Microbiota in Autism Spectrum Disorder: A Systematic Review
Abstract
:1. Introduction
2. Methods
3. Results and Discussion
3.1. Differences in Microbiome between ASD and Non-ASD Patients
3.2. Probiotic Interventions
3.3. Microbiota Transfer Therapy Interventions
3.4. Prebiotic Therapy
3.5. Other Interventions
3.6. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- CDC Diagnostic Criteria|Autism Spectrum Disorder (ASD)|NCBDDD|CDC. Available online: https://www.cdc.gov/ncbddd/autism/hcp-dsm.html (accessed on 13 August 2023).
- Anthony, B.J.; Robertson, H.A.; Verbalis, A.; Myrick, Y.; Troxel, M.; Seese, S.; Anthony, L.G. Increasing Autism Acceptance: The Impact of the Sesame Street “See Amazing in All Children” Initiative. Autism 2020, 24, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Saurman, V.; Margolis, K.G.; Luna, R.A. Autism Spectrum Disorder as a Brain-Gut-Microbiome Axis Disorder. Dig. Dis. Sci. 2020, 65, 818–828. [Google Scholar] [CrossRef] [PubMed]
- Krajmalnik-Brown, R.; Lozupone, C.; Kang, D.-W.; Adams, J.B. Gut Bacteria in Children with Autism Spectrum Disorders: Challenges and Promise of Studying How a Complex Community Influences a Complex Disease. Microb. Ecol. Health Dis. 2015, 26, 26914. [Google Scholar] [CrossRef] [PubMed]
- Arnold, L.E.; Luna, R.A.; Williams, K.; Chan, J.; Parker, R.A.; Wu, Q.; Hollway, J.A.; Jeffs, A.; Lu, F.; Hayes, C.; et al. Probiotics for Gastrointestinal Symptoms and Quality of Life in Autism: A Placebo-Controlled Pilot Trial. J. Child. Adolesc. Psychopharmacol. 2019, 29, 659–669. [Google Scholar] [CrossRef] [PubMed]
- Lewandowska-Pietruszka, Z.; Figlerowicz, M.; Mazur-Melewska, K. The History of the Intestinal Microbiota and the Gut-Brain Axis. Pathogens 2022, 11, 1540. [Google Scholar] [CrossRef] [PubMed]
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The Gut-Brain Axis: Interactions between Enteric Microbiota, Central and Enteric Nervous Systems. Ann. Gastroenterol. 2015, 28, 203–209. [Google Scholar] [PubMed]
- Grimaldi, R.; Gibson, G.R.; Vulevic, J.; Giallourou, N.; Castro-Mejía, J.L.; Hansen, L.H.; Leigh Gibson, E.; Nielsen, D.S.; Costabile, A. A Prebiotic Intervention Study in Children with Autism Spectrum Disorders (ASDs). Microbiome 2018, 6, 133. [Google Scholar] [CrossRef] [PubMed]
- Sanctuary, M.R.; Kain, J.N.; Angkustsiri, K.; German, J.B. Dietary Considerations in Autism Spectrum Disorders: The Potential Role of Protein Digestion and Microbial Putrefaction in the Gut-Brain Axis. Front. Nutr. 2018, 5, 40. [Google Scholar] [CrossRef]
- Santocchi, E.; Guiducci, L.; Fulceri, F.; Billeci, L.; Buzzigoli, E.; Apicella, F.; Calderoni, S.; Grossi, E.; Morales, M.A.; Muratori, F. Gut to Brain Interaction in Autism Spectrum Disorders: A Randomized Controlled Trial on the Role of Probiotics on Clinical, Biochemical and Neurophysiological Parameters. BMC Psychiatry 2016, 16, 183. [Google Scholar] [CrossRef]
- Wang, Y.; Li, N.; Yang, J.-J.; Zhao, D.-M.; Chen, B.; Zhang, G.-Q.; Chen, S.; Cao, R.-F.; Yu, H.; Zhao, C.-Y.; et al. Probiotics and Fructo-Oligosaccharide Intervention Modulate the Microbiota-Gut Brain Axis to Improve Autism Spectrum Reducing Also the Hyper-Serotonergic State and the Dopamine Metabolism Disorder. Pharmacol. Res. 2020, 157, 104784. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A Web and Mobile App for Systematic Reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef] [PubMed]
- Andreo-Martínez, P.; Rubio-Aparicio, M.; Sánchez-Meca, J.; Veas, A.; Martínez-González, A.E. A Meta-Analysis of Gut Microbiota in Children with Autism. J. Autism Dev. Disord. 2022, 52, 1374–1387. [Google Scholar] [CrossRef] [PubMed]
- Iglesias-Vázquez, L.; Van Ginkel Riba, G.; Arija, V.; Canals, J. Composition of Gut Microbiota in Children with Autism Spectrum Disorder: A Systematic Review and Meta-Analysis. Nutrients 2020, 12, 792. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Zhang, M.; Teng, L.; Wang, Y.; Zhu, L. Prebiotics and Probiotics for Autism Spectrum Disorder: A Systematic Review and Meta-Analysis of Controlled Clinical Trials. J. Med. Microbiol. 2022, 71, 001510. [Google Scholar] [CrossRef] [PubMed]
- West, K.A.; Xiaochen, Y.; Rutherford, E.M.; Wee, B.; Choi, J.; Chrisman, B.S.; Dunlap, K.L.; Hannibal, R.L.; Hartono, W.; Lin, M.; et al. Multi-Angle Meta-Analysis of the Gut Microbiome in Autism Spectrum Disorder: A Step toward Understanding Patient Subgroups. Sci. Rep. 2022, 12, 17034. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Xu, X.; Li, J.; Li, F. Association between Gut Microbiota and Autism Spectrum Disorder: A Systematic Review and Meta-Analysis. Front. Psychiatry 2019, 10, 473. [Google Scholar] [CrossRef]
- Zhu, D.; Jin, X.; Guo, P.; Sun, Y.; Zhou, L.; Qing, Y.; Shen, W.; Ji, G. Efficacy of Faecal Microbiota Transplantation for the Treatment of Autism in Children: Meta-Analysis of Randomised Controlled Trials. Evid. Based Complement. Alternat. Med. 2023, 2023, 5993628. [Google Scholar] [CrossRef]
- Martínez-González, A.E.; Andreo-Martínez, P. The Role of Gut Microbiota in Gastrointestinal Symptoms of Children with ASD. Medicina 2019, 55, 408. [Google Scholar] [CrossRef]
- Ng, Q.X.; Loke, W.; Venkatanarayanan, N.; Lim, D.Y.; Soh, A.Y.S.; Yeo, W.S. A Systematic Review of the Role of Prebiotics and Probiotics in Autism Spectrum Disorders. Medicina 2019, 55, 129. [Google Scholar] [CrossRef]
- Patel, M.; Atluri, L.M.; Gonzalez, N.A.; Sakhamuri, N.; Athiyaman, S.; Randhi, B.; Gutlapalli, S.D.; Pu, J.; Zaidi, M.F.; Khan, S. A Systematic Review of Mixed Studies Exploring the Effects of Probiotics on Gut-Microbiome to Modulate Therapy in Children with Autism Spectrum Disorder. Cureus 2022, 14, e32313. [Google Scholar] [CrossRef] [PubMed]
- Srikantha, P.; Mohajeri, M.H. The Possible Role of the Microbiota-Gut-Brain-Axis in Autism Spectrum Disorder. Int. J. Mol. Sci. 2019, 20, 2115. [Google Scholar] [CrossRef] [PubMed]
- Tan, Q.; Orsso, C.E.; Deehan, E.C.; Kung, J.Y.; Tun, H.M.; Wine, E.; Madsen, K.L.; Zwaigenbaum, L.; Haqq, A.M. Probiotics, Prebiotics, Synbiotics, and Fecal Microbiota Transplantation in the Treatment of Behavioral Symptoms of Autism Spectrum Disorder: A Systematic Review. Autism Res. 2021, 14, 1820–1836. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Wang, H.; Lu, W.; Zhai, Q.; Zhang, Q.; Yuan, W.; Gu, Z.; Zhao, J.; Zhang, H.; Chen, W. Potential of Gut Microbiome for Detection of Autism Spectrum Disorder. Microb. Pathog. 2020, 149, 104568. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Fu, X.; Liao, X.; Li, Y. Effects of Gut Microbial-Based Treatments on Gut Microbiota, Behavioral Symptoms, and Gastrointestinal Symptoms in Children with Autism Spectrum Disorder: A Systematic Review. Psychiatry Res. 2020, 293, 113471. [Google Scholar] [CrossRef] [PubMed]
- Zafar, U.; Habib, H. The Link between Autism Spectrum Disorder and Gastrointestinal Microbiota. J. Ayub Med. Coll. Abbottabad 2021, 33, 513–518. [Google Scholar] [PubMed]
- Zhang, J.; Zhu, G.; Wan, L.; Liang, Y.; Liu, X.; Yan, H.; Zhang, B.; Yang, G. Effect of Fecal Microbiota Transplantation in Children with Autism Spectrum Disorder: A Systematic Review. Front. Psychiatry 2023, 14, 1123658. [Google Scholar] [CrossRef]
