Secretome as a Tool to Treat Neurological Conditions: Are We Ready?
Abstract
:1. Introduction
2. Secretome Composition
2.1. Soluble Factors
2.2. Extracellular Vesicles
2.2.1. Exosomes
2.2.2. Microvesicles
2.2.3. miRNAs
3. Advantages of the Therapeutic Use of the Secretome Compared to MSCs
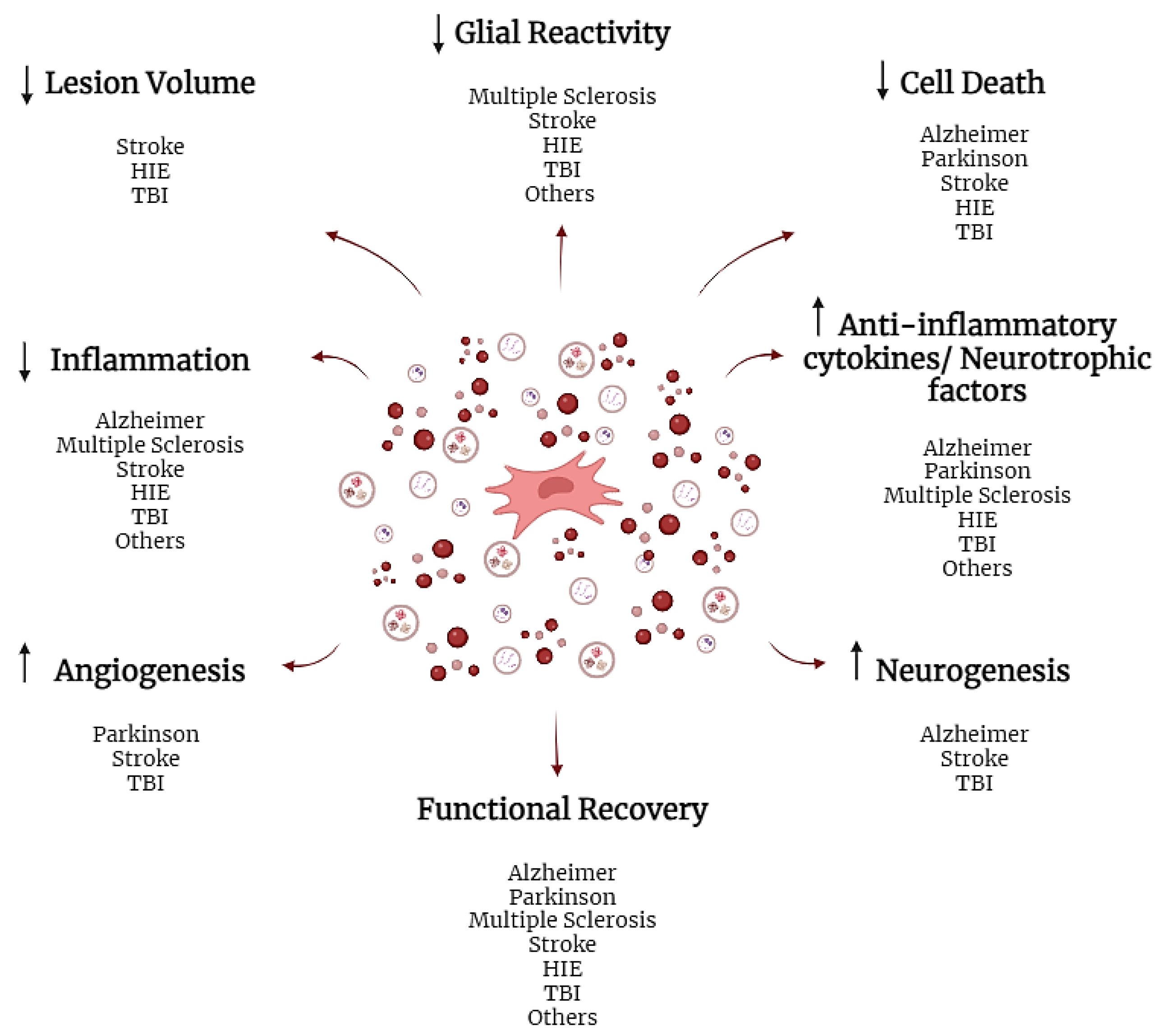
4. The Therapeutic Use of the Secretome in Neurological Diseases
4.1. Clinical Studies
4.2. Preclinical Studies
4.2.1. Alzheimer’s Disease
4.2.2. Multiple Sclerosis
4.2.3. Parkinson’s Disease
4.2.4. Stroke
4.2.5. Hypoxic-Ischemic Encephalopathy
4.2.6. Traumatic Brain Injury
4.2.7. Other Pathologies
5. Preconditioning
5.1. Three-Dimensional Culture and Three-Dimensional Scaffolds
5.2. Pharmacological or Chemical Compounds
5.3. Cytokines and Growth Factors
5.4. Hypoxia
5.5. Other Methods
6. Preparation of the Secretome
Secretome Formulation
7. Current Limitations
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| α-MEM | alpha minimal essential medium |
| AD | Alzheimer’s disease |
| ADSCs | adipose tissue |
| BBB | Blood–brain barrier |
| BDNF | brain-derived neurotrophic factor |
| bFGF | basic fibroblast growth factor |
| BME | Eagle’s basal medium |
| BM | bone marrow |
| CM | conditioned medium |
| CNS | central nervous system |
| DMEM | Dulbecco’s Modified Eagle’s Basal Medium |
| EVs | extracellular vesicles |
| Gal-1 | galectin-1 |
| GDNF | glia-derived growth factor |
| HIE | Hypoxic-ischemic Encephalopathy |
| HGF-1 | hepatocyte growth factor 1 |
| HSP | heat shock protein |
| IFN-Y | interferon |
| IGF-1 | insulin-like growth factor type 1 |
| IL | interleukin |
| IL-1Ra | interleukin 1 receptor antagonist |
| miRNA | Micro-ribonucleic acid |
| mRNA | Messenger ribonucleic acid |
| MSCs | mesenchymal stem cells |
| MVs | microvesicles |
| NGF | nerve growth factor |
| TBI | traumatic brain injury |
| TGF-B | tumor growth factor B |
| TNF-a | tumor necrosis factor α |
| TSG101 | tumor susceptibility protein 101 |
| VEGF | vascular endothelial growth factor |
References
- Fu, X.; Liu, G.; Halim, A.; Ju, Y.; Luo, Q.; Song, A.G. Mesenchymal Stem Cell Migration and Tissue Repair. Cells 2019, 8, 784. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Wang, T.; Ge, Y.; Cai, X.; Wang, J.; Lin, Y. Secreted factors from adipose tissue increase adipogenic differentiation of mesenchymal stem cells. Cell Prolif. 2012, 45, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, L.; Gutierrez-Aranda, I.; Ligero, G.; Rubio, R.; Muñoz-López, M.; García-Pérez, J.L.; Ramos, V.; Real, P.J.; Bueno, C.; Rodríguez, R.; et al. Enrichment of human ESC-derived multipotent mesenchymal stem cells with immunosuppressive and anti-inflammatory properties capable to protect against experimental inflammatory bowel disease. Stem Cells 2011, 29, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Nitzsche, F.; Müller, C.; Lukomska, B.; Jolkkonen, J.; Deten, A.; Boltze, J. Concise Review: MSC Adhesion Cascade-Insights into Homing and Transendothelial Migration. Stem Cells 2017, 35, 1446–1460. [Google Scholar] [CrossRef]
- Vizoso, F.J.; Eiro, N.; Cid, S.; Schneider, J.; Perez-Fernandez, R. Mesenchymal Stem Cell Secretome: Toward Cell-Free Therapeutic Strategies in Regenerative Medicine. Int. J. Mol. Sci. 2017, 18, 1852. [Google Scholar] [CrossRef]
- Timmers, L.; Lim, S.K.; Hoefer, I.E.; Arslan, F.; Lai, R.C.; van Oorschot, A.A.; Goumans, M.J.; Strijder, C.; Sze, S.K.; Choo, A.; et al. Human mesenchymal stem cell-conditioned medium improves cardiac function following myocardial infarction. Stem Cell Res. 2011, 6, 206–214. [Google Scholar] [CrossRef]
- Chang, C.; Yan, J.; Yao, Z.; Zhang, C.; Li, X.; Mao, H.Q. Effects of Mesenchymal Stem Cell-Derived Paracrine Signals and Their Delivery Strategies. Adv. Healthc. Mater. 2021, 10, e2001689. [Google Scholar] [CrossRef]
- Bogatcheva, N.V.; Coleman, M.E. Conditioned Medium of Mesenchymal Stromal Cells: A New Class of Therapeutics. Biochemistry 2019, 84, 1375–1389. [Google Scholar] [CrossRef]
- Muzes, G.; Sipos, F. Mesenchymal Stem Cell-Derived Secretome: A Potential Therapeutic Option for Autoimmune and Immune-Mediated Inflammatory Diseases. Cells 2022, 11, 2300. [Google Scholar] [CrossRef]
- Maqsood, M.; Kang, M.; Wu, X.; Chen, J.; Teng, L.; Qiu, L. Adult mesenchymal stem cells and their exosomes: Sources, characteristics, and application in regenerative medicine. Life Sci. 2020, 256, 118002. [Google Scholar] [CrossRef]
- Hsiao, S.T.; Asgari, A.; Lokmic, Z.; Sinclair, R.; Dusting, G.J.; Lim, S.Y.; Dilley, R.J. Comparative analysis of paracrine factor expression in human adult mesenchymal stem cells derived from bone marrow, adipose, and dermal tissue. Stem Cells Dev. 2012, 21, 2189–2203. [Google Scholar] [CrossRef]
- Amable, P.R.; Teixeira, M.V.; Carias, R.B.; Granjeiro, J.M.; Borojevic, R. Protein synthesis and secretion in human mesenchymal cells derived from bone marrow, adipose tissue and Wharton’s jelly. Stem Cell Res. Ther. 2014, 5, 53. [Google Scholar] [CrossRef]
- Pires, A.O.; Mendes-Pinheiro, B.; Teixeira, F.G.; Anjo, S.I.; Ribeiro-Samy, S.; Gomes, E.D.; Serra, S.C.; Silva, N.A.; Manadas, B.; Sousa, N.; et al. Unveiling the Differences of Secretome of Human Bone Marrow Mesenchymal Stem Cells, Adipose Tissue-Derived Stem Cells, and Human Umbilical Cord Perivascular Cells: A Proteomic Analysis. Stem Cells Dev. 2016, 25, 1073–1083. [Google Scholar] [CrossRef]
- Martins, L.F.; Costa, R.O.; Pedro, J.R.; Aguiar, P.; Serra, S.C.; Teixeira, F.G.; Sousa, N.; Salgado, A.J.; Almeida, R.D. Mesenchymal stem cells secretome-induced axonal outgrowth is mediated by BDNF. Sci. Rep. 2017, 7, 4153. [Google Scholar] [CrossRef]
- Galindo, L.T.; Filippo, T.R.; Semedo, P.; Ariza, C.B.; Moreira, C.M.; Camara, N.O.; Porcionatto, M.A. Mesenchymal stem cell therapy modulates the inflammatory response in experimental traumatic brain injury. Neurol. Res. Int. 2011, 2011, 564089. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, F.G.; Carvalho, M.M.; Neves-Carvalho, A.; Panchalingam, K.M.; Behie, L.A.; Pinto, L.; Sousa, N.; Salgado, A.J. Secretome of mesenchymal progenitors from the umbilical cord acts as modulator of neural/glial proliferation and differentiation. Stem Cell Rev. Rep. 2015, 11, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Kemp, K.; Hares, K.; Mallam, E.; Heesom, K.J.; Scolding, N.; Wilkins, A. Mesenchymal stem cell-secreted superoxide dismutase promotes cerebellar neuronal survival. J. Neurochem. 2010, 114, 1569–1580. [Google Scholar] [CrossRef] [PubMed]
- Baez-Jurado, E.; Vega, G.G.; Aliev, G.; Tarasov, V.V.; Esquinas, P.; Echeverria, V.; Barreto, G.E. Blockade of Neuroglobin Reduces Protection of Conditioned Medium from Human Mesenchymal Stem Cells in Human Astrocyte Model (T98G) Under a Scratch Assay. Mol. Neurobiol. 2018, 55, 2285–2300. [Google Scholar] [CrossRef]
- Szekiova, E.; Slovinska, L.; Blasko, J.; Plsikova, J.; Cizkova, D. The neuroprotective effect of rat adipose tissue-derived mesenchymal stem cell-conditioned medium on cortical neurons using an in vitro model of SCI inflammation. Neurol. Res. 2018, 40, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Hao, P.; Liang, Z.; Piao, H.; Ji, X.; Wang, Y.; Liu, Y.; Liu, R.; Liu, J. Conditioned medium of human adipose-derived mesenchymal stem cells mediates protection in neurons following glutamate excitotoxicity by regulating energy metabolism and GAP-43 expression. Metab. Brain Dis. 2014, 29, 193–205. [Google Scholar] [CrossRef]
- Shen, C.; Lie, P.; Miao, T.; Yu, M.; Lu, Q.; Feng, T.; Li, J.; Zu, T.; Liu, X.; Li, H. Conditioned medium from umbilical cord mesenchymal stem cells induces migration and angiogenesis. Mol. Med. Rep. 2015, 12, 20–30. [Google Scholar] [CrossRef]
- Tran, C.; Damaser, M.S. Stem cells as drug delivery methods: Application of stem cell secretome for regeneration. Adv. Drug Deliv. Rev. 2015, 82–83, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Nickel, W.; Rabouille, C. Mechanisms of regulated unconventional protein secretion. Nat. Rev. Mol. Cell Biol. 2009, 10, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Caccia, D.; Dugo, M.; Callari, M.; Bongarzone, I. Bioinformatics tools for secretome analysis. Biochim. Biophys. Acta 2013, 1834, 2442–2453. [Google Scholar] [CrossRef]
