Alteration in Melanin Content in Retinal Pigment Epithelial Cells upon Hydroquinone Exposure
Abstract
:1. Introduction
2. Results
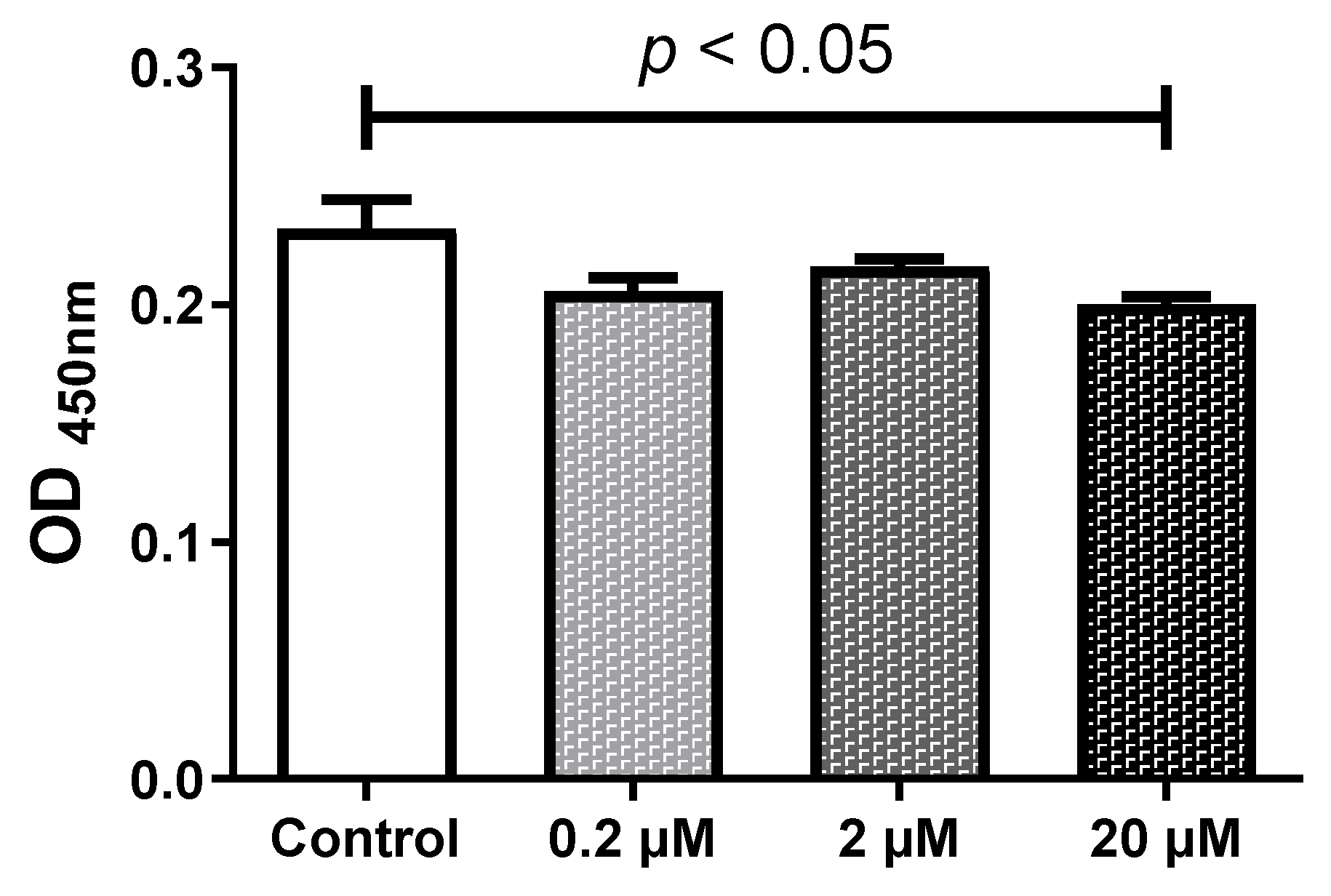
2.1. HQ Below 2 µM Does Not Affect the Cell Viability
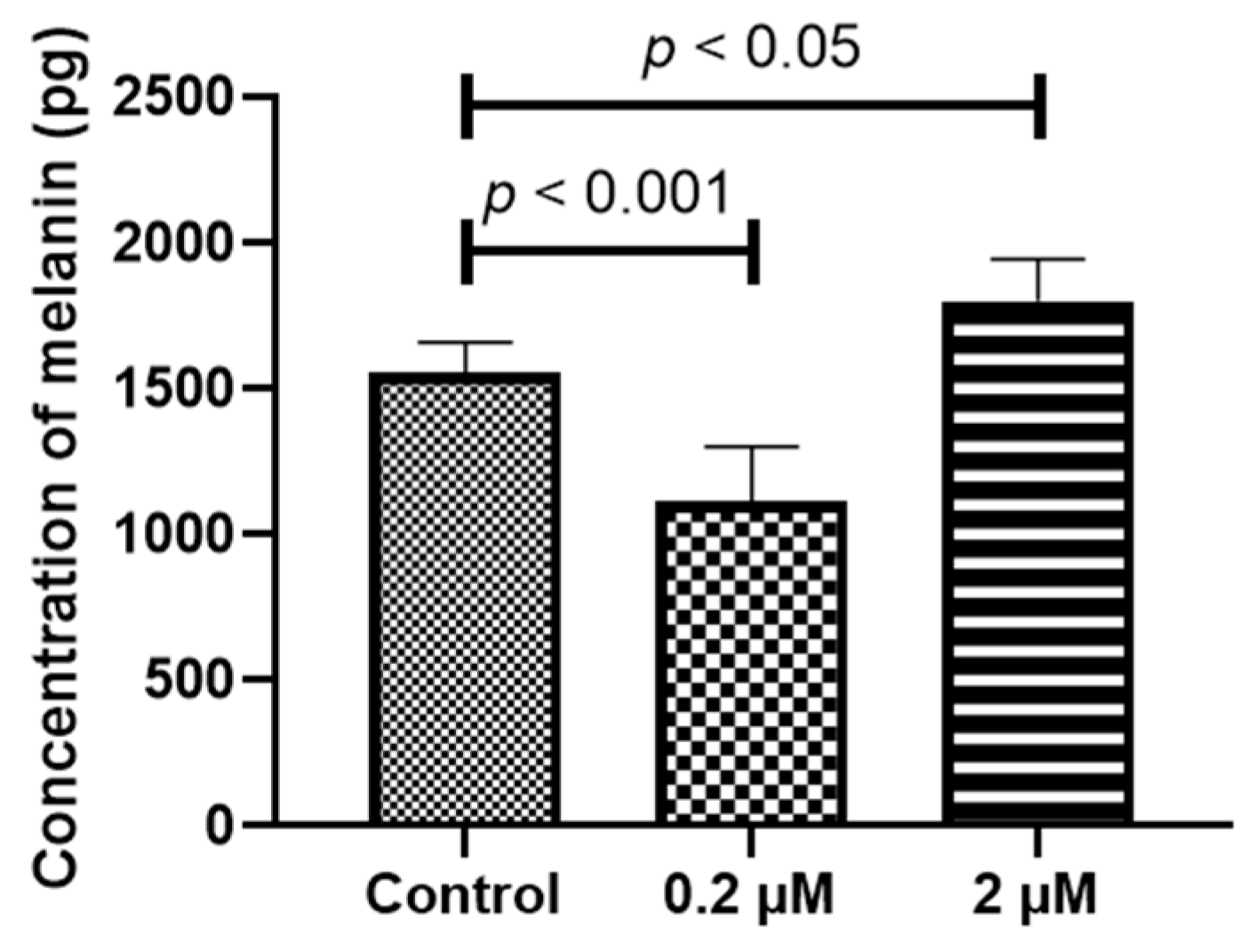
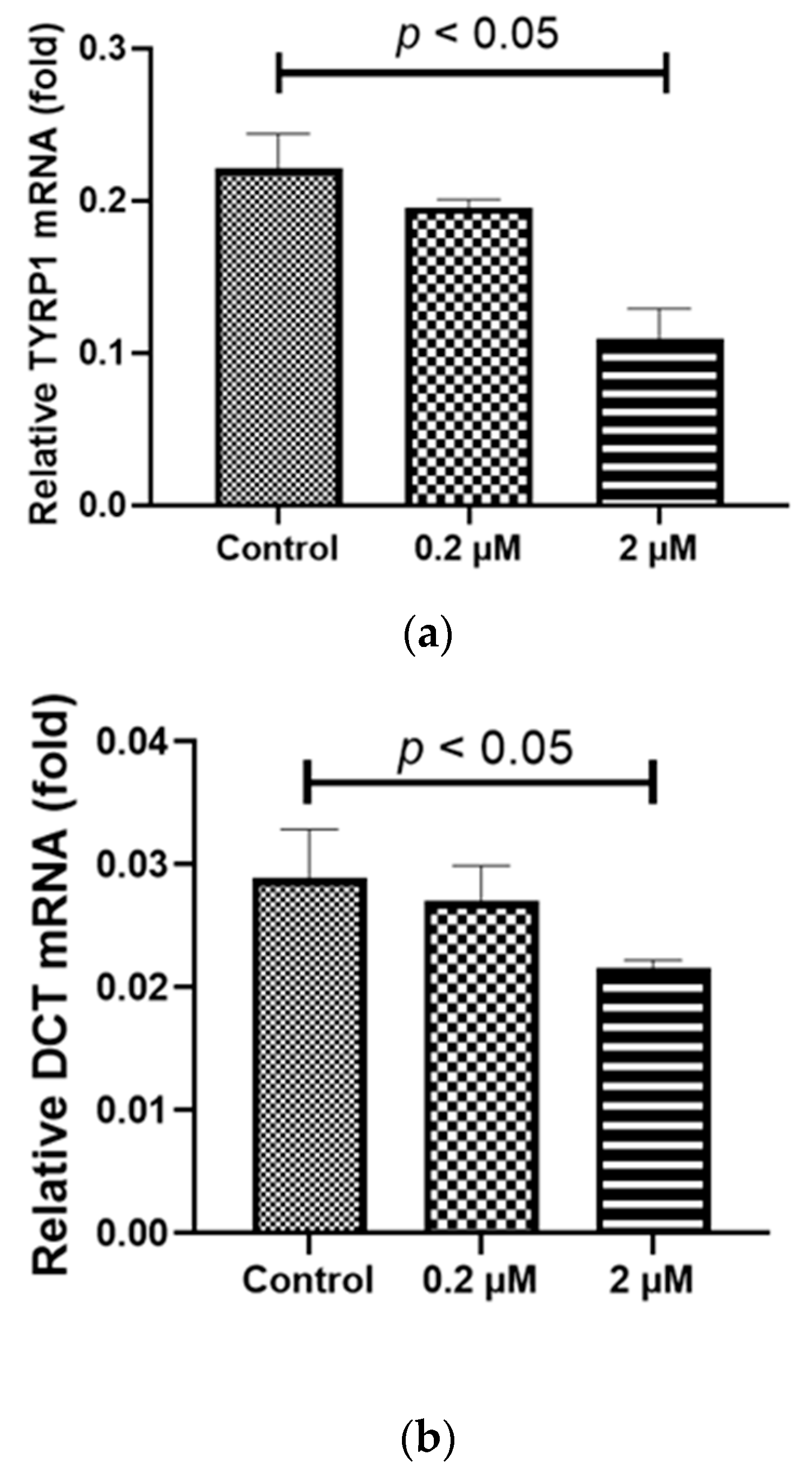
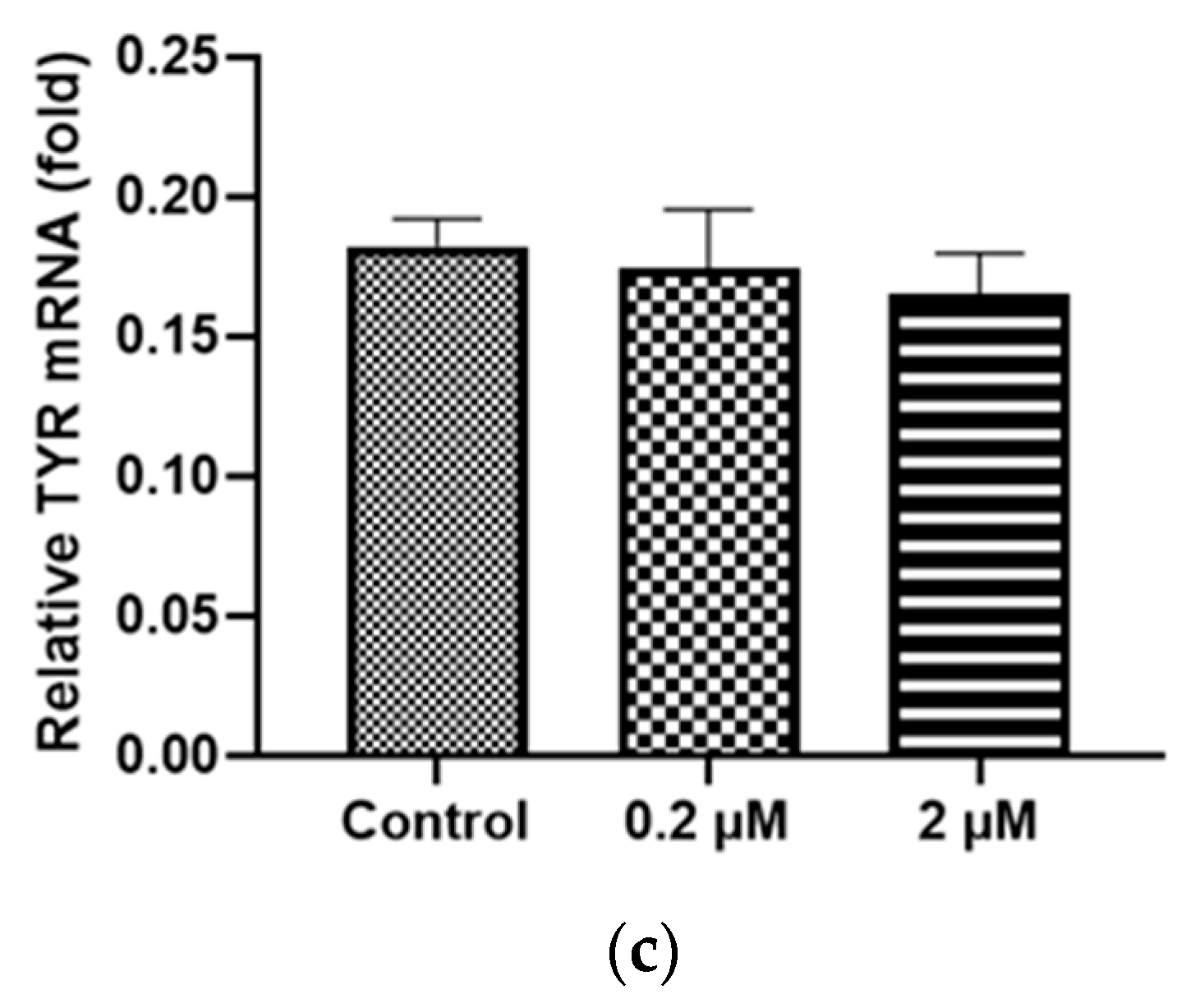
2.2. HQ Exposure for 24 h Reduced Expression of Melanin-Production-Related Genes and Melanin Content, Especially in iPS-RPE Cells
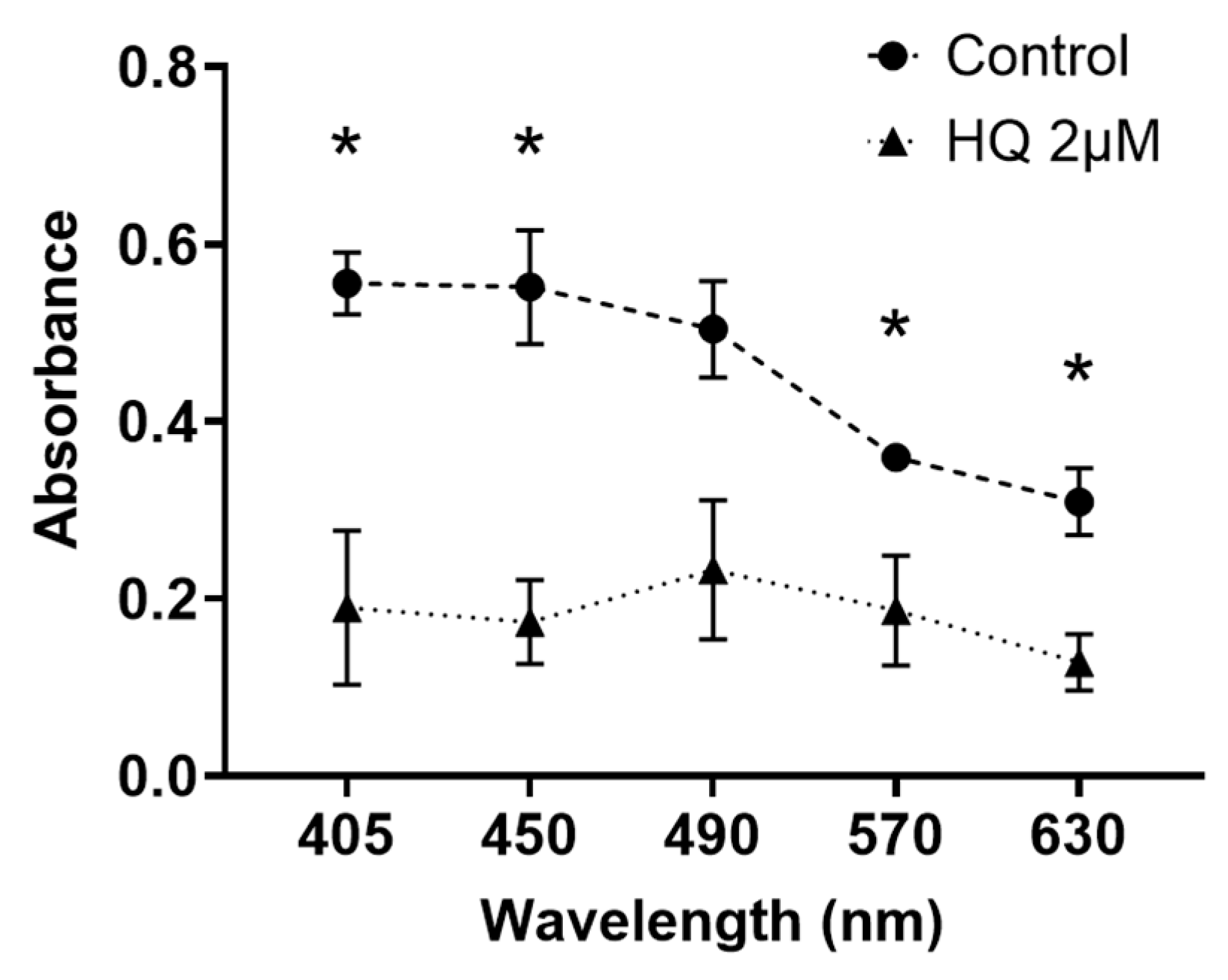
2.3. HQ Exposure for 24 h Reduces Light Transmission through RPE Cells, Particularly in Blue Light
2.4. Prolonged HQ Exposure Paradoxically Increased Melanin Content, but the Expression of Melanin-Production-Related Genes Did Not Change
2.5. Prolonged HQ Exposure Increased the Expression of Melanocortin 1 Receptor (MC1R) mRNA, a Receptor of Melanin Production Cascade
2.6. Agouti Signaling Protein (ASIP) Addition Suppressed the HQ-Induced Re-Elevation in Melanin Production
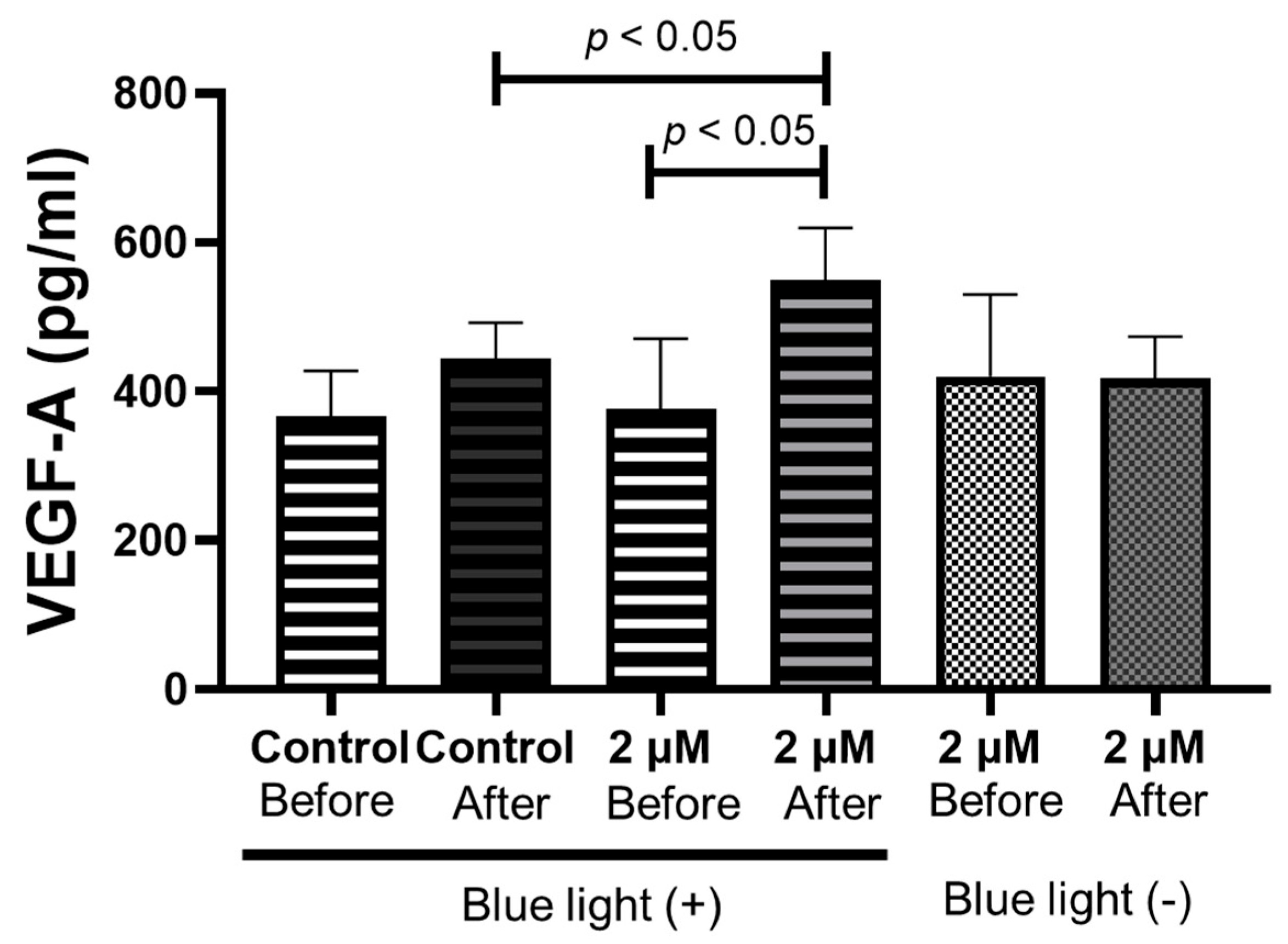
2.7. Blue Light Irradiation of RPE Cells after 1 Week of HQ Addition Significantly Increases Vascular Endothelial Growth Factor (VEGF)-A Secretion
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Measurement of Viable Cell Numbers Using Tetrazolium Salt Cleavage
4.3. Measurement of Melanin-Production-Related Genes
4.4. Measurement of Melanin Protein in the RPE Cells
