A Ubiquitin-Based Module Directing Protein–Protein Interactions in Chloroplasts
Abstract
:1. Introduction
2. Results
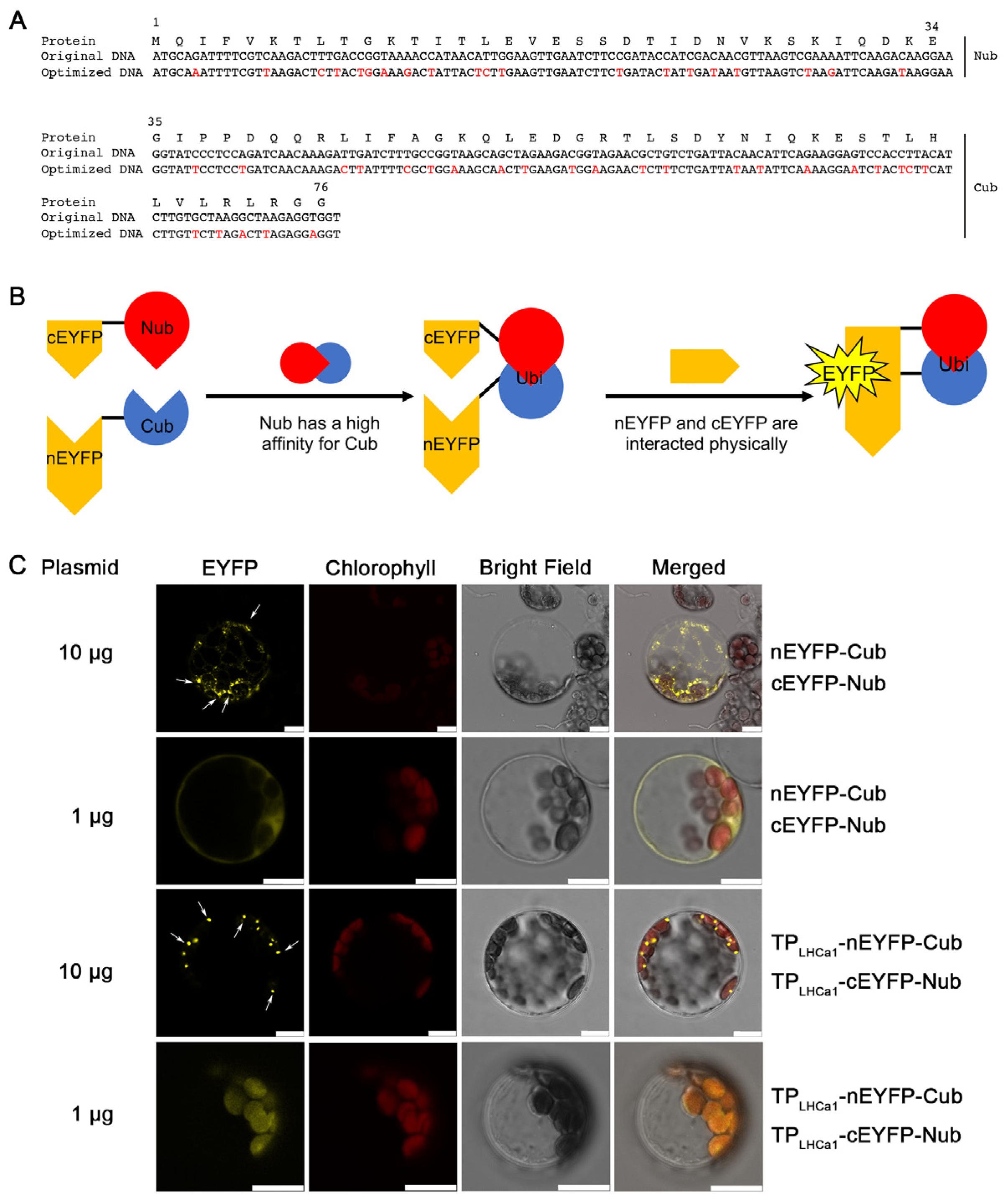
2.1. The Design of a Ubiquitin-Based Module
2.2. Nub–Cub Directs the Tight Interaction of Unrelated Thylakoid Proteins
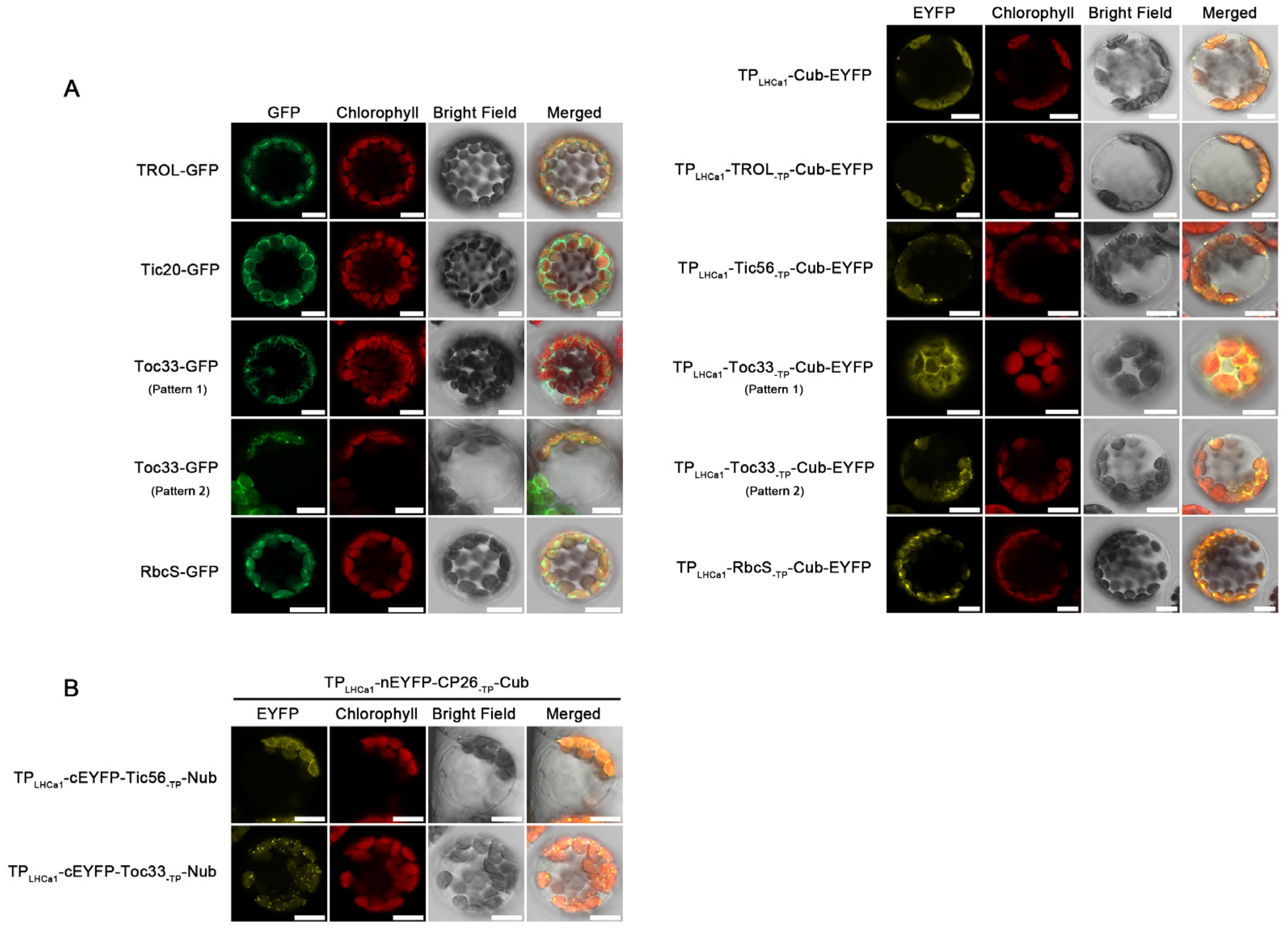
2.3. Impacts of Cub Module on Sub-Chloroplast Localization
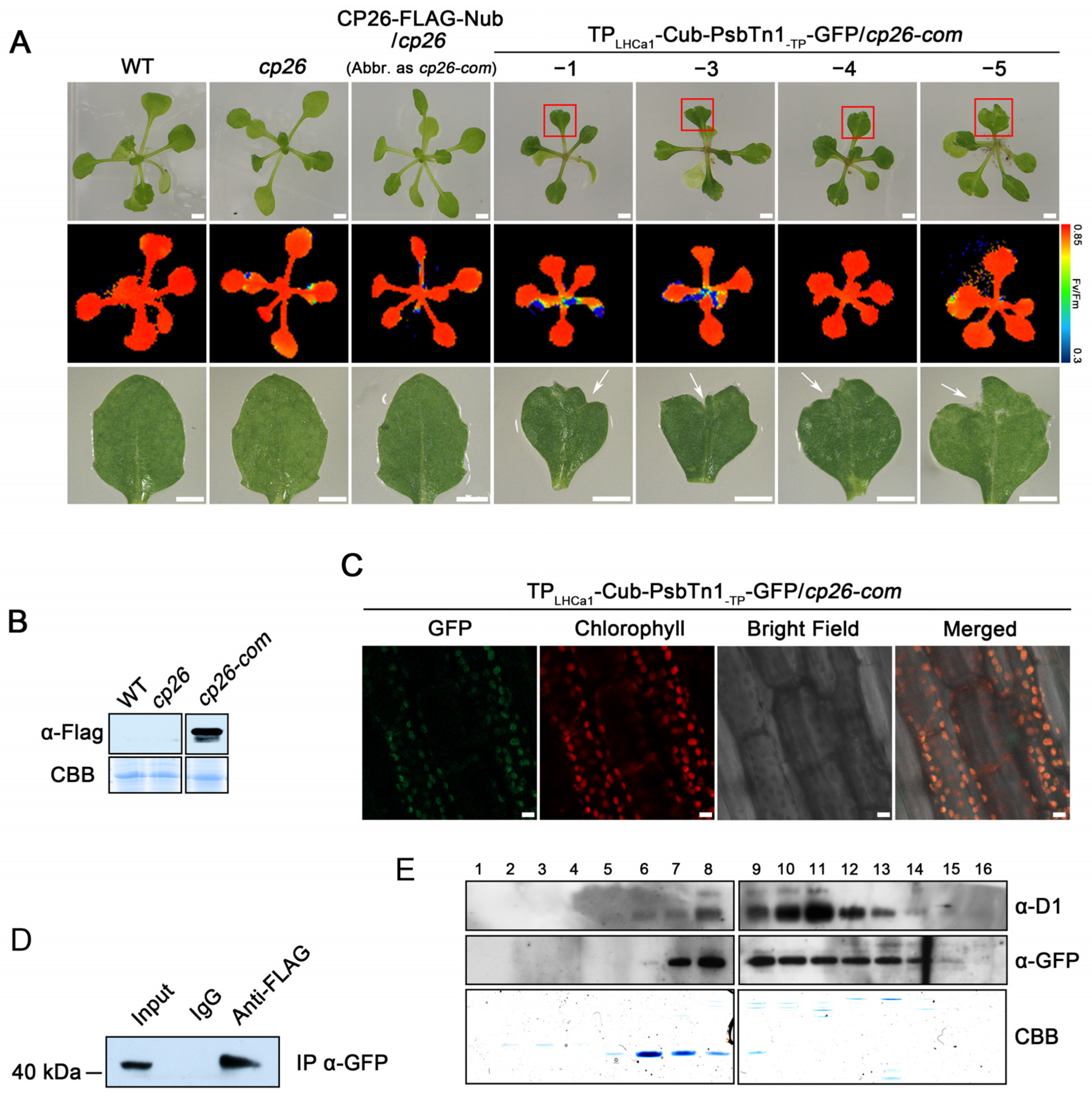
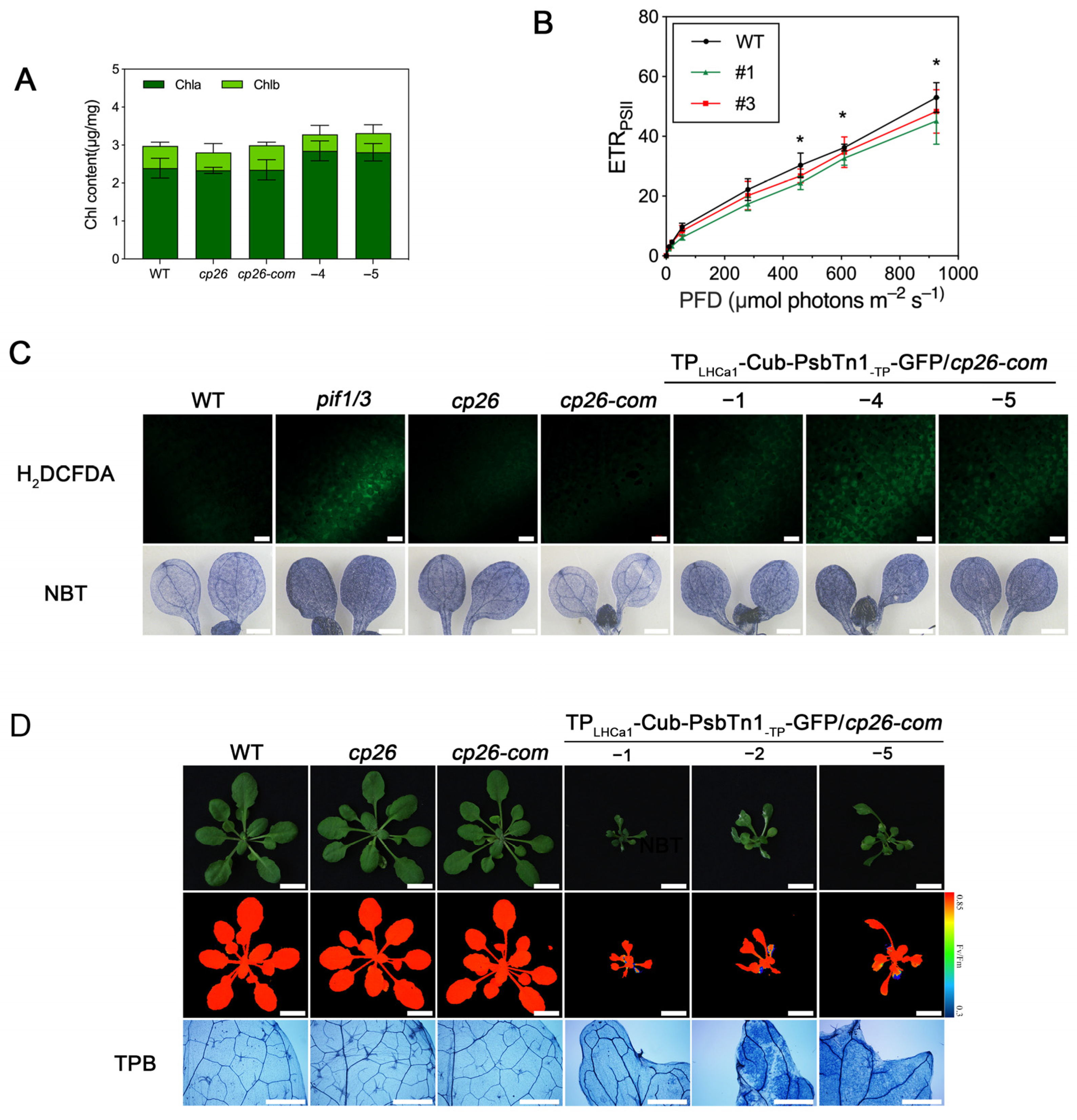
2.4. The Nub–Cub System Guides Chloroplast Protein Interaction in Plants
3. Discussion
4. Materials and Methods
4.1. Plant Material and Growth Conditions
4.2. Codon Optimization
4.3. Plasmid Construction
4.4. Confocal Microscopy Imaging
4.5. Chlorophyll Fluorescence Measurements
4.6. Co-Immunoprecipitation Assay
4.7. The Luciferase Complementation Imaging (LCI) Assay
4.8. ROS and Cell Death Detection
4.9. The Comigration Assay
4.10. Accession Numbers
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shoemaker, B.A.; Panchenko, A.R. Deciphering Protein–Protein Interactions. Part I. Experimental Techniques and Databases. PLoS Comput. Biol. 2007, 3, e42. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.; Chen, R.; Zhu, Y.; Zhang, W.; Mu, W. Interaction between the Anchoring Domain of A-Kinase Anchoring Proteins and the Dimerization and Docking Domain of Protein Kinase A: A Potent Tool for Synthetic Biology. ACS Synth. Biol. 2022, 11, 3154–3162. [Google Scholar] [CrossRef] [PubMed]
- Grünberg, R.; Serrano, L. Strategies for Protein Synthetic Biology. Nucleic Acids. Res. 2010, 38, 2663–2675. [Google Scholar] [CrossRef] [PubMed]
