Graphene-Based Electrochemical Sensing Platform for Rapid and Selective Ferulic Acid Quantification
Abstract
:1. Introduction
2. Results and Discussion
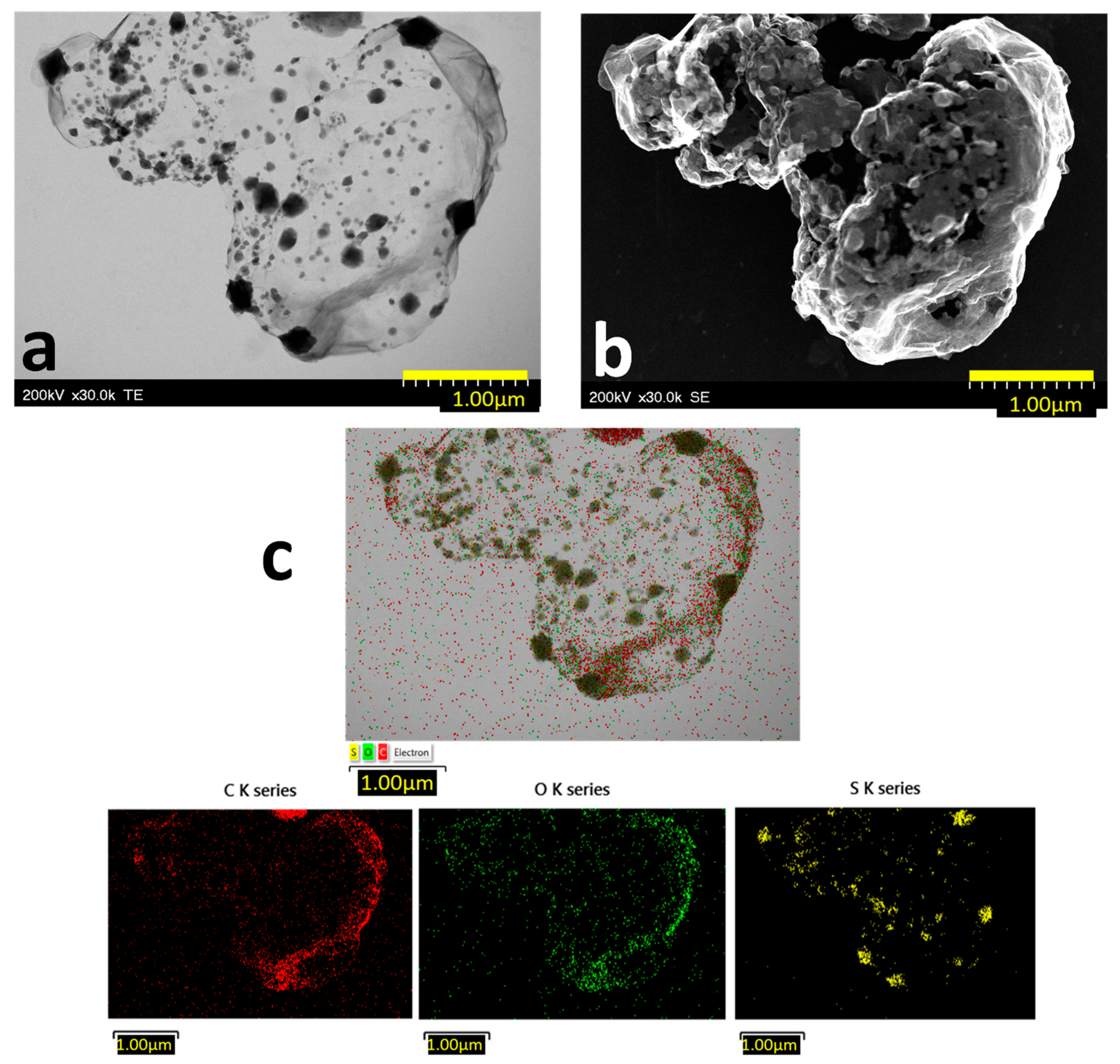
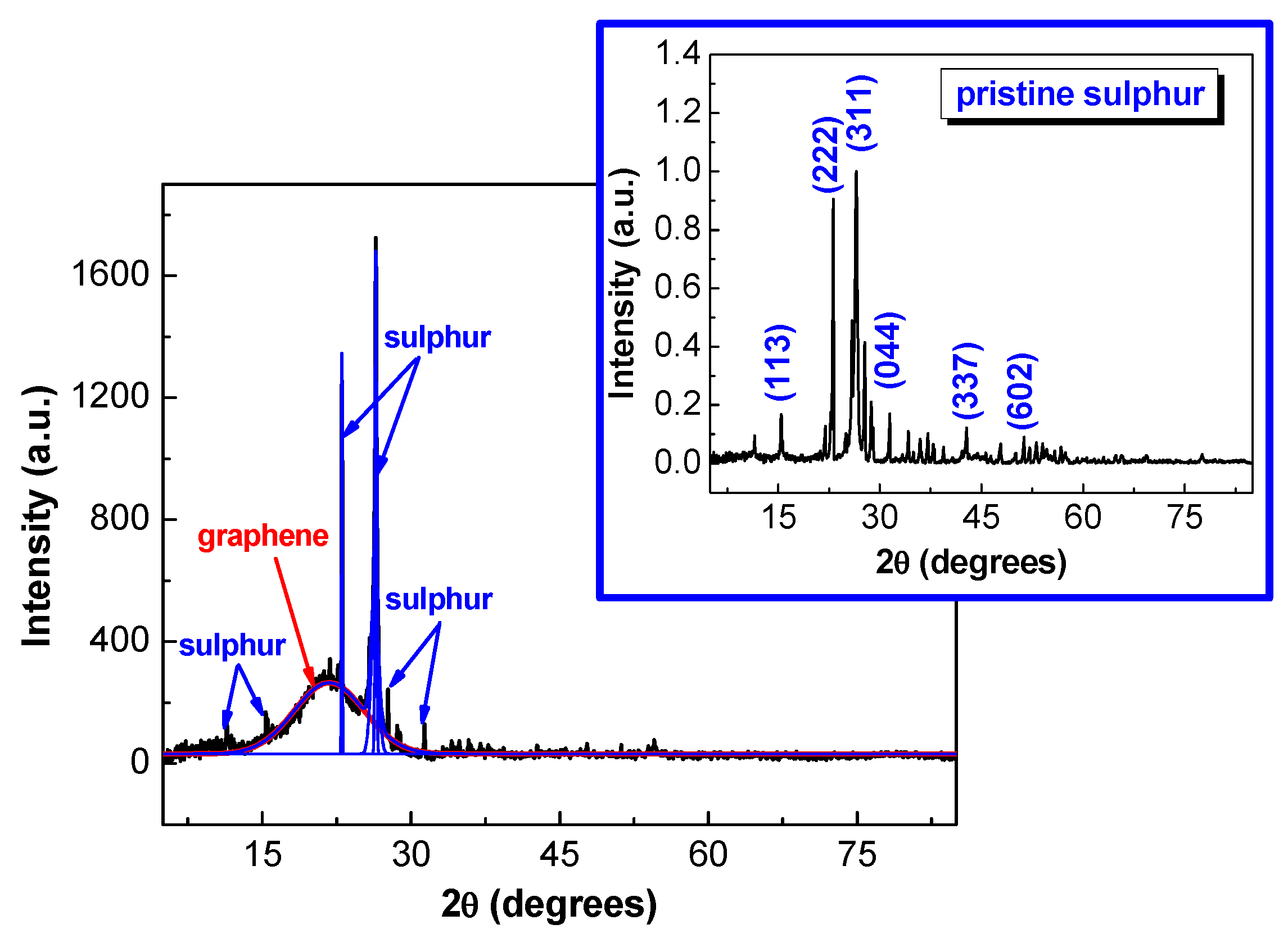
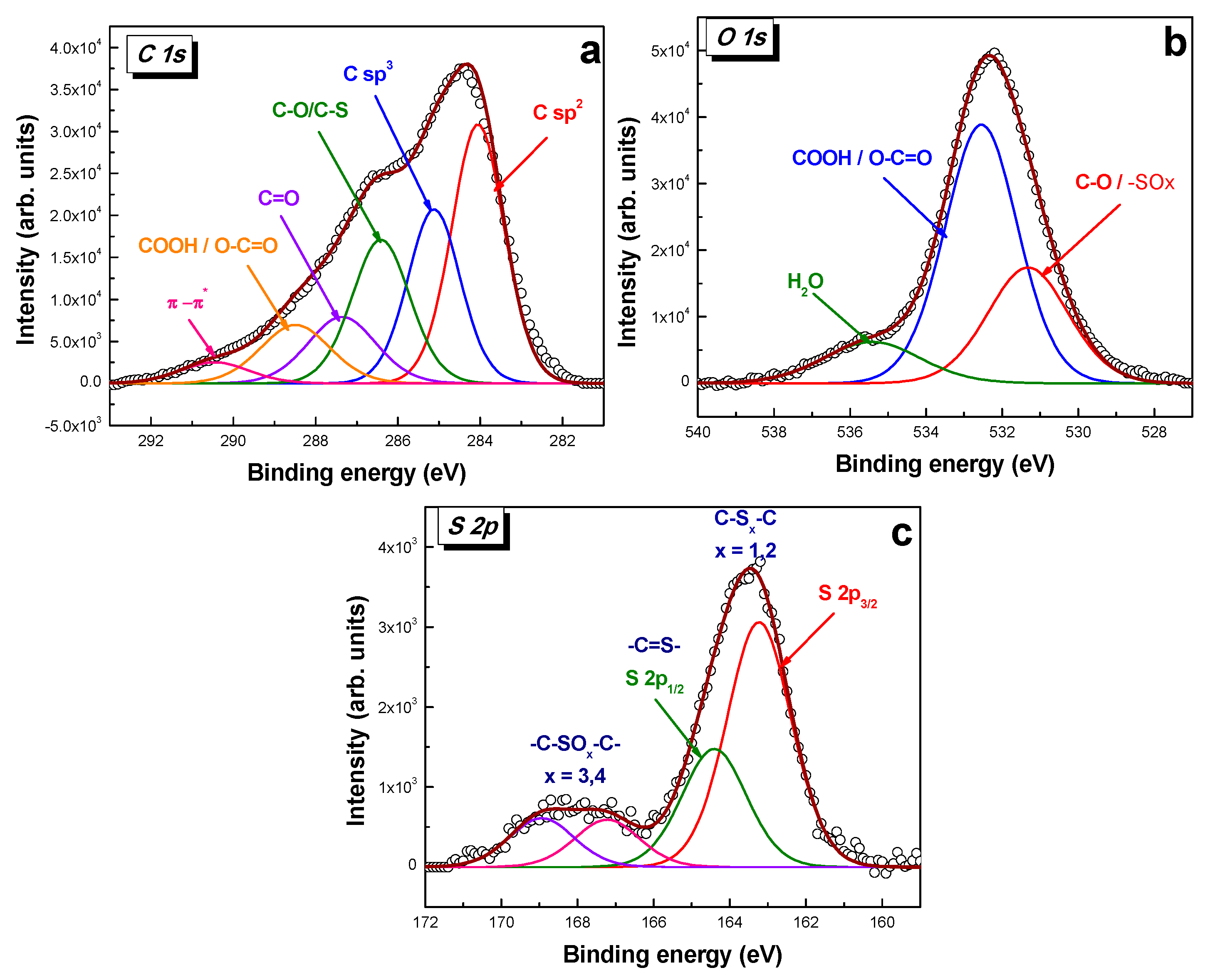
2.1. Morpho-Structural Sulphur-Doped Graphene Characterization
2.2. Development of Electrochemical Detection Protocol for Ferulic Acid Assay on the Surface of Modified Electrode
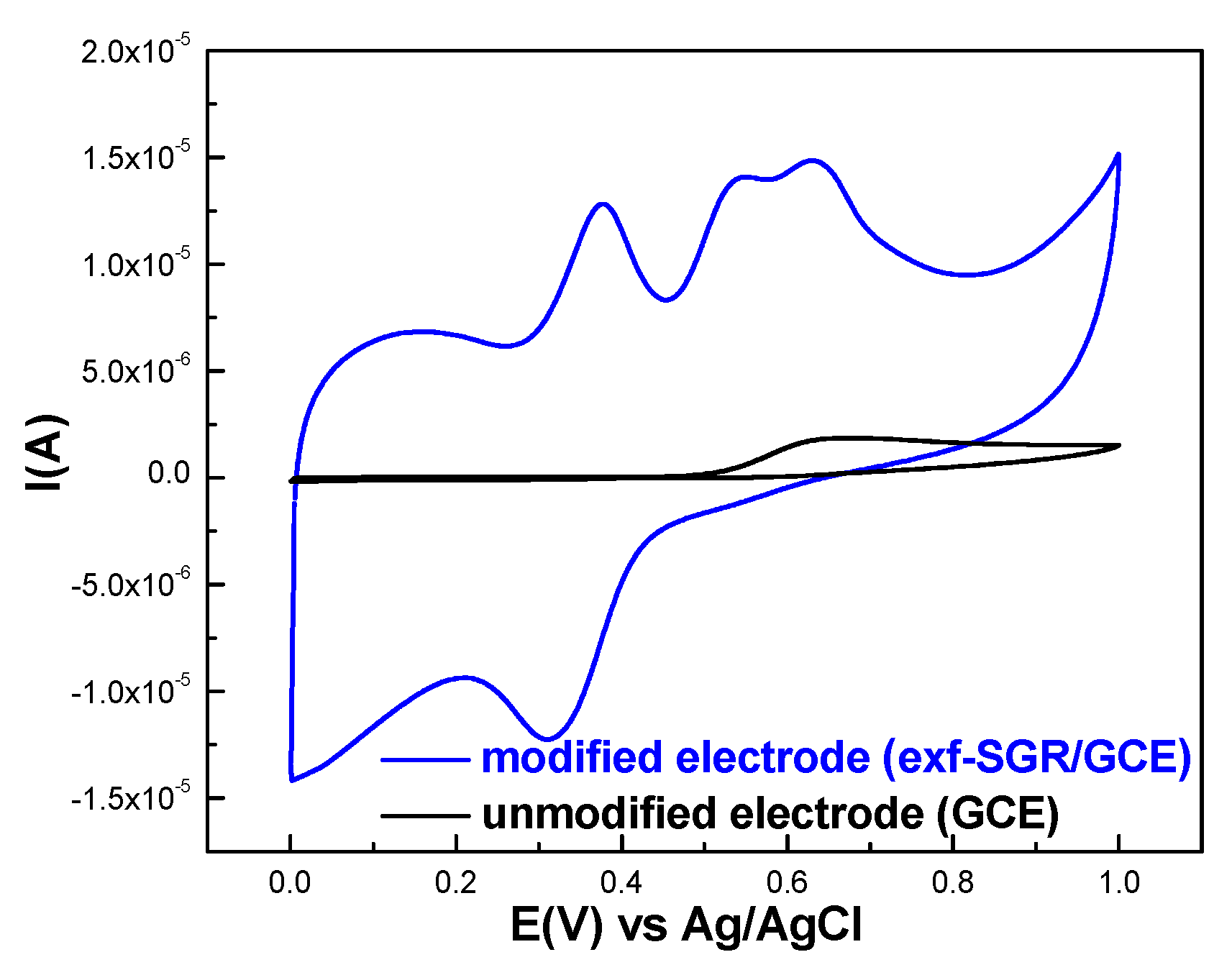
2.2.1. Graphene-Modified Electrode Surface Characterization
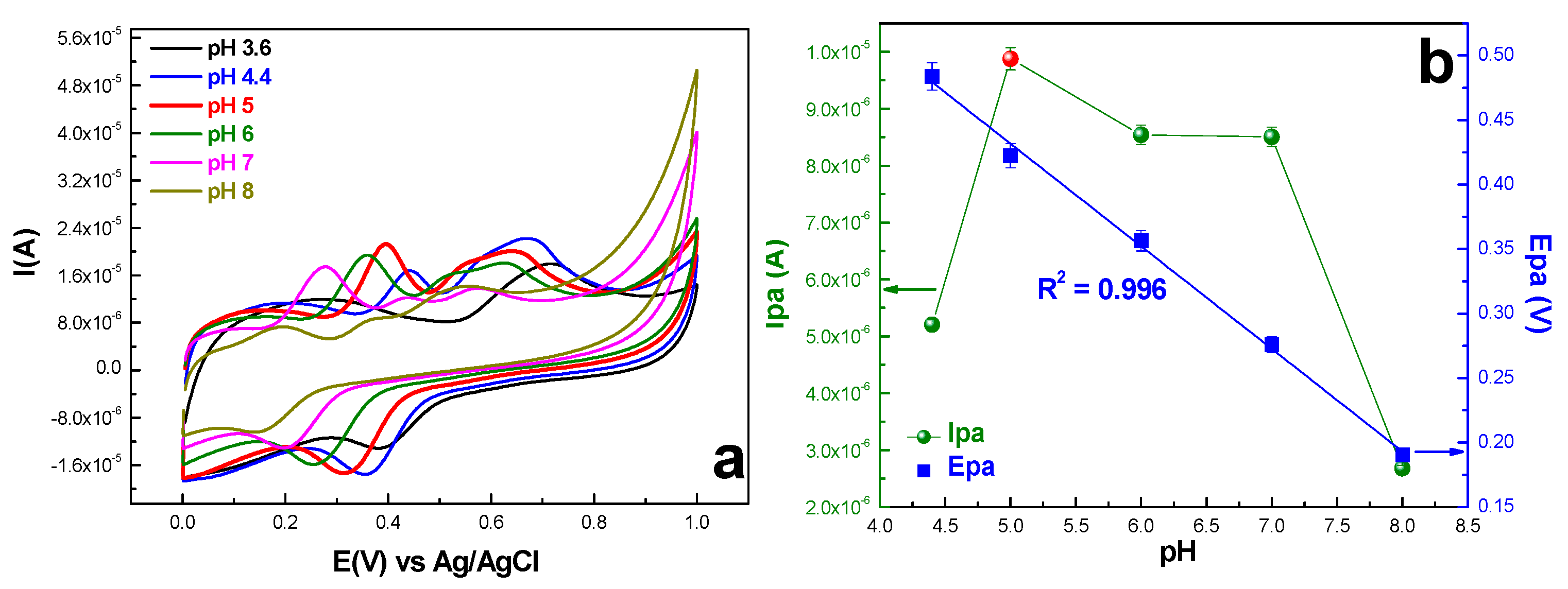
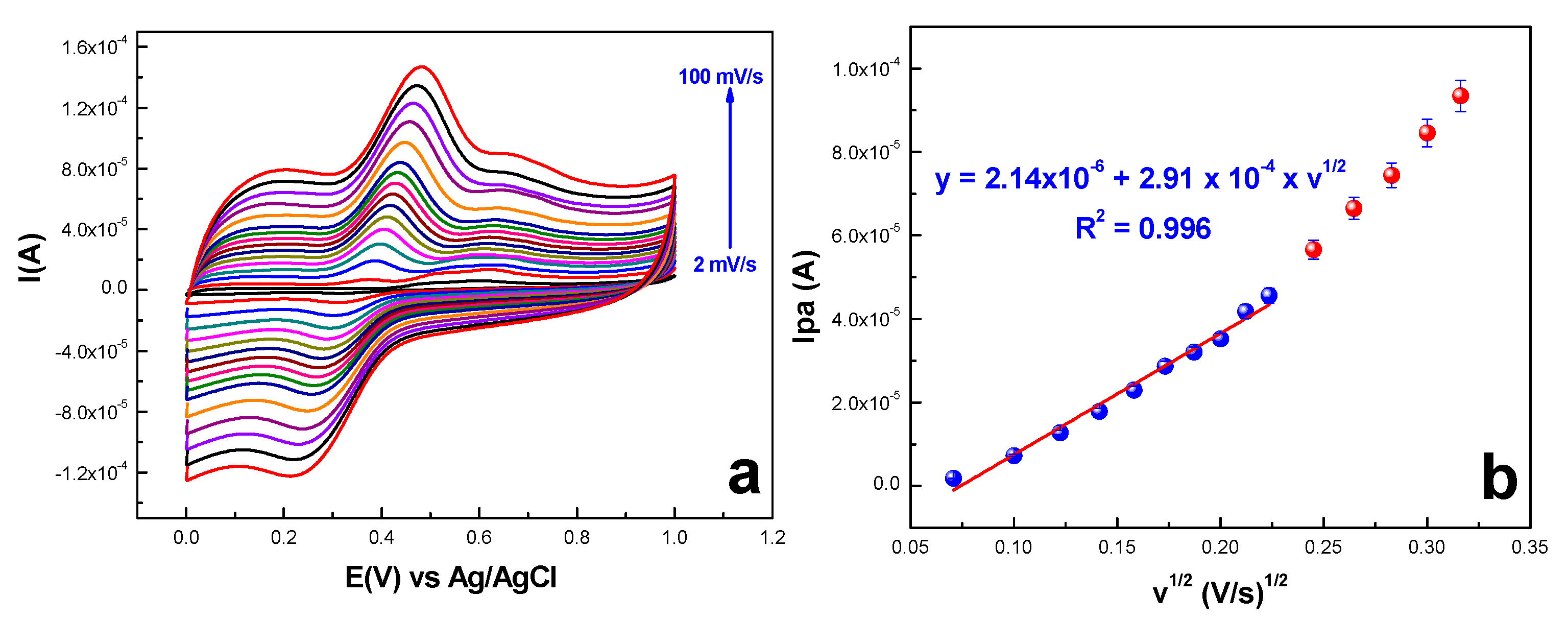
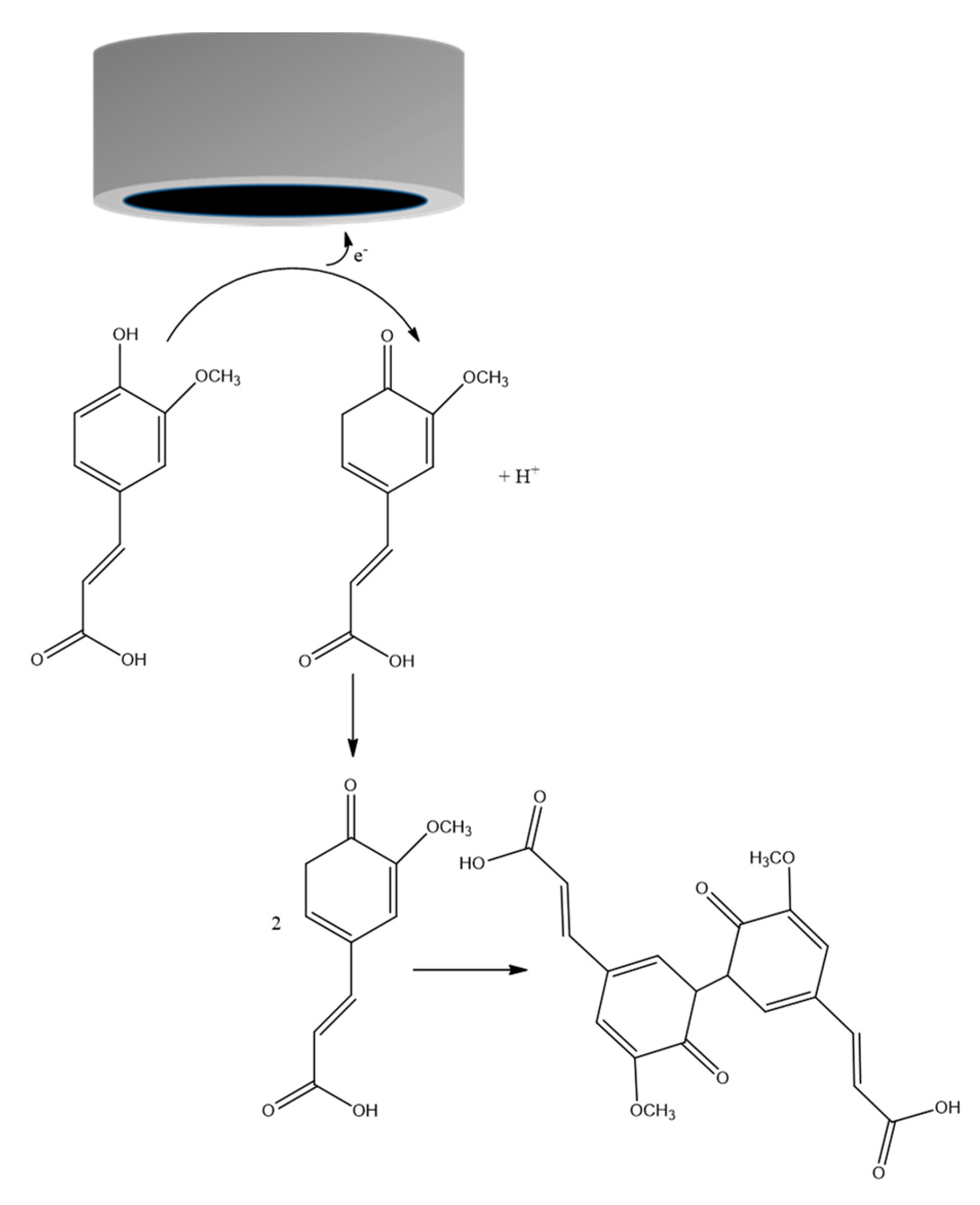
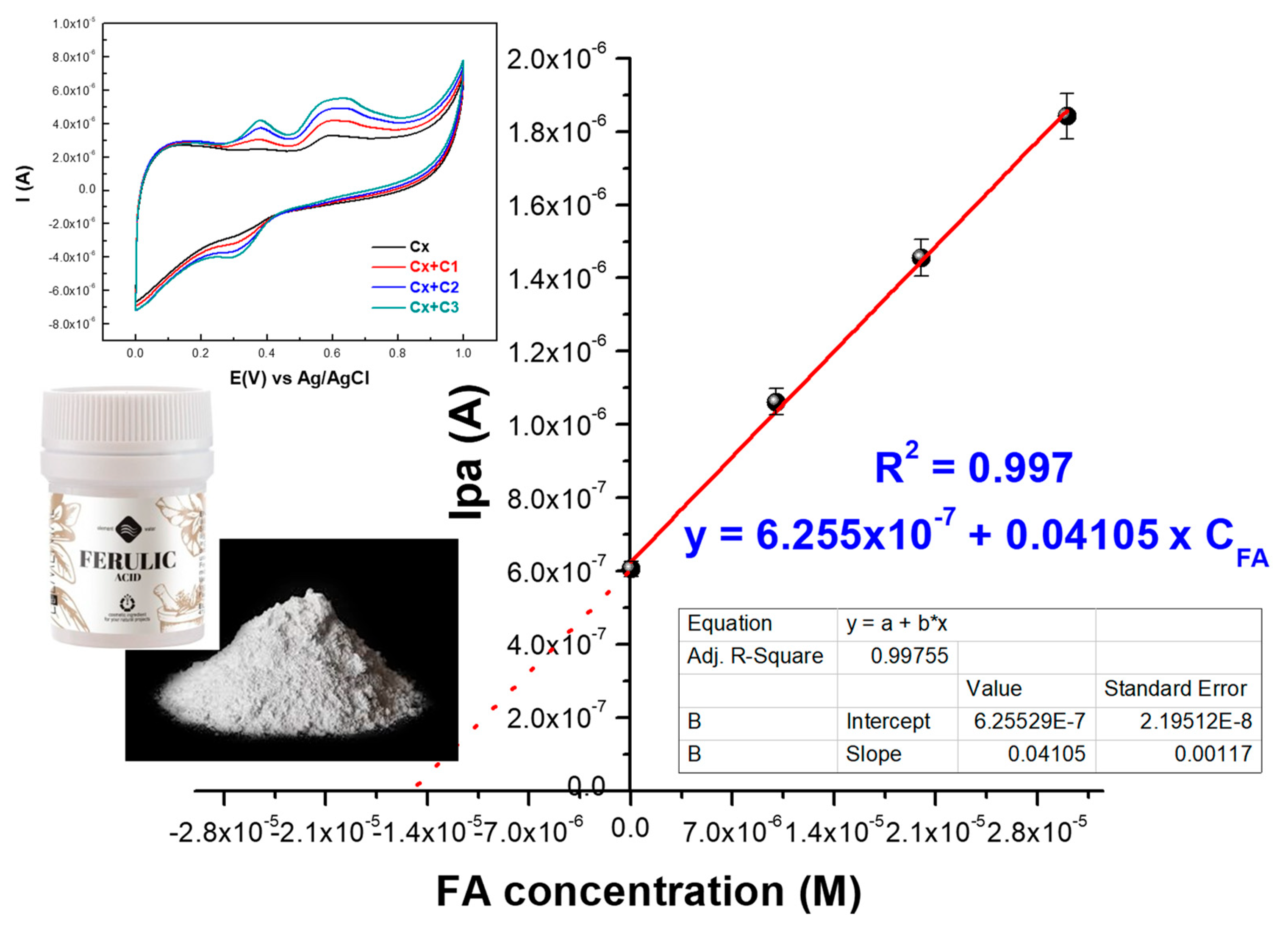
2.2.2. Ferulic Acid Electrochemical Oxidation at the Surface of exf-SGR/GCE
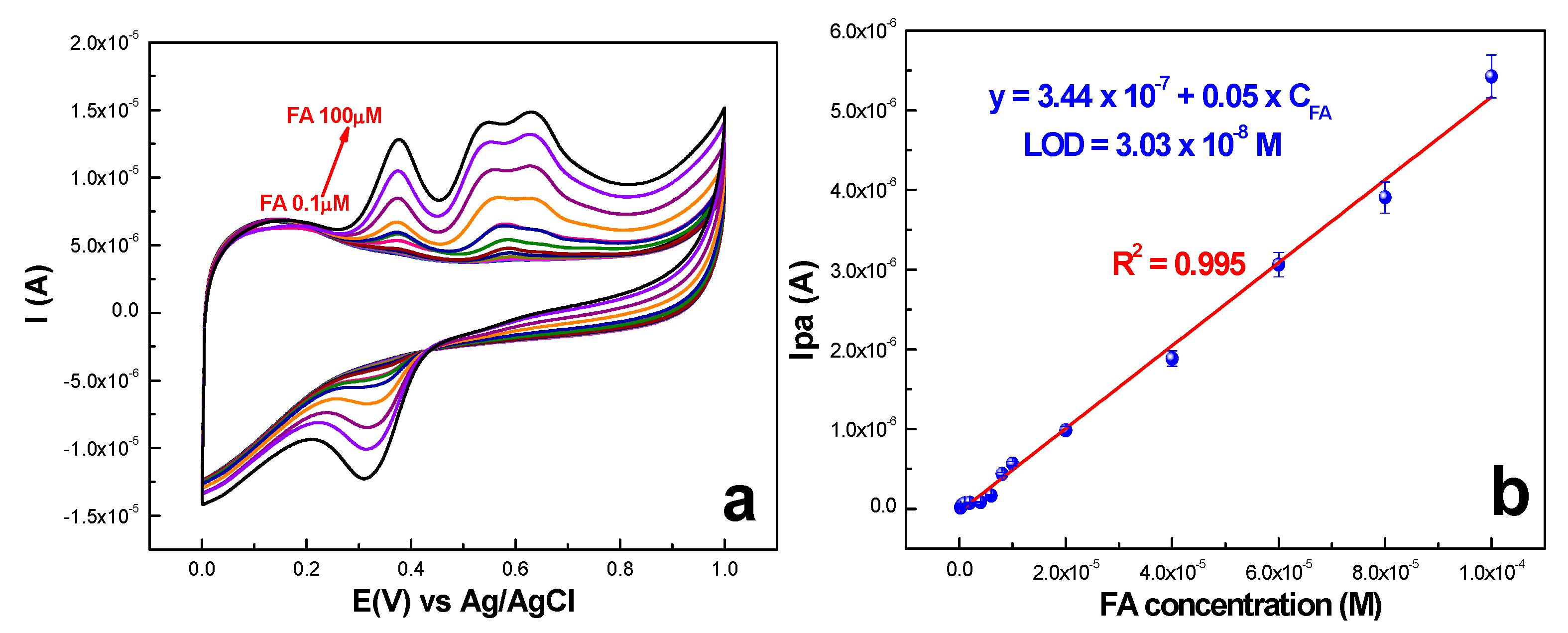
2.2.3. Quantification and Detection Limits
2.2.4. Stability, Reproducibility, and Interference
2.2.5. Real Sample Analysis
3. Materials and Methods
3.1. Reagents
3.2. Sulphur-Doped Graphene Preparation
3.3. Sensor Design
3.4. Apparatus
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gani, A.; Wani, S.M.; Masoodi, F.A.; Hameed, G. Whole-grain cereal bioactive compounds and their health benefits: A Review. J. Food Process. Technol. 2012, 3, 146. [Google Scholar] [CrossRef]
- Zduńska, K.; Dana, A.; Kolodziejczak, A.; Rotsztejn, H. Antioxidant Properties of Ferulic Acid and Its Possible Application. Ski. Pharmacol. Physiol. 2018, 31, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Rice-Evans, C.; Miller, N.; Paganga, G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997, 2, 152–159. [Google Scholar] [CrossRef]