4.5. Measurement of Light Absorbance in the RPE Cell Suspension
4.6. Blue Light Stimulation for RPE Cells
4.7. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.; Klein, R.; Cheng, C.Y.; Wong, T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob. Health 2014, 2, e106–e116. [Google Scholar] [CrossRef] [PubMed]
- Pascolini, D.; Mariotti, S.P. Global estimates of visual impairment: 2010. Br. J. Ophthalmol. 2012, 96, 614–618. [Google Scholar] [CrossRef] [PubMed]
- Congdon, N.; O’Colmain, B.; Klaver, C.C.; Klein, R.; Muñoz, B.; Friedman, D.S.; Kempen, J.; Taylor, H.R.; Mitchell, P.; Eye Diseases Prevalence Research Group. Causes and prevalence of visual impairment among adults in the United States. Arch. Ophthalmol. 2004, 122, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Age-Related Eye Disease Study Research Group. Risk factors associated with age-related macular degeneration: Age-Related Eye Disease Study Report Number 3. Ophthalmology 2000, 107, 2224–2232. [Google Scholar] [CrossRef]
- Boulton, M.E. Studying melanin and lipofuscin in RPE cell culture models. Exp. Eye Res. 2014, 126, 61–67. [Google Scholar] [CrossRef]
- Sarna, T.; Burke, J.M.; Korytowski, W.; Rozanowska, M.; Skumatz, C.M.; Zareba, A.; Zareba, M. Loss of melanin from human RPE with aging: Possible role of melanin photooxidation. Exp. Eye. Res. 2003, 76, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Young, R.W. Pathophysiology of age-related macular degeneration. Surv. Ophthalmol. 1987, 31, 291–306. [Google Scholar] [CrossRef] [PubMed]
