Post-Implantation Inflammatory Responses to Xenogeneic Tissue-Engineered Cartilage Implanted in Rabbit Trachea: The Role of Cultured Chondrocytes in the Modification of Inflammation
Abstract
:1. Introduction
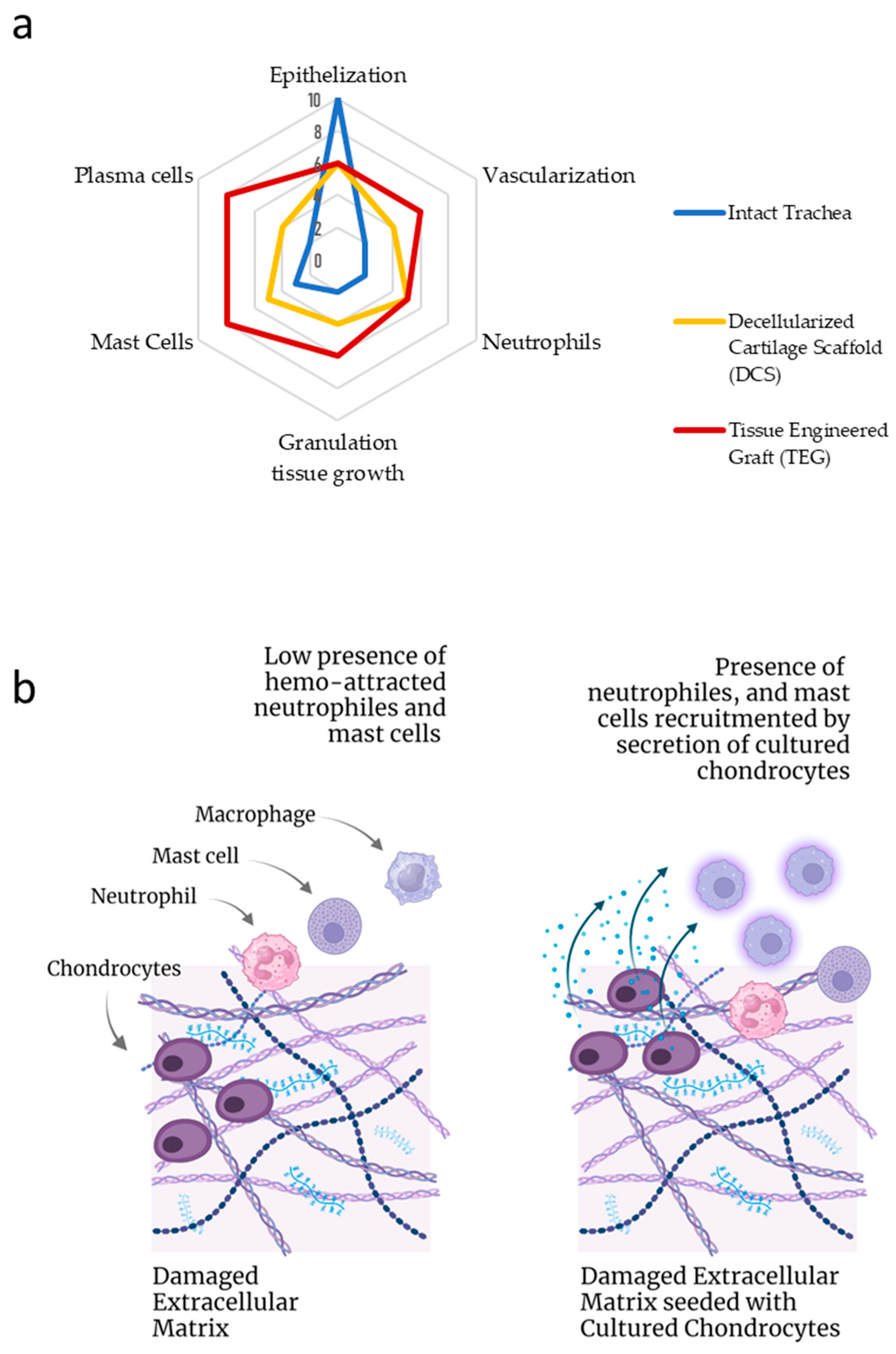
2. Results
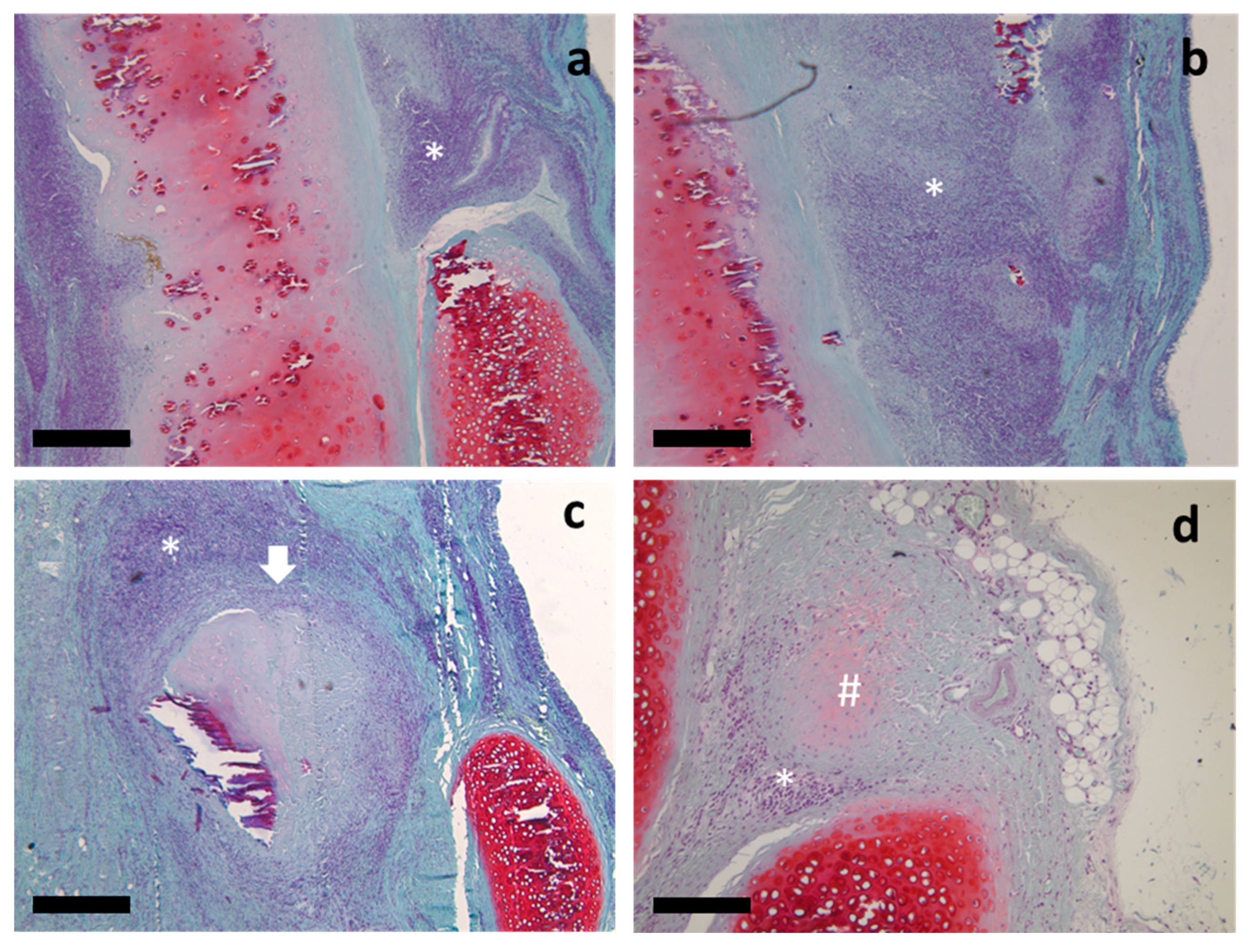
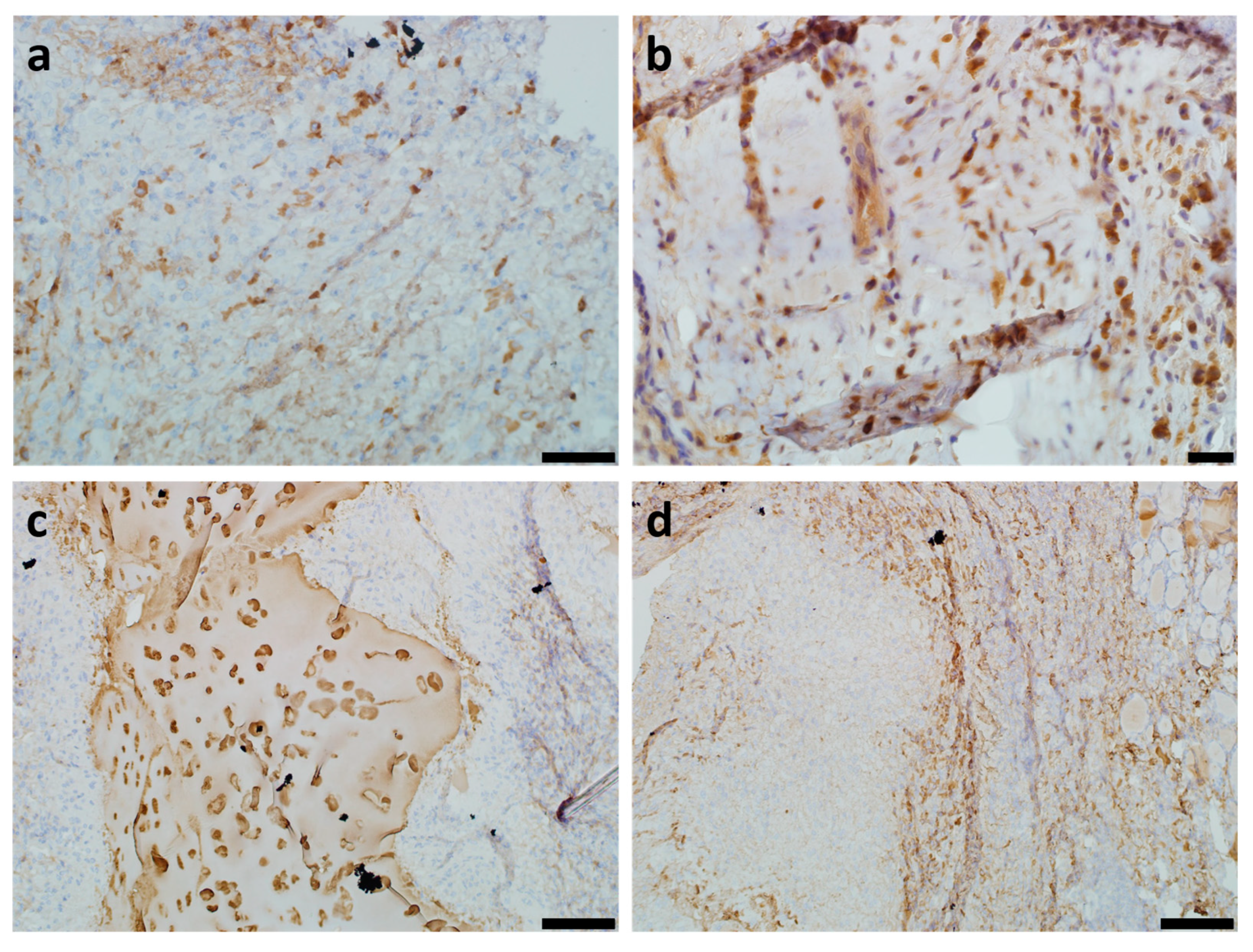
2.1. Histological Study
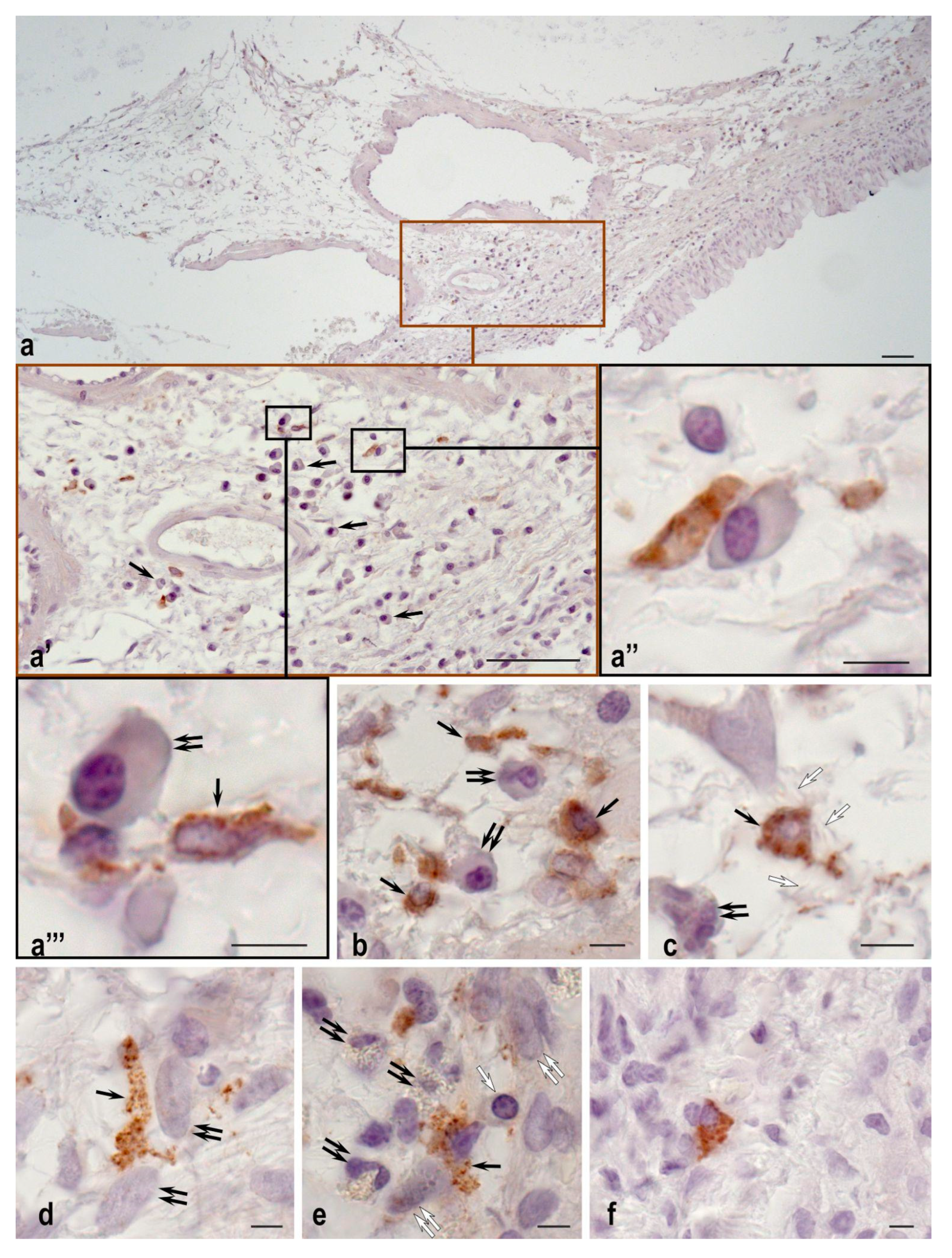
2.2. Plasma Cells Study
2.3. Mast Cell Immunohistochemical Study
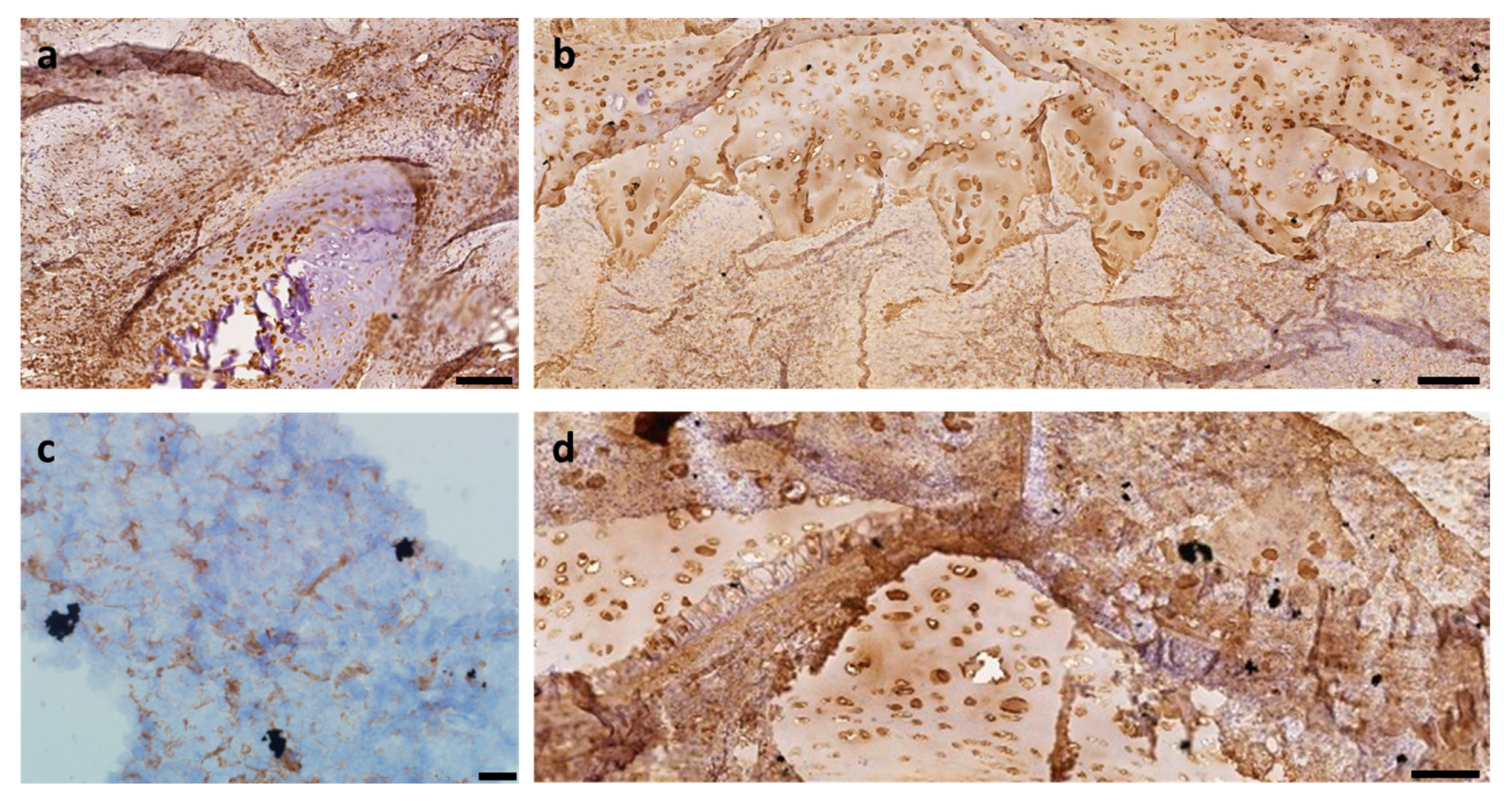
2.4. Vimentin Immunohistochemical Study
2.5. Collagen Type II Immunohistochemical Study
2.6. CD34+ Immunohistochemical Study
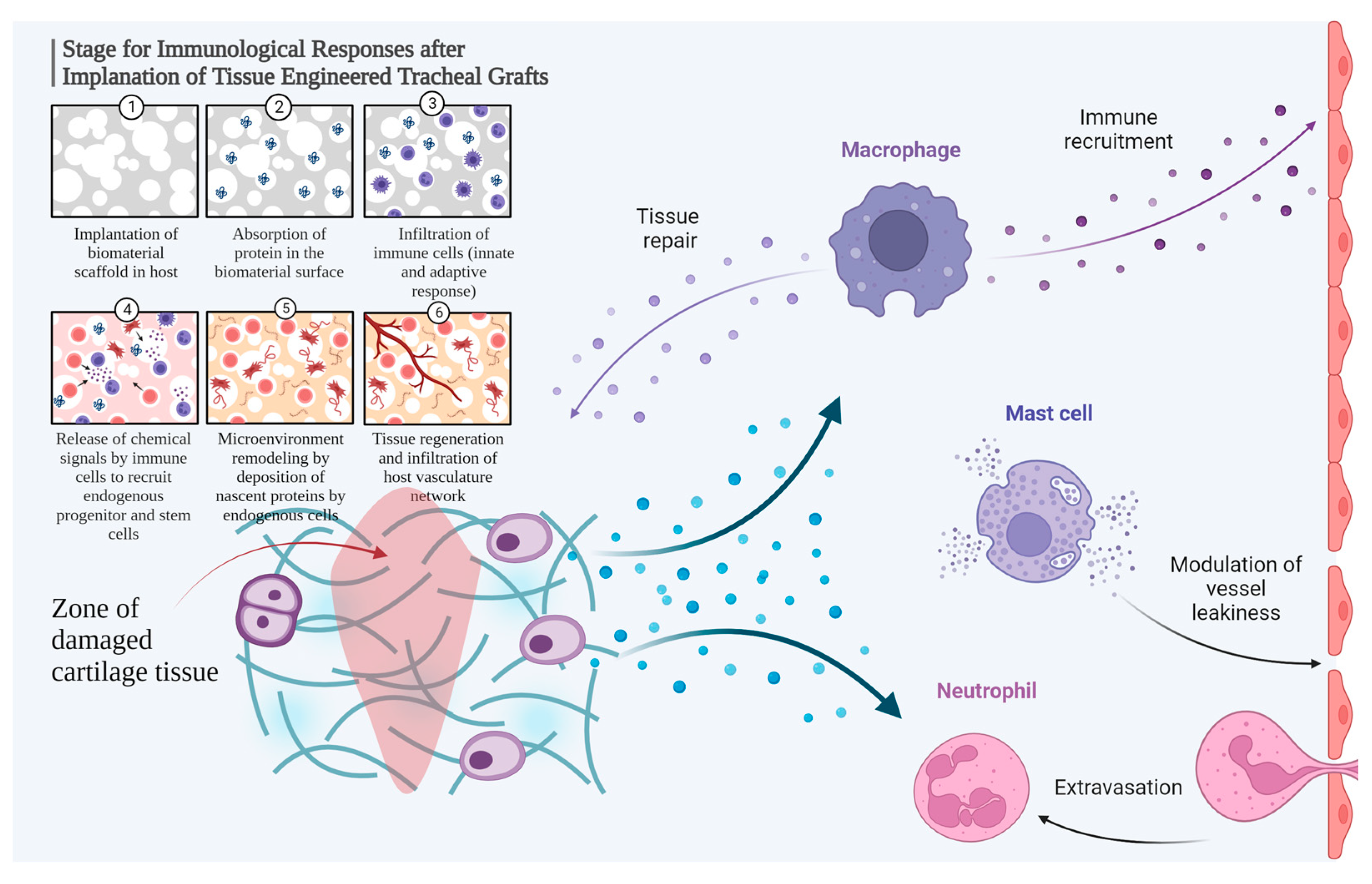
3. Discussion
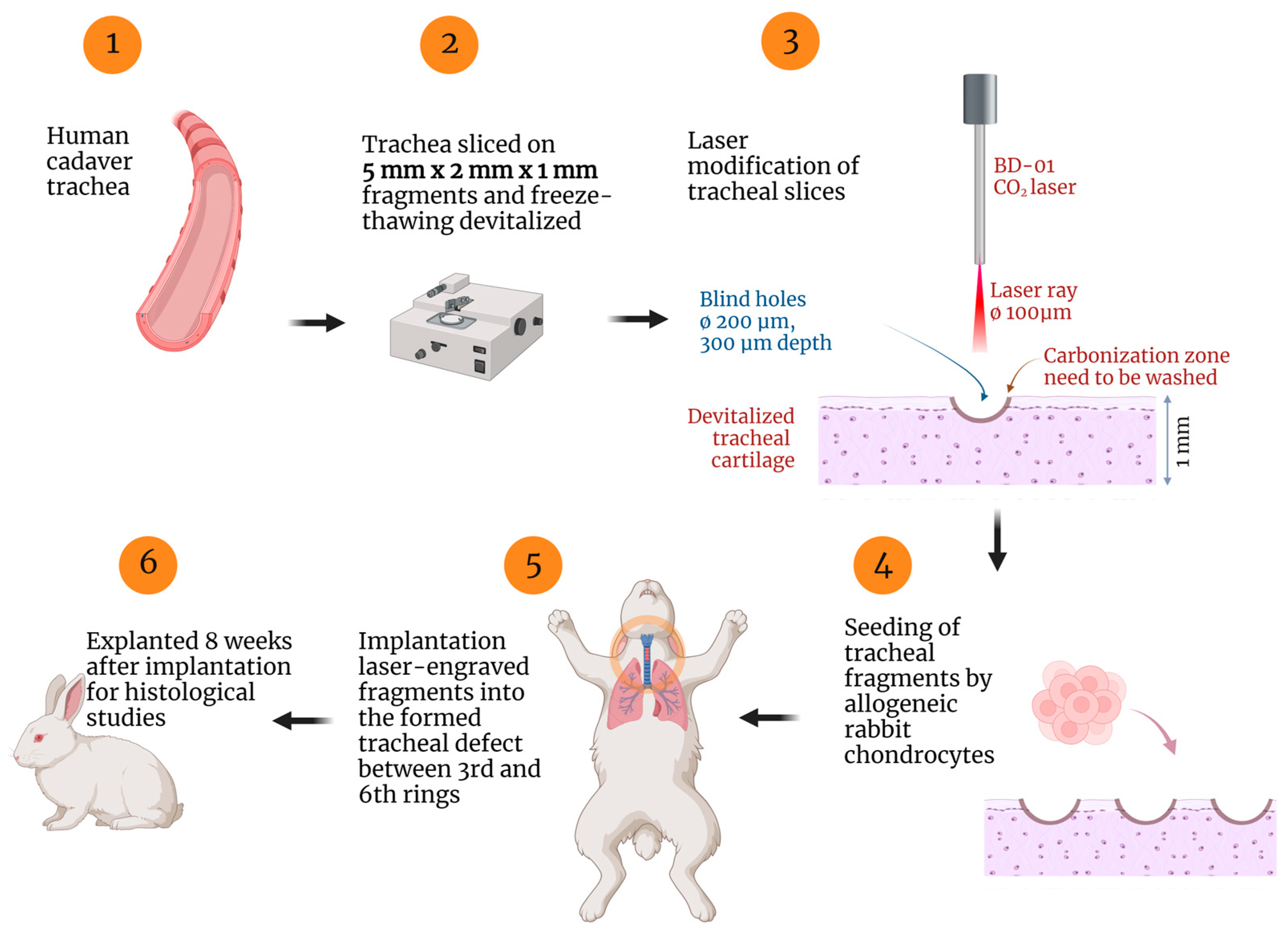
4. Materials and Methods
4.1. Samples Preparation
4.2. Histological and Immunohistochemical Studies
4.3. Microscopy
4.4. Statistics
4.5. Ethics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ochando, J.; Charron, D.; Baptista, P.M.; Uygun, B.E. Immune responses to bioengineered organs. Curr. Opin. Organ Transplant. 2017, 22, 79. [Google Scholar] [CrossRef]
- Dondossola, E.; Friedl, P. Host responses to implants revealed by intravital microscopy. Nat. Rev. Mater. 2022, 7, 6–22. [Google Scholar] [CrossRef]
- Lei, C.; Mei, S.; Zhou, C.; Xia, C. Decellularized tracheal scaffolds in tracheal reconstruction: An evaluation of different techniques. J. Appl. Biomater. Funct. Mater. 2021, 19, 22808000211064948. [Google Scholar] [CrossRef] [PubMed]
- Parmaksiz, M.; Dogan, A.; Odabas, S.; Elçin, A.E.; Elçin, Y.M. Clinical applications of decellularized extracellular matrices for tissue engineering and regenerative medicine. Biomed. Mater. 2016, 11, 022003. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Dang, H.; Xu, Y. Recent advancement of decellularization extracellular matrix for tissue engineering and biomedical application. Artif. Organs 2022, 46, 549–567. [Google Scholar] [CrossRef] [PubMed]
- Meier, R.P.H.; Muller, Y.D.; Balaphas, A.; Morel, P.; Pascual, M.; Seebach, J.D.; Buhler, L.H. Xenotransplantation: Back to the future? Transpl. Int. 2018, 31, 465–477. [Google Scholar] [CrossRef] [PubMed]
- Porzionato, A.; Stocco, E.; Barbon, S.; Grandi, F.; Macchi, V.; De Caro, R. Tissue-engineered grafts from human decellularized extracellular matrices: A systematic review and future perspectives. Int. J. Mol. Sci. 2018, 19, 4117. [Google Scholar] [CrossRef] [PubMed]
- Pippi, R. Post-surgical clinical monitoring of soft tissue wound healing in periodontal and implant surgery. Int. J. Med. Sci. 2017, 14, 721–728. [Google Scholar] [CrossRef]
- Kasravi, M.; Ahmadi, A.; Babajani, A.; Mazloomnejad, R.; Hatamnejad, M.R.; Shariatzadeh, S.; Bahrami, S.; Niknejad, H. Immunogenicity of decellularized extracellular matrix scaffolds: A bottleneck in tissue engineering and regenerative medicine. Biomater. Res. 2023, 27, 10. [Google Scholar] [CrossRef]