- Liu, X.Y.; Wei, M.G.; Liang, J.; Xu, H.H.; Wang, J.J.; Wang, J.; Yang, X.P.; Lv, F.F.; Wang, K.Q.; Duan, J.H.; et al. Injury-preconditioning secretome of umbilical cord mesenchymal stem cells amplified the neurogenesis and cognitive recovery after severe traumatic brain injury in rats. J. Neurochem. 2020, 153, 230–251. [Google Scholar] [CrossRef] [PubMed]
- Waterman, R.S.; Tomchuck, S.L.; Henkle, S.L.; Betancourt, A.M. A new mesenchymal stem cell (MSC) paradigm: Polarization into a pro-inflammatory MSC1 or an Immunosuppressive MSC2 phenotype. PLoS ONE 2010, 5, e10088. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, Y.; Wang, Z.; Gutkind, J.S.; Wang, Z.; Wang, F.; Lu, J.; Niu, G.; Teng, G.; Chen, X. Engineered mesenchymal stem cells with enhanced tropism and paracrine secretion of cytokines and growth factors to treat traumatic brain injury. Stem Cells 2015, 33, 456–467. [Google Scholar] [CrossRef]
- Baraniak, P.R.; McDevitt, T.C. Stem cell paracrine actions and tissue regeneration. Regen. Med. 2010, 5, 121–143. [Google Scholar] [CrossRef]
- Wegmeyer, H.; Bröske, A.M.; Leddin, M.; Kuentzer, K.; Nisslbeck, A.K.; Hupfeld, J.; Wiechmann, K.; Kuhlen, J.; von Schwerin, C.; Stein, C.; et al. Mesenchymal stromal cell characteristics vary depending on their origin. Stem Cells Dev. 2013, 22, 2606–2618. [Google Scholar] [CrossRef]
- Siegel, G.; Kluba, T.; Hermanutz-Klein, U.; Bieback, K.; Northoff, H.; Schäfer, R. Phenotype, donor age and gender affect function of human bone marrow-derived mesenchymal stromal cells. BMC Med. 2013, 11, 146. [Google Scholar] [CrossRef]
- Wilson, A.; Webster, A.; Genever, P. Nomenclature and heterogeneity: Consequences for the use of mesenchymal stem cells in regenerative medicine. Regen. Med. 2019, 14, 595–611. [Google Scholar] [CrossRef] [PubMed]
- Eleuteri, S.; Fierabracci, A. Insights into the Secretome of Mesenchymal Stem Cells and Its Potential Applications. Int. J. Mol. Sci. 2019, 20, 4597. [Google Scholar] [CrossRef]
- Johnson, T.V.; DeKorver, N.W.; Levasseur, V.A.; Osborne, A.; Tassoni, A.; Lorber, B.; Heller, J.P.; Villasmil, R.; Bull, N.D.; Martin, K.R.; et al. Identification of retinal ganglion cell neuroprotection conferred by platelet-derived growth factor through analysis of the mesenchymal stem cell secretome. Brain 2014, 137, 503–519. [Google Scholar] [CrossRef]
- Sze, S.K.; de Kleijn, D.P.; Lai, R.C.; Tan, E.K.W.; Zhao, H.; Yeo, K.S.; Low, T.Y.; Lian, Q.; Lee, C.N.; Mitchell, W.; et al. Elucidating the secretion proteome of human embryonic stem cell-derived mesenchymal stem cells. Mol. Cell. Proteom. 2007, 6, 1680–1689. [Google Scholar] [CrossRef]
- Chouw, A.; Sartika, C.R.; Milanda, T.; Faried, A. Interleukins Profiling in Umbilical Cord Mesenchymal Stem Cell-Derived Secretome. Stem Cells Cloning Adv. Appl. 2022, 15, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Du, Z.; Zhao, L.; Feng, D.; Wei, G.; He, Y.; Tan, J.; Lee, W.H.; Hampel, H.; Dodel, R.; et al. IFATS collection: The conditioned media of adipose stromal cells protect against hypoxia-ischemia-induced brain damage in neonatal rats. Stem Cells 2009, 27, 478–488. [Google Scholar] [CrossRef] [PubMed]
- Chuang, T.J.; Lin, K.C.; Chio, C.C.; Wang, C.C.; Chang, C.P.; Kuo, J.R. Effects of secretome obtained from normoxia-preconditioned human mesenchymal stem cells in traumatic brain injury rats. J. Trauma Acute Care Surg. 2012, 73, 1161–1167. [Google Scholar] [CrossRef]
- Liu, Z.; Cai, H.; Zhang, P.; Li, H.; Liu, H.; Li, Z. Activation of ERK1/2 and PI3K/Akt by IGF-1 on GAP-43 expression in DRG neurons with excitotoxicity induced by glutamate in vitro. Cell. Mol. Neurobiol. 2012, 32, 191–200. [Google Scholar] [CrossRef]
- Teixeira, F.G.; Carvalho, M.M.; Panchalingam, K.M.; Rodrigues, A.J.; Mendes-Pinheiro, B.; Anjo, S.; Manadas, B.; Behie, L.A.; Sousa, N.; Salgado, A.J. Impact of the Secretome of Human Mesenchymal Stem Cells on Brain Structure and Animal Behavior in a Rat Model of Parkinson’s Disease. Stem Cells Transl. Med. 2017, 6, 634–646. [Google Scholar] [CrossRef]
- Del Fattore, A.; Luciano, R.; Pascucci, L.; Goffredo, B.M.; Giorda, E.; Scapaticci, M.; Fierabracci, A.; Muraca, M. Immunoregulatory Effects of Mesenchymal Stem Cell-Derived Extracellular Vesicles on T Lymphocytes. Cell Transplant. 2015, 24, 2615–2627. [Google Scholar] [CrossRef]
- Nakanishi, C.; Nagaya, N.; Ohnishi, S.; Yamahara, K.; Takabatake, S.; Konno, T.; Hayashi, K.; Kawashiri, M.A.; Tsubokawa, T.; Yamagishi, M. Gene and protein expression analysis of mesenchymal stem cells derived from rat adipose tissue and bone marrow. Circ. J. 2011, 75, 2260–2268. [Google Scholar] [CrossRef]
- Liu, Y.; Ren, H.; Wang, J.; Yang, F.; Li, J.; Zhou, Y.; Yuan, X.; Zhu, W.; Shi, X. Prostaglandin E(2) secreted by mesenchymal stem cells protects against acute liver failure via enhancing hepatocyte proliferation. FASEB J. 2019, 33, 2514–2525. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Perez, E.; Gonzalez-Pujana, A.; Igartua, M.; Santos-Vizcaino, E.; Hernandez, R.M. Mesenchymal Stromal Cell Secretome for the Treatment of Immune-Mediated Inflammatory Diseases: Latest Trends in Isolation, Content Optimization and Delivery Avenues. Pharmaceutics 2021, 13, 1802. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.W.; Wei, P.; Zhang, G.J.; Yan, J.X.; Zhang, S.; Liang, J.; Wang, X.L. Intravenous infusion of the exosomes derived from human umbilical cord mesenchymal stem cells enhance neurological recovery after traumatic brain injury via suppressing the NF-κB pathway. Open Life Sci. 2022, 17, 189–201. [Google Scholar] [CrossRef]
- Zhou, O.; You, J.; Xu, X.; Liu, J.; Qiu, H.; Hao, C.; Zou, W.; Wu, W.; Fu, Z.; Tian, D.; et al. Microvesicles Derived from Human Umbilical Cord Mesenchymal Stem Cells Enhance Alveolar Type II Cell Proliferation and Attenuate Lung Inflammation in a Rat Model of Bronchopulmonary Dysplasia. Stem Cells Int. 2022, 2022, 8465294. [Google Scholar] [CrossRef]
- Shiue, S.J.; Rau, R.H.; Shiue, H.S.; Hung, Y.W.; Li, Z.X.; Yang, K.D.; Cheng, J.K. Mesenchymal stem cell exosomes as a cell-free therapy for nerve injury-induced pain in rats. Pain 2019, 160, 210–223. [Google Scholar] [CrossRef]
- Wen, L.; Wang, Y.D.; Shen, D.F.; Zheng, P.D.; Tu, M.D.; You, W.D.; Zhu, Y.R.; Wang, H.; Feng, J.F.; Yang, X.F. Exosomes derived from bone marrow mesenchymal stem cells inhibit neuroinflammation after traumatic brain injury. Neural Regen. Res. 2022, 17, 2717–2724. [Google Scholar] [CrossRef]
- Li, Y.; Yang, Y.Y.; Ren, J.L.; Xu, F.; Chen, F.M.; Li, A. Exosomes secreted by stem cells from human exfoliated deciduous teeth contribute to functional recovery after traumatic brain injury by shifting microglia M1/M2 polarization in rats. Stem Cell Res. Ther. 2017, 8, 198. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chopp, M.; Zhang, Z.G.; Katakowski, M.; Xin, H.; Qu, C.; Ali, M.; Mahmood, A.; Xiong, Y. Systemic administration of cell-free exosomes generated by human bone marrow derived mesenchymal stem cells cultured under 2D and 3D conditions improves functional recovery in rats after traumatic brain injury. Neurochem. Int. 2017, 111, 69–81. [Google Scholar] [CrossRef]
