Beta-Barrel Channel Response to High Electric Fields: Functional Gating or Reversible Denaturation?
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Channel Reconstitution
4.3. Voltage Gating Measurements
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sigworth, F.J. Structural biology: Life’s transistors. Nature 2003, 423, 21–22. [Google Scholar] [CrossRef]
- Swartz, K.J. Sensing voltage across lipid membranes. Nature 2008, 456, 891–897. [Google Scholar] [CrossRef] [PubMed]
- Bezanilla, F. How membrane proteins sense voltage. Nat. Rev. Mol. Cell Biol. 2008, 9, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Bezanilla, F. Gating currents. J. Gen. Physiol. 2018, 150, 911–932. [Google Scholar] [CrossRef] [PubMed]
- Schein, S.J.; Colombini, M.; Finkelstein, A. Reconstitution in planar lipid bilayers of a voltage-dependent anion-selective channel obtained from paramecium mitochondria. J. Membr. Biol. 1976, 30, 99–120. [Google Scholar] [CrossRef] [PubMed]
- Schindler, H.; Rosenbusch, J.P. Matrix protein from Escherichia coli outer membranes forms voltage-controlled channels in lipid bilayers. Proc. Natl. Acad. Sci. USA 1978, 75, 3751–3755. [Google Scholar] [CrossRef] [PubMed]
- Lakey, J.H. Voltage gating in porin channels. FEBS Lett. 1987, 211, 1–4. [Google Scholar] [CrossRef]
- Bainbridge, G.; Gokce, I.; Lakey, J.H. Voltage gating is a fundamental feature of porin and toxin beta-barrel membrane channels. FEBS Lett. 1998, 431, 305–308. [Google Scholar] [CrossRef]
- Robertson, K.M.; Tieleman, D.P. Molecular basis of voltage gating of OmpF porin. Biochem. Cell Biol.-Biochim. Biol. Cell. 2002, 80, 517–523. [Google Scholar] [CrossRef]
- Colombini, M. VDAC structure, selectivity, and dynamics. Biochim. Biophys. Acta-Biomembr. 2012, 1818, 1457–1465. [Google Scholar] [CrossRef]
- Benz, R. Historical Perspective of Pore-Forming Activity Studies of Voltage-Dependent Anion Channel (Eukaryotic or Mitochondrial Porin) Since Its Discovery in the 70th of the Last Century. Front. Physiol. 2021, 12, 734226. [Google Scholar] [CrossRef] [PubMed]
- Ngo, V.A.; Queralt-Martín, M.; Khan, F.; Bergdoll, L.; Abramson, J.; Bezrukov, S.M.; Rostovtseva, T.K.; Hoogerheide, D.P.; Noskov, S.Y. The Single Residue K12 Governs the Exceptional Voltage Sensitivity of Mitochondrial Voltage-Dependent Anion Channel Gating. J. Am. Chem. Soc. 2022, 144, 14564–14577. [Google Scholar] [CrossRef] [PubMed]
- Mayse, L.A.; Movileanu, L. Gating of β-Barrel Protein Pores, Porins, and Channels: An Old Problem with New Facets. Int. J. Mol. Sci. 2023, 24, 12095. [Google Scholar] [CrossRef] [PubMed]
- Sen, K.; Hellman, J.; Nikaido, H. Porin channels in intact cells of Escherichia coli are not affected by Donnan potentials across the outer membrane. J. Biol. Chem. 1988, 263, 1182–1187. [Google Scholar] [CrossRef] [PubMed]
- Engel, A.; Massalski, A.; Schindler, H.; Dorset, D.L.; Rosenbusch, J.P. Porin channel triplets merge into single outlets in Escherichia coli outer membranes. Nature 1985, 317, 643–645. [Google Scholar] [CrossRef]
