.; Navas-Acien, A.; Boineau, R.; Kim, H.; Rosenberg, Y.; Stylianou, M.; Jones, T.L.Z.; Joubert, B.R.; Santella, R.M.; et al. The trial to assess chelation therapy 2 (TACT2): Rationale and design. Am. Heart J. 2022, 252, 1–11. [Google Scholar] [CrossRef]
- Moncla, L.M.; Briend, M.; Bosse, Y.; Mathieu, P. Calcific aortic valve disease: Mechanisms, prevention and treatment. Nat. Rev. Cardiol. 2023, 20, 546–559. [Google Scholar] [CrossRef] [PubMed]
- Gopal, S.; Hauser, J.M.; Mahboobi, S.K. Mechanical Aortic Valve Replacement. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Najahi-Missaoui, W.; Arnold, R.D.; Cummings, B.S. Safe Nanoparticles: Are We There Yet? Int. J. Mol. Sci. 2020, 22, 385. [Google Scholar] [CrossRef]
- Nosoudi, N.; Chowdhury, A.; Siclari, S.; Karamched, S.; Parasaram, V.; Parrish, J.; Gerard, P.; Vyavahare, N. Reversal of Vascular Calcification and Aneurysms in a Rat Model Using Dual Targeted Therapy with EDTA- and PGG-Loaded Nanoparticles. Theranostics 2016, 6, 1975–1987. [Google Scholar] [CrossRef]
- Crick, S.J.; Sheppard, M.N.; Ho, S.Y.; Gebstein, L.; Anderson, R.H. Anatomy of the pig heart: Comparisons with normal human cardiac structure. J. Anat. 1998, 193 Pt 1, 105–119. [Google Scholar] [CrossRef] [PubMed]
- Muller, R.H.; Jacobs, C.; Kayser, O. Nanosuspensions as particulate drug formulations in therapy. Rationale for development and what we can expect for the future. Adv. Drug Deliv. Rev. 2001, 47, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Freeman, R.V.; Otto, C.M. Spectrum of calcific aortic valve disease: Pathogenesis, disease progression, and treatment strategies. Circulation 2005, 111, 3316–3326. [Google Scholar] [CrossRef]
- Sacks, M.S.; Yoganathan, A.P. Heart valve function: A biomechanical perspective. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2007, 362, 1369–1391. [Google Scholar] [CrossRef]
- Balachandran, K.; Sucosky, P.; Jo, H.; Yoganathan, A.P. Elevated cyclic stretch alters matrix remodeling in aortic valve cusps: Implications for degenerative aortic valve disease. Am. J. Physiol. Heart Circ. Physiol. 2009, 296, H756–H764. [Google Scholar] [CrossRef] [PubMed]
- Sucosky, P.; Padala, M.; Elhammali, A.; Balachandran, K.; Jo, H.; Yoganathan, A.P. Design of an ex vivo culture system to investigate the effects of shear stress on cardiovascular tissue. J. Biomech. Eng. 2008, 130, 035001. [Google Scholar] [CrossRef] [PubMed]
- Glasmacher, B.; Deiwick, M.; Reul, H.; Knesch, H.; Keus, D.; Rau, G. A new in vitro test method for calcification of bioprosthetic heart valves. Int. J. Artif. Organs 1997, 20, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Balachandran, K.; Sucosky, P.; Jo, H.; Yoganathan, A.P. Elevated cyclic stretch induces aortic valve calcification in a bone morphogenic protein-dependent manner. Am. J. Pathol. 2010, 177, 49–57. [Google Scholar] [CrossRef]
- Weber, A.; Pfaff, M.; Schottler, F.; Schmidt, V.; Lichtenberg, A.; Akhyari, P. Reproducible In Vitro Tissue Culture Model to Study Basic Mechanisms of Calcific Aortic Valve Disease: Comparative Analysis to Valvular Interstitials Cells. Biomedicines 2021, 9, 474. [Google Scholar] [CrossRef] [PubMed]
