Achilles’ Heel—The Significance of Maintaining Microenvironmental Homeostasis in the Nucleus Pulposus for Intervertebral Discs
Abstract
:1. Introduction
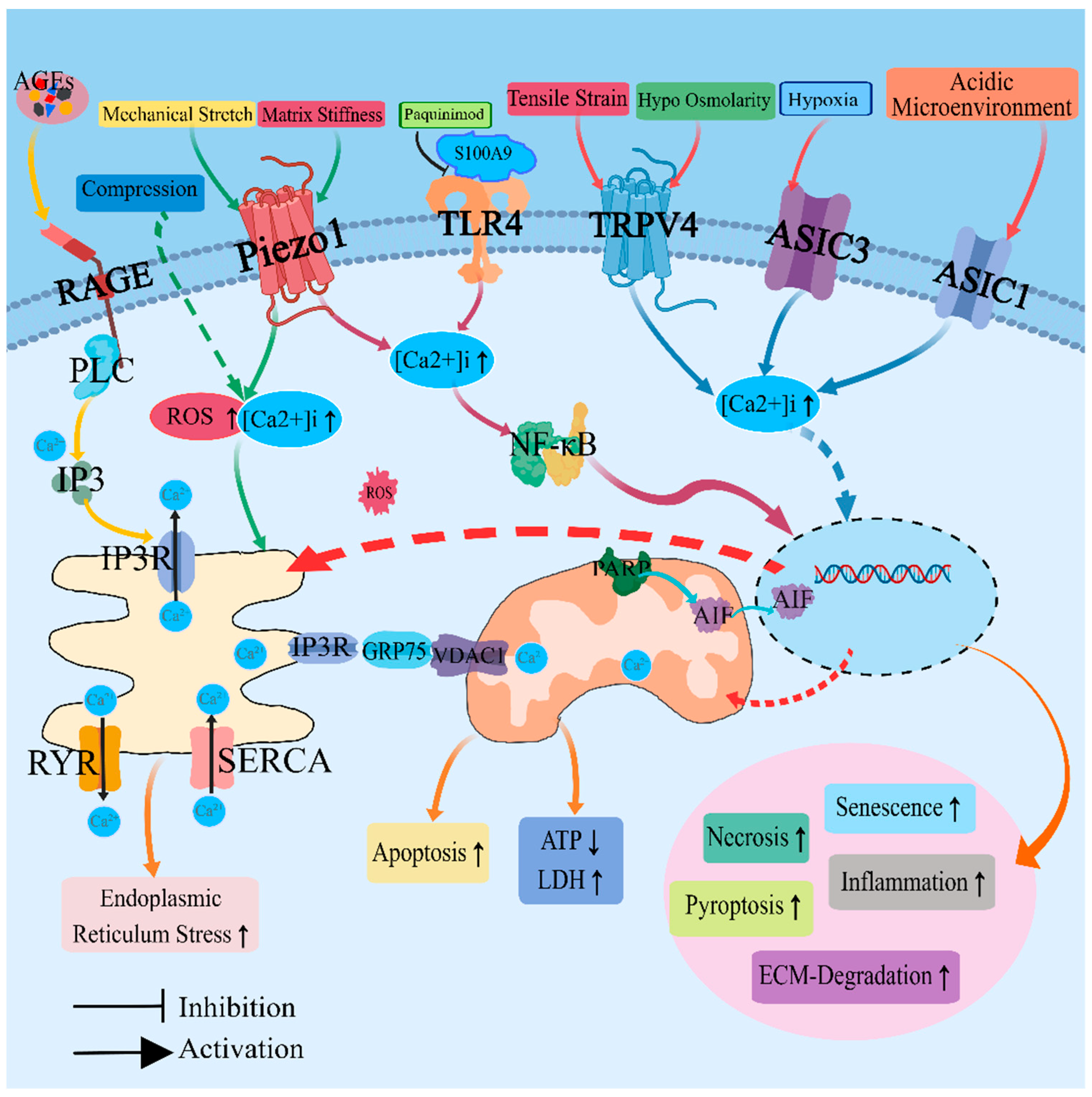
2. Regulation of Calcium Ion Homeostasis and Calcium Ion Channels
3. Revisions in the Acidic Environment and Acid-Sensitive Ion Channels
4. Alterations in Osmotic Pressure and Associated Regulatory Proteins
5. Repartition and Deposition of Metallic Elements
6. Remodeling and Calcification of the Cartilage Endplate
7. Future Directions and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cieza, A.; Causey, K.; Kamenov, K.; Hanson, S.W.; Chatterji, S.; Vos, T. Global estimates of the need for rehabilitation based on the Global Burden of Disease study 2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2021, 396, 2006–2017. [Google Scholar] [CrossRef]
- Guan, S.Y.; Zheng, J.X.; Sam, N.B.; Xu, S.; Shuai, Z.; Pan, F. Global burden and risk factors of musculoskeletal disorders among adolescents and young adults in 204 countries and territories, 1990–2019. Autoimmun. Rev. 2023, 22, 103361. [Google Scholar] [CrossRef] [PubMed]
- Morris, H.; Gonçalves, C.F.; Dudek, M.; Hoyland, J.; Meng, Q.J. Tissue physiology revolving around the clock: Circadian rhythms as exemplified by the intervertebral disc. Ann. Rheum. Dis. 2021, 80, 828–839. [Google Scholar] [CrossRef] [PubMed]
- Frapin, L.; Clouet, J.; Delplace, V.; Fusellier, M.; Guicheux, J.; Le Visage, C. Lessons learned from intervertebral disc pathophysiology to guide rational design of sequential delivery systems for therapeutic biological factors. Adv. Drug Deliv. Rev. 2019, 149–150, 49–71. [Google Scholar] [CrossRef] [PubMed]
- Habib, M.; Hussien, S.; Jeon, O.; Lotz, J.C.; Wu, P.I.; Alsberg, E.; Fields, A.J. Intradiscal treatment of the cartilage endplate for improving solute transport and disc nutrition. Front. Bioeng. Biotechnol. 2023, 11, 1111356. [Google Scholar] [CrossRef]
- Dou, Y.; Sun, X.; Ma, X.; Zhao, X.; Yang, Q. Intervertebral Disk Degeneration: The Microenvironment and Tissue Engineering Strategies. Front. Bioeng. Biotechnol. 2021, 9, 592118. [Google Scholar] [CrossRef]
- Cai, F.; Wang, F.; Hong, X.; Xie, X.H.; Shi, R.; Xie, Z.Y.; Wu, X.T. Acid-sensing ion channel 1a regulates the survival of nucleus pulposus cells in the acidic environment of degenerated intervertebral discs. Iran. J. Basic Med. Sci. 2016, 19, 812–820. [Google Scholar]
- Wang, D.; Zhu, H.; Cheng, W.; Lin, S.; Shao, R.; Pan, H. Effects of hypoxia and ASIC3 on nucleus pulposus cells: From cell behavior to molecular mechanism. Biomed. Pharmacother. 2019, 117, 109061. [Google Scholar] [CrossRef]
- Zhao, C.Q.; Wang, L.M.; Jiang, L.S.; Dai, L.Y. The cell biology of intervertebral disc aging and degeneration. Ageing Res. Rev. 2007, 6, 247–261. [Google Scholar] [CrossRef]
- Chen, F.; Jiang, G.; Liu, H.; Li, Z.; Pei, Y.; Wang, H.; Pan, H.; Cui, H.; Long, J.; Wang, J.; et al. Melatonin alleviates intervertebral disc degeneration by disrupting the IL-1β/NF-κB-NLRP3 inflammasome positive feedback loop. Bone Res. 2020, 8, 10. [Google Scholar] [CrossRef]
- Chen, J.; Zhu, H.; Zhu, Y.; Zhao, C.; Wang, S.; Zheng, Y.; Xie, Z.; Jin, Y.; Song, H.; Yang, L.; et al. Injectable self-healing hydrogel with siRNA delivery property for sustained STING silencing and enhanced therapy of intervertebral disc degeneration. Bioact. Mater. 2022, 9, 29–43. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Peng, Y.; Li, J.; Wang, Z.; Chen, S.; Qing, X.; Pu, F.; Lei, M.; Shao, Z. Reactive Oxygen Species Regulate Endoplasmic Reticulum Stress and ER-Mitochondrial Ca2+ Crosstalk to Promote Programmed Necrosis of Rat Nucleus Pulposus Cells under Compression. Oxid. Med. Cell. Longev. 2021, 2021, 8810698. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Hsieh, M.K.; Hu, Y.J.; Bit, A.; Lai, P.L. Monocarboxylate transporter 1-mediated lactate accumulation promotes nucleus pulposus degeneration under hypoxia in a 3D multilayered nucleus pulposus degeneration model. Eur. Cells Mater. 2022, 43, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Leng, P.; Song, M.; Li, D.; Guo, P.; Xu, X.; Gao, H.; Li, Z.; Li, C.; Zhang, H. Piezo1 activates the NLRP3 inflammasome in nucleus pulposus cell-mediated by Ca2+/NF-kappaB pathway. Int. Immunopharmacol. 2020, 85, 106681. [Google Scholar] [CrossRef]
- Wang, B.; Ke, W.; Wang, K.; Li, G.; Ma, L.; Lu, S.; Xiang, Q.; Liao, Z.; Luo, R.; Song, Y.; et al. Mechanosensitive Ion Channel Piezo1 Activated by Matrix Stiffness Regulates Oxidative Stress-Induced Senescence and Apoptosis in Human Intervertebral Disc Degeneration. Oxid. Med. Cell. Longev. 2021, 2021, 8884922. [Google Scholar] [CrossRef]
- Wu, J.; Chen, Y.; Liao, Z.; Liu, H.; Zhang, S.; Zhong, D.; Qiu, X.; Chen, T.; Su, D.; Ke, X.; et al. Self-amplifying loop of NF-κB and periostin initiated by PIEZO1 accelerates mechano-induced senescence of nucleus pulposus cells and intervertebral disc degeneration. Mol. Ther. J. Am. Soc. Gene Ther. 2022, 30, 3241–3256. [Google Scholar] [CrossRef]
- Kim, M.K.; Ramachandran, R.; Séguin, C.A. Spatiotemporal and functional characterisation of transient receptor potential vanilloid 4 (TRPV4) in the murine intervertebral disc. Eur. Cells Mater. 2021, 41, 194–203. [Google Scholar] [CrossRef]
- Walter, B.A.; Purmessur, D.; Moon, A.; Occhiogrosso, J.; Laudier, D.M.; Hecht, A.C.; Iatridis, J.C. Reduced tissue osmolarity increases TRPV4 expression and pro-inflammatory cytokines in intervertebral disc cells. Eur. Cells Mater. 2016, 32, 123–136. [Google Scholar] [CrossRef]
- Echevarría, M.; Muñoz-Cabello, A.M.; Sánchez-Silva, R.; Toledo-Aral, J.J.; López-Barneo, J. Development of cytosolic hypoxia and hypoxia-inducible factor stabilization are facilitated by aquaporin-1 expression. J. Biol. Chem. 2007, 282, 30207–30215. [Google Scholar] [CrossRef]