- Delcour, A.H. Solute uptake through general porins. Front. Biosci. 2003, 8, d1055–d1071. [Google Scholar] [CrossRef]
- Nikaido, H. Molecular basis of bacterial outer membrane permeability revisited. Microbiol. Mol. Biol. Rev. 2003, 67, 593–656. [Google Scholar] [CrossRef]
- Cowan, S.W.; Schirmer, T.; Rummel, G.; Steiert, M.; Ghosh, R.; Pauptit, R.A.; Jansonius, J.N.; Rosenbusch, J.P. Crystal structures explain functional properties of two E. coli porins. Nature 1992, 358, 727–733. [Google Scholar] [CrossRef]
- Nestorovich, E.M.; Bezrukov, S.M. Voltage-induced “gating” of bacterial porin as reversible protein denaturation. In Proceedings of the Second International Symposium on Fluctuations and Noise, Maspalomas, Spain, 26–28 May 2004; SPIE: Bellingham, WA, USA, 2004; Volume 5467, pp. 42–53. [Google Scholar] [CrossRef]
- Buehler, L.K.; Kusumoto, S.; Zhang, H.; Rosenbusch, J.P. Plasticity of Escherichia coli porin channels. Dependence of their conductance on strain and lipid environment. J. Biol. Chem. 1991, 266, 24446–24450. [Google Scholar] [CrossRef]
- Buehler, L.K.; Rosenbusch, J.P. Single channel behavior of matrix porin of Escherichia coli. Biochem. Biophys. Res. Commun. 1993, 190, 624–629. [Google Scholar] [CrossRef]
- Dela Vega, A.L.; Delcour, A.H. Polyamines decrease Escherichia coli outer membrane permeability. J. Bacteriol. 1996, 178, 3715–3721. [Google Scholar] [CrossRef]
- Van Gelder, P.; Saint, N.; Phale, P.; Eppens, E.F.; Prilipov, A.; van Boxtel, R.; Rosenbusch, J.P.; Tommassen, J. Voltage sensing in the PhoE and OmpF outer membrane porins of Escherichia coli: Role of charged residues. J. Mol. Biol. 1997, 269, 468–472. [Google Scholar] [CrossRef]
- Baslé, A.; Iyer, R.; Delcour, A.H. Subconductance states in OmpF gating. Biochim. Biophys. Acta 2004, 1664, 100–107. [Google Scholar] [CrossRef]
- Babakov, A.V.; Ermishkin, L.N.; Liberman, E.A. Influence of electric field on the capacity of phospholipid membranes. Nature 1966, 210, 953–955. [Google Scholar] [CrossRef]
- Carius, W. Voltage dependence of bilayer membrane capacitance: Harmonic response to ac excitation with dc bias. J. Colloid. Interface Sci. 1976, 57, 301–307. [Google Scholar] [CrossRef]
- Karshikoff, A.; Spassov, V.; Cowan, S.W.; Ladenstein, R.; Schirmer, T. Electrostatic properties of two porin channels from Escherichia coli. J. Mol. Biol. 1994, 240, 372–384. [Google Scholar] [CrossRef]
- Aguilella-Arzo, M.; Garcia-Celma, J.J.; Cervera, J.; Alcaraz, A.; Aguilella, V.M. Electrostatic properties and macroscopic electrodiffusion in OmpF porin and mutants. Bioelectrochemistry 2007, 70, 320–327. [Google Scholar] [CrossRef]
- García-Giménez, E.; Alcaraz, A.; Aguilella-Arzo, M.; Aguilella, V.M. Selectivity of Protein Ion Channels and the Role of Buried Charges. Analytical Solutions, Numerical Calculations, and MD Simulations. J. Phys. Chem. B 2015, 119, 8475–8479. [Google Scholar] [CrossRef]
