Identification of Anti-Neuroinflammatory Bioactive Compounds in Essential Oils and Aqueous Distillation Residues Obtained from Commercial Varieties of Cannabis sativa L.
Abstract
:1. Introduction
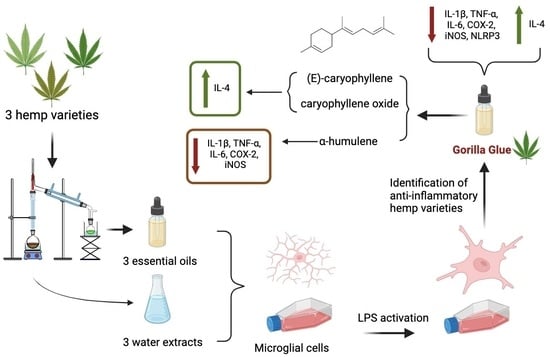
2. Results
2.1. Chemical Constituents of Hemp EOs and ARs
2.2. Potential Cytotoxicity of Hemp EOs and ARs
2.3. Effects of ARs and EOs on LPS-Induced NO Production in BV-2 Cells
2.4. Effects of WS and GG EOs on Pro- and Anti-Inflammatory Mediators in LPS-Activated BV-2 Cells
2.5. GG EO Modulates iNOS, NLRP3, and COX-2 Protein Expression in LPS-Activated BV-2 Cells
2.6. GG EO Counteracts NF-κB Nuclear Translocation
2.7. Effect of GG EO on ROS and GSH Levels
2.8. Modulation of p38 MAPK and Akt by GG EO
2.9. Analysis of the Contribution of (E)-Caryophyllene, Caryophyllene Oxide, and Humulene to the Anti-Inflammatory Activity of GG EO
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Plant Material and Extraction Processes
4.3. Cell Culture and Treatments
4.4. Viability Assay
4.5. Nitric Oxide Production
4.6. Measurement of Intracellular ROS Levels
4.7. Measurement of Reduced GSH Levels
4.8. Real-Time Polymerase Chain Reaction (PCR)
4.9. Western Immunoblotting
4.10. Immunofluorescence Confocal Microscopy
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lall, D.; Baloh, R.H. Microglia and C9orf72 in Neuroinflammation and ALS and Frontotemporal Dementia. J. Clin. Investig. 2017, 127, 3250–3258. [Google Scholar] [CrossRef]
- Boyd, R.J.; Avramopoulos, D.; Jantzie, L.L.; McCallion, A.S. Neuroinflammation Represents a Common Theme amongst Genetic and Environmental Risk Factors for Alzheimer and Parkinson Diseases. J. Neuroinflamm. 2022, 19, 223. [Google Scholar] [CrossRef]
- Saba, J.; Couselo, F.L.; Bruno, J.; Carniglia, L.; Durand, D.; Lasaga, M.; Caruso, C. Neuroinflammation in Huntington’s Disease: A Starring Role for Astrocyte and Microglia. Curr. Neuropharmacol. 2022, 20, 1116–1143. [Google Scholar] [CrossRef]
- Cervellati, C.; Trentini, A.; Pecorelli, A.; Valacchi, G. Inflammation in Neurological Disorders: The Thin Boundary between Brain and Periphery. Antioxid. Redox Signal. 2020, 33, 191–210. [Google Scholar] [CrossRef]
- Nayak, D.; Roth, T.L.; McGavern, D.B. Microglia Development and Function. Annu. Rev. Immunol. 2014, 32, 367–402. [Google Scholar] [CrossRef]
- Subhramanyam, C.S.; Wang, C.; Hu, Q.; Dheen, S.T. Microglia-Mediated Neuroinflammation in Neurodegenerative Diseases. Semin. Cell Dev. Biol. 2019, 94, 112–120. [Google Scholar] [CrossRef]
- Chen, T.; Li, Z.; Li, S.; Zou, Y.; Gao, X.; Shu, S.; Wang, Z. Cycloastragenol Suppresses M1 and Promotes M2 Polarization in LPS-Stimulated BV-2 Cells and Ischemic Stroke Mice. Int. Immunopharmacol. 2022, 113, 109290. [Google Scholar] [CrossRef]
- Mantovani, A.; Biswas, S.K.; Galdiero, M.R.; Sica, A.; Locati, M. Macrophage Plasticity and Polarization in Tissue Repair and Remodelling. J. Pathol. 2013, 229, 176–185. [Google Scholar] [CrossRef]
