Abstract
Gastrointestinal cancer is a common clinical malignant tumor disease that seriously endangers human health and lacks effective treatment methods. As part of the innate immune defense of many organisms, antimicrobial peptides not only have broad-spectrum antibacterial activity but also can specifically kill tumor cells. The positive charge of antimicrobial peptides under neutral conditions determines their high selectivity to tumor cells. In addition, antimicrobial peptides also have unique anticancer mechanisms, such as inducing apoptosis, autophagy, cell cycle arrest, membrane destruction, and inhibition of metastasis, which highlights the low drug resistance and high specificity of antimicrobial peptides. In this review, we summarize the related studies on antimicrobial peptides in the treatment of digestive tract tumors, mainly oral cancer, esophageal cancer, gastric cancer, liver cancer, pancreatic cancer, and colorectal cancer. This paper describes the therapeutic advantages of antimicrobial peptides due to their unique anticancer mechanisms. The length, net charge, and secondary structure of antimicrobial peptides can be modified by design or modification to further enhance their anticancer effects. In summary, as an emerging cancer treatment drug, antimicrobial peptides need to be further studied to realize their application in gastrointestinal cancer diseases.
1. Introduction
Antimicrobial peptides (AMPs) are short amino acid sequences found in bacteria and mammals, typically containing 12 to 50 L-amino acids, with a net positive charge of +2 to +9 at neutral pH [1,2,3]. According to the biosynthetic pathway, AMPs can be divided into two categories: ribosomal synthesis and non-ribosomal synthesis. Post-translational modifications may occur in many AMPs synthesized by ribosomes, resulting in amino acids with nonprotein structures, such as Nisin [4]. Non-ribosomal synthesis involves non-ribosomal peptide synthetases (NRPSs), which are mainly found in bacteria and fungi, such as bacitracin [5]. AMPs are also released from immune cells and epithelial cells in different human organs. Most AMPs share common characteristics, including hydrophobicity, cationic properties, and amphiphilic structures, which determine their broad-spectrum antimicrobial activities against bacteria, fungi, protozoa, and viruses [6,7,8]. This unique molecular structure and antimicrobial activity make AMPs promising as an alternative to antibiotics and widely studied, as antibiotic resistance is currently a major challenge for the effective treatment of bacterial infections. In addition, AMPs have also shown anticancer activity [9,10]. Many AMPs, known as anticancer peptides (ACPs), can destroy the structure of tumor cells or inhibit the proliferation and metastasis of tumor cells and cause little damage to normal cells [11,12]. Compared with current anticancer strategies, AMPs have a lower likelihood of developing resistance during treatment and produce less harmful effects on normal cells [13,14].
Cancer, a tumor or malignancy, is the leading cause of death, affecting nearly 10 million people, and the second leading cause of death in developing countries [15,16]. According to data from the World Health Organization (WHO), the mortality from gastrointestinal tumors accounted for about 35% of all malignant tumor mortality in 2020, mainly including oral cancer, esophageal cancer, gastric cancer, liver cancer, pancreatic cancer, and colorectal cancer, which seriously endanger human health [17]. Surgical resection, radiotherapy, chemotherapy, immunotherapy, and antibody-based molecules are common cancer treatments [18,19,20,21]. These approaches all face challenges and limitations in the field of gastrointestinal cancer treatment. The cure rate is relatively low, and these treatments can affect solid cells, leading to a series of adverse reactions such as severe nausea and vomiting, alopecia, and cardiac toxicity [22,23,24]. With these concerns in mind, researchers and cancer patients are hoping to reduce the burden of cancer with a more specific treatment that has fewer side effects and a lower rate of cancer recurrence [25,26,27].
In recent years, more and more evidence has shown that the high selectivity and low drug resistance of AMPs can effectively inhibit the metastasis and proliferation of cancer cells, which provides a new strategy for cancer treatment [28,29,30]. According to the current study, the use of AMPs in gastrointestinal tumors is an effective approach for the development of novel anticancer drugs (Figure 1). This article reviews the application of AMPs in oral cancer, esophageal cancer, gastric cancer, liver cancer, pancreatic cancer, and colorectal cancer, and provides a new vision and ideas for the development and clinical application of new drugs for digestive tract tumors.
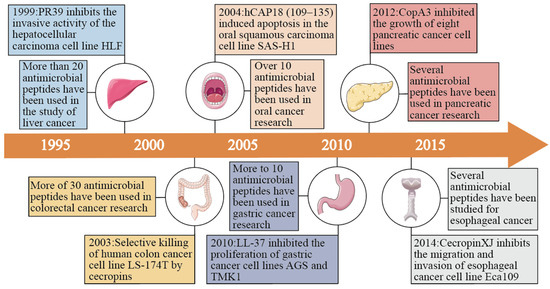
Figure 1.
Early studies of antimicrobial peptides in various gastrointestinal cancers (oral, esophageal, gastric, liver, pancreatic, and colorectal) and the number of antimicrobial peptides studied to date in various gastrointestinal cancers.
2. Antimicrobial Peptides against Gastrointestinal Tumors
Gastrointestinal tumors are tumors that grow on the digestive system, including oral cancer, esophageal cancer, gastric cancer, liver cancer, pancreatic cancer, and colorectal cancer. Recent studies have found that AMPs have anticancer activity against a variety of digestive tract tumor cells. Among them, LL-37, as the only human member of the antimicrobial peptide family, has shown potent anticancer effects, showing antitumor activity against colorectal cancer, gastric cancer, and liver cancer cells [31]. CopA3 has antitumor effects on colorectal cancer, gastric cancer, and pancreatic cancer cells, and cecropin series AMPs showed antitumor effects on gastric cancer, liver cancer, and esophageal cancer cells. In addition, the research progress of various AMPs in gastrointestinal tumors is introduced in the following section.
2.1. Oral Cancer
Oral squamous cell carcinoma (OSCC) is the most common oral cancer, with poor prognosis and high mortality. There are about 300,000 diagnosed cases and 150,000 deaths worldwide every year [32,33,34]. The main treatment for oral squamous cell carcinoma is surgical resection, but the prognosis of survival is not high [35,36,37]. Commonly used first-line chemotherapy drugs include cisplatin, carboplatin, 5-fluorouracil, etc. However, these drugs not only kill cancer cells but also damage normal healthy cells [38,39,40,41]. Considering these factors, it is necessary to find new therapeutic methods for oral squamous cell carcinoma, and some AMPs have emerged as potential drugs for the treatment of oral squamous cell carcinoma (Table 1).

Table 1.
Examples of antimicrobial peptides used in the treatment of oral cancer and their properties.
As early as 2004, it was reported that hCAP18 (109–135), an analog of LL-37, induced apoptosis in SAS-H1 cells through a caspase-independent pathway [46]. KI-21-3, a shortened fragment of LL-37, has obvious oncolytic properties in SCC-4 cells through antiproliferation and caspase-3 apoptotic pathways [45]. Human phthalamide antimicrobial peptide (CAMP) and LL-37 C-terminal deletion mutant (CDEL) were also shown to induce apoptosis of HSC-3 cells through the P53-Bcl-2/BAX signaling pathway [44]. These results all suggest that LL37 and its analogs have varying degrees of influence on the development of oral squamous cell carcinoma and may act as tumor suppressors in oral squamous cells.
Human β-defensin (HBD) is produced by the epithelial cells of many organs. Among the numerous types of HBD, HBD-1, HBD-2, and HBD-3 have been well studied. Among them, HBD-1 could inhibit the proliferation of oral squamous cell carcinoma BHY cells, but BHY cells increased after the stimulation of HBD-2 and -3 [49]. In a further study, Qi Han et al. found that exogenous expression of HBD-1 inhibited the migration and invasion of oral squamous cell carcinoma lines; however, the specific mechanism remains unclear [50]. For HBD-2, Yoshitaka Kamino et al. found that increased HBD-2 expression inhibited SAS cell proliferation and invasion [52].
2.2. Esophageal Cancer
Esophageal cancer is the sixth most common cancer worldwide, with poor prognosis and a low overall survival rate [53]. At present, there are few studies on AMPs in esophageal cancer, mainly several types of cecropin, such as cecropin A, cecropin B, cecropin D, and cecropinXJ (Table 2). Cecropin A, cecropin B, and cecropin D can exert anti-esophageal cancer activities through the mitochondrial apoptosis pathway [54,55,56]. CecropinXJ was found to induce cytoskeletal disruption such as microtubule depolymerization and actin polymerization, as well as to regulate the expression of cytoskeletal protein genes, resulting in cytotoxicity against esophageal cancer Eca109 cells [57]. In addition, Shangjie Liu et al. found that LvHemB1 could also be selectively toxic to esophageal cancer through the mitochondrial apoptosis pathway, and EC190 cell viability decreased by 49.1% after treatment with 50 µg/mL for 24 h, with no significant effect on the proliferation of noncancer cell lines [58]. Compared with cecropin, LvHemB1 has a higher toxicity to EC190 cells.

Table 2.
Examples of antimicrobial peptides used in the treatment of esophageal cancer and their properties.
2.3. Gastric Cancer
Gastric cancer is the fifth most common cancer in the world, accounting for 7.7% of all cancer deaths [59]. Because of the few symptoms caused by the early stage, gastric cancer is usually not diagnosed in time, and metastasis occurs in 80% to 90% of patients with gastric cancer [60,61]. Despite improvements in diagnosis and treatment, the overall survival of patients with gastric cancer is <40% [62]. At present, more than ten AMPs have been studied for the treatment of gastric cancer (Table 3).

Table 3.
Examples of antimicrobial peptides used in the treatment of gastric cancer and their properties.
Melittin, an antimicrobial peptide derived from bee venom, has been extensively studied on a variety of cancer cells. Amir Mahmoodzadeh et al. first reported the toxicity of melittin isolated from Iranian bee venom to gastric cancer AGS cells. Even at very low concentrations (0.5 mg/mL) of melittin treatment (6–24 h), melittin inhibited the proliferation of AGS cells. At the concentration of 1 mg/mL, loose integrity of the cell membrane was observed, which was a marker of cell necrosis and death [74]. Caroline Soliman et al. also studied the transient effect of melittin on gastric cancer AGS cells (within 15 min). They found that swelling, membrane blebbing, and rupture of cells occurred within a few seconds after high-dose melittin treatment and complete cell death occurred within 15 min [70]. The cause of death in most cancer patients is directly related to recurrence and cancer cell metastasis. In the study by Jye-Yu Huang et al., it was shown that melittin can reduce the expression of related proteins and inhibit AGS cell migration and invasion through multiple pathways (Table 3), which indicates that melittin has the potential to treat metastatic gastric cancer [65]. In addition, melittin has been found to induce apoptosis in human gastric cancer cells SGC-7901 by activating the mitochondrial pathway, which is a further understanding of the anticancer mechanism of melittin [61]. However, in the above studies, melittin showed not only anticancer activity but also strong hemolytic activity, which is the biggest challenge in developing melittin as a therapeutic agent for gastric cancer.
2.4. Liver Cancer
Liver cancer is the fourth most common cause of cancer-related death worldwide, and the most common form is hepatocellular carcinoma, with a 5-year survival rate of approximately 18% [76,77]. Sorafenib has been used as a new targeted drug for the treatment of liver cancer since 2007. It is the only systemic treatment drug approved by the FDA for the treatment of advanced unresectable hepatocellular carcinoma [78]. However, it has high toxicity and drug resistance, which may affect the function of normal cells and cause some adverse reactions common to antiangiogenic drugs [79]. Due to the high degree of selective toxicity of AMPs, more than 20 kinds of AMPs derived from humans, insects, animals, and plants and via artificial synthesis have been widely used in the study of liver cancer treatment (Table 4).