- Jeppesen, D.K.; Fenix, A.M.; Franklin, J.L.; Higginbotham, J.N.; Zhang, Q.; Zimmerman, L.J.; Liebler, D.C.; Ping, J.; Liu, Q.; Evans, R.; et al. Reassessment of Exosome Composition. Cell 2019, 177, 428–445.e418. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Zhang, J.; Buller, B.A.; Zhang, Z.G.; Zhang, Y.; Lu, M.; Rosene, D.L.; Medalla, M.; Moore, T.L.; Chopp, M. Exosomes derived from bone marrow mesenchymal stromal cells promote remyelination and reduce neuroinflammation in the demyelinating central nervous system. Exp. Neurol. 2022, 347, 113895. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Ding, J.; Li, Y.; Liu, W.; Ji, J.; Wang, H.; Wang, X. Exosomes derived from PEDF modified adipose-derived mesenchymal stem cells ameliorate cerebral ischemia-reperfusion injury by regulation of autophagy and apoptosis. Exp. Cell Res. 2018, 371, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Baez-Jurado, E.; Hidalgo-Lanussa, O.; Barrera-Bailon, B.; Sahebkar, A.; Ashraf, G.M.; Echeverria, V.; Barreto, G.E. Secretome of Mesenchymal Stem Cells and Its Potential Protective Effects on Brain Pathologies. Mol. Neurobiol. 2019, 56, 6902–6927. [Google Scholar] [CrossRef]
- Harman, R.M.; Marx, C.; Van de Walle, G.R. Translational Animal Models Provide Insight Into Mesenchymal Stromal Cell (MSC) Secretome Therapy. Front. Cell Dev. Biol. 2021, 9, 654885. [Google Scholar] [CrossRef] [PubMed]
- McKelvey, K.J.; Powell, K.L.; Ashton, A.W.; Morris, J.M.; McCracken, S.A. Exosomes: Mechanisms of Uptake. J. Circ. Biomark. 2015, 4, 7. [Google Scholar] [CrossRef] [PubMed]
- Rani, S.; Ryan, A.E.; Griffin, M.D.; Ritter, T. Mesenchymal Stem Cell-derived Extracellular Vesicles: Toward Cell-free Therapeutic Applications. Mol. Ther. J. Am. Soc. Gene Ther. 2015, 23, 812–823. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, X.; Wei, M.; Gao, X.; Zhao, L.; Shi, R.; Sun, W.; Duan, Y.; Yang, G.; Yuan, L. In Vitro and in Vivo RNA Inhibition by CD9-HuR Functionalized Exosomes Encapsulated with miRNA or CRISPR/dCas9. Nano Lett. 2019, 19, 19–28. [Google Scholar] [CrossRef]
- Yáñez-Mó, M.; Siljander, P.R.; Andreu, Z.; Zavec, A.B.; Borràs, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef]
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef]
- Xu, T.; Lin, Y.; Yu, X.; Jiang, G.; Wang, J.; Xu, K.; Fang, J.; Wang, S.; Dai, X. Comparative Effects of Exosomes and Ectosomes Isolated From Adipose-Derived Mesenchymal Stem Cells on Achilles Tendinopathy in a Rat Model. Am. J. Sports Med. 2022, 50, 2740–2752. [Google Scholar] [CrossRef] [PubMed]
- Aqmasheh, S.; Shamsasenjan, K.; Khalaf Adeli, E.; Movassaghpourakbari, A.; Akbarzadehlaleh, P.; Pashoutan Sarvar, D.; Timari, H. Effect of Mesenchymal Stem Cell-derived Microvesicles on Megakaryocytic Differentiation of CD34(+) Hematopoietic Stem Cells. Adv. Pharm. Bull. 2020, 10, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Liao, G.; Wang, X.; Li, L.; Zhang, J.; Chen, Y.; Liu, J.; Liu, S.; Wei, L.; Zhang, W.; et al. Mesenchymal stem cells-microvesicle-miR-451a ameliorate early diabetic kidney injury by negative regulation of P15 and P19. Exp. Biol. Med. 2018, 243, 1233–1242. [Google Scholar] [CrossRef] [PubMed]
- Fiedler, T.; Rabe, M.; Mundkowski, R.G.; Oehmcke-Hecht, S.; Peters, K. Adipose-derived mesenchymal stem cells release microvesicles with procoagulant activity. Int. J. Biochem. Cell Biol. 2018, 100, 49–53. [Google Scholar] [CrossRef]
- Tofiño-Vian, M.; Guillén, M.I.; Pérez Del Caz, M.D.; Silvestre, A.; Alcaraz, M.J. Microvesicles from Human Adipose Tissue-Derived Mesenchymal Stem Cells as a New Protective Strategy in Osteoarthritic Chondrocytes. Cell. Physiol. Biochem. 2018, 47, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Mokarizadeh, A.; Delirezh, N.; Morshedi, A.; Mosayebi, G.; Farshid, A.A.; Mardani, K. Microvesicles derived from mesenchymal stem cells: Potent organelles for induction of tolerogenic signaling. Immunol. Lett. 2012, 147, 47–54. [Google Scholar] [CrossRef]
- Muralidharan-Chari, V.; Clancy, J.W.; Sedgwick, A.; D’Souza-Schorey, C. Microvesicles: Mediators of extracellular communication during cancer progression. J. Cell Sci. 2010, 123, 1603–1611. [Google Scholar] [CrossRef]
- Baglio, S.R.; Rooijers, K.; Koppers-Lalic, D.; Verweij, F.J.; Perez Lanzon, M.; Zini, N.; Naaijkens, B.; Perut, F.; Niessen, H.W.; Baldini, N.; et al. Human bone marrow- and adipose-mesenchymal stem cells secrete exosomes enriched in distinctive miRNA and tRNA species. Stem Cell Res. Ther. 2015, 6, 127. [Google Scholar] [CrossRef]
- Gong, M.; Yu, B.; Wang, J.; Wang, Y.; Liu, M.; Paul, C.; Millard, R.W.; Xiao, D.S.; Ashraf, M.; Xu, M. Mesenchymal stem cells release exosomes that transfer miRNAs to endothelial cells and promote angiogenesis. Oncotarget 2017, 8, 45200–45212. [Google Scholar] [CrossRef]
- Ferguson, S.W.; Wang, J.; Lee, C.J.; Liu, M.; Neelamegham, S.; Canty, J.M.; Nguyen, J. The microRNA regulatory landscape of MSC-derived exosomes: A systems view. Sci. Rep. 2018, 8, 1419. [Google Scholar] [CrossRef]
- Ramot, Y.; Steiner, M.; Morad, V.; Leibovitch, S.; Amouyal, N.; Cesta, M.F.; Nyska, A. Pulmonary thrombosis in the mouse following intravenous administration of quantum dot-labeled mesenchymal cells. Nanotoxicology 2010, 4, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Ben-David, U.; Mayshar, Y.; Benvenisty, N. Large-scale analysis reveals acquisition of lineage-specific chromosomal aberrations in human adult stem cells. Cell Stem Cell 2011, 9, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.G.; Tung, L.; Sekar, R.B.; Chang, C.Y.; Cysyk, J.; Dong, P.; Marbán, E.; Abraham, M.R. Proarrhythmic potential of mesenchymal stem cell transplantation revealed in an in vitro coculture model. Circulation 2006, 113, 1832–1841. [Google Scholar] [CrossRef]
- Breitbach, M.; Bostani, T.; Roell, W.; Xia, Y.; Dewald, O.; Nygren, J.M.; Fries, J.W.; Tiemann, K.; Bohlen, H.; Hescheler, J.; et al. Potential risks of bone marrow cell transplantation into infarcted hearts. Blood 2007, 110, 1362–1369. [Google Scholar] [CrossRef] [PubMed]
- Moll, G.; Alm, J.J.; Davies, L.C.; von Bahr, L.; Heldring, N.; Stenbeck-Funke, L.; Hamad, O.A.; Hinsch, R.; Ignatowicz, L.; Locke, M.; et al. Do cryopreserved mesenchymal stromal cells display impaired immunomodulatory and therapeutic properties? Stem Cells 2014, 32, 2430–2442. [Google Scholar] [CrossRef]
- Ferreira, J.R.; Teixeira, G.Q.; Santos, S.G.; Barbosa, M.A.; Almeida-Porada, G.; Goncalves, R.M. Mesenchymal Stromal Cell Secretome: Influencing Therapeutic Potential by Cellular Pre-conditioning. Front. Immunol. 2018, 9, 2837. [Google Scholar] [CrossRef]
- Harrell, C.R.; Fellabaum, C.; Jovicic, N.; Djonov, V.; Arsenijevic, N.; Volarevic, V. Molecular Mechanisms Responsible for Therapeutic Potential of Mesenchymal Stem Cell-Derived Secretome. Cells 2019, 8, 467. [Google Scholar] [CrossRef]
- Siracusa, R.; Fusco, R.; Cuzzocrea, S. Astrocytes: Role and Functions in Brain Pathologies. Front. Pharmacol. 2019, 10, 1114. [Google Scholar] [CrossRef]
- Shoichet, M.S.; Tate, C.C.; Baumann, M.D.; LaPlaca, M.C. Frontiers in Neuroengineering Strategies for Regeneration and Repair in the Injured Central Nervous System. In Indwelling Neural Implants: Strategies for Contending with the In Vivo Environment; Reichert, W.M., Ed.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2008. [Google Scholar]
- Goldman, L.; Siddiqui, E.M.; Khan, A.; Jahan, S.; Rehman, M.U.; Mehan, S.; Sharma, R.; Budkin, S.; Kumar, S.N.; Sahu, A.; et al. Understanding Acquired Brain Injury: A Review. Biomedicines 2022, 10, 2167. [Google Scholar] [CrossRef]
- Dahbour, S.; Jamali, F.; Alhattab, D.; Al-Radaideh, A.; Ababneh, O.; Al-Ryalat, N.; Al-Bdour, M.; Hourani, B.; Msallam, M.; Rasheed, M.; et al. Mesenchymal stem cells and conditioned media in the treatment of multiple sclerosis patients: Clinical, ophthalmological and radiological assessments of safety and efficacy. CNS Neurosci. Ther. 2017, 23, 866–874. [Google Scholar] [CrossRef]
- Combination of Conditioned Medium and Umbilical Cord-Mesenchymal Stem Cells Therapy for Acute Stroke Infarct. Available online: https://clinicaltrials.gov/study/NCT05008588?cond=Neurologic%20Disorder&intr=MSCs%20conditioned%20medium&term=Neurological%20Disorder&rank=1 (accessed on 4 September 2023).
- Potential Use of Autologous and Allogeneic Mesenchymal Stem Cells in Patients with Multiple System Atrophy. Available online: https://clinicaltrials.gov/study/NCT04876326?cond=brain&intr=secretome&term=Neurological%20Disorder&rank=2 (accessed on 4 September 2023).