- Agarwala, S.; Naik, B.; Ramachandra, N.B. Mucosa-Associated Specific Bacterial Species Disrupt the Intestinal Epithelial Barrier in the Autism Phenome. Brain Behav. Immun. Health 2021, 15, 100269. [Google Scholar] [CrossRef]
- Chiappori, F.; Cupaioli, F.A.; Consiglio, A.; Di Nanni, N.; Mosca, E.; Licciulli, V.F.; Mezzelani, A. Analysis of Faecal Microbiota and Small ncRNAs in Autism: Detection of miRNAs and piRNAs with Possible Implications in Host-Gut Microbiota Cross-Talk. Nutrients 2022, 14, 1340. [Google Scholar] [CrossRef]
- Coretti, L.; Paparo, L.; Riccio, M.P.; Amato, F.; Cuomo, M.; Natale, A.; Borrelli, L.; Corrado, G.; De Caro, C.; Comegna, M.; et al. Gut Microbiota Features in Young Children with Autism Spectrum Disorders. Front. Microbiol. 2018, 9, 3146. [Google Scholar] [CrossRef]
- Ha, S.; Oh, D.; Lee, S.; Park, J.; Ahn, J.; Choi, S.; Cheon, K.-A. Altered Gut Microbiota in Korean Children with Autism Spectrum Disorders. Nutrients 2021, 13, 3300. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Liu, K.; Wei, Z.; Feng, Z.; Chen, J.; Yang, J.; Zhong, Q.; Wan, G.; Kong, X.-J. Serum Oxytocin Level Correlates with Gut Microbiome Dysbiosis in Children with Autism Spectrum Disorder. Front. Neurosci. 2021, 15, 721884. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.-W.; Adams, J.B.; Gregory, A.C.; Borody, T.; Chittick, L.; Fasano, A.; Khoruts, A.; Geis, E.; Maldonado, J.; McDonough-Means, S.; et al. Microbiota Transfer Therapy Alters Gut Ecosystem and Improves Gastrointestinal and Autism Symptoms: An Open-Label Study. Microbiome 2017, 5, 10. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.-W.; Adams, J.B.; Coleman, D.M.; Pollard, E.L.; Maldonado, J.; McDonough-Means, S.; Caporaso, J.G.; Krajmalnik-Brown, R. Long-Term Benefit of Microbiota Transfer Therapy on Autism Symptoms and Gut Microbiota. Sci. Rep. 2019, 9, 5821. [Google Scholar] [CrossRef] [PubMed]
- Niu, M.; Li, Q.; Zhang, J.; Wen, F.; Dang, W.; Duan, G.; Li, H.; Ruan, W.; Yang, P.; Guan, C.; et al. Characterization of Intestinal Microbiota and Probiotics Treatment in Children with Autism Spectrum Disorders in China. Front. Neurol. 2019, 10, 1084. [Google Scholar] [CrossRef] [PubMed]
- Plaza-Díaz, J.; Gómez-Fernández, A.; Chueca, N.; de la Torre-Aguilar, M.J.; Gil, Á.; Perez-Navero, J.L.; Flores-Rojas, K.; Martín-Borreguero, P.; Solis-Urra, P.; Ruiz-Ojeda, F.J.; et al. Autism Spectrum Disorder (ASD) with and without Mental Regression Is Associated with Changes in the Fecal Microbiota. Nutrients 2019, 11, 337. [Google Scholar] [CrossRef] [PubMed]
- Pulikkan, J.; Maji, A.; Dhakan, D.B.; Saxena, R.; Mohan, B.; Anto, M.M.; Agarwal, N.; Grace, T.; Sharma, V.K. Gut Microbial Dysbiosis in Indian Children with Autism Spectrum Disorders. Microb. Ecol. 2018, 76, 1102–1114. [Google Scholar] [CrossRef]
- Qiao, Y.; Wu, M.; Feng, Y.; Zhou, Z.; Chen, L.; Chen, F. Alterations of Oral Microbiota Distinguish Children with Autism Spectrum Disorders from Healthy Controls. Sci. Rep. 2018, 8, 1597. [Google Scholar] [CrossRef]
- Ragusa, M.; Santagati, M.; Mirabella, F.; Lauretta, G.; Cirnigliaro, M.; Brex, D.; Barbagallo, C.; Domini, C.N.; Gulisano, M.; Barone, R.; et al. Potential Associations Among Alteration of Salivary miRNAs, Saliva Microbiome Structure, and Cognitive Impairments in Autistic Children. Int. J. Mol. Sci. 2020, 21, 6203. [Google Scholar] [CrossRef]
- Shaaban, S.Y.; El Gendy, Y.G.; Mehanna, N.S.; El-Senousy, W.M.; El-Feki, H.S.A.; Saad, K.; El-Asheer, O.M. The Role of Probiotics in Children with Autism Spectrum Disorder: A Prospective, Open-Label Study. Nutr. Neurosci. 2018, 21, 676–681. [Google Scholar] [CrossRef]
- Son, J.S.; Zheng, L.J.; Rowehl, L.M.; Tian, X.; Zhang, Y.; Zhu, W.; Litcher-Kelly, L.; Gadow, K.D.; Gathungu, G.; Robertson, C.E.; et al. Comparison of Fecal Microbiota in Children with Autism Spectrum Disorders and Neurotypical Siblings in the Simons Simplex Collection. PLoS ONE 2015, 10, e0137725. [Google Scholar] [CrossRef] [PubMed]
- Strati, F.; Cavalieri, D.; Albanese, D.; De Felice, C.; Donati, C.; Hayek, J.; Jousson, O.; Leoncini, S.; Renzi, D.; Calabrò, A.; et al. New Evidences on the Altered Gut Microbiota in Autism Spectrum Disorders. Microbiome 2017, 5, 24. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; You, Z.; Jia, L.; Wang, F. Autism Spectrum Disorder Is Associated with Gut Microbiota Disorder in Children. BMC Pediatr. 2019, 19, 516. [Google Scholar] [CrossRef] [PubMed]
- Tomova, A.; Husarova, V.; Lakatosova, S.; Bakos, J.; Vlkova, B.; Babinska, K.; Ostatnikova, D. Gastrointestinal Microbiota in Children with Autism in Slovakia. Physiol. Behav. 2015, 138, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Tomova, A.; Soltys, K.; Kemenyova, P.; Karhanek, M.; Babinska, K. The Influence of Food Intake Specificity in Children with Autism on Gut Microbiota. Int. J. Mol. Sci. 2020, 21, 2797. [Google Scholar] [CrossRef] [PubMed]
- Tong, Z.; Zhou, X.; Chu, Y.; Zhang, T.; Zhang, J.; Zhao, X.; Wang, Z.; Ding, R.; Meng, Q.; Ju, J.; et al. Implications of Oral Streptococcal Bacteriophages in Autism Spectrum Disorder. NPJ Biofilms Microbiomes 2022, 8, 91. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Christophersen, C.T.; Sorich, M.J.; Gerber, J.P.; Angley, M.T.; Conlon, M.A. Low Relative Abundances of the Mucolytic Bacterium Akkermansia Muciniphila and Bifidobacterium Spp. in Feces of Children with Autism. Appl. Environ. Microbiol. 2011, 77, 6718–6721. [Google Scholar] [CrossRef]
- Wong, O.W.H.; Lam, A.M.W.; Or, B.P.N.; Mo, F.Y.M.; Shea, C.K.S.; Lai, K.Y.C.; Ma, S.L.; Hung, S.F.; Chan, S.; Kwong, T.N.Y.; et al. Disentangling the Relationship of Gut Microbiota, Functional Gastrointestinal Disorders and Autism: A Case-Control Study on Prepubertal Chinese Boys. Sci. Rep. 2022, 12, 10659. [Google Scholar] [CrossRef]
- Ye, F.; Gao, X.; Wang, Z.; Cao, S.; Liang, G.; He, D.; Wang, L.; Xu, P.; Zhang, Q. Comparison of Gut Microbiota in Autism Spectrum Disorders and Neurotypical Boys in China: A Case-Control Study. Synth. Syst. Biotechnol. 2021, 6, 120–126. [Google Scholar] [CrossRef]
- Zhang, M.; Ma, W.; Zhang, J.; He, Y.; Wang, J. Analysis of Gut Microbiota Profiles and Microbe-Disease Associations in Children with Autism Spectrum Disorders in China. Sci. Rep. 2018, 8, 13981. [Google Scholar] [CrossRef]
- Zhou, R.; Xu, F.; Wang, Y.; Duan, M.; Guo, M.; Zhang, Q.; Zhao, H.; Zheng, H. Changes in the Gut Microbiota of Children with Autism Spectrum Disorder. Autism Res. 2020, 13, 1614–1625. [Google Scholar] [CrossRef] [PubMed]
- Shea, B.J.; Reeves, B.C.; Wells, G.; Thuku, M.; Hamel, C.; Moran, J.; Moher, D.; Tugwell, P.; Welch, V.; Kristjansson, E.; et al. AMSTAR 2: A Critical Appraisal Tool for Systematic Reviews That Include Randomised or Non-Randomised Studies of Healthcare Interventions, or Both. BMJ 2017, 358, j4008. [Google Scholar] [CrossRef] [PubMed]
- ROBINS-I|Cochrane Bias. Available online: https://methods.cochrane.org/bias/risk-bias-non-randomized-studies-interventions (accessed on 13 August 2023).
- RoB 2: A Revised Cochrane Risk-of-Bias Tool for Randomized Trials|Cochrane Bias. Available online: https://methods.cochrane.org/bias/resources/rob-2-revised-cochrane-risk-bias-tool-randomized-trials (accessed on 13 August 2023).
