X-ray and DNA Damage: Limitations of the Dose as a Parameter for In Vitro Studies
Abstract
:1. Introduction
2. Results and Discussion
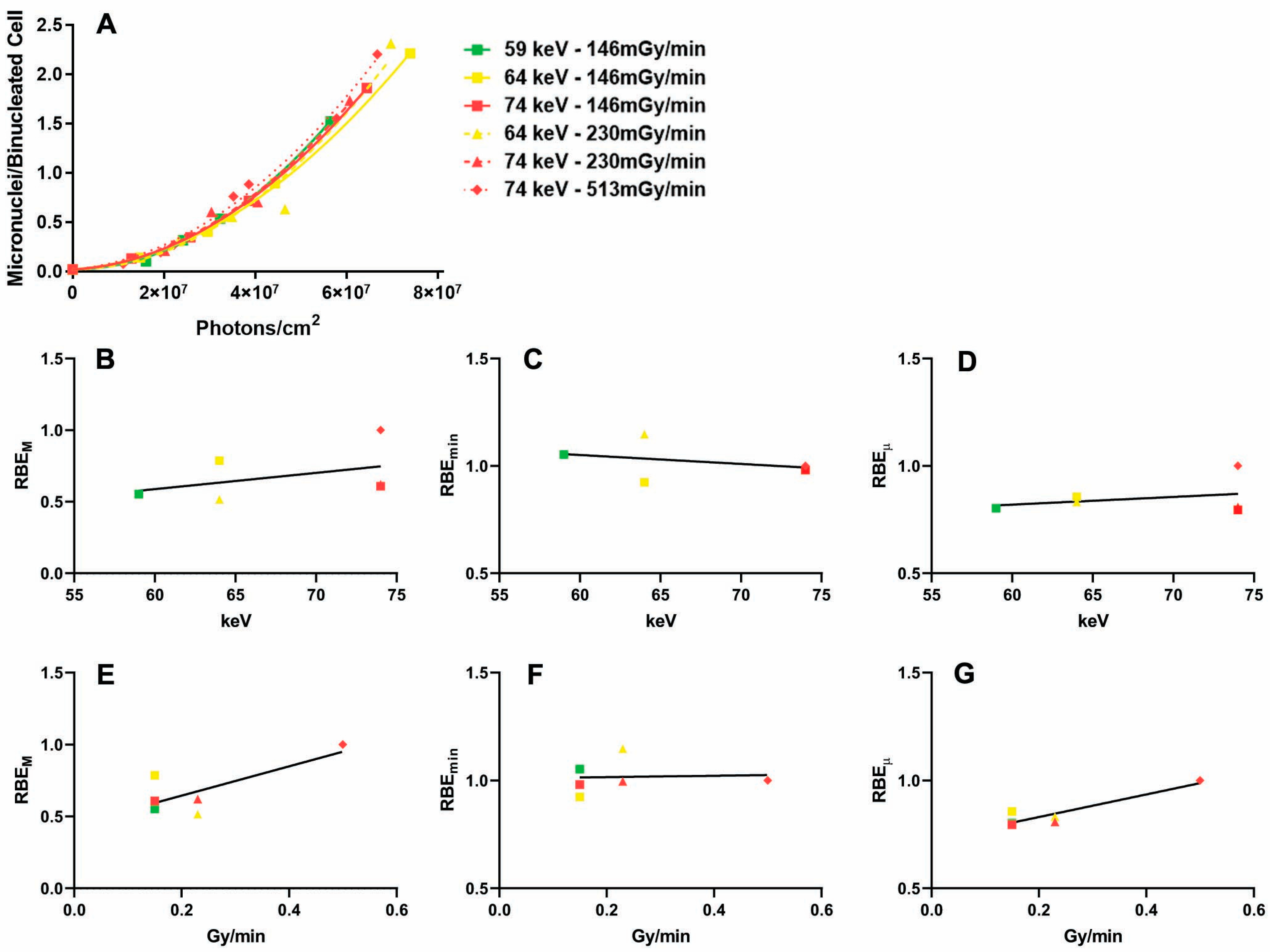
2.1. Influence of the Dose Rate on the Dose–Response Relationship
2.2. Influence of Photon Energy on the Dose–Response Relationship
2.3. Absorbed Dose Versus Fluence
2.4. Incident Photons and DSBs
3. Materials and Methods
3.1. X-Irradiation
3.2. Cell Cultures
3.3. Micronucleus Test
3.4. Clonogenic Assay
3.5. Comet Assay
3.6. Statistical Analyses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wigoder, S.B.; Patten, R.E. Variations in the growth of irradiated bean roots. Br. J. Radiol. 1929, 2, 588–596. [Google Scholar] [CrossRef]
- Cantril, S.T.; Parker, H. The Tolerance Dose; Argonne National Laboratory: Technical Information Division, Oak Ridge Directed Operations: DuPage, IL, USA, 1945. [Google Scholar]
- ICRP. The 2007 Recommendations of the International Commission on Radiological Protection: ICRP Publication 103. Ann. ICRP 2007, 37, 1–332. [Google Scholar]
- Marthinsen, A.B.; Gisetstad, R.; Danielsen, S.; Frengen, J.; Strickert, T.; Lundgren, S. Relative biological effectiveness of photon energies used in brachytherapy and intraoperative radiotherapy techniques for two breast cancer cell lines. Acta Oncol. 2010, 49, 1261–1268. [Google Scholar] [CrossRef]
