Refining Immunogenicity through Intradermal Delivery of Outer Membrane Vesicles against Shigella flexneri in Mice
Abstract
:1. Introduction
2. Results
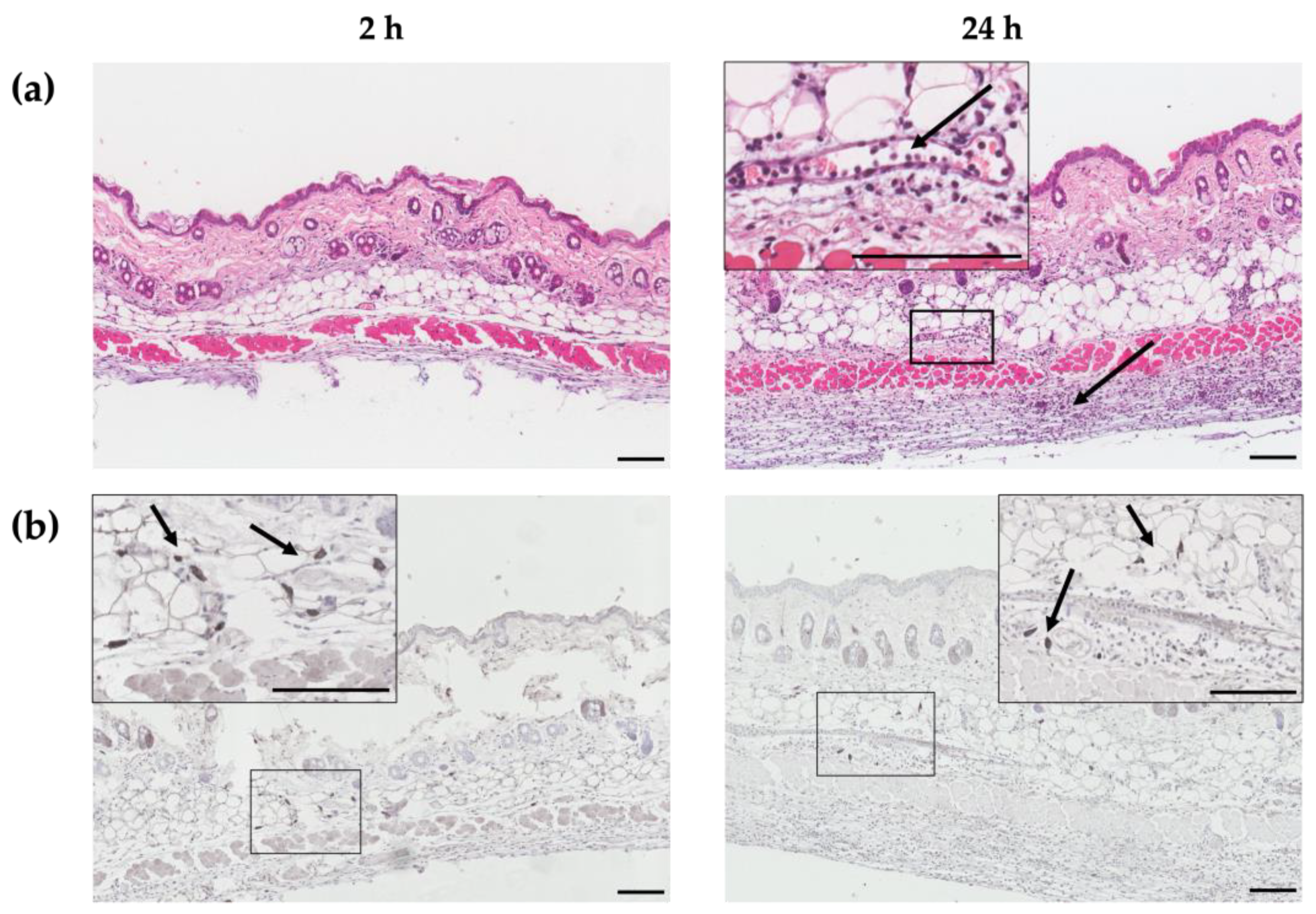
2.1. ID Immunization Recruits Immune Cells into Dermis
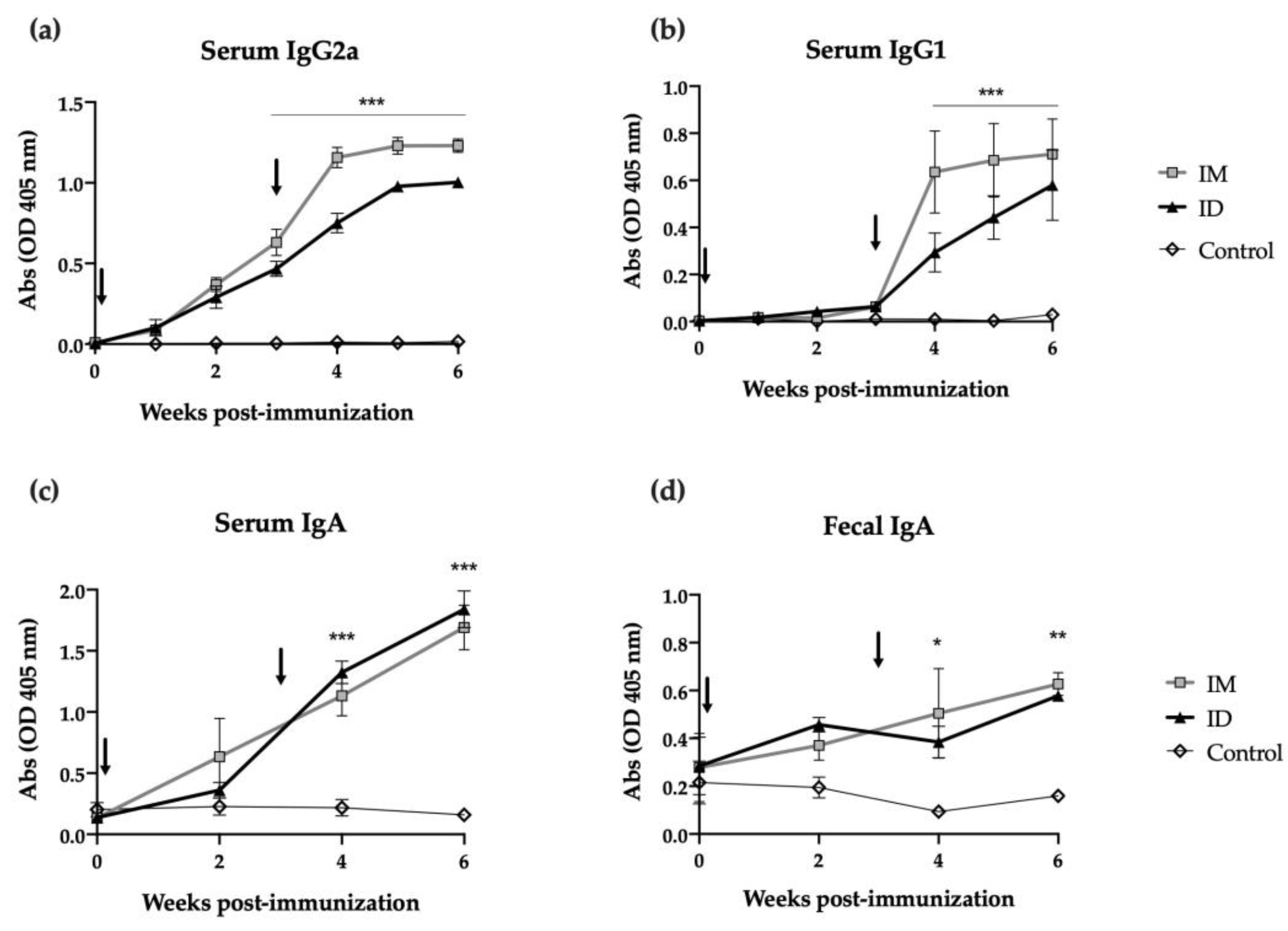
2.2. ID Administered HT-ΔtolR Vaccine Induces Specific B Cell Response in Mice
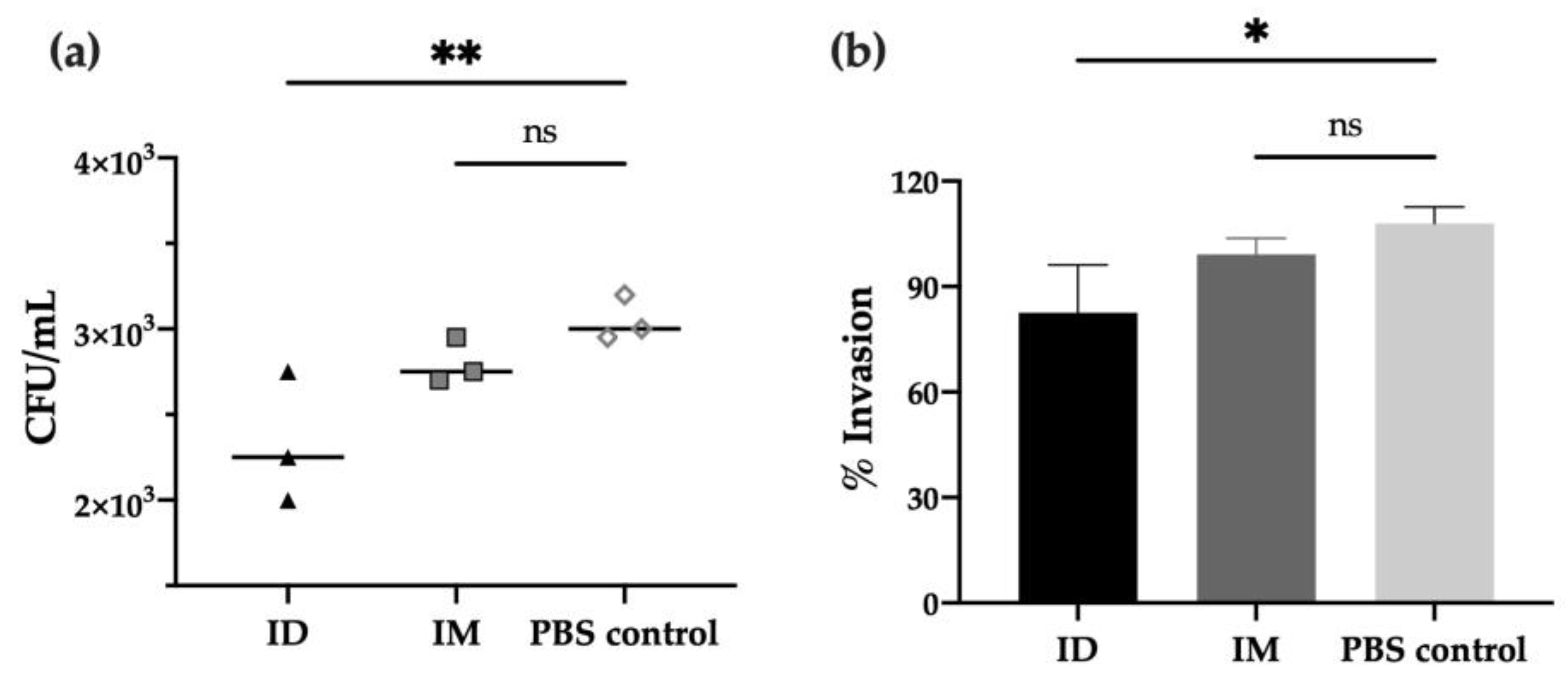
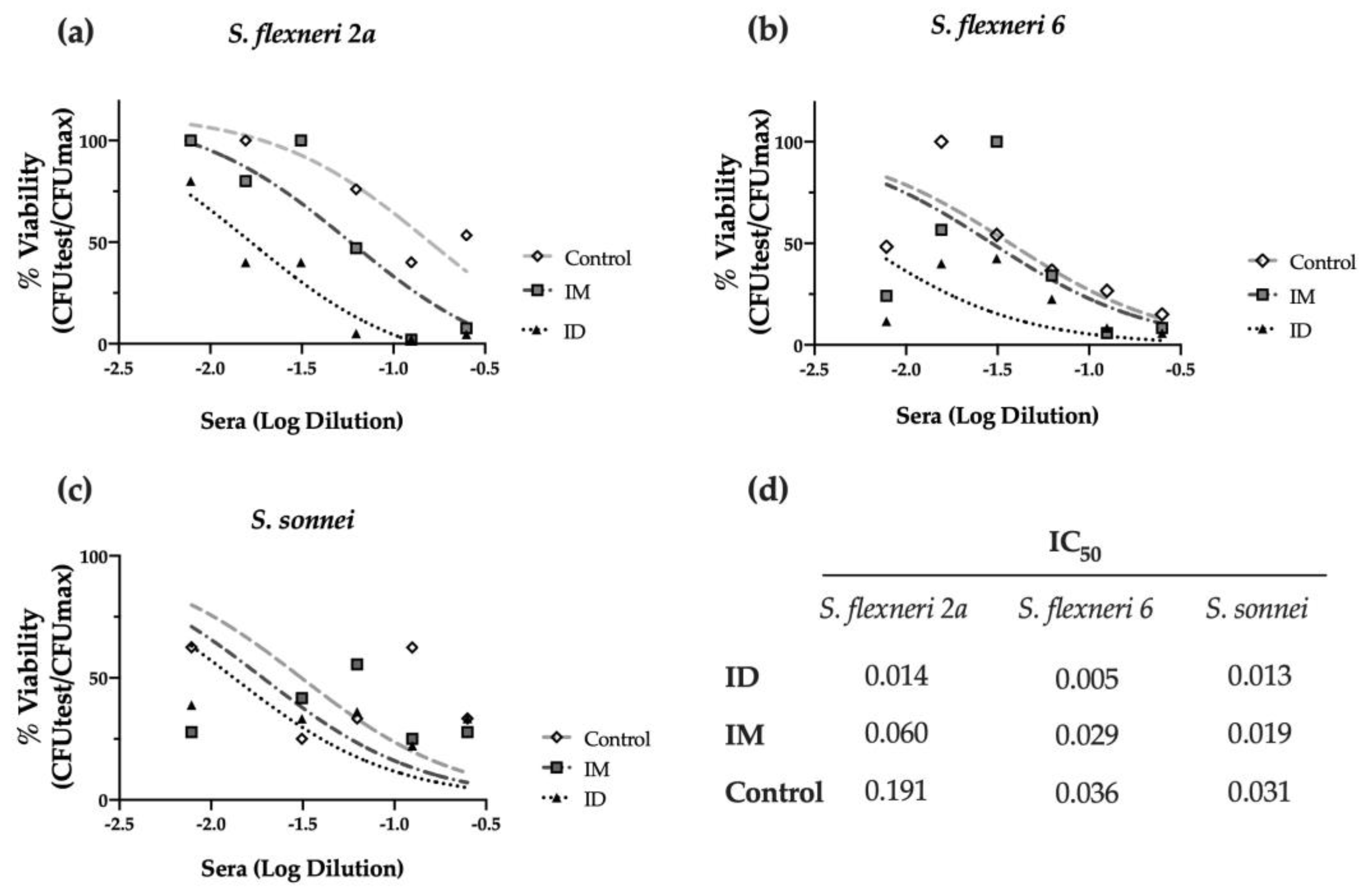
2.3. ID Delivery of HT-ΔtolR Induces Neutralizing Antibodies against Shigella
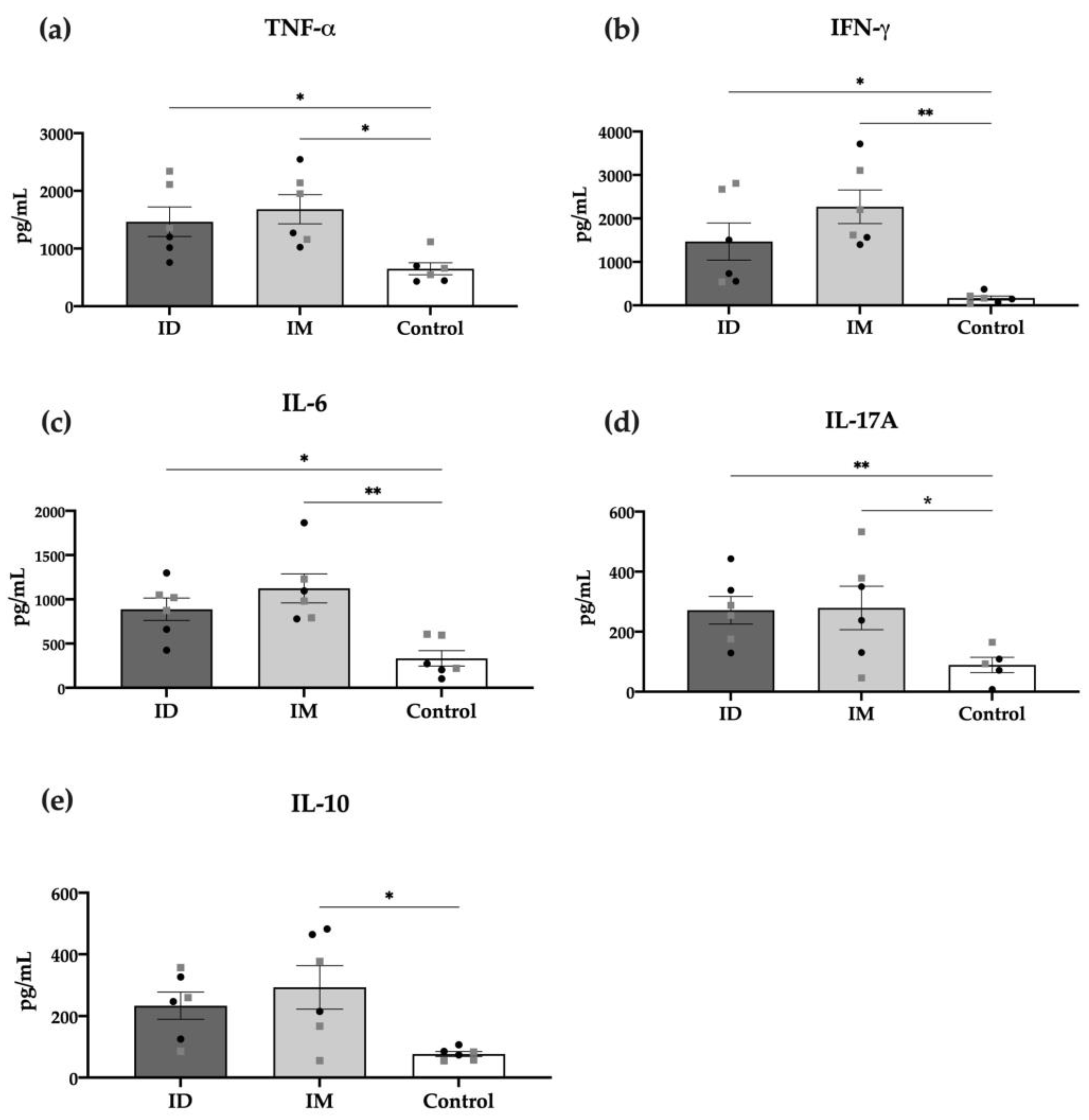
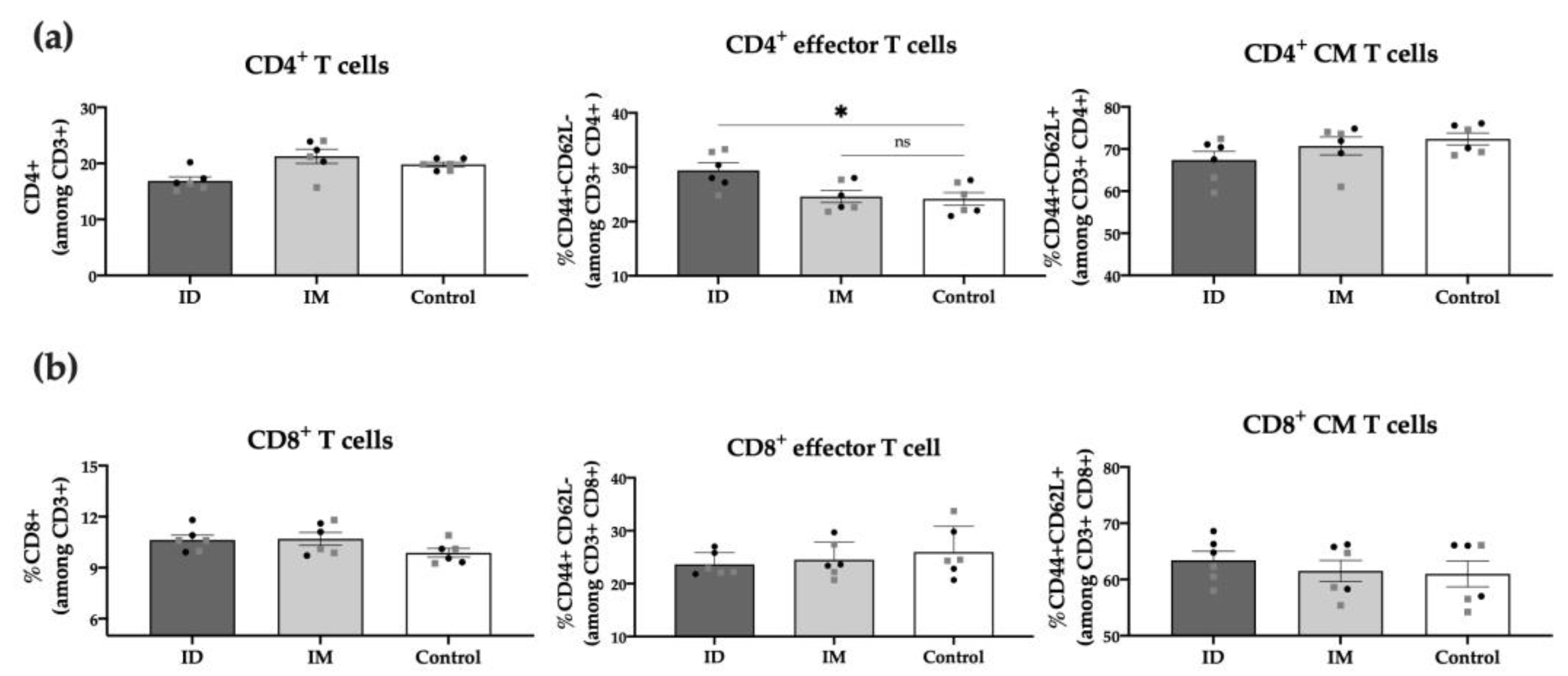
2.4. HT-ΔtolR ID Vaccination Elicits Specific T Cell Response in Mice
3. Discussion
4. Materials and Methods
4.1. Animal Ethics Statement
4.2. Preparation of Outer Membrane Vesicles
4.3. Mice Immunization
4.4. Histology and Immunohistochemistry
4.5. Splenic Cells Processing
4.6. Specific Antibody-Mediated Responses
4.7. Adhesion/Invasion Assay
4.8. Serum Bactericidal Assay
4.9. Cellular Immune Responses
4.10. Flow Cytometry Analysis
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raso, M.M.; Arato, V.; Gasperini, G.; Micoli, F. Toward a Shigella Vaccine: Opportunities and Challenges to Fight an Antimicrobial-Resistant Pathogen. Int. J. Mol. Sci. 2023, 24, 4649. [Google Scholar] [CrossRef]
