Multi-Organ Increase in Norepinephrine Levels after Central Leptin Administration and Diet-Induced Obesity
Abstract
:1. Introduction
2. Results
2.1. Intracerebroventricular Leptin Injection Increases the Concentration of Norepinephrine in Different Tissues
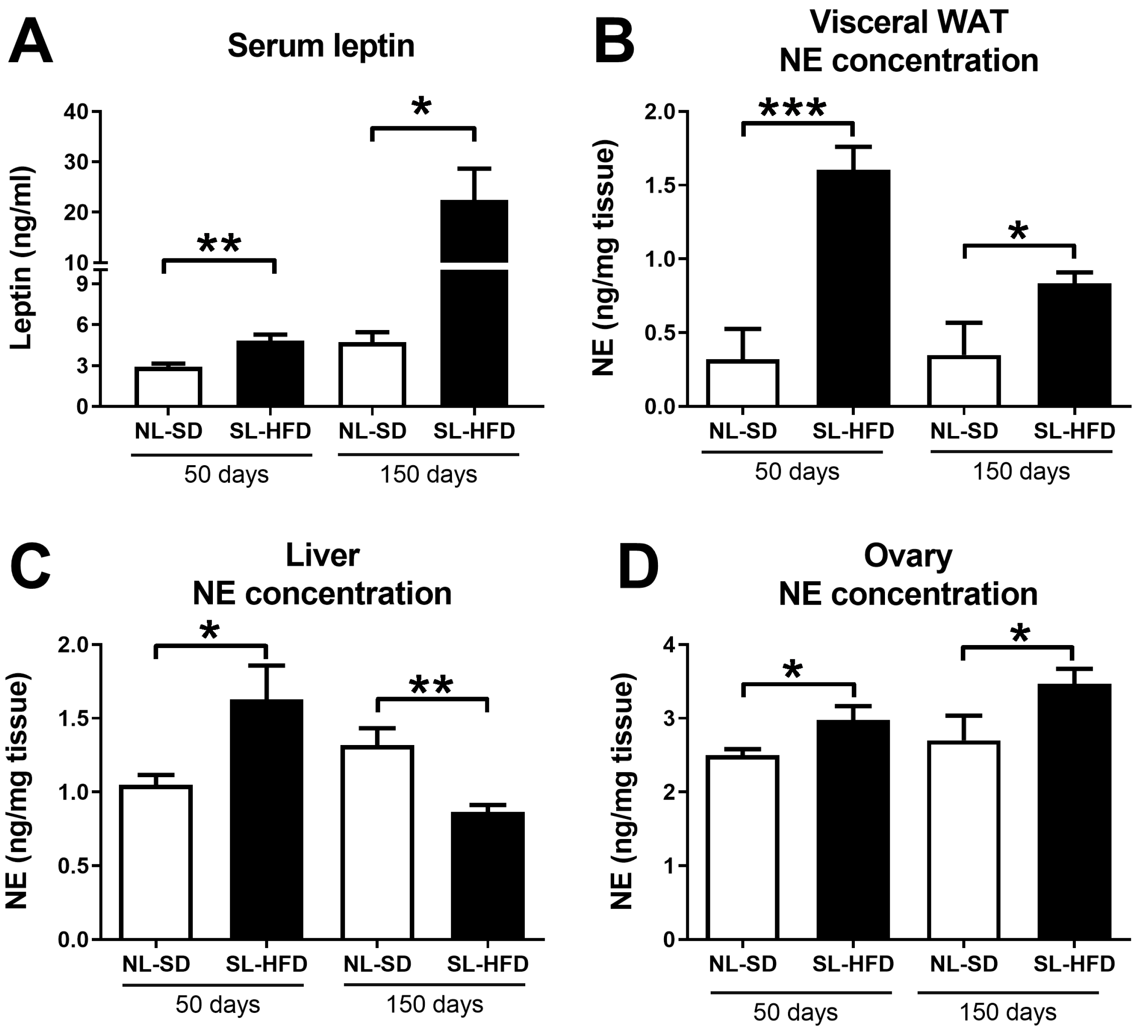
2.2. Obesity Leads to a Simultaneous Increase in Serum Leptin and Norepinephrine in the Liver and Ovaries
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Experimental Design
- Experiment 1:
- Experiment 2:
4.3. Norepinephrine Concentration
5. Conclusions
Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Manfredi-Lozano, M.; Roa, J.; Tena-Sempere, M. Connecting metabolism and gonadal function: Novel central neuropeptide pathways involved in the metabolic control of puberty and fertility. Front. Neuroendocrinol. 2018, 48, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.W.; Elias, C.F. Neuroanatomical Framework of the Metabolic Control of Reproduction. Physiol. Rev. 2018, 98, 2349–2380. [Google Scholar] [CrossRef] [PubMed]
- Navarro, V.M.; Kaiser, U.B. Metabolic influences on neuroendocrine regulation of reproduction. Curr. Opin. Endocrinol. Diabetes Obes. 2013, 20, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Sinha, M.K.; Caro, J.F. Clinical aspects of leptin. Vitam. Horm. 1998, 54, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Dalamaga, M.; Chou, S.H.; Shields, K.; Papageorgiou, P.; Polyzos, S.A.; Mantzoros, C.S. Leptin at the intersection of neuroendocrinology and metabolism: Current evidence and therapeutic perspectives. Cell Metab. 2013, 18, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Lavoie, O.; Michael, N.J.; Caron, A. A critical update on the leptin-melanocortin system. J. Neurochem. 2023, in press. [CrossRef]
- Commins, S.P.; Watson, P.M.; Levin, N.; Beiler, R.J.; Gettys, T.W. Central leptin regulates the UCP1 and ob genes in brown and white adipose tissue via different beta-adrenoceptor subtypes. J. Biol. Chem. 2000, 275, 33059–33067. [Google Scholar] [CrossRef] [PubMed]
- Püschel, G.P. Control of hepatocyte metabolism by sympathetic and parasympathetic hepatic nerves. Anat. Rec. A Discov. Mol. Cell Evol. Biol. 2004, 280, 854–867. [Google Scholar] [CrossRef]
- Vázquez, M.J.; Romero-Ruiz, A.; Tena-Sempere, M. Roles of leptin in reproduction, pregnancy and polycystic ovary syndrome: Consensus knowledge and recent developments. Metabolism 2015, 64, 79–91. [Google Scholar] [CrossRef]
- Chen, X.; Xiao, Z.; Cai, Y.; Huang, L.; Chen, C. Hypothalamic mechanisms of obesity-associated disturbance of hypothalamic-pituitary-ovarian axis. Trends Endocrinol. Metab. 2022, 33, 206–217. [Google Scholar] [CrossRef]