- Frankenberg, D.; Kelnhofer, K.; Bär, K.; Frankenberg-Schwager, M. Enhanced neoplastic transformation by mammography X rays relative to 200 kVp X rays: Indication for a strong dependence on photon energy of the RBE(M) for various end points. Radiat. Res. 2002, 157, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Virsik, R.P.; Harder, D.; Hansmann, I. The RBE of 30 kV X-rays for the induction of dicentric chromosomes in human lymphocytes. Radiat. Environ. Biophys. 1977, 14, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Virsik, R.P.; Harder, D. Analysis of radiation-induced acentric fragments in human G0 lymphocytes. Radiat. Environ. Biophys. 1981, 19, 29–40. [Google Scholar] [CrossRef]
- Schmid, E.; Regulla, D.; Kramer, H.M.; Harder, D. The effect of 29 kV X rays on the dose response of chromosome aberrations in human lymphocytes. Radiat. Res. 2002, 158, 771–777. [Google Scholar] [CrossRef]
- Guerrero-Carbajal, C.; Edwards, A.A.; Lloyd, D.C. Induction of chromosome aberration in human lymphocytes and its dependence on X ray energy. Radiat. Prot. Dosim. 2003, 106, 131–135. [Google Scholar] [CrossRef]
- Hill, M.A. The variation in biological effectiveness of X-rays and gamma rays with energy. Radiat. Prot. Dosim. 2004, 112, 471–481. [Google Scholar] [CrossRef]
- Mestres, M.; Caballín, M.R.; Barrios, L.; Ribas, M.; Barquinero, J.F. RBE of X rays of different energies: A cytogenetic evaluation by FISH. Radiat. Res. 2008, 170, 93–100. [Google Scholar] [CrossRef]
- Russ, S. On the effect of X-rays of different wave-lengths upon some animal tissues.—Proof of differential action. Proc. R. Soc. Lond. B 1923, 95, 131–142. [Google Scholar] [CrossRef]
- Strangeways, T.S.P.; Hopwood, F.L. The effects of X-rays upon mitotic cell division in tissue cultures in vitro. Proc. R. Soc. Lond. B 1926, 100, 283–293. [Google Scholar]
- Ruiz de Almodóvar, J.M.; Bush, C.; Peacock, J.H.; Steel, G.G.; Whitaker, S.J.; McMillan, T.J. Dose-rate effect for DNA damage induced by ionizing radiation in human tumor cells. Radiat. Res. 1994, 138 (Suppl. S1), S93–S96. [Google Scholar] [CrossRef] [PubMed]
- Dikomey, E.; Brammer, I. Relationship between cellular radiosensitivity and non-repaired double-strand breaks studied for different growth states, dose rates and plating conditions in a normal human fibroblast line. Int. J. Radiat. Biol. 2000, 76, 773–781. [Google Scholar]