- Palacio-Mancheno, P.E.; Evashwick-Rogler, T.W.; Laudier, D.M.; Purmessur, D.; Iatridis, J.C. Hyperosmolarity induces notochordal cell differentiation with aquaporin3 upregulation and reduced N-cadherin expression. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2018, 36, 788–798. [Google Scholar] [CrossRef]
- Snuggs, J.W.; Tessier, S.; Bunning, R.A.B.; Shapiro, I.M.; Risbud, M.V.; Le Maitre, C.L. TonEBP regulates the hyperosmotic expression of aquaporin 1 and 5 in the intervertebral disc. Sci. Rep. 2021, 11, 3164. [Google Scholar] [CrossRef] [PubMed]
- Tas, U.; Cayli, S.; Inanir, A.; Ozyurt, B.; Ocakli, S.; Karaca, Z.I.; Sarsilmaz, M. Aquaporin-1 and aquaporin-3 expressions in the intervertebral disc of rats with aging. Balk. Med. J. 2012, 29, 349–353. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhu, Y. Aquaporin-1: A potential membrane channel for facilitating the adaptability of rabbit nucleus pulposus cells to an extracellular matrix environment. J. Orthop. Sci. 2011, 16, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Jing, Y.; Xia, J.; Wang, X.; You, C.; Yan, J. Aquaporin 3 protects against lumbar intervertebral disc degeneration via the Wnt/β-catenin pathway. Int. J. Mol. Med. 2016, 37, 859–864. [Google Scholar] [CrossRef]
- Ding, J.; Zhang, R.; Li, H.; Ji, Q.; Cheng, X.; Thorne, R.F.; Hondermarck, H.; Liu, X.; Shen, C. ASIC1 and ASIC3 mediate cellular senescence of human nucleus pulposus mesenchymal stem cells during intervertebral disc degeneration. Aging 2021, 13, 10703–10723. [Google Scholar] [CrossRef]
- Cai, F.; Hong, X.; Tang, X.; Liu, N.C.; Wang, F.; Zhu, L.; Xie, X.H.; Xie, Z.Y.; Wu, X.T. ASIC1a activation induces calcium-dependent apoptosis of BMSCs under conditions that mimic the acidic microenvironment of the degenerated intervertebral disc. Biosci. Rep. 2019, 39, BSR20192708. [Google Scholar] [CrossRef]
- Chen, C.C.; Wong, C.W. Neurosensory mechanotransduction through acid-sensing ion channels. J. Cell. Mol. Med. 2013, 17, 337–349. [Google Scholar] [CrossRef]
- Cheng, Y.R.; Jiang, B.Y.; Chen, C.C. Acid-sensing ion channels: Dual function proteins for chemo-sensing and mechano-sensing. J. Biomed. Sci. 2018, 25, 46. [Google Scholar] [CrossRef]
- Gilbert, H.T.J.; Hodson, N.; Baird, P.; Richardson, S.M.; Hoyland, J.A. Acidic pH promotes intervertebral disc degeneration: Acid-sensing ion channel -3 as a potential therapeutic target. Sci. Rep. 2016, 6, 37360. [Google Scholar] [CrossRef]
- Li, W.G.; Xu, T.L. ASIC3 channels in multimodal sensory perception. ACS Chem. Neurosci. 2011, 2, 26–37. [Google Scholar] [CrossRef]
- Ruan, N.; Tribble, J.; Peterson, A.M.; Jiang, Q.; Wang, J.Q.; Chu, X.P. Acid-Sensing Ion Channels and Mechanosensation. Int. J. Mol. Sci. 2021, 22, 4810. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Han, L.; Chen, H.; Zhang, S.; Zhang, S.; Zhang, H.; Li, Y.; Tao, H.; Li, J. Sa12b Improves Biological Activity of Human Degenerative Nucleus Pulposus Mesenchymal Stem Cells in a Severe Acid Environment by Inhibiting Acid-Sensitive Ion Channels. Front. Bioeng. Biotechnol. 2022, 10, 816362. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.L.; Zhao, M.D.; Jiang, D.L.; Jin, C.; Liu, H.F.; Xu, M.H.; Hu, W.; Li, X. Involvement of acid-sensing ion channel 1a in matrix metabolism of endplate chondrocytes under extracellular acidic conditions through NF-kappaB transcriptional activity. Cell Stress Chaperones 2016, 21, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; An, R.; Xiang, Q.; Li, G.; Wang, K.; Song, Y.; Liao, Z.; Li, S.; Hua, W.; Feng, X.; et al. Acid-sensing ion channels regulate nucleus pulposus cell inflammation and pyroptosis via the NLRP3 inflammasome in intervertebral disc degeneration. Cell Prolif. 2021, 54, e12941. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.P.; Wu, X.S.; Wang, Z.S.; Xie, Y.Y.; Ge, J.F.; Chen, F.H. Novel Insights into Acid-Sensing Ion Channels: Implications for Degenerative Diseases. Aging Dis. 2016, 7, 491–501. [Google Scholar] [CrossRef]
- Johnson, Z.I.; Shapiro, I.M.; Risbud, M.V. Extracellular osmolarity regulates matrix homeostasis in the intervertebral disc and articular cartilage: Evolving role of TonEBP. Matrix Biol. J. Int. Soc. Matrix Biol. 2014, 40, 10–16. [Google Scholar] [CrossRef]
- Johnson, Z.I.; Doolittle, A.C.; Snuggs, J.W.; Shapiro, I.M.; Le Maitre, C.L.; Risbud, M.V. TNF-α promotes nuclear enrichment of the transcription factor TonEBP/NFAT5 to selectively control inflammatory but not osmoregulatory responses in nucleus pulposus cells. J. Biol. Chem. 2017, 292, 17561–17575. [Google Scholar] [CrossRef]
- Lin, W.; Shi, C.; Wang, W.; Wu, H.; Yang, C.; Wang, A.; Shen, X.; Tian, Y.; Cao, P.; Yuan, W. Osmolarity and calcium regulate connective tissue growth factor (CTGF/CCN2) expression in nucleus pulposus cells. Gene 2019, 704, 15–24. [Google Scholar] [CrossRef]
- Tessier, S.; Tran, V.A.; Ottone, O.K.; Novais, E.J.; Doolittle, A.; DiMuzio, M.J.; Shapiro, I.M.; Risbud, M.V. TonEBP-deficiency accelerates intervertebral disc degeneration underscored by matrix remodeling, cytoskeletal rearrangements, and changes in proinflammatory gene expression. Matrix Biol. J. Int. Soc. Matrix Biol. 2020, 87, 94–111. [Google Scholar] [CrossRef]
- Sharma, P.; Hughes, S.; El Haj, A.; Maffulli, N. Expression of the Two Pore Domain Potassium Channel TREK-1 in_Human Intervertebral Disc Cells. Curr. Stem Cell Res. Ther. 2012, 7, 266–271. [Google Scholar] [CrossRef]
- Grant, M.P.; Epure, L.M.; Bokhari, R.; Roughley, P.; Antoniou, J.; Mwale, F. Human cartilaginous endplate degeneration is induced by calcium and the extracellular calcium-sensing receptor in the intervertebral disc. Eur. Cells Mater. 2016, 32, 137–151. [Google Scholar] [CrossRef] [PubMed]