- Vingerling, J.R.; Hofman, A.; Grobbee, D.E.; de Jong, P.T. Age-related macular degeneration and smoking. The Rotterdam study. Arch. Ophthalmol. 1996, 114, 1193–1196. [Google Scholar] [CrossRef]
- Bertram, K.M.; Baglole, C.J.; Phipps, R.P.; Libby, R.T. Molecular regulation of cigarette smoke induced-oxidative stress in human retinal pigment epithelial cells: Implications for age-related macular degeneration. Am. J. Physiol. Cell Physiol. 2009, 297, C1200–C1210. [Google Scholar] [CrossRef]
- Klein, R.; Klein, B.E.; Linton, K.L.; DeMets, D.L. The Beaver Dam Eye Study: The relation of age-related maculopathy to smoking. Am. J. Epidemiol. 1993, 137, 190–200. [Google Scholar] [CrossRef]
- Bolton, J.L.; Trush, M.A.; Penning, T.M.; Dryhurst, G.; Monks, T.J. Role of quinones in toxicology. Chem. Res. Toxicol. 2000, 13, 135–160. [Google Scholar] [CrossRef]
- Ong, C.N.; Lee, B.L.; Shi, C.Y.; Ong, H.Y.; Lee, H.P. Elevated levels of benzene-related compounds in the urine of cigarette smokers. Int. J. Cancer 1994, 59, 177–180. [Google Scholar] [CrossRef]
- Tsujinaka, H.; Itaya-Hironaka, A.; Yamauchi, A.; Sakuramoto-Tsuchida, S.; Ota, H.; Takeda, M.; Fujimura, T.; Takasawa, S.; Ogata, N. Human retinal pigment epithelial cell proliferation by the combined stimulation of hydroquinone and advanced glycation end-products via up-regulation of VEGF gene. Biochem. Biophys. Rep. 2015, 2, 123–131. [Google Scholar] [CrossRef]