- Bertucci, A.; Smilenov, L.B.; Turner, H.C.; Amundson, S.A.; Brenner, D.J. In vitro RABiT measurement of dose rate effects on radiation induction of micronuclei in human peripheral blood lymphocytes. Radiat. Environ. Biophys. 2016, 55, 53–59. [Google Scholar] [CrossRef]
- Krönig, B.; Friedrich, W. Physikalische und Biologische Grundlagen der Strahlentherapie; Urban and Schwarzenberg: Berlin, Germany, 1918; Volume 3, pp. 211–228. [Google Scholar]
- Canti, R.G.; Spear, F.G. The effect of gamma irradiation on cell division in tissue culture in vitro. Proceedings of the Royal Society of London. Ser. B Contain. Pap. A Biol. Character 1927, 102, 92–101. [Google Scholar]
- Udroiu, I.; Sgura, A. Quantitative relationships between acentric fragments and micronuclei: New models and implications for curve fitting. Int. J. Radiat. Biol. 2020, 96, 197–205. [Google Scholar] [CrossRef]
- Sgura, A.; Antoccia, A.; Cherubini, R.; Dalla Vecchia, M.; Tiveron, P.; Degrassi, F.; Tanzarella, C. Micronuclei, CREST-positive micronuclei and cell inactivation induced in Chinese hamster cells by radiation with different quality. Int. J. Radiat. Biol. 2000, 76, 367–374. [Google Scholar]
