New Insights into Cardiovascular Diseases Treatment Based on Molecular Targets
Abstract
:1. Introduction
2. Dyslipidemia, Its Association with Cardiovascular Disease, and New Insights in Treatment New Insights in Treatment of Dyslipidemia
2.1. Alirocumab and Evolocumab
2.2. Evinacumab
2.3. Bempedoic Acid
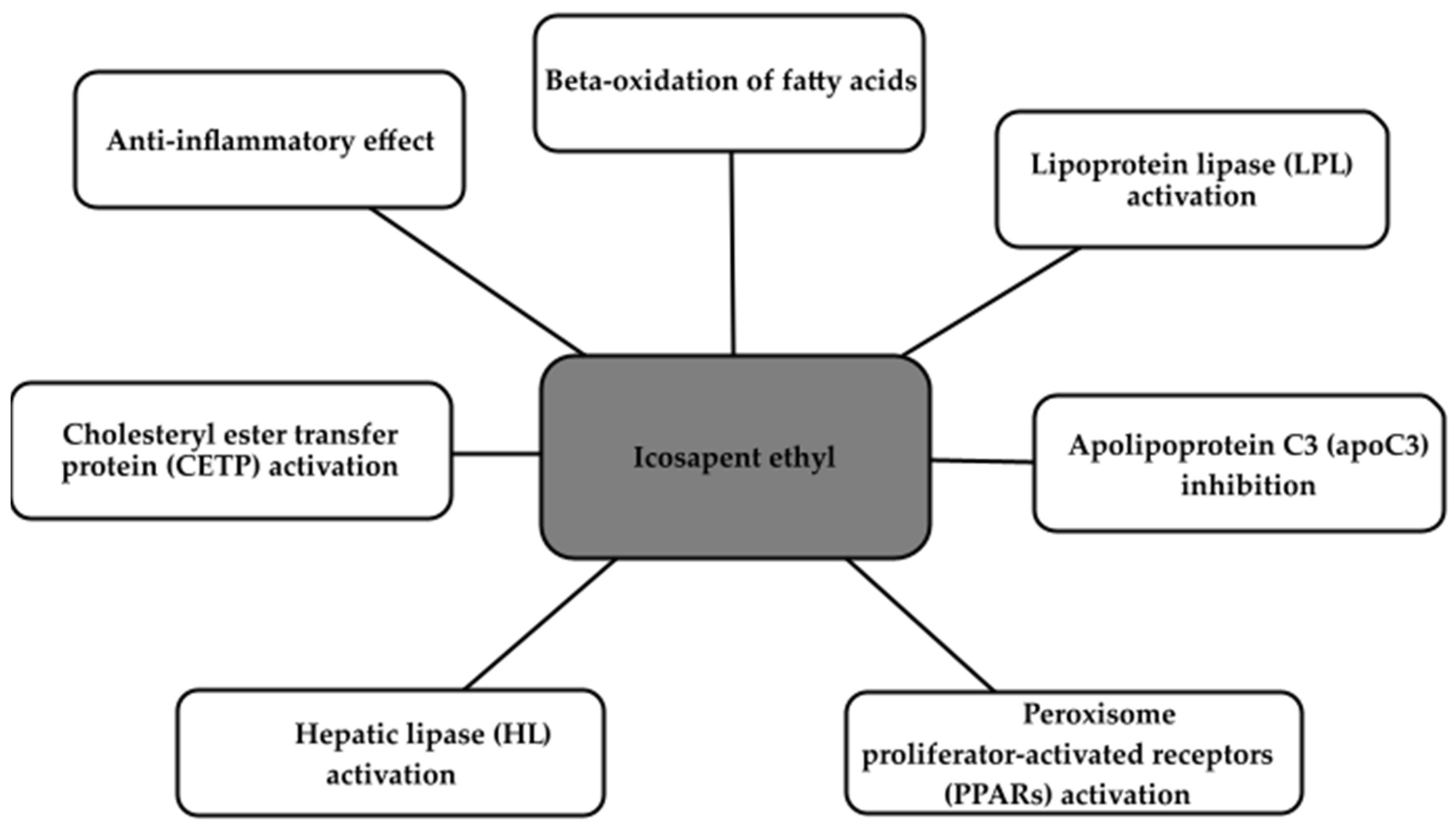
2.4. Icosapent Ethyl
2.5. SiRNA Therapy
2.6. Other Therapy Solutions
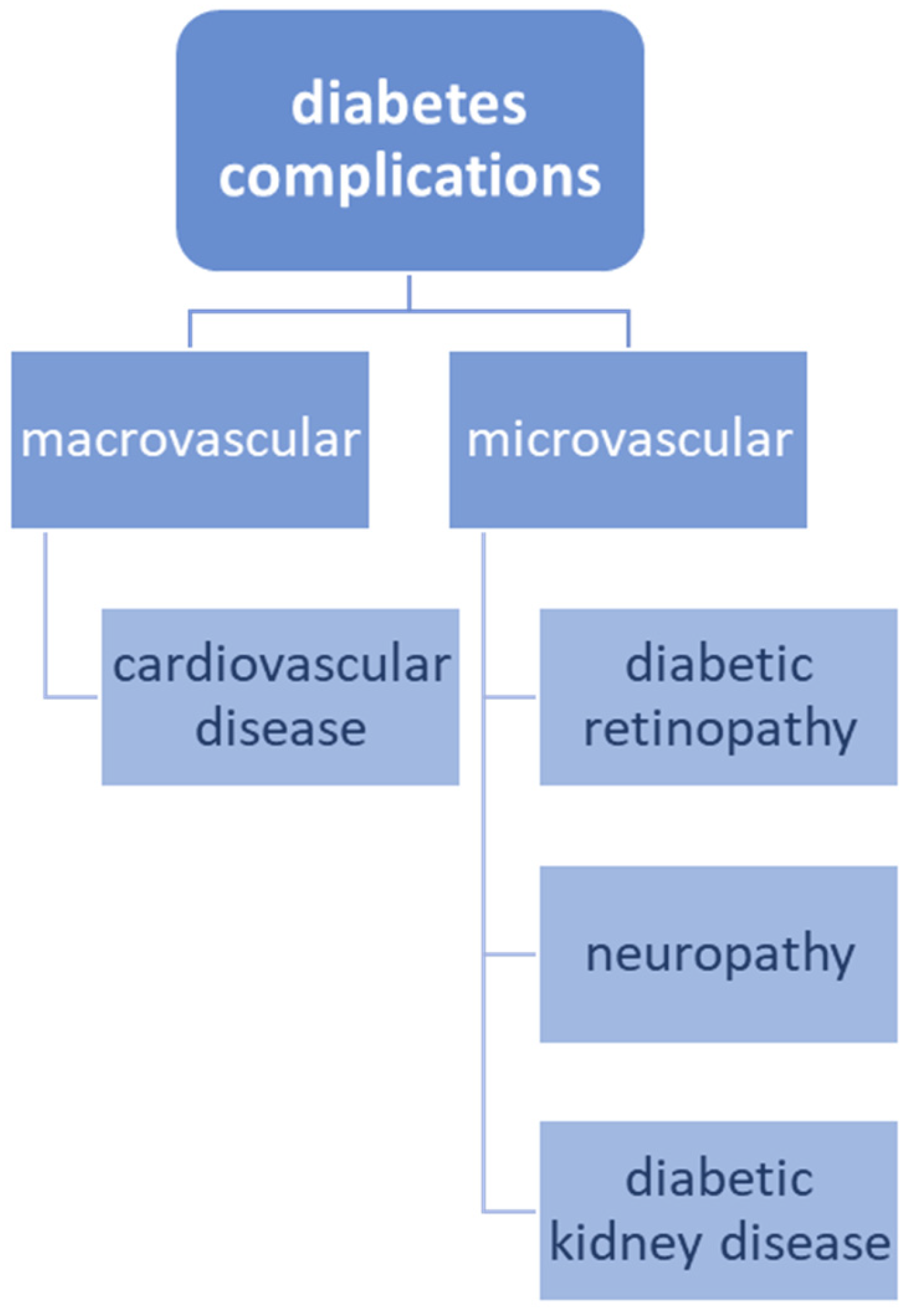
3. The Association between Cardiovascular Disease and Diabetes
4. The Role of SGLT-2 Inhibitors and GLP-1 Receptor Agonists in Diabetes and Cardiovascular Disease
4.1. Sodium-Glucose Co-Transporter-2 (SGLT-2) Inhibitors
4.2. Glucagon-like Peptide-1 (GLP-1) Receptor Agonists
5. The Emerging Significance of VCP/p97 in Cardiovascular Ailments: Fresh Insights and Therapeutic Prospects
5.1. VCP/p97′s Involvement in Cardiovascular Conditions
5.2. VCP/p97 and Ischemia/Reperfusion-Induced Damage
6. Fresh Insights and Novel Perspectives on Exosomes in Cardiovascular Disorders
6.1. Exosomes as Diagnostic Tools in Cardiovascular Ailments
6.2. Exosomes as Therapeutics in Cardiovascular Ailments
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bhatnagar, P.; Wickramasinghe, K.; Wilkins, E.; Townsend, N. Trends in the epidemiology of cardiovascular disease in the UK. Heart 2016, 102, 1945–1952. [Google Scholar] [CrossRef] [PubMed]
- GBD 2017 Causes of Death Collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1736–1788. [Google Scholar] [CrossRef] [PubMed]
- Mensah, G.A.; Roth, G.A.; Fuster, V. The Global Burden of Cardiovascular Diseases and Risk Factors: 2020 and beyond. J. Am. Coll. Cardiol. 2019, 74, 2529–2532. [Google Scholar] [CrossRef] [PubMed]
- Bays, H.E.; Kulkarni, A.; German, C.; Satish, P.; Iluyomade, A.; Dudum, R.; Thakkar, A.; Rifai, M.A.; Mehta, A.; Thobani, A.; et al. Ten things to know about ten cardiovascular disease risk factors—2022. Am. J. Prev. Cardiol. 2022, 10, 100342. [Google Scholar] [CrossRef] [PubMed]
- Kazemnian, H.; Mehrad-Majd, H. Recent advances in the Prevention and Treatment of Chemotherapy–induced Cardiotoxicity. Res. Biotechnol. Environ. Sci. 2023, 2, 24–29. [Google Scholar] [CrossRef]
- Tripathy, D.; Merovci, A.; Basu, R.; Abdul-Ghani, M.; DeFronzo, R.A. Mild Physiologic Hyperglycemia Induces Hepatic Insulin Resistance in Healthy Normal Glucose-Tolerant Participants. J. Clin. Endocrinol. Metab. 2019, 104, 2842–2850. [Google Scholar] [CrossRef] [PubMed]
- Cole, J.B.; Florez, J.C. Genetics of diabetes mellitus and diabetes complications. Nat. Rev. Nephrol. 2020, 16, 377–390. [Google Scholar] [CrossRef]
- Provenzano, M.; Coppolino, G.; De Nicola, L.; Serra, R.; Garofalo, C.; Andreucci, M.; Bolignano, D. Unraveling Cardiovascular Risk in Renal Patients: A New Take on Old Tale. Front. Cell Dev. Biol. 2019, 7, 314. [Google Scholar] [CrossRef]
- Aygun, S.; Tokgozoglu, L. Comparison of Current International Guidelines for the Management of Dyslipidemia. J. Clin. Med. 2022, 11, 7249. [Google Scholar] [CrossRef]