- Chester, A.H.; Sarathchandra, P.; McCormack, A.; Yacoub, M.H. Organ Culture Model of Aortic Valve Calcification. Front. Cardiovasc. Med. 2021, 8, 734692. [Google Scholar] [CrossRef]
- Rathan, S.; Yoganathan, A.P.; O’Neill, C.W. The role of inorganic pyrophosphate in aortic valve calcification. J. Heart Valve Dis. 2014, 23, 387–394. [Google Scholar]
- Nissen, S.E. Concerns about reliability in the Trial to Assess Chelation Therapy (TACT). JAMA 2013, 309, 1293–1294. [Google Scholar] [CrossRef]
- Mathew, R.O.; Schulman-Marcus, J.; Nichols, E.L.; Newman, J.D.; Bangalore, S.; Farkouh, M.; Sidhu, M.S. Chelation Therapy as a Cardiovascular Therapeutic Strategy: The Rationale and the Data in Review. Cardiovasc. Drugs Ther. 2017, 31, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Sultan, S.; Murarka, S.; Jahangir, A.; Mookadam, F.; Tajik, A.J.; Jahangir, A. Chelation therapy in cardiovascular disease: An update. Expert. Rev. Clin. Pharmacol. 2017, 10, 843–854. [Google Scholar] [CrossRef] [PubMed]
- Karamched, S.R.; Nosoudi, N.; Moreland, H.E.; Chowdhury, A.; Vyavahare, N.R. Site-specific chelation therapy with EDTA-loaded albumin nanoparticles reverses arterial calcification in a rat model of chronic kidney disease. Sci. Rep. 2019, 9, 2629. [Google Scholar] [CrossRef] [PubMed]
- Lane, B.A.; Wang, X.; Lessner, S.M.; Vyavahare, N.R.; Eberth, J.F. Targeted Gold Nanoparticles as an Indicator of Mechanical Damage in an Elastase Model of Aortic Aneurysm. Ann. Biomed. Eng. 2020, 48, 2268–2278. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lane, B.A.; Eberth, J.F.; Lessner, S.M.; Vyavahare, N.R. Gold nanoparticles that target degraded elastin improve imaging and rupture prediction in an AngII mediated mouse model of abdominal aortic aneurysm. Theranostics 2019, 9, 4156–4167. [Google Scholar] [CrossRef] [PubMed]
- Rosenbloom, J.; Abrams, W.R.; Mecham, R. Extracellular matrix 4: The elastic fiber. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 1993, 7, 1208–1218. [Google Scholar] [CrossRef]
- Sakai, L.Y.; Keene, D.R.; Engvall, E. Fibrillin, a new 350-kD glycoprotein, is a component of extracellular microfibrils. J. Cell Biol. 1986, 103, 2499–2509. [Google Scholar] [CrossRef]
- Halsey, G.; Sinha, D.; Dhital, S.; Wang, X.; Vyavahare, N. Role of elastic fiber degradation in disease pathogenesis. Biochim. Biophys. Acta Mol. Basis Dis. 2023, 1869, 166706. [Google Scholar] [CrossRef]
- Nosoudi, N.; Chowdhury, A.; Siclari, S.; Parasaram, V.; Karamched, S.; Vyavahare, N. Systemic Delivery of Nanoparticles Loaded with Pentagalloyl Glucose Protects Elastic Lamina and Prevents Abdominal Aortic Aneurysm in Rats. J. Cardiovasc. Transl. Res. 2016, 9, 445–455. [Google Scholar] [CrossRef]
- Geng, B.; Chen, X.; Chi, J.; Li, F.; Yim, W.Y.; Wang, K.; Li, C.; Xie, M.; Zhu, P.; Fan, Z.; et al. Platelet membrane-coated alterbrassicene A nanoparticle inhibits calcification of the aortic valve by suppressing phosphorylation P65 NF-kappaB. Theranostics 2023, 13, 3781–3793. [Google Scholar] [CrossRef]
- Adelnia, H.; Blakey, I.; Little, P.J.; Ta, H.T. Poly(succinimide) nanoparticles as reservoirs for spontaneous and sustained synthesis of poly(aspartic acid) under physiological conditions: Potential for vascular calcification therapy and oral drug delivery. J. Mater. Chem. B 2023, 11, 2650–2662. [Google Scholar] [CrossRef]