- Finkelstein, A.V.; Ptitsin, O.B. Protein Physics; Academic Press: London, UK, 2002; p. 354. [Google Scholar] [CrossRef]
- Frauenfelder, H. Physics of Proteins: An Introduction to Biological Physics and Molecular Biophysics; Series: Biological and Medical Physics, Biomedical Engineering); Springer: New York, NY, USA, 2010; pp. 1–448. [Google Scholar] [CrossRef]
- Saint, N.; Lou, K.L.; Widmer, C.; Luckey, M.; Schirmer, T.; Rosenbusch, J.P. Structural and functional characterization of OmpF porin mutants selected for larger pore size. II. Functional characterization. J. Biol. Chem. 1996, 271, 20676–20680. [Google Scholar] [CrossRef]
- Phale, P.S.; Philippsen, A.; Widmer, C.; Phale, V.P.; Rosenbusch, J.P.; Schirmer, T. Role of charged residues at the OmpF porin channel constriction probed by mutagenesis and simulation. Biochemistry 2001, 40, 6319–6325. [Google Scholar] [CrossRef]
- Nestorovich, E.M.; Rostovtseva, T.K.; Bezrukov, S.M. Residue ionization and ion transport through OmpF channels. Biophys. J. 2003, 85, 3718–3729. [Google Scholar] [CrossRef] [PubMed]
- Colombini, M. Voltage gating in the mitochondrial channel, VDAC. J. Membr. Biol. 1989, 111, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Rappaport, S.M.; Teijido, O.; Hoogerheide, D.P.; Rostovtseva, T.K.; Berezhkovskii, A.M.; Bezrukov, S.M. Conductance hysteresis in the voltage-dependent anion channel. Eur. Biophys. J. 2015, 44, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Zizi, M.; Byrd, C.; Boxus, R.; Colombini, M. The voltage-gating process of the voltage-dependent anion channel is sensitive to ion flow. Biophys. J. 1998, 75, 704–713. [Google Scholar] [CrossRef] [PubMed]
- Mathes, A.; Engelhardt, H. Voltage-dependent closing of porin channels: Analysis of relaxation kinetics. J. Membr. Biol. 1998, 165, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Bezanilla, F.; Taylor, R.E.; Fernández, J.M. Distribution and kinetics of membrane dielectric polarization. 1. Long-term inactivation of gating currents. J. Gen. Physiol. 1982, 79, 21–40. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.P.; Thouta, S.; Cheng, Y.M.; Claydon, T.W. Extracellular protons accelerate hERG channel deactivation by destabilizing voltage sensor relaxation. J. Gen. Physiol. 2019, 151, 231–246. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, S.; Morales, M.J.; Strauss, H.C.; Rasmusson, R.L. A quantitative analysis of the activation and inactivation kinetics of HERG expressed in Xenopus oocytes. J. Physiol. 1997, 502 Pt 1, 45–60. [Google Scholar] [CrossRef]
- Piper, D.R.; Rupp, J.; Sachse, F.B.; Sanguinetti, M.C.; Tristani-Firouzi, M. Cooperative interactions between R531 and acidic residues in the voltage sensing module of hERG1 channels. Cell Physiol. Biochem. 2008, 21, 37–46. [Google Scholar] [CrossRef]