- Sulaiman, R.A. Inherited metabolic disorders and dyslipidaemia. J. Clin. Pathol. 2020, 73, 384–390. [Google Scholar] [CrossRef]
- Ference, B.A.; Yoo, W.; Alesh, I.; Mahajan, N.; Mirowska, K.K.; Mewada, A.; Kahn, J.; Afonso, L.; Williams, K.A., Sr.; Flack, J.M. Effect of Long-Term Exposure to Lower Low-Density Lipoprotein Cholesterol Beginning Early in Life on the Risk of Coronary Heart Disease: A Mendelian Randomization Analysis. J. Am. Coll. Cardiol. 2012, 60, 2631–2639. [Google Scholar] [CrossRef] [PubMed]
- Emerging Risk Factors Collaboration; Di Angelantonio, E.; Gao, P.; Pennells, L.; Kaptoge, S.; Caslake, M.; Thompson, A.; Butterworth, A.S.; Sarwar, N.; Wormser, D.; et al. Lipid-Related Markers and Cardiovascular Disease Prediction. JAMA 2012, 307, 2499–2506. [Google Scholar] [CrossRef]
- Berberich, A.J.; Hegele, R.A. A Modern Approach to Dyslipidemia. Endocr. Rev. 2022, 43, 611–653. [Google Scholar] [CrossRef] [PubMed]
- Chapman, M.J.; Zamorano, J.L.; Parhofer, K.G. Reducing residual cardiovascular risk in Europe: Therapeutic implications of European medicines agency approval of icosapent ethyl/eicosapentaenoic acid. Pharmacol. Ther. 2022, 237, 108172. [Google Scholar] [CrossRef] [PubMed]
- Borghi, C.; Fogacci, F.; Agnoletti, D.; Cicero, A.F.G. Hypertension and Dyslipidemia Combined Therapeutic Approaches. High Blood Press. Cardiovasc. Prev. 2022, 29, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Fulcher, J.; Abeysuriya, N.; Park, L.; Kumar, S.; Di Tanna, G.L.; Wilcox, I.; Keech, A.; Rodgers, A.; Lal, S. Intensive LDL cholesterol-lowering treatment beyond current recommendations for the prevention of major vascular events: A systematic review and meta-analysis of randomised trials including 327 037 participants. Lancet Diabetes Endocrinol. 2020, 8, 36–49. [Google Scholar] [CrossRef]
- Roth, E.M. Alirocumab for low-density lipoprotein cholesterol lowering. Future Cardiol. 2019, 15, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Della Pepa, G.; Bozzetto, L.; Annuzzi, G.; Rivellese, A.A. Alirocumab for the treatment of hypercholesterolaemia. Expert Rev. Clin. Pharmacol. 2017, 10, 571–582. [Google Scholar] [CrossRef]
- Robinson, J.G.; Farnier, M.; Krempf, M.; Bergeron, J.; Luc, G.; Averna, M.; Stroes, E.S.; Langslet, G.; Raal, F.J.; El Shahawy, M.; et al. Efficacy and Safety of Alirocumab in Reducing Lipids and Cardiovascular Events. N. Engl. J. Med. 2015, 372, 1489–1499. [Google Scholar] [CrossRef]
- Alirocumab (Praluent) to Lower LDL-Cholesterol. JAMA 2015, 314, 1284–1285. [CrossRef]
- Huang, H.-C.; Hsu, S.-J.; Chang, C.-C.; Chuang, C.-L.; Hou, M.-C.; Lee, F.-Y. Effects of PCSK-9 Inhibition by Alirocumab Treatments on Biliary Cirrhotic Rats. Int. J. Mol. Sci. 2022, 23, 7378. [Google Scholar] [CrossRef] [PubMed]