- Shin, G.; Lim, S.I. Site-Specific Proximity Ligation Provides Molecular Insights into Biologically Relevant Interfaces of Protein-Protein Interaction. Biochem. Biophys. Res. Commun. 2020, 533, 932–937. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Cao, S.; Liu, M.; Kang, W.; Xia, J. Self-Assembled Multienzyme Nanostructures on Synthetic Protein Scaffolds. ACS Nano 2019, 13, 11343–11352. [Google Scholar] [CrossRef] [PubMed]
- Yanase, T.; Okuda-Shimazaki, J.; Asano, R.; Ikebukuro, K.; Sode, K.; Tsugawa, W. Development of a Versatile Method to Construct Direct Electron Transfer-Type Enzyme Complexes Employing SpyCatcher/SpyTag System. IJMS 2023, 24, 1837. [Google Scholar] [CrossRef]
- Terao, Y.; Kawabata, S.; Nakata, M.; Nakagawa, I.; Hamada, S. Molecular Characterization of a Novel Fibronectin-Binding Protein of Streptococcus pyogenes Strains Isolated from Toxic Shock-like Syndrome Patients. J. Biol. Chem. 2002, 277, 47428–47435. [Google Scholar] [CrossRef]
- Leister, D. Genetic Engineering, Synthetic Biology and the Light Reactions of Photosynthesis. Plant Physiol. 2019, 179, 778–793. [Google Scholar] [CrossRef]
- Batista-Silva, W.; da Fonseca-Pereira, P.; Martins, A.O.; Zsögön, A.; Nunes-Nesi, A.; Araújo, W.L. Engineering Improved Photosynthesis in the Era of Synthetic Biology. Plant Commun. 2020, 1, 100032. [Google Scholar] [CrossRef]
- Lassen, L.M.; Nielsen, A.Z.; Ziersen, B.; Gnanasekaran, T.; Møller, B.L.; Jensen, P.E. Redirecting Photosynthetic Electron Flow into Light-Driven Synthesis of Alternative Products Including High-Value Bioactive Natural Compounds. ACS Synth. Biol. 2014, 3, 1–12. [Google Scholar] [CrossRef]