- Joseph, B.; Venero, J.L. (Eds.) A Brief Overview of Multitalented Microglia. In Microglia: Methods and Protocols; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2013; pp. 3–8. ISBN 978-1-62703-520-0. [Google Scholar]
- Sica, A.; Mantovani, A. Macrophage Plasticity and Polarization: In Vivo Veritas. J. Clin. Investig. 2012, 122, 787–795. [Google Scholar] [CrossRef]
- Gustin, A.; Kirchmeyer, M.; Koncina, E.; Felten, P.; Losciuto, S.; Heurtaux, T.; Tardivel, A.; Heuschling, P.; Dostert, C. NLRP3 Inflammasome Is Expressed and Functional in Mouse Brain Microglia but Not in Astrocytes. PLoS ONE 2015, 10, e0130624. [Google Scholar] [CrossRef]
- Hu, X.; Li, P.; Guo, Y.; Wang, H.; Leak, R.K.; Chen, S.; Gao, Y.; Chen, J. Microglia/Macrophage Polarization Dynamics Reveal Novel Mechanism of Injury Expansion After Focal Cerebral Ischemia. Stroke 2012, 43, 3063–3070. [Google Scholar] [CrossRef]
- Mikita, J.; Dubourdieu-Cassagno, N.; Deloire, M.S.; Vekris, A.; Biran, M.; Raffard, G.; Brochet, B.; Canron, M.-H.; Franconi, J.-M.; Boiziau, C.; et al. Altered M1/M2 Activation Patterns of Monocytes in Severe Relapsing Experimental Rat Model of Multiple Sclerosis. Amelioration of Clinical Status by M2 Activated Monocyte Administration. Mult. Scler. 2011, 17, 2–15. [Google Scholar] [CrossRef]
- Song, G.J.; Suk, K. Pharmacological Modulation of Functional Phenotypes of Microglia in Neurodegenerative Diseases. Front. Aging Neurosci. 2017, 9, 139. [Google Scholar] [CrossRef]
- Andre, C.M.; Hausman, J.-F.; Guerriero, G. Cannabis Sativa: The Plant of the Thousand and One Molecules. Front. Plant Sci. 2016, 7, 19. [Google Scholar] [CrossRef]
- Namdar, D.; Mazuz, M.; Ion, A.; Koltai, H. Variation in the Compositions of Cannabinoid and Terpenoids in Cannabis Sativa Derived from Inflorescence Position along the Stem and Extraction Methods. Ind. Crops Prod. 2018, 113, 376–382. [Google Scholar] [CrossRef]
- Fischedick, J.T.; Hazekamp, A.; Erkelens, T.; Choi, Y.H.; Verpoorte, R. Metabolic Fingerprinting of Cannabis Sativa L., Cannabinoids and Terpenoids for Chemotaxonomic and Drug Standardization Purposes. Phytochemistry 2010, 71, 2058–2073. [Google Scholar] [CrossRef]
- Smeriglio, A.; Trombetta, D.; Alloisio, S.; Cornara, L.; Denaro, M.; Garbati, P.; Grassi, G.; Circosta, C. Promising in Vitro Antioxidant, Anti-Acetylcholinesterase and Neuroactive Effects of Essential Oil from Two Non-Psychotropic Cannabis Sativa L. Biotypes. Phytother. Res. 2020, 34, 2287–2302. [Google Scholar] [CrossRef]
- Wanas, A.S.; Radwan, M.M.; Chandra, S.; Lata, H.; Mehmedic, Z.; Ali, A.; Baser, K.; Demirci, B.; ElSohly, M.A. Chemical Composition of Volatile Oils of Fresh and Air-Dried Buds of Cannabis Chemovars, Their Insecticidal and Repellent Activities. Nat. Prod. Commun. 2020, 15, 1934578X20926729. [Google Scholar] [CrossRef]