- Li, J.; Hara, H.; Wang, Y.; Esmon, C.; Cooper, D.K.; Iwase, H. Evidence for the important role of inflammation in xenotransplantation. J. Inflamm. 2019, 16, 10. [Google Scholar] [CrossRef]
- Stone, K.R.; Walgenbach, A.W.; Abrams, J.T.; Nelson, J.; Gillett, N.; Galili, U. Porcine and bovine cartilage transplants in cynomolgus monkey: I. A model for chronic xenograft rejection. Transplantation 1997, 63, 640–645. [Google Scholar] [CrossRef] [PubMed]
- Stahl, E.C.; Bonvillain, R.W.; Skillen, C.D.; Burger, B.L.; Hara, H.; Lee, W.; Trygg, C.B.; Didier, P.J.; Grasperge, B.F.; Pashos, N.C.; et al. Evaluation of the host immune response to decellularized lung scaffolds derived from α-Gal knockout pigs in a non-human primate model. Biomaterials 2018, 187, 93–104. [Google Scholar] [CrossRef]
- Li, M.; Yin, H.; Yan, Z.; Li, H.; Wu, J.; Wang, Y.; Wei, F.; Tian, G.; Ning, C.; Li, H.; et al. The immune microenvironment in cartilage injury and repair. Acta Biomater. 2022, 140, 23–42. [Google Scholar] [CrossRef] [PubMed]
- Henderson, B.; Pettipher, E.R. Arthritogenic actions of recombinant IL-1 and tumour necrosis factor alpha in the rabbit: Evidence for synergistic interactions between cytokines in vivo. Clin. Exp. Immunol. 1989, 75, 306. [Google Scholar]
- Daghestani, H.N.; Pieper, C.F.; Kraus, V.B. Soluble macrophage biomarkers indicate inflammatory phenotypes in patients with knee osteoarthritis. Arthritis Rheumatol. 2015, 67, 956–965. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Mi, B.B.; Lin, Z.; Hu, Y.Q.; Yu, L.; Zha, K.K.; Panayi, A.C.; Yu, T.; Chen, L.; Liu, Z.-P.; et al. The role of the immune microenvironment in bone, cartilage, and soft tissue regeneration: From mechanism to therapeutic opportunity. Mil Med Res. 2021, 9, 65. [Google Scholar] [CrossRef]
- Ozpinar, E.W.; Frey, A.L.; Cruse, G.; Freytes, D.O. Mast cell–biomaterial interactions and tissue repair. Tissue Eng. Part B Rev. 2021, 27, 590–603. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.; Liu, L.; Rung, S.; Wang, Y.; Ma, Y.; Hu, C.; Zhao, X.; Man, Y.; Qu, Y. Modulation of foreign body reaction and macrophage phenotypes concerning microenvironment. J. Biomed. Mater. Res. Part A 2020, 108, 127–135. [Google Scholar] [CrossRef]
- Popa, E.R.; Harmsen, M.C.; Tio, R.A.; van der Strate, B.W.; Brouwer, L.A.; Schipper, M.; Koerts, J.; De Jongste, M.J.L.; Hazenberg, A.; Hendriks, M.; et al. Circulating CD34+ progenitor cells modulate host angiogenesis and inflammation in vivo. J. Mol. Cell. Cardiol. 2006, 41, 86–96. [Google Scholar] [CrossRef]
- Pu, X.; Zhu, P.; Zhou, X.; He, Y.; Wu, H.; Du, L.; Gong, H.; Sun, X.; Chen, T.; Zhu, J.; et al. CD34+ cell atlas of main organs implicates its impact on fibrosis. Cell. Mol. Life Sci. 2022, 79, 576. [Google Scholar] [CrossRef]
- Marsano, A.; Medeiros da Cunha, C.M.; Ghanaati, S.; Gueven, S.; Centola, M.; Tsaryk, R.; Barbeck, M.; Stuedle, C.; Barbero, A.; Helmrich, U.; et al. Spontaneous in vivo chondrogenesis of bone marrow-derived mesenchymal progenitor cells by blocking vascular endothelial growth factor signaling. Stem Cells Transl. Med. 2016, 5, 1730–1738. [Google Scholar] [CrossRef]