- Weber, C.; Kreuter, J.; Langer, K. Desolvation process and surface characteristics of HSA-nanoparticles. Int. J. Pharm. 2000, 196, 197–200. [Google Scholar] [CrossRef]
- Mesken, J.; Iltzsche, A.; Mulac, D.; Langer, K. Modifying plasmid-loaded HSA-nanoparticles with cell penetrating peptides—Cellular uptake and enhanced gene delivery. Int. J. Pharm. 2017, 522, 198–209. [Google Scholar] [CrossRef]
- Zabirnyk, A.; Perez, M.D.M.; Blasco, M.; Stenslokken, K.O.; Ferrer, M.D.; Salcedo, C.; Vaage, J. A Novel Ex Vivo Model of Aortic Valve Calcification. A Preliminary Report. Front. Pharmacol. 2020, 11, 568764. [Google Scholar] [CrossRef] [PubMed]
- Moorehead, W.R.; Biggs, H.G. 2-Amino-2-methyl-1-propanol as the alkalizing agent in an improved continuous-flow cresolphthalein complexone procedure for calcium in serum. Clin. Chem. 1974, 20, 1458–1460. [Google Scholar] [CrossRef]
- Nitschke, Y.; Weissen-Plenz, G.; Terkeltaub, R.; Rutsch, F. Npp1 promotes atherosclerosis in ApoE knockout mice. J. Cell. Mol. Med. 2011, 15, 2273–2283. [Google Scholar] [CrossRef] [PubMed]
- Buchholz, A.; Stücker, S.; Koßlowski, F.; Lohmann, C.H.; Bertrand, J. High-Resolution Imaging Methods for Identification of Calcium Crystal Types in Osteoarthritis. Gout Urate Cryst. Depos. Dis. 2023, 1, 62–82. [Google Scholar] [CrossRef]

| Nanoparticle System | Hydrodynamic Diameter [nm] | Polydispersity Index | Zeta Potential [mV] | Drug Load [mol DTPA/mol HSA] |
|---|---|---|---|---|
| DTPA-(HSA-NP)-AntiElastinPig DTPA-(HSA-NP)-IgG | 159.7± 16.5 | 0.06 ± 0.01 | −33.7 ± 1.9 | 9.0 ± 3.0 |
| (HSA-PF633P-NP)-AntiElastinPig (HSA-PF633P-NP)-IgG | 169.4 ± 17.9 | 0.04 ± 0.03 | −41.3 ± 6.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feldmann, A.; Nitschke, Y.; Linß, F.; Mulac, D.; Stücker, S.; Bertrand, J.; Buers, I.; Langer, K.; Rutsch, F. Improved Reversion of Calcifications in Porcine Aortic Heart Valves Using Elastin-Targeted Nanoparticles. Int. J. Mol. Sci. 2023, 24, 16471. https://doi.org/10.3390/ijms242216471
Feldmann A, Nitschke Y, Linß F, Mulac D, Stücker S, Bertrand J, Buers I, Langer K, Rutsch F. Improved Reversion of Calcifications in Porcine Aortic Heart Valves Using Elastin-Targeted Nanoparticles. International Journal of Molecular Sciences. 2023; 24(22):16471. https://doi.org/10.3390/ijms242216471
Chicago/Turabian StyleFeldmann, Anja, Yvonne Nitschke, Franziska Linß, Dennis Mulac, Sina Stücker, Jessica Bertrand, Insa Buers, Klaus Langer, and Frank Rutsch. 2023. "Improved Reversion of Calcifications in Porcine Aortic Heart Valves Using Elastin-Targeted Nanoparticles" International Journal of Molecular Sciences 24, no. 22: 16471. https://doi.org/10.3390/ijms242216471
APA StyleFeldmann, A., Nitschke, Y., Linß, F., Mulac, D., Stücker, S., Bertrand, J., Buers, I., Langer, K., & Rutsch, F. (2023). Improved Reversion of Calcifications in Porcine Aortic Heart Valves Using Elastin-Targeted Nanoparticles. International Journal of Molecular Sciences, 24(22), 16471. https://doi.org/10.3390/ijms242216471