- Zheljazkov, V.D.; Sikora, V.; Dincheva, I.; Kačániová, M.; Astatkie, T.; Semerdjieva, I.B.; Latkovic, D. Industrial, CBD, and Wild Hemp: How Different Are Their Essential Oil Profile and Antimicrobial Activity? Molecules 2020, 25, 4631. [Google Scholar] [CrossRef]
- Benelli, G.; Pavela, R.; Petrelli, R.; Cappellacci, L.; Santini, G.; Fiorini, D.; Sut, S.; Dall’Acqua, S.; Canale, A.; Maggi, F. The Essential Oil from Industrial Hemp (Cannabis Sativa L.) by-Products as an Effective Tool for Insect Pest Management in Organic Crops. Ind. Crops Prod. 2018, 122, 308–315. [Google Scholar] [CrossRef]
- Borgonetti, V.; Benatti, C.; Governa, P.; Isoldi, G.; Pellati, F.; Alboni, S.; Tascedda, F.; Montopoli, M.; Galeotti, N.; Manetti, F.; et al. Non-Psychotropic Cannabis Sativa L. Phytocomplex Modulates Microglial Inflammatory Response through CB2 Receptors-, Endocannabinoids-, and NF-κB-Mediated Signaling. Phytother. Res. 2022, 36, 2246–2263. [Google Scholar] [CrossRef]
- Weston-Green, K.; Clunas, H.; Jimenez Naranjo, C. A Review of the Potential Use of Pinene and Linalool as Terpene-Based Medicines for Brain Health: Discovering Novel Therapeutics in the Flavours and Fragrances of Cannabis. Front. Psychiatry 2021, 12, 583211. [Google Scholar] [CrossRef]
- Matešić, N.; Jurina, T.; Benković, M.; Panić, M.; Valinger, D.; Gajdoš Kljusurić, J.; Jurinjak Tušek, A. Microwave-Assisted Extraction of Phenolic Compounds from Cannabis Sativa L.: Optimization and Kinetics Study. Sep. Sci. Technol. 2021, 56, 2047–2060. [Google Scholar] [CrossRef]
- Abdollahi, M.; Sefidkon, F.; Calagari, M.; Mousavi, A.; Mahomoodally, M.F. Impact of Four Hemp (Cannabis Sativa L.) Varieties and Stage of Plant Growth on Yield and Composition of Essential Oils. Ind. Crops Prod. 2020, 155, 112793. [Google Scholar] [CrossRef]
- Ascrizzi, R.; Ceccarini, L.; Tavarini, S.; Flamini, G.; Angelini, L.G. Valorisation of Hemp Inflorescence after Seed Harvest: Cultivation Site and Harvest Time Influence Agronomic Characteristics and Essential Oil Yield and Composition. Ind. Crops Prod. 2019, 139, 111541. [Google Scholar] [CrossRef]
- Nahler, G.; Jones, T.M.; Russo, E.B. Cannabidiol and Contributions of Major Hemp Phytocompounds to the “Entourage Effect”; Possible Mechanisms. J. Altern. Complement. Integr. Med. 2019, 5, 1–16. [Google Scholar] [CrossRef]
- Mazzara, E.; Torresi, J.; Fico, G.; Papini, A.; Kulbaka, N.; Dall’Acqua, S.; Sut, S.; Garzoli, S.; Mustafa, A.M.; Cappellacci, L.; et al. A Comprehensive Phytochemical Analysis of Terpenes, Polyphenols and Cannabinoids, and Micromorphological Characterization of 9 Commercial Varieties of Cannabis Sativa L. Plants 2022, 11, 891. [Google Scholar] [CrossRef]
- Berridge, M.V.; Herst, P.M.; Tan, A.S. Tetrazolium Dyes as Tools in Cell Biology: New Insights into Their Cellular Reduction. Biotechnol. Annu. Rev. 2005, 11, 127–152. [Google Scholar] [CrossRef]