- Vandana, P.; Simkin, D.R.; Hendren, R.L.; Arnold, L.E. Autism Spectrum Disorder and Complementary-Integrative Medicine. Child. Adolesc. Psychiatr. Clin. N. Am. 2023, 32, 469–494. [Google Scholar] [CrossRef] [PubMed]
- Baspinar, B.; Yardimci, H. Gluten-Free Casein-Free Diet for Autism Spectrum Disorders: Can It Be Effective in Solving Behavioural and Gastrointestinal Problems? Eurasian J. Med. 2020, 52, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Knivsberg, A.M.; Reichelt, K.L.; Høien, T.; Nødland, M. A Randomised, Controlled Study of Dietary Intervention in Autistic Syndromes. Nutr. Neurosci. 2002, 5, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Whiteley, P.; Haracopos, D.; Knivsberg, A.-M.; Reichelt, K.L.; Parlar, S.; Jacobsen, J.; Seim, A.; Pedersen, L.; Schondel, M.; Shattock, P. The ScanBrit Randomised, Controlled, Single-Blind Study of a Gluten- and Casein-Free Dietary Intervention for Children with Autism Spectrum Disorders. Nutr. Neurosci. 2010, 13, 87–100. [Google Scholar] [CrossRef] [PubMed]
- El-Salhy, M. Intestinal Bacteria Associated with Irritable Bowel Syndrome and Chronic Fatigue. Neurogastroenterol. Motil. 2023, 35, e14621. [Google Scholar] [CrossRef]
- Transeth, E.L.; Dale, H.F.; Lied, G.A. Comparison of Gut Microbiota Profile in Celiac Disease, Non-Celiac Gluten Sensitivity, and Irritable Bowel Syndrome: A Systematic Review. Turk. J. Gastroenterol. 2020, 31, 735–745. [Google Scholar] [CrossRef]
- Bandini, L.; Curtin, C.; Phillips, S.; Anderson, S.E.; Maslin, M.; Must, A. Changes in Food Selectivity in Children with Autism Spectrum Disorder. J. Autism Dev. Disord. 2017, 47, 439–446. [Google Scholar] [CrossRef]
- Saffouri, G.B.; Shields-Cutler, R.R.; Chen, J.; Yang, Y.; Lekatz, H.R.; Hale, V.L.; Cho, J.M.; Battaglioli, E.J.; Bhattarai, Y.; Thompson, K.J.; et al. Small Intestinal Microbial Dysbiosis Underlies Symptoms Associated with Functional Gastrointestinal Disorders. Nat. Commun. 2019, 10, 2012. [Google Scholar] [CrossRef]
- Wei, L.; Singh, R.; Ro, S.; Ghoshal, U.C. Gut Microbiota Dysbiosis in Functional Gastrointestinal Disorders: Underpinning the Symptoms and Pathophysiology. JGH Open 2021, 5, 976–987. [Google Scholar] [CrossRef]
- Lee, Y.-K. Effects of Diet on Gut Microbiota Profile and the Implications for Health and Disease. Biosci. Microbiota Food Health 2013, 32, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Luna, R.A.; Oezguen, N.; Balderas, M.; Venkatachalam, A.; Runge, J.K.; Versalovic, J.; Veenstra-VanderWeele, J.; Anderson, G.M.; Savidge, T.; Williams, K.C. Distinct Microbiome-Neuroimmune Signatures Correlate with Functional Abdominal Pain in Children with Autism Spectrum Disorder. Cell. Mol. Gastroenterol. Hepatol. 2017, 3, 218–230. [Google Scholar] [CrossRef]
- Garcia-Mazcorro, J.F.; Rivera-Gutierrez, X.; Cobos-Quevedo, O.D.J.; Grube-Pagola, P.; Meixueiro-Daza, A.; Hernandez-Flores, K.; Cabrera-Jorge, F.J.; Vivanco-Cid, H.; Dowd, S.E.; Remes-Troche, J.M. First Insights into the Gut Microbiota of Mexican Patients with Celiac Disease and Non-Celiac Gluten Sensitivity. Nutrients 2018, 10, 1641. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Lin, F.; Yang, F.; Chen, J.; Cai, W.; Zou, T. Gut Microbiome Characteristics of Comorbid Generalized Anxiety Disorder and Functional Gastrointestinal Disease: Correlation with Alexithymia and Personality Traits. Front. Psychiatry 2022, 13, 946808. [Google Scholar] [CrossRef] [PubMed]
- Kumbhare, S.V.; Francis-Lyon, P.A.; Kachru, D.; Uday, T.; Irudayanathan, C.; Muthukumar, K.M.; Ricchetti, R.R.; Singh-Rambiritch, S.; Ugalde, J.; Dulai, P.S.; et al. Digital Therapeutics Care Utilizing Genetic and Gut Microbiome Signals for the Management of Functional Gastrointestinal Disorders: Results from a Preliminary Retrospective Study. Front. Microbiol. 2022, 13, 826916. [Google Scholar] [CrossRef] [PubMed]
- Lasheras, I.; Seral, P.; Latorre, E.; Barroso, E.; Gracia-García, P.; Santabárbara, J. Microbiota and Gut-Brain Axis Dysfunction in Autism Spectrum Disorder: Evidence for Functional Gastrointestinal Disorders. Asian J. Psychiatry 2020, 47, 101874. [Google Scholar] [CrossRef]
- Toubon, G.; Butel, M.-J.; Rozé, J.-C.; Nicolis, I.; Delannoy, J.; Zaros, C.; Ancel, P.-Y.; Aires, J.; Charles, M.A. Early Life Factors Influencing Children Gut Microbiota at 3.5 Years from Two French Birth Cohorts. Microorganisms 2023, 11, 1390. [Google Scholar] [CrossRef]
- Roswall, J.; Olsson, L.M.; Kovatcheva-Datchary, P.; Nilsson, S.; Tremaroli, V.; Simon, M.-C.; Kiilerich, P.; Akrami, R.; Krämer, M.; Uhlén, M.; et al. Developmental trajectory of the healthy human gut microbiota during the first 5 years of life. Cell Host Microbe 2021, 29, 765–776. [Google Scholar] [CrossRef]
- Pinn, D.M.; Aroniadis, O.C.; Brandt, L.J. Is Fecal Microbiota Transplantation (FMT) an Effective Treatment for Patients with Functional Gastrointestinal Disorders (FGID)? Neurogastroenterol. Motil. 2015, 27, 19–29. [Google Scholar] [CrossRef]