- Ricu, M.; Paredes, A.; Greiner, M.; Ojeda, S.R.; Lara, H.E. Functional development of the ovarian noradrenergic innervation. Endocrinology 2008, 149, 50–56. [Google Scholar] [CrossRef]
- Lara, H.E.; Dorfman, M.; Venegas, M.; Luza, S.M.; Luna, S.L.; Mayerhofer, A.; Guimaraes, M.A.; Rosa E Silva, A.A.; Ramírez, V.D. Changes in sympathetic nerve activity of the mammalian ovary during a normal estrous cycle and in polycystic ovary syndrome: Studies on norepinephrine release. Microsc. Res. Tech. 2002, 59, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Lara, H.E.; Porcile, A.; Espinoza, J.; Romero, C.; Luza, S.M.; Fuhrer, J.; Miranda, C.; Roblero, L. Release of norepinephrine from human ovary: Coupling to steroidogenic response. Endocrine 2001, 15, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Lara, H.E.; McDonald, J.K.; Ahmed, C.E.; Ojeda, S.R. Guanethidine-mediated destruction of ovarian sympathetic nerves disrupts ovarian development and function in rats. Endocrinology 1990, 127, 2199–2209. [Google Scholar] [CrossRef] [PubMed]
- Gerendai, I.; Toth, I.E.; Boldogkoi, Z.; Halasz, B. Recent findings on the organization of central nervous system structures involved in the innervation of endocrine glands and other organs; observations obtained by the transneuronal viral double-labeling technique. Endocrine 2009, 36, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Uyama, N.; Geerts, A.; Reynaert, H. Neural connections between the hypothalamus and the liver. Anat. Rec. A Discov. Mol. Cell Evol. Biol. 2004, 280, 808–820. [Google Scholar] [CrossRef] [PubMed]
- Fiedler, J.; Jara, P.; Luza, S.; Dorfman, M.; Grouselle, D.; Rage, F.; Lara, H.E.; Arancibia, S. Cold stress induces metabolic activation of thyrotrophin-releasing hormone-synthesising neurones in the magnocellular division of the hypothalamic paraventricular nucleus and concomitantly changes ovarian sympathetic activity parameters. J. Neuroendocrinol. 2006, 18, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Gao, X.; Pan, X.F.; Zhou, T.; Zhu, C.; Li, F.; Fan, J.G.; Targher, G.; Zhao, J. The hepato-ovarian axis: Genetic evidence for a causal association between non-alcoholic fatty liver disease and polycystic ovary syndrome. BMC Med. 2023, 21, 62. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.J.; Kim, D.; Yim, J.Y.; Kang, J.H.; Han, K.H.; Kim, S.M.; Hwang, K.R.; Ku, S.Y.; Suh, C.S.; Kim, S.H.; et al. Polycystic ovary syndrome with hyperandrogenism as a risk factor for non-obese non-alcoholic fatty liver disease. Aliment. Pharmacol. Ther. 2017, 45, 1403–1412. [Google Scholar] [CrossRef]
- Cox, A.J.; West, N.P.; Cripps, A.W. Obesity, inflammation, and the gut microbiota. Lancet Diabetes Endocrinol. 2015, 3, 207–215. [Google Scholar] [CrossRef]
