TFIIB-Related Protein BRP5/PTF2 Is Required for Both Male and Female Gametogenesis and for Grain Formation in Rice
Abstract
:1. Introduction
2. Results
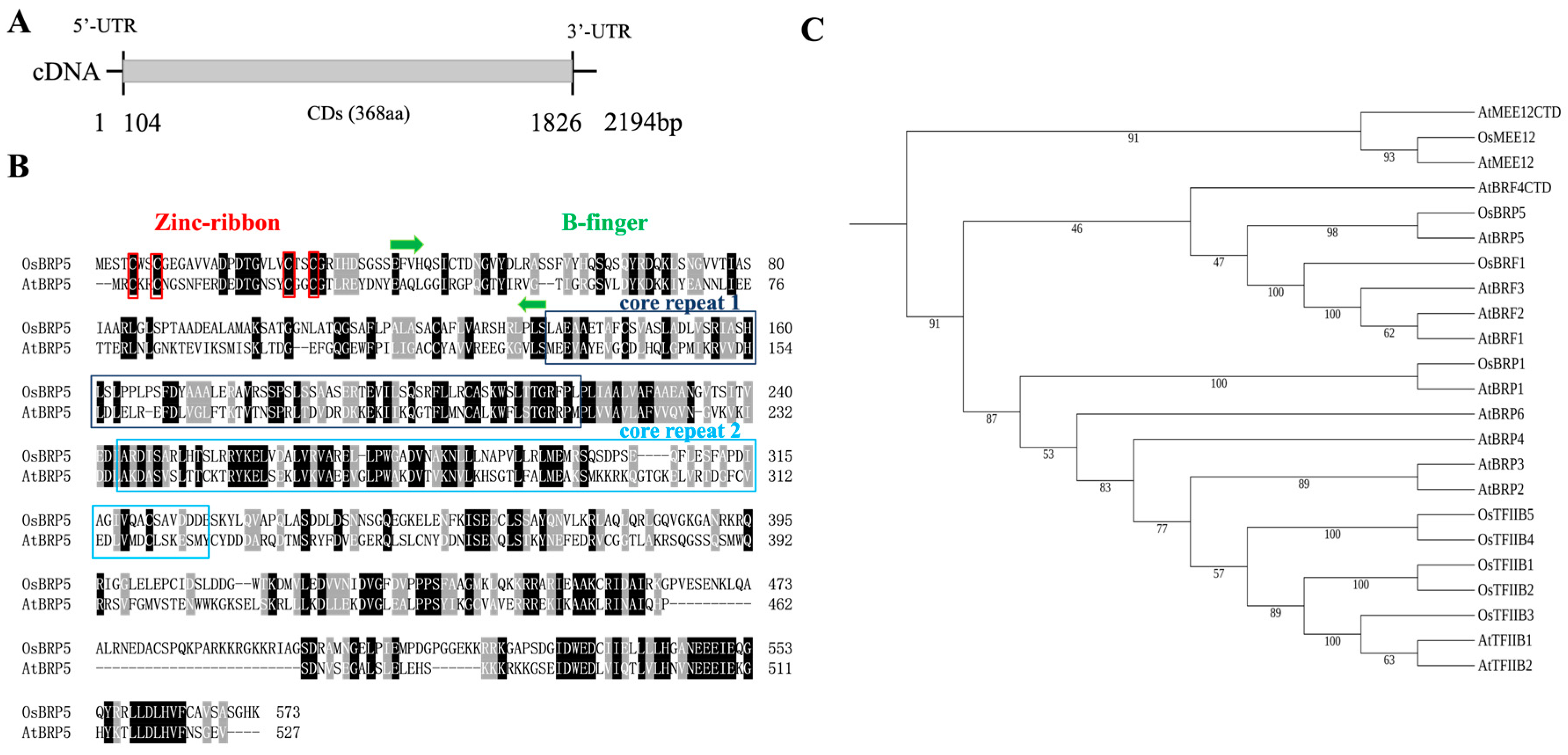
2.1. OsBRP5 Protein Sequence
2.2. Expression and Subcellular Localization of OsBRP5
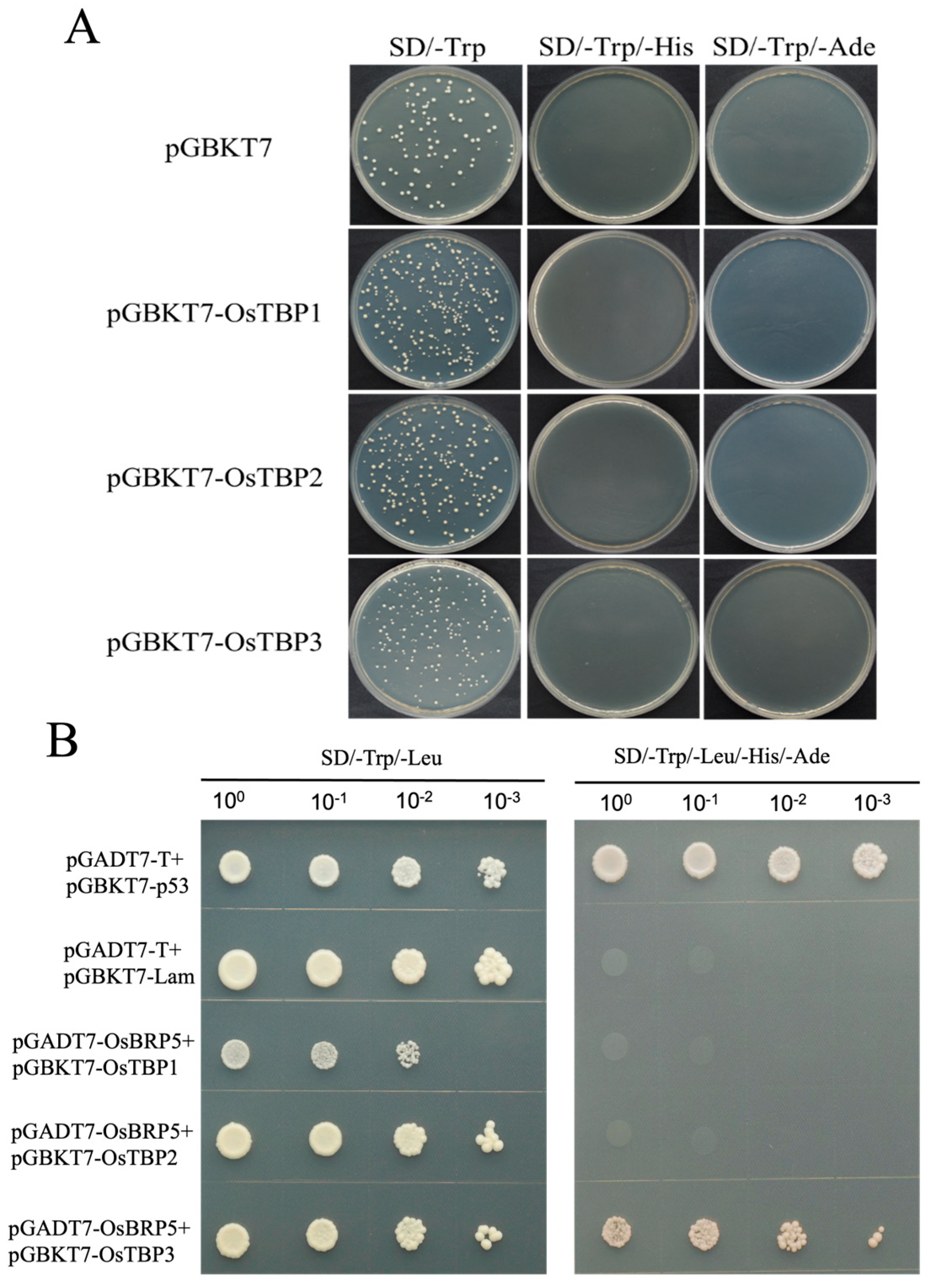
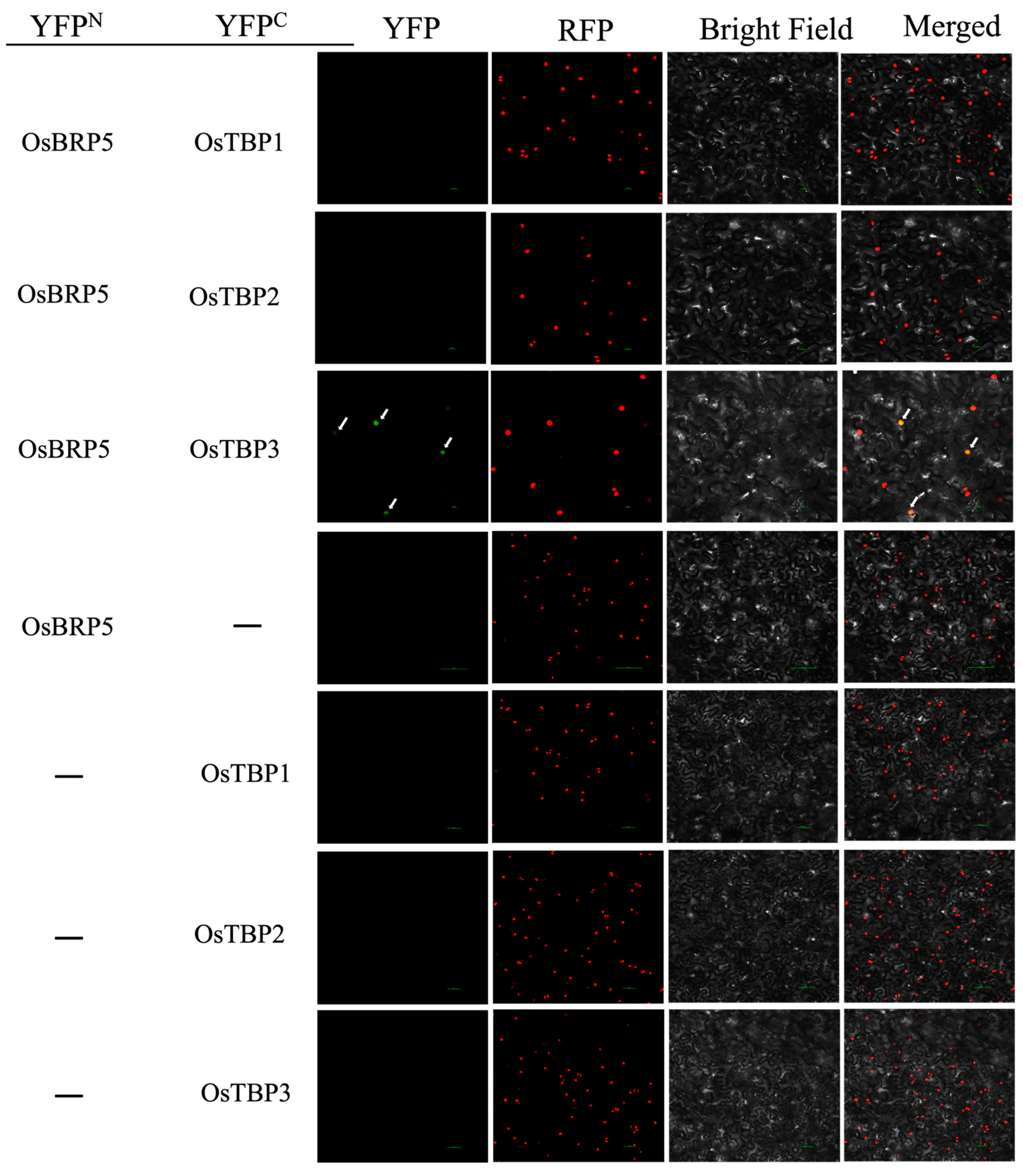
2.3. OsBRP5 Interacts with OsTBPs In Vitro and In Vivo
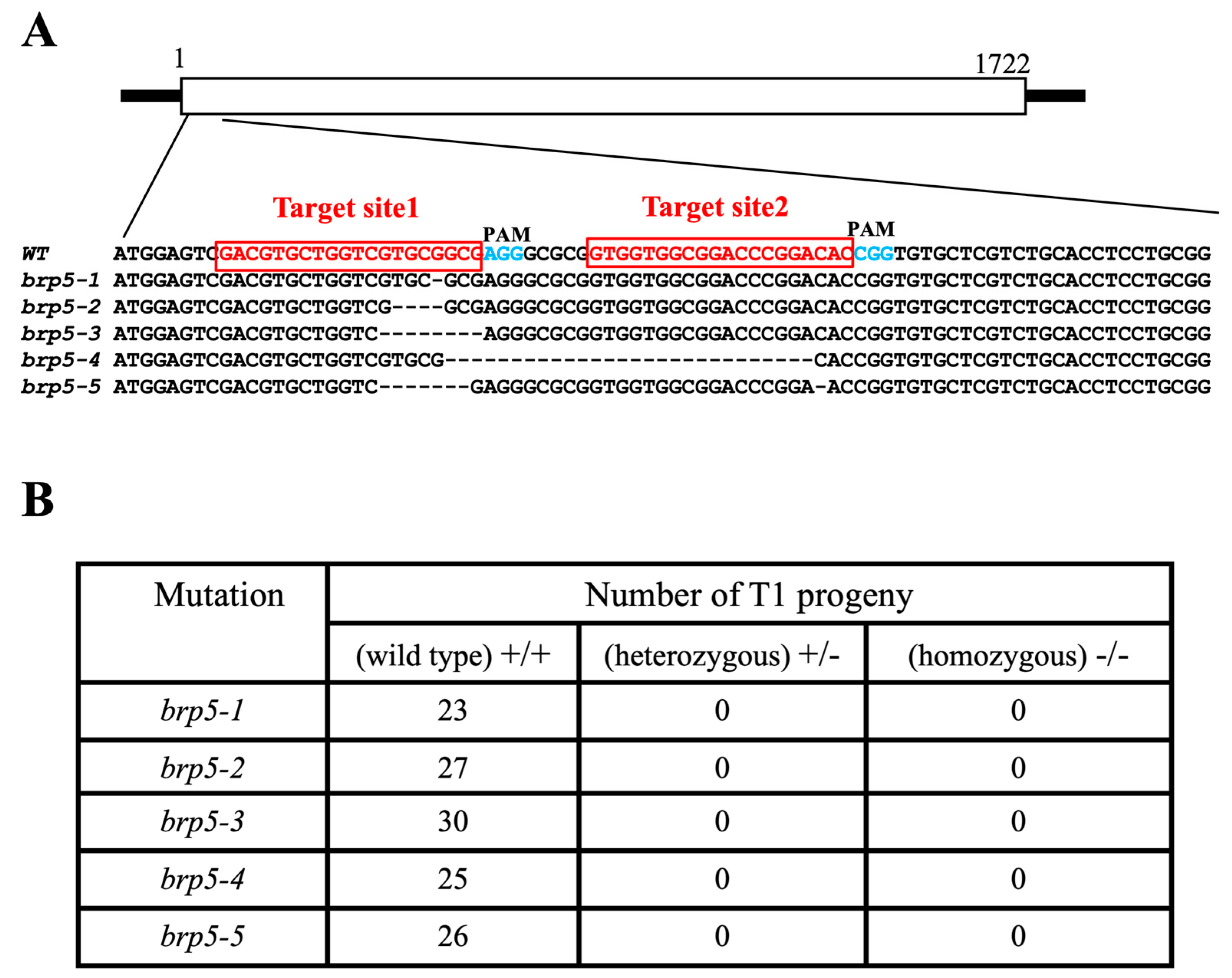
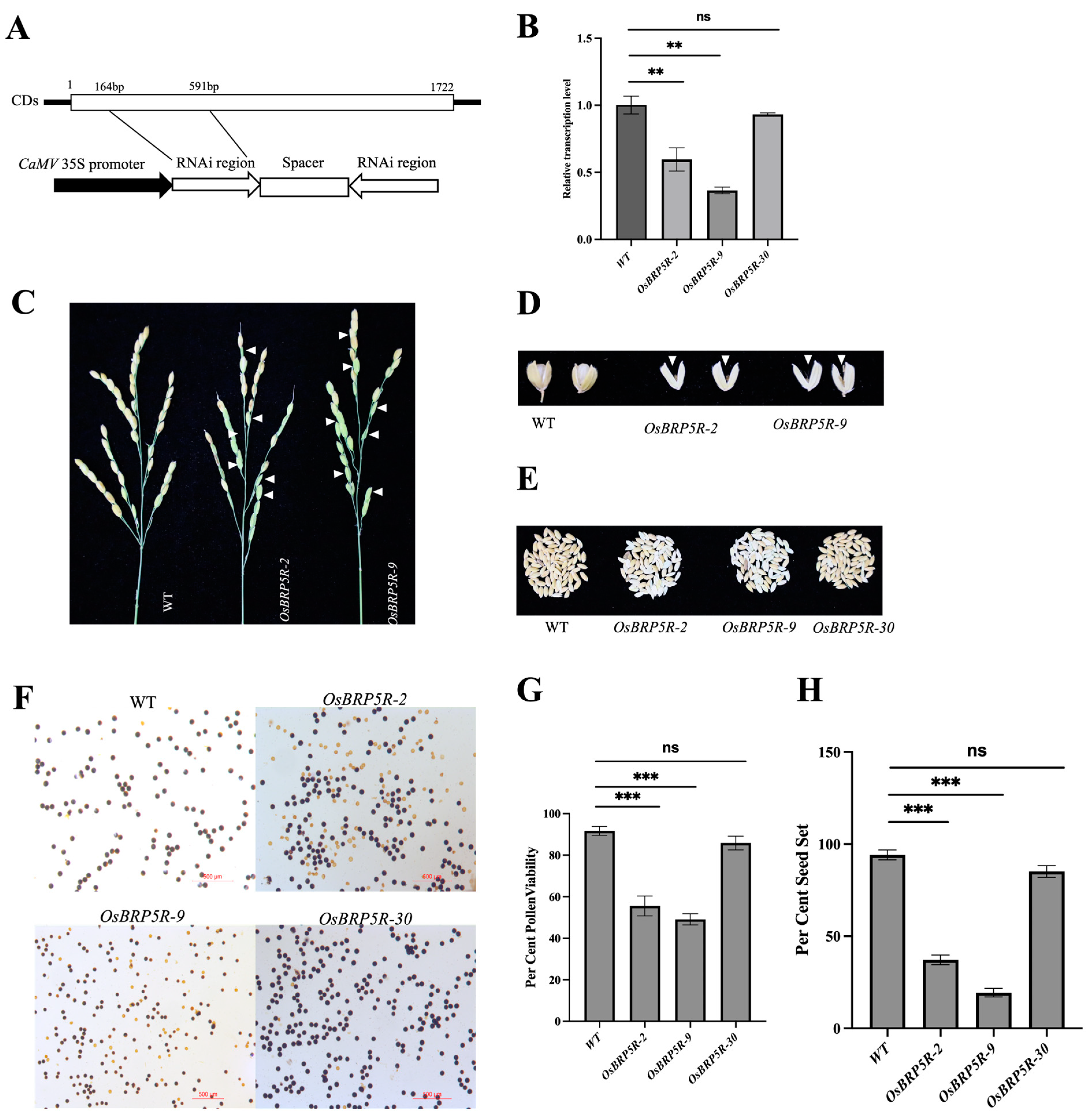
2.4. Generation and Characterization of Rice OsBRP5 Mutants
2.5. Generation and Characterization of Rice OsBRP5 Silencing and Overexpressing Plants
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Protein Sequence Analysis
4.3. GUS Staining
4.4. Subcellular Localization
4.5. Yeast Two-Hybrid (Y2H) Screen and Bimolecular Fluorescence Complementation (BiFC) Assay
4.6. CRISPR/Cas9 Gene Editing and Phenotype Observation
4.7. RNAi and Overexpression of OsBRP5 and Phenotype Observation
4.8. RNA Isolation, cDNA Synthesis, and qRT-PCR Analysis
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vannini, A.; Cramer, P. Conservation between the RNA polymerase I, II, and III transcription initiation machineries. Mol. Cell 2012, 45, 439–446. [Google Scholar] [CrossRef]