Table 4.
Examples of antimicrobial peptides used in the treatment of liver cancer and their properties.
Cecropin is an antimicrobial peptide from Musca domestica. Xiaobao Jin et al. found that cecropin could inhibit the proliferation of human liver cancer BEL-7402 cells in a dose- and time-dependent manner through the extrinsic apoptotic pathway [83]. Further studies showed that cecropin also showed inhibitory potential for liver cancer cell metastasis, and inhibited the adhesion and migration of human liver cancer BEL-7402 cells [82]. Purified cecropin-B also had anti-HCC activity with a semi-inhibitory concentration of 25 µg/mL on HepG2 cells, which was safe for human normal lung WI-38 cells with a cytotoxicity of 0.92% [86]. Cecropin XJ shares 98% of its identity with cecropin B [106]. Lijie Xia et al. found that cecropin XJ could inhibit the proliferation and induce apoptosis of Huh7 cells in vitro through the mitochondrial apoptosis pathway [85]. A cecropin B analogue, cecropin-p17, was also found to exert anti-HCC activity both in vitro and in vivo, which may be related to cell apoptosis [99]. It has been shown that the bacteriocin Nisin can also play a role in HepG2 cell apoptosis through the mitochondrial pathway, and more importantly, Nisin treatment can lead to a reduction in the expression of the EMT transcription factor TWIST1, which can misregulate the sensitivity to drug treatment [96,103].
2.5. Pancreatic Cancer
Pancreatic cancer is a common cause of cancer death worldwide, with a mortality rate of about 4.5% in both men and women [17]. The antimicrobial peptide CopA3, derived from dung beetle defensins, was found to dose-dependently inhibit the growth of human pancreatic cancer MIA-PaCa2 with an IC50 of 61.7 µM [105]. This is the first study on the application of AMPs in pancreatic cancer. CopA3 has potential application in the treatment of pancreatic cancer, but its antitumor mechanism still needs to be further elucidated.
2.6. Colorectal Cancer
Colorectal cancer (CRC) is the third most common type of cancer worldwide, with high morbidity and mortality [107]. Oxaliplatin and fluorouracil are common chemotherapeutic drugs, but oxaliplatin can cause severe peripheral nerve injury, and fluorouracil can also cause adverse gastrointestinal reactions and liver injury [108]. In recent years, dozens of AMPs have been widely used in the treatment of colorectal cancer because of their high specificity and low occurrence of side effects. They have different degrees of cancer-killing effects in various colon cancer cell lines (Table 5).

Table 5.
Examples of antimicrobial peptides used in the treatment of colorectal cancer and their properties.
The antimicrobial peptide LL37 and its residues and analogs present in the human body have been widely studied in colon cancer. Shun X. Ren et al. reported that antimicrobial peptide LL37, which exists in the human body, induces caspase-independent apoptosis by upregulating Bax and Bak and downregulating Bcl-2, leading to nuclear translocation of AIF and EndoG to induce caspase-independent apoptosis and thus inhibiting the occurrence of colon cancer [123]. FK-16, as a 17–32 residue of LL37, also induced AIF-dependent/EndoG-dependent apoptosis and autophagic cell death through the p53-Bax/Bcl-2 cascade commonly observed in colon cancer cells [113,123]. Compared with LL37, FK-16 has a more significant effect on the activity of colon cancer cells, showing better anticancer activity, and the shortened length of FK-16 can also reduce the production cost associated with peptide synthesis. FF/CAP18 is an analog of LL-37; Kengo Kuroda et al. have found that 10 μg/mL FF/CAP18 can induce partial mitochondrial membrane depolarization at the early stage of apoptosis, and high-dose treatment (40 μg/mL) can lead to late apoptosis. Glycolysis and the tricarboxylic acid cycle are inhibited to reduce ATP production, resulting in the absence of most metabolites [140]. As a mimetic of LL-37, CSA-13 could induce cell cycle arrest and inhibit the proliferation of HCT116 cells [130].
In addition, Br-J-I, a halogen-derived antimicrobial peptide isolated from royal honeybee jelly, did not induce cell death by apoptosis or membrane destruction. It had little cytotoxicity against colon cancer cells but showed antibacterial activity against Fusobacterium nucleorum (Fn) [127]. Some studies have reported that Fn is closely related to the occurrence and development of CRC [141,142,143,144]. Therefore, Br-J-I can directly kill Fn to indirectly inhibit colorectal cancer growth [127].
3. Anticancer Mechanisms of Antimicrobial Peptides against Gastrointestinal Tumors
In recent years, AMPs have become a research hotspot for antitumor drugs, and their antitumor mechanisms have been reported in experimental studies of digestive tract tumors. Most AMPs interact with membranes and form special pore channels, possibly through barrel stave-, carpet-, and detergent-“like” mechanisms, that allow AMPs, ions, or other substances to reach intracellular targets, thereby triggering a variety of antitumor mechanisms [145,146], including induction of apoptosis, autophagy, disruption of the cell membrane, arrest of the cell cycle, inhibition of metastasis, and disruption of the cytoskeleton. Among them, the mechanism of cytoskeleton disruption has not been extensively studied. This article explains the following five important anticancer mechanisms of AMPs (Figure 2).
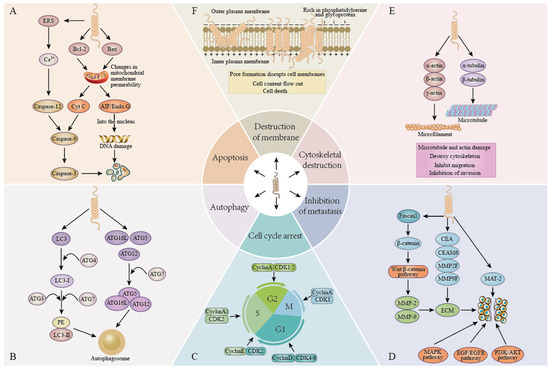
Figure 2.
Anticancer mechanisms of antimicrobial peptides. (A). Apoptosis: the induction of apoptosis in cancer cells by either caspase-dependent or caspase-independent pathways. (B). Autophagy: inducing autophagy in cancer cells by regulating the expression of autophagy markers and autophagy-related proteins. (C). Cell cycle arrest: results in cell cycle arrest by regulating the expression of cyclin and cyclin-dependent kinase (CDK). (D). Inhibition of metastasis: inhibition of cancer cell metastasis by regulating signaling pathways and inhibiting the expression of matrix metalloproteinases. (E). Destruction of the cytoskeleton: by regulating the expression of actin and tubulin, microtubules and microfilaments are damaged and the cytoskeleton is destroyed. (F). Membrane destruction: the pore formation mechanism destroys the cell membrane and causes the cell content to flow out, leading to cell death.
3.1. Cell Apoptosis
Apoptosis is a type Ⅰ programmed cell death process. Two major apoptotic pathways exist, the first being the extrinsic pathway (death receptor pathway), triggered by the CD95 (Fas) death receptor and some members of the tumor necrosis factor-α (TNF-α) receptor superfamily [128]. The second is the endogenous pathway (mitochondrial-mediated pathway), which is further divided into caspase-dependent and caspase-independent pathways. Some studies have found that AMPs can cause mitochondrial dysfunction to exert anticancer activity. Due to the negative charge of mitochondria, AMPs may target mitochondria, destroy the integrity of mitochondrial membranes, or control the permeability of mitochondrial membranes by regulating the ratio of Bax/Bcl-2 and the release of ROS [147,148,149]. This eventually leads to the release of cytochrome C into the cytoplasm, which binds to APAF-1 and caspase-9 to form apoptotic bodies, activates downstream caspase-3, and eventually leads to caspase-dependent apoptosis [147,150]. In addition, when cells are stimulated by internal apoptotic factors, the proapoptotic protein AIF present in the mitochondria is transferred to the nucleus, leading to DNA damage and causing caspase-independent apoptosis. Among them, LL37 and FK-16 induced caspase-independent apoptosis of colon cancer cells by inducing the nuclear translocation of AIF and EndoG through the upregulation of Bax and Bak and the downregulation of Bcl-2 [113,123].
3.2. Autophagy
In the study of AMPs in the treatment of tumors, it is found that there may be an interaction between autophagy and apoptosis. Autophagy is a type Ⅱ programmed death process, which includes four key steps: initiation, nucleation, maturation, and degradation [151]. The formation of autophagy leads to the enzymatic conversion of LC3-Ⅰ to membrane type LC3 (LC3-Ⅱ). As an autophagy marker, LC3-II participates in the formation of autophagosome membranes [152,153]. Beclin 1 plays an important role in autophagy and tumorigenesis. It can mediate the localization of autophagy proteins to phagosomes and induce the formation and maturation of autophagosomes [154,155]. Normally, the expression of beclin 1 is increased during autophagy. LFcinB 25, GW-H1, and bovine lactoferricin B increased LC3-II and beclin-1 at the same time in the early stage of treatment, LC3-II began to decrease in the later stage, beclin-1 increased continuously, and autophagy was inhibited [64,67]. Shun X. Ren et al. found that elimination of autophagy can make FK-16 promote apoptosis; FK-16 activates p53 to upregulate Bax and downregulate Bcl-2 to induce apoptosis; Bcl-2 and Bcl-xL seem to be important factors in autophagy and inhibit autophagy by binding to beclin-1 [113]. KT2 inhibited autophagy by reducing the expression of LC3-I, Atg5, Atg7, Atg16L1, and beclin-1 in cells, but the effect between autophagy and apoptosis was not further studied [120]. The lysosome is an important regulator of autophagy. Jiali Zeng et al. found that M1-8 can colocalize with lysosomes, leading to lysosomal rupture, release cathepsin D (m-CTSD) into the cytoplasm, activate caspases and change mitochondrial membrane potential, and finally induce cell apoptosis [95]. Smp43 and Smp24, two AMPs derived from scorpion venom, increased the expression of autophagosome formation marker LC3A/B-Ⅱ in a dose-dependent manner, and increased autophagy by regulating the PI3K/Akt/mTOR signaling pathway [88,97].
3.3. Cell Cycle Arrest
Some of the currently reported AMPs can inhibit cancer cell proliferation through cell cycle arrest, which includes interphase G1, S, G2, and mitotic M phases. This process is tightly regulated by cyclin and cyclin-dependent kinases (CDKS). When cells in the G0 phase are stimulated, they express cyclin C, cyclin D, and cyclin E. Cyclin D binds to a variety of kinases, mainly CDK4, and cyclin E binds to a variety of kinases, mainly CDK2, and cells enter the S phase and begin DNA synthesis [156,157]. The S and M phases are mainly regulated by cyclin A and cyclin B. Debasish Kumar Dey et al. found that CopA3 treatment inhibited the expression of cyclin and CDK and arrested the G1 phase of the cell cycle [126]. Cyclin E1 is involved in the regulation of G1/S transition, and when its expression is inhibited, it can effectively promote S phase progression. C. Freiburghaus et al. found that lactoferrin inhibited cyclin E1 expression and prolonged the S phase of cancer cells [134]. Cell cycle arrest prevents cells from undergoing mitosis, leading to accumulation of DNA, and DNA content reflects cell cycle arrest. As measured using cell cycle DNA content, both CSA-13 and the bacteriocin enterocin-A arrested cancer cells in the G1 phase, and overexpression of DEFA5 inhibited the G1/S phase of the cell cycle [116,130]. P53 regulates the expression of a series of genes involved in the G1/S and G2/M transitions [158]. Pardaxin may lead to G2/M phase-induced cell arrest through the regulation of p53 and cyclin B1 [42].