- Mellor, S.B.; Vavitsas, K.; Nielsen, A.Z.; Jensen, P.E. Photosynthetic Fuel for Heterologous Enzymes: The Role of Electron Carrier Proteins. Photosynth. Res. 2017, 134, 329–342. [Google Scholar] [CrossRef]
- Cardona, T.; Shao, S.; Nixon, P.J. Enhancing Photosynthesis in Plants: The Light Reactions. Essays Biochem. 2018, 62, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Tognetti, V.B.; Palatnik, J.F.; Fillat, M.F.; Melzer, M.; Hajirezaei, M.-R.; Valle, E.M.; Carrillo, N. Functional Replacement of Ferredoxin by a Cyanobacterial Flavodoxin in Tobacco Confers Broad-Range Stress Tolerance. Plant Cell 2006, 18, 2035–2050. [Google Scholar] [CrossRef] [PubMed]
- Chida, H.; Nakazawa, A.; Akazaki, H.; Hirano, T.; Suruga, K.; Ogawa, M.; Satoh, T.; Kadokura, K.; Yamada, S.; Hakamata, W.; et al. Expression of the Algal Cytochrome C6 Gene in Arabidopsis Enhances Photosynthesis and Growth. Plant Cell Physiol. 2007, 48, 948–957. [Google Scholar] [CrossRef] [PubMed]
- Blanco, N.E.; Ceccoli, R.D.; Segretin, M.E.; Poli, H.O.; Voss, I.; Melzer, M.; Bravo-Almonacid, F.F.; Scheibe, R.; Hajirezaei, M.-R.; Carrillo, N. Cyanobacterial Flavodoxin Complements Ferredoxin Deficiency in Knocked-down Transgenic Tobacco Plants. Plant J. 2011, 65, 922–935. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Takahashi, S.; Badger, M.R.; Shikanai, T. Artificial Remodelling of Alternative Electron Flow by Flavodiiron Proteins in Arabidopsis. Nat. Plants 2016, 2, 16012. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Huang, H.; Cheng, C.; Ho, M.; Ger, M. Constitutive Expression of a Plant Ferredoxin-like Protein (Pflp) Enhances Capacity of Photosynthetic Carbon Assimilation in Rice (Oryza sativa). Transgenic. Res. 2017, 26, 279–289. [Google Scholar] [CrossRef]
- Simkin, A.J.; McAusland, L.; Lawson, T.; Raines, C.A. Overexpression of the RieskeFeS Protein Increases Electron Transport Rates and Biomass Yield. Plant Physiol. 2017, 175, 134–145. [Google Scholar] [CrossRef]
- Johnsson, N.; Varshavsky, A. Split Ubiquitin as a Sensor of Protein Interactions in vivo. Proc. Natl. Acad. Sci. USA 1994, 91, 10340–10344. [Google Scholar] [CrossRef]
- Jurić, S.; Hazler-Pilepić, K.; Tomašić, A.; Lepeduš, H.; Jeličić, B.; Puthiyaveetil, S.; Bionda, T.; Vojta, L.; Allen, J.F.; Schleiff, E.; et al. Tethering of Ferredoxin:NADP+ Oxidoreductase to Thylakoid Membranes Is Mediated by Novel Chloroplast Protein TROL: Tethering of FNR to Thylakoids Is Mediated by TROL. Plant J. 2009, 60, 783–794. [Google Scholar] [CrossRef]
- de Bianchi, S.; Dall’Osto, L.; Tognon, G.; Morosinotto, T.; Bassi, R. Minor Antenna Proteins CP24 and CP26 Affect the Interactions between Photosystem II Subunits and the Electron Transport Rate in Grana Membranes of Arabidopsis. Plant Cell 2008, 20, 1012–1028. [Google Scholar] [CrossRef] [PubMed]
