Antioxidant and Hypoglycemic Potential of Essential Oils in Diabetes Mellitus and Its Complications
Abstract
:1. Introduction
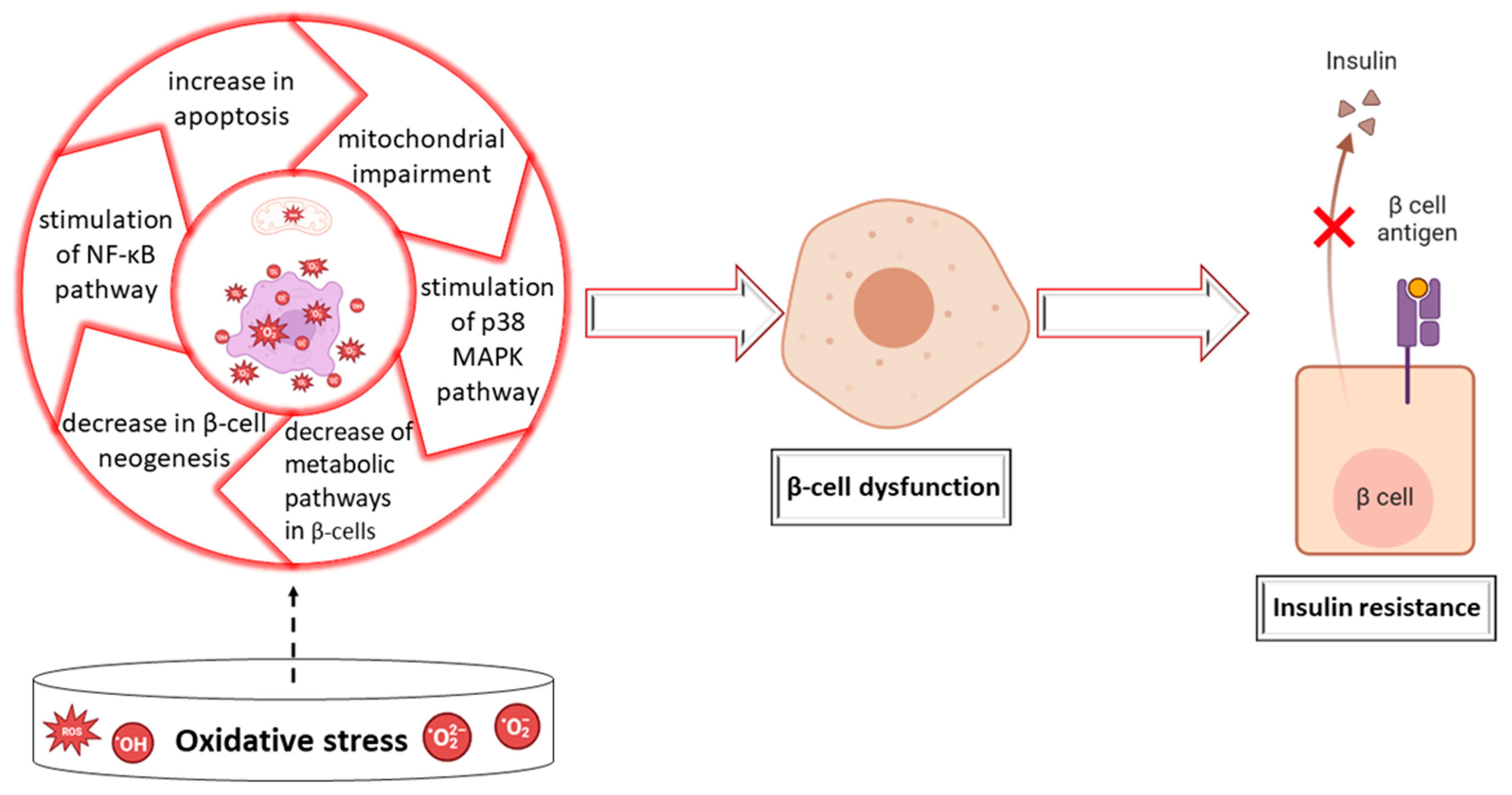
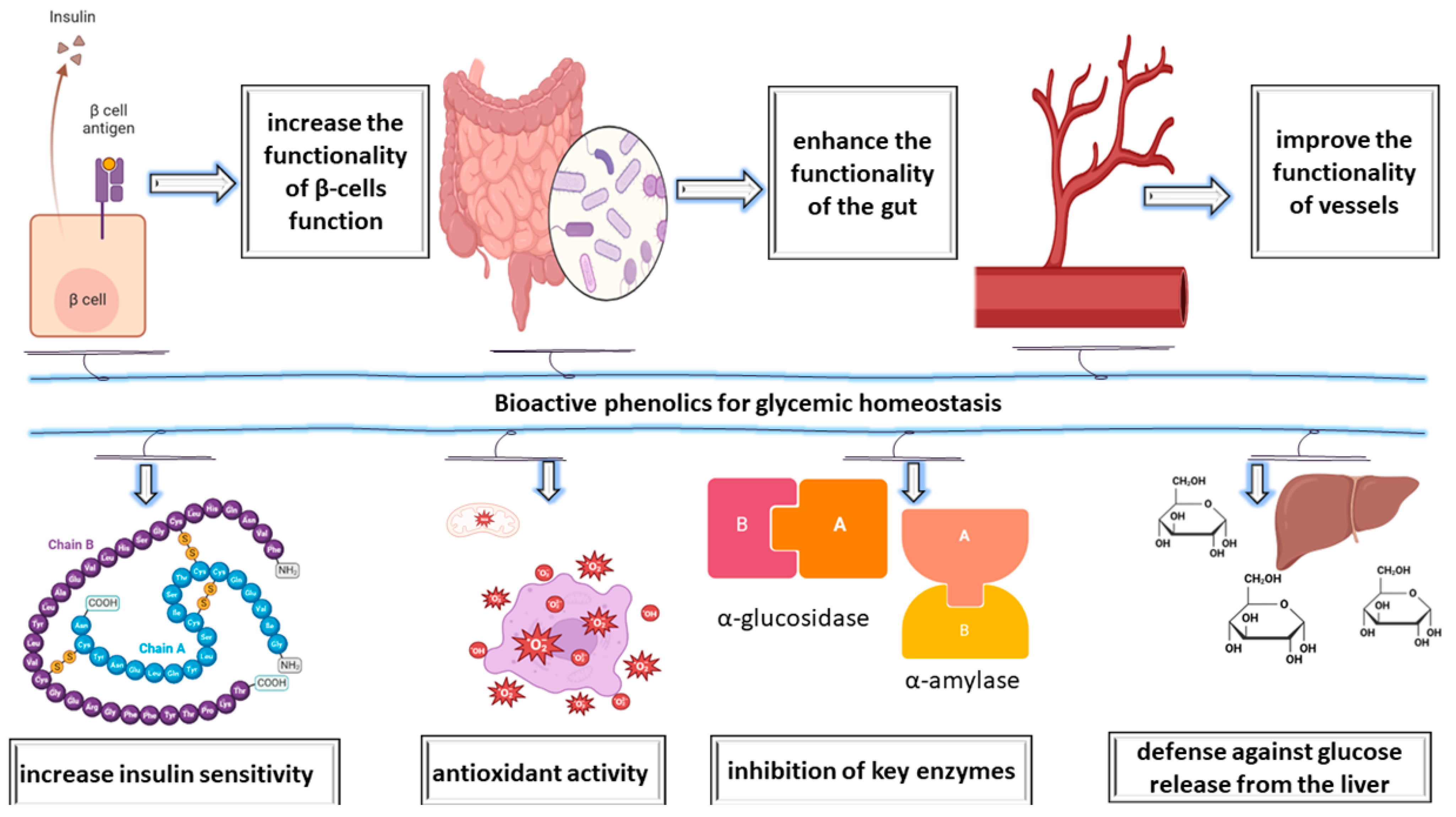
2. Impact of Essential Oils on Oxidative Stress and Enzymes in Diabetes Mellitus
Antioxidant Effects of Plants in Diabetes Mellitus
3. Essential Oil Types Used in Diabetes Mellitus
3.1. Clove Essential Oil
3.2. Peppermint Essential Oil
3.3. Lavender Essential Oil
3.4. Origanum Essential Oil
3.5. Oliveria decumbens, Thymus kotschyanus, Trachyspermum ammi, and Zataria multiflora Essential Oils
3.6. Nigella Sativa Essential Oil
3.7. Salvia Essential Oil
3.8. Citrus Aurantifolia Essential Oil
3.9. Black Pepper Essential Oil
3.10. Rosemary Essential Oil
3.11. Periscaria hydropiper Essential Oil
3.12. Momordica charantia Essential Oil
3.13. Blepharispermum hirtum Essential Oil
3.14. Eucalyptus Essential Oil
3.15. Melissa officinalis Essential Oil
3.16. Rhaponticum acaule Essential Oil
4. Clinical Research on the Applicability of Essential Oils in Glycemic Control and Toxicological Considerations
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Raut, J.S.; Karuppayil, S.M. A Status Review on the Medicinal Properties of Essential Oils. Ind. Crops Prod. 2014, 62, 250–264. [Google Scholar] [CrossRef]
- Cimino, C.; Maurel, O.M.; Musumeci, T.; Bonaccorso, A.; Drago, F.; Souto, E.M.B.; Pignatello, R.; Carbone, C. Essential Oils: Pharmaceutical Applications and Encapsulation Strategies into Lipid-Based Delivery Systems. Pharmaceutics 2021, 13, 327. [Google Scholar] [CrossRef] [PubMed]
- Lakhan, S.E.; Sheafer, H.; Tepper, D. The Effectiveness of Aromatherapy in Reducing Pain: A Systematic Review and Meta-Analysis. Pain Res. Treat. 2016, 2016, 8158693. [Google Scholar] [CrossRef] [PubMed]
- Fung, T.K.H.; Lau, B.W.M.; Ngai, S.P.C.; Tsang, H.W.H. Therapeutic Effect and Mechanisms of Essential Oils in Mood Disorders: Interaction between the Nervous and Respiratory Systems. Int. J. Mol. Sci. 2021, 22, 4844. [Google Scholar] [CrossRef]
- Panda, S.; Sahoo, S.; Tripathy, K.; Singh, Y.D.; Sarma, M.K.; Babu, P.J.; Singh, M.C. Essential Oils and Their Pharmacotherapeutics Applications in Human Diseases. Adv. Tradit. Med. 2022, 22, 1–15. [Google Scholar] [CrossRef]
- Bungau, A.F.; Radu, A.-F.; Bungau, S.G.; Vesa, C.M.; Tit, D.M.; Purza, A.L.; Endres, L.M. Emerging Insights into the Applicability of Essential Oils in the Management of Acne Vulgaris. Molecules 2023, 28, 6395. [Google Scholar] [CrossRef] [PubMed]
- Valussi, M.; Antonelli, M.; Donelli, D.; Firenzuoli, F. Appropriate Use of Essential Oils and Their Components in the Management of Upper Respiratory Tract Symptoms in Patients with COVID-19. J. Herb. Med. 2021, 28, 100451. [Google Scholar] [CrossRef]
- Alves-Silva, J.M.; Zuzarte, M.; Girão, H.; Salgueiro, L. The Role of Essential Oils and Their Main Compounds in the Management of Cardiovascular Disease Risk Factors. Molecules 2021, 26, 3506. [Google Scholar] [CrossRef] [PubMed]
- Siahbalaei, R.; Kavoosi, G.; Shakeri, R. In Vitro Antioxidant and Antidiabetic Activity of Essential Oils Encapsulated in Gelatin-Pectin Particles against Sugar, Lipid and Protein Oxidation and Amylase and Glucosidase Activity. Food Sci. Nutr. 2020, 8, 6457–6466. [Google Scholar] [CrossRef]
- De Blasio, A.; D’Anneo, A.; Lauricella, M.; Emanuele, S.; Giuliano, M.; Pratelli, G.; Calvaruso, G.; Carlisi, D. The Beneficial Effects of Essential Oils in Anti-Obesity Treatment. Int. J. Mol. Sci. 2021, 22, 11832. [Google Scholar] [CrossRef]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, Regional and Country-Level Diabetes Prevalence Estimates for 2021 and Projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef]
- Chávez-Delgado, E.L.; Jacobo-Velázquez, D.A. Essential Oils: Recent Advances on Their Dual Role as Food Preservatives and Nutraceuticals against the Metabolic Syndrome. Foods 2023, 12, 1079. [Google Scholar] [CrossRef] [PubMed]
- Mardani, S.; Nasri, H.; Rafieian-Kopaei, M.; Hajian, S. Herbal Medicine and Diabetic Kidney Disease. J. Nephropharmacol. 2013, 2, 1–2. [Google Scholar] [PubMed]