3.4. Membrane Destruction
Many AMPs are cationic amphiphilic peptides that can bind to negatively charged cell membranes through electrostatic interactions, leading to membrane disruption. The surface of cancer cells is negatively charged because the outer membrane of cancer cells expresses anionic components such as glycoproteins and phosphatidylserine (PS) [159]. PS is a component present in the inner lobe of the plasma membrane of normal mammalian cells. The expression of PS in cancer cells is transferred to the outer lobe of the plasma membrane, resulting in a negative charge on the surface of cancer cells. Therefore, this chemical difference contributes to the electrostatic interaction between the AMPs and cancer cells, rapidly disrupting the cell membrane and causing the flow of cell contents to induce cell death. In addition, the increase in membrane surface area caused by microvilli on the membrane surface of cancer cells and the increase in membrane fluidity caused by reducing the level of lipoprotein in the membrane are more conducive to the binding of AMPs to cancer cells [160,161]. Of course, AMPs also disrupt membrane structure in a dose-dependent manner, with LfcinB (20–25)4, G3, melittin, HDH-LGBP-A1, and HDH-LGBP-A2 disrupting the cell membrane at high concentration levels [43,70,112,121].
3.5. Inhibition of Metastasis
Metastasis is the process by which cancer cells spread from the original tumor cells to nearby tissues or organs. The extracellular matrix (ECM) is an obstacle to tumor invasion and metastasis. In the process of cancer metastasis, a variety of proteases (matrix metalloproteinases (MMPs)) can degrade the ECM, which is conducive to the invasion and metastasis of cancer cells [162,163]. MMP downregulation has been reported to inhibit cancer metastasis [164,165]. At present, the research on inhibiting metastasis mainly focuses on the factors or pathways related to MMPs, among which MMP-2 and MMP-9 play a key role in tumor progression. Lactacin, melittin, cecropin, and rpNK-lysin can all inhibit cancer cell metastasis by downregulating key members of the MMP family [65,82,103,118]. Metastasis-associated protein 2 (MTA 2) is closely related to the progression of various cancers such as liver cancer, gastric cancer, and colorectal cancer [166,167]. Human β-defensin-3 (HBD3) inhibits cancer cell metastasis by downregulating MTA2 [168]. In addition, anti-metastasis mechanisms in cancer are closely related to cell signaling pathways. Human α-defensin 5 (DEFA5) attenuates the downstream signal transduction of the PI3K-AKT pathway by binding to subunits of the PI3K complex, resulting in delayed cell metastasis [166]. Human α-defensin 6 (HD6) inhibits colorectal cancer metastasis by regulating the EGF/EGFR signaling pathway [129]. MMP-2 and MMP-9 are the downstream target genes of the Wnt/β-catenin signaling pathway [169]. rpNK-lysin regulates the Wnt/β-catenin signaling pathway by downregulating Fascin1 and inducing β-catenin degradation and inhibits the expression of downstream target genes MMP-2 and MMP-9 [103].
4. The Effect of Modification and Optimization of Antimicrobial Peptides on Gastrointestinal Tumors
4.1. Peptide Length
Most natural AMPs have relatively long primary sequences, but it is usually the core amino acid fragments in AMPs that have biological activity. Long sequences are usually limited by high production costs and instability of enzymatic degradation. Cecropin B, an antimicrobial peptide composed of 35 amino acids, has shown a wide range of antitumor activities in previous studies, including inhibition of the proliferation of liver cancer cells, gastric cancer cells, and bladder cancer cells [170,171,172]. Chunli Wu et al. synthesized cecropin-p17, an analogue with the same net charge as cecropin B, based on an amphiphilic structural design, which consists of only 17 amino acids. Cecropin-P17 inhibited HepG-2 cells in a time- and dose-dependent manner, and showed low cytotoxicity on human normal liver L02 cells [99]. Elaheh Jamasbi et al. designed a branched chain dimer form of melittin and found that a short sequence melittin monomer was more toxic to gastric cancer cells than a long sequence dimer at low concentrations (1–5 μM) [69]. Musca Domestica Cecropin (MDC) is a linear molecule of 40 amino acids, with M1-8 derived from the N-terminal 1–8 amino acids of MDC. Previously, MDC has been shown to inhibit the growth of hepatocellular carcinoma cells. Jiali Zeng et al. found that M1-8 also showed excellent antiproliferation ability for hepatocellular carcinoma HepG2 cells and significantly inhibited the growth of tumors, indicating that short sequence peptide M1-8 did not seem to have any effect on hepatocellular carcinoma [82,95]. LL37 has an amphiphilic long helical structure spanning 2–31 residues, and FK-16 corresponding to 17–32 residues retains antibacterial and antitumor effects. Shun X. Ren et al. found that the cytotoxicity of short sequence FK16 on colon cancer cells was stronger than that of LL37, which showed better anticancer effects than the full-length peptide [113]. Notably, the effect of FK16 made cancer cells more susceptible to membrane disruption, suggesting that the FK16 fragment is a core functional region of LL37. Yahya Acil et al. found that KI-21-3, a shortened fragment of LL-37, exhibited the same anti-oral cancer mechanism as LL37, and the oncological effect of KI-21-3 was verified in vivo, indicating that the shortening of the peptide length did not affect the effect of KI-21-3, but could effectively solve the problem of high production costs faced with long sequences [45].
4.2. Peptide Charge
The cancer cell membrane is rich in glycoproteins and PS anions, so AMPs with more positive charges may act more effectively on cancer cell membranes. Bo-Hye Nam et al. found that HDH-LGBP-A2, which has one more net positive charge than HDH-LGBP-A1, increased the cytotoxicity of HeLa, A549, and HCT 116 cancer cells by 183.3%, 75%, and 45.5%, respectively, at low concentrations [121]. Several derived peptides of LfcinB have charges above +3. Víctor A. Solarte et al. found that LfcinB (20–25)4, a derived peptide with a net positive charge of +16, exhibited higher cytotoxicity in CAL27 and SCC15 cells, with inhibition rates of 93% and 96%, respectively [43]. Mengyun Ke et al. designed and synthesized a novel peptide Mel-PEP by replacing the valine at the eighth position and the proline at the fourteenth position of MEL with lysine; this modification increased the charge and helicity of the peptide. They found that Mel-PEP exhibited a stronger antiproliferation ability than MEL against liver cancer BEL-7402/5-FU cells [100]. In addition, it has been reported that highly charged amphiphilic peptides do not exhibit significant cytotoxicity [101]. Therefore, the charge does not positively correlate with the anticancer activity of the peptide, and there seems to be a threshold.
4.3. Peptide Secondary Structure
Secondary structure is an important determinant of protein function and activity. Most natural or synthetic AMPs have a certain secondary structure, such as α-helix, which may promote the formation of holes in the membrane of cancer cells, leading to the leakage of cell contents. It may also interfere with phospholipid fluidity and form transient pores in the membrane of cancer cells, prompting AMPs to enter the cell to play a role. The MDC mentioned in the peptide length showed three α-helical structures at residues 1–6, 9–21, and 27–39. M1-8 derived from the N-terminal 1-8 amino acids containing a helix structure in MDC appeared to have no effect on hepatocellular carcinoma HepG2 cells [95]. Therefore, it is speculated that the α-helix structure may be closely related to the anticancer effect. The RWL sequence is the C-terminal trimer of the chicken β-defensin AvBD-4, and H stands for the amino acid sequence GLRPKYS. N. Dong et al. designed H-(RWL) n (n = 1, 2, 3, 4, 5) peptides GL10, GL13, GL16, GL19, and GL22, among which GW10 and GW13 showed a disordered conformation and GW16, GW19, and GW22 showed a secondary structure with an α-helical conformation. Peptides with higher RWL content were richer in their α-helical structure. Compared with peptides GW10 and GW13, peptides with an α-helical conformation (GW16, GW19, and GW22) showed higher cytotoxicity on human hepatocellular carcinoma HepG2 cells [91]. This finding suggests that the abundant α-helical structure may be responsible for the gradual increase in cytotoxicity.
4.4. Combined and Coupled Peptides
At present, there are still challenges in the clinical use of AMPs. Coupling with polymers, coupling with small molecule peptides, or combination with anticancer drugs may overcome the shortcomings of AMPs and improve the therapeutic potential of AMPs. It has been reported that bacteriocin (enterocin-A +enterocin-B), 10 μg/mL HNP 1 and 50 μg/mL lactoferrin, colicin A and colicin E1, TMTP1 and DKK fusion peptide, MELITININ, and BMAP27 coupling peptide all showed significant anticancer activity against gastrointestinal tumors [48,63,66,75,125]. In addition, the combination of gramicidin A (GA) and the anticancer drug doxorubicin (Doxo) also significantly reduced cancer cell viability [139]. This class of combinatorial coupled peptides showed certain synergistic effects, and there may be a subtle relationship between them to inhibit the growth of cancer cells.
5. Advantages of Antimicrobial Peptides in Anti-Gastrointestinal Tumor Treatments
5.1. High Selectivity
AMPs, as short sequence peptides containing amino acids, are a better choice as tumor therapeutic agents compared with antibodies and small molecules because of their high selectivity. There are significant differences in cell membrane composition between healthy cells and cancer cells. Eukaryotic membranes contain large amounts of amphotericin phosphatidylcholine and cholesterol, while cancer cells contain higher amounts of anions such as O-glycosylation mucin, phosphatidylserine, and heparan sulfate [173,174,175]. Cancer cells have a high transmembrane potential compared with normal eukaryotic cells. Therefore, cationic AMPs mainly interact with normal eukaryotic cells via hydrophobic interaction but with cancer cell membranes via electrostatic interaction. For example, CopA3 mediates cell necrosis through specific interactions with cancer cell membrane phosphatidylserine and phosphatidylcholine [176]. Since the eukaryotic cell membrane is rich in cholesterol, this property can increase the cohesion of the lipid bilayer to prevent membrane disruption and can also change membrane fluidity to prevent membrane dissolution [177]. In addition, cancer cells also contain more abundant microvilli compared with healthy cells, which increases the membrane surface area and is more conducive to the interaction of AMPs with cancer cells [171,178].
5.2. Drug Resistance
Currently, conventional chemotherapy remains the preferred treatment for cancer, but its effectiveness often prevents intrinsic or acquired resistance. The mechanism of action of AMPs is less likely to lead to resistance than conventional chemotherapy [179,180,181]. Conventional chemotherapeutic drugs must enter the cell to work effectively, while AMPs can selectively attach to the membrane of cancer cells, thereby destroying the cell membrane and causing the cell content to flow out. Some AMPs exert their effect before entering the cell, and this unique mechanism is the possible reason for reduced resistance [9,182]. In addition, epithelial-to-mesenchymal transition (EMT) was detected to be closely related to drug resistance in hepatocellular carcinoma (HCC) [183]. This process is regulated by the transcription factors ZEB1, TWIST1, and SNAI1. Among them, the downregulation of TWIST1 leads to rapid cell death and increased sensitivity to drug treatment [184,185]. Pelin Balcik-Ercin et al. found that treatment of the hepatocarcinoma cell line HuH-7 with the bacteriocin lactacin resulted in significant inhibition of SNAI1 and TWIST1 expression, which are critical for drug resistance [104].
P-glycoprotein (P-gp) is one of the widely studied MDR proteins, also known as multidrug resistance protein 1 (MDR1), whose main function is to expel chemotherapy drugs from cancer cells [186,187]. Mengyun Ke et al. found that when the novel antimicrobial peptide MEL-pep was used in human 5-FU-resistant HCC cells (BEL-7402/5-FU), it could inhibit the expression of P-gp by inhibiting the PI3K/Akt pathway to improve the sensitivity to 5-FU, which has great potential in the treatment of drug-resistant cancer [100].