- Vojta, L.; Fulgosi, H. Topology of TROL Protein in Thylakoid Membranes of Arabidopsis thaliana. Physiol. Plant. 2019, 166, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Agne, B.; Köhler, D.; Baginsky, S. Protein Import-Independent Functions of Tic56, a Component of the 1-MDa Translocase at the Inner Chloroplast Envelope Membrane. Plant Signal. Behav. 2017, 12, e1284726. [Google Scholar] [CrossRef] [PubMed]
- Gutensohn, M.; Schulz, B.; Nicolay, P.; Flugge, U.-I. Functional Analysis of the Two Arabidopsis Homologues of Toc34, a Component of the Chloroplast Protein Import Apparatus. Plant J. 2000, 23, 771–783. [Google Scholar] [CrossRef] [PubMed]
- Machettira, A.B.; Gross, L.E.; Sommer, M.S.; Weis, B.L.; Englich, G.; Tripp, J.; Schleiff, E. The Localization of Tic20 Proteins in Arabidopsis thaliana Is Not Restricted to the Inner Envelope Membrane of Chloroplasts. Plant Mol. Biol. 2011, 77, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Teresinski, H.J.; Gidda, S.K.; Nguyen, T.N.D.; Howard, N.J.M.; Porter, B.K.; Grimberg, N.; Smith, M.D.; Andrews, D.W.; Dyer, J.M.; Mullen, R.T. An RK/ST C-Terminal Motif Is Required for Targeting of OEP7.2 and a Subset of Other Arabidopsis Tail-Anchored Proteins to the Plastid Outer Envelope Membrane. Plant Cell Physiol. 2019, 60, 516–537. [Google Scholar] [CrossRef] [PubMed]
- Ling, Q.; Li, N.; Jarvis, P. Chloroplast Ubiquitin E3 Ligase SP1: Does It Really Function in Peroxisomes? Plant Physiol. 2017, 175, 586–588. [Google Scholar] [CrossRef]
- Pan, R.; Hu, J. The Arabidopsis E3 Ubiquitin Ligase SP1 Targets to Chloroplasts, Peroxisomes, and Mitochondria. Plant Physiol. 2018, 176, 480–482. [Google Scholar] [CrossRef]