- Cox, M.M.; Doudna, J.; O’Donnell, M. Molecular Biology: Principle and Practice; Freenman and Company: New York, NY, USA, 2012. [Google Scholar]
- Chen, J.; Yang, S.; Fan, B.; Zhu, C.; Chen, Z. The Mediator Complex: A Central Coordinator of Plant Adaptive Responses to Environmental Stresses. Int. J. Mol. Sci. 2022, 23, 6170. [Google Scholar] [CrossRef]
- Knutson, B.A. Emergence and expansion of TFIIB-like factors in the plant kingdom. Gene 2013, 526, 30–38. [Google Scholar] [CrossRef]
- Burton, S.P.; Burton, Z.F. The σ enigma: Bacterial σ factors, archaeal TFB, and eukaryotic TFIIB are homologs. Transcription 2014, 5, e967599. [Google Scholar] [CrossRef]
- Ning, H.; Yang, S.; Fan, B.; Zhu, C.; Chen, Z. Expansion and Functional Diversification of TFIIB-Like Factors in Plants. Int. J. Mol. Sci. 2021, 22, 1078. [Google Scholar] [CrossRef]
- Zhou, J.J.; Liang, Y.; Niu, Q.K.; Chen, L.Q.; Zhang, X.Q.; Ye, D. The Arabidopsis general transcription factor TFIIB1 (AtTFIIB1) is required for pollen tube growth and endosperm development. J. Exp. Bot. 2013, 64, 2205–2218. [Google Scholar] [CrossRef]