- Bett, G.C.; Zhou, Q.; Rasmusson, R.L. Models of HERG gating. Biophys. J. 2011, 101, 631–642. [Google Scholar] [CrossRef]
- Millhauser, G.L.; Salpeter, E.E.; Oswald, R.E. Diffusion models of ion-channel gating and the origin of power-law distributions from single-channel recording. Proc. Natl. Acad. Sci. USA 1988, 85, 1503–1507. [Google Scholar] [CrossRef] [PubMed]
- Sigg, D.; Qian, H.; Bezanilla, F. Kramers’ diffusion theory applied to gating kinetics of voltage-dependent ion channels. Biophys. J. 1999, 76, 782–803. [Google Scholar] [CrossRef] [PubMed]
- Goychuk, I.; Hänggi, P. Ion channel gating: A first-passage time analysis of the Kramers type. Proc. Natl. Acad. Sci. USA 2002, 99, 3552–3556. [Google Scholar] [CrossRef]
- Goychuk, I.; Hänggi, P. The role of conformational diffusion in ion channel gating. Phys. A Stat. Mech. Its Appl. 2003, 325, 9–18. [Google Scholar] [CrossRef]
- Goychuk, I.; Hänggi, P. Fractional diffusion modeling of ion channel gating. Phys. Rev. E Stat. Nonlin Soft Matter Phys. 2004, 70, 051915. [Google Scholar] [CrossRef]
- Haddad, G.A.; Blunck, R. Mode shift of the voltage sensors in Shaker K+ channels is caused by energetic coupling to the pore domain. J. Gen. Physiol. 2011, 137, 455–472. [Google Scholar] [CrossRef]
- Hofmeister, F. Zur Lehre von der Wirkung der Salze. Arch. Für Exp. Pathol. Und Pharmakol. 1888, 24, 247–260. [Google Scholar] [CrossRef]
- He, X.L.; Ewing, A.G. Hofmeister Series: From Aqueous Solution of Biomolecules to Single Cells and Nanovesicles. Chembiochem 2023, 24, e2022006. [Google Scholar] [CrossRef]
- Collins, K.D.; Washabaugh, M.W. The Hofmeister effect and the behaviour of water at interfaces. Q. Rev. Biophys. 1985, 18, 323–422. [Google Scholar] [CrossRef]
- Grigorjev, P.A.; Bezrukov, S.M. Hofmeister effect in ion transport: Reversible binding of halide anions to the roflamycoin channel. Biophys. J. 1994, 67, 2265–2271. [Google Scholar] [CrossRef]
- Gurnev, P.A.; Harries, D.; Parsegian, V.A.; Bezrukov, S.M. The Dynamic Side of the Hofmeister Effect: A Single-Molecule Nanopore Study of Specific Complex Formation. Chemphyschem 2009, 10, 1445–1449. [Google Scholar] [CrossRef] [PubMed]
- Gurnev, P.A.; Roark, T.C.; Petrache, H.I.; Sodt, A.J.; Bezrukov, S.M. Cation-Selective Channel Regulated by Anions According to Their Hofmeister Ranking. Angew. Chem. Int. Ed. Engl. 2017, 56, 3506–3509. [Google Scholar] [CrossRef] [PubMed]
- Dill, E.T.; Holden, M.J.; Colombini, M. Voltage gating in VDAC is markedly inhibited by micromolar quantities of aluminum. J. Membr. Biol. 1987, 99, 187–196. [Google Scholar] [CrossRef]
- Bryant, S.L.; Clark, T.; Thomas, C.A.; Ware, K.S.; Bogard, A.; Calzacorta, C.; Prather, D.; Fologea, D. Insights into the Voltage Regulation Mechanism of the Pore-Forming Toxin Lysenin. Toxins 2018, 10, 334. [Google Scholar] [CrossRef]
- Bogard, A.; Finn, P.W.; Smith, A.R.; Flacau, I.M.; Whiting, R.; Fologea, D. Modulation of Voltage-Gating and Hysteresis of Lysenin Channels by Cu2+ Ions. Int. J. Mol. Sci. 2023, 24, 12996. [Google Scholar] [CrossRef]