- Tuñón, J.; Steg, P.G.; Bhatt, D.L.; Bittner, V.A.; Díaz, R.; Goodman, S.G.; Jukema, J.W.; Kim, Y.-U.; Li, Q.H.; Mueller, C.; et al. Effect of alirocumab on major adverse cardiovascular events according to renal function in patients with a recent acute coronary syndrome: Prespecified analysis from the ODYSSEY OUTCOMES randomized clinical trial. Eur. Heart J. 2020, 41, 4114–4123. [Google Scholar] [CrossRef] [PubMed]
- Sabatine, M.S.; Giugliano, R.P.; Keech, A.C.; Honarpour, N.; Wiviott, S.D.; Murphy, S.A.; Kuder, J.F.; Wang, H.; Liu, T.; Wasserman, S.M.; et al. Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. N. Engl. J. Med. 2017, 376, 1713–1722. [Google Scholar] [CrossRef] [PubMed]
- Kasichayanula, S.; Grover, A.; Emery, M.G.; Gibbs, M.A.; Somaratne, R.; Wasserman, S.M.; Gibbs, J.P. Clinical Pharmacokinetics and Pharmacodynamics of Evolocumab, a PCSK9 Inhibitor. Clin. Pharmacokinet. 2018, 57, 769–779. [Google Scholar] [CrossRef] [PubMed]
- Keating, G.M. Evolocumab: A Review in Hyperlipidemia. Am. J. Cardiovasc. Drugs 2016, 16, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Wiggins, B.S.; Senfield, J.; Kassahun, H.; Lira, A.; Somaratne, R. Evolocumab: Considerations for the Management of Hyperlipidemia. Curr. Atheroscler. Rep. 2018, 20, 17. [Google Scholar] [CrossRef] [PubMed]
- Sabatine, M.S.; Giugliano, R.P.; Wiviott, S.D.; Raal, F.J.; Blom, D.J.; Robinson, J.; Ballantyne, C.M.; Somaratne, R.; Legg, J.; Wasserman, S.M.; et al. Efficacy and Safety of Evolocumab in Reducing Lipids and Cardiovascular Events. N. Engl. J. Med. 2015, 372, 1500–1509. [Google Scholar] [CrossRef]
- Hamilton, P. Evolocumab and clinical outcomes in patients with cardiovascular disease. Ann. Clin. Biochem. 2017, 54, 511. [Google Scholar] [CrossRef]
- Nicholls, S.J.; Kataoka, Y.; Nissen, S.E.; Prati, F.; Windecker, S.; Puri, R.; Hucko, T.; Aradi, D.; Herrman, J.-P.R.; Hermanides, R.S.; et al. Effect of Evolocumab on Coronary Plaque Phenotype and Burden in Statin-Treated Patients Following Myocardial Infarction. JACC Cardiovasc. Imaging 2022, 15, 1308–1321. [Google Scholar] [CrossRef]
- Hirai, K.; Imamura, S.; Hirai, A.; Ookawara, S.; Morishita, Y. Effect of Evolocumab on Vulnerable Coronary Plaques: A Serial Coronary Computed Tomography Angiography Study. J. Clin. Med. 2020, 9, 3338. [Google Scholar] [CrossRef]
- Kersten, S. New insights into angiopoietin-like proteins in lipid metabolism and cardiovascular disease risk. Curr. Opin. Lipidol. 2019, 30, 205–211. [Google Scholar] [CrossRef]
- Su, X.; Peng, D.Q. New Insights into ANGPLT3 in Controlling Lipoprotein Metabolism and Risk of Cardiovascular Diseases. Lipids Health Dis. 2018, 17, 12. [Google Scholar] [CrossRef] [PubMed]
- Ono, M.; Shimizugawa, T.; Shimamura, M.; Yoshida, K.; Noji-Sakikawa, C.; Ando, Y.; Koishi, R.; Furukawa, H. Protein Region Important for Regulation of Lipid Metabolism in Angiopoietin-like 3 (ANGPTL3): ANGPTL3 is cleaved and activated in vivo. J. Biol. Chem. 2003, 278, 41804–41809. [Google Scholar] [CrossRef] [PubMed]