- Stem Cell and Conditioned Medium for Cerebral Palsy. Available online: https://clinicaltrials.gov/study/NCT04314687?cond=brain&intr=conditioned%20medium&term=Neurological%20Disorder&rank=1 (accessed on 4 September 2023).
- Allogenic Mesenchymal Stem Cell Derived Exosome in Patients with Acute Ischemic Stroke. Available online: https://clinicaltrials.gov/study/NCT03384433?cond=Neurologic%20Disorder&intr=exosomes&term=Neurological%20Disorder&rank=3 (accessed on 4 September 2023).
- The Safety and the Efficacy Evaluation of Allogenic Adipose MSC-Exos in Patients with Alzheimer’s Disease. Available online: https://clinicaltrials.gov/study/NCT04388982?cond=Neurologic%20Disorder&intr=MSCs%20exosomes&term=Neurological%20Disorder&rank=1 (accessed on 4 September 2023).
- The Pilot Experimental Study of the Neuroprotective Effects of Exosomes in Extremely Low Birth Weight Infants. Available online: https://clinicaltrials.gov/study/NCT05490173?cond=Neurologic%20Disorder&intr=MSCs%20exosomes&term=Neurological%20Disorder&rank=2 (accessed on 4 September 2023).
- Santamaria, G.; Brandi, E.; Vitola, P.; Grandi, F.; Ferrara, G.; Pischiutta, F.; Vegliante, G.; Zanier, E.R.; Re, F.; Uccelli, A.; et al. Intranasal delivery of mesenchymal stem cell secretome repairs the brain of Alzheimer’s mice. Cell Death Differ. 2021, 28, 203–218. [Google Scholar] [CrossRef] [PubMed]
- Mita, T.; Furukawa-Hibi, Y.; Takeuchi, H.; Hattori, H.; Yamada, K.; Hibi, H.; Ueda, M.; Yamamoto, A. Conditioned medium from the stem cells of human dental pulp improves cognitive function in a mouse model of Alzheimer’s disease. Behav. Brain Res. 2015, 293, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Venugopal, C.; Shobha, K.; Rai, K.S.; Dhanushkodi, A. Neurogenic and cognitive enhancing effects of human dental pulp stem cells and its secretome in animal model of hippocampal neurodegeneration. Brain Res. Bull. 2022, 180, 46–58. [Google Scholar] [CrossRef]
- Wang, X.; Yang, G. Bone marrow mesenchymal stem cells-derived exosomes reduce Aβ deposition and improve cognitive function recovery in mice with Alzheimer’s disease by activating sphingosine kinase/sphingosine-1-phosphate signaling pathway. Cell Biol. Int. 2021, 45, 775–784. [Google Scholar] [CrossRef]
- Liu, S.; Fan, M.; Xu, J.X.; Yang, L.J.; Qi, C.C.; Xia, Q.R.; Ge, J.F. Exosomes derived from bone-marrow mesenchymal stem cells alleviate cognitive decline in AD-like mice by improving BDNF-related neuropathology. J. Neuroinflammation 2022, 19, 35. [Google Scholar] [CrossRef]
- Ding, M.; Shen, Y.; Wang, P.; Xie, Z.; Xu, S.; Zhu, Z.; Wang, Y.; Lyu, Y.; Wang, D.; Xu, L.; et al. Exosomes Isolated From Human Umbilical Cord Mesenchymal Stem Cells Alleviate Neuroinflammation and Reduce Amyloid-Beta Deposition by Modulating Microglial Activation in Alzheimer’s Disease. Neurochem. Res. 2018, 43, 2165–2177. [Google Scholar] [CrossRef]
- Niu, Y.; Wang, X.; Li, M.; Niu, B. Exosomes from human umbilical cord Mesenchymal stem cells attenuates stress-induced hippocampal dysfunctions. Metab. Brain Dis. 2020, 35, 1329–1340. [Google Scholar] [CrossRef]
- Diniz, B.S.; Teixeira, A.L.; Campos, A.C.; Miranda, A.S.; Rocha, N.P.; Talib, L.L.; Gattaz, W.F.; Forlenza, O.V. Reduced serum levels of adiponectin in elderly patients with major depression. J. Psychiatr. Res. 2012, 46, 1081–1085. [Google Scholar] [CrossRef] [PubMed]
- Borhani-Haghighi, M.; Mohamadi, Y. Intranasal administration of conditioned medium derived from mesenchymal stem cells-differentiated oligodendrocytes ameliorates experimental autoimmune encephalomyelitis. J. Chem. Neuroanat. 2020, 106, 101792. [Google Scholar] [CrossRef]
- Xue, C.; Li, X.; Ba, L.; Zhang, M.; Yang, Y.; Gao, Y.; Sun, Z.; Han, Q.; Zhao, R.C. MSC-Derived Exosomes can Enhance the Angiogenesis of Human Brain MECs and Show Therapeutic Potential in a Mouse Model of Parkinson’s Disease. Aging Dis. 2021, 12, 1211–1222. [Google Scholar] [CrossRef]
- Chen, H.X.; Liang, F.C.; Gu, P.; Xu, B.L.; Xu, H.J.; Wang, W.T.; Hou, J.Y.; Xie, D.X.; Chai, X.Q.; An, S.J. Exosomes derived from mesenchymal stem cells repair a Parkinson’s disease model by inducing autophagy. Cell Death Dis. 2020, 11, 288. [Google Scholar] [CrossRef]
- Donnan, G.A.; Fisher, M.; Macleod, M.; Davis, S.M. Stroke. Lancet 2008, 371, 1612–1623. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Hu, J.; Xiang, J.; Gu, Y.; Jin, P.; Hua, F.; Zhang, Z.; Liu, Y.; Zan, K.; Zhang, Z.; et al. Intranasal administration of human umbilical cord mesenchymal stem cells-conditioned medium enhances vascular remodeling after stroke. Brain Res. 2015, 1624, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Sugiyama, M.; Hattori, H.; Wakita, H.; Wakabayashi, T.; Ueda, M. Stem cells from human exfoliated deciduous tooth-derived conditioned medium enhance recovery of focal cerebral ischemia in rats. Tissue Eng. Part. A 2013, 19, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.J.; Tsai, S.K.; Hu, B.R.; Liou, D.Y.; Huang, S.L.; Huang, M.C.; Huang, W.C.; Cheng, H.; Huang, S.S. Recovery of neurological function of ischemic stroke by application of conditioned medium of bone marrow mesenchymal stem cells derived from normal and cerebral ischemia rats. J. Biomed. Sci. 2014, 21, 5. [Google Scholar] [CrossRef] [PubMed]
- Faezi, M.; Nasseri Maleki, S.; Aboutaleb, N.; Nikougoftar, M. The membrane mesenchymal stem cell derived conditioned medium exerts neuroprotection against focal cerebral ischemia by targeting apoptosis. J. Chem. Neuroanat. 2018, 94, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Asgari Taei, A.; Nasoohi, S.; Hassanzadeh, G.; Kadivar, M.; Dargahi, L.; Farahmandfar, M. Enhancement of angiogenesis and neurogenesis by intracerebroventricular injection of secretome from human embryonic stem cell-derived mesenchymal stem cells in ischemic stroke model. Biomed. Pharmacother. 2021, 140, 111709. [Google Scholar] [CrossRef]
- Chen, K.H.; Chen, C.H.; Wallace, C.G.; Yuen, C.M.; Kao, G.S.; Chen, Y.L.; Shao, P.L.; Chen, Y.L.; Chai, H.T.; Lin, K.C.; et al. Intravenous administration of xenogenic adipose-derived mesenchymal stem cells (ADMSC) and ADMSC-derived exosomes markedly reduced brain infarct volume and preserved neurological function in rat after acute ischemic stroke. Oncotarget 2016, 7, 74537–74556. [Google Scholar] [CrossRef]
- Xu, R.; Bai, Y.; Min, S.; Xu, X.; Tang, T.; Ju, S. In vivo Monitoring and Assessment of Exogenous Mesenchymal Stem Cell-Derived Exosomes in Mice with Ischemic Stroke by Molecular Imaging. Int. J. Nanomed. 2020, 15, 9011–9023. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, M.; Liu, H.; Zhu, R.; He, H.; Zhou, Y.; Zhang, Y.; Li, C.; Liang, D.; Zeng, Q.; et al. Bone marrow mesenchymal stem cell-derived exosomes attenuate cerebral ischemia-reperfusion injury-induced neuroinflammation and pyroptosis by modulating microglia M1/M2 phenotypes. Exp. Neurol. 2021, 341, 113700. [Google Scholar] [CrossRef]
- Wang, C.; Börger, V.; Mohamud Yusuf, A.; Tertel, T.; Stambouli, O.; Murke, F.; Freund, N.; Kleinschnitz, C.; Herz, J.; Gunzer, M.; et al. Postischemic Neuroprotection Associated With Anti-Inflammatory Effects by Mesenchymal Stromal Cell-Derived Small Extracellular Vesicles in Aged Mice. Stroke 2022, 53, e14–e18. [Google Scholar] [CrossRef] [PubMed]
- Nalamolu, K.R.; Venkatesh, I.; Mohandass, A.; Klopfenstein, J.D.; Pinson, D.M.; Wang, D.Z.; Veeravalli, K.K. Exosomes Treatment Mitigates Ischemic Brain Damage but Does Not Improve Post-Stroke Neurological Outcome. Cell. Physiol. Biochem. 2019, 52, 1280–1291. [Google Scholar] [CrossRef] [PubMed]
- Dumbrava, D.A.; Surugiu, R.; Börger, V.; Ruscu, M.; Tertel, T.; Giebel, B.; Hermann, D.M.; Popa-Wagner, A. Mesenchymal stromal cell-derived small extracellular vesicles promote neurological recovery and brain remodeling after distal middle cerebral artery occlusion in aged rats. GeroScience 2022, 44, 293–310. [Google Scholar] [CrossRef]
- Doeppner, T.R.; Herz, J.; Görgens, A.; Schlechter, J.; Ludwig, A.K.; Radtke, S.; de Miroschedji, K.; Horn, P.A.; Giebel, B.; Hermann, D.M. Extracellular Vesicles Improve Post-Stroke Neuroregeneration and Prevent Postischemic Immunosuppression. Stem Cells Transl. Med. 2015, 4, 1131–1143. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Pong, K.U.; Yang, F.; Ji, Z.; Lin, J.; Weng, Z.; Tsang, L.L.; Merson, T.D.; Ruan, Y.C.; Wan, C.; et al. Human pluripotent stem cell-derived ectomesenchymal stromal cells promote more robust functional recovery than umbilical cord-derived mesenchymal stromal cells after hypoxic-ischaemic brain damage. Theranostics 2022, 12, 143–166. [Google Scholar] [CrossRef]