- Stiers, P.J.; Stegen, S.; van Gastel, N.; Van Looveren, R.; Torrekens, S.; Carmeliet, G. Inhibition of the Oxygen Sensor PHD2 Enhances Tissue-Engineered Endochondral Bone Formation. J. Bone Miner. Res. 2019, 34, 333–348. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.S.; Luo, W.; Zhu, S.A.; Lei, G.H. T cells in osteoarthritis: Alterations and beyond. Front. Immunol. 2017, 8, 356. [Google Scholar] [CrossRef]
- Shestakova, V.A.; Klabukov, I.D.; Baranovskii, D.S.; Shegay, P.V.; Kaprin, A.D. Assessment of Immunological Responses—A Novel Challenge in Tissue Engineering and Regenerative Medicine. Biomed. Res. Ther. 2022, 9, 5384–5386. [Google Scholar] [CrossRef]
- Asanuma, K.; Nakamura, T.; Iino, T.; Hagi, T.; Sudo, A. Macrophages and vimentin in tissues adjacent to megaprostheses and mesh in reconstructive surgeries. Commun. Integr. Biol. 2022, 15, 168–181. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Shen, Y.; Mohanasundaram, P.; Lindström, M.; Ivaska, J.; Ny, T.; Eriksson, J.E. Vimentin coordinates fibroblast proliferation and keratinocyte differentiation in wound healing via tgf-β-slug signaling. Proc. Natl. Acad. Sci. USA 2016, 113, E4320–E4327. [Google Scholar] [CrossRef] [PubMed]
- Ostrowska-Podhorodecka, Z.; McCulloch, C.A. Vimentin regulates the assembly and function of matrix adhesions. Wound Repair Regen. 2021, 29, 602–612. [Google Scholar] [CrossRef]
- Walker, J.L.; Bleaken, B.M.; Romisher, A.R.; Alnwibit, A.A.; Menko, A.S. In wound repair vimentin mediates the transition of mesenchymal leader cells to a myofibroblast phenotype. Mol. Biol. Cell 2018, 29, 1555–1570. [Google Scholar] [CrossRef]
- Hisanaga, M.; Tsuchiya, T.; Watanabe, H.; Shimoyama, K.; Iwatake, M.; Tanoue, Y.; Maruyama, K.; Yukawa, H.; Sato, K.; Kato, Y.; et al. Adipose-Derived Mesenchymal Stem Cells Attenuate Immune Reactions against Pig Decellularized Bronchi Engrafted into Rat Tracheal Defects. Organogenesis 2023, 19, 2212582. [Google Scholar] [CrossRef]
- Nürnberger, S.; Schneider, C.; Keibl, C.; Schädl, B.; Heimel, P.; Monforte, X.; Teuschl, A.H.; Nalbach, M.; Thurner, P.J.; Grillari, J.; et al. Repopulation of decellularised articular cartilage by laser-based matrix engraving. EBioMedicine 2021, 64, 103196. [Google Scholar] [CrossRef]
- Lossi, L.; D’Angelo, L.; De Girolamo, P.; Merighi, A. Anatomical features for an adequate choice of experimental animal model in biomedicine: II. Small laboratory rodents, rabbit, and pig. Ann. Anat. Anat. Anz. 2016, 204, 11–28. [Google Scholar] [CrossRef]
- Kajbafzadeh, A.M.; Amanpoor, S.; Golestani, M.; Vejdani, K. Human bladder acellular matrix for augmentation cystoplasty in the rabbit. BJU Int. 2004, 93, S75. [Google Scholar] [CrossRef]
- Menon, N.G.; Rodriguez, E.D.; Byrnes, C.K.; Girotto, J.A.; Goldberg, N.H.; Silverman, R.P. Revascularization of human acellular dermis in full-thickness abdominal wall reconstruction in the rabbit model. Ann. Plast. Surg. 2003, 50, 523–527. [Google Scholar] [CrossRef] [PubMed]