- Mlayeh, L.; Chatkaew, S.; Léonetti, M.; Homblé, F. Modulation of plant mitochondrial VDAC by phytosterols. Biophys. J. 2010, 99, 2097–2106. [Google Scholar] [CrossRef]
- Queralt-Martín, M.; Bergdoll, L.; Jacobs, D.; Bezrukov, S.M.; Abramson, J.; Rostovtseva, T.K. Assessing the role of residue E73 and lipid headgroup charge in VDAC1 voltage gating. Biochim. Biophys. Acta Bioenerg. 2019, 1860, 22–29. [Google Scholar] [CrossRef]
- Rostovtseva, T.K.; Kazemi, N.; Weinrich, M.; Bezrukov, S.M. Voltage gating of VDAC is regulated by nonlamellar lipids of mitochondrial membranes. J. Biol. Chem. 2006, 281, 37496–37506. [Google Scholar] [CrossRef]
- Ide, T.; Aoki, T.; Takeuchi, Y.; Yanagida, T. Lysenin forms a voltage-dependent channel in artificial lipid bilayer membranes. Biochem. Biophys. Res. Commun. 2006, 346, 288–292. [Google Scholar] [CrossRef]
- Rostovtseva, T.K.; Bezrukov, S.M. VDAC regulation: Role of cytosolic proteins and mitochondrial lipids. J. Bioenerg. Biomembr. 2008, 40, 163–170. [Google Scholar] [CrossRef]
- Hwang, W.L.; Chen, M.; Cronin, B.; Holden, M.A.; Bayley, H. Asymmetric droplet interface bilayers. J. Am. Chem. Soc. 2008, 130, 5878–5879. [Google Scholar] [CrossRef] [PubMed]
- Tomita, N.; Mohammad, M.M.; Niedzwiecki, D.J.; Ohta, M.; Movileanu, L. Does the lipid environment impact the open-state conductance of an engineered β-barrel protein nanopore? Biochim. Biophys. Acta 2013, 1828, 1057–1065. [Google Scholar] [CrossRef]
- Liko, I.; Degiacomi, M.T.; Lee, S.; Newport, T.D.; Gault, J.; Reading, E.; Hopper, J.T.S.; Housden, N.G.; White, P.; Colledge, M.; et al. Lipid binding attenuates channel closure of the outer membrane protein OmpF. Proc. Natl. Acad. Sci. USA 2018, 115, 6691–6696. [Google Scholar] [CrossRef] [PubMed]
- Perini, D.A.; Alcaraz, A.; Queralt-Martín, M. Lipid Headgroup Charge and Acyl Chain Composition Modulate Closure of Bacterial β-Barrel Channels. Int. J. Mol. Sci. 2019, 20, 674. [Google Scholar] [CrossRef]
- Donoghue, A.; Winterhalter, M.; Gutsmann, T. Influence of Membrane Asymmetry on OmpF Insertion, Orientation and Function. Membranes 2023, 13, 517. [Google Scholar] [CrossRef] [PubMed]
- Steven, A.C.; Heggeler, B.; Müller, R.; Kistler, J.; Rosenbusch, J.P. Ultrastructure of a periodic protein layer in the outer membrane of Escherichia coli. J. Cell Biol. 1977, 72, 292–301. [Google Scholar] [CrossRef]
- Rostovtseva, T.K.; Antonsson, B.; Suzuki, M.; Youle, R.J.; Colombini, M.; Bezrukov, S.M. Bid, but not Bax, regulates VDAC channels. J. Biol. Chem. 2004, 279, 13575–13583. [Google Scholar] [CrossRef]
- Zimmerberg, J.; Parsegian, V.A. Polymer inaccessible volume changes during opening and closing of a voltage-dependent ionic channel. Nature 1986, 323, 36–39. [Google Scholar] [CrossRef]
- Brunen, M.; Engelhardt, H. Asymmetry of orientation and voltage gating of the Acidovorax delafieldii porin Omp34 in lipid bilayers. Eur. J. Biochem. 1993, 212, 129–135. [Google Scholar] [CrossRef]