- Degrassi, F.; Tanzarella, C. Immunofluorescent staining of kinetochores in micronuclei: A new assay for the detection of aneuploidy. Mutat. Res. 1988, 203, 339–345. [Google Scholar] [CrossRef]
- Kellerer, A.M. Electron spectra and the RBE of X rays. Radiat. Res. 2002, 158, 13–22. [Google Scholar] [CrossRef]
- Pimblott, S.M.; LaVerne, J.A. Effect of electron energy on the radiation chemistry of liquid water. Radiat. Res. 1998, 150, 159–169. [Google Scholar] [CrossRef]
- Udroiu, I.; Sgura, A.; Chendi, A.; Lasagni, L.; Bertolini, M.; Fioroni, F.; Piccagli, V.; Moramarco, A.; Romano, M.G.; Fontana, L.; et al. DNA damage in lens epithelial cells exposed to occupationally-relevant X-ray doses and role in cataract formation. Sci. Rep. 2020, 10, 21693. [Google Scholar] [CrossRef]
- Udroiu, I.; Antoccia, A.; Tanzarella, C.; Giuliani, L.; Pacchierotti, F.; Cordelli, E.; Eleuteri, P.; Villani, P.; Sgura, A. Genotoxicity induced by foetal and infant exposure to magnetic fields and modulation of ionising radiation effects. PLoS ONE 2015, 10, e0142259. [Google Scholar] [CrossRef]
- Dale, R.G.; Jones, B. The assessment of RBE effects using the concept of biologically effective dose. Int. J. Radiat. Oncol. Biol. Phys. 1999, 43, 639–645. [Google Scholar] [CrossRef] [PubMed]