- Surin, A.M.; Sharipov, R.R.; Krasil’nikova, I.A.; Boyarkin, D.P.; Lisina, O.Y.; Gorbacheva, L.R.; Avetisyan, A.V.; Pinelis, V.G. Disruption of Functional Activity of Mitochondria during MTT Assay of Viability of Cultured Neurons. Biochem. (Mosc.) 2017, 82, 737–749. [Google Scholar] [CrossRef]
- Stockert, J.C.; Horobin, R.W.; Colombo, L.L.; Blázquez-Castro, A. Tetrazolium Salts and Formazan Products in Cell Biology: Viability Assessment, Fluorescence Imaging, and Labeling Perspectives. Acta Histochem. 2018, 120, 159–167. [Google Scholar] [CrossRef]
- Lo, J.Y.; Kamarudin, M.N.A.; Hamdi, O.A.A.; Awang, K.; Kadir, H.A. Curcumenol Isolated from Curcuma Zedoaria Suppresses Akt-Mediated NF-κB Activation and P38 MAPK Signaling Pathway in LPS-Stimulated BV-2 Microglial Cells. Food Funct. 2015, 6, 3550–3559. [Google Scholar] [CrossRef]
- Shao, Q.; Zhang, X.; Chen, Y.; Zhu, C.-G.; Shi, J.-G.; Yuan, Y.; Chen, N. Anti-Neuroinflammatory Effects of 20C from Gastrodia Elata via Regulating Autophagy in LPS-Activated BV-2 Cells through MAPKs and TLR4/Akt/mTOR Signaling Pathways. Mol. Immunol. 2018, 99, 115–123. [Google Scholar] [CrossRef]
- Song, W.M.; Colonna, M. The Identity and Function of Microglia in Neurodegeneration. Nat. Immunol. 2018, 19, 1048–1058. [Google Scholar] [CrossRef]
- Saitgareeva, A.R.; Bulygin, K.V.; Gareev, I.F.; Beylerli, O.A.; Akhmadeeva, L.R. The Role of Microglia in the Development of Neurodegeneration. Neurol. Sci. 2020, 41, 3609–3615. [Google Scholar] [CrossRef]
- Russo, E.B. The Case for the Entourage Effect and Conventional Breeding of Clinical Cannabis: No “Strain”, No Gain. Front. Plant Sci. 2018, 9, 1969. [Google Scholar] [CrossRef]
- Nallathambi, R.; Mazuz, M.; Namdar, D.; Shik, M.; Namintzer, D.; Vinayaka, A.C.; Ion, A.; Faigenboim, A.; Nasser, A.; Laish, I.; et al. Identification of Synergistic Interaction Between Cannabis-Derived Compounds for Cytotoxic Activity in Colorectal Cancer Cell Lines and Colon Polyps That Induces Apoptosis-Related Cell Death and Distinct Gene Expression. Cannabis Cannabinoid Res. 2018, 3, 120–135. [Google Scholar] [CrossRef]
- Russo, E.B. Taming THC: Potential Cannabis Synergy and Phytocannabinoid-Terpenoid Entourage Effects. Br. J. Pharmacol. 2011, 163, 1344–1364. [Google Scholar] [CrossRef]
- Koltai, H.; Poulin, P.; Namdar, D. Promoting Cannabis Products to Pharmaceutical Drugs. Eur. J. Pharm. Sci. 2019, 132, 118–120. [Google Scholar] [CrossRef]
- Ben-Shabat, S.; Fride, E.; Sheskin, T.; Tamiri, T.; Rhee, M.H.; Vogel, Z.; Bisogno, T.; De Petrocellis, L.; Di Marzo, V.; Mechoulam, R. An Entourage Effect: Inactive Endogenous Fatty Acid Glycerol Esters Enhance 2-Arachidonoyl-Glycerol Cannabinoid Activity. Eur. J. Pharmacol. 1998, 353, 23–31. [Google Scholar] [CrossRef]
- Pannico, A.; Kyriacou, M.C.; El-Nakhel, C.; Graziani, G.; Carillo, P.; Corrado, G.; Ritieni, A.; Rouphael, Y.; De Pascale, S. Hemp Microgreens as an Innovative Functional Food: Variation in the Organic Acids, Amino Acids, Polyphenols, and Cannabinoids Composition of Six Hemp Cultivars. Food Res. Int. 2022, 161, 111863. [Google Scholar] [CrossRef]
