Appropriate Nitrogen form Ratio and UV-A Supplementation Increased Quality and Production in Purple Lettuce (Lactuca sativa L.)
Abstract
:1. Introduction
2. Results
2.1. Effects of Different Nitrogen forms and UV-A Interaction on the Growth of Purple Lettuce
2.2. Effects of Different Nitrogen Forms and UV-A Interactions on Photosynthetic Pigment Content of Purple Lettuce
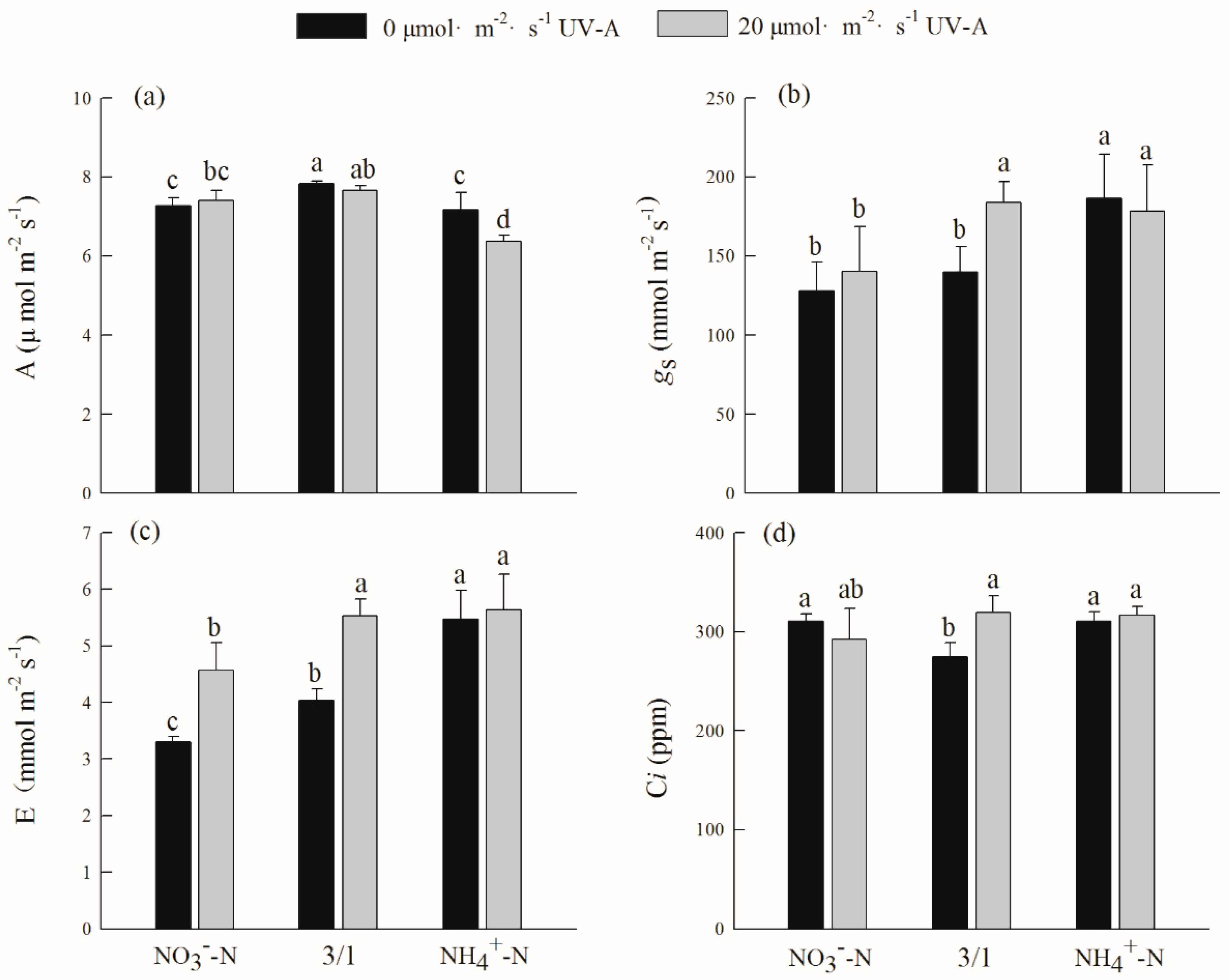
2.3. Effects of Different Nitrogen Forms and UV-A Interactions on Gas Exchange Parameters of Purple Lettuce
2.4. Effects of Different Nitrogen Forms and UV-A Interactions on the Chlorophyll Fluorescence Parameters of Purple Lettuce Leaves
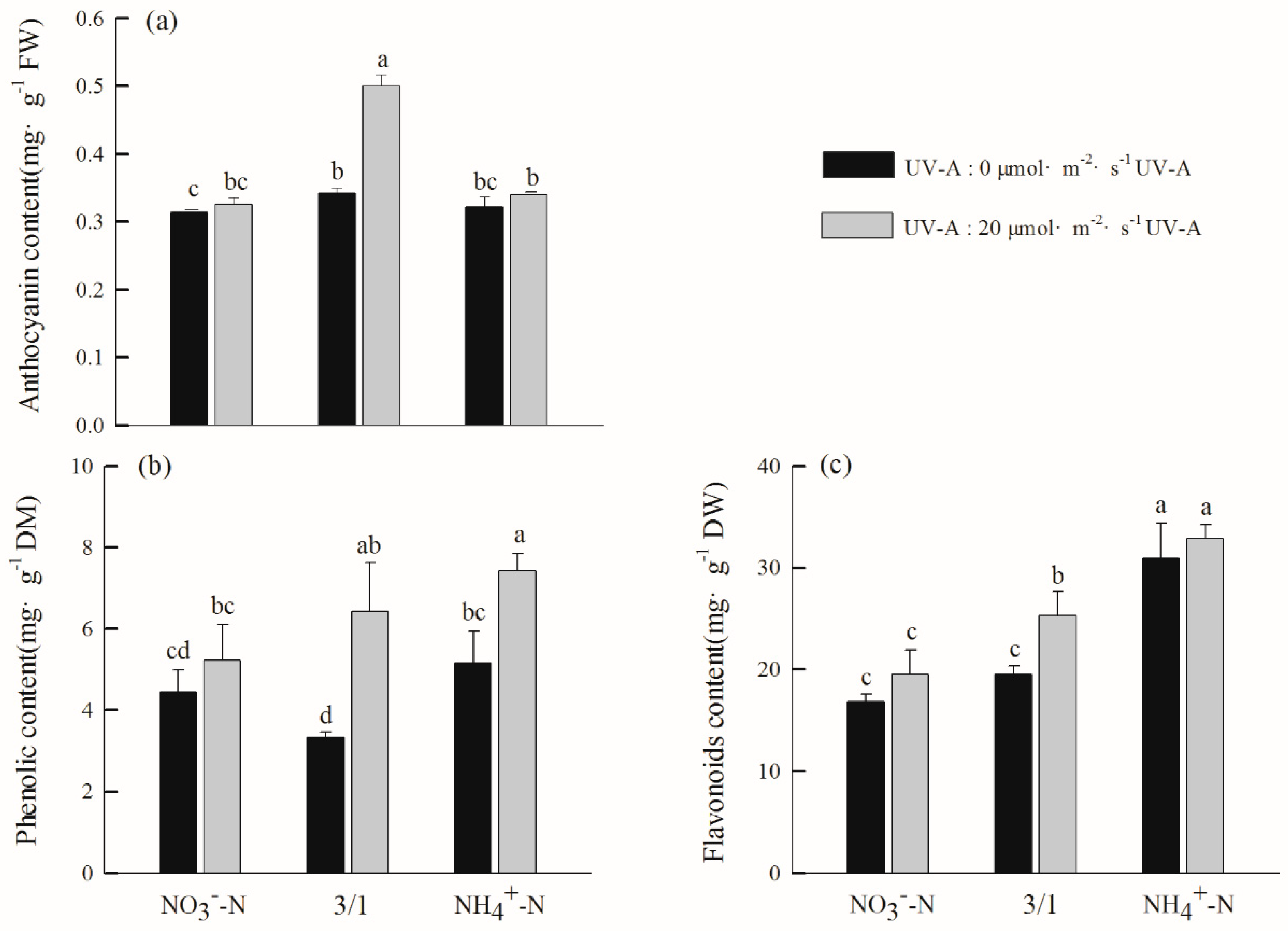
2.5. Effects of Different Nitrogen Forms and UV-A Interactions on Quality of Purple Lettuce
2.6. Effects of Different Nitrogen Forms and UV-A Interactions on Secondary Metabolites of Purple Lettuce
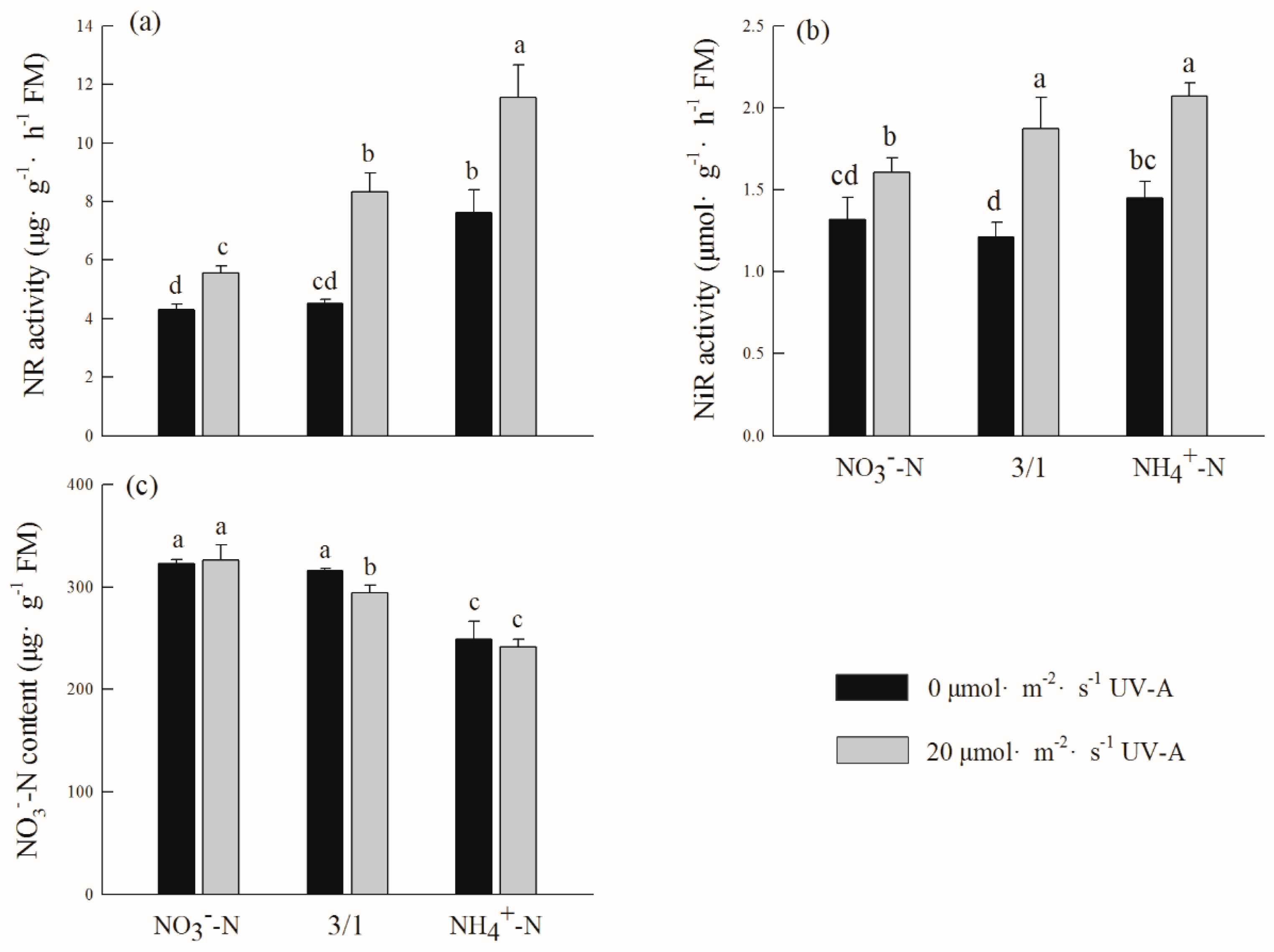
2.7. Effects of Different Ratios of Ammonium Nitrate and UV-A on Nitrate Nitrogen Content and Its Reduction in Purple Lettuce
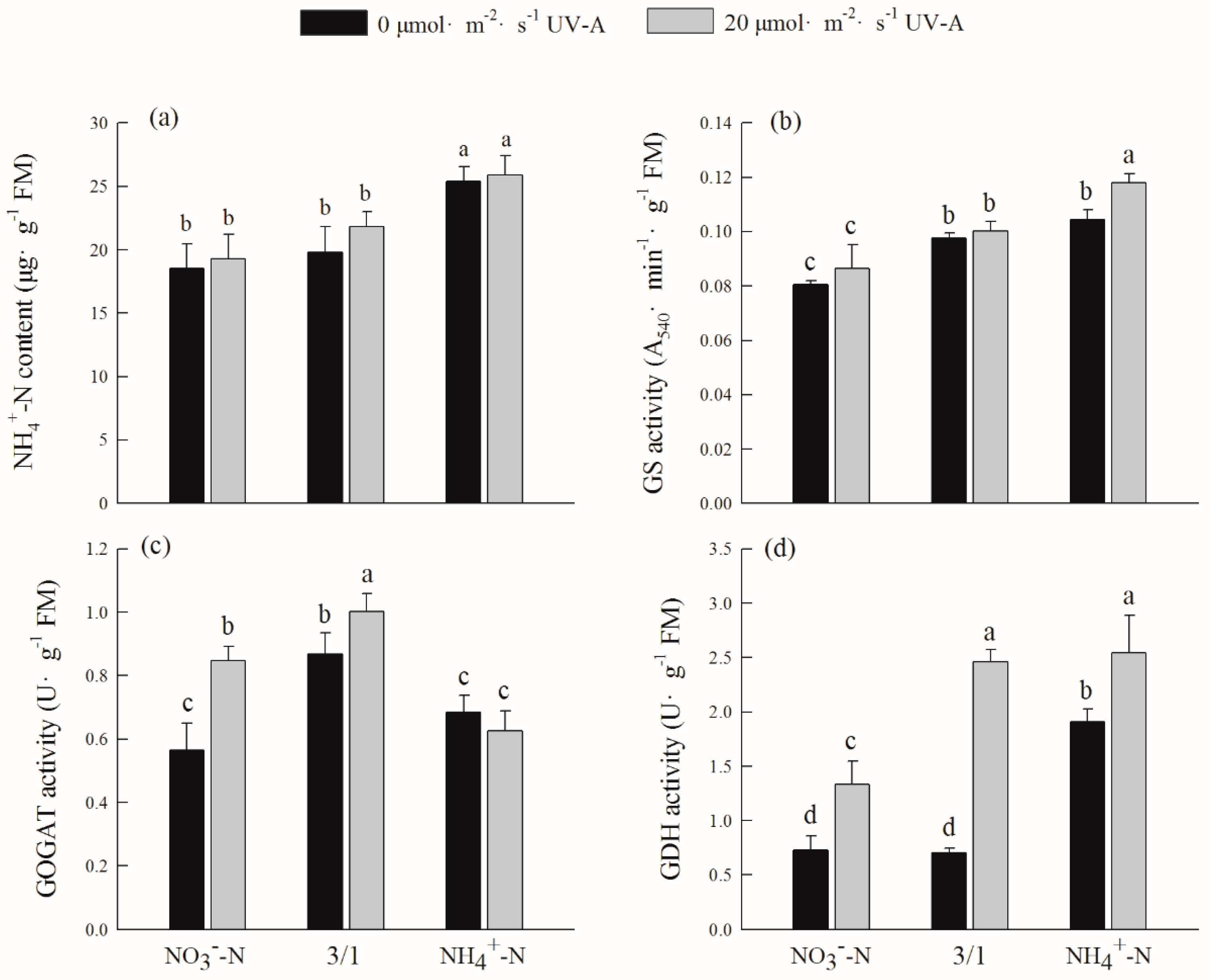
2.8. Effects of Different Ratios of Ammonium Nitrate and UV-A on Ammonium Nitrogen Content and Assimilation of Purple Lettuce
3. Discussion
3.1. Adding 20 µmol m−2 s−1 UV-A to Different Ratios of Ammonium Nitrate Is Beneficial for the Improvement of Photosynthesis and Growth of Purple Leaf Lettuce
3.2. Adding 20 µmol m−2 s−1 UV-A Significantly Reduced the Nitrate Content of NO3−-N/NH4+-N (3/1)-Treated Purple Lettuce and Increased Its Anthocyanin Content
3.3. On the Basis of the Ratio of Ammonium Nitrate, the Addition of 20 µmol m−2 s−1 UV-A Significantly Improved the Nitrogen Assimilation Ability of Each Nitrogen Form Treatment
4. Materials and Methods
4.1. Growing Media and Seeding
4.2. Experimental Design and Growth Environment
4.3. Growth and Photosynthesis Parameters
4.4. Determination of Quality
4.5. Nitrogen Metabolism-Related Enzymes
4.6. Data Processing
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, X.; Gil, M.I.; Yang, Q.; Tomás-Barberán, F.A. Bioactive Compounds in Lettuce: Highlighting the Benefits to Human Health and Impacts of Preharvest and Postharvest Practices. Compr. Rev. Food Sci. Food Saf. 2022, 21, 4–45. [Google Scholar] [CrossRef]