- Carabe-Fernandez, A.; Dale, R.G.; Jones, B. The incorporation of the concept of minimum RBE (RbEmin) into the linear-quadratic model and the potential for improved radiobiological analysis of high-LET treatments. Int. J. Radiat. Biol. 2007, 83, 27–39. [Google Scholar] [CrossRef]
- Little, M.P.; Lambert, B.E. Systematic review of experimental studies on the relative biological effectiveness of tritium. Radiat. Environ. Biophys. 2008, 47, 71–93. [Google Scholar] [CrossRef] [PubMed]
- Mills, C.E.; Thome, C.; Koff, D.; Andrews, D.W.; Boreham, D.R. The relative biological effectiveness of low-dose mammography quality X rays in the human breast MCF-10A cell line. Radiat. Res. 2015, 183, 42–51. [Google Scholar] [CrossRef]
- ICRP. Relative biological effectiveness (RBE), quality factor (Q), and radiation weighting factor (wR): ICRP Publication 92. Ann. ICRP 2003, 33, 1–117. [Google Scholar]
- ICRP. Recommendations of the International Commission on Radiological Protection and of the International Commission on Radiological Units (NBS 47, 1950). Revised by the International Commission on Radiological Protection at the Sixth International Congress of Radiology, London, UK, 1950. Brit. J. Radiol. 1951, 24, 46–53. [Google Scholar]