- Chung, S.; Champagne, E. Ferulic Acid Enhances IgE Binding to Peanut Allergens in Western Blots. Food Chem. 2011, 124, 1639–1642. [Google Scholar] [CrossRef]
- Adeyi, O.E.; Somade, O.T.; Ajayi, B.O.; James, A.S.; Adeboye, T.R.; Olufemi, D.A.; Oyinlola, E.V.; Sanyaolu, E.T.; Mufutau, I.O. The anti-inflammatory effect of ferulic acid is via the modulation of NFκB-TNF-α-IL-6 and STAT1-PIAS1 signaling pathways in 2-methoxyethanol-induced testicular inflammation in rats. Phytomed. Plus 2023, 3, 100464. [Google Scholar] [CrossRef]
- Adom, K.K.; Liu, R.H. Antioxidant Activity of Grains. J. Agric. Food Chem. 2002, 50, 6182–6187. [Google Scholar] [CrossRef] [PubMed]
- Staniforth, V.; Huang, W.-C.; Aravindaram, K.; Yang, N.-S. Ferulic acid, a phenolic phytochemical, inhibits UVB-induced matrix metalloproteinases in mouse skin via posttranslational mechanisms. J. Nutr. Biochem. 2012, 23, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Sgarbossa, A.; Monti, S.; Lenci, F.; Bramanti, E.; Bizzarri, R.; Barone, V. The effects of ferulic acid on β-amyloid fibrillar structures investigated through experimental and computational techniques. Biochim. Biophys. Acta-Gen. Subj. 2013, 1830, 2924–2937. [Google Scholar] [CrossRef]
- Lin, C.-M.; Chiu, J.-H.; Wu, I.-H.; Wang, B.-W.; Pan, C.-M.; Chen, Y.-H. Ferulic acid augments angiogenesis via VEGF, PDGF and HIF-1α. J. Nutr. Biochem. 2010, 21, 627–633. [Google Scholar] [CrossRef]