- Bowen, J.K.; Chaparro, J.M.; McCorkle, A.M.; Palumbo, E.; Prenni, J.E. The Impact of Extraction Protocol on the Chemical Profile of Cannabis Extracts from a Single Cultivar. Sci. Rep. 2021, 11, 21801. [Google Scholar] [CrossRef]
- Angeloni, S.; Freschi, M.; Marrazzo, P.; Hrelia, S.; Beghelli, D.; Juan-García, A.; Juan, C.; Caprioli, G.; Sagratini, G.; Angeloni, C. Antioxidant and Anti-Inflammatory Profiles of Spent Coffee Ground Extracts for the Treatment of Neurodegeneration. Oxid. Med. Cell. Longev. 2021, 2021, 6620913. [Google Scholar] [CrossRef]
- Lund, S.; Christensen, K.V.; Hedtjärn, M.; Mortensen, A.L.; Hagberg, H.; Falsig, J.; Hasseldam, H.; Schrattenholz, A.; Pörzgen, P.; Leist, M. The Dynamics of the LPS Triggered Inflammatory Response of Murine Microglia under Different Culture and in Vivo Conditions. J. Neuroimmunol. 2006, 180, 71–87. [Google Scholar] [CrossRef]
- Thomas, D.M.; Francescutti-Verbeem, D.M.; Kuhn, D.M. Gene Expression Profile of Activated Microglia under Conditions Associated with Dopamine Neuronal Damage. FASEB J. 2006, 20, 515–517. [Google Scholar] [CrossRef]
- Batista, C.R.A.; Gomes, G.F.; Candelario-Jalil, E.; Fiebich, B.L.; de Oliveira, A.C.P. Lipopolysaccharide-Induced Neuroinflammation as a Bridge to Understand Neurodegeneration. Int. J. Mol. Sci. 2019, 20, 2293. [Google Scholar] [CrossRef]
- Orlando, G.; Adorisio, S.; Delfino, D.; Chiavaroli, A.; Brunetti, L.; Recinella, L.; Leone, S.; D’Antonio, M.; Zengin, G.; Acquaviva, A.; et al. Comparative Investigation of Composition, Antifungal, and Anti-Inflammatory Effects of the Essential Oil from Three Industrial Hemp Varieties from Italian Cultivation. Antibiotics 2021, 10, 334. [Google Scholar] [CrossRef]
- Jin, X.; Liu, M.-Y.; Zhang, D.-F.; Zhong, X.; Du, K.; Qian, P.; Gao, H.; Wei, M.-J. Natural Products as a Potential Modulator of Microglial Polarization in Neurodegenerative Diseases. Pharmacol. Res. 2019, 145, 104253. [Google Scholar] [CrossRef]
- Guo, S.; Wang, H.; Yin, Y. Microglia Polarization from M1 to M2 in Neurodegenerative Diseases. Front. Aging Neurosci. 2022, 14, 815347. [Google Scholar] [CrossRef]
- Magni, P.; Ruscica, M.; Dozio, E.; Rizzi, E.; Beretta, G.; Maffei Facino, R. Parthenolide Inhibits the LPS-Induced Secretion of IL-6 and TNF-α and NF-κB Nuclear Translocation in BV-2 Microglia. Phytother. Res. 2012, 26, 1405–1409. [Google Scholar] [CrossRef]
- Zhou, X.; Spittau, B.; Krieglstein, K. TGFβ Signalling Plays an Important Role in IL4-Induced Alternative Activation of Microglia. J. Neuroinflamm. 2012, 9, 210. [Google Scholar] [CrossRef]
- Libermann, T.A.; Baltimore, D. Activation of Interleukin-6 Gene Expression through the NF-Kappa B Transcription Factor. Mol. Cell. Biol. 1990, 10, 2327–2334. [Google Scholar] [CrossRef]