- Bhattarai, N.; Korhonen, E.; Toppila, M.; Koskela, A.; Kaarniranta, K.; Mysore, Y.; Kauppinen, A. Resvega alleviates hydroquinone-induced oxidative stress in ARPE-19 cells. Int. J. Mol. Sci. 2020, 21, 2066. [Google Scholar] [CrossRef]
- Pons, M.; Marin-Castaño, M.E. Cigarette smoke-related hydroquinone dysregulates MCP-1, VEGF and PEDF expression in retinal pigment epithelium in vitro and in vivo. PLoS ONE 2011, 6, e16722. [Google Scholar] [CrossRef]
- Abokyi, S.; Shan, S.W.; Lam, C.H.; Catral, K.P.; Pan, F.; Chan, H.H.; To, C.H.; Tse, D.Y. Targeting lysosomes to reverse hydroquinone-induced autophagy defects and oxidative damage in human retinal pigment epithelial cells. Int. J. Mol. Sci. 2021, 22, 9042. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Patil, J.A.; Gramajo, A.L.; Seigel, G.M.; Kuppermann, B.D.; Kenney, C.M. Effects of hydroquinone on retinal and vascular cells in vitro. Indian J. Ophthalmol. 2012, 60, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, A.; d’Ischia, M.; Misuraca, G.; Prota, G. Mechanism of inhibition of melanogenesis by hydroquinone. Biochim. Biophys. Acta 1991, 1073, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Frenk, E. Treatment of melasma with depigmenting agents. In Melasma: New Approaches to Treatment; Martin Dunitz Ltd.: London, UK, 1995; pp. 9–15. [Google Scholar]