- Yang, G.-W.; Jiang, J.-S.; Lu, W.-Q. Ferulic Acid Exerts Anti-Angiogenic and Anti-Tumor Activity by Targeting Fibroblast Growth Factor Receptor 1-Mediated Angiogenesis. Int. J. Mol. Sci. 2015, 16, 24011–24031. [Google Scholar] [CrossRef]
- El-Gogary, R.I.; Nasr, M.; Rahsed, L.A.; Hamzawy, M.A. Ferulic acid nanocapsules as a promising treatment modality for colorectal cancer: Preparation and in vitro/in vivo appraisal. Life Sci. 2022, 298, 120500. [Google Scholar] [CrossRef] [PubMed]
- Borges, A.; Ferreira, C.; Simões, M.; Wang, M.; Tao, L.; Xu, H.; Alexandri, M.; Papapostolou, H.; Vlysidis, A.; Gardeli, C.; et al. Antibacterial Activity and Mode of Action of Ferulic and Gallic Acids against Pathogenic Bacteria. Microb. Drug Resist. 2013, 19, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Zhang, X.; Sun, Y.; Yang, M.; Song, K.; Zheng, Z.; Chen, Y.; Liu, X.; Jia, Z.; Dong, R.; et al. Antimicrobial Activity of Ferulic Acid against Cronobacter sakazakii and Possible Mechanism of Action. Foodborne Pathog. Dis. 2016, 13, 196–204. [Google Scholar] [CrossRef]
- Savtschenko, A.; Dhein, S.; Rauwald, H. The antiarrhythmic effects of lavandulifolioside and ferulic acid from Leonurus cardiaca extracts on cardiac electrophysiology. Z. Phytother. 2013, 34, P25. [Google Scholar] [CrossRef]
- Pandi, A.; Raghu, M.H.; Chandrashekar, N.; Kalappan, V.M. Cardioprotective effects of Ferulic acid against various drugs and toxic agents. Beni-Suef Univ. J. Basic Appl. Sci. 2022, 11, 92. [Google Scholar] [CrossRef]