- Kim, M.J.; Moon, Y.; Tou, J.C.; Mou, B.; Waterland, N.L. Nutritional Value, Bioactive Compounds and Health Benefits of Lettuce (Lactuca sativa L.). J. Food Compos. Anal. 2016, 49, 19–34. [Google Scholar] [CrossRef]
- Bian, Z.H.; Yang, Q.C.; Liu, W.K. Effects of Light Quality on the Accumulation of Phytochemicals in Vegetables Produced in Controlled Environments: A Review: Effects of Light on Vegetable Phytochemicals. J. Sci. Food Agric. 2015, 95, 869–877. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Guo, Z.; Jiang, X.; Ahammed, G.J.; Zhou, Y. Light Regulation of Horticultural Crop Nutrient Uptake and Utilization. Hortic. Plant J. 2021, 7, 367–379. [Google Scholar] [CrossRef]
- Li, Y.; Wu, L.; Jiang, H.; He, R.; Song, S.; Su, W.; Liu, H. Supplementary Far-Red and Blue Lights Influence the Biomass and Phytochemical Profiles of Two Lettuce Cultivars in Plant Factory. Molecules 2021, 26, 7405. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Liang, X.; Dai, P.; Chen, Y.; Zhang, Y.; Zhang, M.; Lu, L.; Jin, C.; Lin, X. Alteration of Phenolic Composition in Lettuce (Lactuca sativa L.) by Reducing Nitrogen Supply Enhances Its Anti-Proliferative Effects on Colorectal Cancer Cells. Int. J. Mol. Sci. 2019, 20, 4205. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Chen, Y.; Xu, H.; Liang, X.; Hu, Y.; Jin, C.; Lu, L.; Lin, X. Short-Term Nitrate Limitation Prior to Harvest Improves Phenolic Compound Accumulation in Hydroponic-Cultivated Lettuce (Lactuca sativa L.) without Reducing Shoot Fresh Weight. J. Agric. Food Chem. 2018, 66, 10353–10361. [Google Scholar] [CrossRef]
- Liu, C.W.; Sung, Y.; Chen, B.C.; Lai, H.Y. Effects of Nitrogen Fertilizers on the Growth and Nitrate Content of Lettuce (Lactuca sativa L.). Int. J. Environ. Res. Public Health 2014, 11, 4427–4440. [Google Scholar] [CrossRef]
- Zhang, Y.; Zha, L.; Liu, W.; Zhou, C.; Shao, M.; Yang, Q. LED Light Quality of Continuous Light before Harvest Affects Growth and Asa Metabolism of Hydroponic Lettuce Grown under Increasing Doses of Nitrogen. Plants 2021, 10, 176. [Google Scholar] [CrossRef]
- Zhang, T.; Shi, Y.; Piao, F.; Sun, Z. Effects of Different LED Sources on the Growth and Nitrogen Metabolism of Lettuce. Plant Cell Tissue Organ Cult. (PCTOC) 2018, 134, 231–240. [Google Scholar] [CrossRef]
- Miller, A.J.; Cramer, M.D. Root Nitrogen Acquisition and Assimilation. Plant Soil 2005, 274, 1–36. [Google Scholar] [CrossRef]
