Lactoferrin Alleviates Ethanol-Induced Injury via Promoting Nrf2 Nuclear Translocation in BRL-3A Rat Liver Cells
Abstract
:1. Introduction
2. Results
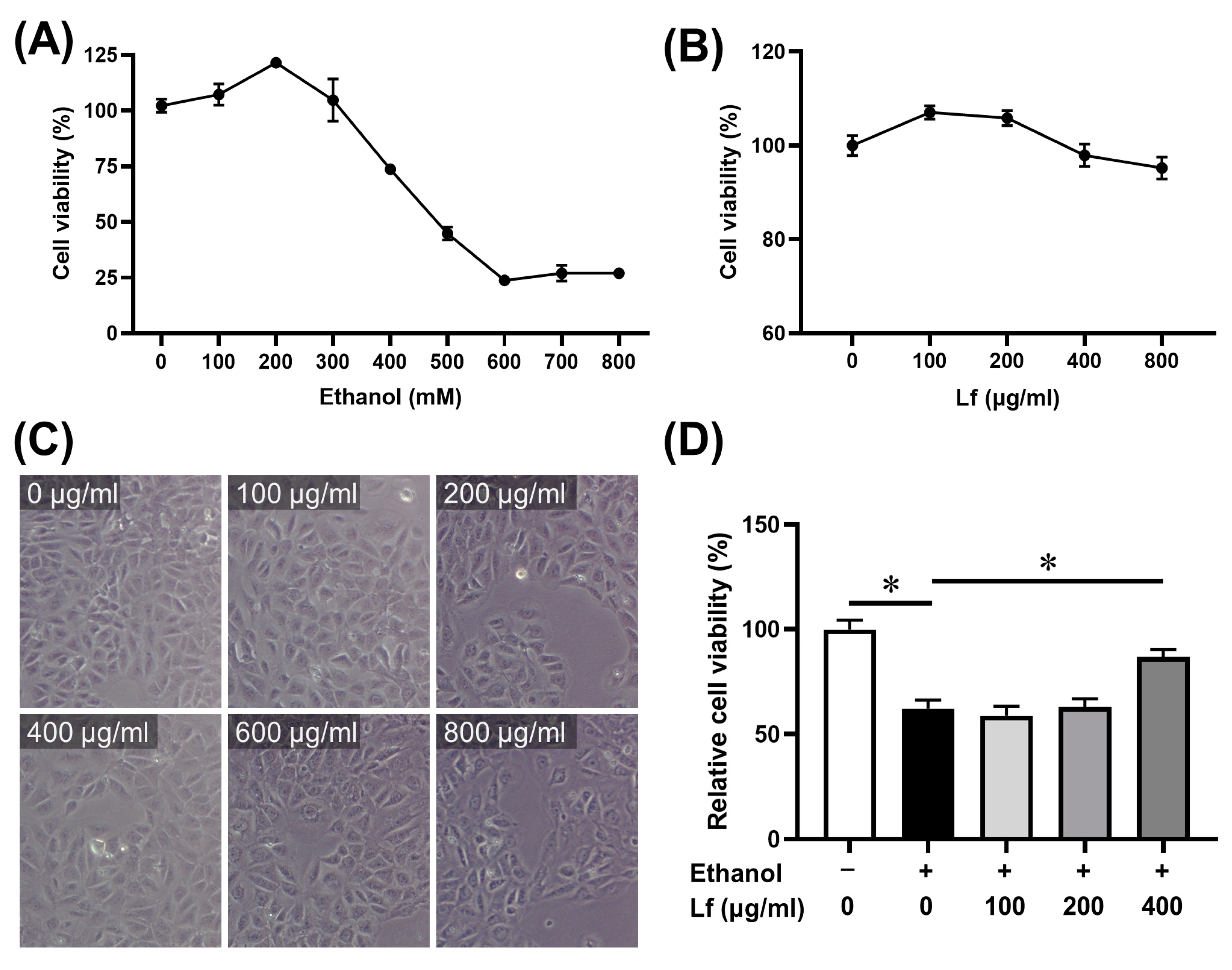