- Khalil, I.A.; Troeger, C.; Blacker, B.F.; Rao, P.C.; Brown, A.; Atherly, D.E.; Brewer, T.G.; Engmann, C.M.; Houpt, E.R.; Kang, G.; et al. Morbidity and Mortality Due to Shigella and Enterotoxigenic Escherichia Coli Diarrhoea: The Global Burden of Disease Study 1990–2016. Lancet Infect. Dis. 2018, 18, 1229–1240. [Google Scholar] [CrossRef]
- Eurepean Centre for Disease Prevention and Control. Annual Epidemiological Report for 2020: Shigellosis; ECDC: Stockholm, Sweden, 2022. [Google Scholar]
- Centers for Disease Control and Prevention; National Center for Emerging and Zoonotic Infectious Diseases (NCEZID); Division of Global Migration and Quarantine (DGMQ). Shigella—Shigellosis. CDC: Atlanta, GA, USA, 2017. Available online: https://www.cdc.gov/shigella/general-information.html (accessed on 29 September 2023).
- Ranjbar, R.; Farahani, A. Shigella: Antibiotic-Resistance Mechanisms and New Horizons for Treatment. Infect. Drug Resist. 2019, 12, 3137–3167. [Google Scholar] [CrossRef]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, Research, and Development of New Antibiotics: The WHO Priority List of Antibiotic-Resistant Bacteria and Tuberculosis. Lancet Infect Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- Livio, S.; Strockbine, N.A.; Panchalingam, S.; Tennant, S.M.; Barry, E.M.; Marohn, M.E.; Antonio, M.; Hossain, A.; Mandomando, I.; Ochieng, J.B.; et al. Shigella Isolates from the Global Enteric Multicenter Study Inform Vaccine Development. Clin. Infect. Dis. 2014, 59, 933–941. [Google Scholar] [CrossRef]