- Qin, Z.; Zhang, X.; Zhang, X.; Xin, W.; Li, J.; Hu, Y. The Arabidopsis transcription factor IIB-related protein BRP4 is involved in the regulation of mitotic cell-cycle progression during male gametogenesis. J. Exp. Bot. 2014, 65, 2521–2531. [Google Scholar] [CrossRef]
- Chen, Y.H.; Li, H.J.; Shi, D.Q.; Yuan, L.; Liu, J.; Sreenivasan, R.; Baskar, R.; Grossniklaus, U.; Yang, W.C. The central cell plays a critical role in pollen tube guidance in Arabidopsis. Plant Cell. 2007, 19, 3563–3577. [Google Scholar] [CrossRef]
- Li, H.J.; Zhu, S.S.; Zhang, M.X.; Wang, T.; Liang, L.; Xue, Y.; Shi, D.Q.; Liu, J.; Yang, W.C. Arabidopsis CBP1 Is a Novel Regulator of Transcription Initiation in Central Cell-Mediated Pollen Tube Guidance. Plant Cell. 2015, 27, 2880–2893. [Google Scholar] [CrossRef]
- Zhang, K.; Yang, W.; Yu, H.; Fu, C.; Liu, X.; Liu, J. Double mutation of BRF1 and BRF2 leads to sterility in Arabidopsis thaliana. Biochem. Biophys. Res. Commun. 2019, 516, 969–975. [Google Scholar] [CrossRef]
- Niu, Q.K.; Liang, Y.; Zhou, J.J.; Dou, X.Y.; Gao, S.C.; Chen, L.Q.; Zhang, X.Q.; Ye, D. Pollen-expressed transcription factor 2 encodes a novel plant-specific TFIIB-related protein that is required for pollen germination and embryogenesis in Arabidopsis. Mol. Plant 2013, 6, 1091–1108. [Google Scholar] [CrossRef]