2.1. The Dosage of Ethanol and Lf
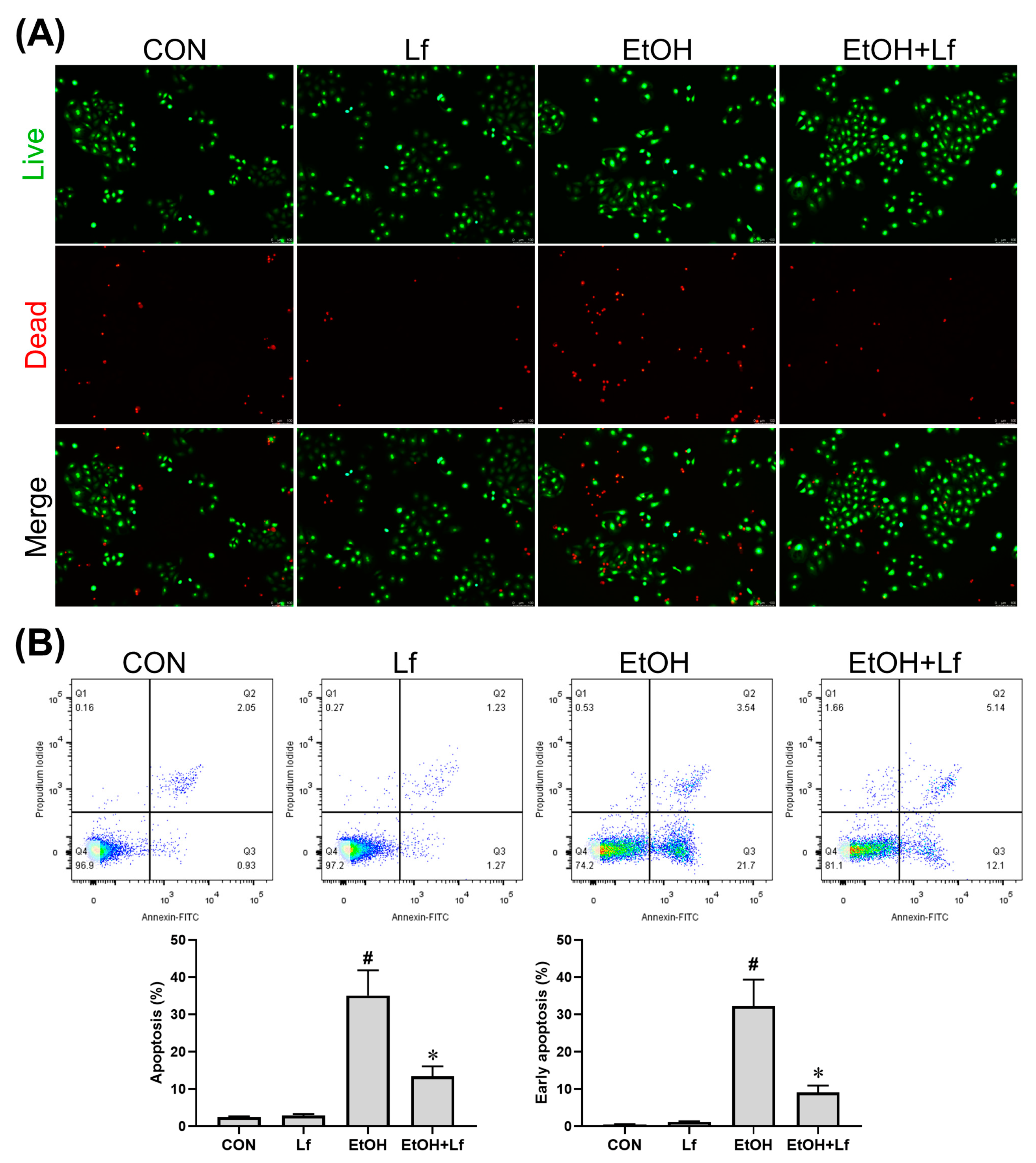
2.2. Effects of Lf on Cell Death and Apoptosis
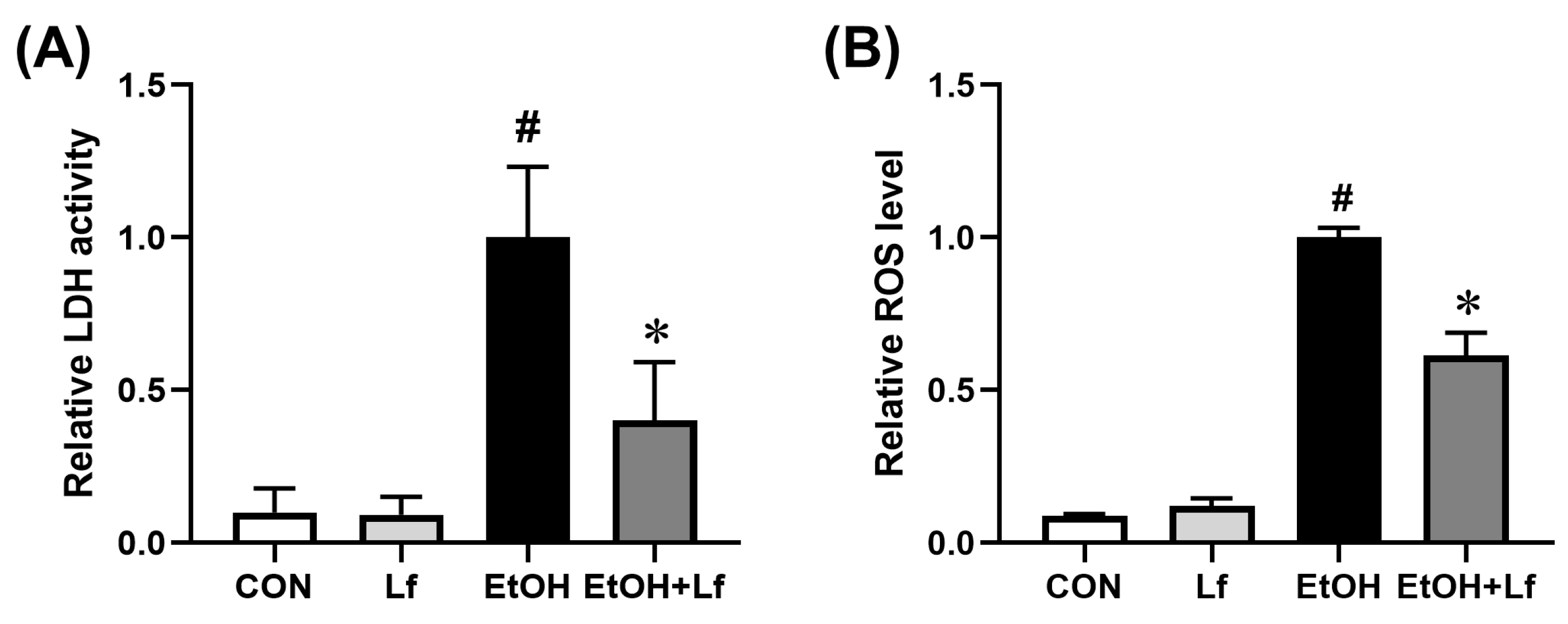
2.3. Protective Effects of Lf on Ethanol-Induced Injury in BRL-3A Cells