- Barry, E.M.; Pasetti, M.F.; Sztein, M.B.; Fasano, A.; Kotloff, K.L.; Levine, M.M. Progress and Pitfalls in Shigella Vaccine Research. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 245–255. [Google Scholar] [CrossRef]
- MacLennan, C.A.; Grow, S.; Ma, L.F.; Steele, A.D. The Shigella Vaccines Pipeline. Vaccines 2022, 10, 1376. [Google Scholar] [CrossRef]
- Ledesma-Feliciano, C.; Chapman, R.; Hooper, J.W.; Elma, K.; Zehrung, D.; Brennan, M.B.; Spiegel, E.K. Improved DNA Vaccine Delivery with Needle-Free Injection Systems. Vaccines 2023, 11, 280. [Google Scholar] [CrossRef]
- Gamazo, C.; Pastor, Y.; Larrañeta, E.; Berzosa, M.; Irache, J.M.; Donnelly, R.F. Understanding the Basis of Transcutaneous Vaccine Delivery. Ther. Deliv. 2019, 10, 63–80. [Google Scholar] [CrossRef]
- Qasim, M.; Wrage, M.; Nüse, B.; Mattner, J. Shigella Outer Membrane Vesicles as Promising Targets for Vaccination. Int. J. Mol. Sci. 2022, 23, 994. [Google Scholar] [CrossRef]
- Pastor, Y.; Camacho, A.I.; Zúñiga-Ripa, A.; Merchán, A.; Rosas, P.; Irache, J.M.; Gamazo, C. Towards a Subunit Vaccine from a Shigella flexneri ΔtolR Mutant. Vaccine 2018, 36, 7509–7519. [Google Scholar] [CrossRef] [PubMed]
- Camacho, A.I.; Irache, J.M.; de Souza, J.; Sánchez-Gómez, S.; Gamazo, C. Nanoparticle-Based Vaccine for Mucosal Protection against Shigella flexneri in Mice. Vaccine 2013, 31, 3288–3294. [Google Scholar] [CrossRef] [PubMed]