- Zhang, X.; Lin, D.; Jiang, R.; Li, H.; Wan, J.; Li, H. Ferulic acid exerts antitumor activity and inhibits metastasis in breast cancer cells by regulating epithelial to mesenchymal transition. Oncol. Rep. 2016, 36, 271–278. [Google Scholar] [CrossRef]
- Alazzouni, A.S.; Dkhil, M.A.; Gadelmawla, M.H.; Gabri, M.S.; Farag, A.H.; Hassan, B.N. Ferulic acid as anticarcinogenic agent against 1,2-dimethylhydrazine induced colon cancer in rats. J. King Saud Univ.-Sci. 2021, 33, 101354. [Google Scholar] [CrossRef]
- Wang, H.; Sun, X.; Zhang, N.; Ji, Z.; Ma, Z.; Fu, Q.; Qu, R.; Ma, S. Ferulic acid attenuates diabetes-induced cognitive impairment in rats via regulation of PTP1B and insulin signaling pathway. Physiol. Behav. 2017, 182, 93–100. [Google Scholar] [CrossRef]
- Ghosh, S.; Chowdhury, S.; Sarkar, P.; Sil, P.C. Ameliorative role of ferulic acid against diabetes associated oxidative stress induced spleen damage. Food Chem. Toxicol. 2018, 118, 272–286. [Google Scholar] [CrossRef]
- Fu, W.; Amenyogbe, E.; Yang, E.; Luo, J.; Huang, J.-S.; Xie, R.-T.; Chen, G. Effects of dietary supplementation of ferulic acid on growth performance, antioxidant ability, non-specific immunity, hepatic morphology and genes expression related to growth and immunity in juvenile hybrid grouper (Epinephelus fuscoguttatus♀ × Epinephelus polyphekadion♂). Aquaculture 2022, 552, 737988. [Google Scholar] [CrossRef]