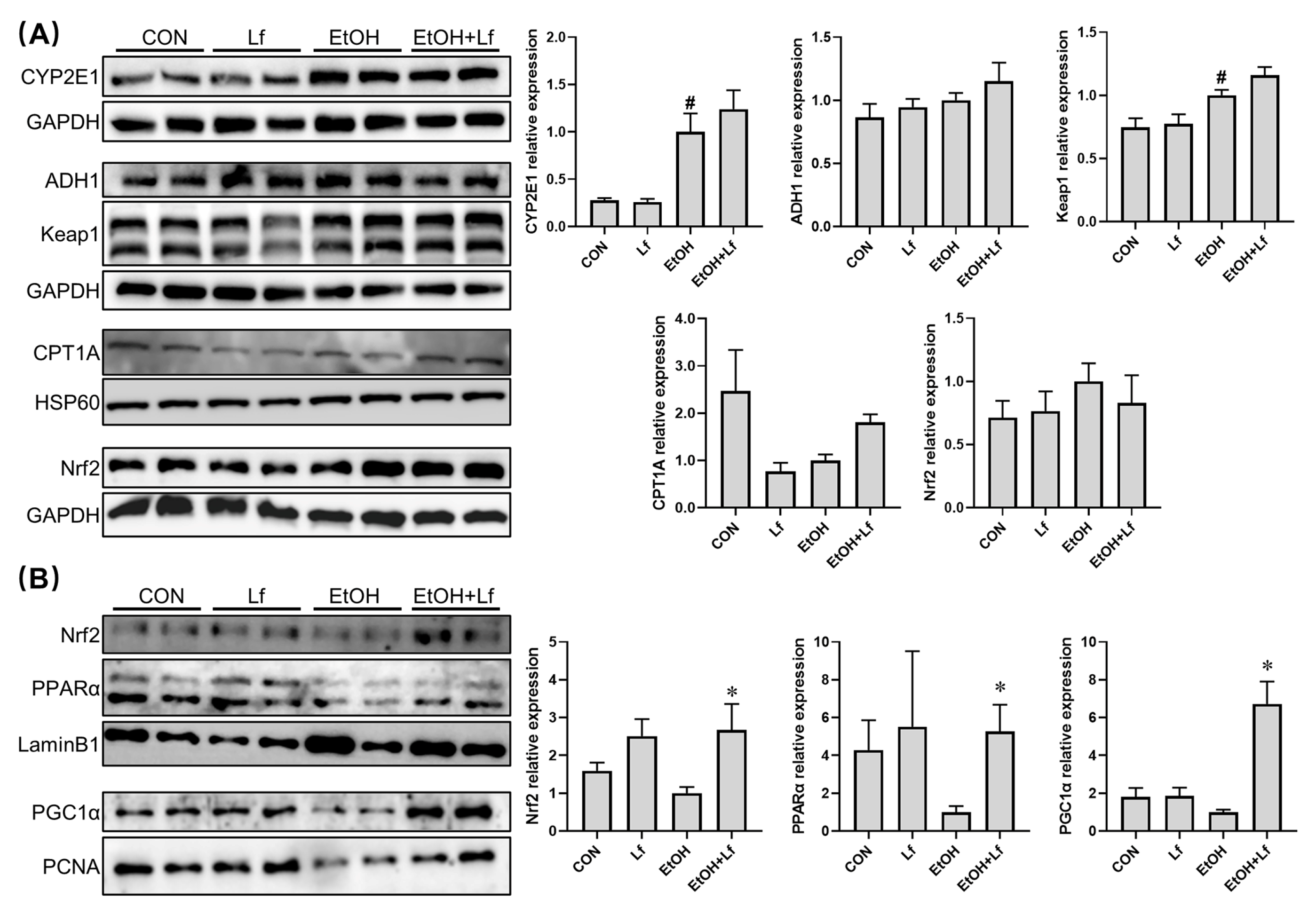
2.4. Effects of Lf on Cytoplasmic and Nuclear Protein Expression in BRL-3A Cells
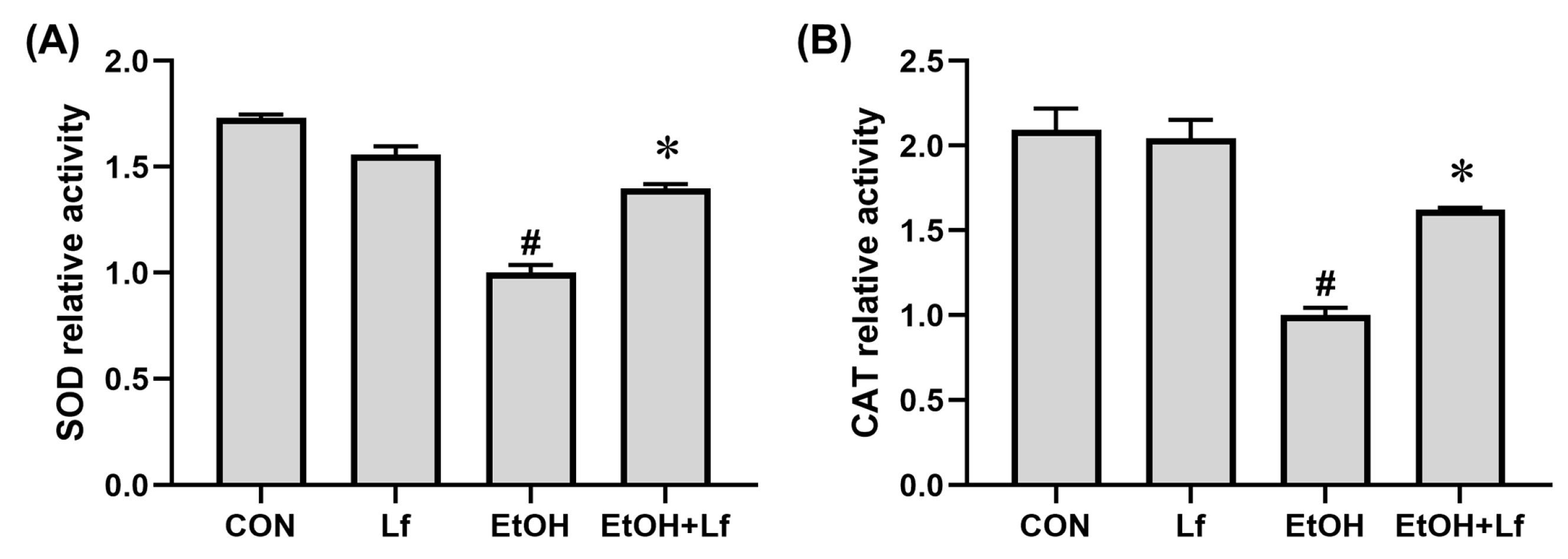