- Ophelders, D.R.; Wolfs, T.G.; Jellema, R.K.; Zwanenburg, A.; Andriessen, P.; Delhaas, T.; Ludwig, A.K.; Radtke, S.; Peters, V.; Janssen, L.; et al. Mesenchymal Stromal Cell-Derived Extracellular Vesicles Protect the Fetal Brain After Hypoxia-Ischemia. Stem Cells Transl. Med. 2016, 5, 754–763. [Google Scholar] [CrossRef]
- Thomi, G.; Joerger-Messerli, M.; Haesler, V.; Muri, L.; Surbek, D.; Schoeberlein, A. Intranasally Administered Exosomes from Umbilical Cord Stem Cells Have Preventive Neuroprotective Effects and Contribute to Functional Recovery after Perinatal Brain Injury. Cells 2019, 8, 855. [Google Scholar] [CrossRef]
- Shu, J.; Jiang, L.; Wang, M.; Wang, R.; Wang, X.; Gao, C.; Xia, Z. Human bone marrow mesenchymal stem cells-derived exosomes protect against nerve injury via regulating immune microenvironment in neonatal hypoxic-ischemic brain damage model. Immunobiology 2022, 227, 152178. [Google Scholar] [CrossRef]
- Thomi, G.; Surbek, D.; Haesler, V.; Joerger-Messerli, M.; Schoeberlein, A. Exosomes derived from umbilical cord mesenchymal stem cells reduce microglia-mediated neuroinflammation in perinatal brain injury. Stem Cell Res. Ther. 2019, 10, 105. [Google Scholar] [CrossRef]
- Drommelschmidt, K.; Serdar, M.; Bendix, I.; Herz, J.; Bertling, F.; Prager, S.; Keller, M.; Ludwig, A.K.; Duhan, V.; Radtke, S.; et al. Mesenchymal stem cell-derived extracellular vesicles ameliorate inflammation-induced preterm brain injury. Brain Behav. Immun. 2017, 60, 220–232. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, G.; Wei, P.; Zhong, L.; Chen, Y.; Zhang, J.; Chen, X.; Zhou, L. Three-dimensional-printed collagen/chitosan/secretome derived from HUCMSCs scaffolds for efficient neural network reconstruction in canines with traumatic brain injury. Regen. Biomater. 2022, 9, rbac043. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.P.; Chio, C.C.; Cheong, C.U.; Chao, C.M.; Cheng, B.C.; Lin, M.T. Hypoxic preconditioning enhances the therapeutic potential of the secretome from cultured human mesenchymal stem cells in experimental traumatic brain injury. Clin. Sci. 2013, 124, 165–176. [Google Scholar] [CrossRef]
- Liu, X.Y.E.; Park, E.; Barretto, T.; Liu, E.; Ferrier, G.A.; Tavakkoli, J.; Baker, A.J. Effect of Human Umbilical Cord Perivascular Cell-Conditioned Media in an Adult Zebrafish Model of Traumatic Brain Injury. Zebrafish 2020, 17, 177–186. [Google Scholar] [CrossRef]
- Williams, A.M.; Bhatti, U.F.; Brown, J.F.; Biesterveld, B.E.; Kathawate, R.G.; Graham, N.J.; Chtraklin, K.; Siddiqui, A.Z.; Dekker, S.E.; Andjelkovic, A.; et al. Early single-dose treatment with exosomes provides neuroprotection and improves blood-brain barrier integrity in swine model of traumatic brain injury and hemorrhagic shock. J. Trauma Acute Care Surg. 2020, 88, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.K.; Nishida, H.; An, S.Y.; Shetty, A.K.; Bartosh, T.J.; Prockop, D.J. Chromatographically isolated CD63+CD81+ extracellular vesicles from mesenchymal stromal cells rescue cognitive impairments after TBI. Proc. Natl. Acad. Sci. USA 2016, 113, 170–175. [Google Scholar] [CrossRef]
- Zhang, Y.; Chopp, M.; Meng, Y.; Katakowski, M.; Xin, H.; Mahmood, A.; Xiong, Y. Effect of exosomes derived from multipluripotent mesenchymal stromal cells on functional recovery and neurovascular plasticity in rats after traumatic brain injury. J. Neurosurg. 2015, 122, 856–867. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Y.; Chopp, M.; Zhang, Z.G.; Mahmood, A.; Xiong, Y. Mesenchymal Stem Cell-Derived Exosomes Improve Functional Recovery in Rats After Traumatic Brain Injury: A Dose-Response and Therapeutic Window Study. Neurorehabilit. Neural Repair 2020, 34, 616–626. [Google Scholar] [CrossRef]
- Ni, H.; Yang, S.; Siaw-Debrah, F.; Hu, J.; Wu, K.; He, Z.; Yang, J.; Pan, S.; Lin, X.; Ye, H.; et al. Exosomes Derived From Bone Mesenchymal Stem Cells Ameliorate Early Inflammatory Responses Following Traumatic Brain Injury. Front. Neurosci. 2019, 13, 14. [Google Scholar] [CrossRef]
- Chen, Y.; Li, J.; Ma, B.; Li, N.; Wang, S.; Sun, Z.; Xue, C.; Han, Q.; Wei, J.; Zhao, R.C. MSC-derived exosomes promote recovery from traumatic brain injury via microglia/macrophages in rat. Aging 2020, 12, 18274–18296. [Google Scholar] [CrossRef]
- Zhuang, Z.; Liu, M.; Luo, J.; Zhang, X.; Dai, Z.; Zhang, B.; Chen, H.; Xue, J.; He, M.; Xu, H.; et al. Exosomes derived from bone marrow mesenchymal stem cells attenuate neurological damage in traumatic brain injury by alleviating glutamate-mediated excitotoxicity. Exp. Neurol. 2022, 357, 114182. [Google Scholar] [CrossRef]
- Cui, L.; Luo, W.; Jiang, W.; Li, H.; Xu, J.; Liu, X.; Wang, B.; Wang, J.; Chen, G. Human umbilical cord mesenchymal stem cell-derived exosomes promote neurological function recovery in rat after traumatic brain injury by inhibiting the activation of microglia and astrocyte. Regen. Ther. 2022, 21, 282–287. [Google Scholar] [CrossRef]
- Williams, A.M.; Higgins, G.A.; Bhatti, U.F.; Biesterveld, B.E.; Dekker, S.E.; Kathawate, R.G.; Tian, Y.; Wu, Z.; Kemp, M.T.; Wakam, G.K.; et al. Early treatment with exosomes following traumatic brain injury and hemorrhagic shock in a swine model promotes transcriptional changes associated with neuroprotection. J. Trauma Acute Care Surg. 2020, 89, 536–543. [Google Scholar] [CrossRef]
- Williams, A.M.; Wu, Z.; Bhatti, U.F.; Biesterveld, B.E.; Kemp, M.T.; Wakam, G.K.; Vercruysse, C.A.; Chtraklin, K.; Siddiqui, A.Z.; Pickell, Z.; et al. Early single-dose exosome treatment improves neurologic outcomes in a 7-day swine model of traumatic brain injury and hemorrhagic shock. J. Trauma Acute Care Surg. 2020, 89, 388–396. [Google Scholar] [CrossRef]
- Campos, J.; Guerra-Gomes, S.; Serra, S.C.; Baltazar, G.; Oliveira, J.F.; Teixeira, F.G.; Salgado, A.J. Astrocyte signaling impacts the effects of human bone marrow mesenchymal stem cells secretome application into the hippocampus: A proliferation and morphometrical analysis on astrocytic cell populations. Brain Res. 2020, 1732, 146700. [Google Scholar] [CrossRef]
- Cizkova, D.; Cubinkova, V.; Smolek, T.; Murgoci, A.N.; Danko, J.; Vdoviakova, K.; Humenik, F.; Cizek, M.; Quanico, J.; Fournier, I.; et al. Localized Intrathecal Delivery of Mesenchymal Stromal Cells Conditioned Medium Improves Functional Recovery in a Rat Model of Spinal Cord Injury. Int. J. Mol. Sci. 2018, 19, 870. [Google Scholar] [CrossRef]
- Gama, K.B.; Santos, D.S.; Evangelista, A.F.; Silva, D.N.; de Alcantara, A.C.; Dos Santos, R.R.; Soares, M.B.P.; Villarreal, C.F. Conditioned Medium of Bone Marrow-Derived Mesenchymal Stromal Cells as a Therapeutic Approach to Neuropathic Pain: A Preclinical Evaluation. Stem Cells Int. 2018, 2018, 8179013. [Google Scholar] [CrossRef]
- Nose, N.; Nogami, S.; Koshino, K.; Chen, X.; Werner, R.A.; Kashima, S.; Rowe, S.P.; Lapa, C.; Fukuchi, K.; Higuchi, T. [18F]FDG-labelled stem cell PET imaging in different route of administrations and multiple animal species. Sci. Rep. 2021, 11, 10896. [Google Scholar] [CrossRef]
- Zhang, Y.; Chopp, M.; Liu, X.S.; Katakowski, M.; Wang, X.; Tian, X.; Wu, D.; Zhang, Z.G. Exosomes Derived from Mesenchymal Stromal Cells Promote Axonal Growth of Cortical Neurons. Mol. Neurobiol. 2017, 54, 2659–2673. [Google Scholar] [CrossRef]
- Cone, A.S.; Yuan, X.; Sun, L.; Duke, L.C.; Vreones, M.P.; Carrier, A.N.; Kenyon, S.M.; Carver, S.R.; Benthem, S.D.; Stimmell, A.C.; et al. Mesenchymal stem cell-derived extracellular vesicles ameliorate Alzheimer’s disease-like phenotypes in a preclinical mouse model. Theranostics 2021, 11, 8129–8142. [Google Scholar] [CrossRef]
- Chen, C.; Xu, H.H.; Liu, X.Y.; Zhang, Y.S.; Zhong, L.; Wang, Y.W.; Xu, L.; Wei, P.; Chen, Y.X.; Liu, P.; et al. 3D printed collagen/silk fibroin scaffolds carrying the secretome of human umbilical mesenchymal stem cells ameliorated neurological dysfunction after spinal cord injury in rats. Regen. Biomater. 2022, 9, rbac014. [Google Scholar] [CrossRef]
- Dai, Y.; Li, W.D.; Zhong, M.; Chen, J.; Cheng, Q.; Liu, Y.X.; Li, T.Y. The paracrine effect of cobalt chloride on BMSCs during cognitive function rescue in the HIBD rat. Behav. Brain Res. 2017, 332, 99–109. [Google Scholar] [CrossRef]
- Tsai, L.K.; Wang, Z.; Munasinghe, J.; Leng, Y.; Leeds, P.; Chuang, D.M. Mesenchymal stem cells primed with valproate and lithium robustly migrate to infarcted regions and facilitate recovery in a stroke model. Stroke 2011, 42, 2932–2939. [Google Scholar] [CrossRef]
- Shen, H.; Gu, X.; Wei, Z.Z.; Wu, A.; Liu, X.; Wei, L. Combinatorial intranasal delivery of bone marrow mesenchymal stem cells and insulin-like growth factor-1 improves neurovascularization and functional outcomes following focal cerebral ischemia in mice. Exp. Neurol. 2021, 337, 113542. [Google Scholar] [CrossRef]