- van Wijk, K.J. Protein Maturation and Proteolysis in Plant Plastids, Mitochondria, and Peroxisomes. Annu. Rev. Plant Biol. 2015, 66, 75–111. [Google Scholar] [CrossRef]
- Shi, L.; Schröder, W.P. The Low Molecular Mass Subunits of the Photosynthetic Supracomplex, Photosystem II. Biochim. Biophys. Acta 2004, 1608, 75–96. [Google Scholar] [CrossRef]
- Chen, Y.; Yuan, S.; Lezhneva, L.; Meurer, J.; Schwenkert, S.; Mamedov, F.; Schröder, W.P. The Low Molecular Mass Photosystem II Protein PsbTn Is Important for Light Acclimation. Plant Physiol. 2019, 179, 1739–1753. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Su, X.; Cao, P.; Liu, X.; Chang, W.; Li, M.; Zhang, X.; Liu, Z. Structure of Spinach Photosystem II–LHCII Supercomplex at 3.2 Å Resolution. Nature 2016, 534, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R. ROS Are Good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Hatlem, D.; Trunk, T.; Linke, D.; Leo, J.C. Catching a SPY: Using the SpyCatcher-SpyTag and Related Systems for Labeling and Localizing Bacterial Proteins. IJMS 2019, 20, 2129. [Google Scholar] [CrossRef] [PubMed]
- Varshavsky, A. The Ubiquitin System. Trends Biochem. Sci. 1997, 22, 383–387. [Google Scholar] [CrossRef] [PubMed]
- Cline, K.; Dabney-Smith, C. Plastid Protein Import and Sorting: Different Paths to the Same Compartments. Curr. Opin. Plant Biol. 2008, 11, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Ouyang, M.; Lu, D.; Zheng, C.; Zhang, L. Protein Sorting within Chloroplasts. Trends Cell Biol. 2021, 31, 9–16. [Google Scholar] [CrossRef]