- Horchani, F.; Hajri, R.; Aschi-Smiti, S. Effect of Ammonium or Nitrate Nutrition on Photosynthesis, Growth, and Nitrogen Assimilation in Tomato Plants. J. Plant Nutr. Soil Sci. 2010, 173, 610–617. [Google Scholar] [CrossRef]
- Mensinga, T.T.; Speijers, G.J.A.; Meulenbelt, J. Health Implications of Exposure to Environmental Nitrogenous Compounds. Toxicol. Rev. 2003, 22, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Wanlai, Z.; Wenke, L.; Qichang, Y. Reducing Nitrate Content in Lettuce by Pre-Harvest Continuous Light Delivered by Red and Blue Light-Emitting Diodes. J. Plant Nutr. 2013, 36, 481–490. [Google Scholar] [CrossRef]
- Lin, K.H.; Huang, M.Y.; Huang, W.D.; Hsu, M.H.; Yang, Z.W.; Yang, C.M. The Effects of Red, Blue, and White Light-Emitting Diodes on the Growth, Development, and Edible Quality of Hydroponically Grown Lettuce (Lactuca sativa L. Var. Capitata). Sci. Hortic. 2013, 150, 86–91. [Google Scholar] [CrossRef]
- Chen, X.; Xue, X.; Guo, W.; Wang, L.; Qiao, X. Growth and Nutritional Properties of Lettuce Affected by Mixed Irradiation of White and Supplemental Light Provided by Light-Emitting Diode. Sci. Hortic. 2016, 200, 111–118. [Google Scholar] [CrossRef]
- Verdaguer, D.; Jansen, M.A.K.; Llorens, L.; Morales, L.O.; Neugart, S. UV-A Radiation Effects on Higher Plants: Exploring the Known Unknown. Plant Sci. 2017, 255, 72–81. [Google Scholar] [CrossRef]
- Mao, P.; Duan, F.; Zheng, Y.; Yang, Q. Blue and UV-A Light Wavelengths Positively Affected Accumulation Profiles of Healthy Compounds in Pak-choi. J. Sci. Food Agric. 2021, 101, 1676–1684. [Google Scholar] [CrossRef]
- Chen, Y.; Fanourakis, D.; Tsaniklidis, G.; Aliniaeifard, S.; Yang, Q.; Li, T. Low UVA Intensity during Cultivation Improves the Lettuce Shelf-Life, an Effect That Is Not Sustained at Higher Intensity. Postharvest Biol. Technol. 2021, 172, 111376. [Google Scholar] [CrossRef]
- Liang, Y.; Cossani, C.M.; Sadras, V.O.; Yang, Q.; Wang, Z. The Interaction between Nitrogen Supply and Light Quality Modulates Plant Growth and Resource Allocation. Front. Plant Sci. 2022, 13, 864090. [Google Scholar] [CrossRef]
- Yang, X.; Hu, J.; Wang, Z.; Huang, T.; Xiang, Y.; Zhang, L.; Peng, J.; Tomas-Barberan, F.A.; Yang, Q. Pre-Harvest Nitrogen Limitation and Continuous Lighting Improve the Quality and Flavor of Lettuce (Lactuca sativa L.) under Hydroponic Conditions in Greenhouse. J. Agric. Food Chem. 2023, 71, 710–720. [Google Scholar] [CrossRef] [PubMed]