- Dawood, M.A.; Metwally, A.E.-S.; El-Sharawy, M.E.; Ghozlan, A.M.; Abdel-Latif, H.M.; Van Doan, H.; Ali, M.A. The influences of ferulic acid on the growth performance, haemato-immunological responses, and immune-related genes of Nile tilapia (Oreochromis niloticus) exposed to heat stress. Aquaculture 2020, 525, 735320. [Google Scholar] [CrossRef]
- Nile, S.H.; Ko, E.Y.; Kim, D.H.; Keum, Y.-S. Screening of ferulic acid related compounds as inhibitors of xanthine oxidase and cyclooxygenase-2 with anti-inflammatory activity. Rev. Bras. Farmacogn. 2016, 26, 50–55. [Google Scholar] [CrossRef]
- Truzzi, F.; Valerii, M.C.; Tibaldi, C.; Zhang, Y.; Abduazizova, V.; Spisni, E.; Dinelli, G. Are Supplements Safe? Effects of Gallic and Ferulic Acids on In Vitro Cell Models. Nutrients 2020, 12, 1591. [Google Scholar] [CrossRef] [PubMed]
- Flores, N.; Sirés, I.; Garrido, J.A.; Centellas, F.; Rodríguez, R.M.; Cabot, P.L.; Brillas, E. Degradation of trans-ferulic acid in acidic aqueous medium by anodic oxidation, electro-Fenton and photoelectro-Fenton. J. Hazard. Mater. 2016, 319, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.-H.; Chan, K.; Leung, K.; Chan, C.-L.; Zhao, Z.-Z.; Jiang, Z.-H. Assay of free ferulic acid and total ferulic acid for quality assessment of Angelica sinensis. J. Chromatogr. A 2005, 1068, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Su, S.; Cui, W.; Liu, P.; Duan, J.