- Foyer, C.H. Reactive Oxygen Species, Oxidative Signaling and the Regulation of Photosynthesis. Environ. Exp. Bot. 2018, 154, 134–142. [Google Scholar] [CrossRef]
- Gray, J.; Janick-Buckner, D.; Buckner, B.; Close, P.S.; Johal, G.S. Light-Dependent Death of Maize Lls1 Cells Is Mediated by Mature Chloroplasts. Plant Physiol. 2002, 130, 1894–1907. [Google Scholar] [CrossRef]
- Pružinská, A.; Tanner, G.; Anders, I.; Roca, M.; Hörtensteiner, S. Chlorophyll Breakdown: Pheophorbide a Oxygenase Is a Rieske-Type Iron-Sulfur Protein, Encoded by the Accelerated Cell Death 1 Gene. Proc. Natl. Acad. Sci. USA 2003, 100, 15259–15264. [Google Scholar] [CrossRef]
- Citovsky, V.; Lee, L.; Vyas, S.; Glick, E.; Chen, M.; Vainstein, A.; Gafni, Y.; Gelvin, S.B.; Tzfira, T. Subcellular Localization of Interacting Proteins by Bimolecular Fluorescence Complementation in Planta. J. Mol. Biol. 2006, 362, 1120–1131. [Google Scholar] [CrossRef] [PubMed]
- Kovtun, Y.; Chiu, W.; Tena, G.; Sheen, J. Functional Analysis of Oxidative Stress-Activated Mitogen-Activated Protein Kinase Cascade in Plants. Proc. Natl. Acad. Sci. USA 2000, 97, 2940–2945. [Google Scholar] [CrossRef] [PubMed]
- Ji, D.; Li, Q.; Guo, Y.; An, W.; Manavski, N.; Meurer, J.; Chi, W. NADP+ Supply Adjusts the Synthesis of Photosystem I in Arabidopsis Chloroplasts. Plant Physiol. 2022, 189, 2128–2143. [Google Scholar] [CrossRef] [PubMed]
- Bai, Z.; Zhang, J.; Ning, X.; Guo, H.; Xu, X.; Huang, X.; Wang, Y.; Hu, Z.; Lu, C.; Zhang, L.; et al. A Kinase–Phosphatase–Transcription Factor Module Regulates Adventitious Root Emergence in Arabidopsis Root–Hypocotyl Junctions. Mol. Plant 2020, 13, 1162–1177. [Google Scholar] [CrossRef]