- Kotsiras, A.; Olympios, C.M.; Drosopoulos, J.; Passam, H.C. Effects of Nitrogen Form and Concentration on the Distribution of Ions within Cucumber Fruits. Sci. Hortic. 2002, 95, 175–183. [Google Scholar] [CrossRef]
- Tabatabaei, S.J.; Fatemi, L.S.; Fallahi, E. Effect of Ammonium: Nitrate Ratio on Yield, Calcium Concentration, and Photosynthesis Rate in Strawberry. J. Plant Nutr. 2006, 29, 1273–1285. [Google Scholar] [CrossRef]
- Smith, V.R. Effect of Nutrients on CO2 Assimilation by Mosses on a Sub-Antarctic Island. New Phytol. 1993, 123, 693–697. [Google Scholar] [CrossRef]
- Britto, D.T.; Kronzucker, H.J. NH4+ Toxicity in Higher Plants: A Critical Review. J. Plant Physiol. 2002, 159, 567–584. [Google Scholar] [CrossRef]
- Lugert, I.; Gerendás, J.; Brück, H.; Sattelmacher, B. Influence of N Form on Growth and Water Status of Tomato Plants. In Plant Nutrition; Horst, W.J., Schenk, M.K., Bürkert, A., Claassen, N., Flessa, H., Frommer, W.B., Goldbach, H., Olfs, H.-W., Römheld, V., Sattelmacher, B., et al., Eds.; Springer: Dordrecht, The Netherlands, 2001; pp. 306–307. [Google Scholar] [CrossRef]
- Johkan, M.; Shoji, K.; Goto, F.; Hahida, S.; Yoshihara, T. Effect of Green Light Wavelength and Intensity on Photomorphogenesis and Photosynthesis in Lactuca sativa. Environ. Exp. Bot. 2012, 75, 128–133. [Google Scholar] [CrossRef]
- Hall, N.P.; Reggiani, R.; Franklin, J.; Keys, A.J.; Lea, P.J. An Investigation into the Interaction between Nitrogen Nutrition, Photosynthesis and Photorespiration. Photosynth. Res. 1984, 5, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Barickman, T.C.; Kopsell, D.A. Nitrogen Form and Ratio Impact Swiss Chard (Beta vulgaris Subsp. Cicla) Shoot Tissue Carotenoid and Chlorophyll Concentrations. Sci. Hortic. 2016, 204, 99–105. [Google Scholar] [CrossRef]
- Takács, E.; Técsi, L. Effects of NO3−/NH4+ Ratio on Photosynthetic Rate, Nitrate Reductase Activity and Chloroplast Ultrastructure in Three Cultivars of Red Pepper (Capsicum annuum L.). J. Plant Physiol. 1992, 140, 298–305. [Google Scholar] [CrossRef]
- Demmig-Adams, B. Photoprotection and Other Responses of Plants to High Light Stress. Annu. Rev. Plant Biol. 1992, 43, 599–626. [Google Scholar] [CrossRef]