- Pastor, Y.; Larrañeta, E.; Erhard, Á.; Quincooces, G.; Peñuelas, I.; Irache, J.M.; Donnelly, R.; Gamazo, C. Dissolving Microneedles for Intradermal Vaccination against Shigellosis. Vaccines 2019, 7, 159. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Das, S.; Howlader, D.R.; Zheng, Q.; Ratnakaram, S.S.K.; Whittier, S.K.; Picking, W.D.; Picking, W.L. L-DBF Elicits Cross Protection Against Different Serotypes of Shigella spp. Front. Trop. Dis. 2021, 2, 729731. [Google Scholar] [CrossRef]
- Migliore, A.; Gigliucci, G.; Di Marzo, R.; Russo, D.; Mammucari, M. Intradermal Vaccination: A Potential Tool in the Battle against the COVID-19 Pandemic? Risk Manag. Healthc. Policy 2021, 14, 2079–2087. [Google Scholar] [CrossRef] [PubMed]
- Heine, S.J.; Diaz-McNair, J.; Andar, A.U.; Drachenberg, C.B.; van de Verg, L.; Walker, R.; Picking, W.L.; Pasetti, M.F. Intradermal Delivery of Shigella IpaB and IpaD Type III Secretion Proteins: Kinetics of Cell Recruitment and Antigen Uptake, Mucosal and Systemic Immunity, and Protection across Serotypes. J. Immunol. 2014, 192, 1630–1640. [Google Scholar] [CrossRef] [PubMed]
- León, Y.; Zapata, L.; Molina, R.E.; Okanovič, G.; Gómez, L.A.; Daza-Castro, C.; Flores-Concha, M.; Reyes, J.L.; Oñate, A.A. Intranasal Immunization of Mice with Multiepitope Chimeric Vaccine Candidate Based on Conserved Autotransporters SiGa, Pic and Sap, Confers Protection against Shigella flexneri. Vaccines 2020, 8, 563. [Google Scholar] [CrossRef]