- Dina, E.F.; Nashwa, R.K.; Nemr, W.A. Histologic Evaluations of Xenotransplanted Rabbit Knees by In Vitro-Propagated Human Amniotic Epithelial Cells: A Preclinical Study. Exp. Clin. Transplant. 2020, 18, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Ayşan, E.; Yiğit Düzköylü, İ.C.; Büyükpınarbaşılı, N. Xenotransplantation of human cryopreserved parathyroid tissue isolated from parathyroid adenomas to normocalcemic rabbits. Turk. J. Surg. 2017, 33, 91. [Google Scholar] [CrossRef] [PubMed]
- Porzionato, A.; Sfriso, M.M.; Pontini, A.; Macchi, V.; Petrelli, L.; Pavan, P.G.; Natali, A.N.; Bassetto, F.; Vindigni, V.; De Caro, R. Decellularized Human Skeletal Muscle as Biologic Scaffold for Reconstructive Surgery. Int. J. Mol. Sci. 2015, 16, 14808–14831. [Google Scholar] [CrossRef]
- Porzionato, A.; Sfriso, M.M.; Pontini, A.; Macchi, V.; Buompensiere, M.I.; Petrelli, L.; Bassetto, F.; Vindigni, V.; De Caro, R. Development of small-diameter vascular grafts through Decellularization of human blood vessels. J. Biomater. Tissue Eng. 2017, 7, 101–110. [Google Scholar] [CrossRef]
- Balyasin, M.V.; Baranovsky, D.S.; Demchenko, A.G.; Fayzullin, A.L.; Krasilnikova, O.A.; Klabukov, I.D.; Krasheninnikov, M.E.; Lyundup, A.V.; Parshin, V.D. Experimental orthotopic implantation of tissue-engineered tracheal graft created based on devitalized scaffold seeded with mesenchymal and epithelial cells. Vestnik Transplantologii i Iskusstvennykh Organov 2019, 21, 96–107. [Google Scholar] [CrossRef]
- Baranovskii, D.; Demner, J.; Nürnberger, S.; Lyundup, A.; Red, H.; Hilpert, M.; Pigeot, S.; Krasheninnikov, M.; Krasilnikova, O.; Klabukov, I.; et al. Engineering of Tracheal Grafts Based on Recellularization of Laser-Engraved Human Airway Cartilage Substrates. Cartilage 2022, 13, 19476035221075951. [Google Scholar] [CrossRef]
- Butterworth, K.T. Evolution of the supermodel: Progress in modelling radiotherapy response in mice. Clin. Oncol. 2019, 31, 272–282. [Google Scholar] [CrossRef]
- Tsumura, R.; Koga, Y.; Hamada, A.; Kuwata, T.; Sasaki, H.; Doi, T.; Aikawa, K.; Ohashi, A.; Katano, I.; Ikarashi, Y.; et al. Report of the use of patient-derived xenograft models in the development of anticancer drugs in Japan. Cancer Sci. 2020, 111, 3386. [Google Scholar] [CrossRef]
- Derman, I.D.; Singh, Y.P.; Saini, S.; Nagamine, M.; Banerjee, D.; Ozbolat, I.T. Bioengineering and Clinical Translation of Human Lung and its Components. Adv. Biol. 2023, 7, 2200267. [Google Scholar] [CrossRef]
- Baranovskii, D.S.; Klabukov, I.D.; Arguchinskaya, N.V.; Yakimova, A.O.; Kisel, A.A.; Yatsenko, E.M.; Ivanov, S.A.; Shegay, P.V.; Kaprin, A.D. Adverse events, side effects and complications in mesenchymal stromal cell-based therapies. Stem Cell Investig. 2022, 9, 7. [Google Scholar] [CrossRef]