- Dooley, T. Topical skin depigmentation agents. J. Dermatol. Treat. 1997, 8, 275–283. [Google Scholar] [CrossRef]
- Curto, E.V.; Kwong, C.; Hermersdörfer, H.; Glatt, H.; Santis, C.; Virador, V.; Hearing, V.J.; Dooley, T.P. Inhibitors of mammalian melanocyte tyrosinase: In vitro comparisons of alkyl esters of gentisic acid with other putative inhibitors. Biochem. Pharmacol. 1999, 57, 663–672. [Google Scholar] [CrossRef]
- D’Mello, S.A.; Finlay, G.J.; Baguley, B.C.; Askarian-Amiri, M.E. Signaling pathways in melanogenesis. Int. J. Mol. Sci. 2016, 17, 1144. [Google Scholar] [CrossRef]
- Wolf Horrell, E.M.; Boulanger, M.C.; D’Orazio, J.A. Melanocortin 1 receptor: Structure, function, and regulation. Front. Genet. 2016, 7, 95. [Google Scholar] [CrossRef]
- Suzuki, I.; Tada, A.; Ollmann, M.M.; Barsh, G.S.; Im, S.; Lamoreux, M.L.; Hearing, V.J.; Nordlund, J.J.; Abdel-Malek, Z.A. Agouti signaling protein inhibits melanogenesis and the response of human melanocytes to alpha-melanotropin. J. Investig. Dermatol. 1997, 108, 838–842. [Google Scholar] [CrossRef]
- Sviderskaya, E.V.; Hill, S.P.; Balachandar, D.; Barsh, G.S.; Bennett, D.C. Agouti signaling protein and other factors modulating differentiation and proliferation of immortal melanoblasts. Dev. Dyn. 2001, 221, 373–379. [Google Scholar] [CrossRef]