- Arora, A.; Behl, T.; Sehgal, A.; Singh, S.; Sharma, N.; Bhatia, S.; Sobarzo-Sanchez, E.; Bungau, S. Unravelling the Involvement of Gut Microbiota in Type 2 Diabetes Mellitus. Life Sci. 2021, 273, 119311. [Google Scholar] [CrossRef]
- Leyva-López, N.; Gutiérrez-Grijalva, E.P.; Vazquez-Olivo, G.; Heredia, J.B. Essential Oils of Oregano: Biological Activity beyond Their Antimicrobial Properties. Molecules 2017, 22, 989. [Google Scholar] [CrossRef]
- Kumar, S.; Behl, T.; Sachdeva, M.; Sehgal, A.; Kumari, S.; Kumar, A.; Kaur, G.; Yadav, H.N.; Bungau, S. Implicating the Effect of Ketogenic Diet as a Preventive Measure to Obesity and Diabetes Mellitus. Life Sci. 2021, 264, 1–35. [Google Scholar] [CrossRef] [PubMed]
- Rasouli, H.; Hosseini-Ghazvini, S.M.-B.; Adibi, H.; Khodarahmi, R. Differential α-Amylase/α-Glucosidase Inhibitory Activities of Plant-Derived Phenolic Compounds: A Virtual Screening Perspective for the Treatment of Obesity and Diabetes. Food Funct. 2017, 8, 1942–1954. [Google Scholar] [CrossRef]
- Wysham, C.; Shubrook, J. Beta-Cell Failure in Type 2 Diabetes: Mechanisms, Markers, and Clinical Implications. Postgrad. Med. 2020, 132, 676–686. [Google Scholar] [CrossRef] [PubMed]
- Roep, B.O.; Thomaidou, S.; van Tienhoven, R.; Zaldumbide, A. Type 1 Diabetes Mellitus as a Disease of the β-Cell (Do Not Blame the Immune System?). Nat. Rev. Endocrinol. 2021, 17, 150–161. [Google Scholar] [CrossRef]
- Abdollahi, M.; Farshchi, A.; Nikfar, S.; Seyedifar, M. Effect of Chromium on Glucose and Lipid Profiles in Patients with Type 2 Diabetes; a Meta-Analysis Review of Randomized Trials. J. Pharm. Pharm. Sci. 2013, 16, 99–114. [Google Scholar] [CrossRef]
- Robertson, R.P. Oxidative Stress and Impaired Insulin Secretion in Type 2 Diabetes. Curr. Opin. Pharmacol. 2006, 6, 615–619. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Sathyapalan, T.; Atkin, S.L.; Sahebkar, A. Molecular Mechanisms Linking Oxidative Stress and Diabetes Mellitus. Oxid. Med. Cell. Longev. 2020, 2020, 8609213. [Google Scholar] [CrossRef] [PubMed]
- Drews, G.; Krippeit-Drews, P.; Düfer, M. Oxidative Stress and Beta-Cell Dysfunction. Pflugers Arch. 2010, 460, 703–718. [Google Scholar] [CrossRef]
- Gerber, P.A.; Rutter, G.A. The Role of Oxidative Stress and Hypoxia in Pancreatic Beta-Cell Dysfunction in Diabetes Mellitus. Antioxid. Redox Signal. 2017, 26, 501–518. [Google Scholar] [CrossRef] [PubMed]
- Robertson, R.P.; Harmon, J.S. Pancreatic Islet Beta-Cell and Oxidative Stress: The Importance of Glutathione Peroxidase. FEBS Lett. 2007, 581, 3743–3748. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, H. Oxidative Stress in Pancreatic Beta Cell Regeneration. Oxid. Med. Cell. Longev. 2017, 2017, 1930261. [Google Scholar] [CrossRef]
- Paz, K.; Hemi, R.; LeRoith, D.; Karasik, A.; Elhanany, E.; Kanety, H.; Zick, Y. A Molecular Basis for Insulin Resistance. Elevated Serine/Threonine Phosphorylation of IRS-1 and IRS-2 Inhibits Their Binding to the Juxtamembrane Region of the Insulin Receptor and Impairs Their Ability to Undergo Insulin-Induced Tyrosine Phosphorylation. J. Biol. Chem. 1997, 272, 29911–29918. [Google Scholar] [CrossRef]
- Kim, J.K.; Kim, Y.J.; Fillmore, J.J.; Chen, Y.; Moore, I.; Lee, J.; Yuan, M.; Li, Z.W.; Karin, M.; Perret, P.; et al. Prevention of Fat-Induced Insulin Resistance by Salicylate. J. Clin. Investig. 2001, 108, 437–446. [Google Scholar] [CrossRef]
- Nishikawa, T.; Edelstein, D.; Du, X.L.; Yamagishi, S.; Matsumura, T.; Kaneda, Y.; Yorek, M.A.; Beebe, D.; Oates, P.J.; Hammes, H.P.; et al. Normalizing Mitochondrial Superoxide Production Blocks Three Pathways of Hyperglycaemic Damage. Nature 2000, 404, 787–790. [Google Scholar] [CrossRef]
- Gitea, M.A.; Bungau, S.G.; Gitea, D.; Pasca, B.M.; Purza, A.L.; Radu, A.-F. Evaluation of the Phytochemistry-Therapeutic Activity Relationship for Grape Seeds Oil. Life 2023, 13, 178. [Google Scholar] [CrossRef]
- Baydar, N.G.; Özkan, G.; Sağdiç, O. Total Phenolic Contents and Antibacterial Activities of Grape (Vitis vinifera L.) Extracts. Food Control 2004, 15, 335–339. [Google Scholar] [CrossRef]
- Djeridane, A.; Yousfi, M.; Nadjemi, B.; Boutassouna, D.; Stocker, P.; Vidal, N. Antioxidant Activity of Some Algerian Medicinal Plants Extracts Containing Phenolic Compounds. Food Chem. 2006, 97, 654–660. [Google Scholar] [CrossRef]