2.5. Effects of Lf on AntiOxidant Enzyme Activities
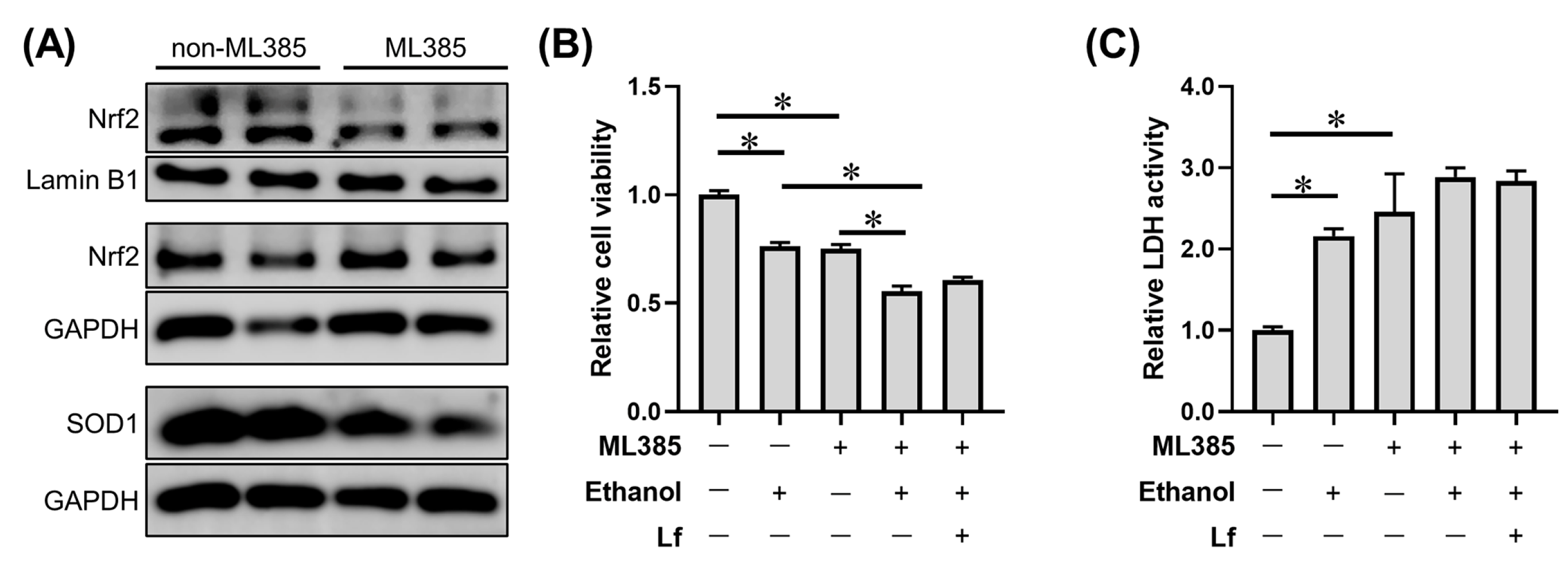
2.6. Effects of ML385 on the Protective Role of Lf in Ethanol-Induced Cell Injury
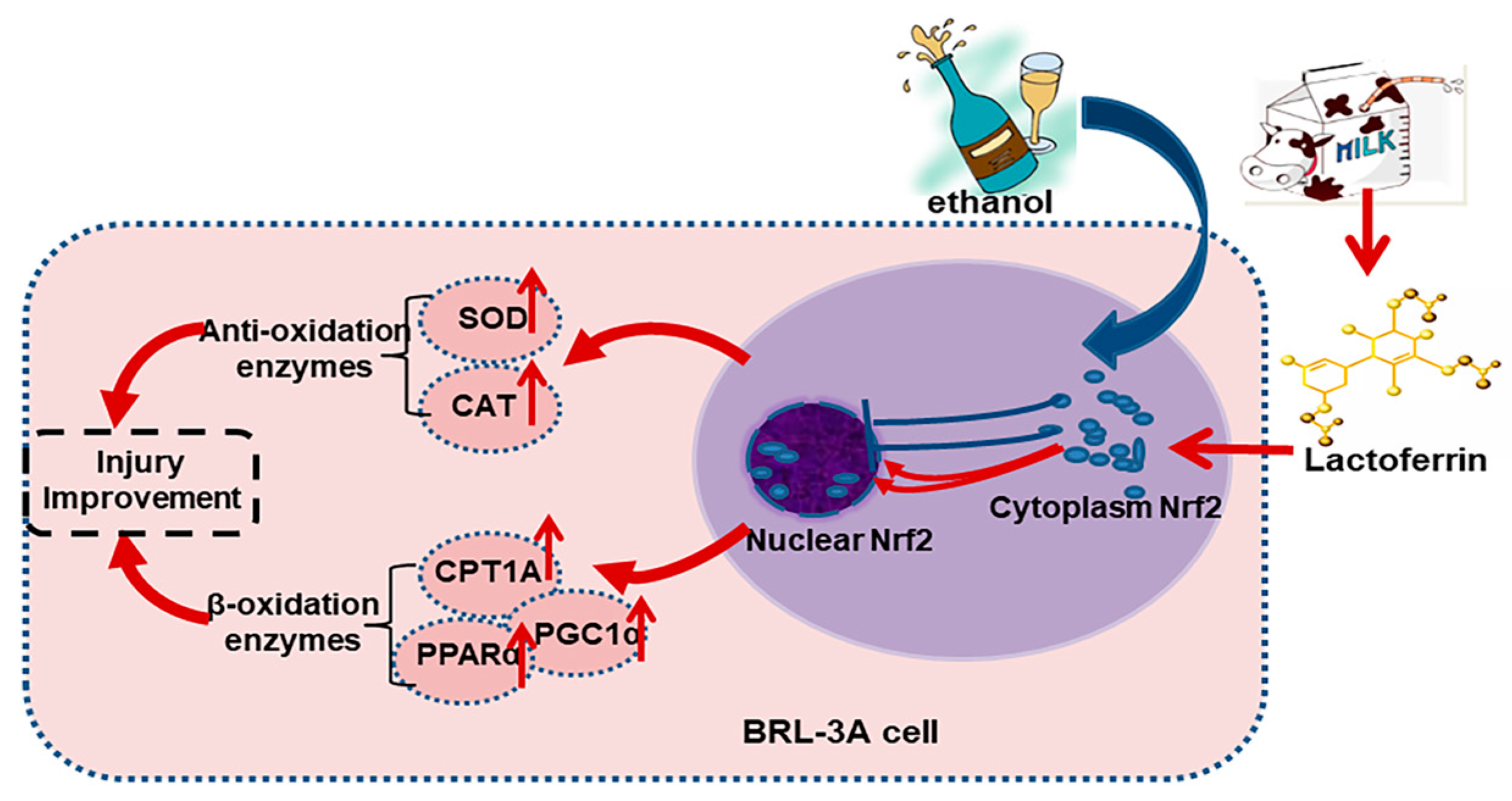
3. Discussion
4. Materials and Methods
4.1. Cells and Reagents
4.2. Cell Culture
4.3. Measurement of Cell Viability
4.4. Measurement of Cytotoxicity
4.5. Cell Treatment
- Control (CON) group: complete medium.