- Soares, C.M.; Björkstén, J.; Tapia, O. L3 loop-mediated mechanisms of pore closing in porin: A molecular dynamics perturbation approach. Protein Eng. 1995, 8, 5–12. [Google Scholar] [CrossRef]
- Watanabe, M.; Rosenbusch, J.; Schirmer, T.; Karplus, M. Computer simulations of the OmpF porin from the outer membrane of Escherichia coli. Biophys. J. 1997, 72, 2094–2102. [Google Scholar] [CrossRef] [PubMed]
- Acharya, A.; Ghai, I.; Piselli, C.; Prajapati, J.D.; Benz, R.; Winterhalter, M.; Kleinekathöfer, U. Conformational Dynamics of Loop L3 in OmpF: Implications toward Antibiotic Translocation and Voltage Gating. J. Chem. Inf. Model. 2023, 63, 910–927. [Google Scholar] [CrossRef]
- Bredin, J.; Saint, N.; Malléa, M.; Dé, E.; Molle, G.; Pagès, J.M.; Simonet, V. Alteration of pore properties of Escherichia coli OmpF induced by mutation of key residues in anti-loop 3 region. Biochem. J. 2002, 363, 521–528. [Google Scholar] [CrossRef]
- Bezanilla, F.; Armstrong, C.M. Gating Currents of the Sodium Channels: Three Ways to Block Them. Science 1974, 183, 753–754. [Google Scholar] [CrossRef] [PubMed]
- Hille, B. Ion Channels in Excitable Membranes; Sinauer Associates: Sunderland, MA, USA, 2001. [Google Scholar] [CrossRef]
- Yellen, G. The voltage-gated potassium channels and their relatives. Nature 2002, 419, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Payandeh, J.; Scheuer, T.; Zheng, N.; Catterall, W.A. The crystal structure of a voltage-gated sodium channel. Nature 2011, 475, 353–358. [Google Scholar] [CrossRef]
- Vargas, E.; Yarov-Yarovoy, V.; Khalili-Araghi, F.; Catterall, W.A.; Klein, M.L.; Tarek, M.; Lindahl, E.; Schulten, K.; Perozo, E.; Bezanilla, F.; et al. An emerging consensus on voltage-dependent gating from computational modeling and molecular dynamics simulations. J. Gen. Physiol. 2012, 140, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Wisedchaisri, G.; Tonggu, L.; McCord, E.; El-Din, T.M.G.; Wang, L.G.; Zheng, N.; Catterall, W.A. Resting-State Structure and Gating Mechanism of a Voltage-Gated Sodium Channel. Cell 2019, 178, 993–1003. [Google Scholar] [CrossRef]
- Catacuzzeno, L.; Franciolini, F. The 70-year search for the voltage-sensing mechanism of ion channels. J. Physiol. 2022, 600, 3227–3247. [Google Scholar] [CrossRef]
- Mandala, V.S.; MacKinnon, R. Voltage-sensor movements in the Eag Kv channel under an applied electric field. Proc. Natl. Acad. Sci. USA 2022, 119, e2214151119. [Google Scholar] [CrossRef]
- Nikaido, H.; Neidhardt, F.C. Outer Membrane in Escherichia coli and Salmonella: Cellular and Molecular Biology. Eds. FC Neidhardt, R. Curtiss III, JL Ingraham, EC Lin, KB Low. Jr., B. Magasanik, WS Reznikoff, M. Riley, M. Schaechter, HE Umbarger, 2nd edn., Washington, DC, Amer’ Microbiol. Mol. Biol. Rev. 1996, 67, 29–47. [Google Scholar]