- Grant, M.P.; VanderSchee, C.R.; Chou, H.; Bolt, A.; Epure, L.M.; Kuter, D.; Antoniou, J.; Bohle, S.; Mann, K.K.; Mwale, F. Tungsten accumulates in the intervertebral disc and vertebrae stimulating disc degeneration and upregulating markers of inflammation and pain. Eur. Cells Mater. 2021, 41, 517–530. [Google Scholar] [CrossRef] [PubMed]
- Martins, D.E.; Medeiros, V.P.; Demerov, G.F.; Accardo, C.M.; Paredes-Gamero, E.J.; Wajchenberg, M.; Reginato, R.D.; Nader, H.B.; Puertas, E.B.; Faloppa, F. Ionic and biochemical characterization of bovine intervertebral disk. Connect. Tissue Res. 2016, 57, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.F.; Jiang, L.B.; Ma, Y.Q.; Xu, J.; Gu, H.J.; Wu, X.H.; Li, X.L.; Dong, J. Decreased Zn2+ Influx Underlies the Protective Role of Hypoxia in Rat Nucleus Pulposus Cells. Biol. Trace Elem. Res. 2015, 168, 196–205. [Google Scholar]
- Yuan, F.L.; Xu, R.S.; Ye, J.X.; Zhao, M.D.; Ren, L.J.; Li, X. Apoptotic bodies from endplate chondrocytes enhance the oxidative stress-induced mineralization by regulating PPi metabolism. J. Cell. Mol. Med. 2019, 23, 3665–3675. [Google Scholar] [CrossRef]
- Wang, W.; Jing, X.; Du, T.; Ren, J.; Liu, X.; Chen, F.; Shao, Y.; Sun, S.; Yang, G.; Cui, X. Iron overload promotes intervertebral disc degeneration via inducing oxidative stress and ferroptosis in endplate chondrocytes. Free Radic. Biol. Med. 2022, 190, 234–246. [Google Scholar] [CrossRef]
- Berridge, M.J.; Bootman, M.D.; Roderick, H.L. Calcium signalling: Dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 2003, 4, 517–529. [Google Scholar] [CrossRef]
- Hofer, A.M.; Gerbino, A.; Caroppo, R.; Curci, S. The extracellular calcium-sensing receptor and cell-cell signaling in epithelia. Cell Calcium 2004, 35, 297–306. [Google Scholar] [CrossRef]
- Chen, Y.F.; Chen, Y.T.; Chiu, W.T.; Shen, M.R. Remodeling of calcium signaling in tumor progression. J. Biomed. Sci. 2013, 20, 23. [Google Scholar] [CrossRef]
- Pagliaro, L.; Marchesini, M.; Roti, G. Targeting oncogenic Notch signaling with SERCA inhibitors. J. Hematol. Oncol. 2021, 14, 8. [Google Scholar] [CrossRef]
- Palmieri, L.; Papaleo, V.; Porcelli, V.; Scarcia, P.; Gaita, L.; Sacco, R.; Hager, J.; Rousseau, F.; Curatolo, P.; Manzi, B.; et al. Altered calcium homeostasis in autism-spectrum disorders: Evidence from biochemical and genetic studies of the mitochondrial aspartate/glutamate carrier AGC1. Mol. Psychiatry 2010, 15, 38–52. [Google Scholar] [CrossRef] [PubMed]
- Banday, A.A.; Lokhandwala, M.F. Oxidative stress causes renal angiotensin II type 1 receptor upregulation, Na+/H+ exchanger 3 overstimulation, and hypertension. Hypertension 2011, 57, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Reyes Gaido, O.E.; Nkashama, L.J.; Schole, K.L.; Wang, Q.; Umapathi, P.; Mesubi, O.O.; Konstantinidis, K.; Luczak, E.D.; Anderson, M.E. CaMKII as a Therapeutic Target in Cardiovascular Disease. Annu. Rev. Pharmacol. Toxicol. 2023, 63, 249–272. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Gu, H.O.; Jung, Y.; Jung, Y.; Seo, S.Y.; Hong, J.H.; Hong, I.S.; Lee, D.H.; Kim, O.H.; Oh, B.C. Candesartan, an angiotensin-II receptor blocker, ameliorates insulin resistance and hepatosteatosis by reducing intracellular calcium overload and lipid accumulation. Exp. Mol. Med. 2023, 55, 910–925. [Google Scholar] [CrossRef]
- Lia, A.; Sansevero, G.; Chiavegato, A.; Sbrissa, M.; Pendin, D.; Mariotti, L.; Pozzan, T.; Berardi, N.; Carmignoto, G.; Fasolato, C.; et al. Rescue of astrocyte activity by the calcium sensor STIM1 restores long-term synaptic plasticity in female mice modelling Alzheimer’s disease. Nat. Commun. 2023, 14, 1590. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Xu, X.; Liu, D.; Gao, J.; Gao, Y.; Wu, X.; Sheng, H.; Li, Q.; Mi, J. The delta subunit of the GABA(A) receptor is necessary for the GPT2-promoted breast cancer metastasis. Theranostics 2023, 13, 1355–1369. [Google Scholar] [CrossRef]
- Li, F.; He, C.; Yao, H.; Zhao, Y.; Ye, X.; Zhou, S.; Zou, J.; Li, Y.; Li, J.; Chen, S.; et al. Glutamate from nerve cells promotes perineural invasion in pancreatic cancer by regulating tumor glycolysis through HK2 mRNA-m6A modification. Pharmacol. Res. 2023, 187, 106555. [Google Scholar] [CrossRef]
- Bartok, A.; Weaver, D.; Golenár, T.; Nichtova, Z.; Katona, M.; Bánsághi, S.; Alzayady, K.J.; Thomas, V.K.; Ando, H.; Mikoshiba, K.; et al. IP3 receptor isoforms differently regulate ER-mitochondrial contacts and local calcium transfer. Nat. Commun. 2019, 10, 3726. [Google Scholar] [CrossRef]
- Csordás, G.; Weaver, D.; Hajnóczky, G. Endoplasmic Reticulum-Mitochondrial Contactology: Structure and Signaling Functions. Trends Cell Biol. 2018, 28, 523–540. [Google Scholar] [CrossRef]
- Chemaly, E.R.; Troncone, L.; Lebeche, D. SERCA control of cell death and survival. Cell Calcium 2018, 69, 46–61. [Google Scholar] [CrossRef]
- Patterson, R.L.; Boehning, D.; Snyder, S.H. Inositol 1,4,5-trisphosphate receptors as signal integrators. Annu. Rev. Biochem. 2004, 73, 437–465. [Google Scholar] [CrossRef] [PubMed]
- Prole, D.L.; Taylor, C.W. Structure and Function of IP3 Receptors. Cold Spring Harb. Perspect. Biol. 2019, 11, a035063. [Google Scholar] [CrossRef] [PubMed]
- Abu-Omar, N.; Das, J.; Szeto, V.; Feng, Z.P. Neuronal Ryanodine Receptors in Development and Aging. Mol. Neurobiol. 2018, 55, 1183–1192. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.J.; Wang, Y.J.; Zhang, M.; Zhang, P.; Liang, H.; Bai, H.J.; Yu, X.J.; Yang, H.T. Functional expression of the Ca2+ signaling machinery in human embryonic stem cells. Acta Pharmacol. Sin. 2017, 38, 1663–1672. [Google Scholar] [CrossRef] [PubMed]