- Lf group: complete medium containing 400 μg/mL of Lf.
- Ethanol model (EtOH) group: complete medium containing 400 mM of ethanol.
- Lf+EtOH group: complete medium containing 400 mM of ethanol and 400 μg/mL of Lf.
4.6. Measurement of Live/Dead Cells
4.7. Measurement of Cell Apoptosis
4.8. Measurements of Intracellular ROS Level and Antioxidant Enzyme Activity
4.9. Western Blots
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Asrani, S.K.; Devarbhavi, H.; Eaton, J.; Kamath, P.S. Burden of liver diseases in the world. J. Hepatol. 2019, 70, 151–171. [Google Scholar] [CrossRef] [PubMed]
- Singal, A.K.; Bataller, R.; Ahn, J.; Kamath, P.S.; Shah, V.H. ACG Clinical Guideline: Alcoholic Liver Disease. Am. J. Gastroenterol. 2018, 113, 175–194. [Google Scholar] [CrossRef] [PubMed]
- Elzoghby, A.O.; Abdelmoneem, M.A.; Hassanin, I.A.; Abd Elwakil, M.M.; Elnaggar, M.A.; Mokhtar, S.; Fang, J.Y.; Elkhodairy, K.A. Lactoferrin, a multi-functional glycoprotein: Active therapeutic, drug nanocarrier & targeting ligand. Biomaterials 2020, 263, 120355. [Google Scholar]
- Wang, B.; Timilsena, Y.P.; Blanch, E.; Adhikari, B. Lactoferrin: Structure, Function, Denaturation and Digestion. Crit. Rev. Food Sci. Nutr. 2019, 59, 580–596. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; He, Q.; Yang, H.; Du, Y.; Yu, K.; Yang, J.; Tong, X.; Guo, Y.; Xu, J.; Qin, L. Daily Dose of Bovine Lactoferrin Prevents Ethanol-Induced Liver Injury and Death in Male Mice by Regulating Hepatic Alcohol Metabolism and Modulating Gut Microbiota. Mol. Nutr. Food Res. 2021, 65, e2100253. [Google Scholar] [CrossRef]
- Li, D.; Hu, Z.; He, Q.; Guo, Y.; Chong, Y.; Xu, J.; Qin, L. Lactoferrin Alleviates Acute Alcoholic Liver Injury by Improving Redox-Stress Response Capacity in Female C57BL/6J Mice. J. Agric. Food Chem. 2021, 69, 14856–14867. [Google Scholar] [CrossRef]
- Li, D.M.; Wu, Y.X.; Hu, Z.Q.; Wang, T.C.; Zhang, L.L.; Zhou, Y.; Tong, X.; Xu, J.Y.; Qin, L.Q. Lactoferrin Prevents Chronic Alcoholic Injury by Regulating Redox Balance and Lipid Metabolism in Female C57BL/6J Mice. Antioxidants 2022, 11, 1508. [Google Scholar] [CrossRef]
- Abdel Baky, N.A.; Al-Najjar, A.H.; Elariny, H.A.; Sallam, A.S.; Mohammed, A.A. Pramipexole and Lactoferrin ameliorate Cyclophosphamide-Induced haemorrhagic cystitis via targeting Sphk1/S1P/MAPK, TLR-4/NF-kappaB, and NLRP3/caspase-1/IL-1beta signalling pathways and modulating the Nrf2/HO-1 pathway. Int. Immunopharmacol. 2022, 112, 109282. [Google Scholar] [CrossRef]
- Essam, R.M.; Saadawy, M.A.; Gamal, M.; Abdelsalam, R.M.; El-Sahar, A.E. Lactoferrin averts neurological and behavioral impairments of thioacetamide-induced hepatic encephalopathy in rats via modulating HGMB1/TLR-4/MyD88/Nrf2 pathway. Neuropharmacology 2023, 236, 109575. [Google Scholar] [CrossRef]
- Hu, P.; Zhao, F.; Wang, J.; Zhu, W. Lactoferrin attenuates lipopolysaccharide-stimulated inflammatory responses and barrier impairment through the modulation of NF-kappaB/MAPK/Nrf2 pathways in IPEC-J2 cells. Food Funct. 2020, 11, 8516–8526. [Google Scholar] [CrossRef]
- Mohamed, O.S.; Abdel Baky, N.A.; Sayed-Ahmed, M.M.; Al-Najjar, A.H. Lactoferrin alleviates cyclophosphamide induced-nephropathy through suppressing the orchestration between Wnt4/beta-catenin and ERK1/2/NF-kappaB signaling and modulating klotho and Nrf2/HO-1 pathway. Life Sci. 2023, 319, 121528. [Google Scholar] [CrossRef] [PubMed]