-A.; Guo, J.; Li, Z.; Shang, E.; Qian, D.; Huang, Z. Simultaneous determination of paeoniflorin, albiflorin, ferulic acid, tetrahydropalmatine, protopine, typhaneoside, senkyunolide I in Beagle dogs plasma by UPLC–MS/MS and its application to a pharmacokinetic study after Oral Administration of Shaofu Zhuyu Decoction. J. Chromatogr. B 2014, 962, 75–81. [Google Scholar] [CrossRef]
- Jadhav, A.P.; Kareparamban, J.A.; Nikam, P.H.; Kadam, V.J. Spectrophotometric Estimation of Ferulic Acid from Ferula asafoetida by Folin—Ciocalteu’s Reagent. Der. Pharm. Sin. 2012, 3, 680–684. [Google Scholar]
- Ostojić, J.; Herenda, S.; Bešić, Z.; Miloš, M.; Galić, B. Advantages of an Electrochemical Method Compared to the Spectrophotometric Kinetic Study of Peroxidase Inhibition by Boroxine Derivative. Molecules 2017, 22, 1120. [Google Scholar] [CrossRef]
- Li, L.-J.; Yu, L.-B.; Chen, Q.-F.; Cheng, H.; Wu, F.-M.; Wu, J.-L.; Kong, H.-X. Determination of Ferulic Acid Based on L-Cysteine Self-assembled Modified Gold Electrode Coupling Irreversible Biamperometry. Chin. J. Anal. Chem. 2007, 35, 933–937. [Google Scholar] [CrossRef]
- Bounegru, A.V.; Apetrei, C. Simultaneous Determination of Caffeic Acid and Ferulic Acid Using a Carbon Nanofiber-Based Screen-Printed Sensor. Sensors 2022, 22, 4689. [Google Scholar] [CrossRef]
- Xia, Z.; Zhang, Y.; Li, Q.; Du, H.; Gui, G.; Zhao, G. Electrochemical Determination of Ferulic Acid in Pinellia Ternata Based on GOs/MWCNTs Nanocomposite Modified Electrode. Int. J. Electrochem. Sci. 2020, 15, 559–566. [Google Scholar] [CrossRef]