- Schwartz, M.W.; Seeley, R.J.; Zeltser, L.M.; Drewnowski, A.; Ravussin, E.; Redman, L.M.; Leibel, R.L. Obesity Pathogenesis: An Endocrine Society Scientific Statement. Endocr. Rev. 2017, 38, 267–296. [Google Scholar] [CrossRef]
- Lee, E.; Korf, H.; Vidal-Puig, A. An adipocentric perspective on the development and progression of non-alcoholic fatty liver disease. J. Hepatol. 2023, in press. [CrossRef]
- Joham, A.E.; Norman, R.J.; Stener-Victorin, E.; Legro, R.S.; Franks, S.; Moran, L.J.; Boyle, J.; Teede, H.J. Polycystic ovary syndrome. Lancet Diabetes Endocrinol. 2022, 10, 668–680. [Google Scholar] [CrossRef] [PubMed]
- Lansdown, A.; Rees, D.A. The sympathetic nervous system in polycystic ovary syndrome: A novel therapeutic target? Clin. Endocrinol. 2012, 77, 791–801. [Google Scholar] [CrossRef] [PubMed]
- Carnagarin, R.; Tan, K.; Adams, L.; Matthews, V.B.; Kiuchi, M.G.; Marisol Lugo Gavidia, L.; Lambert, G.W.; Lambert, E.A.; Herat, L.Y.; Schlaich, M.P. Metabolic Dysfunction-Associated Fatty Liver Disease (MAFLD)-A Condition Associated with Heightened Sympathetic Activation. Int. J. Mol. Sci. 2021, 22, 4241. [Google Scholar] [CrossRef] [PubMed]
- Brito, N.A.; Brito, M.N.; Bartness, T.J. Differential sympathetic drive to adipose tissues after food deprivation, cold exposure or glucoprivation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 294, R1445–R1452. [Google Scholar] [CrossRef] [PubMed]
- Sipe, L.M.; Yang, C.; Ephrem, J.; Garren, E.; Hirsh, J.; Deppmann, C.D. Differential sympathetic outflow to adipose depots is required for visceral fat loss in response to calorie restriction. Nutr. Diabetes 2017, 7, e260. [Google Scholar] [CrossRef] [PubMed]
- Barria, A.; Leyton, V.; Ojeda, S.R.; Lara, H.E. Ovarian steroidal response to gonadotropins and beta-adrenergic stimulation is enhanced in polycystic ovary syndrome: Role of sympathetic innervation. Endocrinology 1993, 133, 2696–2703. [Google Scholar] [CrossRef] [PubMed]
- Riquelme, R.; Ruz, F.; Mayerhofer, A.; Lara, H.E. Role of ovarian sympathetic nerves and cholinergic local system during cold stress. J. Endocrinol. 2019, 242, 115–124. [Google Scholar] [CrossRef]
- Bernuci, M.P.; Szawka, R.E.; Helena, C.V.; Leite, C.M.; Lara, H.E.; Anselmo-Franci, J.A. Locus coeruleus mediates cold stress-induced polycystic ovary in rats. Endocrinology 2008, 149, 2907–2916. [Google Scholar] [CrossRef]
- Dorfman, M.; Arancibia, S.; Fiedler, J.L.; Lara, H.E. Chronic intermittent cold stress activates ovarian sympathetic nerves and modifies ovarian follicular development in the rat. Biol. Reprod. 2003, 68, 2038–2043. [Google Scholar] [CrossRef]