6. Conclusions and Prospects
Cancer is the main cause of death in the world population. The burden of cancer is increasing, and the prevention and treatment of cancer are facing a serious challenge. Traditional cancer therapies have certain drawbacks, such as drug side effects, low specificity, and drug resistance of cancer cells. Therefore, the development and use of AMPs have become a new means for the treatment of cancer. In this review, we discuss the anti-gastrointestinal tumor mechanisms of AMPs, their limitations as anticancer drugs, their specificity to tumor cells, and their sensitivity. Due to their specificity and sensitivity to tumor cells, some AMPs have been shown to have potential therapeutic effects in different types of gastrointestinal tumors.
Although the anticancer potential of many AMPs has been proved, few AMPs have been used in clinical treatment, and the clinical application and development of AMPs still face great challenges. First, the yield of natural AMPs is low and the extraction procedure is complex, while the high price of synthetic AMPs is not suitable for their commercial development. AMP can be abundantly expressed in heterologous expression systems of microbial cells, and heterologous expression technology holds promise for improving AMP production. Second, AMP is exceptionally sensitive to degradation by proteases and is easily cleaved by proteases in vivo and rapidly excreted from the kidney, resulting in its short half-life. Chemical modification methods such as sequence manipulation, net charge, and secondary structure are beneficial for solving the limitations of AMPs, such as poor stability, low bioavailability, and proteolytic enzyme degradation. The combination of AMPs and AMPs, AMPs and currently used chemotherapy drugs, and AMPs and nanocarriers can also help to improve the pharmacokinetics, half-life, bioavailability, and targeting specificity of AMPs, and reduce the side effects in patients.
In conclusion, AMPs are potential drugs for cancer treatment, but more comprehensive and in-depth research is needed to make them better and more efficient for clinical application.
Author Contributions
Investigation, Q.L.; writing—original draft, Q.L.; formal analysis, Q.L., L.W., D.H., Y.W., X.L., Y.Y., Z.C., Z.D. and Y.L.; writing—review and editing, Y.S. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the National Natural Science Foundation of China (grant NO. 31860607).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article. The data presented in this study are available in insert article. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Raheem, N.; Straus, S.K. Mechanisms of Action for Antimicrobial Peptides With Antibacterial and Antibiofilm Functions. Front. Microbiol. 2019, 10, 2866. [Google Scholar] [CrossRef]
- Piotrowska, U.; Sobczak, M.; Oledzka, E. Current state of a dual behaviour of antimicrobial peptides-Therapeutic agents and promising delivery vectors. Chem. Biol. Drug Des. 2017, 90, 1079–1093. [Google Scholar] [CrossRef] [PubMed]
- Hancock, R.E.; Falla, T.; Brown, M. Cationic bactericidal peptides. Adv. Microb. Physiol. 1995, 37, 135–175. [Google Scholar] [CrossRef] [PubMed]
- Entian, K.D.; de Vos, W.M. Genetics of subtilin and nisin biosyntheses: Biosynthesis of lantibiotics. Antonie Van Leeuwenhoek 1996, 69, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Nakano, M.M.; Zuber, P. Molecular biology of antibiotic production in Bacillus. Crit. Rev. Biotechnol. 1990, 10, 223–240. [Google Scholar] [CrossRef] [PubMed]
- Ganz, T. Defensins: Antimicrobial peptides of innate immunity. Nat. Rev. Immunol. 2003, 3, 710–720. [Google Scholar] [CrossRef] [PubMed]
- Brogden, K.A. Antimicrobial peptides: Pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 2005, 3, 238–250. [Google Scholar] [CrossRef]
- Zhang, G.; Sunkara, L.T. Avian antimicrobial host defense peptides: From biology to therapeutic applications. Pharmaceuticals 2014, 7, 220–247. [Google Scholar] [CrossRef]
- Hoskin, D.W.; Ramamoorthy, A. Studies on anticancer activities of antimicrobial peptides. Biochim. Biophys. Acta 2008, 1778, 357–375. [Google Scholar] [CrossRef]
- Baindara, P.; Korpole, S.; Grover, V. Bacteriocins: Perspective for the development of novel anticancer drugs. Appl. Microbiol. Biotechnol. 2018, 102, 10393–10408. [Google Scholar] [CrossRef]
- Tornesello, A.L.; Borrelli, A.; Buonaguro, L.; Buonaguro, F.M.; Tornesello, M.L. Antimicrobial Peptides as Anticancer Agents: Functional Properties and Biological Activities. Molecules 2020, 25, 2850. [Google Scholar] [CrossRef]
- Bakare, O.O.; Gokul, A.; Wu, R.; Niekerk, L.A.; Klein, A.; Keyster, M. Biomedical Relevance of Novel Anticancer Peptides in the Sensitive Treatment of Cancer. Biomolecules 2021, 11, 1120. [Google Scholar] [CrossRef] [PubMed]
- Sledge, G.W.; Mamounas, E.P.; Hortobagyi, G.N.; Burstein, H.J.; Goodwin, P.J.; Wolff, A.C. Past, present, and future challenges in breast cancer treatment. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2014, 32, 1979–1986. [Google Scholar] [CrossRef] [PubMed]
- Saido-Sakanaka, H.; Ishibashi, J.; Momotani, E.; Amano, F.; Yamakawa, M. In vitro and in vivo activity of antimicrobial peptides synthesized based on the insect defensin. Peptides 2004, 25, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Saxena, M.; van der Burg, S.H.; Melief, C.J.M.; Bhardwaj, N. Therapeutic cancer vaccines. Nat. Rev. Cancer 2021, 21, 360–378. [Google Scholar] [CrossRef] [PubMed]
- Yavari, B.; Mahjub, R.; Saidijam, M.; Raigani, M.; Soleimani, M. The Potential Use of Peptides in Cancer Treatment. Curr. Protein Pept. Sci. 2018, 19, 759–770. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef] [PubMed]
- Karunakaran, M.; Barreto, S.G. Surgery for pancreatic cancer: Current controversies and challenges. Future Oncol. 2021, 17, 5135–5162. [Google Scholar] [CrossRef]
- Baskar, R.; Lee, K.A.; Yeo, R.; Yeoh, K.W. Cancer and radiation therapy: Current advances and future directions. Int. J. Med. Sci. 2012, 9, 193–199. [Google Scholar] [CrossRef]
- Furue, H. Chemotherapy cancer treatment during the past sixty years. Gan Kagaku Ryoho Cancer Chemother. 2003, 30, 1404–1411. [Google Scholar]
- Wu, Y.; Yang, Z.; Cheng, K.; Bi, H.; Chen, J. Small molecule-based immunomodulators for cancer therapy. Acta Pharm. Sin. B 2022, 12, 4287–4308. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.; Walton, R.; Kataria, S.P. Chemotherapy-Induced Nausea and Vomiting: Pathogenesis, Recommendations, and New Trends. Cancer Treat. Res. Commun. 2021, 26, 100278. [Google Scholar] [CrossRef] [PubMed]
- Freites-Martinez, A.; Shapiro, J.; Goldfarb, S.; Nangia, J.; Jimenez, J.J.; Paus, R.; Lacouture, M.E. Hair disorders in patients with cancer. J. Am. Acad. Dermatol. 2019, 80, 1179–1196. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, J. Adverse cardiac effects of cancer therapies: Cardiotoxicity and arrhythmia. Nat. Rev. Cardiol. 2020, 17, 474–502. [Google Scholar] [CrossRef] [PubMed]
- Helmy, K.Y.; Patel, S.A.; Nahas, G.R.; Rameshwar, P. Cancer immunotherapy: Accomplishments to date and future promise. Ther. Deliv. 2013, 4, 1307–1320. [Google Scholar] [CrossRef] [PubMed]
- Saghatchian, M.; Lesur, A. Management of side effects related to adjuvant hormone therapy in young women with breast cancer. Bull. Du Cancer 2019, 106, S37–S42. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, J.; Ma, X.; Tan, L.; Yan, Y.; Xue, C.; Hui, B.; Liu, R.; Ma, H.; Ren, J. A Review of Neoadjuvant Chemoradiotherapy for Locally Advanced Rectal Cancer. Int. J. Biol. Sci. 2016, 12, 1022–1031. [Google Scholar] [CrossRef]
- Papo, N.; Seger, D.; Makovitzki, A.; Kalchenko, V.; Eshhar, Z.; Degani, H.; Shai, Y. Inhibition of tumor growth and elimination of multiple metastases in human prostate and breast xenografts by systemic inoculation of a host defense-like lytic peptide. Cancer Res. 2006, 66, 5371–5378. [Google Scholar] [CrossRef]
- Lehmann, J.; Retz, M.; Sidhu, S.S.; Suttmann, H.; Sell, M.; Paulsen, F.; Harder, J.; Unteregger, G.; Stöckle, M. Antitumor activity of the antimicrobial peptide magainin II against bladder cancer cell lines. Eur. Urol. 2006, 50, 141–147. [Google Scholar] [CrossRef]
- Mader, J.S.; Hoskin, D.W. Cationic antimicrobial peptides as novel cytotoxic agents for cancer treatment. Expert Opin. Investig. Drugs 2006, 15, 933–946. [Google Scholar] [CrossRef]
- Dürr, U.H.; Sudheendra, U.S.; Ramamoorthy, A. LL-37, the only human member of the cathelicidin family of antimicrobial peptides. Biochim. Biophys. Acta 2006, 1758, 1408–1425. [Google Scholar] [CrossRef] [PubMed]
- Chandler, K.B.; Alamoud, K.A.; Stahl, V.L.; Nguyen, B.C.; Kartha, V.K.; Bais, M.V.; Nomoto, K.; Owa, T.; Monti, S.; Kukuruzinska, M.A.; et al. β-Catenin/CBP inhibition alters epidermal growth factor receptor fucosylation status in oral squamous cell carcinoma. Mol. Omics 2020, 16, 195–209. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Jiang, S.; Xiao, Y.; He, Y.; Ren, T.; Jiang, L.; Liu, R.; Chen, Q. SOX2-dependent expression of dihydroorotate dehydrogenase regulates oral squamous cell carcinoma cell proliferation. Int. J. Oral Sci. 2021, 13, 3. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.G.; Baek, C.H.; Chaturvedi, P.; Gallagher, R.; Kowalski, L.P.; Leemans, C.R.; Warnakulasuriya, S.; Nguyen, S.A.; Day, T.A. Update on oral and oropharyngeal cancer staging-International perspectives. World J. Otorhinolaryngol. Head Neck Surg. 2020, 6, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Montero, P.H.; Patel, S.G. Cancer of the oral cavity. Surg. Oncol. Clin. N. Am. 2015, 24, 491–508. [Google Scholar] [CrossRef] [PubMed]