- Arias-Salvatierra, D.; Silbergeld, E.K.; Acosta-Saavedra, L.C.; Calderon-Aranda, E.S. Role of Nitric Oxide Produced by iNOS through NF-κB Pathway in Migration of Cerebellar Granule Neurons Induced by Lipopolysaccharide. Cell. Signal. 2011, 23, 425–435. [Google Scholar] [CrossRef]
- Kim, B.-W.; Koppula, S.; Hong, S.-S.; Jeon, S.-B.; Kwon, J.-H.; Hwang, B.-Y.; Park, E.-J.; Choi, D.-K. Regulation of Microglia Activity by Glaucocalyxin-A: Attenuation of Lipopolysaccharide-Stimulated Neuroinflammation through NF-κB and P38 MAPK Signaling Pathways. PLoS ONE 2013, 8, e55792. [Google Scholar] [CrossRef]
- Janpaijit, S.; Lertpatipanpong, P.; Sillapachaiyaporn, C.; Baek, S.J.; Charoenkiatkul, S.; Tencomnao, T.; Sukprasansap, M. Anti-Neuroinflammatory Effects of Cleistocalyx Nervosum Var. Paniala Berry-Seed Extract in BV-2 Microglial Cells via Inhibition of MAPKs/NF-κB Signaling Pathway. Heliyon 2022, 8, e11869. [Google Scholar] [CrossRef]
- Awasthi, A.; Raju, M.B.; Rahman, M.A. Current Insights of Inhibitors of P38 Mitogen-Activated Protein Kinase in Inflammation. Med. Chem. 2021, 17, 555–575. [Google Scholar] [CrossRef]
- Lee, J.K.; Kim, N.-J. Recent Advances in the Inhibition of P38 MAPK as a Potential Strategy for the Treatment of Alzheimer’s Disease. Molecules 2017, 22, 1287. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, M.; He, S.; Xu, Q.; Du, C.; Zhu, H.; Lin, T.; Wang, G.; Tian, W.; Chen, H. Polyhydric Stigmastane-Type Steroids Derivative from Vernonia Amygdalina and Their Anti-Neuroinflammatory Activity. Pharmaceuticals 2022, 15, 1160. [Google Scholar] [CrossRef]
- Dang, T.K.; Hong, S.-M.; Dao, V.T.; Tran, P.T.T.; Tran, H.T.; Do, G.H.; Hai, T.N.; Nguyet Pham, H.T.; Kim, S.Y. Anti-Neuroinflammatory Effects of Alkaloid-Enriched Extract from Huperzia Serrata on Lipopolysaccharide-Stimulated BV-2 Microglial Cells. Pharm. Biol. 2023, 61, 135–143. [Google Scholar] [CrossRef]
- Zhou, L.-T.; Wang, K.-J.; Li, L.; Li, H.; Geng, M. Pinocembrin Inhibits Lipopolysaccharide-Induced Inflammatory Mediators Production in BV2 Microglial Cells through Suppression of PI3K/Akt/NF-κB Pathway. Eur. J. Pharmacol. 2015, 761, 211–216. [Google Scholar] [CrossRef]
- Zhao, D.; Kwon, S.-H.; Chun, Y.S.; Gu, M.-Y.; Yang, H.O. Anti-Neuroinflammatory Effects of Fucoxanthin via Inhibition of Akt/NF-κB and MAPKs/AP-1 Pathways and Activation of PKA/CREB Pathway in Lipopolysaccharide-Activated BV-2 Microglial Cells. Neurochem. Res. 2017, 42, 667–677. [Google Scholar] [CrossRef]
- Solleiro-Villavicencio, H.; Rivas-Arancibia, S. Effect of Chronic Oxidative Stress on Neuroinflammatory Response Mediated by CD4+T Cells in Neurodegenerative Diseases. Front. Cell. Neurosci. 2018, 12, 114. [Google Scholar] [CrossRef]
- Pellegrini, M.; Palmieri, S.; Ricci, A.; Serio, A.; Paparella, A.; Lo Sterzo, C. In Vitro Antioxidant and Antimicrobial Activity of Cannabis Sativa L. Cv ‘Futura 75’ Essential Oil. Nat. Prod. Res. 2021, 35, 6020–6024. [Google Scholar] [CrossRef]