- Reeskamp, L.F.; Tromp, T.R.; Stroes, E.S. The next generation of triglyceride-lowering drugs: Will reducing apolipoprotein C-III or angiopoietin like protein 3 reduce cardiovascular disease? Curr. Opin. Lipidol. 2020, 31, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Kosmas, C.E.; Bousvarou, M.D.; Sourlas, A.; Papakonstantinou, E.J.; Peña Genao, E.; Echavarria Uceta, R.; Guzman, E. Angiopoietin-Like Protein 3 (ANGPTL3) Inhibitors in the Management of Refractory Hypercholesterolemia. Clin. Pharmacol. 2022, 14, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, F.; Mansfield, B.S.; Raal, F.J. ANGPTL3 as a Drug Target in Hyperlipidemia and Atherosclerosis. Curr. Atheroscler. Rep. 2022, 24, 959–967. [Google Scholar] [CrossRef] [PubMed]
- Rosenson, R.S.; Burgess, L.J.; Ebenbichler, C.F.; Baum, S.J.; Stroes, E.S.G.; Ali, S.; Khilla, N.; Hamlin, R.; Pordy, R.; Dong, Y.; et al. Evinacumab in Patients with Refractory Hypercholesterolemia. N. Engl. J. Med. 2020, 383, 2307–2319. [Google Scholar] [CrossRef] [PubMed]
- Akoumianakis, I.; Zvintzou, E.; Kypreos, K.; Filippatos, T.D. ANGPTL3 and Apolipoprotein C-III as Novel Lipid-Lowering Targets. Curr. Atheroscler. Rep. 2021, 23, 20. [Google Scholar] [CrossRef]
- Ahmad, Z.; Pordy, R.; Rader, D.J.; Gaudet, D.; Ali, S.; Gonzaga-Jauregui, C.; Ponda, M.P.; Shumel, B.; Banerjee, P.; Dunbar, R.L. Inhibition of Angiopoietin-Like Protein 3 With Evinacumab in Subjects with High and Severe Hypertriglyceridemia. J. Am. Coll. Cardiol. 2021, 78, 193–195. [Google Scholar] [CrossRef]
- Burke, A.C.; Telford, D.E.; Huff, M.W. Bempedoic acid: Effects on lipoprotein metabolism and atherosclerosis. Curr. Opin. Lipidol. 2019, 30, 1–9. [Google Scholar] [CrossRef]
- Nicholls, S.; Lincoff, A.M.; Bays, H.E.; Cho, L.; Grobbee, D.E.; Kastelein, J.J.; Libby, P.; Moriarty, P.M.; Plutzky, J.; Ray, K.K.; et al. Rationale and design of the CLEAR-outcomes trial: Evaluating the effect of bempedoic acid on cardiovascular events in patients with statin intolerance. Am. Heart J. 2021, 235, 104–112. [Google Scholar] [CrossRef]
- Marrs, J.C.; Anderson, S.L. Bempedoic acid for the treatment of dyslipidemia. Drugs Context 2020, 9, 1–9. [Google Scholar] [CrossRef]
- Ballantyne, C.M.; Bays, H.; Catapano, A.L.; Goldberg, A.; Ray, K.K.; Saseen, J.J. Role of Bempedoic Acid in Clinical Practice. Cardiovasc. Drugs Ther. 2021, 35, 853–864. [Google Scholar] [CrossRef] [PubMed]
- Agarwala, A.; Goldberg, A.C. Bempedoic acid: A promising novel agent for LDL-C lowering. Futur. Cardiol. 2020, 16, 361–371. [Google Scholar] [CrossRef]
- National Institute for Health and Care Exellence. Icosapent Ethyl with Statin Therapy for Reducing the Risk of CardiovascularEvents in People with Raised Triglycerides. Technology Appraisal Guidance. Published: 13 July 2022. Available online: https://www.nice.org.uk/guidance/ta805 (accessed on 1 September 2023).
- European Medicines Agency. Vazkepa International Non-Proprietary Name: Icosapent Ethyl. Available online: https://www.ema.europa.eu/en/documents/assessment-report/vazkepa-epar-public-assessment-report_en.pdf (accessed on 1 September 2023).