- Hamazaki, M.; Sawada, T.; Yamamura, T.; Maeda, K.; Mizutani, Y.; Ishikawa, E.; Furune, S.; Yamamoto, K.; Ishikawa, T.; Kakushima, N.; et al. Fecal Microbiota Transplantation in the Treatment of Irritable Bowel Syndrome: A Single-Center Prospective Study in Japan. BMC Gastroenterol. 2022, 22, 342. [Google Scholar] [CrossRef]
- Chinna Meyyappan, A.; Forth, E.; Wallace, C.J.K.; Milev, R. Effect of Fecal Microbiota Transplant on Symptoms of Psychiatric Disorders: A Systematic Review. BMC Psychiatry 2020, 20, 299. [Google Scholar] [CrossRef]
- Vulevic, J.; Tzortzis, G.; Juric, A.; Gibson, G.R. Effect of a Prebiotic Galactooligosaccharide Mixture (B-GOS®) on Gastrointestinal Symptoms in Adults Selected from a General Population Who Suffer with Bloating, Abdominal Pain, or Flatulence. Neurogastroenterol. Motil. 2018, 30, e13440. [Google Scholar] [CrossRef]
- Johnstone, N.; Milesi, C.; Burn, O.; van den Bogert, B.; Nauta, A.; Hart, K.; Sowden, P.; Burnet, P.W.J.; Cohen Kadosh, K. Anxiolytic Effects of a Galacto-Oligosaccharides Prebiotic in Healthy Females (18–25 Years) with Corresponding Changes in Gut Bacterial Composition. Sci. Rep. 2021, 11, 8302. [Google Scholar] [CrossRef]
- Chandwe, K.; Kelly, P. Colostrum Therapy for Human Gastrointestinal Health, and Disease. Nutrients 2021, 13, 1956. [Google Scholar] [CrossRef]
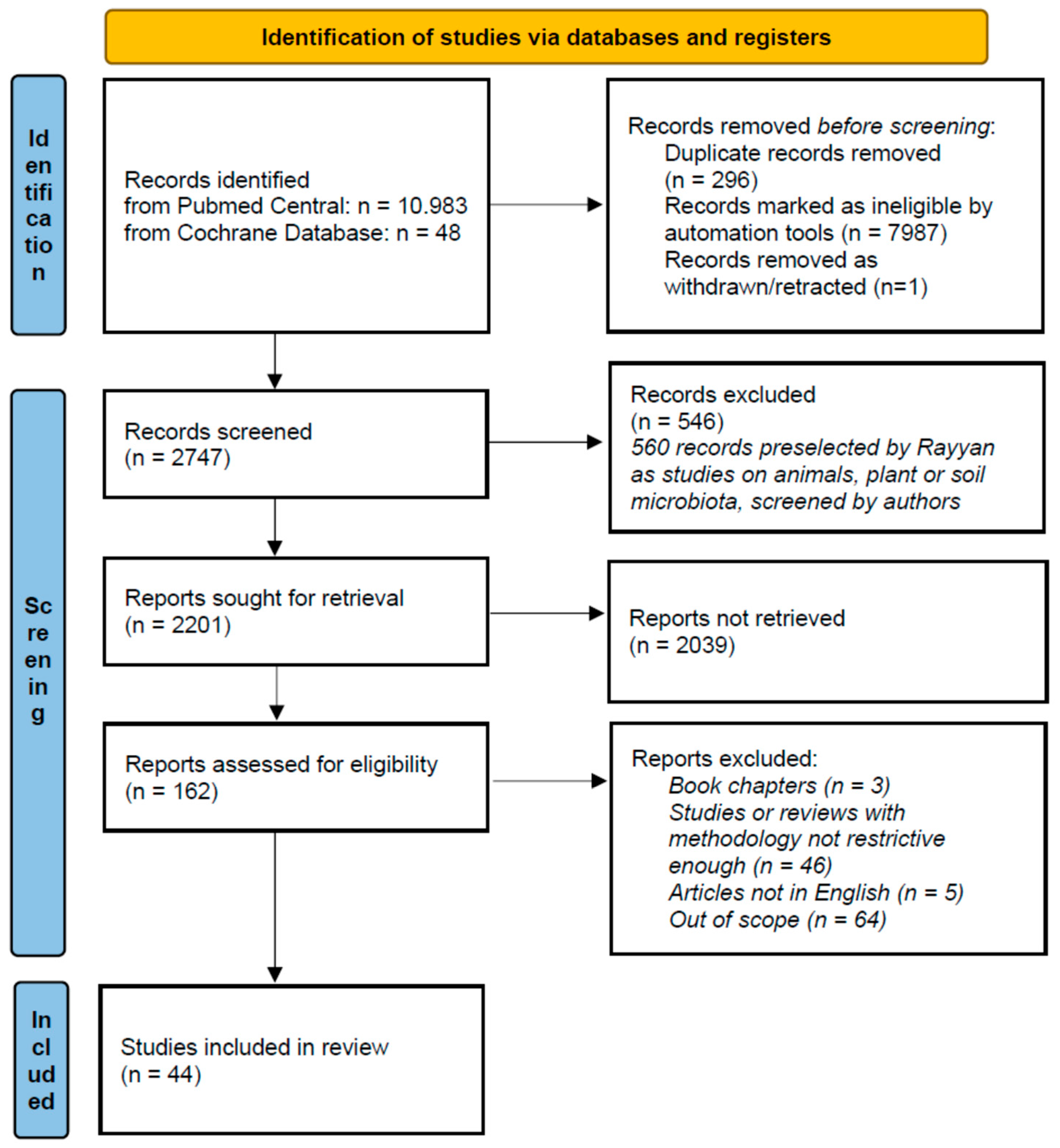
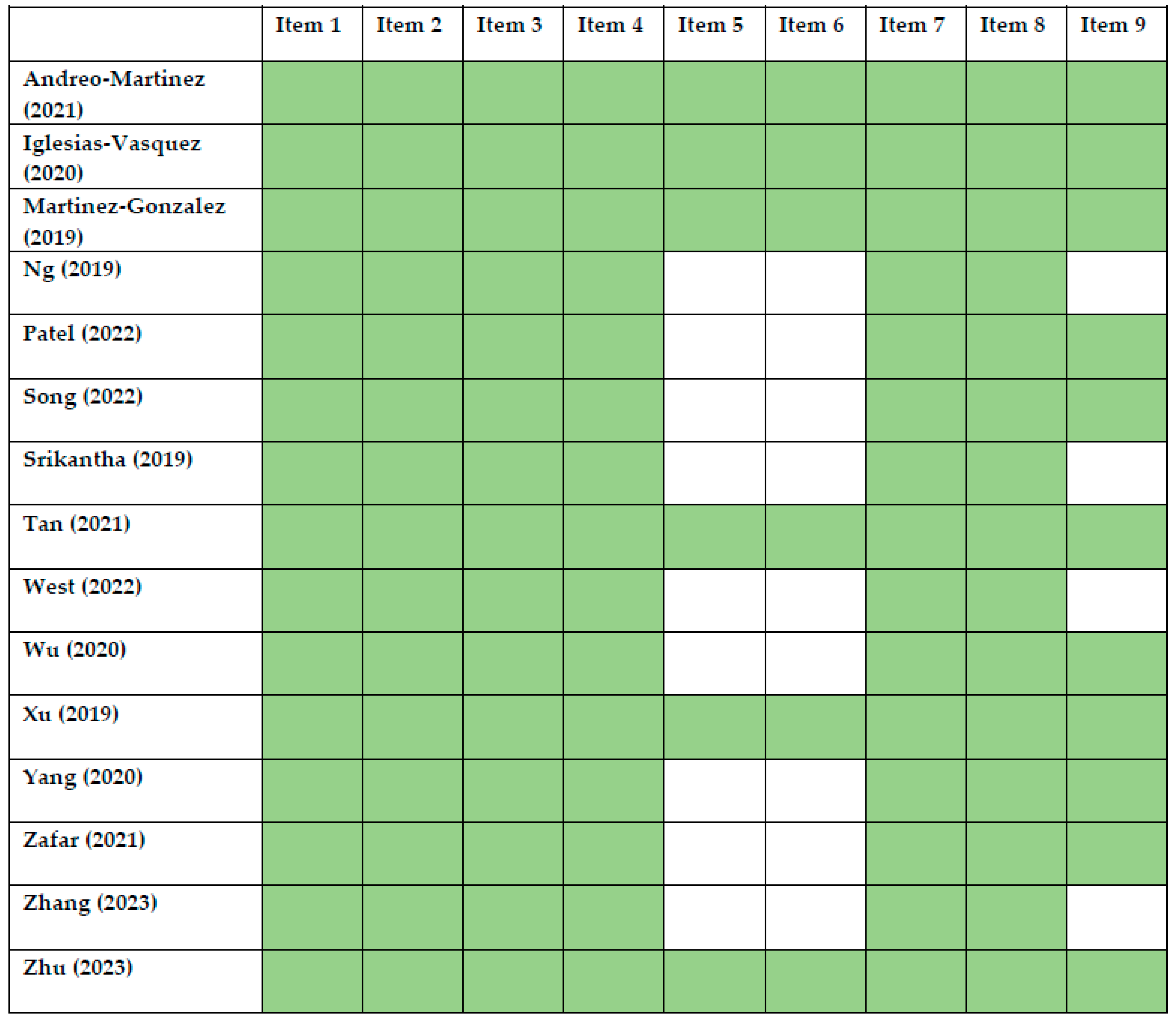
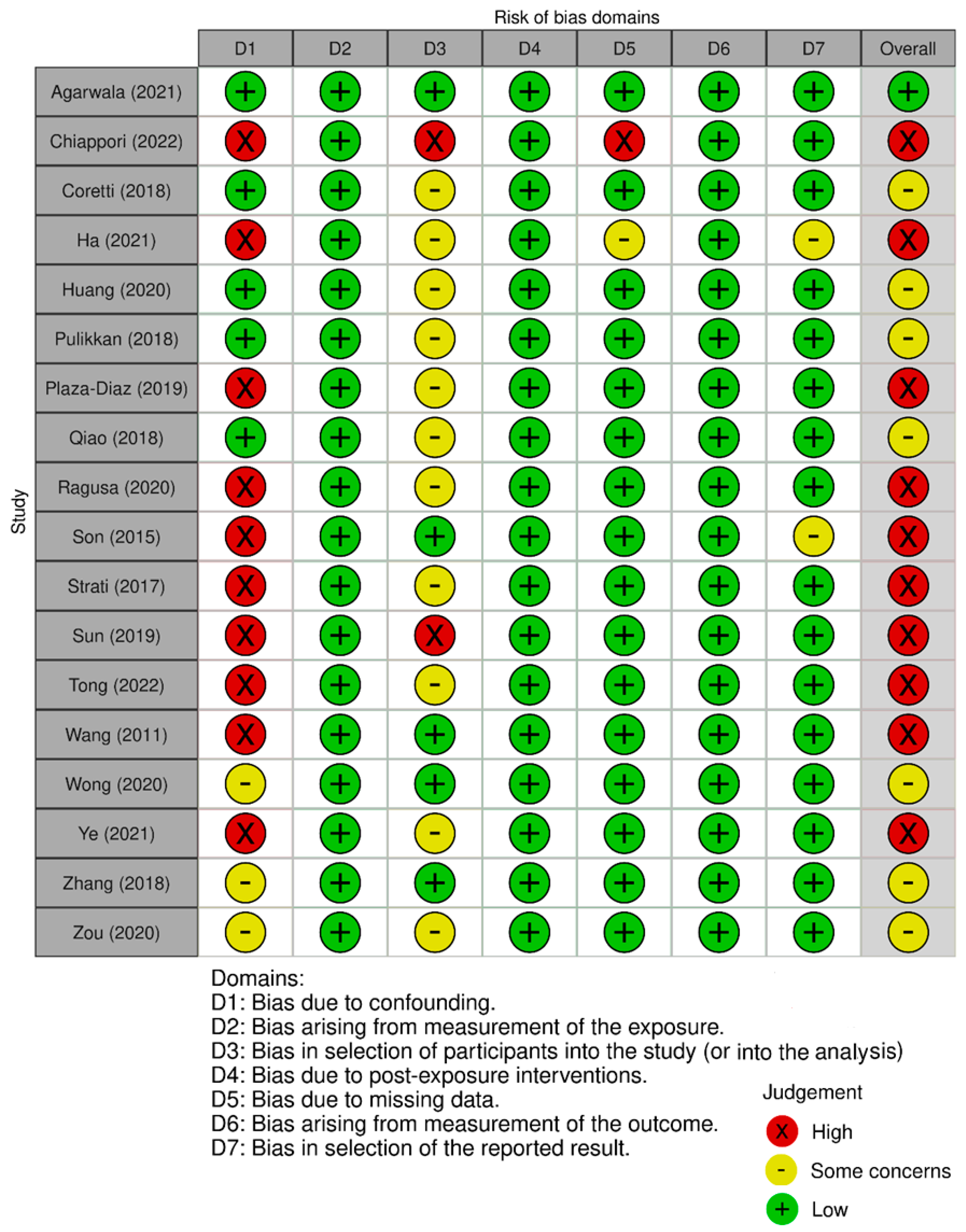

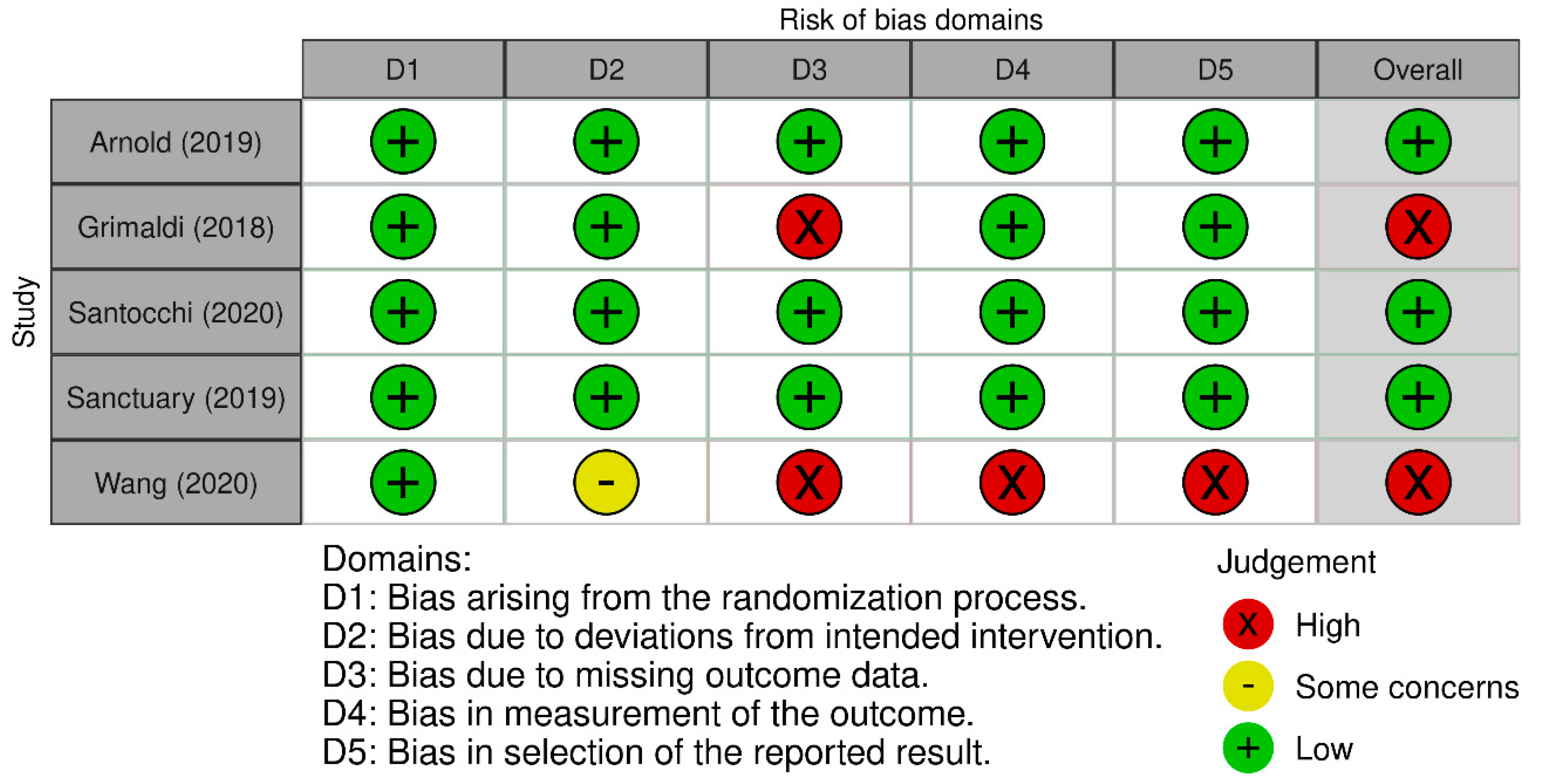
| Study | Country | ASD (n) | ASD Age (Years) | Healthy Control (n) | Healthy Control Age (Years) | Microbiota Assessment Method | Study Type | Microbiota Assessment —Other Important Results | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Behavior | GI Symptoms | Microbiota Composition | ||||||||
| Agarwala (2021) [29] | India | 30 | – | 30 | – | 16s rRNA | Retrospective | – | – | – |
| Chiappori (2022) [30] | Italy | 6 | 6–17 | 6 | 10–20 | 16s rRNA | case-controlled | – | – | – |
| Coretti (2018) [31] | Italy | 11 | 2.92 ± 0.48 | 14 | 2.92 ± 0.70 | 16s rRNA | case-controlled | – | – | – |
| Ha (2021) [32] | South Korea | 54 | 7.0 ± 2.1 | 38 | 6.0 ± 1.7 | 16s rRNA | case-controlled | weak correlation of microbiota composition with SRS | – | – |
| Huang (2020) [33] | China | 39 | 4.74 ± 1.12 | 44 (healthy control) 38 (mother control) | 5.11 ± 0.95 (healthy control) 34.12 ± 5.07 (mother control) | 16s rRNA | case-controlled | – | – | – |
| Pulikkan (2018) [38] | India | 30 | 3–16 | 24 | 3.5–16 | 16s rRNA | case-controlled | – | – | – |
| Plaza-Diaz (2019) [37] | Spain | 54 | 2–6 | 57 | 2–6 | 16s rRNA | case-controlled | – | – | – |
| Son (2015) [42] | USA | 66 | 10.3 ± 1.8 | 37 (siblings) | 10.0 ± 1.8 | 16s rRNA | case-controlled | – | correlation of FGID with: - Firmicutes: Asteroleplasma - Proteobacteria: Thalassospira, Burkholderia, Comamonadaceae - Fusobacteria: Fusobacteriales - Bacteroidetes: Prevotellaceae - Actinobacteria: Mobiluncus | – |
| Strati (2017) [43] | Italy | 40 | 5–17 | 40 | 3.6–12 | 16s rRNA | case-controlled | – | negative correlation of constipation with Gemmiger, Ruminococcus positive correlation of constipation with Escherichia/Shigella, Clostridium cluster XVIII | – |
| Sun (2019) [44] | China | 9 | 3–12 | 6 | 3–12 | 16s rRNA | case-controlled | – | – | – |
| Tomova (2020) [46] | Slovakia | 46 | 4.0–8.5 | 16 | 2.8–9.15 | 16s rRNA | case-controlled | – | – | – |
| Wang (2011) [48] | Australia | 23 | 3–17 | 31 (22—siblings, 9—healthy independent) | siblings: 4.5–18.5 healthy independent: 3.5–15 | qPCR | case-controlled | – | – | comparable levels of sulfate-reducing bacteria in ASD and neurotypical children |
| Wong (2020) [49] | China | 92 | 8.43 ± 1.54 | 112 | 8.12 ± 1.99 | 16s rRNA | case-controlled | – | – | – |
| Ye (2021) [50] | China | 71 | 4.28 ± 1.52 | 18 | 4.62 ± 1.29 | 16s rRNA | case-controlled | – | – | strains selected for ASD prediction: Prevotella buccae, Bifidobacterium longum, Streptococcus thermophilus, Enterobacter cloacae, Klebsiella oxytoca, Eubacterium hallii, Clostridium ramosum, Erysipelotrichaceae bacterium 6_1_45, Eubacterium siraeum, Lautropia mirabilis |
| Zhang (2018) [51] | China | 35 | 4.9 ± 1.5 | 6 | 4.6 ± 1.1 | 16s rRNA | case-controlled | – | – | – |
| Zou (2020) [52] | China | 48 | 5 (2–7) | 48 | 4 | 16s rRNA | case-controlled | – | – | – |
| Study | Country | ASD (n) | ASD Age (Years) | Healthy Control (n) | Healthy Control Age (Years) | Microbiota Assessment Method | Study Type | Microbiota Assessment—Behavior |
|---|---|---|---|---|---|---|---|---|
| Qiao (2018) [39] | China | 32 (salivary) 26 | 10.02 ± 1.43 (salivary) 10.15 ± 1.35 (dental) | 27 (salivary) 26 (dental) | 10.19 ± 0.59 (salivary) 10.37 ± 0.66 (dental) | 16s rRNA | case-controlled | – |
| Ragusa (2020) [40] | Italy | 76 | 7 ± 1.5 | 39 | 6.75 ± 1.51 | RT-PCR | case-controlled | positive correlation: Moryella—VIQ, Ralstonia—ADI-A negative correlation: Moryella—ADI-D, Saccharibacteria—ADI-B and ADI-C, Weeksellaceae and Ralstonia—VIQ, PIQ, TIQ significant predictors of Tannerella abundance: VIQ, PIQ, TIQ, ADI-C, ADOS-A |
| Tong (2022) [47] | China | 26 | 4.13 ± 0.95 | 26 | 4.04 ± 0.89 | 16s rRNA | case-controlled | – |