- Omura, K. Current status of oral cancer treatment strategies: Surgical treatments for oral squamous cell carcinoma. Int. J. Clin. Oncol. 2014, 19, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Day, T.A.; Davis, B.K.; Gillespie, M.B.; Joe, J.K.; Kibbey, M.; Martin-Harris, B.; Neville, B.; Reed, S.G.; Richardson, M.S.; Rosenzweig, S.; et al. Oral cancer treatment. Curr. Treat. Options Oncol. 2003, 4, 27–41. [Google Scholar] [CrossRef]
- Rivera, C. Essentials of oral cancer. Int. J. Clin. Exp. Pathol. 2015, 8, 11884–11894. [Google Scholar]
- Huang, S.H.; O’Sullivan, B. Oral cancer: Current role of radiotherapy and chemotherapy. Med. Oral Patol. Oral Y Cir. Bucal 2013, 18, e233–e240. [Google Scholar] [CrossRef]
- Donnelly, J.G. Pharmacogenetics in cancer chemotherapy: Balancing toxicity and response. Ther. Drug Monit. 2004, 26, 231–235. [Google Scholar] [CrossRef]
- Hartner, L. Chemotherapy for Oral Cancer. Dent. Clin. N. Am. 2018, 62, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Cui, Z.; Li, Y.H.; Hsu, W.H.; Lee, B.H. In Vitro and in Vivo Anticancer Activity of Pardaxin against Proliferation and Growth of Oral Squamous Cell Carcinoma. Mar. Drugs 2015, 14, 2. [Google Scholar] [CrossRef] [PubMed]
- Solarte, V.A.; Rosas, J.E.; Rivera, Z.J.; Arango-Rodríguez, M.L.; García, J.E.; Vernot, J.P. A Tetrameric Peptide Derived from Bovine Lactoferricin Exhibits Specific Cytotoxic Effects against Oral Squamous-Cell Carcinoma Cell Lines. BioMed Res. Int. 2015, 2015, 630179. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Ji, S.; Si, J.; Zhang, X.; Wang, X.; Guo, Y.; Zou, X. Human cathelicidin antimicrobial peptide suppresses proliferation, migration and invasion of oral carcinoma HSC-3 cells via a novel mechanism involving caspase-3 mediated apoptosis. Mol. Med. Rep. 2020, 22, 5243–5250. [Google Scholar] [CrossRef] [PubMed]
- Açil, Y.; Torz, K.; Gülses, A.; Wieker, H.; Gerle, M.; Purcz, N.; Will, O.M.; Eduard Meyer, J.; Wiltfang, J. An experimental study on antitumoral effects of KI-21-3, a synthetic fragment of antimicrobial peptide LL-37, on oral squamous cell carcinoma. J. Cranio-Maxillofac. Surg. 2018, 46, 1586–1592. [Google Scholar] [CrossRef] [PubMed]
- Okumura, K.; Itoh, A.; Isogai, E.; Hirose, K.; Hosokawa, Y.; Abiko, Y.; Shibata, T.; Hirata, M.; Isogai, H. C-terminal domain of human CAP18 antimicrobial peptide induces apoptosis in oral squamous cell carcinoma SAS-H1 cells. Cancer Lett. 2004, 212, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Musrati, A.A.; Tervahartiala, T.; Gürsoy, M.; Könönen, E.; Fteita, D.; Sorsa, T.; Uitto, V.J.; Gürsoy, U.K. Human neutrophil peptide-1 affects matrix metalloproteinase-2, -8 and -9 secretions of oral squamous cell carcinoma cell lines in vitro. Arch. Oral Biol. 2016, 66, 1–7. [Google Scholar] [CrossRef]
- McKeown, S.T.; Lundy, F.T.; Nelson, J.; Lockhart, D.; Irwin, C.R.; Cowan, C.G.; Marley, J.J. The cytotoxic effects of human neutrophil peptide-1 (HNP1) and lactoferrin on oral squamous cell carcinoma (OSCC) in vitro. Oral Oncol. 2006, 42, 685–690. [Google Scholar] [CrossRef]
- Winter, J.; Pantelis, A.; Reich, R.; Martini, M.; Kraus, D.; Jepsen, S.; Allam, J.P.; Novak, N.; Wenghoefer, M. Human beta-defensin-1, -2, and -3 exhibit opposite effects on oral squamous cell carcinoma cell proliferation. Cancer Investig. 2011, 29, 196–201. [Google Scholar] [CrossRef]
- Han, Q.; Wang, R.; Sun, C.; Jin, X.; Liu, D.; Zhao, X.; Wang, L.; Ji, N.; Li, J.; Zhou, Y.; et al. Human beta-defensin-1 suppresses tumor migration and invasion and is an independent predictor for survival of oral squamous cell carcinoma patients. PLoS ONE 2014, 9, e91867. [Google Scholar] [CrossRef]
- Hou, D.; Hu, F.; Mao, Y.; Yan, L.; Zhang, Y.; Zheng, Z.; Wu, A.; Forouzanfar, T.; Pathak, J.L.; Wu, G. Cationic antimicrobial peptide NRC-03 induces oral squamous cell carcinoma cell apoptosis via CypD-mPTP axis-mediated mitochondrial oxidative stress. Redox Biol. 2022, 54, 102355. [Google Scholar] [CrossRef] [PubMed]
- Kamino, Y.; Kurashige, Y.; Uehara, O.; Sato, J.; Nishimura, M.; Yoshida, K.; Arakawa, T.; Nagayasu, H.; Saitoh, M.; Abiko, Y. HBD-2 is downregulated in oral carcinoma cells by DNA hypermethylation, and increased expression of hBD-2 by DNA demethylation and gene transfection inhibits cell proliferation and invasion. Oncol. Rep. 2014, 32, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Murphy, G.; McCormack, V.; Abedi-Ardekani, B.; Arnold, M.; Camargo, M.C.; Dar, N.A.; Dawsey, S.M.; Etemadi, A.; Fitzgerald, R.C.; Fleischer, D.E.; et al. International cancer seminars: A focus on esophageal squamous cell carcinoma. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2017, 28, 2086–2093. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Lv, D.; Wang, X.; Wang, Y.; Hou, C.; Gao, K.; Guo, X. Inhibitory effects of Bombyx mori antimicrobial peptide cecropins on esophageal cancer cells. Eur. J. Pharmacol. 2020, 887, 173434. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Martín, F.; Herrera-León, C.; D’Amelio, N. Molecular basis of the anticancer, apoptotic and antibacterial activities of Bombyx mori Cecropin A. Arch. Biochem. Biophys. 2022, 715, 109095. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Martín, F.; Herrera-León, C.; D’Amelio, N. Bombyx mori Cecropin D could trigger cancer cell apoptosis by interacting with mitochondrial cardiolipin. Biochim. Biophys. Acta Biomembr. 2022, 1864, 184003. [Google Scholar] [CrossRef]
- Xia, L.; Wu, Y.; Kang, S.; Ma, J.; Yang, J.; Zhang, F. CecropinXJ, a silkworm antimicrobial peptide, induces cytoskeleton disruption in esophageal carcinoma cells. Acta Biochim. Biophys. Sin. 2014, 46, 867–876. [Google Scholar] [CrossRef]
- Liu, S.; Aweya, J.J.; Zheng, L.; Zheng, Z.; Huang, H.; Wang, F.; Yao, D.; Ou, T.; Zhang, Y. LvHemB1, a novel cationic antimicrobial peptide derived from the hemocyanin of Litopenaeus vannamei, induces cancer cell death by targeting mitochondrial voltage-dependent anion channel 1. Cell Biol. Toxicol. 2022, 38, 87–110. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Gao, J.; Zhang, X.; Meng, Q.; Jin, H.; Zhu, Z.; Wang, Z.; Qian, W.; Zhang, L.; Liu, Y.; Min, M.; et al. Linked Color Imaging Can Improve Detection Rate of Early Gastric Cancer in a High-Risk Population: A Multi-Center Randomized Controlled Clinical Trial. Dig. Dis. Sci. 2021, 66, 1212–1219. [Google Scholar] [CrossRef]
- Kong, G.M.; Tao, W.H.; Diao, Y.L.; Fang, P.H.; Wang, J.J.; Bo, P.; Qian, F. Melittin induces human gastric cancer cell apoptosis via activation of mitochondrial pathway. World J. Gastroenterol. 2016, 22, 3186–3195. [Google Scholar] [CrossRef] [PubMed]
- Karimi, P.; Islami, F.; Anandasabapathy, S.; Freedman, N.D.; Kamangar, F. Gastric cancer: Descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol. Biomark. Prev. 2014, 23, 700–713. [Google Scholar] [CrossRef] [PubMed]
- Fathizadeh, H.; Saffari, M.; Esmaeili, D.; Moniri, R.; Mahabadi, J.A. Anticancer Effect of Enterocin A-Colicin E1 Fusion Peptide on the Gastric Cancer Cell. Probiotics Antimicrob. Proteins 2021, 13, 1443–1451. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.R.; Chen, P.W.; Chen, Y.L.; Hsu, H.C.; Lin, C.C.; Chen, W.J. Bovine lactoferricin B induces apoptosis of human gastric cancer cell line AGS by inhibition of autophagy at a late stage. J. Dairy Sci. 2013, 96, 7511–7520. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.Y.; Peng, S.F.; Chueh, F.S.; Chen, P.Y.; Huang, Y.P.; Huang, W.W.; Chung, J.G. Melittin suppresses epithelial-mesenchymal transition and metastasis in human gastric cancer AGS cells via regulating Wnt/BMP associated pathway. Biosci. Biotechnol. Biochem. 2021, 85, 2250–2262. [Google Scholar] [CrossRef] [PubMed]
- Ankaiah, D.; Palanichamy, E.; Antonyraj, C.B.; Ayyanna, R.; Perumal, V.; Ahamed, S.I.B.; Arul, V. Cloning, overexpression, purification of bacteriocin enterocin-B and structural analysis, interaction determination of enterocin-A, B against pathogenic bacteria and human cancer cells. Int. J. Biol. Macromol. 2018, 116, 502–512. [Google Scholar] [CrossRef]
- Pan, W.R.; Chen, Y.L.; Hsu, H.C.; Chen, W.J. Antimicrobial peptide GW-H1-induced apoptosis of human gastric cancer AGS cell line is enhanced by suppression of autophagy. Mol. Cell. Biochem. 2015, 400, 77–86. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, I.W.; Shin, Y.P.; Park, H.J.; Lee, Y.S.; Lee, I.H.; Kim, M.A.; Yun, E.Y.; Nam, S.H.; Ahn, M.Y.; et al. Enantiomeric CopA3 dimer peptide suppresses cell viability and tumor xenograft growth of human gastric cancer cells. Tumor Biol. 2016, 37, 3237–3245. [Google Scholar] [CrossRef]
- Jamasbi, E.; Lucky, S.S.; Li, W.; Hossain, M.A.; Gopalakrishnakone, P.; Separovic, F. Effect of dimerized melittin on gastric cancer cells and antibacterial activity. Amino Acids 2018, 50, 1101–1110. [Google Scholar] [CrossRef]
- Soliman, C.; Eastwood, S.; Truong, V.K.; Ramsland, P.A.; Elbourne, A. The membrane effects of melittin on gastric and colorectal cancer. PLoS ONE 2019, 14, e0224028. [Google Scholar] [CrossRef]