- Liu, L.-J.; Gao, X.; Zhang, P.; Feng, S.-L.; Hu, F.-D.; Li, Y.-D.; Wang, C.-M. Ultrasensitive Detection of Ferulic Acid Using Poly(diallyldimethylammonium chloride) Functionalized Graphene-Based Electrochemical Sensor. J. Anal. Methods Chem. 2014, 2014, 424790. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, Y.; Fan, Y.; Li, J.; Wang, X.; Wang, Y.; Fan, Y.; Li, J. Voltammetric determination of ferulic acid using a GC electrode modified with poly-aspartic acid film. J. Tongji Univ. 2011, 39, 1084–1098. (In Chinese) [Google Scholar] [CrossRef]
- Abdel-Hamid, R.; Newair, E.F. Voltammetric Determination of Ferulic Acid Using Polypyrrole-Multiwalled Carbon Nanotubes Modified Electrode with Sample Application. Nanomaterials 2015, 5, 1704–1715. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Gou, Y.; Gao, X.; Zhang, P.; Chen, W.; Feng, S.; Hu, F.; Li, Y. Electrochemically reduced graphene oxide-based electrochemical sensor for the sensitive determination of ferulic acid in A. sinensis and biological samples. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 42, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Ziyatdinova, G.; Zhupanova, A.; Davletshin, R. Simultaneous Determination of Ferulic Acid and Vanillin in Vanilla Extracts Using Voltammetric Sensor Based on Electropolymerized Bromocresol Purple. Sensors 2021, 22, 288. [Google Scholar] [CrossRef]
- Bounegru, A.V.; Apetrei, C. Development of a Novel Electrochemical Biosensor Based on Carbon Nanofibers–Gold Nanoparticles–Tyrosinase for the Detection of Ferulic Acid in Cosmetics. Sensors 2020, 20, 6724. [Google Scholar] [CrossRef] [PubMed]