- Jung, I.; Lee, D.Y.; Lee, M.Y.; Kwon, H.; Rhee, E.J.; Park, C.Y.; Oh, K.W.; Lee, W.Y.; Park, S.W.; Park, S.E. Autonomic Imbalance Increases the Risk for Non-alcoholic Fatty Liver Disease. Front. Endocrinol. 2021, 12, 752944. [Google Scholar] [CrossRef]
- Hurr, C.; Simonyan, H.; Morgan, D.A.; Rahmouni, K.; Young, C.N. Liver sympathetic denervation reverses obesity-induced hepatic steatosis. J. Physiol. 2019, 597, 4565–4580. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, S.; Ashraf, N.; Yilmaz, G.; Harmancey, R. Crosstalk between beta-adrenergic and insulin signaling mediates mechanistic target of rapamycin hyperactivation in liver of high-fat diet-fed male mice. Physiol. Rep. 2021, 9, e14958. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Yang, L.; Wang, G.; Liu, J.; Zhao, X.; Wang, Y.; Li, J.; Yang, J. Metabolic stress drives sympathetic neuropathy within the liver. Cell Metab. 2021, 33, 666–675 e664. [Google Scholar] [CrossRef] [PubMed]
- Yildirir, A.; Aybar, F.; Kabakci, G.; Yarali, H.; Oto, A. Heart rate variability in young women with polycystic ovary syndrome. Ann. Noninvasive Electrocardiol. 2006, 11, 306–312. [Google Scholar] [CrossRef]
- Lara, H.E.; Ferruz, J.L.; Luza, S.; Bustamante, D.A.; Borges, Y.; Ojeda, S.R. Activation of ovarian sympathetic nerves in polycystic ovary syndrome. Endocrinology 1993, 133, 2690–2695. [Google Scholar] [CrossRef]
- Rosa-E-Silva, A.; Guimaraes, M.A.; Padmanabhan, V.; Lara, H.E. Prepubertal administration of estradiol valerate disrupts cyclicity and leads to cystic ovarian morphology during adult life in the rat: Role of sympathetic innervation. Endocrinology 2003, 144, 4289–4297. [Google Scholar] [CrossRef]
- Peng, Y.; Yang, H.; Song, J.; Feng, D.; Na, Z.; Jiang, H.; Meng, Y.; Shi, B.; Li, D. Elevated Serum Leptin Levels as a Predictive Marker for Polycystic Ovary Syndrome. Front. Endocrinol. 2022, 13, 845165. [Google Scholar] [CrossRef]
- Guzelkas, I.; Orbak, Z.; Doneray, H.; Ozturk, N.; Sagsoz, N. Serum kisspeptin, leptin, neuropeptide Y, and neurokinin B levels in adolescents with polycystic ovary syndrome. J. Pediatr. Endocrinol. Metab. 2022, 35, 481–487. [Google Scholar] [CrossRef]
- Dawood, A.S.; Elgergawy, A.; Elhalwagy, A. Circulating Levels of Vitamin D. J. Hum. Reprod. Sci. 2018, 11, 343–347. [Google Scholar] [CrossRef]
- van Houten, E.L.; Kramer, P.; McLuskey, A.; Karels, B.; Themmen, A.P.; Visser, J.A. Reproductive and metabolic phenotype of a mouse model of PCOS. Endocrinology 2012, 153, 2861–2869. [Google Scholar] [CrossRef]
- EU Parliament; Council of the European Union. Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the Protection of Animals Used for Scientific Purposes. Off. J. Eur. Union 2010, 276, 33–79. [Google Scholar]