| Study | Country | ASD (n) | ASD Age (Years) | Healthy Control (n) | Healthy Control age (years) | Microbiota Assessment method | Study Type | Intervention Type | Intervention Length | Microbiota Assessment— Other Important Results | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Behavior | GI Symptoms | Microbiota Composition | ||||||||||
| Arnold (2019) [5] | USA | 10 (6—probiotic first, then placebo, 4—placebo first, then probiotic) | 3–12 | 0 | – | 16S rRNA | RCT, placebo-controlled, double-blind, parallel | Probiotic (Lactobacillus casei, L. plantarum, L. acidophilus, L. delbrueckii subsp. bulgaricus, Bifidobacterium longum, B. infantis, B. breve, Streptococcus salivarius ssp. thermophilus) | 8 weeks of probiotics or placebo, 3 weeks of washout, 8 weeks of a crossover treatment | statistically important improvement: ABC, SRS, CSHQ, PSI-SF non-statistically important improvement: PRAS-ASD | non-statistically important improvement: Peds-QL GI module correlation of relative abundance of Lactobacillus and PEDS-QL | – |
| Grimaldi (2018) [8] | UK | 30 (18—non-restrictive diet, 12—exclusion diet) | 4–11 (mean 7.7) | – | – | 16s rRNA | RCT, double-blind, parallel | B-GOS | 6 weeks | B-GOS: - some children showed improvement in sleep patterns - significant improvement in social behavior of children on restrictive diet | lower baseline FGID in the children on restrictive diet (abdominal pain, bowel movement) B-GOS: - non-statistically important improvement in FGID, | unrestricted diet group: increase in Bifidobacterium spp., Ruminococcus spp., Lachnospiraceae (Coprococcus spp., Dorea formicigenerans, Oribacterium spp.), Eubacterium dolchum, Saccharibacteria, Mogibacteriaceae restricted diet group: Bifidobacterium adolescentis and Bifidobacterium longum the most abundant in Bifidobacterium spp.—the latter predominant |
| Kang (2017) [34] | USA | 18 | 7–16 | 20 | 7–16 | 16s rRNA | nRCT, cohort, case-controlled | MTT | 2 weeks of antibiotic treatment and cleansing bowels, MTT for 7–8 weeks | significant improvement in behavior (PGI-II, CARS, VABS-II) | significant improvement in GI symptoms: abdominal pain, indigestion, diarrhea, constipation (GSRS) | increase in the abundance of Bifidobacterium, Prevotella, Desulfovibrio (two former—persistent increase) |
| Kang (2019) [35] | USA | 18 | 7–16 | – | – | 16s rRNA | nRCT, cohort, case-controlled | MTT—follow-up after 2 years | 2 weeks of antibiotic treatment and cleansing bowels, MTT for 7–8 weeks; follow-up after 2 years | – | – | – |
| Niu (2019) [36] | China | 114; 37—probiotic, 28—without probiotic | 3–8 (mean 4.5) | 40 | 3–8 (mean 4.2) | PCR | nRCT, open-label, case-controlled | Probiotics | 4 weeks | improvement in behavior (ATEC), mood, eating pattern abnormalities, sleep quality | improvement | – |
| Sanctuary (2019) [9] | USA | 8 | 6.8 ± 2.4 (3.9–10.9) | – | – | 16s rRNA | RCT, double-blind, placebo controlled, parallel | Prebiotic (bovine colostrum product) only or pre- and probiotic (bovine colostrum product + Bifidobacterium infantis) | 5 weeks of probiotic + prebiotic supplementation, 2 weeks of washout, 5 weeks of prebiotic only supplementation | BCP only: - improvement in irritability, stereotypy, hyperactivity, lethargy—ABC, ABAS-II, RBS-R BCP + probiotic: - improvement in lethargy (ABC) | BCP only: - 87.5% of patients—some improvement in GI symptoms (QPGS-RIII, CHARGE-GIH) - GI symptoms improvement greater, according to parents BCP + probiotic: - 100% of patients—some improvement in GI symptoms (QPGS-RIII, CHARGE-GIH) | four microbiota enterotypes—high in: Prevotella, Bifidobacterium, Bacteroides, mixed no or inconsistent change in enterotype after intervention |
| Santocchi (2020) [10] | Italy | 63 | 4.15 ± 1.08 | – | – | – | RCT, double blind, parallel, factorial, efficacy controlled | Probiotic: Streptococcus thermophilus, Bifidobacterium breve, B. longum, B. infantis, Lactobacillus acidophilus, L. plantarum, L. para-casei, L. delbrueckii subsp. bulgaricu | 6 months | no statistically significant difference in the total ADOS-CSS if analyzed in children both with and without GI symptoms in children without GI symptoms: the total ADOS-CSS decreased in probiotic group and increased in placebo group in children with GI symptoms: improvement in adaptive functioning (receptive, domestic, and coping skills, sensory profile—VABS-II subscales) | in children with GI symptoms: improvement (total GSI, 6-GSI, stool smell, flatulence) | – |
| Shaaban (2017) [41] | Egypt | 30 | 5–9 | 30 | 5–9 | RT-PCR, qPCR | nRCT, prospective, open-label | Probiotic (Lactobacillus acidophilus, L. rhamnosus, Bifidobacterium longum) | 3 months | improvement in speech/language/communication, sociability, sensory/cognitive awareness, and health/physical/behavior in ATEC | improvement in 6-GSI (especially constipation, stool consistency, flatulence, abdominal pain) | increase in Bifidobacterium and Lactobacillus |