- Luo, R.; Song, Y.; Liao, Z.; Yin, H.; Zhan, S.; Wang, K.; Li, S.; Li, G.; Ma, L.; Lu, S.; et al. Impaired calcium homeostasis via advanced glycation end products promotes apoptosis through endoplasmic reticulum stress in human nucleus pulposus cells and exacerbates intervertebral disc degeneration in rats. FEBS J. 2019, 286, 4356–4373. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Wang, K.; Zhao, J.; Luo, Y. Serum calcium concentration as an indicator of intervertebral disk degeneration prognosis. Biol. Trace Elem. Res. 2013, 154, 333–337. [Google Scholar] [CrossRef]
- Zhang, Y.; Qian, X.; Yang, X.; Niu, R.; Song, S.; Zhu, F.; Zhu, C.; Peng, X.; Chen, F. ASIC1a induces synovial inflammation via the Ca2+/NFATc3/ RANTES pathway. Theranostics 2020, 10, 247–264. [Google Scholar] [CrossRef]
- Hong, S.J.; Dawson, T.M.; Dawson, V.L. Nuclear and mitochondrial conversations in cell death: PARP-1 and AIF signaling. Trends Pharmacol. Sci. 2004, 25, 259–264. [Google Scholar] [CrossRef]
- Joza, N.; Susin, S.A.; Daugas, E.; Stanford, W.L.; Cho, S.K.; Li, C.Y.; Sasaki, T.; Elia, A.J.; Cheng, H.Y.; Ravagnan, L.; et al. Essential role of the mitochondrial apoptosis-inducing factor in programmed cell death. Nature 2001, 410, 549–554. [Google Scholar] [CrossRef]
- Chen, Z.; Jiao, Y.; Zhang, Y.; Wang, Q.; Wu, W.; Zheng, J.; Li, J. G-Protein Coupled Receptor 35 Induces Intervertebral Disc Degeneration by Mediating the Influx of Calcium Ions and Upregulating Reactive Oxygen Species. Oxid. Med. Cell. Longev. 2022, 2022, 5469220. [Google Scholar] [CrossRef]
- Goldin, A.; Beckman, J.A.; Schmidt, A.M.; Creager, M.A. Advanced glycation end products: Sparking the development of diabetic vascular injury. Circulation 2006, 114, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Adamopoulos, C.; Mihailidou, C.; Grivaki, C.; Papavassiliou, K.A.; Kiaris, H.; Piperi, C.; Papavassiliou, A.G. Systemic effects of AGEs in ER stress induction in vivo. Glycoconj. J. 2016, 33, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Dargan, S.L.; Parker, I. Buffer kinetics shape the spatiotemporal patterns of IP3-evoked Ca2+ signals. J. Physiol. 2003, 553 Pt 3, 775–788. [Google Scholar] [CrossRef] [PubMed]
- Randhawa, P.K.; Jaggi, A.S. TRPV4 channels: Physiological and pathological role in cardiovascular system. Basic Res. Cardiol. 2015, 110, 54. [Google Scholar] [CrossRef]
- White, J.P.; Cibelli, M.; Urban, L.; Nilius, B.; McGeown, J.G.; Nagy, I. TRPV4: Molecular Conductor of a Diverse Orchestra. Physiol. Rev. 2016, 96, 911–973. [Google Scholar] [CrossRef]
- Ge, J.; Li, W.; Zhao, Q.; Li, N.; Chen, M.; Zhi, P.; Li, R.; Gao, N.; Xiao, B.; Yang, M. Architecture of the mammalian mechanosensitive Piezo1 channel. Nature 2015, 527, 64–69. [Google Scholar] [CrossRef]
- Coste, B.; Xiao, B.; Santos, J.S.; Syeda, R.; Grandl, J.; Spencer, K.S.; Kim, S.E.; Schmidt, M.; Mathur, J.; Dubin, A.E.; et al. Piezo proteins are pore-forming subunits of mechanically activated channels. Nature 2012, 483, 176–181. [Google Scholar] [CrossRef]
- Chu, Y.C.; Lim, J.; Lai, C.H.; Tseng, M.C.; Chu, Y.S.; Wang, J.L. Elevation of Intra-Cellular Calcium in Nucleus Pulposus Cells with Micro-Pipette-Guided Ultrasound. Ultrasound Med. Biol. 2021, 47, 1775–1784. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, R.; Lu, S.; Zhang, W.; Wang, D.; Ge, Y.; Jiang, F.; Qin, X.; Liu, Y. S100 Calcium Binding Protein A16 Promotes Cell Proliferation by triggering LATS1 ubiquitin degradation mediated by CUL4A ligase to inhibit Hippo pathway in Glioma development. Int. J. Biol. Sci. 2023, 19, 2034–2052. [Google Scholar] [CrossRef]
- Lukanidin, E.; Sleeman, J.P. Building the niche: The role of the S100 proteins in metastatic growth. Semin. Cancer Biol. 2012, 22, 216–225. [Google Scholar] [CrossRef]
- Kovačić, M.; Mitrović-Ajtić, O.; Beleslin-Čokić, B.; Djikić, D.; Subotički, T.; Diklić, M.; Leković, D.; Gotić, M.; Mossuz, P.; Čokić, V.P. TLR4 and RAGE conversely mediate pro-inflammatory S100A8/9-mediated inhibition of proliferation-linked signaling in myeloproliferative neoplasms. Cell. Oncol. 2018, 41, 541–553. [Google Scholar] [CrossRef] [PubMed]
- Golubinskaya, V.; Puttonen, H.; Fyhr, I.M.; Rydbeck, H.; Hellström, A.; Jacobsson, B.; Nilsson, H.; Mallard, C.; Sävman, K. Expression of S100A Alarmins in Cord Blood Monocytes Is Highly Associated With Chorioamnionitis and Fetal Inflammation in Preterm Infants. Front. Immunol. 2020, 11, 1194. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.Y.; Raftery, M.J.; Goyette, J.; Hsu, K.; Geczy, C.L. Oxidative modifications of S100 proteins: Functional regulation by redox. J. Leukoc. Biol. 2009, 86, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Emberley, E.D.; Murphy, L.C.; Watson, P.H. S100 proteins and their influence on pro-survival pathways in cancer. Biochem. Cell Biol. Biochim. Biol. Cell. 2004, 82, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Song, R.; Wang, Z.; Jing, Z.; Wang, S.; Ma, J. S100A8/A9 in Inflammation. Front. Immunol. 2018, 9, 1298. [Google Scholar] [CrossRef]
- Guo, S.; Su, Q.; Wen, J.; Zhu, K.; Tan, J.; Fu, Q.; Sun, G. S100A9 induces nucleus pulposus cell degeneration through activation of the NF-κB signaling pathway. J. Cell. Mol. Med. 2021, 25, 4709–4720. [Google Scholar] [CrossRef]
- Zheng, J.; Wang, J.; Liu, H.; Chen, F.; Wang, H.; Chen, S.; Xie, J.; Zheng, Z.; Li, Z. Alarmins S100A8/A9 promote intervertebral disc degeneration and inflammation-related pain in a rat model through toll-like receptor-4 and activation of the NF-κB signaling pathway. Osteoarthr. Cartil. 2022, 30, 998–1011. [Google Scholar] [CrossRef]