- Hellinen, L.; Hagström, M.; Knuutila, H.; Ruponen, M.; Urtti, A.; Reinisalo, M. Characterization of artificially re-pigmented ARPE-19 retinal pigment epithelial cell model. Sci. Rep. 2019, 9, 13761. [Google Scholar] [CrossRef] [PubMed]
- Nasti, T.H.; Timares, L. MC1R, eumelanin and pheomelanin: Their role in determining the susceptibility to skin cancer. Photochem. Photobiol. 2015, 91, 188–200. [Google Scholar] [CrossRef] [PubMed]
- Halder, R.M.; Richards, G.M. Topical agents used in the management of hyperpigmentation. Ski. Ther. Lett. 2004, 9, 1–3. [Google Scholar]
- Narimatsu, T.; Negishi, K.; Miyake, S.; Hirasawa, M.; Osada, H.; Kurihara, T.; Tsubota, K.; Ozawa, Y. Blue light-induced inflammatory marker expression in the retinal pigment epithelium-choroid of mice and the protective effect of a yellow intraocular lens material in vivo. Exp. Eye Res. 2015, 132, 48–51. [Google Scholar] [CrossRef]
- Ito, Y.; Ito, M.; Iwase, T.; Kataoka, K.; Yamada, K.; Yasuda, S.; Ito, H.; Takeuchi, J.; Nakano, Y.; Fujita, A.; et al. Prevalence of and factors associated with dilated choroidal vessels beneath the retinal pigment epithelium among the Japanese. Sci. Rep. 2021, 11, 11278. [Google Scholar] [CrossRef]
- Talhout, R.; Schulz, T.; Florek, E.; van Benthem, J.; Wester, P.; Opperhuizen, A. Hazardous compounds in tobacco smoke. Int. J. Environ. Res. Public Health 2011, 8, 613–628. [Google Scholar] [CrossRef]