- Lieberman, L.A. Outer Membrane Vesicles: A Bacterial-Derived Vaccination System. Front. Microbiol. 2022, 13, 1029146. [Google Scholar] [CrossRef]
- Launay, O.; Lewis, D.J.M.; Anemona, A.; Loulergue, P.; Leahy, J.; Sciré, A.S.; Maugard, A.; Marchetti, E.; Zancan, S.; Huo, Z.; et al. Safety Profile and Immunologic Responses of a Novel Vaccine Against Shigella sonnei Administered Intramuscularly, Intradermally and Intranasally: Results From Two Parallel Randomized Phase 1 Clinical Studies in Healthy Adult Volunteers in Europe. eBioMedicine 2017, 22, 164–172. [Google Scholar] [CrossRef]
- Pastor, Y.; Camacho, A.; Gil, A.G.; Ramos, R.; López De Ceráin, A.; Peñuelas, I.; Irache, J.M.; Gamazo, C. Effective Protection of Mice against Shigella flexneri with a New Self-Adjuvant Multicomponent Vaccine. J. Med. Microbiol. 2017, 66, 946–958. [Google Scholar] [CrossRef]
- Kaminski, R.W.; Pasetti, M.F.; Aguilar, A.O.; Clarkson, K.A.; Rijpkema, S.; Bourgeois, A.L.; Cohen, D.; Feavers, I.; Maclennan, C.A. Consensus Report on Shigella Controlled Human Infection Model: Immunological Assays. Clin. Infect. Dis. 2019, 69, S596–S601. [Google Scholar] [CrossRef]