| Tomova (2014) [45] | Slovakia | 10 | 2–9 | 19 (10—non-autistic siblings, 10—non-autistic independent controls) | siblings: 5–17 independent controls: 2–11 | RT-PCR | nRCT, prospective, open-label, controlled | Probiotic: 3 strains of Lactobacillus, 2 strains of Bifidobacteria, 1 strain of Streptococcus | 4 months | positive correlation between severity of core symptoms and higher Clostridia and Desulfovibrio levels, and lower Bacteroidetes/Firmicutes ratio | positive correlation between severity of GI symptoms and lower Clostridia and Desulfovibrio levels, and lower Bacteroidetes/Firmicutes ratio | siblings vs. independent controls: - ↑ Firmicutes - ↓ Bacteroidetes - ↓ Bacteroidetes/ Firmicutes ratio Probiotics: - increase in Bacteroidetes/Firmicutes ratio - lower Lactobacillus absolute amount, higher relative amount - lower Desulfovibrio level |
| Wang (2020) [11] | China | 26 | 2–8 | 24 | 2–8 | 16s rRNA | RCT, case-controlled, placebo-controlled, double-blind | Probiotic (Bifidobacterium infantis, B. lactis, Lactobacillus rhamnosus, L. paracasei) | 30 days | a significant decrease in severity of autistic symptoms—ATEC (speech/language/communication and sociability categories) | – | – |
| Study | Country | Articles Included (n) | ASD (n) | Healthy Control (n) | Method | Microbiota Assessment | ||
|---|---|---|---|---|---|---|---|---|
| Behavior | GI Symptoms | Microbiota Composition | ||||||
| Andreo-Martinez (2021) [14] | Spain | 18 | 642 | 356 | MA | Bacteroides—positive correlation with ASD severity | – | – |
| Iglesias-Vasquez (2020) [15] | Spain | 18 | 493 | 404 | SR/MA | – | – | – |
| Martinez-Gonzalez (2019) [20] | Spain | 16 | – | – | SR | – | – | – |
| Ng (2019) [21] | Singapore | 8 | 544 | – | SR | Probiotics: - L. plantarum (39 patients): no differences in behavior - L. acidophilus (22 patients): improvement in concentration - L. acidophilus, L. rhamnosus, B. longum (30 patients): improved behavior - L. rhamnosus, B. animalis, B. lactis (342 patients): worse behavior after probiotics - L. acidophilus, L. casei, L. delbrueckii, B. longum, B. bifidum (33 patients): improvement in behavior B-GOS (30 patients): - no difference in sleep pattern | Probiotics: - L. acidophilus, L. casei, L. delbrueckii, B. longum, B. bifidum (33 patients): improvement in GI symptoms - L. plantarum (39 patients): no differences in GI symptoms - L. acidophilus, L. rhamnosus, B. longum (30 patients): improved GI symptoms Bovine colostrum (8 patients): - ↓ GI symptoms B-GOS (30 patients): - ↓ GI discomfort with no difference in GI symptoms | Probiotics: - L. plantarum (39 patients): ↓ Clostridium after probiotics - L. acidophilus, L. rhamnosus, B. longum (30 patients): ↑ Bifidobacterium after probiotics - Lactobacillus (3 strains), Bifidobacteria (2 strains), Streptococcus (1 strain) (29) patients: ↓ Bifidobacterium, Lactobacillus |
| Patel (2022) [22] | USA | 9 | 710 | – | SR | – | - gut dysbiosis connected with the severity and prevalence of GI symptoms - probiotics: improvement in GI symptoms and behavior - prebiotics: equivocal efficacy | – |
| Song (2022) [16] | China | 3 | 144 (74 pro- and prebiotic, 34 placebo) | MA | - core symptoms: 2 studies—no significant improvement, 1 study—improvement in speech/language/communication - behavior: improvement in 2 studies | improvement in 2 studies | – | |
| Srikantha (2019) [23] | Switzerland | 136 | – | – | SR | - ↓ diversity correlated with the severity of GI symptoms - ↑ Clostridium spp. (C. perfingens) correlated with higher severity of symptoms (CARS) Probiotics: - Lactobacillus + Bifidobacterium: improvement in PGI-III Vancomycin therapy: - improvement in behavioral difficulties during antibiotic therapy, but not after its end (weak evidence) | MTT: - improvement in GI symptoms Probiotics: improvement in GI symptoms | Probiotics: - Lactobacillus + Bifidobacterium + Streptococcus: ↑ Bacteroidetes and Bacteroidetes/Firmicutes ratio - Lactobacillus + Bifidobacterium: -↑ diversity - ↑ Bifidobacterium, Prevotella, Desulfovibrio |
| Tan (2021) [24] | Canada | 13 | 481 | – | SR | Probiotics: - no influence, 4 RCTs - positive influence, 5 papers (1 RCT, 4 non-RCTs) Pre- and synbiotics: - positive influence, 4 papers (3 RCTs, 1 non-RCT) MTT: - positive influence, 1 non-RCT study in 2 papers | Probiotics: - no influence, 4 papers (3 RCTs, 1 non-RCT) - positive influence, 2 non-RCTs Pre- and synbiotics: - no influence, 1 RCT - positive influence, 2 papers (1 RCT, 1 non-RCT) MTT: - positive influence, 1 non-RCT study in 2 papers | – |
| West (2022) [17] | USA | 13 | – | – | MA | – | – | – |
| Wu (2020) [25] | China | 5 | 169 | 128 | MA | – | – | Prevotella, Roseburia, Ruminococcus, Megasphaera, Catenibacterium—potential biomarkers of ASD (in forest analysis machine learning) |
| Xu (2019) [18] | China | 9 | 254 | 167 | SR, MA | – | – | – |
| Zafar (2021) [27] | Pakistan | 8 (6 original papers + 2 SRs including 153 studies) | 330 | 178 | SR | – | – | – |
| Zhang (2023) [28] | China | 5 | 150 | – | SR | MTT: - improvement in CARS, ABC, VABS-II, sleep disturbances | MTT: - improvement - ↓ Eubacterium coprostanoligenes correlated with GI symptoms | MTT: - ↑ Bifidobacterium, Prevotella, Desulfovibrio, Roseburia, Ruminococcus, Faecalibacterium |
| Zhou (2023) [52] | China | 9 (5 RCTs included in MA) | 186 | 150 | MA | MTT: ABC and CARS significantly ↓ | – | – |