- Chen, C.N.; Chang, C.C.; Lai, H.S.; Jeng, Y.M.; Chen, C.I.; Chang, K.J.; Lee, P.H.; Lee, H. Connective tissue growth factor inhibits gastric cancer peritoneal metastasis by blocking integrin α3β1-dependent adhesion. Gastric Cancer Off. J. Int. Gastric Cancer Assoc. Jpn. Gastric Cancer Assoc. 2015, 18, 504–515. [Google Scholar] [CrossRef]
- Fu, M.; Peng, D.; Lan, T.; Wei, Y.; Wei, X. Multifunctional regulatory protein connective tissue growth factor (CTGF): A potential therapeutic target for diverse diseases. Acta Pharm. Sin. B 2022, 12, 1740–1760. [Google Scholar] [CrossRef]
- Pi, L.; Robinson, P.M.; Jorgensen, M.; Oh, S.H.; Brown, A.R.; Weinreb, P.H.; Trinh, T.L.; Yianni, P.; Liu, C.; Leask, A.; et al. Connective tissue growth factor and integrin αvβ6: A new pair of regulators critical for ductular reaction and biliary fibrosis in mice. Hepatology 2015, 61, 678–691. [Google Scholar] [CrossRef]
- Wei, J.L.; Fu, W.; Hettinghouse, A.; He, W.J.; Lipson, K.E.; Liu, C.J. Role of ADAMTS-12 in Protecting Against Inflammatory Arthritis in Mice By Interacting With and Inactivating Proinflammatory Connective Tissue Growth Factor. Arthritis Rheumatol. 2018, 70, 1745–1756. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Stefanoska, D.; Grad, S.; Alini, M.; Peroglio, M. Direct and Intervertebral Disc-Mediated Sensitization of Dorsal Root Ganglion Neurons by Hypoxia and Low pH. Neurospine 2020, 17, 42–59. [Google Scholar] [CrossRef] [PubMed]
- Gold, M.S.; Gebhart, G.F. Nociceptor sensitization in pain pathogenesis. Nat. Med. 2010, 16, 1248–1257. [Google Scholar] [CrossRef] [PubMed]
- Umans, B.D.; Liberles, S.D. Neural Sensing of Organ Volume. Trends Neurosci. 2018, 41, 911–924. [Google Scholar] [CrossRef]
- Kim, J.W.; Jeon, N.; Shin, D.E.; Lee, S.Y.; Kim, M.; Han, D.H.; Shin, J.Y.; Lee, S. Regeneration in Spinal Disease: Therapeutic Role of Hypoxia-Inducible Factor-1 Alpha in Regeneration of Degenerative Intervertebral Disc. Int. J. Mol. Sci. 2021, 22, 5281. [Google Scholar] [CrossRef]
- Diamant, B.; Karlsson, J.; Nachemson, A. Correlation between lactate levels and pH in discs of patients with lumbar rhizopathies. Experientia 1968, 24, 1195–1196. [Google Scholar] [CrossRef]
- Nachemson, A. Intradiscal measurements of pH in patients with lumbar rhizopathies. Acta Orthop. Scand. 1969, 40, 23–42. [Google Scholar] [CrossRef]
- Kitano, T.; Zerwekh, J.E.; Usui, Y.; Edwards, M.L.; Flicker, P.L.; Mooney, V. Biochemical changes associated with the symptomatic human intervertebral disk. Clin. Orthop. Relat. Res. 1993, 293, 372–377. [Google Scholar] [CrossRef]
- Kim, J.W.; An, H.J.; Yeo, H.; Jeong, Y.; Lee, H.; Lee, J.; Nam, K.; Lee, J.; Shin, D.E.; Lee, S. Activation of Hypoxia-Inducible Factor-1α Signaling Pathway Has the Protective Effect of Intervertebral Disc Degeneration. Int. J. Mol. Sci. 2021, 22, 11355. [Google Scholar] [CrossRef]
- Wu, W.; Zhang, X.; Hu, X.; Wang, X.; Sun, L.; Zheng, X.; Jiang, L.; Ni, X.; Xu, C.; Tian, N.; et al. Lactate down-regulates matrix systhesis and promotes apoptosis and autophagy in rat nucleus pulposus cells. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2014, 32, 253–261. [Google Scholar] [CrossRef]
- Liu, J.; Tao, H.; Wang, H.; Dong, F.; Zhang, R.; Li, J.; Ge, P.; Song, P.; Zhang, H.; Xu, P.; et al. Biological Behavior of Human Nucleus Pulposus Mesenchymal Stem Cells in Response to Changes in the Acidic Environment During Intervertebral Disc Degeneration. Stem Cells Dev. 2017, 26, 901–911. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Liang, H.; Lee, S.M.; Li, Z.; Zhang, J.; Fei, Q. Isolation and identification of stem cells from degenerated human intervertebral discs and their migration characteristics. Acta Biochim. Biophys. Sin. 2017, 49, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; He, J.; Zhang, G.; Chen, H.; Luo, Z.; Deng, B.; Zhou, Y.; Kang, X. Role of Caspase Family in Intervertebral Disc Degeneration and Its Therapeutic Prospects. Biomolecules 2022, 12, 1074. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.W.; Zhou, J.W.; Zhang, G.Z.; Luo, Z.B.; Li, L.; Kang, X.W. Emerging role and therapeutic implication of mTOR signalling in intervertebral disc degeneration. Cell Prolif. 2022, 56, e13338. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wu, F.R.; Xu, R.S.; Hu, W.; Jiang, D.L.; Ji, C.; Chen, F.H.; Yuan, F.L. Acid-sensing ion channel 1a-mediated calcium influx regulates apoptosis of endplate chondrocytes in intervertebral discs. Expert Opin. Ther. Targets 2014, 18, 1–14. [Google Scholar] [CrossRef]
- Hernández, C.; Konno, K.; Salceda, E.; Vega, R.; Zaharenko, A.J.; Soto, E. Sa12b Peptide from Solitary Wasp Inhibits ASIC Currents in Rat Dorsal Root Ganglion Neurons. Toxins 2019, 11, 585. [Google Scholar] [CrossRef]
- Han, L.; Wang, Z.; Chen, H.; Li, J.; Zhang, S.; Zhang, S.; Shao, S.; Zhang, Y.; Shen, C.; Tao, H. Sa12b-Modified Functional Self-Assembling Peptide Hydrogel Enhances the Biological Activity of Nucleus Pulposus Mesenchymal Stem Cells by Inhibiting Acid-Sensing Ion Channels. Front. Cell Dev. Biol. 2022, 10, 822501. [Google Scholar] [CrossRef]
- Wagner, K.; Unger, L.; Salman, M.M.; Kitchen, P.; Bill, R.M.; Yool, A.J. Signaling Mechanisms and Pharmacological Modulators Governing Diverse Aquaporin Functions in Human Health and Disease. Int. J. Mol. Sci. 2022, 23, 1388. [Google Scholar] [CrossRef]
- Beitz, E.; Golldack, A.; Rothert, M.; von Bülow, J. Challenges and achievements in the therapeutic modulation of aquaporin functionality. Pharmacol. Ther. 2015, 155, 22–35. [Google Scholar] [CrossRef]
- Choi, S.Y.; Lee-Kwon, W.; Kwon, H.M. The evolving role of TonEBP as an immunometabolic stress protein. Nat. Rev. Nephrol. 2020, 16, 352–364. [Google Scholar] [CrossRef]