- Shimanovich, A.A.; Buskirk, A.D.; Heine, S.J.; Blackwelder, W.C.; Wahid, R.; Kotloff, K.L.; Pasetti, M.F. Functional and Antigen-Specific Serum Antibody Levels as Correlates of Protection against Shigellosis in a Controlled Human Challenge Study. Clin. Vaccine Immunol. 2017, 24, e00412-16. [Google Scholar] [CrossRef] [PubMed]
- Pavlinac, P.B.; McQuade, E.T.R.; Platts-Mills, J.A.; Kotloff, K.L.; Deal, C.; Giersing, B.K.; Isbrucker, R.A.; Kang, G.; Ma, L.F.; Maclennan, C.A.; et al. Pivotal Shigella Vaccine Efficacy Trials—Study Design Considerations from a Shigella Vaccine Trial Design Working Group. Vaccines 2022, 10, 489. [Google Scholar] [CrossRef] [PubMed]
- Bhaumik, U.; Halder, P.; Howlader, D.R.; Banerjee, S.; Maiti, S.; Dutta, S.; Koley, H. A Tetravalent Shigella Outer Membrane Vesicles Based Candidate Vaccine Offered Cross-Protection against All the Serogroups of Shigella in Adult Mice. Microbes Infect. 2023, 25, 105100. [Google Scholar] [CrossRef]
- Gasperini, G.; Raso, M.M.; Schiavo, F.; Aruta, M.G.; Ravenscroft, N.; Bellich, B.; Cescutti, P.; Necchi, F.; Rappuoli, R.; Micoli, F. Rapid Generation of Shigella flexneri GMMA Displaying Natural or New and Cross-Reactive O-Antigens. NPJ Vaccines 2022, 7, 69. [Google Scholar] [CrossRef]
- Samassa, F.; Ferrari, M.L.; Husson, J.; Mikhailova, A.; Porat, Z.; Sidaner, F.; Brunner, K.; Teo, T.H.; Frigimelica, E.; Tinevez, J.Y.; et al. Shigella Impairs Human T Lymphocyte Responsiveness by Hijacking Actin Cytoskeleton Dynamics and T Cell Receptor Vesicular Trafficking. Cell. Microbiol. 2020, 22, e13166. [Google Scholar] [CrossRef] [PubMed]