- Zhang, M.P.; Zhang, C.J.; Yu, G.H.; Jiang, Y.Z.; Strasser, R.J.; Yuan, Z.Y.; Yang, X.S.; Chen, G.X. Changes in Chloroplast Ultrastructure, Fatty Acid Components of Thylakoid Membrane and Chlorophyll a Fluorescence Transient in Flag Leaves of a Super-High-Yield Hybrid Rice and Its Parents during the Reproductive Stage. J. Plant Physiol. 2010, 167, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Ghorbel, M.; Brini, F.; Sharma, A.; Landi, M. Role of jasmonic acid in plants: The molecular point of view. Plant Cell Rep. 2021, 40, 1471–1494. [Google Scholar] [CrossRef] [PubMed]
- Ximenes, M.; Rath, S.; Reyes, F. Polarographic Determination of Nitrate in Vegetables. Talanta 2000, 51, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Nedunchezhian, N.; Annamabinathan, K. Induction of Heat Shock-like Proteins in Vigna sinemsis Seedlings Growing under Ultraviolet-B (280–320 nm) Enhanced Radiation. Physiol. Plant. 1992, 85, 503–506. [Google Scholar] [CrossRef]
- Tsormpatsidis, E.; Henbest, R.G.C.; Davis, F.J.; Battey, N.H.; Hadley, P.; Wagstaffe, A. UV Irradiance as a Major Influence on Growth, Development and Secondary Products of Commercial Importance in Lollo Rosso Lettuce ‘Revolution’ Grown under Polyethylene Films. Environ. Exp. Bot. 2008, 63, 232–239. [Google Scholar] [CrossRef]
- Copley, R.R.; Letunic, I.; Bork, P. Genome and Protein Evolution in Eukaryotes. Curr. Opin. Chem. Biol. 2002, 6, 39–45. [Google Scholar] [CrossRef]
- Hirner, A.A.; Veit, S.; Seitz, H.U. Regulation of Anthocyanin Biosynthesis in UV-A-Irradiated Cell Cultures of Carrot and in Organs of Intact Carrot Plants. Plant Sci. 2001, 161, 315–322. [Google Scholar] [CrossRef]
- Jampeetong, A.; Brix, H. Nitrogen Nutrition of Salvinia Natans: Effects of Inorganic Nitrogen Form on Growth, Morphology, Nitrate Reductase Activity and Uptake Kinetics of Ammonium and Nitrate. Aquat. Bot. 2009, 90, 67–73. [Google Scholar] [CrossRef]
- Kumar, A.; Sinha, R.P.; Häder, D.P. Effect of UV-B on Enzymes of Nitrogen Metabolism in the Cyanobacterium Nostoc calcicola. J. Plant Physiol. 1996, 148, 86–91. [Google Scholar] [CrossRef]
- Poonnachit, U.; Darnell, R. Effect of Ammonium and Nitrate on Ferric Chelate Reductase and Nitrate Reductase in Vaccinium Species. Ann. Bot. 2004, 93, 399–405. [Google Scholar] [CrossRef]
- Leleu, O.; Vuylsteker, C. Unusual Regulatory Nitrate Reductase Activity in Cotyledons of Brassica Napus Seedlings: Enhancement of Nitrate Reductase Activity by Ammonium Supply. J. Exp. Bot. 2004, 55, 815–823. [Google Scholar] [CrossRef] [PubMed]