| Yang (2020) [26] | China | 16 | 376 | 66 | SR | Probiotics: - improvement in ASD core symptoms comparable to placebo, 3 papers - greater improvement after probiotics only in comparison to probiotics + prebiotics, 1 paper - improvement in irritability (ATEC), 2 papers - improvement in concentration and carrying out orders, 1 paper Prebiotics: - improvement in sociability, 1 paper Pre- and probiotics combined: - improvement in behavior (CARS, SRS, VABS-II, ABC), 2 papers MTT: - ↓ CARS, 1 paper Vitamin A supplementation: - no influence on ASD core symptoms Streptococcus, Alistipes putredinis, Bacteroides, Clostridium—positive correlation with ASD symptoms Coprococcus—negative correlation with CARS Lactobacillus—correlation with Peds-QL | Probiotics: - no improvement, 2 papers - non-statistically important improvement, 2 papers - statistically important improvement, 1 paper Prebiotics: - improvement, 1 paper Pre- and probiotics combined: - improvement, 2 papers MTT: - statistically significant improvement, 2 papers Blautia wexlerae—positive correlation with the frequency of stool passage Clostridium leptum, Eubacterium sp. Marseille—negative correlation with the frequency of stool passage | Probiotics: - effective, 8 papers - ineffective, 1 paper - ↑ Bifidobacteria, Lactobacillus (relative amount), Enterococci - ↓ Firmicutes, Desulfovibrio, Clostridium, Lactobacillus (absolute amount) Prebiotics: - ↓ a-diversity - ↑ Bifidobacteriales - ↓ Clostridium MTT: - ↑ Bifidobacterium, Prevotella, Desulfovibrio - ↓ Bacteroides fragilis Vitamin A supplementation: - ↑ Bacteroidetes/Bacteroidales and Bacteroidetes/Firmicutes ratio |
| Phylum/Genus | Agarwala (2021) [29] | Arnold (2019) [5] | Chiappori (2022) [30] | Coretti (2018) [31] | Grimaldi (2018) [8] | Ha (2021) [32] | Huang (2020) [33] | Kang (2017) [34] | Niu (2019) [36] | Pulikkan (2018) [38] | Plaza-Diaz (2019) [37] | Sanctuary (2019) [9] | Shaaban (2017) [41] | Son (2015) [42] | Strati (2017) [43] | Sun (2019) [44] | Tomova (2014) [45] | Tomova (2020) [46] | Wang (2011) [48] | Wang (2020) [11] | Wong (2020) [49] | Ye (2021) [50] | Zhang (2018) [51] | Zou (2020) [52] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Actinobacteria | ↓ | ↑ | ↑ | ↓ | ↑ | ↓ | ↓ng | ↑ | ↑ | |||||||||||||||
| Actinomyces | ↓ | |||||||||||||||||||||||
| Bifidobacterium | ↓ | ↓ | ↓ | ↓ed ↑nd | ↑ | ↓ | ↓ | ↑ | ↓ | ↓ | ↑ sc | ↓sc, ic | ↓ | ↓ | ↑ | |||||||||
| Collinsella | = | ↑ | ↑ | ↓ng | ||||||||||||||||||||
| Corynebacterium | = | ↓ | ||||||||||||||||||||||
| Eggerthella | ↓ | ↓ | ↑nd | |||||||||||||||||||||
| Nitriliruptor | ↑ | |||||||||||||||||||||||
| Bacteroidetes | ↓ | ↑ed | ↓ | ↓ | = | ↓ | ↑ | ↓ | ↑ | |||||||||||||||
| Alistipes | ↓ | ↓ | ↓ng | |||||||||||||||||||||
| Barnesiella | ↓ | |||||||||||||||||||||||
| Odoribacter | ↑ | |||||||||||||||||||||||
| Parabacteroides | = | ↓ | ↑ | ↓ | ↓ | ↑nfs | ↓ng | |||||||||||||||||
| Prevotella | ↑ | ↑ | ↓ | ↓ | = | ↑fs | = | ↑ | ||||||||||||||||
| Bacillota (Firmicutes) | ↓ | ↑ed | = | ↑3,4 ↓5,6 | ↑ | ↑ | ↑3,7,8 ↓6 | ↓9 ↑5 | ↑g | ↑/ ↓5 | ↓/↑5,6 | |||||||||||||
| Acidaminococcus | ↑ | |||||||||||||||||||||||
| Anaerophilum | ↑fs | |||||||||||||||||||||||
| Anaerostipes | ↑ | |||||||||||||||||||||||
| Blautia | = | ↓ | ↑ng | ↑ | ↑ | |||||||||||||||||||
| Butyricicoccus | ↓ | |||||||||||||||||||||||
| Butyrivirio | ↑ | |||||||||||||||||||||||
| Christensenella | ↓ | |||||||||||||||||||||||
| Cloacibacillus | ↑ | |||||||||||||||||||||||
| Clostridium | ↓10 | ↑ | ↑sc | ↑fs | = | ↓ng | ↑ | |||||||||||||||||
| Coprococcus | ↑ | |||||||||||||||||||||||
| Dehalobacterium | ↑nd | |||||||||||||||||||||||
| Dialister | ↓ | ↓ | ↓ | ↑g | ↓ | ↓ | ||||||||||||||||||
| Dorea | ↑ | ↑ng | ||||||||||||||||||||||
| Eisenbergiella | ↑ | |||||||||||||||||||||||
| Enterococcus | = | ↑ | = | |||||||||||||||||||||
| Eubacterium | ↓ | ↑ | ||||||||||||||||||||||
| Faecalibacterium | ↓ | ↓ | ↑ | ↑ed | ↓ | = | ↑ | ↓ | ↑ | |||||||||||||||
| Filifactor | ||||||||||||||||||||||||
| Flavonifactor | ↓ | |||||||||||||||||||||||
| Fusicatenibacter | ↑g | |||||||||||||||||||||||
| Gemella | ↓ | |||||||||||||||||||||||
| Lactobacillus | = | ↑ | ↑ | ↑ | ↑ | |||||||||||||||||||
| Lactostreptococcus | ↑nd | |||||||||||||||||||||||
| Lachnospira | ↓ | ↓ | ↑ | ↑ | ||||||||||||||||||||
| Limosilactobacillus | ↑ | |||||||||||||||||||||||
| Megasphaera | ↑ | ↓ | ||||||||||||||||||||||
| Mitsuokella | ↑ | |||||||||||||||||||||||
| Oscillospira | ↑ | ↑ | ||||||||||||||||||||||
| Phascolarctobacterium | = | ↓ | ↓g | |||||||||||||||||||||
| Streptococcus | ↓ | ↓ | ↓ | ↑nd | ↓ | ↓ | ↑ | |||||||||||||||||
| Roseburia | = | ↑ed | ↓ | ↓ | ↓ | ↓ | ↑ | |||||||||||||||||
| Ruminococcus | = | ↑ | ↑ | ↓ | ↑ | ↑ | ||||||||||||||||||
| Ruminiclostridium | ↓ | |||||||||||||||||||||||
| Sarcina | ↑ | |||||||||||||||||||||||
| Turicibacter | ↑g | |||||||||||||||||||||||
| Veillonella | ↑ | ↓ | ↓ | ↓ | ||||||||||||||||||||
| Fusobacteriota | = | ↑ | ||||||||||||||||||||||
| Cetobacterium | ↑ | |||||||||||||||||||||||
| Fusobacterium | ↑ | |||||||||||||||||||||||
| Lentisphaerota | ↑ | |||||||||||||||||||||||