- Sarker, P.; Mily, A.; Ara, A.; Haque, F.; Maier, N.; Wierzba, T.F.; Walker, R.I.; Venkatesan, M.M.; Raqib, R. Functional Antibodies and Innate Immune Responses to WRSS1, a Live Oral Shigella sonnei Vaccine Candidate, in Bangladeshi Adults and Children. J. Infect. Dis. 2021, 224, S829–S839. [Google Scholar] [CrossRef]
- Berlanda Scorza, F.; Colucci, A.M.; Maggiore, L.; Sanzone, S.; Rossi, O.; Ferlenghi, I.; Pesce, I.; Caboni, M.; Norais, N.; Di Cioccio, V.; et al. High Yield Production Process for Shigella Outer Membrane Particles. PLoS ONE 2012, 7, e35616. [Google Scholar] [CrossRef]
- Mitra, S.; Sinha, R.; Mitobe, J.; Koley, H. Development of a Cost-Effective Vaccine Candidate with Outer Membrane Vesicles of a TolA-Disrupted Shigella boydii Strain. Vaccine 2016, 34, 1839–1846. [Google Scholar] [CrossRef]
- Brunner, K.; Samassa, F.; Sansonetti, P.J.; Phalipon, A. Shigella-Mediated Immunosuppression in the Human Gut: Subversion Extends from Innate to Adaptive Immune Responses. Hum. Vaccin. Immunother. 2019, 15, 1317–1325. [Google Scholar] [CrossRef]
- Anam, K.; Endharti, A.T.; Poeranto, S.; Sujuti, H.; Hidayati, D.Y.N.; Prawiro, S.R. Shigella flexneri Vaccine Development: Oral Administration of Peptides Derived from the 49.8 KDa Pili Protein Subunit Activates the Intestinal