- Sykiotis, G.P. Keap1/Nrf2 Signaling Pathway. Antioxidants 2021, 10, 828. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Xiao, J.H. The Keap1-Nrf2 System: A Mediator between Oxidative Stress and Aging. Oxid. Med. Cell. Longev. 2021, 2021, 6635460. [Google Scholar] [CrossRef]
- Dunn, W.; Shah, V.H. Pathogenesis of Alcoholic Liver Disease. Clin. Liver Dis. 2016, 20, 445–456. [Google Scholar] [CrossRef]
- Nagappan, A.; Kim, J.H.; Jung, D.Y.; Jung, M.H. Cryptotanshinone from the Salvia miltiorrhiza Bunge Attenuates Ethanol-Induced Liver Injury by Activation of AMPK/SIRT1 and Nrf2 Signaling Pathways. Int. J. Mol. Sci. 2019, 21, 265. [Google Scholar] [CrossRef]
- Balusikova, K.; Kovar, J. Alcohol dehydrogenase and cytochrome P450 2E1 can be induced by long-term exposure to ethanol in cultured liver HEP-G2 cells. Vitr. Cell. Dev. Biol. Anim. 2013, 49, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, K.; Swaminathan, K.; Kumar, S.M.; Chatterjee, S.; Clemens, D.L.; Dey, A. Elevated glutathione level does not protect against chronic alcohol mediated apoptosis in recombinant human hepatoma cell line VL-17A over-expressing alcohol metabolizing enzymes—Alcohol dehydrogenase and Cytochrome P450 2E1. Toxicol. Vitr. 2011, 25, 969–978. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Geng, X.C.; Wang, J.F.; Miao, Y.F.; Lu, Y.L.; Li, B. The HepaRG cell line, a superior in vitro model to L-02, HepG2 and hiHeps cell lines for assessing drug-induced liver injury. Cell Biol. Toxicol. 2016, 32, 37–59. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, Y.; Zheng, C.; Shen, C. Investigation of Cross-Contamination and Misidentification of 278 Widely Used Tumor Cell Lines. PLoS ONE 2017, 12, e0170384. [Google Scholar] [CrossRef]
- Ye, F.; Chen, C.; Qin, J.; Liu, J.; Zheng, C. Genetic profiling reveals an alarming rate of cross-contamination among human cell lines used in China. FASEB J. 2015, 29, 4268–4672. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, J.; Shen, S.; Tong, Q.; Ma, X.; Lin, L. SIRT3 promotes lipophagy and chaperon-mediated autophagy to protect hepatocytes against lipotoxicity. Cell Death Differ. 2020, 27, 329–344. [Google Scholar] [CrossRef] [PubMed]
- Okuda, T.; Haseba, T.; Katsuyama, M.; Maruyama, M.; Akimoto, T.; Igarashi, T.; Ohno, Y. Metabolic pharmacokinetics of early chronic alcohol consumption mediated by liver alcohol dehydrogenases 1 and 3 in mice. J. Gastroenterol. Hepatol. 2018, 33, 1912–1919. [Google Scholar] [CrossRef]
- Louvet, A.; Mathurin, P. Alcoholic liver disease: Mechanisms of injury and targeted treatment. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 231–242. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhai, Y.; Chen, J.; Xu, X.; Wang, H. Kaempferol Ameliorates Oxygen-Glucose Deprivation/Reoxygenation-Induced Neuronal Ferroptosis by Activating Nrf2/SLC7A11/GPX4 Axis. Biomolecules 2021, 11, 923. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Venkannagari, S.; Oh, K.H.; Zhang, Y.Q.; Rohde, J.M.; Liu, L.; Nimmagadda, S.; Sudini, K.; Brimacombe, K.R.; Gajghate, S.; et al. Small Molecule Inhibitor of NRF2 Selectively Intervenes Therapeutic Resistance in KEAP1-Deficient NSCLC Tumors. ACS Chem. Biol. 2016, 11, 3214–3225. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Shu, Y.; Wang, L.; Li, G.; Zhang, S.; Gu, W.; Sun, Y.; Hua, W.; Huang, L.; Chen, F.; et al. The protective effect of Veronica ciliata Fisch. Extracts on relieving oxidative stress-induced liver injury via activating AMPK/p62/Nrf2 pathway. J. Ethnopharmacol. 2021, 270, 113775. [Google Scholar] [CrossRef]