- Vergalli, J.; Bodrenko, I.V.; Masi, M.; Moynié, L.; Acosta-Gutiérrez, S.; Naismith, J.H.; Davin-Regli, A.; Ceccarelli, M.; van den Berg, B.; Winterhalter, M.; et al. Porins and small-molecule translocation across the outer membrane of Gram-negative bacteria. Nat. Rev. Microbiol. 2020, 18, 164–176. [Google Scholar] [CrossRef] [PubMed]
- Lakey, J.H.; Lea, E.J.; Pattus, F. OmpC mutants which allow growth on maltodextrins show increased channel size and greater voltage sensitivity. FEBS Lett. 1991, 278, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Colombini, M.; Liu, P.; Dee, C. Triplin: Mechanistic Basis for Voltage Gating. Int. J. Mol. Sci. 2023, 24, 11473. [Google Scholar] [CrossRef] [PubMed]
- Simonet, V.C.; Baslé, A.; Klose, K.E.; Delcour, A.H. The Vibrio cholerae porins OmpU and OmpT have distinct channel properties. J. Biol. Chem. 2003, 278, 17539–17545. [Google Scholar] [CrossRef] [PubMed]
- Todt, J.C.; Rocque, W.J.; McGroarty, E.J. Effects of pH on bacterial porin function. Biochemistry 1992, 31, 10471–10478. [Google Scholar] [CrossRef]
- Iyer, R.; Delcour, A.H. Complex inhibition of OmpF and OmpC bacterial porins by polyamines. J. Biol. Chem. 1997, 272, 18595–18601. [Google Scholar] [CrossRef]
- Benarroch, J.M.; Asally, M. The Microbiologist’s Guide to Membrane Potential Dynamics. Trends Microbiol. 2020, 28, 304–314. [Google Scholar] [CrossRef]
- Alegun, O.; Pandeya, A.; Cui, J.; Ojo, I.; Wei, Y. Donnan Potential across the Outer Membrane of Gram-Negative Bacteria and Its Effect on the Permeability of Antibiotics. Antibiotics 2021, 10, 701. [Google Scholar] [CrossRef]
- Kralj, J.M.; Hochbaum, D.R.; Douglass, A.D.; Cohen, A.E. Electrical spiking in Escherichia coli probed with a fluorescent voltage-indicating protein. Science 2011, 333, 345–348. [Google Scholar] [CrossRef]
- Rostovtseva, T.K.; Sheldon, K.L.; Hassanzadeh, E.; Monge, C.; Saks, V.; Bezrukov, S.M.; Sackett, D.L. Tubulin binding blocks mitochondrial voltage-dependent anion channel and regulates respiration. Proc. Natl. Acad. Sci. USA 2008, 105, 18746–18751. [Google Scholar] [CrossRef] [PubMed]
- Rostovtseva, T.K.; Gurnev, P.A.; Protchenko, O.; Hoogerheide, D.P.; Yap, T.L.; Philpott, C.C.; Lee, J.C.; Bezrukov, S.M. α-Synuclein Shows High Affinity Interaction with Voltage-dependent Anion Channel, Suggesting Mechanisms of Mitochondrial Regulation and Toxicity in Parkinson Disease. J. Biol. Chem. 2015, 290, 18467–18477. [Google Scholar] [CrossRef] [PubMed]
- Rostovtseva, T.K.; Bezrukov, S.M.; Hoogerheide, D.P. Regulation of Mitochondrial Respiration by VDAC Is Enhanced by Membrane-Bound Inhibitors with Disordered Polyanionic C-Terminal Domains. Int. J. Mol. Sci. 2021, 22, 7358. [Google Scholar] [CrossRef] [PubMed]
- Colombini, M. VDAC: The channel at the interface between mitochondria and the cytosol. Mol. Cell. Biochem. 2004, 256, 107–115. [Google Scholar] [CrossRef]