- Ramírez, C.; Cáceres-del-Carpio, J.; Chu, J.; Moustafa, M.T.; Chwa, M.; Limb, G.A.; Kuppermann, B.D.; Kenney, M.C. Brimonidine can prevent in vitro hydroquinone damage on retinal pigment epithelium cells and retinal Müller cells. J. Ocul. Pharmacol. Ther. 2016, 32, 102–108. [Google Scholar] [CrossRef]
- Moustafa, M.T.; Ramirez, C.; Schneider, K.; Atilano, S.R.; Limb, G.A.; Kuppermann, B.D.; Kenney, M.C. Protective effects of memantine on hydroquinone-treated human retinal pigment epithelium cells and human retinal Müller cells. J. Ocul. Pharmacol. Ther. 2017, 33, 610–619. [Google Scholar] [CrossRef]
- Dunn, K.C.; Aotaki-Keen, A.E.; Putkey, F.R.; Hjelmeland, L.M. ARPE-19, a human retinal pigment epithelial cell line with differentiated properties. Exp. Eye Res. 1996, 62, 155–169. [Google Scholar] [CrossRef] [PubMed]
- Gamm, D.M.; Melvan, J.N.; Shearer, R.L.; Pinilla, I.; Sabat, G.; Svendsen, C.N.; Wright, L.S. A novel serum-free method for culturing human prenatal retinal pigment epithelial cells. Investig. Ophthalmol. Vis. Sci. 2008, 49, 788–799. [Google Scholar] [CrossRef] [PubMed]
- Masui, T.; Ota, I.; Itaya-Hironaka, A.; Takeda, M.; Kasai, T.; Yamauchi, A.; Sakuramoto-Tsuchida, S.; Mikami, S.; Yane, K.; Takasawa, S.; et al. Expression of REG III and prognosis in head and neck cancer. Oncol. Rep. 2013, 30, 573–578. [Google Scholar] [CrossRef]