- Keogh, R.C.; Deverall, B.J.; McLeod, S. Comparison of Histological and Physiological Responses to Phakopsora pachyrhizi in Resistant and Susceptible Soybean. Trans. Br. Mycol. Soc. 1980, 74, 329–333. [Google Scholar] [CrossRef]
- Peng, L.; Ma, J.; Chi, W.; Guo, J.; Zhu, S.; Lu, Q.; Lu, C.; Zhang, L. Low Psii Accumulation1 Is Involved in Efficient Assembly of Photosystem II in Arabidopsis thaliana. Plant Cell 2006, 18, 955–969. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.; Li, Q.; Ji, D.; Tian, L.; Meurer, J.; Chi, W. A Ubiquitin-Based Module Directing Protein–Protein Interactions in Chloroplasts. Int. J. Mol. Sci. 2023, 24, 16673. https://doi.org/10.3390/ijms242316673
Guo Y, Li Q, Ji D, Tian L, Meurer J, Chi W. A Ubiquitin-Based Module Directing Protein–Protein Interactions in Chloroplasts. International Journal of Molecular Sciences. 2023; 24(23):16673. https://doi.org/10.3390/ijms242316673
Chicago/Turabian StyleGuo, Yinjie, Qiuxin Li, Daili Ji, Lijin Tian, Jörg Meurer, and Wei Chi. 2023. "A Ubiquitin-Based Module Directing Protein–Protein Interactions in Chloroplasts" International Journal of Molecular Sciences 24, no. 23: 16673. https://doi.org/10.3390/ijms242316673
APA StyleGuo, Y., Li, Q., Ji, D., Tian, L., Meurer, J., & Chi, W. (2023). A Ubiquitin-Based Module Directing Protein–Protein Interactions in Chloroplasts. International Journal of Molecular Sciences, 24(23), 16673. https://doi.org/10.3390/ijms242316673