- Choi, S.Y.; Lim, S.W.; Salimi, S.; Yoo, E.J.; Lee-Kwon, W.; Lee, H.H.; Lee, J.H.; Mitchell, B.D.; Sanada, S.; Parsa, A.; et al. Tonicity-Responsive Enhancer-Binding Protein Mediates Hyperglycemia-Induced Inflammation and Vascular and Renal Injury. J. Am. Soc. Nephrol. JASN 2018, 29, 492–504. [Google Scholar] [CrossRef] [PubMed]
- Halterman, J.A.; Kwon, H.M.; Leitinger, N.; Wamhoff, B.R. NFAT5 expression in bone marrow-derived cells enhances atherosclerosis and drives macrophage migration. Front. Physiol. 2012, 3, 313. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Suh, J.H.; Choi, S.Y.; Kang, H.J.; Lee, H.H.; Ye, B.J.; Lee, G.R.; Jung, S.W.; Kim, C.J.; Lee-Kwon, W.; et al. Tonicity-responsive enhancer-binding protein promotes hepatocellular carcinogenesis, recurrence and metastasis. Gut 2019, 68, 347–358. [Google Scholar] [CrossRef]
- Ye, B.J.; Lee, H.H.; Yoo, E.J.; Lee, C.Y.; Lee, J.H.; Kang, H.J.; Jeong, G.W.; Park, H.; Lee-Kwon, W.; Choi, S.Y.; et al. TonEBP in dendritic cells mediates pro-inflammatory maturation and Th1/Th17 responses. Cell Death Dis. 2020, 11, 421. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.Z.; Liu, M.Q.; Chen, H.W.; Wu, Z.L.; Gao, Y.C.; Ma, Z.J.; He, X.G.; Kang, X.W. NF-κB signalling pathways in nucleus pulposus cell function and intervertebral disc degeneration. Cell Prolif. 2021, 54, e13057. [Google Scholar] [CrossRef]
- Urban, J.P.; McMullin, J.F. Swelling pressure of the lumbar intervertebral discs: Influence of age, spinal level, composition, and degeneration. Spine 1988, 13, 179–187. [Google Scholar] [CrossRef]
- Gantenbein-Ritter, B.; Chan, S.C. The evolutionary importance of cell ratio between notochordal and nucleus pulposus cells: An experimental 3-D co-culture study. Eur. Spine J. Off. Publ. Eur. Spine Soc. Eur. Spinal Deform. Soc. Eur. Sect. Cerv. Spine Res. Soc. 2012, 21 (Suppl. 6), S819–S825. [Google Scholar] [CrossRef]
- McCann, M.R.; Bacher, C.A.; Séguin, C.A. Exploiting notochord cells for stem cell-based regeneration of the intervertebral disc. J. Cell Commun. Signal. 2011, 5, 39–43. [Google Scholar] [CrossRef]
- Chen, J.; Yan, W.; Setton, L.A. Molecular phenotypes of notochordal cells purified from immature nucleus pulposus. Eur. Spine J. Off. Publ. Eur. Spine Soc. Eur. Spinal Deform. Soc. Eur. Sect. Cerv. Spine Res. Soc. 2006, 15 (Suppl. 3), S303–S811. [Google Scholar] [CrossRef]
- Staszkiewicz, R.; Bryś, K.; Gładysz, D.; Gralewski, M.; Garczarek, M.; Gadzieliński, M.; Wieczorek, J.; Marcol, W.; Ostenda, A.; Grabarek, B.O. Changes in Elements and Relationships among Elements in Intervertebral Disc Degeneration. Int. J. Environ. Res. Public. Health 2022, 19, 9042. [Google Scholar] [CrossRef]
- Vivier, D.; Bennis, K.; Lesage, F.; Ducki, S. Perspectives on the Two-Pore Domain Potassium Channel TREK-1 (TWIK-Related K(+) Channel 1). A Novel Therapeutic Target? J. Med. Chem. 2016, 59, 5149–5157. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.L.; Chen, Y.J.; Zhang, G.Z.; Xie, Q.Q.; Wang, K.P.; Yang, X.; Liu, T.C.; Wang, Z.Q.; Zhao, G.H.; Zhang, H.H. SKI knockdown suppresses apoptosis and extracellular matrix degradation of nucleus pulposus cells via inhibition of the Wnt/β-catenin pathway and ameliorates disc degeneration. Apoptosis Int. J. Program. Cell Death 2022, 27, 133–148. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Xing, H.; Tang, R.; Qian, S.; He, S.; Hu, Q.; Zhang, N. The preconditioning of lithium promotes mesenchymal stem cell-based therapy for the degenerated intervertebral disc via upregulating cellular ROS. Stem Cell Res. Ther. 2021, 12, 239. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Li, C.; Shen, B.; Chen, X.; Hu, T.; Wu, D. Is intervertebral disc degeneration associated with reduction in serum ferritin? Eur. Spine J. Off. Publ. Eur. Spine Soc. Eur. Spinal Deform. Soc. Eur. Sect. Cerv. Spine Res. Soc. 2022, 31, 2950–2959. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, B.; Zhao, X.; Li, X.; Lou, Z.; Chen, X.; Zhang, F. Iron deficiency accelerates intervertebral disc degeneration through affecting the stability of DNA polymerase epsilon complex. Am. J. Transl. Res. 2018, 10, 3430–3442. [Google Scholar]
- Lu, S.; Song, Y.; Luo, R.; Li, S.; Li, G.; Wang, K.; Liao, Z.; Wang, B.; Ke, W.; Xiang, Q.; et al. Ferroportin-Dependent Iron Homeostasis Protects against Oxidative Stress-Induced Nucleus Pulposus Cell Ferroptosis and Ameliorates Intervertebral Disc Degeneration In Vivo. Oxid. Med. Cell. Longev. 2021, 2021, 6670497. [Google Scholar] [CrossRef]
- Ling, Z.; Li, L.; Chen, Y.; Hu, H.; Zhao, X.; Wilson, J.; Qi, Q.; Liu, D.; Wei, F.; Chen, X.; et al. Changes of the end plate cartilage are associated with intervertebral disc degeneration: A quantitative magnetic resonance imaging study in rhesus monkeys and humans. J. Orthop. Transl. 2020, 24, 23–31. [Google Scholar] [CrossRef]
- Ashinsky, B.G.; Bonnevie, E.D.; Mandalapu, S.A.; Pickup, S.; Wang, C.; Han, L.; Mauck, R.L.; Smith, H.E.; Gullbrand, S.E. Intervertebral Disc Degeneration Is Associated With Aberrant Endplate Remodeling and Reduced Small Molecule Transport. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2020, 35, 1572–1581. [Google Scholar] [CrossRef]
- Wen, T.; Wang, Z.; Chen, X.; Ren, Y.; Lu, X.; Xing, Y.; Lu, J.; Chang, S.; Zhang, X.; Shen, Y.; et al. Structural basis for activation and allosteric modulation of full-length calcium-sensing receptor. Sci. Adv. 2021, 7, eabg1483. [Google Scholar] [CrossRef]
- Li, F.; Sun, X.; Wang, Y.; Gao, L.; Shi, J.; Sun, K. Development and Validation of a Novel Nomogram to Predict the Risk of Intervertebral Disc Degeneration. Mediat. Inflamm. 2022, 2022, 3665934. [Google Scholar] [CrossRef]
- Zhou, J.; Mi, J.; Peng, Y.; Han, H.; Liu, Z. Causal Associations of Obesity With the Intervertebral Degeneration, Low Back Pain, and Sciatica: A Two-Sample Mendelian Randomization Study. Front. Endocrinol. 2021, 12, 740200. [Google Scholar] [CrossRef] [PubMed]