- Xiao, J.; Lv, Y.; Lin, B.; Tipoe, G.L.; Youdim, M.B.; Xing, F.; Liu, Y. A novel antioxidant multitarget iron chelator M30 protects hepatocytes against ethanol-induced injury. Oxid. Med. Cell Longev. 2015, 2015, 607271. [Google Scholar] [CrossRef]
- Shini, V.S.; Udayarajan, C.T.; Nisha, P. A comprehensive review on lactoferrin: A natural multifunctional glycoprotein. Food Funct. 2022, 13, 11954–11972. [Google Scholar] [CrossRef]
- Seitz, H.K. The role of cytochrome P4502E1 in the pathogenesis of alcoholic liver disease and carcinogenesis. Chem. Biol. Interact. 2020, 316, 108918. [Google Scholar] [CrossRef]
- Hu, P.; Liu, Y.; Li, S.; Zhao, Y.; Gu, H.; Zong, Q.; Ahmed, A.A.; Bao, W.; Liu, H.Y.; Cai, D. Lactoferrin Relieves Deoxynivalenol-Induced Oxidative Stress and Inflammatory Response by Modulating the Nrf2/MAPK Pathways in the Liver. J. Agric. Food Chem. 2023, 71, 8182–8191. [Google Scholar] [CrossRef]
- Zakharova, E.T.; Sokolov, A.V.; Pavlichenko, N.N.; Kostevich, V.A.; Abdurasulova, I.N.; Chechushkov, A.V.; Voynova, I.V.; Elizarova, A.Y.; Kolmakov, N.N.; Bass, M.G.; et al. Erythropoietin and Nrf2: Key factors in the neuroprotection provided by apo-lactoferrin. Biometals 2018, 31, 425–443. [Google Scholar] [CrossRef] [PubMed]
- Donato, M.T.; Tolosa, L.; Gomez-Lechon, M.J. Culture and Functional Characterization of Human Hepatoma HepG2 Cells. Methods Mol. Biol. 2015, 1250, 77–93. [Google Scholar] [PubMed]
- Thomas, J.P.; Geiger, P.G.; Girotti, A.W. Lethal damage to endothelial cells by oxidized low density lipoprotein: Role of selenoperoxidases in cytoprotection against lipid hydroperoxide- and iron-mediated reactions. J. Lipid. Res. 1993, 34, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Liu, F.; Wang, X.; Li, X. Apple Polyphenol Extract Alleviates High-Fat-Diet-Induced Hepatic Steatosis in Male C57BL/6 Mice by Targeting LKB1/AMPK Pathway. J. Agric. Food Chem. 2019, 67, 12208–12218. [Google Scholar] [CrossRef]
- Li, D.; Cui, Y.; Wang, X.; Liu, F.; Li, X. Apple Polyphenol Extract Improves High-Fat Diet-Induced Hepatic Steatosis by Regulating Bile Acid Synthesis and Gut Microbiota in C57BL/6 Male Mice. J. Agric. Food Chem. 2021, 69, 6829–6841. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, D.; Ding, L.; Yan, Y.; Xing, Y.; Xu, J.; Qin, L. Lactoferrin Alleviates Ethanol-Induced Injury via Promoting Nrf2 Nuclear Translocation in BRL-3A Rat Liver Cells. Int. J. Mol. Sci. 2023, 24, 16848. https://doi.org/10.3390/ijms242316848
Li D, Ding L, Yan Y, Xing Y, Xu J, Qin L. Lactoferrin Alleviates Ethanol-Induced Injury via Promoting Nrf2 Nuclear Translocation in BRL-3A Rat Liver Cells. International Journal of Molecular Sciences. 2023; 24(23):16848. https://doi.org/10.3390/ijms242316848
Chicago/Turabian StyleLi, Deming, Li Ding, Yilin Yan, Yifei Xing, Jiaying Xu, and Liqiang Qin. 2023. "Lactoferrin Alleviates Ethanol-Induced Injury via Promoting Nrf2 Nuclear Translocation in BRL-3A Rat Liver Cells" International Journal of Molecular Sciences 24, no. 23: 16848. https://doi.org/10.3390/ijms242316848
APA StyleLi, D., Ding, L., Yan, Y., Xing, Y., Xu, J., & Qin, L. (2023). Lactoferrin Alleviates Ethanol-Induced Injury via Promoting Nrf2 Nuclear Translocation in BRL-3A Rat Liver Cells. International Journal of Molecular Sciences, 24(23), 16848. https://doi.org/10.3390/ijms242316848