- Lea, P.J.; Miflin, B.J. Alternative Route for Nitrogen Assimilation in Higher Plants. Nature 1974, 251, 614–616. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, H.S.; Singh, R.P. Role and Regulation of L-Glutamate Dehydrogenase Activity in Higher Plants. Phytochemistry 1987, 26, 597–610. [Google Scholar] [CrossRef]
- Clegg, K.M. The Application of the Anthrone Reagent to the Estimation of Starch in Cereals. J. Sci. Food Agric. 1956, 7, 40–44. [Google Scholar] [CrossRef]
- Sedmakand, J.J.; Grossberg, S.E. A Rapid, Sensitive, and Versatile Assay for Protein Using Coomassie Brilliant Blue G250. Anal. Biochem. 1977, 79, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Arya, S.P.; Mahajan, M.; Jain, P. Photometric Methods for the Determination of Vitamin C. Anal. Sci. 1998, 14, 889–895. [Google Scholar] [CrossRef]
- Yemm, E.W.; Cocking, E.C.; Ricketts, R.E. The Determination of Amino-Acids with Ninhydrin. Analyst 1955, 80, 209. [Google Scholar] [CrossRef]
- Cataldo, D.A.; Maroon, M.; Schrader, L.E.; Youngs, V.L. Rapid Colorimetric Determination of Nitrate in Plant Tissue by Nitration of Salicylic Acid. Commun. Soil Sci. Plant Anal. 1975, 6, 71–80. [Google Scholar] [CrossRef]
- Solórzano, L. Determination of Ammonia in Natural Waters by the Phenolhypochlorite Method. Limnol. Oceanogr. 1969, 14, 799–801. [Google Scholar] [CrossRef]
- Lin, C.C.; Kao, C.H. Disturbed Ammonium Assimilation Is Associated with Growth Inhibition of Roots in Rice Seedlings Caused by NaCl. Plant Growth Regul. 1996, 18, 233–238. [Google Scholar] [CrossRef]
- Singh, R.P.; Srivastava, H.S. Regulation of Gliitainate Dehydrogenase Activity by Amino Adds in Maize Seedlings. Physiol. Plant. 1983, 57, 549–554. [Google Scholar] [CrossRef]
| UV-A | NO3−/NH4+ | Stem Length (cm) | Stem Diameter (mm) | Leaf Area (cm2) | Fresh Weight (g) | Dry Weight (g) |
|---|---|---|---|---|---|---|
| 0 | NO3−-N | 8.53 ± 0.99 b | 1.096 ± 0.089 a | 1727.61 ± 116.03 b | 75.98 ± 6.29 b | 3.68 ± 0.45 c |
| 3/1 | 7.56 ± 0.23 b | 1.127 ± 0.023 a | 2220.05 ± 320.04 a | 84.76 ± 10.66 b | 4.17 ± 0.5 bc | |
| NH4+-N | 2.94 ± 0.74 c | 0.85 ± 0.054 b | 482.40 ± 23.82 c | 15.49 ± 0.07 c | 1.37 ± 0.06 d | |
| NO3−-N | 11.05 ± 0.69 a | 1.065 ± 0.084 a | 2423.76 ± 22.39 a | 98.43 ± 7.31 a | 4.62 ± 0.38 ab | |