- Nafis, A.; Kasrati, A.; Jamali, C.A.; Mezrioui, N.; Setzer, W.; Abbad, A.; Hassani, L. Antioxidant Activity and Evidence for Synergism of Cannabis Sativa (L.) Essential Oil with Antimicrobial Standards. Ind. Crops Prod. 2019, 137, 396–400. [Google Scholar] [CrossRef]
- Zengin, G.; Menghini, L.; Di Sotto, A.; Mancinelli, R.; Sisto, F.; Carradori, S.; Cesa, S.; Fraschetti, C.; Filippi, A.; Angiolella, L.; et al. Chromatographic Analyses, In Vitro Biological Activities, and Cytotoxicity of Cannabis Sativa L. Essential Oil: A Multidisciplinary Study. Molecules 2018, 23, 3266. [Google Scholar] [CrossRef]
- Askari, V.R.; Shafiee-Nick, R. The Protective Effects of β-Caryophyllene on LPS-Induced Primary Microglia M1/M2 Imbalance: A Mechanistic Evaluation. Life Sci. 2019, 219, 40–73. [Google Scholar] [CrossRef]
- Rossi, M.; Freschi, M.; de Camargo Nascente, L.; Salerno, A.; de Melo Viana Teixeira, S.; Nachon, F.; Chantegreil, F.; Soukup, O.; Prchal, L.; Malaguti, M.; et al. Sustainable Drug Discovery of Multi-Target-Directed Ligands for Alzheimer’s Disease. J Med. Chem. 2021, 64, 4972–4990. [Google Scholar] [CrossRef]
- Barbalace, M.C.; Zallocco, L.; Beghelli, D.; Ronci, M.; Scortichini, S.; Digiacomo, M.; Macchia, M.; Mazzoni, M.R.; Fiorini, D.; Lucacchini, A.; et al. Antioxidant and Neuroprotective Activity of Extra Virgin Olive Oil Extracts Obtained from Quercetano Cultivar Trees Grown in Different Areas of the Tuscany Region (Italy). Antioxidants 2021, 10, 421. [Google Scholar] [CrossRef]
- Marrazzo, P.; Angeloni, C.; Freschi, M.; Lorenzini, A.; Prata, C.; Maraldi, T.; Hrelia, S. Combination of Epigallocatechin Gallate and Sulforaphane Counteracts In Vitro Oxidative Stress and Delays Stemness Loss of Amniotic Fluid Stem Cells. Oxid. Med. Cell. Longev. 2018, 2018, 5263985. [Google Scholar] [CrossRef]
- Angeloni, C.; Teti, G.; Barbalace, M.C.; Malaguti, M.; Falconi, M.; Hrelia, S. 17β-Estradiol Enhances Sulforaphane Cardioprotection against Oxidative Stress. J. Nutr. Biochem. 2017, 42, 26–36. [Google Scholar] [CrossRef]
- Lenzi, M.; Cocchi, V.; Novaković, A.; Karaman, M.; Sakač, M.; Mandić, A.; Pojić, M.; Barbalace, M.C.; Angeloni, C.; Hrelia, P.; et al. Meripilus Giganteus Ethanolic Extract Exhibits Pro-Apoptotic and Anti-Proliferative Effects in Leukemic Cell Lines. BMC Complement. Altern. Med. 2018, 18, 300. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing Real-Time PCR Data by the Comparative C(T) Method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Angeloni, C.; Malaguti, M.; Rizzo, B.; Barbalace, M.C.; Fabbri, D.; Hrelia, S. Neuroprotective Effect of Sulforaphane against Methylglyoxal Cytotoxicity. Chem. Res. Toxicol. 2015, 28, 1234–1245. [Google Scholar] [CrossRef]









| Compound | Hemp Varieties | ||
|---|---|---|---|
| White Shark | Lemon Conti Kush New | Gorilla Glue | |