- Tundis, R.; Loizzo, M.R.; Menichini, F. Natural Products as Alpha-Amylase and Alpha-Glucosidase Inhibitors and Their Hypoglycaemic Potential in the Treatment of Diabetes: An Update. Mini Rev. Med. Chem. 2010, 10, 315–331. [Google Scholar] [CrossRef]
- Li, J.J.; Lee, S.H.; Kim, D.K.; Jin, R.; Jung, D.-S.; Kwak, S.-J.; Kim, S.H.; Han, S.H.; Lee, J.E.; Moon, S.J.; et al. Colchicine Attenuates Inflammatory Cell Infiltration and Extracellular Matrix Accumulation in Diabetic Nephropathy. Am. J. Physiol. Renal Physiol. 2009, 297, F200–F209. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Sun, H.; Wu, L.; Guo, X.; Dou, H.; Tso, M.O.M.; Zhao, L.; Li, S. Baicalein Reduces Inflammatory Process in a Rodent Model of Diabetic Retinopathy. Investig. Ophthalmol. Vis. Sci. 2009, 50, 2319–2327. [Google Scholar] [CrossRef]
- Das Gupta, P.; De, A. Diabetes Mellitus and Its Herbal Treatment. Int. J. Res. Pharm. Biomed. Sci. 2012, 3, 706–721. [Google Scholar]
- Shamim Hossain, M.; Hassan, N.; Kumar Dash, B.; Ashrafuzzaman Sapon, M.; Kumer Sen, M.; Mamun-or-Rashid, A. A Review on Medicinal Plants with Antidiabetic Activity. J. Pharmacogn. Phytochem. 2014, 3, 149–159. [Google Scholar]
- Lee, J.; Noh, S.; Lim, S.; Kim, B. Plant Extracts for Type 2 Diabetes: From Traditional Medicine to Modern Drug Discovery. Antioxidants 2021, 10, 81. [Google Scholar] [CrossRef]
- Evans, P.; Halliwell, B. Micronutrients: Oxidant/Antioxidant Status. Br. J. Nutr. 2001, 85, S67–S74. [Google Scholar] [CrossRef]
- Bounous, G. Whey Protein Concentrate (WPC) and Glutathione Modulation in Cancer Treatment. Anticancer Res. 2000, 20, 4785–4792. [Google Scholar]
- Noguchi, N.; Niki, E. Phenolic Antioxidants: A Rationale for Design and Evaluation of Novel Antioxidant Drug for Atherosclerosis. Free Radic. Biol. Med. 2000, 28, 1538–1546. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, D.; Christopher, A.; Shetty, K. Phenolic Bioactives From Plant-Based Foods for Glycemic Control. Front. Endocrinol. 2022, 12, 727503. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, P.K.; Doble, M. A Target Based Therapeutic Approach towards Diabetes Mellitus Using Medicinal Plants. Curr. Diabetes Rev. 2008, 4, 291–308. [Google Scholar] [CrossRef] [PubMed]
- Millán, I.; Desco, M.D.C.; Torres-Cuevas, I.; Pérez, S.; Pulido, I.; Mena-Mollá, S.; Mataix, J.; Asensi, M.; Ortega, Á.L. Pterostilbene Prevents Early Diabetic Retinopathy Alterations in a Rabbit Experimental Model. Nutrients 2019, 12, 82. [Google Scholar] [CrossRef] [PubMed]
- Tunç, M.T.; Koca, İ. Ohmic Heating Assisted Hydrodistillation of Clove Essential Oil. Ind. Crops Prod. 2019, 141, 111763. [Google Scholar] [CrossRef]
- Zare, M.; Razzaghi, S. Eugenol Enrichment of Clove Bud Essential Oil Using Different Microwave-Assisted Distillation Methods. Food Sci. Technol. Res. 2017, 23, 385–394. [Google Scholar]
- Nait Irahal, I.; Darif, D.; Guenaou, I.; Hmimid, F.; Azzahra Lahlou, F.; Ez-Zahra Ousaid, F.; Abdou-Allah, F.; Aitsi, L.; Akarid, K.; Bourhim, N. Therapeutic Potential of Clove Essential Oil in Diabetes: Modulation of Pro-Inflammatory Mediators, Oxidative Stress and Metabolic Enzyme Activities. Chem. Biodivers. 2023, 20, e202201169. [Google Scholar] [CrossRef] [PubMed]
- Irahal, I.N.; Guenaou, I.; Lahlou, F.A.; Hmimid, F.; Bourhim, N. Syzygium Aromaticum Bud (Clove) Essential Oil Is a Novel and Safe Aldose Reductase Inhibitor: In Silico, In Vitro, and In Vivo Evidence. Hormones 2022, 21, 229–240. [Google Scholar] [CrossRef]
- Zhao, H.; Ren, S.; Yang, H.; Tang, S.; Guo, C.; Liu, M.; Tao, Q.; Ming, T.; Xu, H. Peppermint Essential Oil: Its Phytochemistry, Biological Activity, Pharmacological Effect and Application. Biomed. Pharmacother. 2022, 154, 113559. [Google Scholar] [CrossRef]
- Abdellatief, S.A.; Beheiry, R.R.; El-Mandrawy, S.A.M. Peppermint Essential Oil Alleviates Hyperglycemia Caused by Streptozotocin- Nicotinamide-Induced Type 2 Diabetes in Rats. Biomed. Pharmacother. 2017, 95, 990–999. [Google Scholar] [CrossRef]