Immune Response in Mice. Vet. World 2022, 15, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Boero, E.; Vezzani, G.; Micoli, F.; Pizza, M.; Rossi, O. Functional Assays to Evaluate Antibody-Mediated Responses against Shigella: A Review. Front. Cell. Infect. Microbiol. 2023, 13, 1171213. [Google Scholar] [CrossRef] [PubMed]
- Necchi, F.; Saul, A.; Rondini, S. Development of a High-Throughput Method to Evaluate Serum Bactericidal Activity Using Bacterial ATP Measurement as Survival Readout. PLoS ONE 2017, 12, e0172163. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pastor, Y.; Calvo, A.; Salvador-Erro, J.; Gamazo, C. Refining Immunogenicity through Intradermal Delivery of Outer Membrane Vesicles against Shigella flexneri in Mice. Int. J. Mol. Sci. 2023, 24, 16910. https://doi.org/10.3390/ijms242316910
Pastor Y, Calvo A, Salvador-Erro J, Gamazo C. Refining Immunogenicity through Intradermal Delivery of Outer Membrane Vesicles against Shigella flexneri in Mice. International Journal of Molecular Sciences. 2023; 24(23):16910. https://doi.org/10.3390/ijms242316910
Chicago/Turabian StylePastor, Yadira, Alba Calvo, Josune Salvador-Erro, and Carlos Gamazo. 2023. "Refining Immunogenicity through Intradermal Delivery of Outer Membrane Vesicles against Shigella flexneri in Mice" International Journal of Molecular Sciences 24, no. 23: 16910. https://doi.org/10.3390/ijms242316910