- Wu, Z.; Ding, Z.; Cheng, B.; Cui, Z. The inhibitory effect of human DEFA5 in growth of gastric cancer by targeting BMI1. Cancer Sci. 2021, 112, 1075–1083. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.K.; Sung, J.J.; To, K.F.; Yu, L.; Li, H.T.; Li, Z.J.; Chu, K.M.; Yu, J.; Cho, C.H. The host defense peptide LL-37 activates the tumor-suppressing bone morphogenetic protein signaling via inhibition of proteasome in gastric cancer cells. J. Cell. Physiol. 2010, 223, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.L.; Xia, L.J.; Li, J.Y.; Zhang, F.C. CecropinXJ inhibits the proliferation of human gastric cancer BGC823 cells and induces cell death in vitro and in vivo. Int. J. Oncol. 2015, 46, 2181–2193. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mahmoodzadeh, A.; Zarrinnahad, H.; Bagheri, K.P.; Moradia, A.; Shahbazzadeh, D. First report on the isolation of melittin from Iranian honey bee venom and evaluation of its toxicity on gastric cancer AGS cells. J. Chin. Med. Assoc. JCMA 2015, 78, 574–583. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Xi, L.; Luo, D.; Liu, R.; Li, S.; Liu, Y.; Fan, L.; Ye, S.; Yang, W.; Yang, S.; et al. Anti-tumor effects of the peptide TMTP1-GG-D(KLAKLAK)(2) on highly metastatic cancers. PLoS ONE 2012, 7, e42685. [Google Scholar] [CrossRef] [PubMed]
- McGlynn, K.A.; Petrick, J.L.; El-Serag, H.B. Epidemiology of Hepatocellular Carcinoma. Hepatology 2021, 73 (Suppl. S1), 4–13. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA A Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Vogel, A.; Meyer, T.; Sapisochin, G.; Salem, R.; Saborowski, A. Hepatocellular carcinoma. Lancet 2022, 400, 1345–1362. [Google Scholar] [CrossRef]
- Tang, W.; Chen, Z.; Zhang, W.; Cheng, Y.; Zhang, B.; Wu, F.; Wang, Q.; Wang, S.; Rong, D.; Reiter, F.P.; et al. The mechanisms of sorafenib resistance in hepatocellular carcinoma: Theoretical basis and therapeutic aspects. Signal Transduct. Target. Ther. 2020, 5, 87. [Google Scholar] [CrossRef]
- Li, X.; Song, W.; Zhang, M.; Zhao, P. Human β-defensin 1 Functions as a Tumor Suppressor via ER Stress-triggered JNK pathway in Hepatocellular Carcinoma. J. Balk. Union Oncol. 2021, 26, 1365–1372. [Google Scholar]
- Al Kashgry, N.A.T.; Abulreesh, H.H.; El-Sheikh, I.A.; Almaroai, Y.A.; Salem, R.; Mohamed, I.; Waly, F.R.; Osman, G.; Mohamed, M.S.M. Utilization of a recombinant defensin from Maize (Zea mays L.) as a potential antimicrobial peptide. AMB Express 2020, 10, 208. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.B.; Mei, H.F.; Pu, Q.H.; Shen, J.; Lu, X.M.; Chu, F.J.; Zhu, J.Y. Effects of Musca domestica cecropin on the adhesion and migration of human hepatocellular carcinoma BEL-7402 cells. Biol. Pharm. Bull. 2013, 36, 938–943. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Mei, H.; Li, X.; Ma, Y.; Zeng, A.H.; Wang, Y.; Lu, X.; Chu, F.; Wu, Q.; Zhu, J. Apoptosis-inducing activity of the antimicrobial peptide cecropin of Musca domestica in human hepatocellular carcinoma cell line BEL-7402 and the possible mechanism. Acta Biochim. Biophys. Sin. 2010, 42, 259–265. [Google Scholar] [CrossRef]
- Lu, J.; Chen, Z.W. Isolation, characterization and anti-cancer activity of SK84, a novel glycine-rich antimicrobial peptide from Drosophila virilis. Peptides 2010, 31, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Wu, Y.; Ma, J.I.; Yang, J.; Zhang, F. The antibacterial peptide from Bombyx mori cecropinXJ induced growth arrest and apoptosis in human hepatocellular carcinoma cells. Oncol. Lett. 2016, 12, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Okasha, H.; Nasr, S.M.; Samir, S. Recombinant Expression of Cec-B Peptide in Escherichia coli with a Significant Anticancer Effect on Hepatocellular Carcinoma. Curr. Pharm. Biotechnol. 2021, 22, 1235–1245. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Li, J.H.; Yu, C.Y.; Lin, C.J.; Chiu, P.H.; Chen, P.W.; Lin, C.C.; Chen, W.J. Novel cationic antimicrobial peptide GW-H1 induced caspase-dependent apoptosis of hepatocellular carcinoma cell lines. Peptides 2012, 36, 257–265. [Google Scholar] [CrossRef]
- Chai, J.; Yang, W.; Gao, Y.; Guo, R.; Peng, Q.; Abdel-Rahman, M.A.; Xu, X. Antitumor Effects of Scorpion Peptide Smp43 through Mitochondrial Dysfunction and Membrane Disruption on Hepatocellular Carcinoma. J. Nat. Prod. 2021, 84, 3147–3160. [Google Scholar] [CrossRef]
- Liu, S.; Aweya, J.J.; Zheng, L.; Wang, F.; Zheng, Z.; Zhong, M.; Lun, J.; Zhang, Y. A Litopenaeus vannamei Hemocyanin-Derived Antimicrobial Peptide (Peptide B11) Attenuates Cancer Cells’ Proliferation. Molecules 2018, 23, 3202. [Google Scholar] [CrossRef]
- Hossain, M.A.; Guilhaudis, L.; Sonnevend, A.; Attoub, S.; van Lierop, B.J.; Robinson, A.J.; Wade, J.D.; Conlon, J.M. Synthesis, conformational analysis and biological properties of a dicarba derivative of the antimicrobial peptide, brevinin-1BYa. Eur. Biophys. J. 2011, 40, 555–564. [Google Scholar] [CrossRef]
- Dong, N.; Zhu, X.; Lv, Y.F.; Ma, Q.Q.; Jiang, J.G.; Shan, A.S. Cell specificity and molecular mechanism of antibacterial and antitumor activities of carboxyl-terminal RWL-tagged antimicrobial peptides. Amino Acids 2014, 46, 2137–2154. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Zhou, C.; Hou, X.; Liu, Y.; Wang, Z.; Peng, X.; Zhang, Z.; Wang, R.; Kong, D. Molecular characterization and bioactivity evaluation of two novel bombinin peptides from the skin secretion of Oriental fire-bellied toad, Bombina orientalis. Amino Acids 2018, 50, 241–253. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Bian, D.; Li, W.; Xie, Y.; Li, X.; Lv, J.; Tang, R. Host defense peptide LL-37 is involved in the regulation of cell proliferation and production of pro-inflammatory cytokines in hepatocellular carcinoma cells. Amino Acids 2021, 53, 471–484. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Zhang, T.; Sun, L.; Luo, Y.; Liu, D.H.; Xie, S.T.; Song, X.Y.; Wang, G.F.; Chen, X.L.; Zhou, B.C.; et al. Calpain, Atg5 and Bak play important roles in the crosstalk between apoptosis and autophagy induced by influx of extracellular calcium. Apoptosis 2013, 18, 435–451. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Wang, J.; Wu, J.; Deng, R.; Zhang, L.; Chen, Q.; Wang, J.; Jin, X.; Gui, S.; Xu, Y.; et al. A novel antimicrobial peptide M1-8 targets the lysosomal pathway to inhibit autolysosome formation and promote apoptosis in liver cancer cells. J. Cell. Mol. Med. 2023, 27, 340–352. [Google Scholar] [CrossRef] [PubMed]
- Zainodini, N.; Hajizadeh, M.R.; Mirzaei, M.R. Evaluation of Apoptotic Gene Expression in Hepatoma Cell Line (HepG2) Following Nisin Treatment. Asian Pac. J. Cancer Prev. APJCP 2021, 22, 1413–1419. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Guo, R.; Chai, J.; Wu, J.; Liu, J.; Chen, X.; Abdel-Rahman, M.A.; Xia, H.; Xu, X. Smp24, a Scorpion-Venom Peptide, Exhibits Potent Antitumor Effects against Hepatoma HepG2 Cells via Multi-Mechanisms In Vivo and In Vitro. Toxins 2022, 14, 717. [Google Scholar] [CrossRef]
- Ohtake, T.; Fujimoto, Y.; Ikuta, K.; Saito, H.; Ohhira, M.; Ono, M.; Kohgo, Y. Proline-rich antimicrobial peptide, PR-39 gene transduction altered invasive activity and actin structure in human hepatocellular carcinoma cells. Br. J. Cancer 1999, 81, 393–403. [Google Scholar] [CrossRef][Green Version]
- Wu, C.; Geng, X.; Wan, S.; Hou, H.; Yu, F.; Jia, B.; Wang, L. Cecropin-P17, an analog of Cecropin B, inhibits human hepatocellular carcinoma cell HepG-2 proliferation via regulation of ROS, Caspase, Bax, and Bcl-2. J. Pept. Sci. Off. Publ. Eur. Pept. Soc. 2015, 21, 661–668. [Google Scholar] [CrossRef]
- Ke, M.; Dong, J.; Wang, Y.; Zhang, J.; Zhang, M.; Wu, Z.; Lv, Y.; Wu, R. MEL-pep, an analog of melittin, disrupts cell membranes and reverses 5-fluorouracil resistance in human hepatocellular carcinoma cells. Int. J. Biochem. Cell Biol. 2018, 101, 39–48. [Google Scholar] [CrossRef]
- Ma, Z.; Yang, J.; Han, J.; Gao, L.; Liu, H.; Lu, Z.; Zhao, H.; Bie, X. Insights into the Antimicrobial Activity and Cytotoxicity of Engineered α-Helical Peptide Amphiphiles. J. Med. Chem. 2016, 59, 10946–10962. [Google Scholar] [CrossRef] [PubMed]
- Judge, C.J.; Reyes-Aviles, E.; Conry, S.J.; Sieg, S.S.; Feng, Z.; Weinberg, A.; Anthony, D.D. HBD-3 induces NK cell activation, IFN-γ secretion and mDC dependent cytolytic function. Cell. Immunol. 2015, 297, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Fan, K.; Sun, N.; Yin, W.; Sun, Y.; Sun, P.; Jahejo, A.R.; Li, H. Recombinant porcine NK-lysin inhibits the invasion of hepatocellular carcinoma cells in vitro. Int. J. Biol. Macromol. 2019, 140, 1249–1259. [Google Scholar] [CrossRef]
- Balcik-Ercin, P.; Sever, B. An investigation of bacteriocin nisin anti-cancer effects and FZD7 protein interactions in liver cancer cells. Chem. Biol. Interact. 2022, 366, 110152. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.W.; Kim, S.J.; Kwon, Y.N.; Yun, E.Y.; Ahn, M.Y.; Kang, D.C.; Hwang, J.S. Effects of the synthetic coprisin analog peptide, CopA3 in pathogenic microorganisms and mammalian cancer cells. J. Microbiol. Biotechnol. 2012, 22, 156–158. [Google Scholar] [CrossRef] [PubMed]
- Jin-Yao, L.I.; Fu-Chun, Z.; Zheng-Hai, M.A. Prokaryotic expression of cecropin gene isolated from the silkworm Bombyx mori Xinjiang race and antibacterial activity of fusion cecropin. Acta Entomol. Sin. 2004, 47, 407–411. [Google Scholar]
- Farinha, P.; Pinho, J.O.; Matias, M.; Gaspar, M.M. Nanomedicines in the treatment of colon cancer: A focus on metallodrugs. Drug Deliv. Transl. Res. 2022, 12, 49–66. [Google Scholar] [CrossRef] [PubMed]
- Haraldsdottir, S.; Einarsdottir, H.M.; Smaradottir, A.; Gunnlaugsson, A.; Halfdanarson, T.R. Colorectal cancer-review. Laeknabladid 2014, 100, 75–82. [Google Scholar] [CrossRef]
- Anju, A.; Smitha, C.K.; Preetha, K.; Boobal, R.; Rosamma, P. Molecular characterization, recombinant expression and bioactivity profile of an antimicrobial peptide, Ss-arasin from the Indian mud crab, Scylla serrata. Fish Shellfish Immunol. 2019, 88, 352–358. [Google Scholar] [CrossRef]
- De Giani, A.; Bovio, F.; Forcella, M.; Fusi, P.; Sello, G.; Di Gennaro, P. Identification of a bacteriocin-like compound from Lactobacillus plantarum with antimicrobial activity and effects on normal and cancerogenic human intestinal cells. AMB Express 2019, 9, 88. [Google Scholar] [CrossRef]