- Nicholls, S.J. PCSK9 inhibitors and reduction in cardiovascular events: Current evidence and future perspectives. Kardiologia Polska 2023, 81, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Laffin, L.J.; Bruemmer, D.; Garcia, M.; Brennan, D.M.; McErlean, E.; Jacoby, D.S.; Michos, E.D.; Ridker, P.M.; Wang, T.Y.; Watson, K.E.; et al. Comparative Effects of Low-Dose Rosuvastatin, Placebo, and Dietary Supplements on Lipids and Inflammatory Biomarkers. J. Am. Coll. Cardiol. 2023, 81, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Scheen, A.J.; Wallemacq, C.; Lancellotti, P. Le médicament du mois. L’inclisiran (Leqvio®), hypocholestérolémiant puissant inhibant la synthèse de PCSK9 par la technique innovante de l’ARN interférent [Inclisiran (Leqvio®), a potent cholesterol-lowering agent by inhibiting PCSK9 using small interfering RNA-based innovative therapy]. Rev. Med. Liege 2022, 77, 745–751. [Google Scholar] [PubMed]
- Alshaer, W.; Zureigat, H.; Al Karaki, A.; Al-Kadash, A.; Gharaibeh, L.; Hatmal, M.M.; Aljabali, A.A.; Awidi, A. siRNA: Mechanism of action, challenges, and therapeutic approaches. Eur. J. Pharmacol. 2021, 905, 174178. [Google Scholar] [CrossRef]
- Zhang, M.M.; Bahal, R.; Rasmussen, T.P.; Manautou, J.E.; Zhong, X.B. The growth of siRNA-based therapeutics: Updated clinical studies. Biochem. Pharmacol. 2021, 189, 114432. [Google Scholar] [CrossRef]
- Agrawal, N.; Dasaradhi, P.V.N.; Mohmmed, A.; Malhotra, P.; Bhatnagar, R.K.; Mukherjee, S.K. RNA Interference: Biology, Mechanism, and Applications. Microbiol. Mol. Biol. Rev. 2003, 67, 657–685. [Google Scholar] [CrossRef]
- Springer, A.D.; Dowdy, S.F. GalNAc-siRNA Conjugates: Leading the Way for Delivery of RNAi Therapeutics. Nucleic Acid. Ther. 2018, 28, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Dec, A.; Niemiec, A.; Wojciechowska, E.; Maligłówka, M.; Bułdak, Ł.; Bołdys, A.; Okopień, B. Inclisiran—A Revolutionary Addition to a Cholesterol-Lowering Therapy. Int. J. Mol. Sci. 2023, 24, 6858. [Google Scholar] [CrossRef]
- Ranasinghe, P.; Addison, M.L.; Dear, J.W.; Webb, D.J. Small interfering RNA: Discovery, pharmacology and clinical development—An introductory review. Br. J. Pharmacol. 2022, 180, 2697–2720. [Google Scholar] [CrossRef] [PubMed]
- Padda, I.S.; Mahtani, A.U.; Parmar, M. Small Interfering RNA (siRNA) Therapy. 3 June 2023. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, January 2023. [Google Scholar]
- Feingold, K.R. Lipoprotein Apheresis. 2023 Feb 19. In Endotext [Internet]; Feingold, K.R., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., Hofland, J., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Yu, Y.; Chen, L.; Zhang, H.; Fu, Z.; Liu, Q.; Zhao, H.; Liu, Y.; Chen, Y. Association Between Familial Hypercholesterolemia and Risk of Cardiovascular Events and Death in Different Cohorts: A Meta-Analysis of 1.1 Million Subjects. Front. Cardiovasc. Med. 2022, 9, 860196. [Google Scholar] [CrossRef] [PubMed]
- Enas, E.A.; Kuruvila, A.; Khanna, P.; Pitchumoni, C.S.; Mohan, V. Benefits & risks of statin therapy for primary prevention of cardiovascular disease in Asian Indians—A population with the highest risk of premature coronary artery disease & diabetes. Indian J. Med. Res. 2013, 138, 461–491. [Google Scholar] [PubMed]
- Banach, M.; Kaźmierczak, J.; Mitkowski, P.; Wita, K.; Broncel, M.; Gąsior, M.; Gierlotka, M.; Gil, R.; Jankowski, P.; Niewada, M.; et al. Which patients at risk of cardiovascular disease might benefit the most from inclisiran? Polish experts’ opinion. The compromise between EBM and possibilities in healthcare. Arch. Med. Sci. 2022, 18, 569–576. [Google Scholar] [CrossRef]
- Ray, K.K.; Troquay, R.P.T.; Visseren, F.L.J.; Leiter, L.A.; Scott Wright, R.S.; Vikarunnessa, S.; Talloczy, Z.; Zang, X.; Maheux, P.; Lesogor, A.; et al. Long-term efficacy and safety of inclisiran in patients with high cardiovascular risk and elevated LDL cholesterol (ORION-3): Results from the 4-year open-label extension of the ORION-1 trial. Lancet Diabetes Endocrinol. 2023, 11, 109–119. [Google Scholar] [CrossRef]
- Ray, K.K.; Raal, F.J.; Kallend, D.G.; Jaros, M.J.; Koenig, W.; Leiter, L.A.; Landmesser, U.; Schwartz, G.G.; Lawrence, D.; Friedman, A.; et al. Inclisiran and cardiovascular events: A patient-level analysis of phase III trials. Eur. Heart J. 2023, 44, 129–138. [Google Scholar] [CrossRef]
- Katsiki, N.; Vrablik, M.; Banach, M.; Gouni-Berthold, I. Inclisiran, Low-Density Lipoprotein Cholesterol and Lipoprotein (a). Pharmaceuticals 2023, 16, 577. [Google Scholar] [CrossRef]
- Lakhin, A.V.; Tarantul, V.Z.; Gening, L.V. Aptamers: Problems, solutions and prospects. Acta Naturae 2013, 5, 34–43. [Google Scholar] [CrossRef]
- Klapak, D.; Broadfoot, S.; Penner, G.; Singh, A.; Inapuri, E. Development of novel aptamers for low-density lipoprotein particle quantification. PLoS ONE 2018, 13, e0205460. [Google Scholar] [CrossRef] [PubMed]