- Kantaros, A. 3D Printing in Regenerative Medicine: Technologies and Resources Utilized. Int. J. Mol. Sci. 2022, 23, 14621. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Cendal, A.I.; Gómez-Seoane, I.; de Toro-Santos, F.J.; Fuentes-Boquete, I.M.; Señarís-Rodríguez, J.; Díaz-Prado, S.M. Biomedical Applications of the Biopolymer Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV): Drug Encapsulation and Scaffold Fabrication. Int. J. Mol. Sci. 2023, 24, 11674. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Lindsay, S.; Gurbaxani, S.; Crawford, A.; Claeyssens, F. Elastomeric Porous Poly(glycerol sebacate) Methacrylate (PGSm) Microspheres as 3D Scaffolds for Chondrocyte Culture and Cartilage Tissue Engineering. Int. J. Mol. Sci. 2023, 24, 10445. [Google Scholar] [CrossRef]
- Klabukov, I.; Balyasin, M.; Krasilnikova, O.; Tenchurin, T.; Titov, A.; Krasheninnikov, M.; Mudryak, D.; Sulina, Y.; Shepelev, A.; Chvalun, S.; et al. Angiogenic Modification of Microfibrous Polycaprolactone by pCMV-VEGF165 Plasmid Promotes Local Vascular Growth after Implantation in Rats. Int. J. Mol. Sci. 2023, 24, 1399. [Google Scholar] [CrossRef] [PubMed]
- Pina, S.; Ribeiro, V.P.; Marques, C.F.; Maia, F.R.; Silva, T.H.; Reis, R.L.; Oliveira, J.M. Scaffolding strategies for tissue engineering and regenerative medicine applications. Materials 2019, 12, 1824. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.; Lu, Y.; Li, J.; Shi, H. The biological properties of the decellularized tracheal scaffolds and 3D printing biomimetic materials: A comparative study. J. Biomed. Mater. Res. Part A 2022, 110, 1062–1076. [Google Scholar] [CrossRef]
- Das, P.; Mishra, R.; Devi, B.K.; Rajesh, K.; Basak, P.; Roy, M.; Roy, P.; Lahiri, D.; Nandi, S.K. Decellularized xenogenic cartilage extracellular matrix (ECM) scaffolds for the reconstruction of osteochondral defects in rabbits. J. Mater. Chem. B 2021, 9, 4873–4894. [Google Scholar] [CrossRef]
- Henn, D.; Chen, K.; Fehlmann, T.; Trotsyuk, A.A.; Sivaraj, D.; Maan, Z.N.; Bonham, C.A.; Barrera, J.A.; Mays, C.J.; Greco, A.H.; et al. Xenogeneic skin transplantation promotes angiogenesis and tissue regeneration through activated Trem2+ macrophages. Sci. Adv. 2021, 7, eabi4528. [Google Scholar] [CrossRef] [PubMed]
- Pelttari, K.; Mumme, M.; Barbero, A.; Martin, I. Nasal chondrocytes as a neural crest-derived cell source for regenerative medicine. Curr. Opin. Biotechnol. 2017, 9, 4873–4894. [Google Scholar] [CrossRef] [PubMed]
- Willers, C.; Chen, J.; Wood, D.; Xu, J.; Zheng, M.H. Autologous chondrocyte implantation with collagen bioscaffold for the treatment of osteochondral defects in rabbits. Tissue Eng. 2005, 11, 1065–1076. [Google Scholar] [CrossRef] [PubMed]