- Marcu, L.G.; Bezak, E.; Sykes, P.J. Radiation Research Journals Need to Stipulate Minimal Dosimetry Requirements for Publishing Research Using X-Radiation Exposures. Radiat Res. 2022, 198, 204–206. [Google Scholar] [CrossRef]
- Trompier, F.; DeWerd, L.A.; Poirier, Y.; Dos Santos, M.; Sheng, K.; Kunugi, K.A.; Winters, T.A.; DiCarlo, A.L.; Satyamitra, M. Minimum reporting standards should be expected for preclinical radiobiology irradiators and dosimetry in the published literature. Int. J. Radiat. Biol. 2023, in press. [CrossRef] [PubMed]

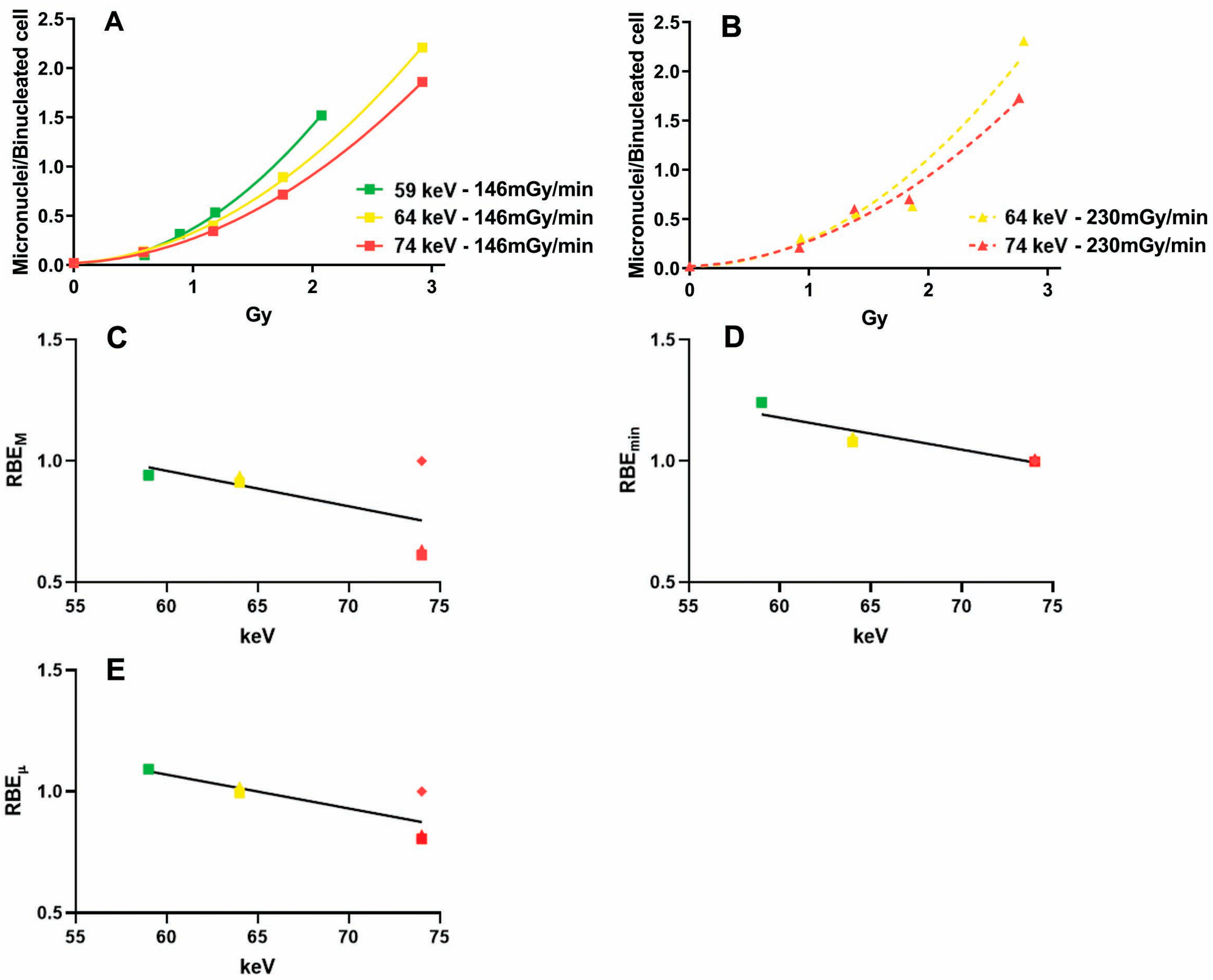
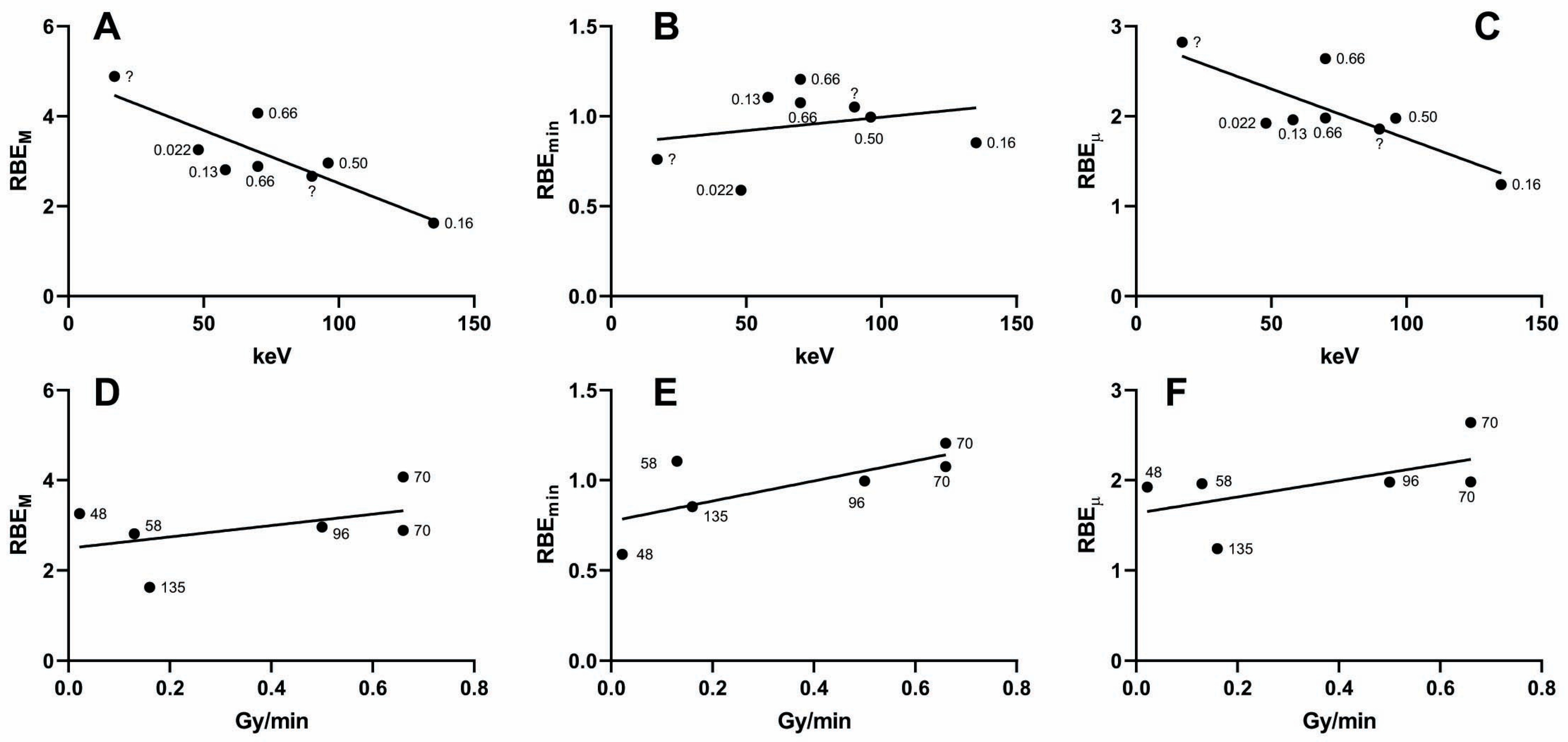
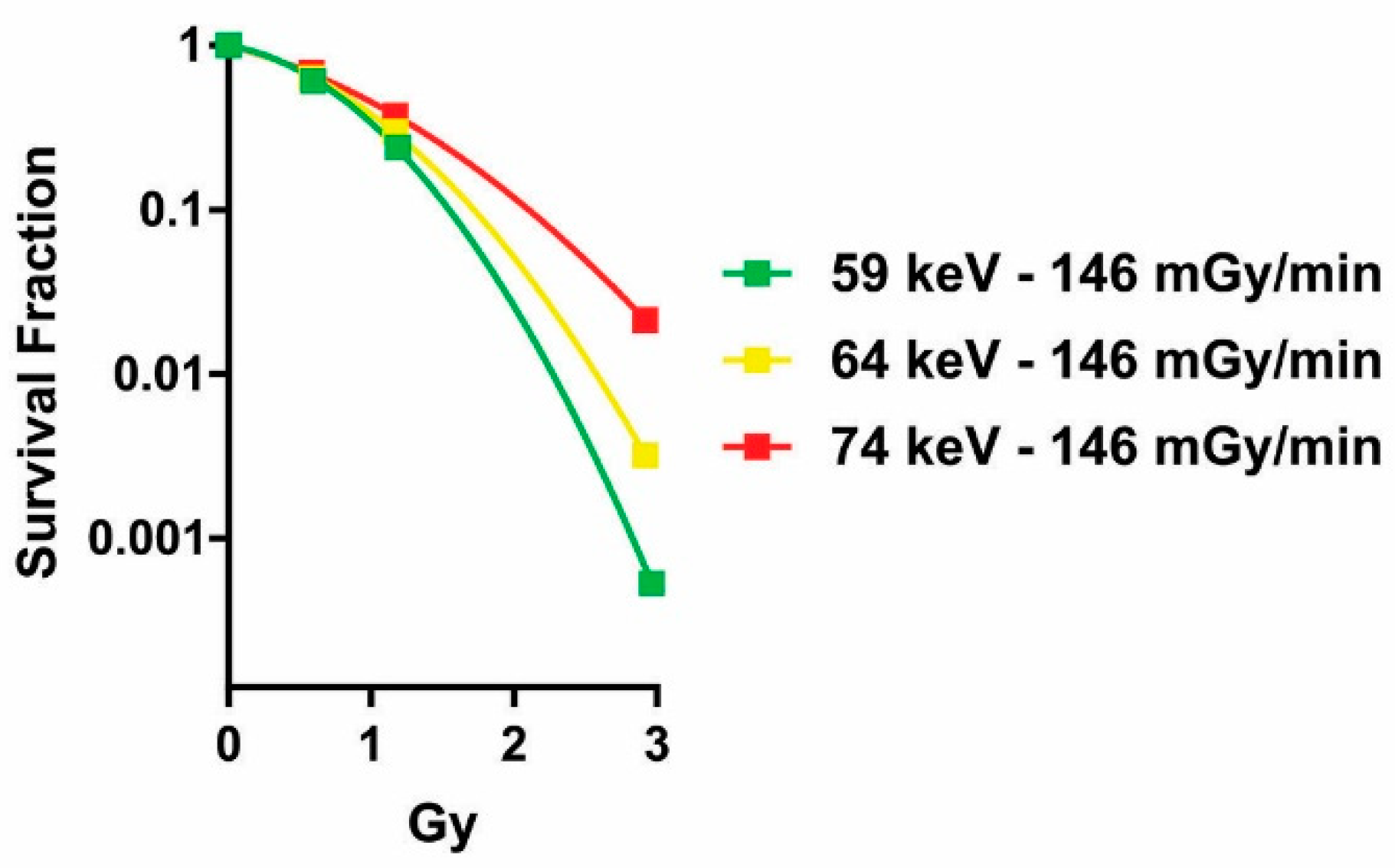
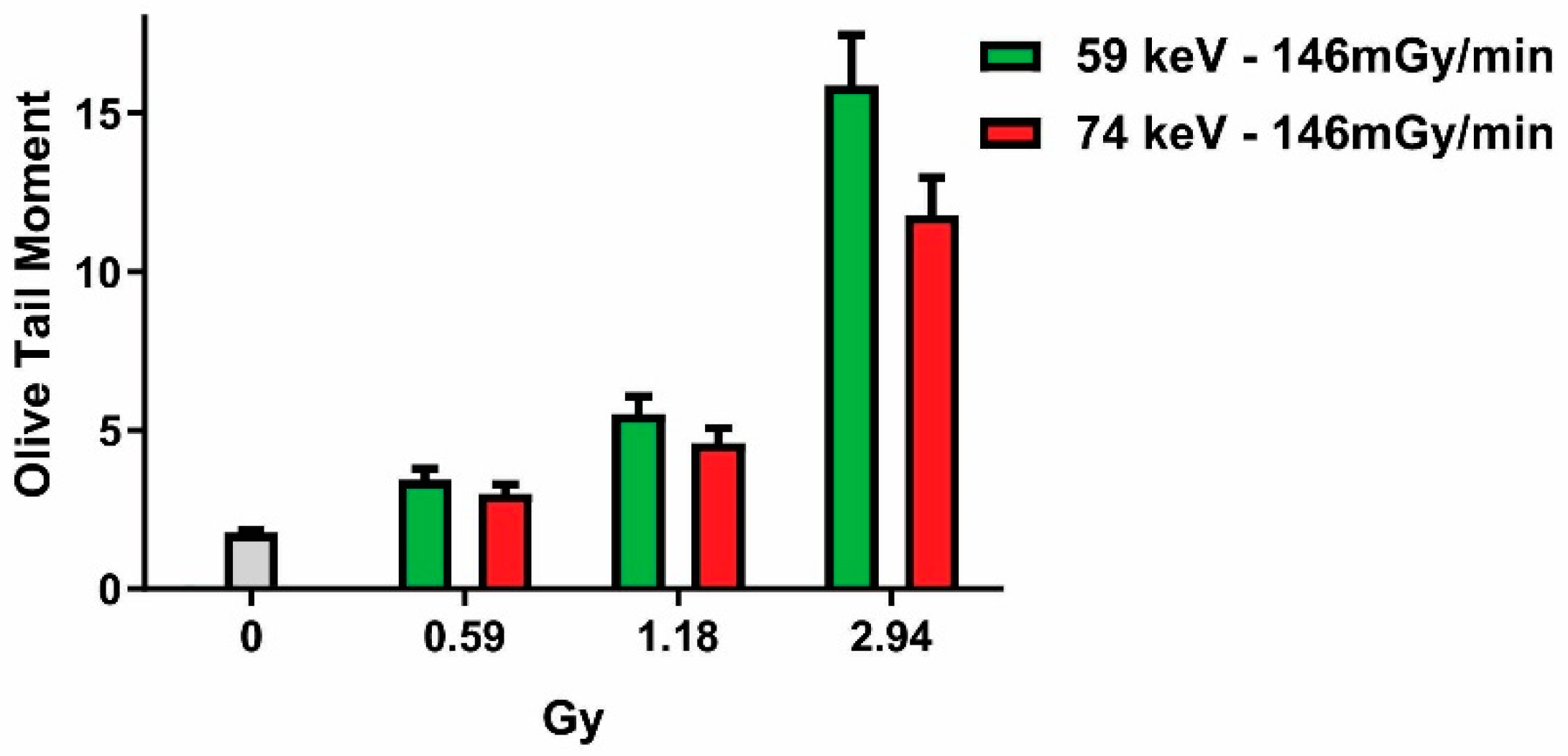
| Cell Line 1 | Photon Energy (keV) | Dose Rate (Gy/min) | α | β | RBEM 2 | RBEmin 2 | RBEµ 2 | Reference |
|---|---|---|---|---|---|---|---|---|
| B3 | 74 | 0.51 | 0.085 | 0.199 | 1 | 1 | 1 | This study |
| B3 | 74 | 0.23 | 0.054 | 0.202 | 0.64 | 1.01 | 0.82 | This study |
| B3 | 64 | 0.23 | 0.08 | 0.240 | 0.94 | 1.10 | 1.02 | This study |
| HFFF2 | 74 | 0.51 | 0.061 | 0.017 | 1 | 1 | 1 | [19] |