- Kang, S.J.; Ji, H.Y.; Lee, B.J. Anticancer activity of undecapeptide analogues derived from antimicrobial peptide, brevinin-1EMa. Arch. Pharmacal Res. 2012, 35, 791–799. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, S.; Tomeh, M.A.; Wilkinson, R.N.; Hill, C.; Brown, S.; Zhao, X. Designed Antitumor Peptide for Targeted siRNA Delivery into Cancer Spheroids. ACS Appl. Mater. Interfaces 2021, 13, 49713–49728. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.X.; Shen, J.; Cheng, A.S.; Lu, L.; Chan, R.L.; Li, Z.J.; Wang, X.J.; Wong, C.C.; Zhang, L.; Ng, S.S.; et al. FK-16 derived from the anticancer peptide LL-37 induces caspase-independent apoptosis and autophagic cell death in colon cancer cells. PLoS ONE 2013, 8, e63641. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.; Lee, J.K.; Park, E.; Seo, C.H.; Luchian, T.; Park, Y. Antitumor activity of HPA3P through RIPK3-dependent regulated necrotic cell death in colon cancer. Oncotarget 2018, 9, 7902–7917. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.; Park, S.C.; Hahm, K.S.; Park, Y. A helix-PXXP-helix peptide with antibacterial activity without cytotoxicity against MDRPA-infected mice. Biomaterials 2014, 35, 1025–1039. [Google Scholar] [CrossRef] [PubMed]
- Ankaiah, D.; Esakkiraj, P.; Perumal, V.; Ayyanna, R.; Venkatesan, A. Probiotic characterization of Enterococcus faecium por1: Cloning, over expression of Enterocin-A and evaluation of antibacterial, anti-cancer properties. J. Funct. Foods 2018, 38, 280–292. [Google Scholar] [CrossRef]
- Avaiyarasi, N.D.; Ravindran, A.D.; Venkatesh, P.; Arul, V. Invitro selection, characterization and cytotoxic effect of bacteriocin of Lactobacillus sakei GM3 isolated from goat milk. Food Control 2016, 69, 124–133. [Google Scholar] [CrossRef]
- Norouzi, Z.; Salimi, A.; Halabian, R.; Fahimi, H. Nisin, a potent bacteriocin and anti-bacterial peptide, attenuates expression of metastatic genes in colorectal cancer cell lines. Microb. Pathog. 2018, 123, 183–189. [Google Scholar] [CrossRef]
- Chen, Y.C.; Tsai, T.L.; Ye, X.H.; Lin, T.H. Anti-proliferative effect on a colon adenocarcinoma cell line exerted by a membrane disrupting antimicrobial peptide KL15. Cancer Biol. Ther. 2015, 16, 1172–1183. [Google Scholar] [CrossRef]
- Maraming, P.; Klaynongsruang, S.; Boonsiri, P.; Peng, S.F.; Daduang, S.; Leelayuwat, C.; Pientong, C.; Chung, J.G.; Daduang, J. The cationic cell-penetrating KT2 peptide promotes cell membrane defects and apoptosis with autophagy inhibition in human HCT 116 colon cancer cells. J. Cell. Physiol. 2019, 234, 22116–22129. [Google Scholar] [CrossRef]
- Nam, B.H.; Moon, J.Y.; Park, E.H.; Kong, H.J.; Kim, Y.O.; Kim, D.G.; Kim, W.J.; An, C.M.; Seo, J.K. Antimicrobial and Antitumor Activities of Novel Peptides Derived from the Lipopolysaccharide- and β-1,3-Glucan Binding Protein of the Pacific Abalone Haliotis discus hannai. Mar. Drugs 2016, 14, 227. [Google Scholar] [CrossRef] [PubMed]
- Panjeta, A.; Preet, S. Anticancer potential of human intestinal defensin 5 against 1, 2-dimethylhydrazine dihydrochloride induced colon cancer: A therapeutic approach. Peptides 2020, 126, 170263. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.X.; Cheng, A.S.; To, K.F.; Tong, J.H.; Li, M.S.; Shen, J.; Wong, C.C.; Zhang, L.; Chan, R.L.; Wang, X.J.; et al. Host immune defense peptide LL-37 activates caspase-independent apoptosis and suppresses colon cancer. Cancer Res. 2012, 72, 6512–6523. [Google Scholar] [CrossRef] [PubMed]
- Maijaroen, S.; Klaynongsruang, S.; Roytrakul, S.; Konkchaiyaphum, M.; Taemaitree, L.; Jangpromma, N. An Integrated Proteomics and Bioinformatics Analysis of the Anticancer Properties of RT2 Antimicrobial Peptide on Human Colon Cancer (Caco-2) Cells. Molecules 2022, 27, 1426. [Google Scholar] [CrossRef] [PubMed]
- Saleh, R.O.; Essia, I.N.A.; Jasim, S.A. The Anticancer Effect of a Conjugated Antimicrobial Peptide Against Colorectal Cancer (CRC) Cells. J. Gastrointest. Cancer 2022, 54, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Dey, D.K.; Kang, S.C. CopA3 peptide induces permanent cell-cycle arrest in colorectal cancer cells. Mech. Ageing Dev. 2021, 196, 111497. [Google Scholar] [CrossRef] [PubMed]
- Jia, F.; Yu, Q.; Wang, R.; Zhao, L.; Yuan, F.; Guo, H.; Shen, Y.; He, F. Optimized Antimicrobial Peptide Jelleine-I Derivative Br-J-I Inhibits Fusobacterium Nucleatum to Suppress Colorectal Cancer Progression. Int. J. Mol. Sci. 2023, 24, 1469. [Google Scholar] [CrossRef]
- Tsai, T.L.; Li, A.C.; Chen, Y.C.; Liao, Y.S.; Lin, T.H. Antimicrobial peptide m2163 or m2386 identified from Lactobacillus casei ATCC 334 can trigger apoptosis in the human colorectal cancer cell line SW480. Tumour Biol. J. Int. Soc. Oncodevelopmental Biol. Med. 2015, 36, 3775–3789. [Google Scholar] [CrossRef]
- Wei, P.L.; Lin, J.C.; Hung, C.S.; Makondi, P.T.; Batzorig, U.; Chang, T.C.; Huang, C.Y.; Chang, Y.J. Human α-defensin 6 (HD6) suppresses CRC proliferation and metastasis through abolished EGF/EGFR signaling pathway. Int. J. Med. Sci. 2022, 19, 34–46. [Google Scholar] [CrossRef]
- Kuroda, K.; Fukuda, T.; Okumura, K.; Yoneyama, H.; Isogai, H.; Savage, P.B.; Isogai, E. Ceragenin CSA-13 induces cell cycle arrest and antiproliferative effects in wild-type and p53 null mutant HCT116 colon cancer cells. Anti Cancer Drugs 2013, 24, 826–834. [Google Scholar] [CrossRef]
- Kuroda, K.; Fukuda, T.; Yoneyama, H.; Katayama, M.; Isogai, H.; Okumura, K.; Isogai, E. Anti-proliferative effect of an analogue of the LL-37 peptide in the colon cancer derived cell line HCT116 p53+/+ and p53. Oncol. Rep. 2012, 28, 829–834. [Google Scholar] [CrossRef]
- Gu, Q.Q.; He, S.W.; Liu, L.H.; Wang, G.H.; Hao, D.F.; Liu, H.M.; Wang, C.B.; Li, C.; Zhang, M.; Li, N.Q. A teleost bactericidal permeability-increasing protein-derived peptide that possesses a broad antibacterial spectrum and inhibits bacterial infection as well as human colon cancer cells growth. Dev. Comp. Immunol. 2021, 118, 103995. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, S.; Ghollasi, M.; Hosseini, H.M. The apoptotic impact of nisin as a potent bacteriocin on the colon cancer cells. Microb. Pathog. 2017, 111, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Freiburghaus, C.; Janicke, B.; Lindmark-Månsson, H.; Oredsson, S.M.; Paulsson, M.A. Lactoferricin treatment decreases the rate of cell proliferation of a human colon cancer cell line. J. Dairy Sci. 2009, 92, 2477–2484. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, X.; Li, Y.; Lan, X.; Leung, P.H.; Li, J.; Li, G.; Xie, M.; Han, Y.; Lin, X. Composite Membranes of Recombinant Silkworm Antimicrobial Peptide and Poly (L-lactic Acid) (PLLA) for biomedical application. Sci. Rep. 2016, 6, 31149. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Lönnerdal, B. Bovine lactoferrin and lactoferricin exert antitumor activities on human colorectal cancer cells (HT-29) by activating various signaling pathways. Biochem. Cell Biol. 2017, 95, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Varas, M.A.; Muñoz-Montecinos, C.; Kallens, V.; Simon, V.; Allende, M.L.; Marcoleta, A.E.; Lagos, R. Exploiting Zebrafish Xenografts for Testing the in vivo Antitumorigenic Activity of Microcin E492 Against Human Colorectal Cancer Cells. Front. Microbiol. 2020, 11, 405. [Google Scholar] [CrossRef]
- Okasha, H.; Samir, S.; Nasr, S.M. Purified recombinant human Chromogranin A N46 peptide with remarkable anticancer effect on human colon cancer cells. Bioorg. Chem. 2021, 115, 105266. [Google Scholar] [CrossRef]
- Raileanu, M.; Popescu, A.; Bacalum, M. Antimicrobial Peptides as New Combination Agents in Cancer Therapeutics: A Promising Protocol against HT-29 Tumoral Spheroids. Int. J. Mol. Sci. 2020, 21, 6964. [Google Scholar] [CrossRef]
- Kuroda, K.; Fukuda, T.; Isogai, H.; Okumura, K.; Krstic-Demonacos, M.; Isogai, E. Antimicrobial peptide FF/CAP18 induces apoptotic cell death in HCT116 colon cancer cells via changes in the metabolic profile. Int. J. Oncol. 2015, 46, 1516–1526. [Google Scholar] [CrossRef]
- Mima, K.; Nishihara, R.; Qian, Z.R.; Cao, Y.; Sukawa, Y.; Nowak, J.A.; Yang, J.; Dou, R.; Masugi, Y.; Song, M.; et al. Fusobacterium nucleatum in colorectal carcinoma tissue and patient prognosis. Gut 2016, 65, 1973–1980. [Google Scholar] [CrossRef] [PubMed]
- Cochrane, K.; Robinson, A.V.; Holt, R.A.; Allen-Vercoe, E. A survey of Fusobacterium nucleatum genes modulated by host cell infection. Microb. Genom. 2020, 6, e000300. [Google Scholar] [CrossRef] [PubMed]
- Haruki, K.; Kosumi, K.; Hamada, T.; Twombly, T.S.; Väyrynen, J.P.; Kim, S.A.; Masugi, Y.; Qian, Z.R.; Mima, K.; Baba, Y.; et al. Association of autophagy status with amount of Fusobacterium nucleatum in colorectal cancer. J. Pathol. 2020, 250, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, Y.; Zhang, J.; Cao, P.; Su, W.; Deng, Y.; Zhan, N.; Fu, X.; Huang, Y.; Dong, W. Fusobacterium nucleatum Promotes Metastasis in Colorectal Cancer by Activating Autophagy Signaling via the Upregulation of CARD3 Expression. Theranostics 2020, 10, 323–339. [Google Scholar] [CrossRef] [PubMed]
- Epand, R.M.; Vogel, H.J. Diversity of antimicrobial peptides and their mechanisms of action. Biochim. Biophys. Acta 1999, 1462, 11–28. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Kizhakkedathu, J.N.; Straus, S.K. Antimicrobial Peptides: Diversity, Mechanism of Action and Strategies to Improve the Activity and Biocompatibility In Vivo. Biomolecules 2018, 8, 4. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, J.C.; Waterhouse, N.J.; Juin, P.; Evan, G.I.; Green, D.R. The coordinate release of cytochrome c during apoptosis is rapid, complete and kinetically invariant. Nat. Cell Biol. 2000, 2, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Gross, A.; McDonnell, J.M.; Korsmeyer, S.J. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 1999, 13, 1899–1911. [Google Scholar] [CrossRef]