- Losurdo, M.; Pedrazzoli, M.; D’Agostino, C.; Elia, C.A.; Massenzio, F.; Lonati, E.; Mauri, M.; Rizzi, L.; Molteni, L.; Bresciani, E.; et al. Intranasal delivery of mesenchymal stem cell-derived extracellular vesicles exerts immunomodulatory and neuroprotective effects in a 3xTg model of Alzheimer’s disease. Stem Cells Transl. Med. 2020, 9, 1068–1084. [Google Scholar] [CrossRef]
- Cunningham, C.J.; Wong, R.; Barrington, J.; Tamburrano, S.; Pinteaux, E.; Allan, S.M. Systemic conditioned medium treatment from interleukin-1 primed mesenchymal stem cells promotes recovery after stroke. Stem Cell Res. Ther. 2020, 11, 32. [Google Scholar] [CrossRef]
- Cui, G.H.; Wu, J.; Mou, F.F.; Xie, W.H.; Wang, F.B.; Wang, Q.L.; Fang, J.; Xu, Y.W.; Dong, Y.R.; Liu, J.R.; et al. Exosomes derived from hypoxia-preconditioned mesenchymal stromal cells ameliorate cognitive decline by rescuing synaptic dysfunction and regulating inflammatory responses in APP/PS1 mice. FASEB J. 2018, 32, 654–668. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Fraser, J.L.; Lu, Z.Y.; Hu, X.; Yu, S.P. Transplantation of hypoxia preconditioned bone marrow mesenchymal stem cells enhances angiogenesis and neurogenesis after cerebral ischemia in rats. Neurobiol. Dis. 2012, 46, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Bicer, M.; Cottrell, G.S.; Widera, D. Impact of 3D cell culture on bone regeneration potential of mesenchymal stromal cells. Stem Cell Res. Ther. 2021, 12, 31. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Ge, J.; Zhou, Y.; Wang, S.; Zhao, R.C.; Wu, Y. Three-dimensional spheroid-cultured mesenchymal stem cells devoid of embolism attenuate brain stroke injury after intra-arterial injection. Stem Cells Dev. 2014, 23, 978–989. [Google Scholar] [CrossRef]
- Linares, G.R.; Chiu, C.T.; Scheuing, L.; Leng, Y.; Liao, H.M.; Maric, D.; Chuang, D.M. Preconditioning mesenchymal stem cells with the mood stabilizers lithium and valproic acid enhances therapeutic efficacy in a mouse model of Huntington’s disease. Exp. Neurol. 2016, 281, 81–92. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, S.; Li, T.; Yuan, L.; Liu, H.; Wang, X.; Wang, F.; Wang, S.; Hao, A.; Liu, D.; et al. Preconditioning of bone marrow mesenchymal stem cells with hydrogen sulfide improves their therapeutic potential. Oncotarget 2016, 7, 58089–58104. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chu, L.; Fang, Y.; Yang, Y.; Qu, T.; Zhang, J.; Yin, Y.; Gu, J. Preconditioning of bone marrow-derived mesenchymal stromal cells by tetramethylpyrazine enhances cell migration and improves functional recovery after focal cerebral ischemia in rats. Stem Cell Res. Ther. 2017, 8, 112. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.N.; Chen, L.F.; Huang, X.J.; Wu, K.; Ding, S.D.; Wang, W.K.; Wang, B.; Smith, C.; Ren, C.H.; Ni, H.Q.; et al. Calpain inhibitor MDL28170 improves the transplantation-mediated therapeutic effect of bone marrow-derived mesenchymal stem cells following traumatic brain injury. Stem Cell Res. Ther. 2019, 10, 96. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.E.; Sung, S.I.; Chang, Y.S.; Ahn, S.Y.; Sung, D.K.; Park, W.S. Thrombin Preconditioning Enhances Therapeutic Efficacy of Human Wharton’s Jelly-Derived Mesenchymal Stem Cells in Severe Neonatal Hypoxic Ischemic Encephalopathy. Int. J. Mol. Sci. 2019, 20, 2477. [Google Scholar] [CrossRef]
- Noh, J.H.; Jeong, J.S.; Park, S.J.; Jin Jung, K.; Lee, B.S.; Kim, W.J.; Han, J.S.; Cho, M.K.; Sung, D.K.; Ahn, S.Y.; et al. Preclinical assessment of thrombin-preconditioned human Wharton’s jelly-derived mesenchymal stem cells for neonatal hypoxic-ischaemic brain injury. J. Cell. Mol. Med. 2021, 25, 10430–10440. [Google Scholar] [CrossRef]
- Tang, Y.L.; Han, L.L.; Bai, X.C.; Liang, X.N.; Zhao, J.; Huang, F.; Wang, J. Intranasal Delivery of Bone Marrow Stromal Cells Preconditioned with Fasudil to Treat a Mouse Model of Parkinson’s Disease. Neuropsychiatr. Dis. Treat. 2020, 16, 249–262. [Google Scholar] [CrossRef]
- Wise, R.M.; Harrison, M.A.A.; Sullivan, B.N.; Al-Ghadban, S.; Aleman, S.J.; Vinluan, A.T.; Monaco, E.R.; Donato, U.M.; Pursell, I.A.; Bunnell, B.A. Short-Term Rapamycin Preconditioning Diminishes Therapeutic Efficacy of Human Adipose-Derived Stem Cells in a Murine Model of Multiple Sclerosis. Cells 2020, 9, 2218. [Google Scholar] [CrossRef]
- Chen, J.; Lin, X.; Yao, C.; Bingwa, L.A.; Wang, H.; Lin, Z.; Jin, K.; Zhuge, Q.; Yang, S. Transplantation of Roxadustat-preconditioned bone marrow stromal cells improves neurological function recovery through enhancing grafted cell survival in ischemic stroke rats. CNS Neurosci. Ther. 2022, 28, 1519–1531. [Google Scholar] [CrossRef]
- Maadawi, Z.M.E. Conditioned Medium Derived from Salidroside-Pretreated Mesenchymal Stem Cell Culture Ameliorates Mouse Lipopolysaccharide-Induced Cerebral Neuroinflammation- Histological and Immunohistochemical Study. Int. J. Stem Cells 2017, 10, 60–68. [Google Scholar] [CrossRef]
- Umeda, K.; Kotake, Y.; Miyara, M.; Ishida, K.; Sanoh, S.; Ohta, S. Methoxychlor and fenvalerate induce neuronal death by reducing GluR2 expression. J. Toxicol. Sci. 2016, 41, 255–264. [Google Scholar] [CrossRef]
- Boroujeni, F.B.; Pasbakhsh, P.; Mortezaee, K.; Pirhajati, V.; Alizadeh, R.; Aryanpour, R.; Madadi, S.; Kashani, I.R. Intranasal delivery of SDF-1 alpha-preconditioned bone marrow mesenchymal cells improves remyelination in the cuprizone-induced mouse model of multiple sclerosis. Cell Biol. Int. 2020, 44, 499–511. [Google Scholar] [CrossRef]
- Jha, K.A.; Gentry, J.; Del Mar, N.A.; Reiner, A.; Sohl, N.; Gangaraju, R. Adipose Tissue-Derived Mesenchymal Stem Cell Concentrated Conditioned Medium Alters the Expression Pattern of Glutamate Regulatory Proteins and Aquaporin-4 in the Retina after Mild Traumatic Brain Injury. J. Neurotrauma 2021, 38, 1702–1716. [Google Scholar] [CrossRef]
- Jha, K.A.; Pentecost, M.; Lenin, R.; Klaic, L.; Elshaer, S.L.; Gentry, J.; Russell, J.M.; Beland, A.; Reiner, A.; Jotterand, V.; et al. Concentrated Conditioned Media from Adipose Tissue Derived Mesenchymal Stem Cells Mitigates Visual Deficits and Retinal Inflammation Following Mild Traumatic Brain Injury. Int. J. Mol. Sci. 2018, 19, 2016. [Google Scholar] [CrossRef]
- Quintanilla, M.E.; Ezquer, F.; Morales, P.; Santapau, D.; Berrios-Carcamo, P.; Ezquer, M.; Herrera-Marschitz, M.; Israel, Y. Intranasal mesenchymal stem cell secretome administration markedly inhibits alcohol and nicotine self-administration and blocks relapse-intake: Mechanism and translational options. Stem Cell Res. Ther. 2019, 10, 205. [Google Scholar] [CrossRef]
- Farfán, N.; Carril, J.; Redel, M.; Zamorano, M.; Araya, M.; Monzón, E.; Alvarado, R.; Contreras, N.; Tapia-Bustos, A.; Quintanilla, M.E.; et al. Intranasal Administration of Mesenchymal Stem Cell Secretome Reduces Hippocampal Oxidative Stress, Neuroinflammation and Cell Death, Improving the Behavioral Outcome Following Perinatal Asphyxia. Int. J. Mol. Sci. 2020, 21, 7800. [Google Scholar] [CrossRef]
- Xu, H.; Jia, Z.; Ma, K.; Zhang, J.; Dai, C.; Yao, Z.; Deng, W.; Su, J.; Wang, R.; Chen, X. Protective effect of BMSCs-derived exosomes mediated by BDNF on TBI via miR-216a-5p. Med. Sci. Monit. 2020, 26, e920855. [Google Scholar] [CrossRef]
- Karagyaur, M.; Dzhauari, S.; Basalova, N.; Aleksandrushkina, N.; Sagaradze, G.; Danilova, N.; Malkov, P.; Popov, V.; Skryabina, M.; Efimenko, A.; et al. MSC Secretome as a Promising Tool for Neuroprotection and Neuroregeneration in a Model of Intracerebral Hemorrhage. Pharmaceutics 2021, 13, 2031. [Google Scholar] [CrossRef]
- Jha, K.A.; Pentecost, M.; Lenin, R.; Gentry, J.; Klaic, L.; Del Mar, N.; Reiner, A.; Yang, C.H.; Pfeffer, L.M.; Sohl, N.; et al. TSG-6 in conditioned media from adipose mesenchymal stem cells protects against visual deficits in mild traumatic brain injury model through neurovascular modulation. Stem Cell Res. Ther. 2019, 10, 318. [Google Scholar] [CrossRef] [PubMed]
- Verkman, A.S.; Ruiz-Ederra, J.; Levin, M.H. Functions of aquaporins in the eye. Prog. Retin. Eye Res. 2008, 27, 420–433. [Google Scholar] [CrossRef]
- Sun, Y.; Hu, B.; Wang, Y.; Li, Z.; Wu, J.; Yang, Y.; Wei, Y.; Peng, X.; Chen, H.; Chen, R.; et al. miR-216a-5p inhibits malignant progression in small cell lung cancer: Involvement of the Bcl-2 family proteins. Cancer Manag. Res. 2018, 10, 4735–4745. [Google Scholar] [CrossRef]
- Liu, J.; He, J.; Huang, Y.; Ge, L.; Xiao, H.; Zeng, L.; Jiang, Z.; Lu, M.; Hu, Z. Hypoxia-preconditioned mesenchymal stem cells attenuate microglial pyroptosis after intracerebral hemorrhage. Ann. Transl. Med. 2021, 9, 1362. [Google Scholar] [CrossRef]