| 20 | 3/1 | 10.93 ± 0.68 a | 1.071 ± 0.045 a | 2494.38 ± 379.57 a | 100.71 ± 5.99 a | 5.06 ± 0.72 a |
| NH4+-N | 4.19 ± 0.17 c | 0.938 ± 0.068 b | 744.33 ± 124.74 c | 20.83 ± 1.46 c | 1.84 ± 0.17 d | |
| Effects | UV-A | *** | NS | ** | *** | ** |
| N | *** | *** | *** | *** | *** | |
| UV-A × N | * | NS | NS | NS | NS |
| UV-A | NO3−/NH4+ | Chlorophyll a (mg·g−1 FM) | Chlorophyll b (mg·g−1 FM) | Chlorophyll a + b (mg·g−1 FM) | Chlorophyll a/b (mg·g−1 FM) | Carotenoids (mg·g−1 FM) |
|---|---|---|---|---|---|---|
| 0 | NO3−-N | 1.093 ± 0.219 e | 0.318 ± 0.099 c | 1.411 ± 0.318 e | 3.515 ± 0.367 a | 0.157 ± 0.024 b |
| 3/1 | 3.646 ± 0.341 ab | 1.232 ± 0.085 ab | 4.878 ± 0.388 ab | 2.964 ± 0.263 ab | 0.712 ± 0.114 a | |
| NH4+-N | 1.481 ± 0.741 de | 0.635 ± 0.503 c | 2.116 ± 1.244 de | 2.701 ± 0.738 b | 0.238 ± 0.179 b | |
| NO3−-N | 2.208 ± 0.624 cd | 0.759 ± 0.143 bc | 2.968 ± 0.766 cd | 2.877 ± 0.263 ab | 0.496 ± 0.121 ab | |
| 20 | 3/1 | 4.592 ± 0.629 a | 1.605 ± 0.403 a | 6.197 ± 0.959 a | 2.952 ± 0.591 ab | 0.733 ± 0.390 a |
| NH4+-N | 2.821 ± 0.387 bc | 1.158 ± 0.229 ab | 3.979 ± 0.519 bc | 2.478 ± 0.428 b | 0.465 ± 0.246 ab | |
| UV-A | ** | ** | ** | NS | NS | |
| Effects | N | *** | ** | *** | NS | * |
| UV-A × N | NS | NS | NS | NS | NS |
| UV-A | NO3−/NH4+ | Fv/Fm | Fv’/Fm’ | ΦPSII | qP | NPQ | ETR |
|---|---|---|---|---|---|---|---|
| 0 | NO3−-N | 0.874 ± 0.03 ab | 0.790 ± 0.03 a | 0.598 ± 0.09 bc | 0.756 ± 0.105 b | 0.499 ± 0.29 bc | 198.71 ± 31.64 bc |
| 3/1 | 0.891 ± 0.01 a | 0.810 ± 0.01 a | 0.718 ± 0.01 a | 0.886 ± 0.03 a | 0.710 ± 0.18 ab | 238.25 ± 3.46 a | |
| NH4+-N | 0.877 ± 0.01 ab | 0.826 ± 0.03 a | 0.683 ± 0.02 ab | 0.828 ± 0.03 ab | 1.001 ± 0.24 a | 226.87 ± 6.51 ab | |
| NO3−-N | 0.855 ± 0.01 bc | 0.809 ± 0.01 a | 0.520 ± 0.03 cd | 0.643 ± 0.03 c | 0.492 ± 0.16 bc | 172.75 ± 9.88 cd | |
| 20 | 3/1 | 0.853 ± 0.01 bc | 0.792 ± 0.02 a | 0.589 ± 0.02 c | 0.743 ± 0.01 b | 0.465 ± 0.13 bc | 195.44 ± 7.51 c |
| NH4+-N | 0.838 ± 0.02 c | 0.791 ± 0.02 a | 0.475 ± 0.05 d | 0.599 ± 0.05 c | 0.183 ± 0.13 c | 157.62 ± 16.32 d | |
| Effects | UV-A | ** | NS | *** | *** | ** | *** |
| N | NS | NS | * | ** | NS | * | |
| UV-A × N | NS | NS | NS | NS | * | NS |
| UV-A | NO3−/NH4+ | Soluble Sugar (mg·g−1 FM) | Soluble Protein (mg·g−1 FM) | Free Amino Acid (mg·g−1 FM) | Vc (mg·g−1 FM) | Nitrate (mg·g−1 FM) |