- Porcelli, A.M.; Ghelli, A.; Zanna, C.; Pinton, P.; Rizzuto, R.; Rugolo, M. pH difference across the outer mitochondrial membrane measured with a green fluorescent protein mutant. Biochem. Biophys. Res. Commun. 2005, 326, 799–804. [Google Scholar] [CrossRef]
- Lemeshko, V.V. Electrical control of the cell energy metabolism at the level of mitochondrial outer membrane. Biochim. Biophys. Acta Biomembr. 2021, 1863, 183493. [Google Scholar] [CrossRef]
- Blaustein, R.O.; Koehler, T.M.; Collier, R.J.; Finkelstein, A. Anthrax toxin: Channel-forming activity of protective antigen in planar phospholipid bilayers. Proc. Natl. Acad. Sci. USA 1989, 86, 2209–2213. [Google Scholar] [CrossRef]
- Samartzidou, H.; Delcour, A.H. E. coli PhoE porin has an opposite voltage-dependence to the homologous OmpF. EMBO J. 1998, 17, 93–100. [Google Scholar] [CrossRef]
- Engelhardt, H.; Heinz, C.; Niederweis, M. A tetrameric porin limits the cell wall permeability of Mycobacterium smegmatis. J. Biol. Chem. 2002, 277, 37567–37572. [Google Scholar] [CrossRef]
- Fologea, D.; Krueger, E.; Lee, R.; Naglak, M.; Mazur, Y.; Henry, R.; Salamo, G. Controlled gating of lysenin pores. Biophys. Chem. 2010, 146, 25–29. [Google Scholar] [CrossRef]
- Montal, M.; Mueller, P. Formation of bimolecular membranes from lipid monolayers and a study of their electrical properties. Proc. Natl. Acad. Sci. USA 1972, 69, 3561–3566. [Google Scholar] [CrossRef] [PubMed]






| Empirical Findings | In Favor of Field-Induced Denaturation | In Favor of Functional Gating | Source |
|---|---|---|---|
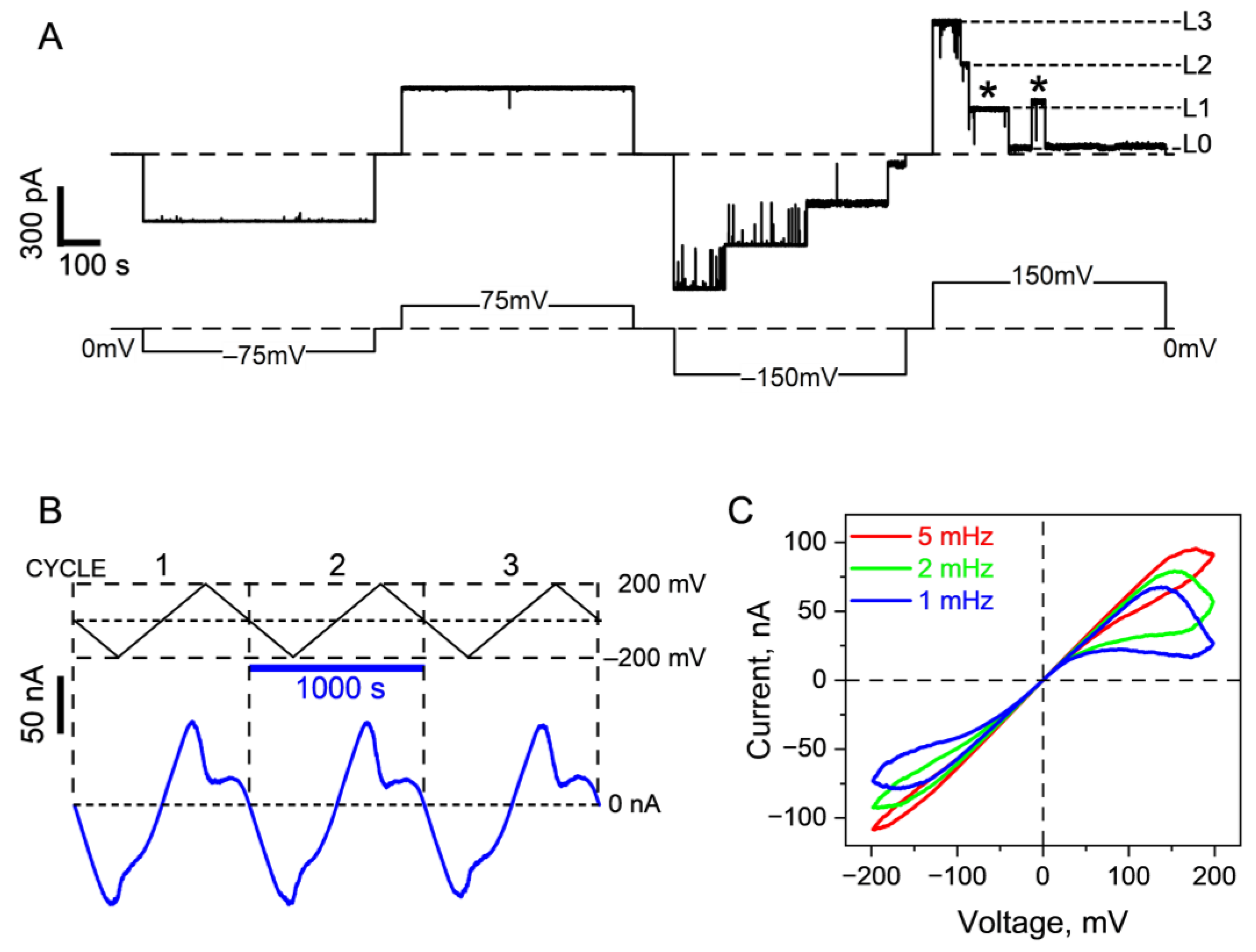
| Closing by strong electric fields | Yes | Maybe | Refs. [6,20,21,23], present study, Figure 1 and Figure 2 |
| Response to voltages of either polarity | Yes | Unlikely | Refs. [6,20], present study, Figure 1 |
| A complex array of voltage gating memory effects | Yes | Maybe | Ref. [19], present study, Figure 2, Figure 3 and Figures S1–S3 |
| Multiplicity of closed state conformations | Yes | Unlikely | Refs. [19,24], present study, Figure 4 |
| Effects of salts along the Hofmeister series | Yes | Maybe | Ref. [19], present study, Figure 5 and Figure S3 |
| Open conformation is stabilized in clusters | Yes | Unlikely | Present study, Figure 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nestorovich, E.M.; Bezrukov, S.M. Beta-Barrel Channel Response to High Electric Fields: Functional Gating or Reversible Denaturation? Int. J. Mol. Sci. 2023, 24, 16655. https://doi.org/10.3390/ijms242316655
Nestorovich EM, Bezrukov SM. Beta-Barrel Channel Response to High Electric Fields: Functional Gating or Reversible Denaturation? International Journal of Molecular Sciences. 2023; 24(23):16655. https://doi.org/10.3390/ijms242316655
Chicago/Turabian StyleNestorovich, Ekaterina M., and Sergey M. Bezrukov. 2023. "Beta-Barrel Channel Response to High Electric Fields: Functional Gating or Reversible Denaturation?" International Journal of Molecular Sciences 24, no. 23: 16655. https://doi.org/10.3390/ijms242316655