- Seoane-Collazo, P.; Lopez, M. Analyzing AMPK Function in the Hypothalamus. Methods Mol. Biol. 2018, 1732, 433–448. [Google Scholar] [CrossRef] [PubMed]
- Heras, V.; Castellano, J.M.; Fernandois, D.; Velasco, I.; Rodriguez-Vazquez, E.; Roa, J.; Vazquez, M.J.; Ruiz-Pino, F.; Rubio, M.; Pineda, R.; et al. Central Ceramide Signaling Mediates Obesity-Induced Precocious Puberty. Cell Metab. 2020, 32, 951–966.e8. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Garrido, M.A.; Castellano, J.M.; Ruiz-Pino, F.; Garcia-Galiano, D.; Manfredi-Lozano, M.; Leon, S.; Romero-Ruiz, A.; Dieguez, C.; Pinilla, L.; Tena-Sempere, M. Metabolic programming of puberty: Sexually dimorphic responses to early nutritional challenges. Endocrinology 2013, 154, 3387–3400. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Garrido, M.A.; Ruiz-Pino, F.; Pozo-Salas, A.I.; Castellano, J.M.; Vazquez, M.J.; Luque, R.M.; Tena-Sempere, M. Early overnutrition sensitizes the growth hormone axis to the impact of diet-induced obesity via sex-divergent mechanisms. Sci. Rep. 2020, 10, 13898. [Google Scholar] [CrossRef]
- Barroso, A.; Santos-Marcos, J.A.; Perdices-Lopez, C.; Vega-Rojas, A.; Sanchez-Garrido, M.A.; Krylova, Y.; Molina-Abril, H.; Ohlsson, C.; Perez-Martinez, P.; Poutanen, M.; et al. Neonatal exposure to androgens dynamically alters gut microbiota architecture. J. Endocrinol. 2020, 247, 69–85. [Google Scholar] [CrossRef]
- Castellano, J.M.; Bentsen, A.H.; Sanchez-Garrido, M.A.; Ruiz-Pino, F.; Romero, M.; Garcia-Galiano, D.; Aguilar, E.; Pinilla, L.; Dieguez, C.; Mikkelsen, J.D.; et al. Early metabolic programming of puberty onset: Impact of changes in postnatal feeding and rearing conditions on the timing of puberty and development of the hypothalamic kisspeptin system. Endocrinology 2011, 152, 3396–3408. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernandois, D.; Vázquez, M.J.; Barroso, A.; Paredes, A.H.; Tena-Sempere, M.; Cruz, G. Multi-Organ Increase in Norepinephrine Levels after Central Leptin Administration and Diet-Induced Obesity. Int. J. Mol. Sci. 2023, 24, 16909. https://doi.org/10.3390/ijms242316909
Fernandois D, Vázquez MJ, Barroso A, Paredes AH, Tena-Sempere M, Cruz G. Multi-Organ Increase in Norepinephrine Levels after Central Leptin Administration and Diet-Induced Obesity. International Journal of Molecular Sciences. 2023; 24(23):16909. https://doi.org/10.3390/ijms242316909
Chicago/Turabian StyleFernandois, Daniela, María Jesús Vázquez, Alexia Barroso, Alfonso H. Paredes, Manuel Tena-Sempere, and Gonzalo Cruz. 2023. "Multi-Organ Increase in Norepinephrine Levels after Central Leptin Administration and Diet-Induced Obesity" International Journal of Molecular Sciences 24, no. 23: 16909. https://doi.org/10.3390/ijms242316909