|---|---|---|---|---|---|---|
| 0 | NO3−-N | 5.237 ± 0.48 b | 5.073 ± 0.24 b | 0.154 ± 0.004 c | 1.578 ± 0.01 c | 0.252 ± 0.03 a |
| 3/1 | 6.395 ± 0.22 a | 5.159 ± 0.34 b | 0.229 ± 0.026 a | 1.612 ± 0.02 b | 0.214 ± 0.02 b | |
| NO3−-N | 6.621 ± 0.23 a | 5.769 ± 0.05 a | 0.226 ± 0.017 a | 1.596 ± 0.02 bc | 0.199 ± 0.01 bc | |
| 20 | NO3−-N | 6.273 ± 0.13 a | 5.088 ± 0.05 b | 0.189 ± 0.008 b | 1.620 ± 0.02 b | 0.219 ± 0.02 b |
| 3/1 | 6.282 ± 0.20 a | 5.198 ± 0.11 b | 0.230 ± 0.001 a | 1.677 ± 0.01 a | 0.178 ± 0.01 c | |
| NO3−-N | 6.687 ± 0.25 a | 5.759 ± 0.08 a | 0.220 ± 0.007 a | 1.649 ± 0.01 a | 0.171 ± 0.02 c |
| Nutrient Source | NO3−/NH4+ | ||
|---|---|---|---|
| NO3− | NO3−/NH4+ = 3:1 | NH4+ | |
| KNO3 | 3 | 0 | 0 |
| Ca(NO3)2 | 2 | 2.625 | 0 |
| KH2PO4 | 1 | 0 | 0 |
| NH4H2PO4 | 0 | 1 | 1 |
| MgSO4 | 1 | 1 | 1 |
| K2SO4 | 0 | 2 | 2 |
| CaCl2 | 0.625 | 0 | 2.625 |
| (NH4)2SO4 | 0 | 0.375 | 3 |
| Light Quality Ratio W:R:B:UV-A | NO3−/NH4+ |
|---|---|
| 150:120:30:0 | NO3−-N |
| NO3−-N/NH4+-N = 3/1 | |
| NH4+-N | |
| 130:120:30:20 | NO3−-N |
| NO3−-N/NH4+-N = 3/1 | |
| NH4+-N |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, B.; Mao, P.; Yang, Q.; Qin, H.; Xu, Y.; Zheng, Y.; Li, Q. Appropriate Nitrogen form Ratio and UV-A Supplementation Increased Quality and Production in Purple Lettuce (Lactuca sativa L.). Int. J. Mol. Sci. 2023, 24, 16791. https://doi.org/10.3390/ijms242316791
Liu B, Mao P, Yang Q, Qin H, Xu Y, Zheng Y, Li Q. Appropriate Nitrogen form Ratio and UV-A Supplementation Increased Quality and Production in Purple Lettuce (Lactuca sativa L.). International Journal of Molecular Sciences. 2023; 24(23):16791. https://doi.org/10.3390/ijms242316791
Chicago/Turabian StyleLiu, Binbin, Pengpeng Mao, Qi Yang, Hengshan Qin, Yaliang Xu, Yinjian Zheng, and Qingming Li. 2023. "Appropriate Nitrogen form Ratio and UV-A Supplementation Increased Quality and Production in Purple Lettuce (Lactuca sativa L.)" International Journal of Molecular Sciences 24, no. 23: 16791. https://doi.org/10.3390/ijms242316791
APA StyleLiu, B., Mao, P., Yang, Q., Qin, H., Xu, Y., Zheng, Y., & Li, Q. (2023). Appropriate Nitrogen form Ratio and UV-A Supplementation Increased Quality and Production in Purple Lettuce (Lactuca sativa L.). International Journal of Molecular Sciences, 24(23), 16791. https://doi.org/10.3390/ijms242316791