- Kostrewa, D.; Zeller, M.E.; Armache, K.J.; Seizl, M.; Leike, K.; Thomm, M.; Cramer, P. RNA polymerase II-TFIIB structure and mechanism of transcription initiation. Nature 2009, 462, 323–330. [Google Scholar] [CrossRef]
- Ha, I.; Lane, W.S.; Reinberg, D. Cloning of a human gene encoding the general transcription initiation factor IIB. Nature 1991, 352, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Hawley, D. Transcriptional activation: Enter TFIIB. Trends Biochem Sci. Sep. 1991, 16, 317–318. [Google Scholar] [CrossRef]
- Tsai, F.T.; Sigler, P.B. Structural basis of preinitiation complex assembly on human pol II promoters. EMBO J. 2000, 19, 25–36. [Google Scholar] [CrossRef]
- Dawe, R.K. Meiotic chromosome organization and segregation in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1998, 49, 371–395. [Google Scholar] [CrossRef]
- Kleckner, N. Chiasma formation: Chromatin/axis interplay and the role(s) of the synaptonemal complex. Chromosoma 2006, 115, 175–194. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.B.; Bhalla, P.L. Control of male germ cell development in flowering plants. BioEssays 2007, 29, 1124–1132. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.B.; Bhalla, P.L.; Russell, S.D. Molecular repertoire of flowering plant male germ cells. Sex. Plant Reprod. 2008, 21, 27–36. [Google Scholar] [CrossRef]
- Nonomura, K.; Miyoshi, K.; Eiguchi, M.; Suzuki, T.; Miyao, A.; Hirochika, H.; Kurata, N. The MSP1 gene is necessary to restrict the number of cells entering into male and female sporogenesis and to initiate anther wall formation in rice. Plant Cell 2003, 15, 1728–1739. [Google Scholar] [CrossRef]
- Zhao, X.; De Palma, J.; Oane, R.; Gamuyao, R.; Luo, M.; Chaudhury, A.; Hervé, P.; Xue, Q.; Bennett, J. OsTDL1A binds to the LRR domain of rice receptor kinase MSP1, and is required to limit sporocyte numbers. Plant J. 2008, 54, 375–387. [Google Scholar] [CrossRef]
- Aya, K.; Ueguchi-Tanaka, M.; Kndo, M.; Hamada, K.; Yano, K.; Nishimura, M.; Matsuoka, M. Gibberellin modulates anther development in rice via the transcriptional regulation of GAMYB. Plant Cell 2011, 21, 1453–1472. [Google Scholar] [CrossRef]