| EO | (g/100 g) | ||
| α-pinene | 8.53 | 3.24 | 0.74 |
| β-pinene | 3.59 | 2.78 | 0.82 |
| myrcene | 20.28 | 11.14 | 7.16 |
| limonene | 9.17 | 5.97 | 6.79 |
| terpinolene | 9.51 | 30.47 | 0.27 |
| (E)-caryophyllene | 10.79 | 10.40 | 18.94 |
| humulene | 2.96 | 3.04 | 4.86 |
| caryophyllene oxide | 3.52 | 3.34 | 6.62 |
| CBD | 5.54 | 2.91 | 3.06 |
| AR | (g/100 g) | ||
| cannabisin B | 0.71 | 0.35 | 0.30 |
| luteolin-C-hexoside-O-rutinoside | 0.23 | 0.50 | 2.51 |
| rutin | 7.93 | 0.35 | 0.75 |
| luteolin-hexoside-hexoside | 0.21 | 2.35 | 0.21 |
| apigenin-hexoside-glucuronide | 1.12 | 0.32 | 0.79 |
| Gene | 5′-Forward-3′ | 5′-Reverse-3′ |
|---|---|---|
| IL-1β | GTTCCCATTAGACAACTGCACTACAG | GTCGTTGCTTGGTTCTCCTTGTA |
| TNF-α | CCCCAAAGGGATGAGAAGTTC | CCTCCACTTGGTGGTTTGCT |
| iNOS | CCTCCTCCACCCTACCAAGT | CACCCAAAGTGCTTCAGTCA |
| COX-2 | TGGGGTGATGAGCAACTATT | AAGGAGCTCTGGGTCAAACT |
| Nlrp3 | GATGCTGGAATTAGACAACTG | GTACATTTCACCCAACTGTAG |
| IL-6 | TCCTTCAGAGAGATACAGAAAC | TTCTGTGACTCCAGCTTATC |
| IL-4 | CTGGATTCATCGATAAGCTG | TTTGCATGATGCTCTTTAGG |
| MRC1 | GTTATGAAAGGCAAGGATGG | ATCAGTGAAGGTGGATAGAG |
| GAPDH | ACCACAGTCCATGCCATCAC | TCCACCACCCTGTTGCTGTA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbalace, M.C.; Freschi, M.; Rinaldi, I.; Mazzara, E.; Maraldi, T.; Malaguti, M.; Prata, C.; Maggi, F.; Petrelli, R.; Hrelia, S.; et al. Identification of Anti-Neuroinflammatory Bioactive Compounds in Essential Oils and Aqueous Distillation Residues Obtained from Commercial Varieties of Cannabis sativa L. Int. J. Mol. Sci. 2023, 24, 16601. https://doi.org/10.3390/ijms242316601
Barbalace MC, Freschi M, Rinaldi I, Mazzara E, Maraldi T, Malaguti M, Prata C, Maggi F, Petrelli R, Hrelia S, et al. Identification of Anti-Neuroinflammatory Bioactive Compounds in Essential Oils and Aqueous Distillation Residues Obtained from Commercial Varieties of Cannabis sativa L. International Journal of Molecular Sciences. 2023; 24(23):16601. https://doi.org/10.3390/ijms242316601
Chicago/Turabian StyleBarbalace, Maria Cristina, Michela Freschi, Irene Rinaldi, Eugenia Mazzara, Tullia Maraldi, Marco Malaguti, Cecilia Prata, Filippo Maggi, Riccardo Petrelli, Silvana Hrelia, and et al. 2023. "Identification of Anti-Neuroinflammatory Bioactive Compounds in Essential Oils and Aqueous Distillation Residues Obtained from Commercial Varieties of Cannabis sativa L." International Journal of Molecular Sciences 24, no. 23: 16601. https://doi.org/10.3390/ijms242316601
APA StyleBarbalace, M. C., Freschi, M., Rinaldi, I., Mazzara, E., Maraldi, T., Malaguti, M., Prata, C., Maggi, F., Petrelli, R., Hrelia, S., & Angeloni, C. (2023). Identification of Anti-Neuroinflammatory Bioactive Compounds in Essential Oils and Aqueous Distillation Residues Obtained from Commercial Varieties of Cannabis sativa L. International Journal of Molecular Sciences, 24(23), 16601. https://doi.org/10.3390/ijms242316601