- Doss, F.; Menard, J.; Hauschild, M.; Kreutzer, H.J.; Mittlmeier, T.; Müller-Steinhardt, M.; Müller, B. Elevated IL-6 levels in the synovial fluid of osteoarthritis patients stem from plasma cells. Scand. J. Rheumatol. 2007, 36, 136–139. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, R.; Das, P.; Joardar, S.N.; Biswas, B.K.; Batabyal, S.; Das, P.K.; Nandi, S.K. Novel decellularized animal conchal cartilage graft for application in human patient. J. Tissue Eng. Regen. Med. 2019, 13, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Klabukov, I.D.; Krasilnikova, O.A.; Baranovskii, D.S.; Ivanov, S.A.; Shegay, P.V.; Kaprin, A.D. Comment on: Regenerative medicine, organ bioengineering and transplantation. Br. J. Surg. 2021, 108, e386. [Google Scholar] [CrossRef]
- Gyöngyösi, M. Cell-Free Approaches and Therapeutic Biomolecules for Cardiac Regeneration. Biomolecules 2021, 11, 161. [Google Scholar] [CrossRef]
- Li, Y.; Xu, Y.; Liu, Y.; Wang, Z.; Chen, W.; Duan, L.; Gu, D. Decellularized cartilage matrix scaffolds with laser-machined micropores for cartilage regeneration and articular cartilage repair. Mater. Sci. Eng. C 2019, 105, 110139. [Google Scholar] [CrossRef]
- Dzemeshkevich, S.L. Organ “bioengineering”, stem cells and scientific integrity in surgery. Clin. Exp. Surg. Petrovsk. J. 2015, 1, 80–85. [Google Scholar]
- Damiano, G.; Palumbo, V.D.; Fazzotta, S.; Curione, F.; Lo Monte, G.; Brucato, V.M.B.; Lo Monte, A.I. Current strategies for tracheal replacement: A review. Life 2021, 11, 618. [Google Scholar] [CrossRef]
- von Bomhard, A.; Elsaesser, A.; Riepl, R.; Pippich, K.; Faust, J.; Schwarz, S.; Koerber, L.; Breiter, R.; Rotter, N. Cartilage regeneration using decellularized cartilage matrix: Long-term comparison of subcutaneous and intranasal placement in a rabbit model. J. Cranio-Maxillofac. Surg. 2019, 47, 682–694. [Google Scholar] [CrossRef] [PubMed]
- Atiakshin, D.; Buchwalow, I.; Samoilova, V.; Tiemann, M. Tryptase as a polyfunctional component of mast cells. Histochem. Cell Biol. 2018, 149, 461–477. [Google Scholar] [CrossRef] [PubMed]
- Lyons, J.J.; Yi, T. Mast cell tryptases in allergic inflammation and immediate hypersensitivity. Curr. Opin. Immunol. 2021, 72, 94–106. [Google Scholar] [CrossRef] [PubMed]
- Hellman, L.; Akula, S.; Fu, Z.; Wernersson, S. Mast Cell and Basophil Granule Proteases—In Vivo Targets and Function. Front. Immunol. 2022, 13, 918305. [Google Scholar] [CrossRef]
- Vitte, J. Human mast cell tryptase in biology and medicine. Mol. Immunol. 2015, 63, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Caughey, G.H. Mast cell proteases as protective and inflammatory mediators. Adv. Exp. Med. Biol. 2011, 716, 212–234. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Korthuis, R.J. Mast Cell Proteases and Inflammation. Drug Discov. Today Dis. Models 2011, 8, 47–55. [Google Scholar] [CrossRef]
- Krystel-Whittemore, M.; Dileepan, K.N.; Wood, J.G. Mast cell: A multi-functional master cell. Front. Immunol. 2016, 6, 620. [Google Scholar] [CrossRef]
- Aponte-López, A.; Muñoz-Cruz, S. Mast Cells in the Tumor Microenvironment. Adv. Exp. Med. Biol. 2020, 1273, 159–173. [Google Scholar] [CrossRef]
- Elieh Ali Komi, D.; Wöhrl, S.; Bielory, L. Mast Cell Biology at Molecular Level: A Comprehensive Review. Clin. Rev. Allergy Immunol. 2020, 58, 342–365. [Google Scholar] [CrossRef]