- Oh, J.S.; Ha, Y.; An, S.S.; Khan, M.; Pennant, W.A.; Kim, H.J.; Yoon, D.H.; Lee, M.; Kim, K.N. Hypoxia-preconditioned adipose tissue-derived mesenchymal stem cell increase the survival and gene expression of engineered neural stem cells in a spinal cord injury model. Neurosci. Lett. 2010, 472, 215–219. [Google Scholar] [CrossRef]
- Sun, J.; Wei, Z.Z.; Gu, X.; Zhang, J.Y.; Zhang, Y.; Li, J.; Wei, L. Intranasal delivery of hypoxia-preconditioned bone marrow-derived mesenchymal stem cells enhanced regenerative effects after intracerebral hemorrhagic stroke in mice. Exp. Neurol. 2015, 272, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Chau, M.J.; Deveau, T.C.; Gu, X.; Kim, Y.S.; Xu, Y.; Yu, S.P.; Wei, L. Delayed and repeated intranasal delivery of bone marrow stromal cells increases regeneration and functional recovery after ischemic stroke in mice. BMC Neurosci. 2018, 19, 20. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.W.; Qiu, Y.R.; Fu, Y.; Liu, J.; He, Z.J.; Huang, Z.T. Transplantation with hypoxia-preconditioned mesenchymal stem cells suppresses brain injury caused by cardiac arrest-induced global cerebral ischemia in rats. J. Neurosci. Res. 2017, 95, 2059–2070. [Google Scholar] [CrossRef]
- Wei, N.; Yu, S.P.; Gu, X.; Taylor, T.M.; Song, D.; Liu, X.F.; Wei, L. Delayed intranasal delivery of hypoxic-preconditioned bone marrow mesenchymal stem cells enhanced cell homing and therapeutic benefits after ischemic stroke in mice. Cell Transplant. 2013, 22, 977–991. [Google Scholar] [CrossRef]
- Ma, H.; Lam, P.K.; Tong, C.S.W.; Lo, K.K.Y.; Wong, G.K.C.; Poon, W.S. The neuroprotection of hypoxic adipose tissue-derived mesenchymal stem cells in experimental traumatic brain injury. Cell Transplant. 2019, 28, 874–884. [Google Scholar] [CrossRef]
- Xia, C.; Braunstein, Z.; Toomey, A.C.; Zhong, J.; Rao, X. S100 Proteins As an Important Regulator of Macrophage Inflammation. Front. Immunol. 2017, 8, 1908. [Google Scholar] [CrossRef] [PubMed]
- Sreejit, G.; Flynn, M.C.; Patil, M.; Krishnamurthy, P.; Murphy, A.J.; Nagareddy, P.R. S100 family proteins in inflammation and beyond. Adv. Clin. Chem. 2020, 98, 173–231. [Google Scholar] [CrossRef]
- Jiang, R.H.; Wu, C.J.; Xu, X.Q.; Lu, S.S.; Zu, Q.Q.; Zhao, L.B.; Wang, J.; Liu, S.; Shi, H.B. Hypoxic conditioned medium derived from bone marrow mesenchymal stromal cells protects against ischemic stroke in rats. J. Cell Physiol. 2019, 234, 1354–1368. [Google Scholar] [CrossRef]
- Xu, C.; Diao, Y.F.; Wang, J.; Liang, J.; Xu, H.H.; Zhao, M.L.; Zheng, B.; Luan, Z.; Wang, J.J.; Yang, X.P.; et al. Intravenously Infusing the Secretome of Adipose-Derived Mesenchymal Stem Cells Ameliorates Neuroinflammation and Neurological Functioning After Traumatic Brain Injury. Stem Cells Dev. 2020, 29, 222–234. [Google Scholar] [CrossRef]
- Liu, X.; Wang, J.; Wang, P.; Zhong, L.; Wang, S.; Feng, Q.; Wei, X.; Zhou, L. Hypoxia-pretreated mesenchymal stem cell-derived exosomes-loaded low-temperature extrusion 3D-printed implants for neural regeneration after traumatic brain injury in canines. Front. Bioeng. Biotechnol. 2022, 10, 1025138. [Google Scholar] [CrossRef]
- Nalamolu, K.R.; Venkatesh, I.; Mohandass, A.; Klopfenstein, J.D.; Pinson, D.M.; Wang, D.Z.; Kunamneni, A.; Veeravalli, K.K. Exosomes Secreted by the Cocultures of Normal and Oxygen-Glucose-Deprived Stem Cells Improve Post-stroke Outcome. Neuromolecular Med. 2019, 21, 529–539. [Google Scholar] [CrossRef]
- Jiang, X.; Savchenko, O.; Li, Y.; Qi, S.; Yang, T.; Zhang, W.; Chen, J. A Review of Low-Intensity Pulsed Ultrasound for Therapeutic Applications. IEEE Trans. Biomed. Eng. 2019, 66, 2704–2718. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Mao, X.; Wang, D.; Xia, S. Preconditioned MSCs Alleviate Cerebral Ischemia-Reperfusion Injury in Rats by Improving the Neurological Function and the Inhibition of Apoptosis. Brain Sci. 2022, 12, 631. [Google Scholar] [CrossRef]
- Ye, Y.C.; Chang, Z.H.; Wang, P.; Wang, Y.W.; Liang, J.; Chen, C.; Wang, J.J.; Sun, H.T.; Wang, Y.; Li, X.H. Infarct-preconditioning exosomes of umbilical cord mesenchymal stem cells promoted vascular remodeling and neurological recovery after stroke in rats. Stem Cell Res. Ther. 2022, 13, 378. [Google Scholar] [CrossRef]
- Ning, G.Z.; Song, W.Y.; Xu, H.; Zhu, R.S.; Wu, Q.L.; Wu, Y.; Zhu, S.B.; Li, J.Q.; Wang, M.; Qu, Z.G.; et al. Bone marrow mesenchymal stem cells stimulated with low-intensity pulsed ultrasound: Better choice of transplantation treatment for spinal cord injury: Treatment for SCI by LIPUS-BMSCs transplantation. CNS Neurosci. Ther. 2019, 25, 496–508. [Google Scholar] [CrossRef]
- Ren, L.; Chang, M.J.; Zhang, Z.; Dhaka, A.; Guo, Z.; Cao, Y.Q. Quantitative Analysis of Mouse Dural Afferent Neurons Expressing TRPM8, VGLUT3, and NF200. Headache 2018, 58, 88–101. [Google Scholar] [CrossRef]
- Nakamura, S.; Yamada, Y.; Baba, S.; Kato, H.; Kogami, H.; Takao, M.; Matsumoto, N.; Ueda, M. Culture medium study of human mesenchymal stem cells for practical use of tissue engineering and regenerative medicine. Biomed. Mater. Eng. 2008, 18, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Van der Valk, J.; Brunner, D.; De Smet, K.; Fex Svenningsen, A.; Honegger, P.; Knudsen, L.E.; Lindl, T.; Noraberg, J.; Price, A.; Scarino, M.L.; et al. Optimization of chemically defined cell culture media—replacing fetal bovine serum in mammalian in vitro methods. Toxicol. Vitr. 2010, 24, 1053–1063. [Google Scholar] [CrossRef] [PubMed]
- Cherian, D.S.; Bhuvan, T.; Meagher, L.; Heng, T.S.P. Biological Considerations in Scaling Up Therapeutic Cell Manufacturing. Front. Pharmacol. 2020, 11, 654. [Google Scholar] [CrossRef] [PubMed]
- Arora, M. Cell Culture Media: A Review. Mater. Methods 2013, 3, 24. [Google Scholar] [CrossRef]
- Gottipamula, S.; Muttigi, M.S.; Kolkundkar, U.; Seetharam, R.N. Serum-free media for the production of human mesenchymal stromal cells: A review. Cell Prolif. 2013, 46, 608–627. [Google Scholar] [CrossRef] [PubMed]
- Gwam, C.; Mohammed, N.; Ma, X. Stem cell secretome, regeneration, and clinical translation: A narrative review. Ann. Transl. Med. 2021, 9, 70. [Google Scholar] [CrossRef]
- Venugopal, C.; Shamir, C.; Senthilkumar, S.; Babu, J.V.; Sonu, P.K.; Nishtha, K.J.; Rai, K.S.; Shobha, K.; Dhanushkodi, A. Dosage and Passage Dependent Neuroprotective Effects of Exosomes Derived from Rat Bone Marrow Mesenchymal Stem Cells: An In Vitro Analysis. Curr. Gene Ther. 2017, 17, 379–390. [Google Scholar] [CrossRef]
- Liu, Q.; Rojas-Canales, D.M.; Divito, S.J.; Shufesky, W.J.; Stolz, D.B.; Erdos, G.; Sullivan, M.L.; Gibson, G.A.; Watkins, S.C.; Larregina, A.T.; et al. Donor dendritic cell-derived exosomes promote allograft-targeting immune response. J. Clin. Investig. 2016, 126, 2805–2820. [Google Scholar] [CrossRef]
- Drago, D.; Cossetti, C.; Iraci, N.; Gaude, E.; Musco, G.; Bachi, A.; Pluchino, S. The stem cell secretome and its role in brain repair. Biochimie 2013, 95, 2271–2285. [Google Scholar] [CrossRef]
- Liesveld, J.L.; Sharma, N.; Aljitawi, O.S. Stem cell homing: From physiology to therapeutics. Stem Cells 2020, 38, 1241–1253. [Google Scholar] [CrossRef]
- Ezquer, F.; Quintanilla, M.E.; Morales, P.; Santapau, D.; Ezquer, M.; Kogan, M.J.; Salas-Huenuleo, E.; Herrera-Marschitz, M.; Israel, Y. Intranasal delivery of mesenchymal stem cell-derived exosomes reduces oxidative stress and markedly inhibits ethanol consumption and post-deprivation relapse drinking. Addict. Biol. 2019, 24, 994–1007. [Google Scholar] [CrossRef]
- Helwa, I.; Cai, J.; Drewry, M.D.; Zimmerman, A.; Dinkins, M.B.; Khaled, M.L.; Seremwe, M.; Dismuke, W.M.; Bieberich, E.; Stamer, W.D.; et al. A Comparative Study of Serum Exosome Isolation Using Differential Ultracentrifugation and Three Commercial Reagents. PLoS ONE 2017, 12, e0170628. [Google Scholar] [CrossRef]
- Zhang, Y.; Bi, J.; Huang, J.; Tang, Y.; Du, S.; Li, P. Exosome: A Review of Its Classification, Isolation Techniques, Storage, Diagnostic and Targeted Therapy Applications. Int. J. Nanomed. 2020, 15, 6917–6934. [Google Scholar] [CrossRef]
- Li, P.; Kaslan, M.; Lee, S.H.; Yao, J.; Gao, Z. Progress in Exosome Isolation Techniques. Theranostics 2017, 7, 789–804. [Google Scholar] [CrossRef]
- Gholizadeh, S.; Shehata Draz, M.; Zarghooni, M.; Sanati-Nezhad, A.; Ghavami, S.; Shafiee, H.; Akbari, M. Microfluidic approaches for isolation, detection, and characterization of extracellular vesicles: Current status and future directions. Biosens. Bioelectron. 2017, 91, 588–605. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Bucan, V.; Baehre, H.; von der Ohe, J.; Otte, A.; Hass, R. Acquisition of new tumor cell properties by MSC-derived exosomes. Int. J. Oncol. 2015, 47, 244–252. [Google Scholar] [CrossRef]


| Source | Model | Priming | Outcomes | Ref. |