- Batiha, G.E.-S.; Teibo, J.O.; Wasef, L.; Shaheen, H.M.; Akomolafe, A.P.; Teibo, T.K.A.; Al-Kuraishy, H.M.; Al-Garbeeb, A.I.; Alexiou, A.; Papadakis, M. A Review of the Bioactive Components and Pharmacological Properties of Lavandula Species. Naunyn. Schmiedebergs. Arch. Pharmacol. 2023, 396, 877–900. [Google Scholar] [CrossRef] [PubMed]
- Sebai, H.; Selmi, S.; Rtibi, K.; Souli, A.; Gharbi, N.; Sakly, M. Lavender (Lavandula stoechas L.) Essential Oils Attenuate Hyperglycemia and Protect against Oxidative Stress in Alloxan-Induced Diabetic Rats. Lipids Health Dis. 2013, 12, 189. [Google Scholar] [CrossRef]
- Sarikurkcu, C.; Zengin, G.; Oskay, M.; Uysal, S.; Ceylan, R.; Aktumsek, A. Composition, Antioxidant, Antimicrobial and Enzyme Inhibition Activities of Two Origanum vulgare Subspecies (Subsp. vulgare and Subsp. hirtum) Essential Oils. Ind. Crops Prod. 2015, 70, 178–184. [Google Scholar] [CrossRef]
- Ramadan, M.F. Nutritional Value, Functional Properties and Nutraceutical Applications of Black Cumin (Nigella sativa L.): An Overview. Int. J. Food Sci. Technol. 2007, 42, 1208–1218. [Google Scholar] [CrossRef]
- Kanter, M.; Meral, I.; Yener, Z.; Ozbek, H.; Demir, H. Partial Regeneration/Proliferation of the Beta-Cells in the Islets of Langerhans by Nigella sativa L. in Streptozotocin-Induced Diabetic Rats. Tohoku J. Exp. Med. 2003, 201, 213–219. [Google Scholar] [CrossRef]
- Sultan, M.T.; Butt, M.S.; Karim, R.; Zia-Ul-Haq, M.; Batool, R.; Ahmad, S.; Aliberti, L.; De Feo, V. Nigella sativa Fixed and Essential Oil Supplementation Modulates Hyperglycemia and Allied Complications in Streptozotocin-Induced Diabetes Mellitus. Evid.-Based Complement. Altern. Med. 2014, 2014, 826380. [Google Scholar] [CrossRef]
- Ahmad, A.; Husain, A.; Mujeeb, M.; Khan, S.A.; Najmi, A.K.; Siddique, N.A.; Damanhouri, Z.A.; Anwar, F. A Review on Therapeutic Potential of Nigella sativa: A Miracle Herb. Asian Pac. J. Trop. Biomed. 2013, 3, 337–352. [Google Scholar] [CrossRef] [PubMed]
- Khader, M.; Eckl, P.M. Thymoquinone: An Emerging Natural Drug with a Wide Range of Medical Applications. Iran. J. Basic Med. Sci. 2014, 17, 950–957. [Google Scholar]
- Abdelrazek, H.M.A.; Kilany, O.E.; Muhammad, M.A.A.; Tag, H.M.; Abdelazim, A.M. Black Seed Thymoquinone Improved Insulin Secretion, Hepatic Glycogen Storage, and Oxidative Stress in Streptozotocin-Induced Diabetic Male Wistar Rats. Oxid. Med. Cell. Longev. 2018, 2018, 8104165. [Google Scholar] [CrossRef]
- Rahmani, A.; Niknafs, B.; Naseri, M.; Nouri, M.; Tarighat-Esfanjani, A. Effect of Nigella sativa Oil on Oxidative Stress, Inflammatory, and Glycemic Control Indices in Diabetic Hemodialysis Patients: A Randomized Double-Blind, Controlled Trial. Evid.-Based Complement. Altern. Med. 2022, 2022, 2753294. [Google Scholar] [CrossRef]
- Balbaa, M.; El-Zeftawy, M.; Ghareeb, D.; Taha, N.; Mandour, A.W. Nigella sativa Relieves the Altered Insulin Receptor Signaling in Streptozotocin-Induced Diabetic Rats Fed with a High-Fat Diet. Oxid. Med. Cell. Longev. 2016, 2016, 2492107. [Google Scholar] [CrossRef]
- Kooshki, A.; Tofighiyan, T.; Rastgoo, N.; Rakhshani, M.H.; Miri, M. Effect of Nigella sativa Oil Supplement on Risk Factors for Cardiovascular Diseases in Patients with Type 2 Diabetes Mellitus. Phyther. Res. 2020, 34, 2706–2711. [Google Scholar] [CrossRef]
- El-Dakhakhny, M.; Mady, N.; Lembert, N.; Ammon, H.P.T. The Hypoglycemic Effect of Nigella sativa Oil Is Mediated by Extrapancreatic Actions. Planta Med. 2002, 68, 465–466. [Google Scholar] [CrossRef]
- Lu, Y.; Foo, L.Y. Polyphenolics of Salvia—A Review. Phytochemistry 2002, 59, 117–140. [Google Scholar] [CrossRef]
- Grdiša, M.; Jug-Dujaković, M.; Lončarić, M.; Carović-Stanko, K.; Ninčević Runjić, T.; Liber, Z.; Radosavljevic, I.; Šatović, Z. Dalmatian Sage (Salvia officinalis L.): A Review of Biochemical Contents, Medical Properties and Genetic Diversity. Agric. Conspec. Sci. 2015, 80, 69–78. [Google Scholar]
- Alarcon-Aguilar, F.J.; Roman-Ramos, R.; Flores-Saenz, J.L.; Aguirre-Garcia, F. Investigation on the Hypoglycaemic Effects of Extracts of Four Mexican Medicinal Plants in Normal and Alloxan-Diabetic Mice. Phytother. Res. 2002, 16, 383–386. [Google Scholar] [CrossRef]
- Eidi, M.; Eidi, A.; Zamanizadeh, H. Effect of Salvia officinalis L. Leaves on Serum Glucose and Insulin in Healthy and Streptozotocin-Induced Diabetic Rats. J. Ethnopharmacol. 2005, 100, 310–313. [Google Scholar] [CrossRef]