- Komarova, N.; Panova, O.; Titov, A.; Kuznetsov, A. Aptamers Targeting Cardiac Biomarkers as an Analytical Tool for the Diagnostics of Cardiovascular Diseases: A Review. Biomedicines 2022, 10, 1085. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.M.; Giacca, M. Small non-coding RNA therapeutics for cardiovascular disease. Eur. Heart J. 2022, 43, 4548–4561. [Google Scholar] [CrossRef] [PubMed]
- Kronenberg, F.; Mora, S.; Stroes, E.S.G.; Ference, B.A.; Arsenault, B.J.; Berglund, L.; Dweck, M.R.; Koschinsky, M.; Lambert, G.; Mach, F.; et al. Lipoprotein(a) in atherosclerotic cardiovascular disease and aortic stenosis: A European Atherosclerosis Society consensus statement. Eur. Heart J. 2022, 43, 3925–3946. [Google Scholar] [CrossRef] [PubMed]
- Karwatowska-Prokopczuk, E.; Lesogor, A.; Yan, J.-H.; Hurh, E.; Hoenlinger, A.; Margolskee, A.; Xia, S.; Tsimikas, S. Efficacy and safety of pelacarsen in lowering Lp(a) in healthy Japanese subjects. J. Clin. Lipidol. 2023, 17, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, S.J.; Nissen, S.E.; Fleming, C.; Urva, S.; Suico, J.; Berg, P.H.; Linnebjerg, H.; Ruotolo, G.; Turner, P.K.; Michael, L.F. Muvalaplin, an Oral Small Molecule Inhibitor of Lipoprotein(a) Formation: A Randomized Clinical Trial. JAMA 2023, 330, 1042–1053. [Google Scholar] [CrossRef]
- Virani, S.S.; Alonso, A.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics—2020 Update: A Report from the American Heart Association. Circulation 2020, 141, e139–e596. [Google Scholar] [CrossRef] [PubMed]
- Kaze, A.D.; Santhanam, P.; Erqou, S.; Bertoni, A.G.; Ahima, R.S.; Echouffo-Tcheugui, J.B. Microvascular disease and cardiovascular outcomes among individuals with type 2 diabetes. Diabetes Res. Clin. Pract. 2021, 176, 108859. [Google Scholar] [CrossRef]
- Teo, K.K.; Rafiq, T. Cardiovascular Risk Factors and Prevention: A Perspective from Developing Countries. Can. J. Cardiol. 2021, 37, 733–743. [Google Scholar] [CrossRef]
- Cheung, N.; Wang, J.J.; Klein, R.; Couper, D.J.; Sharrett, A.R.; Wong, T.Y. Diabetic Retinopathy and the Risk of Coronary Heart Disease. Diabetes Care 2007, 30, 1742–1746. [Google Scholar] [CrossRef]
- Verma, S.; Wanner, C.; Zwiener, I.; Ofstad, A.P.; George, J.T.; Fitchett, D.; Zinman, B. Influence of Microvascular Disease on Cardiovascular Events in Type 2 Diabetes. J. Am. Coll. Cardiol. 2019, 73, 2780–2782. [Google Scholar] [CrossRef] [PubMed]
- Henning, R.J.; Duntas, L.; Kolovou, G.; Ussher, J.R.; Sutendra, G.; Jaswal, J.S.; Tkáč, I.; Gotthardová, I.; Jamaluddin, J.L.; Huri, H.Z.; et al. Type-2 diabetes mellitus and cardiovascular disease. Futur. Cardiol. 2018, 14, 491–509. [Google Scholar] [CrossRef]
- Goldin, A.; Beckman, J.A.; Schmidt, A.M.; Creager, M.A. Advanced Glycation End Products: Sparking the Development of Diabetic Vascular Injury. Circulation 2006, 114, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Khalid, M.; Petroianu, G.; Adem, A. Advanced Glycation End Products and Diabetes Mellitus: Mechanisms and Perspectives. Biomolecules 2022, 12, 542. [Google Scholar] [CrossRef] [PubMed]
- Perrone, A.; Giovino, A.; Benny, J.; Martinelli, F. Advanced Glycation End Products (AGEs): Biochemistry, Signaling, Analytical Methods, and Epigenetic Effects. Oxidative Med. Cell. Longev. 2020, 2020, 3818196. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.; Heerspink, H.J.L.; Cuthbertson, D.J.; Wilding, J.P.H. SGLT2 inhibitors and GLP-1 receptor agonists: Established and emerging indications. Lancet 2021, 398, 262–276. [Google Scholar] [CrossRef] [PubMed]
- van der Aart-van der Beek, A.B.; de Boer, R.A.; Heerspink, H.J.L. Kidney and heart failure outcomes associated with SGLT2 inhibitor use. Nat. Rev. Nephrol. 2022, 18, 294–306. [Google Scholar] [CrossRef]
- Cowie, M.R.; Fisher, M. SGLT2 inhibitors: Mechanisms of cardiovascular benefit beyond glycaemic control. Nat. Rev. Cardiol. 2020, 17, 761–772. [Google Scholar] [CrossRef]
- Thorvaldsen, T.; Ferrannini, G.; Mellbin, L.; Benson, L.; Cosentino, F.; Mcmurray, J.J.; Dahlström, U.; Lund, L.H.; Savarese, G. Eligibility for Dapagliflozin and Empagliflozin in a Real-world Heart Failure Population. J. Card. Fail. 2022, 28, 1050–1062. [Google Scholar] [CrossRef]
- Wright, E.M. SGLT2 Inhibitors: Physiology and Pharmacology. Kidney360 2021, 2, 2027–2037. [Google Scholar] [CrossRef]
- Nauck, M.A.; Quast, D.R.; Wefers, J.; Pfeiffer, A.F.H. The evolving story of incretins (GIP and GLP-1) in metabolic and cardiovascular disease: A pathophysiological update. Diabetes, Obes. Metab. 2021, 23, 5–29. [Google Scholar] [CrossRef] [PubMed]
- Nauck, M.A.; Meier, J.J. Incretin hormones: Their role in health and disease. Diabetes Obes. Metab. 2018, 20 (Suppl. S1), 5–21. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wang, M.; Wen, Z.; Lu, Z.; Cui, L.; Fu, C.; Xue, H.; Liu, Y.; Zhang, Y. GLP-1 Receptor Agonists: Beyond Their Pancreatic Effects. Front. Endocrinol. 2021, 12, 721135. [Google Scholar] [CrossRef] [PubMed]