- Jin, P.; Xing, Y.; Xiao, B.; Wei, Y.; Yan, K.; Zhao, J.; Tian, W. Diabetes and intervertebral disc degeneration: A Mendelian randomization study. Front. Endocrinol. 2023, 14, 1100874. [Google Scholar] [CrossRef] [PubMed]
- Russo, F.; Ambrosio, L.; Ngo, K.; Vadalà, G.; Denaro, V.; Fan, Y.; Sowa, G.; Kang, J.D.; Vo, N. The Role of Type I Diabetes in Intervertebral Disc Degeneration. Spine 2019, 44, 1177–1185. [Google Scholar] [CrossRef] [PubMed]
- Nasto, L.A.; Ngo, K.; Leme, A.S.; Robinson, A.R.; Dong, Q.; Roughley, P.; Usas, A.; Sowa, G.A.; Pola, E.; Kang, J.; et al. Investigating the role of DNA damage in tobacco smoking-induced spine degeneration. Spine J. Off. J. N. Am. Spine Soc. 2014, 14, 416–423. [Google Scholar] [CrossRef]
- Vo, N.; Wang, D.; Sowa, G.; Witt, W.; Ngo, K.; Coelho, P.; Bedison, R.; Byer, B.; Studer, R.; Lee, J.; et al. Differential effects of nicotine and tobacco smoke condensate on human annulus fibrosus cell metabolism. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2011, 29, 1585–1591. [Google Scholar] [CrossRef]
- Wang, D.; Nasto, L.A.; Roughley, P.; Leme, A.S.; Houghton, A.M.; Usas, A.; Sowa, G.; Lee, J.; Niedernhofer, L.; Shapiro, S.; et al. Spine degeneration in a murine model of chronic human tobacco smokers. Osteoarthr. Cartil. 2012, 20, 896–905. [Google Scholar] [CrossRef]
- Wang, S.Z.; Fan, W.M.; Jia, J.; Ma, L.Y.; Yu, J.B.; Wang, C. Is exclusion of leukocytes from platelet-rich plasma (PRP) a better choice for early intervertebral disc regeneration? Stem Cell Res. Ther. 2018, 9, 199. [Google Scholar] [CrossRef]
- Yang, H.; Yuan, C.; Wu, C.; Qian, J.; Shi, Q.; Li, X.; Zhu, X.; Zou, J. The role of TGF-β1/Smad2/3 pathway in platelet-rich plasma in retarding intervertebral disc degeneration. J. Cell. Mol. Med. 2016, 20, 1542–1549. [Google Scholar] [CrossRef]
- Akeda, K.; Ohishi, K.; Takegami, N.; Sudo, T.; Yamada, J.; Fujiwara, T.; Niimi, R.; Matsumoto, T.; Nishimura, Y.; Ogura, T.; et al. Platelet-Rich Plasma Releasate versus Corticosteroid for the Treatment of Discogenic Low Back Pain: A Double-Blind Randomized Controlled Trial. J. Clin. Med. 2022, 11, 304. [Google Scholar] [CrossRef]
- Huang, X.; Chen, D.; Liang, C.; Shi, K.; Zhou, X.; Zhang, Y.; Li, Y.; Chen, J.; Xia, K.; Shu, J.; et al. Swelling-Mediated Mechanical Stimulation Regulates Differentiation of Adipose-Derived Mesenchymal Stem Cells for Intervertebral Disc Repair Using Injectable UCST Microgels. Adv. Healthc. Mater. 2023, 12, e2201925. [Google Scholar] [CrossRef]
- Ying, L.; Liang, C.; Zhang, Y.; Wang, J.; Wang, C.; Xia, K.; Shi, K.; Yu, C.; Yang, B.; Xu, H.; et al. Enhancement of nucleus pulposus repair by glycoengineered adipose-derived mesenchymal cells. Biomaterials 2022, 283, 121463. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Song, Y.; Liao, Z.; Wang, K.; Luo, R.; Lu, S.; Zhao, K.; Feng, X.; Liang, H.; Ma, L.; et al. Bone-derived mesenchymal stem cells alleviate compression-induced apoptosis of nucleus pulposus cells by N6 methyladenosine of autophagy. Cell Death Dis. 2020, 11, 103. [Google Scholar] [CrossRef] [PubMed]
- Kumar, H.; Ha, D.H.; Lee, E.J.; Park, J.H.; Shim, J.H.; Ahn, T.K.; Kim, K.T.; Ropper, A.E.; Sohn, S.; Kim, C.H.; et al. Safety and tolerability of intradiscal implantation of combined autologous adipose-derived mesenchymal stem cells and hyaluronic acid in patients with chronic discogenic low back pain: 1-year follow-up of a phase I study. Stem Cell Res. Ther. 2017, 8, 262. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.; Zeng, Z.; Fang, B.; Tao, M.; Gu, C.; Zheng, L.; Wang, Y.; Shi, Y.; Fang, C.; Mei, S.; et al. Mesenchymal stem cell-derived exosomes ameliorate intervertebral disc degeneration via anti-oxidant and anti-inflammatory effects. Free Radic. Biol. Med. 2019, 143, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, J.; Zhang, Y.; Liu, W.; Ni, W.; Huang, X.; Yuan, J.; Zhao, B.; Xiao, H.; Xue, F. Mesenchymal stem cells-derived exosomes ameliorate intervertebral disc degeneration through inhibiting pyroptosis. J. Cell. Mol. Med. 2020, 24, 11742–11754. [Google Scholar] [CrossRef] [PubMed]
- Capoor, M.N.; Ruzicka, F.; Machackova, T.; Jancalek, R.; Smrcka, M.; Schmitz, J.E.; Hermanova, M.; Sana, J.; Michu, E.; Baird, J.C.; et al. Prevalence of Propionibacterium acnes in Intervertebral Discs of Patients Undergoing Lumbar Microdiscectomy: A Prospective Cross-Sectional Study. PLoS ONE 2016, 11, e0161676. [Google Scholar] [CrossRef]
- Lin, Y.; Jiao, Y.; Yuan, Y.; Zhou, Z.; Zheng, Y.; Xiao, J.; Li, C.; Chen, Z.; Cao, P. Propionibacterium acnes induces intervertebral disc degeneration by promoting nucleus pulposus cell apoptosis via the TLR2/JNK/mitochondrial-mediated pathway. Emerg. Microbes Infect. 2018, 7, 1. [Google Scholar] [CrossRef]
- He, D.; Zhou, M.; Bai, Z.; Wen, Y.; Shen, J.; Hu, Z. Propionibacterium acnes induces intervertebral disc degeneration by promoting nucleus pulposus cell pyroptosis via NLRP3-dependent pathway. Biochem. Biophys. Res. Commun. 2020, 526, 772–779. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, L.; He, D.; Zhang, Y.; Bao, J.; Gao, W.; Cheng, W.; Zhu, C.; Jin, H.; Zhang, W.; et al. Systemic pharmacology reveal the mechanism by which the Qiangjin Zhuanggu Qufeng mixture inhibits LPS-induced pyroptosis of rat nucleus pulposus cells. Phytomed. Int. J. Phytother. Phytopharm. 2023, 119, 154998. [Google Scholar] [CrossRef]
- Wang, X.; Tan, Y.; Liu, F.; Wang, J.; Liu, F.; Zhang, Q.; Li, J. Pharmacological network analysis of the functions and mechanism of kaempferol from Du Zhong in intervertebral disc degeneration (IDD). J. Orthop. Transl. 2023, 39, 135–146. [Google Scholar] [CrossRef]