- Li, N.; Zhang, D.S.; Liu, H.S.; Yin, C.S.; Li, X.X.; Liang, W.Q.; Yuan, Z.; Xu, B.; Chu, H.W.; Wang, J.; et al. The rice tapetum degeneration retardation gene is required for tapetum degradation and anther development. Plant Cell 2006, 18, 2999–3014. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Forno, D.A.; Cook, J.H.; Gomez, K.A. Routine procedures for growing rice plants in culture solution. In Laboratory Manual for Physiological Studies of Rice; Yoshida, S., Forno, D.A., Cook, J.H., Gomez, K.A., Eds.; International Rice Research Institute: Los Banos, Philippines, 1976; pp. 61–66. [Google Scholar]
- Xu, L.; Zhao, H.; Ruan, W.; Deng, M.; Wang, F.; Peng, J.; Luo, J.; Chen, Z. ABNORMAL INFLORESCENCE MERISTEM1 Functions in Salicylic Acid Biosynthesis to Maintain Proper Reactive Oxygen Species Levels for Root Meristem Activity in Rice. Plant Cell 2017, 29, 560–574. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Chen, J.; Ding, Y.; Huang, Q.; Chen, G.; Ulhassan, Z.; Zhang, X.Q.; Ye, D. Genome-wide investigation, and expression profiling of LOR gene family in rapeseed under salinity and ABA stress. Front. Plant Sci. 2023, 14, 1197781. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Bio. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef]