| HFFF2 | 74 | 0.23 | 0.043 | 0.016 | 0.7 | 0.99 | 0.84 | This study |
| HFFF2 | 64 | 0.23 | 0.056 | 0.02 | 0.92 | 1.09 | 1.01 | This study |
| Cl-1 | 74 | 0.7 | 0.05 | 0.022 | 1 | 1 | 1 | [20] |
| Cl-1 | 68 | 0.7 | 0.056 | 0.025 | 1.12 | 1.06 | 1.09 | [21] |
| Dose Rate | Peak Potential | Peak Potential | Dose Rate | |
| 146 mGy/min | 100 kVp | 100 kVp | 146 mGy/min | |
| 120 kVp | 120 kVp | 146 mGy/min | ||
| 168 kVp | 230 mGy/min | |||
| 230 mGy/min | 120 kVp | 168 kVp | 146 mGy/min | |
| 168 kVp | 230 mGy/min | |||
| 513 mGy/min | 168 kVp | 513 mGy/min |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Udroiu, I.; Sgura, A. X-ray and DNA Damage: Limitations of the Dose as a Parameter for In Vitro Studies. Int. J. Mol. Sci. 2023, 24, 16643. https://doi.org/10.3390/ijms242316643
Udroiu I, Sgura A. X-ray and DNA Damage: Limitations of the Dose as a Parameter for In Vitro Studies. International Journal of Molecular Sciences. 2023; 24(23):16643. https://doi.org/10.3390/ijms242316643
Chicago/Turabian StyleUdroiu, Ion, and Antonella Sgura. 2023. "X-ray and DNA Damage: Limitations of the Dose as a Parameter for In Vitro Studies" International Journal of Molecular Sciences 24, no. 23: 16643. https://doi.org/10.3390/ijms242316643