|---|---|---|---|---|
| hP-MSCs | Stroke | 3D culture/scaffolds | Functional recovery; ↓lesion volume; ↓cell death; ↑anti-inflammatory cytokines/neurotrophic factors; ↑neuronal differentiation; ↑angiogenesis; | [146] |
| hUC-CM | SCI | 3D culture/scaffolds | Functional recovery; ↑angiogenesis; ↑remyelination | [137] |
| hUC-CM | TBI | 3D culture/scaffolds | Functional recovery; ↓lesion volume; ↓cell death; ↓inflammation; ↓glial reactivity; ↑angiogenesis; ↑neurogenesis; ↑remyelination | [118] |
| hBM-MSCs-Evs | AD | 3D culture/scaffolds | ↓Glial reactivity | [136] |
| hBM-MSC-Ex | TBI | 3D culture/scaffolds | Functional recovery; ↓inflammation; ↑angiogenesis; ↑neurogenesis | [49] |
| rBM-MSC | Stroke | Valproate and/or lithium chloride | Functional recovery; ↓lesion volume; ↑angiogenesis; ↑neurogenesis; ↑homing | [139] |
| hADSCs | MS | Rapamycin | ↑Anti-inflammatory cytokines | [154] |
| hWJ-MSC | HIE | Thrombin | Functional recovery; ↓lesion volume; ↓cell death; ↓glial reactivity | [151] |
| hWJ-MSC | HIE | Thrombin | NA | [152] |
| rBM-MSCs | HI | Tetramethylpyrazine | Functional recovery; ↑angiogenesis; ↑homing | [149] |
| rBM-MSCs | Huntington | Valproate and/or lithium chloride | Functional recovery; ↓cell death | [147] |
| rBM-MSCs | PD | Fasudil | Functional recovery; ↑neurotrophic factors; ↑angiogenesis; | [153] |
| rBM-MSCs | Stroke | Hydrogen sulfide | Functional recovery; ↓lesion volume; ↓cell death; ↑neurotrophic factors | [148] |
| rBM-MSCs | Stroke | Roxadustat (FG-4592) | Functional recovery; ↓cell death; ↓inflammation; ↓glial reactivity; | [155] |
| rBM-MSCs | TBI | Calpain inhibitor (MDL28170) | Functional recovery; ↓lesion volume; ↓inflammation; ↓glial reactivity; ↑anti-inflammatory cytokines | [150] |
| rBMSC-CM | HIE | Cobalt chloride | Functional recovery; ↓cell death | [138] |
| rBMSC-CM | Neuroinflammation (induced by LPS) | Salidroside | ↓Cell death; ↓inflammation; ↓glial reactivity | [156] |
| rBM-MSC | Stroke | IGF-1 | Functional recovery; ↑angiogenesis;↑neurogenesis | [140] |
| rBM-MSCs | MS | SDF-1α | Functional recovery; ↓glial reactivity; ↑homing | [158] |
| hASC-CM | PA | TNF-α and IFN-γ | Functional recovery; ↓cell death; ↓inflammation; ↓glial reactivity; ↓oxidative Stress; | [162] |
| hASC-CM | TBI | TNF-α and IFN-γ | ↓Inflammation; ↓glial reactivity; ↓loss of visual acuity | [160] |
| hASC-CM | TBI | TNF-α and IFN-γ | ↓Inflammation; ↓glial reactivity; ↓loss of visual acuity; | [165] |
| hASC-CM | TBI | TNF-α and IFN-γ | ↓inflammation; ↓excitoxicity | [159] |
| rASC-CM or hASC-CM | ICH | BDNF | ↓Lesion volume; ↓glial reactivity | [164] |
| rBMSC-CM | Stroke | IL-1α | Functional recovery; ↓lesion volume; ↑body weight | [142] |
| hBM-MSC-Evs | AD | TNF-α and IFN-γ | ↓Inflammation; ↓glial reactivity; ↑anti-inflammatory cytokines | [141] |
| rBM-MSC-Ex | TBI | BDNF | Functional recovery; ↓cell death; ↑neurogenesis | [163] |
| hADSCs | SCI | Hypoxia | ↓Cell death; ↑neurogenesis | [169] |
| hOM-MSCs | ICH | Hypoxia | ↓Cell death; ↓inflammation; ↓glial reactivity | [168] |
| rADMSCs | TBI | Hypoxia | Functional recovery; ↓cell death; ↓inflammation; ↑anti-inflammatory cytokines/neurotrophic factors; | [174] |
| rBM-MSCs | Brain injury caused by cardiac arrest | Hypoxia | ↓Inflammation; ↑homing | [172] |
| rBM-MSCs | ICH | Hypoxia | Functional recovery; ↓lesion volume; ↑neurotrophic Factors; ↑neurogenesis; | [170] |
| rBM-MSCs | Stroke | Hypoxia | Functional recovery; ↓inflammation; ↑neurotrophic factors; ↑angiogenesis; ↑neurogenesis; ↑differentiation | [144] |
| rBM-MSCs | Stroke | Hypoxia | Functional recovery; ↓lesion volume; ↓cell death; ↑homing | [173] |
| rBM-MSCs | Stroke | Hypoxia | Functional recovery; ↑neurotrophic factors; ↑angiogenesis; ↑neurogenesis; | [171] |
| hASC-CM | TBI | Hypoxia | Functional recovery; ↓inflammation; ↓glial reactivity; ↓cell death | [178] |
| hBMSC-CM | TBI | Hypoxia | Functional recovery; ↓lesion volume; ↓cell death | [119] |
| rBMSC-CM | Stroke | Hypoxia | Functional recovery; ↓cell death; ↑angiogenesis | [177] |
| rBM-MSC-Ex | AD | Hypoxia | Functional recovery; ↓inflammation; ↓glial reactivity; ↑anti-inflammatory cytokines/neurotrophic factors | [143] |
| hUC-MSCs-Ex | Stroke | Hypoxia | Functional recovery; ↑body weight | [180] |
| hUC-MSCs-Ex | TBI | Hypoxia | Functional recovery; ↓cell death; ↓inflammation; ↑angiogenesis; ↑neurogenesis | [179] |
| rBM-MSCs | SCI | Low-intensity pulsed ultrasound | Functional recovery; ↓lesion volume; ↓glial reactivity; ↑neurotrophic factors | [184] |
| rBM-MSCs | Stroke | Ischemic brain tissue extract | ↓Lesion volume; ↓cell death; ↑anti-inflammatory cytokines/neurotrophic Factors; | [181] |
| hUC-CM | TBI | Traumatically injured brain tissue extract | Functional recovery; ↓cell death; ↑neurotrophic factors; ↑neurogenesis; ↑homing; | [25] |
| hUC-MSCs-Ex | Stroke | Cerebral infarct tissue extracts | Functional recovery; ↓lesion volume; ↓cell death; | [183] |
| Source | Model | Priming | Medium | Density | Conditioning Time | Concentration | T Volume Administered | N° Administrations | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| rBMSC | AD | No | Serum-free RPMI | 1 × 106 cells | 24 h | Ultrafiltration using centrifugal filters (unspecified) | 25 μL | 4 or 8 | [88] |
| hBMSC | AD | No | Neurobasal-A | 2.4 × 104 cells/mL | 24 h | INA | 4 μL or 8 μL | 1 or 2 | [39] |
| SHED, BMSC and FibroMSC | AD | No | Serum-free DMEM | 1 × 104 cells/cm2 | 48 h | INA | 400 μL | 8 | [89] |
| hWJMSC (differentiate into Ols) | EAE | No | Serum-free DMEM/F12 | INA | 72 h | 100-fold with ultrafiltration using 3 kDa cut-off | 140 μL | 14 | [96] |
| rASC | HIE | No | BME | 4 × 106 cells/cm2 | 24 h | 250-fold by 10,000 cut-off | 10 μL | 1 | [36] |
| PSC-EMSC | HIE | No | Serum-free α-MEM | 9.2 × 104 cells/cm2 | 24 h | Removal of ions and molecules below 1kDa | 84 μL | 7 | [112] |
| hDPSC/hBMSC | Hippocampal neurodegeneration | No | Serum-free basal | NA | 24 h | Not concentrated or diluted | 8 μL | 1 | [90] |
| hBMSC | Impaired astrocytic exocytosis (transgenic model) | No | Neurobasal A | 4.0 × 103 cells/cm2 | 24 h | INA | 0.5 μL | 2 | [131] |
| hUCMSC | NA | No | Serum-free DMEM | 1 × 104 cells/cm2 | 72 h | 10-fold by 3 kDa cut-off | 200 µL | 1 | [21] |
| hUCPVC | NA | No | Neurobasal A | 4.0 × 103 cells/cm2 | 24 h | INA | 0.5 μL | 1 | [16] |
| rBMSC | Neuropathic pain | No | Serum-free DMEM | 7 × 106 cells | 24 h | 15-fold using ultrafiltration units (unspecified) | 100 μL | 1 | [133] |
| rBMSC | SCI | No | DMEM with low glucose and with/FBS | 5 × 103 cells/cm2 | 24 h | INA | 480 µL | 16 | [132] |
| hASC | Stroke | No | Serum-free α-MEM | INA | 48 h | INA | 0.5 μL | 1 | [103] |
| rBMSC | Stroke | No | DMEM/F12 supplemented with 2% LE rat serum | INA | 24 h | 10-fold by 5 kDa cut-off | INA | 1 | [102] |
| hESCMSC | Stroke | No | DMEM containing 0.05% human serum albumin and 2 mM L-glutamine, without FBS | INA | 24 h | 100-fold with 3 kDa cut-off | 5 μL or 10 μL | 1 or 2 | [104] |
| hSHEDMSC, hBMSC | Stroke | No | Serum-free DMEM | 4 × 105 cells/cm2 | 48 h | INA | 100 μL | 13 | [101] |
| hUCMSC | Stroke | No | Serum-free DMEM/F12 | 1 × 104 cells/cm2 | 24 h | INA | 140 μL | 14 | [100] |
| hBMSC | TBI | No | Serum-free DMEM | 2 × 106 cells/cm2 | 24 h | 25-fold by 3 kDa cut-off | ≈275 μL | INA | [37] |
| hBMSC | TBI | No | Serum-free DMEM | 2 × 106 cells/cm2 | 24 h | 25-fold by 3 kDa cut-off | ≈1699 μL | 6 | [119] |
| hUCMSC | TBI | No | Serum-free, low-glucose DMEM | 2 × 106cells | 24 h | 100 kDa cut-off (unspecified) | 3 μL | 1 | [25] |
| hUCPVC | TBI | No | Serum-free EBM-2 | 2.5 × 103 cells/cm2 | 72 h | NA | 4 μL | 1 | [120] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silva, A.V.; Serrenho, I.; Araújo, B.; Carvalho, A.M.; Baltazar, G. Secretome as a Tool to Treat Neurological Conditions: Are We Ready? Int. J. Mol. Sci. 2023, 24, 16544. https://doi.org/10.3390/ijms242216544
da Silva AV, Serrenho I, Araújo B, Carvalho AM, Baltazar G. Secretome as a Tool to Treat Neurological Conditions: Are We Ready? International Journal of Molecular Sciences. 2023; 24(22):16544. https://doi.org/10.3390/ijms242216544
Chicago/Turabian Styleda Silva, Andreia Valente, Inês Serrenho, Beatriz Araújo, Alexandre Martins Carvalho, and Graça Baltazar. 2023. "Secretome as a Tool to Treat Neurological Conditions: Are We Ready?" International Journal of Molecular Sciences 24, no. 22: 16544. https://doi.org/10.3390/ijms242216544
APA Styleda Silva, A. V., Serrenho, I., Araújo, B., Carvalho, A. M., & Baltazar, G. (2023). Secretome as a Tool to Treat Neurological Conditions: Are We Ready? International Journal of Molecular Sciences, 24(22), 16544. https://doi.org/10.3390/ijms242216544