- Hemmer, A.; Maiter, D.; Buysschaert, M.; Preumont, V. Long-Term Effects of GLP-1 Receptor Agonists in Type 2 Diabetic Patients: A Retrospective Real-Life Study in 131 Patients. Diabetes Metab. Syndr. 2019, 13, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Assaggaf, H.M.; Naceiri Mrabti, H.; Rajab, B.S.; Attar, A.A.; Alyamani, R.A.; Hamed, M.; El Omari, N.; El Menyiy, N.; Hazzoumi, Z.; Benali, T.; et al. Chemical Analysis and Investigation of Biological Effects of Salvia officinalis Essential Oils at Three Phenological Stages. Molecules 2022, 27, 5157. [Google Scholar] [CrossRef] [PubMed]
- Belhadj, S.; Hentati, O.; Hammami, M.; Ben Hadj, A.; Boudawara, T.; Dammak, M.; Zouari, S.; El Feki, A. Metabolic Impairments and Tissue Disorders in Alloxan-Induced Diabetic Rats Are Alleviated by Salvia officinalis L. Essential Oil. Biomed. Pharmacother. 2018, 108, 985–995. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Zhao, S.; Ning, Z.; Zeng, H.; Shu, Y.; Tao, O.; Xiao, C.; Lu, C.; Liu, Y. Citrus Fruits as a Treasure Trove of Active Natural Metabolites That Potentially Provide Benefits for Human Health. Chem. Cent. J. 2015, 9, 68. [Google Scholar] [CrossRef]
- Kelebek, H.; Selli, S. Determination of Volatile, Phenolic, Organic Acid and Sugar Components in a Turkish Cv. Dortyol (Citrus sinensis L. Osbeck) Orange Juice. J. Sci. Food Agric. 2011, 91, 1855–1862. [Google Scholar] [CrossRef] [PubMed]
- González-Molina, E.; Domínguez-Perles, R.; Moreno, D.A.; García-Viguera, C. Natural Bioactive Compounds of Citrus Limon for Food and Health. J. Pharm. Biomed. Anal. 2010, 51, 327–345. [Google Scholar] [CrossRef] [PubMed]
- Dosoky, N.S.; Setzer, W.N. Biological Activities and Safety of Citrus Spp. Essential Oils. Int. J. Mol. Sci. 2018, 19, 1966. [Google Scholar] [CrossRef] [PubMed]
- Jantan, I.; Ahmad, A.S.; Ahmad, A.R.; Ali, N.A.M.; Ayop, N. Chemical Composition of Some Citrus Oils from Malaysia. J. Essent. Oil Res. 2011, 8, 627–632. [Google Scholar] [CrossRef]
- Ibrahim, F.A.; Usman, L.A.; Akolade, J.O.; Idowu, O.A.; Abdulazeez, A.T.; Amuzat, A.O. Antidiabetic Potentials of Citrus Aurantifolia Leaf Essential Oil. Drug Res. (Stuttg). 2019, 69, 201–206. [Google Scholar] [CrossRef]
- Oboh, G.; Ademosun, A.O.; Odubanjo, O.V.; Akinbola, I.A. Antioxidative Properties and Inhibition of Key Enzymes Relevant to Type-2 Diabetes and Hypertension by Essential Oils from Black Pepper. Adv. Pharmacol. Sci. 2013, 2013, 926047. [Google Scholar] [CrossRef] [PubMed]
- González-Trujano, M.E.; Peña, E.I.; Martínez, A.L.; Moreno, J.; Guevara-Fefer, P.; Déciga-Campos, M.; López-Muñoz, F.J. Evaluation of the Antinociceptive Effect of Rosmarinus officinalis L. Using Three Different Experimental Models in Rodents. J. Ethnopharmacol. 2007, 111, 476–482. [Google Scholar] [CrossRef]
- Martínez, A.L.; González-Trujano, M.E.; Pellicer, F.; López-Muñoz, F.J.; Navarrete, A. Antinociceptive Effect and GC/MS Analysis of Rosmarinus officinalis L. Essential Oil from Its Aerial Parts. Planta Med. 2009, 75, 508–511. [Google Scholar] [CrossRef]
- Selmi, S.; Rtibi, K.; Grami, D.; Sebai, H.; Marzouki, L. Rosemary (Rosmarinus officinalis) Essential Oil Components Exhibit Anti-Hyperglycemic, Anti-Hyperlipidemic and Antioxidant Effects in Experimental Diabetes. Pathophysiology 2017, 24, 297–303. [Google Scholar] [CrossRef]
- Fareed, S.A.; Yousef, E.M.; Abd El-Moneam, S.M. Assessment of Effects of Rosemary Essential Oil on the Kidney Pathology of Diabetic Adult Male Albino Rats. Cureus 2023, 15, e35736. [Google Scholar] [CrossRef]
- Mahnashi, M.H.; Alqahtani, Y.S.; Alyami, B.A.; Alqarni, A.O.; Ayaz, M.; Ghufran, M.; Ullah, F.; Sadiq, A.; Ullah, I.; Haq, I.U.; et al. Phytochemical Analysis, α-Glucosidase and Amylase Inhibitory, and Molecular Docking Studies on Persicaria Hydropiper L. Leaves Essential Oils. Evid. Based. Complement. Alternat. Med. 2022, 2022, 7924171. [Google Scholar] [CrossRef] [PubMed]
- Palamthodi, S.; Lele, S.S. Nutraceutical Applications of Gourd Family Vegetables: Benincasa Hispida, Lagenaria Siceraria and Momordica Charantia. Biomed. Prev. Nutr. 2014, 4, 15–21. [Google Scholar] [CrossRef]