- Andrikou, E.; Tsioufis, C.; Andrikou, I.; Leontsinis, I.; Tousoulis, D.; Papanas, N. GLP-1 receptor agonists and cardiovascular outcome trials: An update. Hell. J. Cardiol. 2019, 60, 347–351. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, A.F.; Green, J.B.; Janmohamed, S.; D’Agostino, R.B.; Granger, C.B.; Jones, N.P.; Leiter, L.A.; Rosenberg, A.E.; Sigmon, K.N.; Somerville, M.C.; et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): A double-blind, randomised placebo-controlled trial. Lancet 2018, 392, 1519–1529. [Google Scholar] [CrossRef] [PubMed]
- Marso, S.P.; Bain, S.C.; Consoli, A.; Eliaschewitz, F.G.; Jódar, E.; Leiter, L.A.; Lingvay, I.; Rosenstock, J.; Seufert, J.; Warren, M.L.; et al. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 1834–1844. [Google Scholar] [CrossRef] [PubMed]
- Marso, S.P.; Daniels, G.H.; Brown-Frandsen, K.; Kristensen, P.; Mann, J.F.E.; Nauck, M.A.; Nissen, S.E.; Pocock, S.; Poulter, N.R.; Ravn, L.S.; et al. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 311–322. [Google Scholar] [CrossRef]
- Pedrosa, M.R.; Franco, D.R.; Gieremek, H.W.; Vidal, C.M.; Bronzeri, F.; Rocha, A.d.C.; Cara, L.G.d.C.; Fogo, S.L.; Eliaschewitz, F.G. GLP-1 Agonist to Treat Obesity and Prevent Cardiovascular Disease: What Have We Achieved so Far? Curr. Atheroscler. Rep. 2022, 24, 867–884. [Google Scholar] [CrossRef]
- Gouveia, M.; Xia, K.; Colón, W.; Vieira, S.I.; Ribeiro, F. Protein aggregation, cardiovascular diseases, and exercise training: Where do we stand? Ageing Res. Rev. 2017, 40, 1–10. [Google Scholar] [CrossRef]
- Brody, M.J.; Vanhoutte, D.; Bakshi, C.V.; Liu, R.; Correll, R.N.; Sargent, M.A.; Molkentin, J.D. Disruption of valosin-containing protein activity causes cardiomyopathy and reveals pleiotropic functions in cardiac homeostasis. J. Biol. Chem. 2019, 294, 8918–8929. [Google Scholar] [CrossRef]
- Sun, X.; Qiu, H. Valosin-Containing Protein, a Calcium-Associated ATPase Protein, in Endoplasmic Reticulum and Mitochondrial Function and Its Implications for Diseases. Int. J. Mol. Sci. 2020, 21, 3842. [Google Scholar] [CrossRef] [PubMed]
- Lejay, A.; Fang, F.; John, R.; Van, J.A.D.; Barr, M.; Thaveau, F.; Chakfe, N.; Geny, B.; Scholey, J.W. Ischemia reperfusion injury, ischemic conditioning and diabetes mellitus. J. Mol. Cell. Cardiol. 2015, 91, 11–22. [Google Scholar] [CrossRef]
- Hausenloy, D.J.; Yellon, D.M. Myocardial ischemia-reperfusion injury: A neglected therapeutic target. J. Clin. Investig. 2013, 123, 92–100. [Google Scholar] [CrossRef]
- Eltzschig, H.K.; Eckle, T. Primary cilia can both mediate and suppress Hedgehog pathway-dependent tumorigenesis. Nat. Med. 2009, 15, 1055–1061. [Google Scholar] [CrossRef]
- Donato, M.; Evelson, P.; Gelpi, R.J. Protecting the heart from ischemia/reperfusion injury. Curr. Opin. Cardiol. 2017, 32, 784–790. [Google Scholar] [CrossRef]
- Bolli, R. Cardioprotective Function of Inducible Nitric Oxide Synthase and Role of Nitric Oxide in Myocardial Ischemia and Preconditioning: An Overview of a Decade of Research. J. Mol. Cell. Cardiol. 2001, 33, 1897–1918. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Ge, L.; Niu, L.; Lian, X.; Ma, H.; Pang, L. The Dual Role of Inducible Nitric Oxide Synthase in Myocardial Ischemia/Reperfusion Injury: Friend or Foe? Oxidative Med. Cell. Longev. 2018, 2018, 1–7. [Google Scholar] [CrossRef]
- Lizano, P.; Rashed, E.; Stoll, S.; Zhou, N.; Wen, H.; Hays, T.T.; Qin, G.; Xie, L.-H.; Depre, C.; Qiu, H. The valosin-containing protein is a novel mediator of mitochondrial respiration and cell survival in the heart in vivo. Sci. Rep. 2017, 7, 46324. [Google Scholar] [CrossRef]
- Lizano, P.; Rashed, E.; Kang, H.; Dai, H.; Sui, X.; Yan, L.; Qiu, H.; Depre, C. The valosin-containing protein promotes cardiac survival through the inducible isoform of nitric oxide synthase. Cardiovasc. Res. 2013, 99, 685–693. [Google Scholar] [CrossRef]
- Mailleux, F.; Beauloye, C.; Balligand, J.-L.; Horman, S.; Bertrand, L. Studying the role of AMPK in cardiac hypertrophy and protein synthesis. Methods Mol. Biol. 2018, 1732, 321–342. [Google Scholar] [CrossRef]
- Sluijter, J.P.G.; Davidson, S.M.; Boulanger, C.M.; Buzás, E.I.; De Kleijn, D.P.V.; Engel, F.B.; Giricz, Z.; Hausenloy, D.J.; Kishore, R.; Lecour, S.; et al. Extracellular vesicles in diagnostics and therapy of the ischaemic heart: Position Paper from the Working Group on Cellular Biology of the Heart of the European Society of Cardiology. Cardiovasc. Res. 2018, 114, 19–34. [Google Scholar] [CrossRef]
- Lawson, C.; Vicencio, J.M.; Yellon, D.M.; Davidson, S.M. Microvesicles and exo somes: New players in metabolic and cardiovascular disease. J. Endocrinol. 2016, 228, R57–R71. [Google Scholar] [CrossRef] [PubMed]