- Yang, S.; Li, L.; Zhu, L.; Zhang, C.; Li, Z.; Guo, Y.; Nie, Y.; Luo, Z. Aucubin inhibits IL-1β- or TNF-α-induced extracellular matrix degradation in nucleus pulposus cell through blocking the miR-140-5p/CREB1 axis. J. Cell. Physiol. 2019, 234, 13639–13648. [Google Scholar] [CrossRef]
- Dai, F.; Yu, P.; Yu, Z.; Jiang, H.; Ma, Z.; Liu, J. Yiqi Huoxue Recipe Delayed Intervertebral Disc Degeneration by Activating Autophagy. Front. Pharmacol. 2021, 12, 705747. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Martin, J.T.; Elliott, D.M.; Smith, L.J.; Mauck, R.L. Phenotypic stability, matrix elaboration and functional maturation of nucleus pulposus cells encapsulated in photocrosslinkable hyaluronic acid hydrogels. Acta Biomater. 2015, 12, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Du, J.; Peng, P.; Cheng, R.; Lin, J.; Xu, C.; Yang, H.; Cui, W.; Mao, H.; Li, Y.; et al. Regulation of the inflammatory cycle by a controllable release hydrogel for eliminating postoperative inflammation after discectomy. Bioact. Mater. 2021, 6, 146–157. [Google Scholar] [CrossRef]
- Luo, J.; Darai, A.; Pongkulapa, T.; Conley, B.; Yang, L.; Han, I.; Lee, K.B. Injectable bioorthogonal hydrogel (BIOGEL) accelerates tissue regeneration in degenerated intervertebral discs. Bioact. Mater. 2023, 23, 551–562. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Huang, L.; Xia, Q.; Liu, Z.; Huang, Y.; Jiang, Y.; Wang, J.; Ding, H.; Zhu, C.; Song, Y.; et al. Injectable mesoporous bioactive glass/sodium alginate hydrogel loaded with melatonin for intervertebral disc regeneration. Mater. Today Biol. 2023, 22, 100731. [Google Scholar] [CrossRef] [PubMed]


| Cellular Phenotype | Stimulating Factor | Protein Involved in the Process | Roles of Elevated Ca2+ | Measures to Mitigate Effects Caused by Ca2+ Elevation | References |
|---|---|---|---|---|---|
| Rat-NPCS | Ionomycin | — | [Ca2+]i↑ CCN2↑ | BAPTA-AM FK506 and CsA | [38] |
| Bovine-NPCS | Micro-pipette ultrasound | ASIC3 GPCRs | [Ca2+]i↑ | Amiloride APETx2 GPAnt-2 U73122 | [78] |
| HNPCS | Compression loading Zaprinst H2O2 | GPR35↑ | [Ca2+]i↑ | GRP35-siRNA | [70] |
| Bovine-NPCS | GSK101 Hypo-osmotic environment | TRPV4↑ | [Ca2+]i↑ | — | [18] |
| Mice-annulus fibrosus cells | GSK101 | TRPV4↑ | [Ca2+]i↑ | GSK2193874 Y-27632 | [17] |
| HNPCS | Matrix stiffness | Piezo1↑ | [Ca2+]i↑ ERS↑ Apoptosis↑ Senescence↑ | Piezo1-siRNA | [15] |
| HNPCS | Mechanical stretch | Piezo1↑ | [Ca2+]i↑ NLRP3, ASC, caspase-1, and IL-1β↑ NF-κB↑ | BAPTA-AM | [14] |
| Rat-BMSC | Culture media with pH 6.0 | ASIC1a↑ calpain↑ | Proliferation↓ Mitochondrial apoptosis↑ | PcTX1 calpeptin cyclosporine-A | [26] |
| HNP-MSCS | Culture media with pH 6.2 | ASIC1 ASIC2 ASIC3 ASIC4 | [Ca2+]i↑ | Sa12b Amiloride | [32] |
| Human-synovial fibroblasts | Culture media with pH 6.0 | ASIC1a↑ NFATc3↑ | RANTES, IL-8, MIP-1a, ICAM-1, sTNF-RI, and sTNF-RII↑ | ASIC1a-shRNA PcTx-1 extracellular calcium chelator- EGTA | [67] |
| HNPCS | AGEs | IP3R↑ RyR↑ SERCA↓ | [Ca2+]i↑ [Ca2+]er↓ ERS↑ Apoptosis↑ | U73122 Xec Rya | [65] |
| Rat-NPCS | Excessive compression loading | IP3R–GRP75–VDAC1 Complex↑ | [Ca2+]m↑ ERS↑ HMGB1↑ LDH↑ ATP↓ C-PARP ↑ Nu-AIF↑ | 4-PBA Xestospongin-C VDAC1-siRNAGRP75-siRNA Ruthenium red (RR) NAC | [12] |
| Cellular Phenotype | Stimulating Factor | Quantity of ASICs | ASICs Inhibition | Roles of Blocking ASICs | References |
|---|---|---|---|---|---|
| HNPCS | Culture media with different pH levels | ASIC1a↑ | PcTx1 | LDH↓ Apoptosis↓ Senescence↓ | [7] |
| HNPCS | Culture media with different pH levels | ASIC3↑ | APETx2 | IL -1β, IL -6, NGF, and BDNF↓ | [29] |
| HNPCS | Different concentrations of lactate | ASIC1a↑ ASIC3↑ | Amiloride PcTx1 APETx2 | ASIC1, ASIC3↓ NLRP3, caspase-1, and IL-1β↓ Pyroptosis↓ ROS, LDH↓ | [34] |
| HNP-MSCS | Culture media with pH 6.6 | ASIC1↑ ASIC3↑ | Amiloride PcTx1 APETx2 | ASIC1, ASIC3↓ Proliferation↑ Recovery of the cell cycle↑ IL-6, IL-8↓ p53, p21, p27, rb1, and p16↓ Senescence↓ Collagen II, Aggrecan↑ MMP-3, MMP-9↓ | [25] |
| HNP-MSCS | Culture media with different pH levels | ASIC1↑ ASIC2↑ ASIC3↑ ASIC4↑ | Amiloride | Proliferation↑ Apoptosis↓ Inhibited the expression of ASICs Notch1, Jagged, Nanog, and Oct4↑ Collagen I, Collagen II, Aggrecan, and SOX-9↑ | [101] |
| HNP-MSCS | Culture media with pH 6.2 | — | Sa12b RAD-Sa12b hydrogels Amiloride | Proliferation↑ Apoptosis↓ ASIC1, ASIC2, ASIC3, and ASIC4↓ Collagen II, Aggrecan, and SOX-9↑ Oct1, Nanog, Jagged, and Notch12↑ Influx of Ca2+↓ Collagen I↓ P-ERK↓ | [32,107] |
| Rabbit-NPCS | 2% oxygen Overexpression of ASIC3 | ASIC3↑ | shRNA-ASIC3 | ASIC3↓ Proliferation↑ Apoptosis↓ Autophagy↓ LC3↓ HIF-1α↓ ERK1/2 and MAPK↓ | [8] |
| Rat-endplate chondrocytes | Culture media with pH 6.0 | ASIC1a↑ | ASIC1a-siRNA PcTx1 | Collagen II, Aggrecan↑ MMP-1, MMP-9, and MMP-13↓ NF-κB↓ Apoptosis↓ Influx of Ca2+↓ | [33,105] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, Z.; Wei, Z.; Zhang, G.; Chen, H.; Li, L.; Kang, X. Achilles’ Heel—The Significance of Maintaining Microenvironmental Homeostasis in the Nucleus Pulposus for Intervertebral Discs. Int. J. Mol. Sci. 2023, 24, 16592. https://doi.org/10.3390/ijms242316592
Luo Z, Wei Z, Zhang G, Chen H, Li L, Kang X. Achilles’ Heel—The Significance of Maintaining Microenvironmental Homeostasis in the Nucleus Pulposus for Intervertebral Discs. International Journal of Molecular Sciences. 2023; 24(23):16592. https://doi.org/10.3390/ijms242316592
Chicago/Turabian StyleLuo, Zhangbin, Ziyan Wei, Guangzhi Zhang, Haiwei Chen, Lei Li, and Xuewen Kang. 2023. "Achilles’ Heel—The Significance of Maintaining Microenvironmental Homeostasis in the Nucleus Pulposus for Intervertebral Discs" International Journal of Molecular Sciences 24, no. 23: 16592. https://doi.org/10.3390/ijms242316592