- Lee, D.S.; Chen, L.J.; Li, C.Y.; Liu, Y.; Tan, X.L.; Lu, B.R.; Li, J.; Gan, S.X.; Kang, S.G.; Suh, H.S.; et al. The Bsister MADS gene FST determines ovule patterning and development of the zygotic embryo and endosperm. PLoS ONE 2013, 8, e58748. [Google Scholar] [CrossRef]
- Chakrabarty, R.; Banerjee, R.; Chung, S.M.; Farman, M.; Citovsky, V.; Hogenhout, S.A.; Tzfira, T.; Goodin, M. pSITE vectors for stable integration or transient expression of autofluorescent protein fusions in plants: Probing Nicotiana benthamiana-virus interactions. Mol. Plant Microbe Interact. 2007, 20, 740–750. [Google Scholar] [CrossRef] [PubMed]
- Gietz, D.; Jean, A.S.; Woods, R.A.; Schiestl, R.H. Improved method for high efficiency transformation of intact yeast cells. Nucleic Acids Res. 1992, 20, 1425. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, X.; Li, K.; Wang, D.; Ding, Y.; Liu, X.; Luo, J.; Fang, C. A simple and efficient cloning system for CRISPR/Cas9-mediated genome editing in rice. PeerJ 2020, 8, e8491. [Google Scholar] [CrossRef]
- Waadt, R.; Schmidt, L.K.; Lohse, M.; Hashimoto, K.; Bock, R.; Kudla, J. Multicolor bimolecular fluorescence complementation reveals simultaneous formation of alternative CBL/CIPK complexes in planta. Plant J. 2008, 56, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Prasad, P.; Boote, K.; Allen, L.; Sheehy, J.; Thomas, J. Species, ecotype and cultivar differences in spikelet fertility and harvest index of rice in response to high temperature stress. Field Crop. Res. 2006, 95, 398–411. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, G.; Hu, H.; Chen, X.; Chen, J.; Wang, S.; Ning, H.; Zhu, C.; Yang, S. TFIIB-Related Protein BRP5/PTF2 Is Required for Both Male and Female Gametogenesis and for Grain Formation in Rice. Int. J. Mol. Sci. 2023, 24, 16473. https://doi.org/10.3390/ijms242216473
Chen G, Hu H, Chen X, Chen J, Wang S, Ning H, Zhu C, Yang S. TFIIB-Related Protein BRP5/PTF2 Is Required for Both Male and Female Gametogenesis and for Grain Formation in Rice. International Journal of Molecular Sciences. 2023; 24(22):16473. https://doi.org/10.3390/ijms242216473
Chicago/Turabian StyleChen, Guangna, Hongliang Hu, Xinhui Chen, Jialuo Chen, Siyi Wang, He Ning, Cheng Zhu, and Su Yang. 2023. "TFIIB-Related Protein BRP5/PTF2 Is Required for Both Male and Female Gametogenesis and for Grain Formation in Rice" International Journal of Molecular Sciences 24, no. 22: 16473. https://doi.org/10.3390/ijms242216473