- Kuwabara, Y.; Ono, K.; Horie, T.; Nishi, H.; Nagao, K.; Kinoshita, M.; Watanabe, S.; Baba, O.; Kojima, Y.; Shizuta, S.; et al. Increased MicroRNA-1 and MicroRNA-133a Levels in Serum of Patients with Cardiovascular Disease Indicate Myocardial Damage. Circ. Cardiovasc. Genet. 2011, 4, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Cheow, E.S.H.; Cheng, W.C.; Lee, C.N.; de Kleijn, D.; Sorokin, V.; Sze, S.K. Plasma-derived Extracellular Vesicles Contain Predictive Biomarkers and Potential Therapeutic Targets for Myocardial Ischemic (MI) Injury. Mol. Cell. Proteom. 2016, 15, 2628–2640. [Google Scholar] [CrossRef] [PubMed]
- Vegter, E.L.; van der Meer, P.; de Windt, L.J.; Pinto, Y.M.; Voors, A.A. MicroRNAs in heart failure: From biomarker to target for therapy. Eur. J. Heart Fail. 2016, 18, 457–468. [Google Scholar] [CrossRef] [PubMed]
- Fleissner, F.; Goerzig, Y.; Haverich, A.; Thum, T. Microvesicles as novel bio markers and therapeutic targets in transplantation medicine. Am. J. Transplant. 2012, 12, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Gao, L.; Zimmerman, M.C.; Zucker, I.H. Myocardial infarc tion-induced microRNA-enriched exosomes contribute to cardiac Nrf2 dysregulation in chronic heart failure. Am. J. Physiol. Heart Circ. Physiol. 2018, 314, 928–939. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Knowlton, A.A. HSP60 trafficking in adult cardiac myocytes: Role of the exosomal pathway. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, H3052–H3056. [Google Scholar] [CrossRef]
- Goren, Y.; Kushnir, M.; Zafrir, B.; Tabak, S.; Lewis, B.S.; Amir, O. Serum levels of microRNAs in patients with heart failure. Eur. J. Heart Fail. 2012, 14, 147–154. [Google Scholar] [CrossRef]
- Creemers, E.E.; Tijsen, A.J.; Pinto, Y.M. Circulating microRNAs: Novel biomarkers and extracellular communicators in cardiovascular disease? Circ. Res. 2012, 110, 483–495. [Google Scholar] [CrossRef]
- Duan, P.; Tan, J.; Miao, Y.; Zhang, Q. Potential role of exosomes in the pathophysiology, diagnosis, and treatment of hypoxic diseases. Am. J. Transl. Res. 2019, 11, 1184–1201. [Google Scholar] [PubMed]
- Heallen, T.R.; Kadow, Z.A.; Kim, J.H.; Wang, J.; Martin, J.F. Stimulating Cardiogenesis as a Treatment for Heart Failure. Circ. Res. 2019, 124, 1647–1657. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.-N.; Cores, J.; Huang, K.; Cui, X.-L.; Luo, L.; Zhang, J.-Y.; Li, T.-S.; Qian, L.; Cheng, K. Concise Review: Is Cardiac Cell Therapy Dead? Embarrassing Trial Outcomes and New Directions for the Future. STEM CELLS Transl. Med. 2018, 7, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Adamiak, M.; Sahoo, S. Exosomes in Myocardial Repair: Advances and Challenges in the Development of Next-Generation Therapeutics. Mol. Ther. 2018, 26, 1635–1643. [Google Scholar] [CrossRef] [PubMed]
- Singla, D.K. Stem cells and exosomes in cardiac repair. Curr. Opin. Pharmacol. 2016, 27, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Lamichhane, T.N.; Sokic, S.; Schardt, J.S.; Raiker, R.S.; Lin, J.W.; Jay, S.M. Emerging roles for extra cellular vesicles in tissue engineering and regenerative medicine. Tissue Eng. Part B Rev. 2015, 21, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Bjørge, I.M.; Kim, S.Y.; Mano, J.F.; Kalionis, B.; Chrzanowski, W. Extracellular vesicles, exosomes and shedding vesicles in regenerative medicine—A new paradigm for tissue repair. Biomater. Sci. 2017, 6, 60–78. [Google Scholar] [CrossRef]
- Zhu, L.-P.; Tian, T.; Wang, J.-Y.; He, J.-N.; Chen, T.; Pan, M.; Xu, L.; Zhang, H.-X.; Qiu, X.-T.; Li, C.-C.; et al. Hypoxia-elicited mesenchymal stem cell-derived exosomes facilitates cardiac repair through miR-125b-mediated prevention of cell death in myocardial infarction. Theranostics 2018, 8, 6163–6177. [Google Scholar] [CrossRef]
- Lai, R.C.; Arslan, F.; Lee, M.M.; Sze, N.S.K.; Choo, A.; Chen, T.S.; Salto-Tellez, M.; Timmers, L.; Lee, C.N.; El Oakley, R.M.; et al. Exosome secreted by MSC re duces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010, 4, 214–222. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wojtasińska, A.; Kućmierz, J.; Tokarek, J.; Dybiec, J.; Rodzeń, A.; Młynarska, E.; Rysz, J.; Franczyk, B. New Insights into Cardiovascular Diseases Treatment Based on Molecular Targets. Int. J. Mol. Sci. 2023, 24, 16735. https://doi.org/10.3390/ijms242316735
Wojtasińska A, Kućmierz J, Tokarek J, Dybiec J, Rodzeń A, Młynarska E, Rysz J, Franczyk B. New Insights into Cardiovascular Diseases Treatment Based on Molecular Targets. International Journal of Molecular Sciences. 2023; 24(23):16735. https://doi.org/10.3390/ijms242316735
Chicago/Turabian StyleWojtasińska, Armanda, Joanna Kućmierz, Julita Tokarek, Jill Dybiec, Anna Rodzeń, Ewelina Młynarska, Jacek Rysz, and Beata Franczyk. 2023. "New Insights into Cardiovascular Diseases Treatment Based on Molecular Targets" International Journal of Molecular Sciences 24, no. 23: 16735. https://doi.org/10.3390/ijms242316735