- Costa Neto, H.; Andrade, A.L.D.L.; Carmo, A.F.D.; Freitas, R.A.; Galvão, H.C. Involvement of tryptase-positive mast cells and angiogenesis in the growth of inflammatory odontogenic cysts. Braz. Oral Res. 2021, 35, e061. [Google Scholar] [CrossRef]
- Ribatti, D. Mast cells as therapeutic target in cancer. Eur. J. Pharmacol. 2016, 778, 152–157. [Google Scholar] [CrossRef]
- Ammendola, M.; Currò, G.; Laface, C.; Zuccalà, V.; Memeo, R.; Luposella, F.; Laforgia, M.; Zizzo, N.; Zito, A.; Loisi, D.; et al. Mast Cells Positive for c-Kit Receptor and Tryptase Correlate with Angiogenesis in Cancerous and Adjacent Normal Pancreatic Tissue. Cells 2021, 10, 444. [Google Scholar] [CrossRef] [PubMed]
- Welle, M. Development, significance, and heterogeneity of mast cells with particular regard to the mast cell-specific proteases chymase and tryptase. J. Leukoc. Biol. 1997, 61, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Atiakshin, D.; Buchwalow, I.; Tiemann, M. Mast cells and collagen fibrillogenesis. Histochem. Cell Biol. 2020, 154, 21–40. [Google Scholar] [CrossRef] [PubMed]
- Lucena, F.; McDougall, J.J. Protease Activated Receptors and Arthritis. Int. J. Mol. Sci. 2021, 22, 9352. [Google Scholar] [CrossRef]
- Steinhoff, M.; Buddenkotte, J.; Shpacovitch, V.; Rattenholl, A.; Moormann, C.; Vergnolle, N.; Luger, T.A.; Hollenberg, M.D. Proteinase-activated receptors: Transducers of proteinase-mediated signaling in inflammation and immune response. Endocr. Rev. 2005, 26, 1–43. [Google Scholar] [CrossRef]
- Merluzzi, S.; Frossi, B.; Gri, G.; Parusso, S.; Tripodo, C.; Pucillo, C. Mast cells enhance proliferation of B lymphocytes and drive their differentiation toward IgA-secreting plasma cells. Blood 2010, 115, 2810–2817. [Google Scholar] [CrossRef] [PubMed]
- Fujihara, Y.; Takato, T.; Hoshi, K. Immunological response to tissue-engineered cartilage derived from auricular chondrocytes and a PLLA scaffold in transgenic mice. Biomaterials 2010, 31, 1227–1234. [Google Scholar] [CrossRef]
- Buchwalow, I.B.; Boecker, W. Immunohistochemistry: Basics and Methods, 1st ed.; Springer: Heidelberg, Germany; Dordrecht, The Netherlands; London, UK; New York, NY, USA, 2010. [Google Scholar] [CrossRef]
- Buchwalow, I.; Samoilova, V.; Boecker, W.; Tiemann, M. Non-specific binding of antibodies in immunohistochemistry: Fallacies and facts. Sci. Rep. 2011, 1, 28. [Google Scholar] [CrossRef]
- Bankhead, P.; Loughrey, M.B.; Fernández, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G.; et al. QuPath: Open source software for digital pathology image analysis. Sci. Rep. 2017, 7, 16878. [Google Scholar] [CrossRef] [PubMed]
| Parametre | Tissue-Engineered Constructs (TEG), Experimental Group | Decellularized Unseeded Tracheal Cartilage (DCS), Control Group |
|---|---|---|
| Fibrosis | Focal fibrosis | N/R |
| Leukocyte infiltration | Lower presence of leukocytes | Extensive leukocyte infiltration in the submucosal layer |