- Jia, S.; Shen, M.; Zhang, F.; Xie, J. Recent Advances in Momordica Charantia: Functional Components and Biological Activities. Int. J. Mol. Sci. 2017, 18, 2555. [Google Scholar] [CrossRef] [PubMed]
- Lo, H.-Y.; Li, C.-C.; Chen, F.-Y.; Chen, J.-C.; Hsiang, C.-Y.; Ho, T.-Y. Gastro-Resistant Insulin Receptor-Binding Peptide from Momordica Charantia Improved the Glucose Tolerance in Streptozotocin-Induced Diabetic Mice via Insulin Receptor Signaling Pathway. J. Agric. Food Chem. 2017, 65, 9266–9274. [Google Scholar] [CrossRef]
- Lo, H.-Y.; Ho, T.-Y.; Li, C.-C.; Chen, J.-C.; Liu, J.-J.; Hsiang, C.-Y. A Novel Insulin Receptor-Binding Protein from Momordica Charantia Enhances Glucose Uptake and Glucose Clearance In Vitro and In Vivo through Triggering Insulin Receptor Signaling Pathway. J. Agric. Food Chem. 2014, 62, 8952–8961. [Google Scholar] [CrossRef]
- Mariammal, B.G.V.; Devarajan, D.W.; Jerrin, R.; Viswanathan, S.; Siddikuzzaman; Gopal, R. In Vivo Treatment Efficacy of Essential Oil Isolated from Seeds of Momordica Charantia in Streptozotocin-Induced Diabetes Mellitus. Recent Pat. Biotechnol. 2021, 15, 316–331. [Google Scholar] [CrossRef]
- Shah, M.; Al-Housni, S.K.; Khan, F.; Ullah, S.; Al-Sabahi, J.N.; Khan, A.; Al-Yahyaei, B.E.M.; Al-Ruqaishi, H.; Rehman, N.U.; Al-Harrasi, A. First Report on Comparative Essential Oil Profile of Stem and Leaves of Blepharispermum Hirtum Oliver and Their Antidiabetic and Anticancer Effects. Metabolites 2022, 12, 907. [Google Scholar] [CrossRef]
- Jerbi, A.; Derbali, A.; Elfeki, A.; Kammoun, M. Essential Oil Composition and Biological Activities of Eucalyptus Globulus Leaves Extracts from Tunisia. J. Essent. Oil Bear. Plants 2017, 20, 438–448. [Google Scholar] [CrossRef]
- Canadanović-Brunet, J.; Cetković, G.; Djilas, S.; Tumbas, V.; Bogdanović, G.; Mandić, A.; Markov, S.; Cvetković, D.; Canadanović, V. Radical Scavenging, Antibacterial, and Antiproliferative Activities of Melissa officinalis L. Extracts. J. Med. Food 2008, 11, 133–143. [Google Scholar] [CrossRef]
- Aharizad, S.; Rahimi, M.H.; Moghaddam, M.; Mohebalipour, N. Study of Genetic Diversity in Lemon Balm (Melissa officinalis L.) Populations Based on Morphological Traits and Essential Oils Content. Ann. Biol. Res. 2012, 2012, 5748–5753. [Google Scholar]
- Chung, M.J.; Cho, S.-Y.; Bhuiyan, M.J.H.; Kim, K.H.; Lee, S.-J. Anti-Diabetic Effects of Lemon Balm (Melissa officinalis) Essential Oil on Glucose- and Lipid-Regulating Enzymes in Type 2 Diabetic Mice. Br. J. Nutr. 2010, 104, 180–188. [Google Scholar] [CrossRef]
- Hasanein, P.; Riahi, H. Antinociceptive and Antihyperglycemic Effects of Melissa officinalis Essential Oil in an Experimental Model of Diabetes. Med. Princ. Pract. 2015, 24, 47–52. [Google Scholar] [CrossRef]
- Bendimerad-Mouttas, F.; Beghdad, M.C.; El Haci, I.A.; Soualem, Z.; Belarbi, M.; Bekkara, F.A. Bioactive Compounds and Antioxidant Activity of Rhaponticum acaule (L.) DC. Nat. Prod. Res. 2020, 34, 1553–1557. [Google Scholar] [CrossRef] [PubMed]
- Mosbah, H.; Chahdoura, H.; Kammoun, J.; Hlila, M.B.; Louati, H.; Hammami, S.; Flamini, G.; Achour, L.; Selmi, B. Rhaponticum acaule (L) DC Essential Oil: Chemical Composition, In Vitro Antioxidant and Enzyme Inhibition Properties. BMC Complement. Altern. Med. 2018, 18, 79. [Google Scholar] [CrossRef] [PubMed]
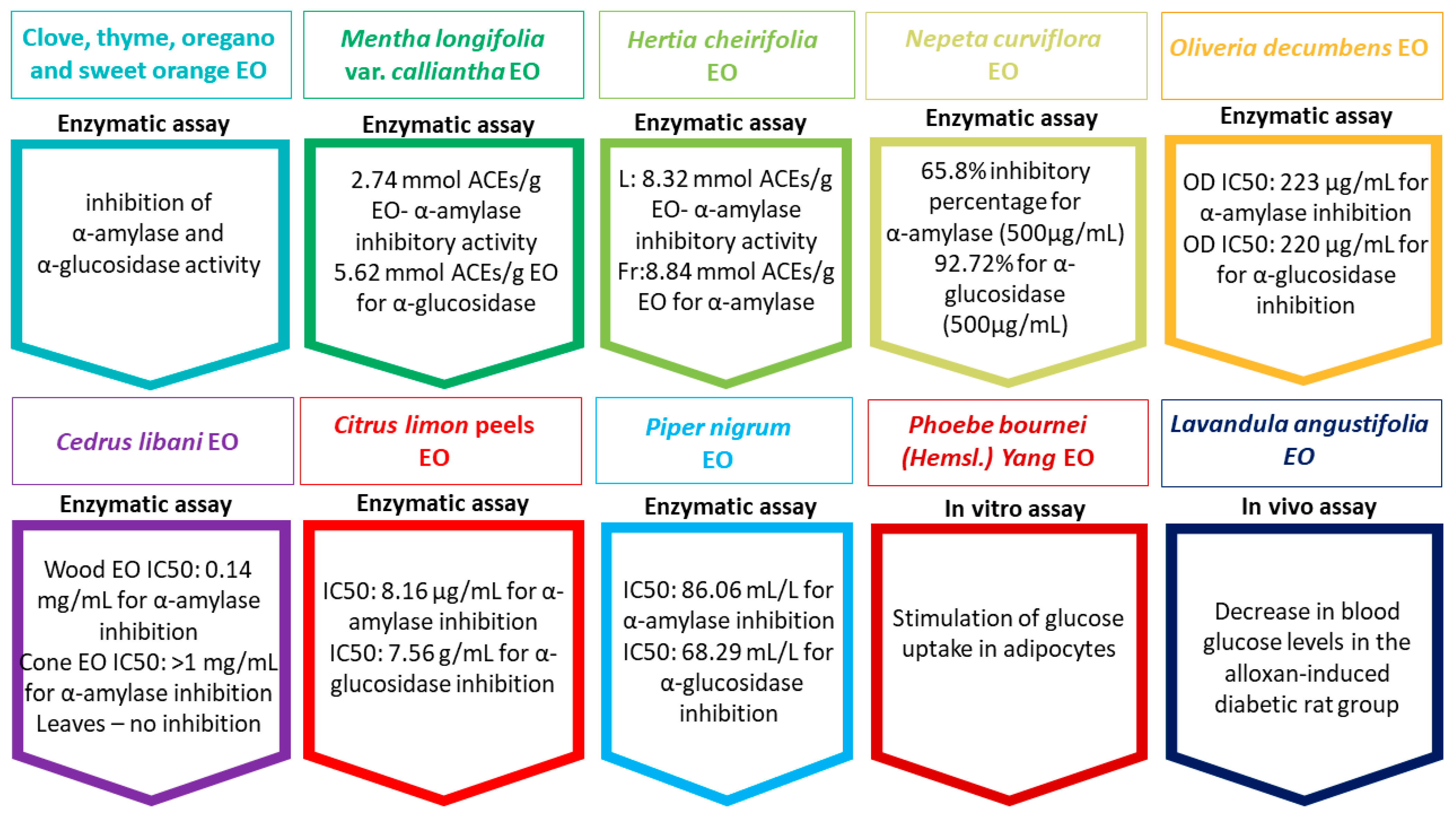
- Radünz, M.; Mota Camargo, T.; dos Santos Hackbart, H.C.; Inchauspe Correa Alves, P.; Radünz, A.L.; Avila Gandra, E.; da Rosa Zavareze, E. Chemical Composition and in Vitro Antioxidant and Antihyperglycemic Activities of Clove, Thyme, Oregano, and Sweet Orange Essential Oils. LWT 2021, 138, 110632. [Google Scholar] [CrossRef]
- Asghari, B.; Zengin, G.; Bahadori, M.B.; Abbas-Mohammadi, M.; Dinparast, L. Amylase, Glucosidase, Tyrosinase, and Cholinesterases Inhibitory, Antioxidant Effects, and GC-MS Analysis of Wild Mint (Mentha longifolia var. calliantha) Essential Oil: A Natural Remedy. Eur. J. Integr. Med. 2018, 22, 44–49. [Google Scholar] [CrossRef]