| Proteobacteria (Pseudomonadota) | ↑ | ↑ | = | ↑ | ↑ | ↑ | ↑fs | ↑ | ↓ | |||||||||||||||
| Citrobacter | ↓ | ↑ | ↓ | |||||||||||||||||||||
| Constrictibacter | ↑ | |||||||||||||||||||||||
| Dichelobacter | ↑ | |||||||||||||||||||||||
| Diaphorobacter | ↓ | |||||||||||||||||||||||
| Enterobacter | ↑ | |||||||||||||||||||||||
| Escherichia | = | ↓ | ↓ | ↓ | ||||||||||||||||||||
| Haemophilus | = | ↓ | ||||||||||||||||||||||
| Klebsiella | ↑ | ↑ | ||||||||||||||||||||||
| Nitratireductor | ↓ | |||||||||||||||||||||||
| Phyllobacterium | ↑ | |||||||||||||||||||||||
| Providencia | ↓ | |||||||||||||||||||||||
| Salmonella | ↑fs | |||||||||||||||||||||||
| Shigella | ↓ng | ↓ | ||||||||||||||||||||||
| Sutterella | ↑ | ↑ | ||||||||||||||||||||||
| Saccharibacteria | = | |||||||||||||||||||||||
| Thermodesulfobacteriota | ↑ | ↑ | ||||||||||||||||||||||
| Bilophila | ↓ | |||||||||||||||||||||||
| Desulfovibrio | = | ↑sc | ||||||||||||||||||||||
| Verrucomicrobiota | = | |||||||||||||||||||||||
| Akkermansia | ↓ | ↓ | ↑nd | ↓ic | ↓ | ↓ | ↓ | |||||||||||||||||
| Bacteroidetes/ Firmicutes ratio | ↑ | ↓ | ↑ | ↓ | = | ↓ | ↓ | ↑ | ↓ | |||||||||||||||
| Overall bacterial diversity | = | ↑ | ↓ | ↓ | ||||||||||||||||||||
| Escherichia/ Shigella ratio | ↑ | ↑fs |
| Phylum/Genus | Andreo-Martinez (2021) [14] | Iglesias-Vasquez (2020) [15] | Martinez-Gonzales (2019) [20] | Srikantha (2019) [23] | West (2022) [17] | Xu (2019) [18] | Zafar (2021) [27] |
|---|---|---|---|---|---|---|---|
| Actinobacteria | ↑ (2) | ||||||
| Actinomyces | ↑b | ||||||
| Bifidobacterium | ↓ | ↓ | ↓ | ↓ | ↓ (2) | ||
| Collinsella | ↓ | ↑ (1) | |||||
| Bacteroidetes | ↑ | ↓ (2)/↑ (1) | |||||
| Alistipes | ↓ (1) | ||||||
| Bacteroides | (↑) | ↑ | ↓b | ↓ng | ↓ | ||
| Barnesiella | ↑ | ||||||
| Odoribacter | ↑ | ||||||
| Parabacteroides | ↑ | ↑ | ↓ | ↑ (1)/↓ (1) | |||
| Porphyromonas | ↑ | ||||||
| Prevotella | (↓) | ↓g | ↑/↓b | ↑ (2)/↓ (3) | |||
| Bacillota (Firmicutes) | ↑ | ↑ (1) | |||||
| Acidaminococcus | ↓ (1) | ||||||
| Anaerophilum | ↑ | ||||||
| Clostridium | (↑) | ↑ | ↑ | ↓ng | = | ↑ (3) | |
| Coprococcus | ↓ | ||||||
| Dialister | ↓ (1) | ||||||
| Dorea | ↑ | ↑ (1) | |||||
| Enterococcus | ↓ | ↓ | |||||
| Faecalibacterium | ↑ | ↑/↑b | ↑ | ||||
| Flavonifactor | ↓ (1) | ||||||
| Granulicatella | ↓ | ||||||
| Lactobacillus | (↑) | = | ↑ | ↑ (1)/↓ (2) | |||
| Lachnospira | (↓) | ↑b | ↑ (1)/↓ (1) | ||||
| Lactococcus | ↓ | ||||||
| Masilloclostridium | ↓ng | ||||||
| Oscillospira | ↓/↑b | ↑ (1) | |||||
| Peptostreptococcus | ↑b | ↑ | |||||
| Phascolarctobacterium | ↑ | ||||||
| Sporobacter | ↓ | ||||||
| Staphylococcus | ↓ | ||||||
| Streptococcus | ↓ | ↓g | ↓/↓b | ↑ (2)/↓ (1) | |||
| Subdoligranulum | ↓ | ||||||
| Roseburia | (↓) | ↑ | |||||
| Ruminococcus | (↓) | ↑b | ↑ | ↑ (1)/↓ (1) | |||
| Turicibacter | ↑ | ||||||
| Tyzzerella | ↓ | ||||||
| Veillonella | ↓g | ↑ (2) | |||||
| Fusobacteriota | |||||||
| Fusobacterium | ↓ | ↓ (1) | |||||
| Lentisphaerota | |||||||
| Proteobacteria (Pseudomonadota) | ↑ | ↑b | ↑ (3) | ||||
| Aeromonas | ↑ | ||||||
| Burkholderia | ↑b | ||||||
| Devosia | ↓b | ↓ | |||||
| Neisseria | ↓b | ||||||
| Parasutterella | ↑ | ||||||
| Pseudomonas | ↑ | ||||||
| Ralstonia | ↑b | ||||||
| Tenericutes | ↑ | ||||||
| Enterobacter | ↑ | ↓ (1) | |||||
| Escherichia | ↓/↓b | ↓ | ↑ (1) | ||||
| Shigella | ↓ (1) | ||||||
| Sutterella | (↑) | ↑b | ↑ (2) | ||||
| Thermodesulfobacteriota | |||||||
| Bilophila | ↓ (1) | ||||||
| Desulfovibrio | ↑ | ||||||
| Verrucomicrobiota | ↓ | ↓ (1) | |||||
| Akkermansia | ↑ | ↓ | ↓ (1) | ||||
| Bacteroidetes/ Firmicutes ratio | ↓ | ↑ |
| Phylum/Genus | Qiao (2018) [39] | Ragusa (2020) [40] | Tong (2022) [47] |
|---|---|---|---|
| Actinobacteria | ↓ | ↑ | |
| Actinomyces | ↓s | ||
| Rothia | ↑d/↓s | ↑ | |
| Bacteroidetes | ↓ | ↑ | |
| Alloprevotella | ↓ s, d | ||
| Porphyromonas | ↓ s | ||
| Prevotella | ↓ d/= s | ||
| Tannerella | ↓ | ||
| Bacillota (Firmicutes) | ↓ | ↓ | |
| Filifactor | ↑ | ||
| Moryella | ↓ | ||
| Peptostreptococcus | ↓ s, d | ||
| Selenomonas | ↓ d | ||
| Solobacterium | ↓ s, d | ||
| Streptococcus | ↑ d | ↑ | |
| Fusobacteriota | ↓ | ↓ | |
| Fusobacterium | ↓ s, d | ||
| Leptotrichia | ↓ s, d | ||
| Proteobacteria (Pseudomonadota) | ↑ | ↑ | |
| Actinobacillus | ↑ | ||
| Aggregatibacter | ↑ | ||
| Haemophilus | ↑ s | ↑ | |
| Ralstonia | ↑ | ||
| Saccharibacteria | ↓ | ||
| Overall bacterial diversity | ↓ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lewandowska-Pietruszka, Z.; Figlerowicz, M.; Mazur-Melewska, K. Microbiota in Autism Spectrum Disorder: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 16660. https://doi.org/10.3390/ijms242316660
Lewandowska-Pietruszka Z, Figlerowicz M, Mazur-Melewska K. Microbiota in Autism Spectrum Disorder: A Systematic Review. International Journal of Molecular Sciences. 2023; 24(23):16660. https://doi.org/10.3390/ijms242316660
Chicago/Turabian StyleLewandowska-Pietruszka, Zuzanna, Magdalena Figlerowicz, and Katarzyna Mazur-Melewska. 2023. "Microbiota in Autism Spectrum Disorder: A Systematic Review" International Journal of Molecular Sciences 24, no. 23: 16660. https://doi.org/10.3390/ijms242316660