| Mast cell count | 13 cells per 1 mm2 | 5 cells per 1 mm2 |
| Mast cell co-localization | More frequent co-localization of mast cells and plasma cells | More frequent co-localization of mast cells and fibroblasts |
| Plasma cell count | 294 cells per 1 mm2 | 50 cells per 1 mm2 |
| Relative content of plasma cells | 0.49% | 0.29% |
| Vimentin | 0.19% (SD 0.05%) More pronounced vimentin staining | 0.13% (SD 0.08%) Weaker vimentin staining. |
| Collagen type II | 0.54% (SD 0.25%) Collagen-positive TEG matrix and neoformed cartilage tissue in the area of TEG implantation | 0.34% (SD 0.19%) Collagen staining is poorly expressed or absent; mild synthesis of collagen II in the peripheral part of the scaffold only |
| CD34+ cells | 0.18% (SD 0.01%) Distinct positive response; newly formed vessels in the submucosal layer and angiogenesis in the granulation tissue | 0.10% (SD 0.03%) Angiogenesis was observed in the submucosal layer only; no angiogenesis was observed or weakly expressed in the scaffold area |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klabukov, I.; Atiakshin, D.; Kogan, E.; Ignatyuk, M.; Krasheninnikov, M.; Zharkov, N.; Yakimova, A.; Grinevich, V.; Pryanikov, P.; Parshin, V.; et al. Post-Implantation Inflammatory Responses to Xenogeneic Tissue-Engineered Cartilage Implanted in Rabbit Trachea: The Role of Cultured Chondrocytes in the Modification of Inflammation. Int. J. Mol. Sci. 2023, 24, 16783. https://doi.org/10.3390/ijms242316783
Klabukov I, Atiakshin D, Kogan E, Ignatyuk M, Krasheninnikov M, Zharkov N, Yakimova A, Grinevich V, Pryanikov P, Parshin V, et al. Post-Implantation Inflammatory Responses to Xenogeneic Tissue-Engineered Cartilage Implanted in Rabbit Trachea: The Role of Cultured Chondrocytes in the Modification of Inflammation. International Journal of Molecular Sciences. 2023; 24(23):16783. https://doi.org/10.3390/ijms242316783
Chicago/Turabian StyleKlabukov, Ilya, Dmitri Atiakshin, Evgenia Kogan, Michael Ignatyuk, Mikhail Krasheninnikov, Nickolay Zharkov, Anna Yakimova, Vyacheslav Grinevich, Pavel Pryanikov, Vladimir Parshin, and et al. 2023. "Post-Implantation Inflammatory Responses to Xenogeneic Tissue-Engineered Cartilage Implanted in Rabbit Trachea: The Role of Cultured Chondrocytes in the Modification of Inflammation" International Journal of Molecular Sciences 24, no. 23: 16783. https://doi.org/10.3390/ijms242316783
APA StyleKlabukov, I., Atiakshin, D., Kogan, E., Ignatyuk, M., Krasheninnikov, M., Zharkov, N., Yakimova, A., Grinevich, V., Pryanikov, P., Parshin, V., Sosin, D., Kostin, A. A., Shegay, P., Kaprin, A. D., & Baranovskii, D. (2023). Post-Implantation Inflammatory Responses to Xenogeneic Tissue-Engineered Cartilage Implanted in Rabbit Trachea: The Role of Cultured Chondrocytes in the Modification of Inflammation. International Journal of Molecular Sciences, 24(23), 16783. https://doi.org/10.3390/ijms242316783