- Rahali, N.; Mehdi, S.; Younsi, F.; Boussaid, M.; Messaoud, C. Antioxidant, α-Amylase, and Acetylcholinesterase Inhibitory Activities of Hertia Cheirifolia Essential Oils: Influence of Plant Organs and Seasonal Variation. Int. J. Food Prop. 2017, 20, 1637–1651. [Google Scholar] [CrossRef]
- Jaradat, N.; Al-Maharik, N.; Abdallah, S.; Shawahna, R.; Mousa, A.; Qtishat, A. Nepeta Curviflora Essential Oil: Phytochemical Composition, Antioxidant, Anti-Proliferative and Anti-Migratory Efficacy against Cervical Cancer Cells, and α-Glucosidase, α-Amylase and Porcine Pancreatic Lipase Inhibitory Activities. Ind. Crops Prod. 2020, 158, 112946. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Saab, A.M.; Statti, G.A.; Menichini, F. Composition and Alpha-Amylase Inhibitory Effect of Essential Oils from Cedrus Libani. Fitoterapia 2007, 78, 323–326. [Google Scholar] [CrossRef]
- Oboh, G.; Olasehinde, T.A.; Ademosun, A.O. Inhibition of Enzymes Linked to Type-2 Diabetes and Hypertension by Essential Oils from Peels of Orange and Lemon. Int. J. Food Prop. 2017, 20, S586–S594. [Google Scholar] [CrossRef]
- Ding, W.; Liping, N.; Xing, H.; Wei, Z.; Zhoua, Q.; Nong, R.; Chen, J. Essential Oil Extracted from Leaf of Phoebe Bournei (Hemsl.) Yang: Chemical Constituents, Antitumor, Antibacterial, Hypoglycemic Activities. Nat. Prod. Res. 2020, 34, 2524–2527. [Google Scholar] [CrossRef]
- Ahmad, B.; Masud, T.; Uppal, A.; Naveed, A. Effects of Nigella sativa Oil on Some Blood Parameters in Type 2 Diabetes Mellitus Patients. Asian J. Chem. 2009, 21, 5373–5381. [Google Scholar]
- Jafari, S.; Sattari, R.; Ghavamzadeh, S. Evaluation the Effect of 50 and 100 Mg Doses of Cuminum cyminum Essential Oil on Glycemic Indices, Insulin Resistance and Serum Inflammatory Factors on Patients with Diabetes Type II: A Double-Blind Randomized Placebo-Controlled Clinical Trial. J. Tradit. Complement. Med. 2017, 7, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Samani Keihan, G.; Gharib, M.H.; Momeni, A.; Hemati, Z.; Sedighin, R. A Comparison Between the Effect of Cuminum cyminum and Vitamin E on the Level of Leptin, Paraoxonase 1, HbA1c and Oxidized LDL in Diabetic Patients. Int. J. Mol. Cell. Med. 2016, 5, 229–235. [Google Scholar] [PubMed]
- Morovati, A.; Pourghassem Gargari, B.; Sarbakhsh, P. Effects of Cumin (Cuminum cyminum L.) Essential Oil Supplementation on Metabolic Syndrome Components: A Randomized, Triple-Blind, Placebo-Controlled Clinical Trial. Phytother. Res. 2019, 33, 3261–3269. [Google Scholar] [CrossRef]
- Jafari, T.; Mahmoodnia, L.; Tahmasebi, P.; Memarzadeh, M.R.; Sedehi, M.; Beigi, M.; Fallah, A.A. Effect of Cumin (Cuminum cyminum) Essential Oil Supplementation on Metabolic Profile and Serum Leptin in Pre-Diabetic Subjects: A Randomized Double-Blind Placebo-Controlled Clinical Trial. J. Funct. Foods 2018, 47, 416–422. [Google Scholar] [CrossRef]
- Taghizadeh, M.; Memarzadeh, M.R.; Asemi, Z.; Esmaillzadeh, A. Effect of the Cumin cyminum L. Intake on Weight Loss, Metabolic Profiles and Biomarkers of Oxidative Stress in Overweight Subjects: A Randomized Double-Blind Placebo-Controlled Clinical Trial. Ann. Nutr. Metab. 2015, 66, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Heghes, S.C.; Filip, L.; Vostinaru, O.; Mogosan, C.; Miere, D.; Iuga, C.A.; Moldovan, M. Essential Oil-Bearing Plants From Balkan Peninsula: Promising Sources for New Drug Candidates for the Prevention and Treatment of Diabetes Mellitus and Dyslipidemia. Front. Pharmacol. 2020, 11, 989. [Google Scholar] [CrossRef]
- Wojtunik-Kulesza, K.A. Toxicity of Selected Monoterpenes and Essential Oils Rich in These Compounds. Molecules 2022, 27, 1716. [Google Scholar] [CrossRef]
- Burkhard, P.R.; Burkhardt, K.; Haenggeli, C.A.; Landis, T. Plant-Induced Seizures: Reappearance of an Old Problem. J. Neurol. 1999, 246, 667–670. [Google Scholar] [CrossRef] [PubMed]
- Malekmohammad, K.; Rafieian-kopaei, M.; Sardari, S.; Sewell, R. Toxicological Effects of Mentha x Piperita (Peppermint): A Review. Toxin Rev. 2019, 40, 1–15. [Google Scholar]
- European Medicines Agency. Assessment Report on Mentha x Piperita L., Folium and Aetheroleum. EMA/HMPC/522409/2013. Available online: https://www.ema.europa.eu/en/documents/herbal-report/assessment-report-mentha-x-piperita-l-folium-aetheroleum-revision-1_en.pdf (accessed on 14 November 2023).