- De Kroon, A.I.; Dolis, D.; Mayer, A.; Lill, R.; de Kruijff, B. Phospholipid composition of highly purified mitochondrial outer membranes of rat liver and Neurospora crassa. Is cardiolipin present in the mitochondrial outer membrane? Biochim. Biophys. Acta 1997, 1325, 108–116. [Google Scholar] [CrossRef]
- Yang, Y.; Yu, Y.; Wang, J.; Li, Y.; Li, Y.; Wei, J.; Zheng, T.; Jin, M.; Sun, Z. Silica nanoparticles induced intrinsic apoptosis in neuroblastoma SH-SY5Y cells via CytC/Apaf-1 pathway. Environ. Toxicol. Pharmacol. 2017, 52, 161–169. [Google Scholar] [CrossRef]
- Feng, Y.; He, D.; Yao, Z.; Klionsky, D.J. The machinery of macroautophagy. Cell Res. 2014, 24, 24–41. [Google Scholar] [CrossRef] [PubMed]
- Kraya, A.A.; Piao, S.; Xu, X.; Zhang, G.; Herlyn, M.; Gimotty, P.; Levine, B.; Amaravadi, R.K.; Speicher, D.W. Identification of secreted proteins that reflect autophagy dynamics within tumor cells. Autophagy 2015, 11, 60–74. [Google Scholar] [CrossRef] [PubMed]
- Tanida, I.; Ueno, T.; Kominami, E. LC3 and Autophagy. Methods Mol. Biol. 2008, 445, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Fass, E.; Shvets, E.; Degani, I.; Hirschberg, K.; Elazar, Z. Microtubules support production of starvation-induced autophagosomes but not their targeting and fusion with lysosomes. J. Biol. Chem. 2006, 281, 36303–36316. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Miao, G.; Xue, X.; Guo, X.; Yuan, C.; Wang, Z.; Zhang, G.; Chen, Y.; Feng, D.; Hu, J.; et al. The Vici Syndrome Protein EPG5 Is a Rab7 Effector that Determines the Fusion Specificity of Autophagosomes with Late Endosomes/Lysosomes. Mol. Cell 2016, 63, 781–795. [Google Scholar] [CrossRef]
- Chu, C.; Geng, Y.; Zhou, Y.; Sicinski, P. Cyclin E in normal physiology and disease states. Trends Cell Biol. 2021, 31, 732–746. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Greenwood, J.; Jones, A.W.; Nurse, P. Core control principles of the eukaryotic cell cycle. Nature 2022, 607, 381–386. [Google Scholar] [CrossRef]
- Engeland, K. Cell cycle arrest through indirect transcriptional repression by p53: I have a DREAM. Cell Death Differ. 2018, 25, 114–132. [Google Scholar] [CrossRef]
- Mulder, K.C.; Lima, L.A.; Miranda, V.J.; Dias, S.C.; Franco, O.L. Current scenario of peptide-based drugs: The key roles of cationic antitumor and antiviral peptides. Front. Microbiol. 2013, 4, 321. [Google Scholar] [CrossRef]
- Silvestro, L.; Gupta, K.; Weiser, J.N.; Axelsen, P.H. The concentration-dependent membrane activity of cecropin A. Biochemistry 1999, 38, 3850. [Google Scholar] [CrossRef]
- Chaudhary, J.; Munshi, M. Scanning electron microscopic analysis of breast aspirates. Cytopathology 1995, 6, 162–167. [Google Scholar] [CrossRef]
- Barlow, M.; Edelman, M.; Glick, R.D.; Steinberg, B.M.; Soffer, S.Z. Celecoxib inhibits invasion and metastasis via a cyclooxygenase 2-independent mechanism in an in vitro model of Ewing sarcoma. J. Pediatr. Surg. 2012, 47, 1223–1227. [Google Scholar] [CrossRef] [PubMed]
- Leeming, D.J.; Bay-Jensen, A.C.; Vassiliadis, E.; Larsen, M.R.; Henriksen, K.; Karsdal, M.A. Post-translational modifications of the extracellular matrix are key events in cancer progression: Opportunities for biochemical marker development. Biomarkers 2011, 16, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zeng, Z.; Wang, S.; Li, T.; Mastriani, E.; Li, Q.-H.; Bao, H.-X.; Zhou, Y.-J.; Wang, X.; Liu, Y.; et al. Main components of pomegranate, ellagic acid and luteolin, inhibit metastasis of ovarian cancer by down-regulating MMP2 and MMP9. Cancer Biol. Ther. 2017, 18, 990–999. [Google Scholar] [CrossRef] [PubMed]
- Mali, A.V.; Wagh, U.V.; Hegde, M.V.; Chandorkar, S.S.; Surve, S.V.; Patole, M.V. In vitro anti-metastatic activity of enterolactone, a mammalian lignan derived from flax lignan, and down-regulation of matrix metalloproteinases in MCF-7 and MDA MB 231 cell lines. Indian J. Cancer 2012, 49, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Hamatsu, T.; Rikimaru, T.; Yamashita, Y.; Aishima, S.; Tanaka, S.; Shirabe, K.; Shimada, M.; Toh, Y.; Sugimachi, K. The role of MTA1 gene expression in human hepatocellular carcinoma. Oncol. Rep. 2003, 10, 599–604. [Google Scholar] [PubMed]
- Toh, Y.; Oki, E.; Oda, S.; Tokunaga, E.; Ohno, S.; Maehara, Y.; Nicolson, G.L.; Sugimachi, K. Overexpression of the MTA1 gene in gastrointestinal carcinomas: Correlation with invasion and metastasis. Int. J. Cancer 1997, 74, 459–463. [Google Scholar] [CrossRef]
- Uraki, S.; Sugimoto, K.; Shiraki, K.; Tameda, M.; Inagaki, Y.; Ogura, S.; Kasai, C.; Nojiri, K.; Yoneda, M.; Yamamoto, N.; et al. Human β-defensin-3 inhibits migration of colon cancer cells via downregulation of metastasis-associated 1 family, member 2 expression. Int. J. Oncol. 2014, 45, 1059–1064. [Google Scholar] [CrossRef]
- Kessenbrock, K.; Plaks, V.; Werb, Z. Matrix metalloproteinases: Regulators of the tumor microenvironment. Cell 2010, 141, 52–67. [Google Scholar] [CrossRef]
- Jin, X.B.; Wang, Y.J.; Liang, L.L.; Pu, Q.H.; Shen, J.; Lu, X.M.; Chu, F.J.; Zhu, J.Y. Cecropin suppresses human hepatocellular carcinoma BEL- 7402 cell growth and survival in vivo without side-toxicity. Asian Pac. J. Cancer Prev. APJCP 2014, 15, 5433–5436. [Google Scholar] [CrossRef]
- Chan, S.C.; Hui, L.; Chen, H.M. Enhancement of the cytolytic effect of anti-bacterial cecropin by the microvilli of cancer cells. Anticancer Res. 1998, 18, 4467–4474. [Google Scholar] [PubMed]
- Suttmann, H.; Retz, M.; Paulsen, F.; Harder, J.; Zwergel, U.; Kamradt, J.; Wullich, B.; Unteregger, G.; Stöckle, M.; Lehmann, J. Antimicrobial peptides of the Cecropin-family show potent antitumor activity against bladder cancer cells. BMC Urol. 2008, 8, 5. [Google Scholar] [CrossRef] [PubMed]
- Yoon, W.H.; Park, H.D.; Lim, K.; Hwang, B.D. Effect of O-glycosylated mucin on invasion and metastasis of HM7 human colon cancer cells. Biochem. Biophys. Res. Commun. 1996, 222, 694–699. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Chen, Y.W.; Zhang, L.; Gong, X.G.; Zhou, Y.; Shang, D.J. Melanoma cell surface-expressed phosphatidylserine as a therapeutic target for cationic anticancer peptide, temporin-1CEa. J. Drug Target. 2016, 24, 548–556. [Google Scholar] [CrossRef] [PubMed]
- Fadnes, B.; Rekdal, O.; Uhlin-Hansen, L. The anticancer activity of lytic peptides is inhibited by heparan sulfate on the surface of the tumor cells. BMC Cancer 2009, 9, 183. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, I.W.; Kim, S.H.; Yun, E.Y.; Nam, S.H.; Ahn, M.Y.; Kang, D.C.; Hwang, J.S. Anticancer activity of CopA3 dimer peptide in human gastric cancer cells. BMB Rep. 2015, 48, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Dathe, M.; Wieprecht, T. Structural features of helical antimicrobial peptides: Their potential to modulate activity on model membranes and biological cells. Biochim. Biophys. Acta 1999, 1462, 71–87. [Google Scholar] [CrossRef]
- Kolata, G.B. Microvilli: A major difference between normal and cancer cells? Science 1975, 188, 819–820. [Google Scholar] [CrossRef]
- Deslouches, B.; Steckbeck, J.D.; Craigo, J.K.; Doi, Y.; Burns, J.L.; Montelaro, R.C. Engineered cationic antimicrobial peptides to overcome multidrug resistance by ESKAPE pathogens. Antimicrob. Agents Chemother. 2015, 59, 1329–1333. [Google Scholar] [CrossRef]
- Qin, Y.; Qin, Z.D.; Chen, J.; Cai, C.G.; Li, L.; Feng, L.Y.; Wang, Z.; Duns, G.J.; He, N.Y.; Chen, Z.S.; et al. From Antimicrobial to Anticancer Peptides: The Transformation of Peptides. Recent Pat. Anti-Cancer Drug Discov. 2019, 14, 70–84. [Google Scholar] [CrossRef]
- Aaghaz, S.; Gohel, V.; Kamal, A. Peptides as Potential Anticancer Agents. Curr. Top. Med. Chem. 2019, 19, 1491–1511. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.R.; Yan, J.X.; Zhang, B.Z.; Song, J.J.; Jia, P.F.; Wang, R. Novel mode of action of polybia-MPI, a novel antimicrobial peptide, in multi-drug resistant leukemic cells. Cancer Lett. 2009, 278, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Giannelli, G.; Koudelkova, P.; Dituri, F.; Mikulits, W. Role of epithelial to mesenchymal transition in hepatocellular carcinoma. J. Hepatol. 2016, 65, 798–808. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.; Feng, X.; Cao, J.G. Twist in hepatocellular carcinoma: Pathophysiology and therapeutics. Hepatol. Int. 2015, 9, 399–405. [Google Scholar] [CrossRef]
- Merle, P.; de la Monte, S.; Kim, M.; Herrmann, M.; Tanaka, S.; Von Dem Bussche, A.; Kew, M.C.; Trepo, C.; Wands, J.R. Functional consequences of frizzled-7 receptor overexpression in human hepatocellular carcinoma. Gastroenterology 2004, 127, 1110–1122. [Google Scholar] [CrossRef]
- González, M.L.; Vera, D.M.A.; Laiolo, J.; Joray, M.B.; Maccioni, M.; Palacios, S.M.; Molina, G.; Lanza, P.A.; Gancedo, S.; Rumjanek, V.; et al. Mechanism Underlying the Reversal of Drug Resistance in P-Glycoprotein-Expressing Leukemia Cells by Pinoresinol and the Study of a Derivative. Front. Pharmacol. 2017, 8, 205. [Google Scholar] [CrossRef]
- Smyth, M.J.; Krasovskis, E.; Sutton, V.R.; Johnstone, R.W. The drug efflux protein, P-glycoprotein, additionally protects drug-resistant tumor cells from multiple forms of caspase-dependent apoptosis. Proc. Natl. Acad. Sci. USA 1998, 95, 7024–7029. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).