- Kyotani, Y.; Ota, H.; Itaya-Hironaka, A.; Yamauchi, A.; Sakuramoto-Tsuchida, S.; Zhao, J.; Ozawa, K.; Nagayama, K.; Ito, S.; Takasawa, S.; et al. Intermittent hypoxia induces the proliferation of rat vascular smooth muscle cell with the increases in epidermal growth factor family and erbB2 receptor. Exp. Cell Res. 2013, 319, 3042–3050. [Google Scholar] [CrossRef] [PubMed]
- Ota, H.; Itaya-Hironaka, A.; Yamauchi, A.; Sakuramoto-Tsuchida, S.; Miyaoka, T.; Fujimura, T.; Tsujinaka, H.; Yoshimoto, K.; Nakagawara, K.; Tamaki, S.; et al. Pancreatic β cell proliferation by intermittent hypoxia via up-regulation of Reg family genes and HGF gene. Life Sci. 2013, 93, 664–672. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishiyama, T.; Tsujinaka, H.; Ueda, T.; Ogata, N. Alteration in Melanin Content in Retinal Pigment Epithelial Cells upon Hydroquinone Exposure. Int. J. Mol. Sci. 2023, 24, 16801. https://doi.org/10.3390/ijms242316801
Nishiyama T, Tsujinaka H, Ueda T, Ogata N. Alteration in Melanin Content in Retinal Pigment Epithelial Cells upon Hydroquinone Exposure. International Journal of Molecular Sciences. 2023; 24(23):16801. https://doi.org/10.3390/ijms242316801
Chicago/Turabian StyleNishiyama, Takeyuki, Hiroki Tsujinaka, Tetsuo Ueda, and Nahoko Ogata. 2023. "Alteration in Melanin Content in Retinal Pigment Epithelial Cells upon Hydroquinone Exposure" International Journal of Molecular Sciences 24, no. 23: 16801. https://doi.org/10.3390/ijms242316801