- Tavakkoli, A.; Mahdian, V.; Razavi, B.M.; Hosseinzadeh, H. Review on Clinical Trials of Black Seed (Nigella sativa) and Its Active Constituent, Thymoquinone. J. Pharmacopunct. 2017, 20, 179–193. [Google Scholar]
- Hadi, A.; Mohammadi, H.; Hadi, Z.; Roshanravan, N.; Kafeshani, M. Cumin (Cuminum cyminum L.) Is a Safe Approach for Management of Lipid Parameters: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Phytother. Res. 2018, 32, 2146–2154. [Google Scholar] [CrossRef] [PubMed]
- Kaltenhäuser, J.; Kneuer, C.; Marx-Stoelting, P.; Niemann, L.; Schubert, J.; Stein, B.; Solecki, R. Relevance and Reliability of Experimental Data in Human Health Risk Assessment of Pesticides. Regul. Toxicol. Pharmacol. 2017, 88, 227–237. [Google Scholar] [CrossRef]
| Description of the Study | Results/Observations | Ref. |
|---|---|---|
| Nigella saliva EO | ||
| Clinical randomized study including 41 patients with T2DM consumed black cumin EO for 40 days (adjusting the daily dose so that it was the same as of the oil extracted from 0.7 g of Nigella sativa seeds), followed by a placebo for 40 more days. Blood samples were collected a jeun on days 0, 40, and 80 of the research, in the case of each subject. | After EO administration, a significant decrease in fasting glucose and an increase in AST and INS levels vs. baseline levels were found. INS and glucose levels reversed after the placebo period. Blood urea and ALT and the number of platelets and of leukocytes did not change in the two periods vs. the baseline values. It resulted that this EO may have a role in the treatment of T2DM, ensuring good hepatic and renal safety. | [103] |
| Cuminum cyminumL. EO | ||
| Clinical randomized double-blind study, 60 days, including 99 patients; 33 subjects were selected/included in each of the 3 groups: 1st group, one C. cyminum capsule (100 mg/day); 2nd group, one C. cyminum capsule each (50 mg/day); 3rd group placebo as control. A single blood sample was taken before/after 60 days of treatment. | HOMA-IR was considerably greater in the first two groups but reduced in the 3rd group. Mean of the FBS, HbA1c, and the serum levels of INS were significantly diminished. Upon completion of the study, in all the three groups, the mean serum levels of hsCRP and TNF-α were significantly decreased, and that of adiponectin was significantly higher. | [104] |
| Clinical randomized double-blind study, 90 days, including 95 T2DM patients distributed in each of the 3 groups: 1st group received capsule form of EO (25 mg/day); 2nd group, vitamin E (800 IU—150 mg); 3rd group, placebo (gelatin capsules) as control. A single blood sample was taken before/after 90 days of treatment. | First group had reduced values in blood glucose, HbA1c, ox-LDL, leptin, and triglyceride, ApoA1 and paraoxonase1 were increased. ApoA1, ApoB, blood glucose, HbA1c, leptin, lipid profile, oxLDL, and paraoxonase1 were determined, resulting decreasing in oxLDL, and significantly higher values for paraoxonase 1 in the second group at the end of the study. EO vs. vitamin E had stronger impact, being more efficient in diabetic index reduction. | [105] |
| Clinical randomized triple-blind trial, 56 days, 56 patients (between 18 and 60 years old), diagnosed with MetS, received 75 mg EO or placebo gelatin capsules 3 times a day. A single blood sample was taken before/after 56 days of treatment. | In patients with MetS, the results revealed that EO administration has an effect only on DBP among all MetS components. | [106] |
| Multi-center randomized, placebo-controlled, double-blind parallel-group clinical trial, including 54 pre-diabetic patients >19 years, distributed in each of the two groups: first group, that received capsule form of cumin EO (75 mg/day) 10 weeks vs. placebo as control. | Improved INS sensitivity (HOMA-IR, fasting serum INS, and QUICKI), lipid profile, and anthropometric parameters among individuals at risk for developing diabetes were observed following treatment with cumin EO. HbA1C, FPG, and leptin blood levels did not significantly improve, however. | [107] |
| Randomized double-blind placebo-controlled clinical, including 78 overweight subjects distributed in each of the 3 groups:: 1st group, one C. cyminum capsule (100 mg/day); 2nd group, orlistat120 capsule; 3rd group placebo as control (8 weeks). | When comparing orlistat, placebo, and Cuminum cyminum L., investigators found that QUICKI was significantly increased, while blood INS levels and HOMA-B were considerably decreased. Overweight patients who received C. cyminum L. experienced the same reductions in weight and body mass index as those who took orlistat120, also observing improvements in INS metabolism vs. placebo and orlistat120 group. | [108] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bungau, S.G.; Vesa, C.M.; Bustea, C.; Purza, A.L.; Tit, D.M.; Brisc, M.C.; Radu, A.-F. Antioxidant and Hypoglycemic Potential of Essential Oils in Diabetes Mellitus and Its Complications. Int. J. Mol. Sci. 2023, 24, 16501. https://doi.org/10.3390/ijms242216501
Bungau SG, Vesa CM, Bustea C, Purza AL, Tit DM, Brisc MC, Radu A-F. Antioxidant and Hypoglycemic Potential of Essential Oils in Diabetes Mellitus and Its Complications. International Journal of Molecular Sciences. 2023; 24(22):16501. https://doi.org/10.3390/ijms242216501
Chicago/Turabian StyleBungau, Simona Gabriela, Cosmin Mihai Vesa, Cristian Bustea, Anamaria Lavinia Purza, Delia Mirela Tit, Mihaela Cristina Brisc, and Andrei-Flavius Radu. 2023. "Antioxidant and Hypoglycemic Potential of Essential Oils in Diabetes Mellitus and Its Complications" International Journal of Molecular Sciences 24, no. 22: 16501. https://doi.org/10.3390/ijms242216501
APA StyleBungau, S. G., Vesa, C. M., Bustea, C., Purza, A. L., Tit, D. M., Brisc, M. C., & Radu, A.-F. (2023). Antioxidant and Hypoglycemic Potential of Essential Oils in Diabetes Mellitus and Its Complications. International Journal of Molecular Sciences, 24(22), 16501. https://doi.org/10.3390/ijms242216501