- Vilian, A.T.E.; Chen, S.-M. Preparation of carbon nanotubes decorated with manganese dioxide nanoparticles for electrochemical determination of ferulic acid. Microchim. Acta 2014, 182, 1103–1111. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef]
- Balandin, A.A.; Ghosh, S.; Bao, W.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Superior Thermal Conductivity of Single-Layer Graphene. Nano Lett. 2008, 8, 902–907. [Google Scholar] [CrossRef]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Sun, G.; Routh, P.; Kim, D.-H.; Huang, W.; Chen, P. Heteroatom-doped graphene materials: Syntheses, properties and applications. Chem. Soc. Rev. 2014, 43, 7067–7098. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Theerthagiri, J.; Nithyadharseni, P.; Arunachalam, P.; Balaji, D.; Kumar, A.M.; Madhavan, J.; Mittal, V.; Choi, M.Y. Heteroatom-doped graphene-based materials for sustainable energy applications: A review. Renew. Sustain. Energy Rev. 2021, 143, 110849. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, B.X.; Yu, Y.B.; Chen, B.-Y.; Hong, J.-M. Sulfur doped-graphene for enhanced acetaminophen degradation via electro-catalytic activation: Efficiency and mechanism. Sci. Total Environ. 2020, 715, 136730. [Google Scholar] [CrossRef] [PubMed]
- Gamez-Mendoza, L.; Terban, M.W.; Billinge, S.J.L.; Martinez-Inesta, M. Modelling and validation of particle size distributions of supported nanoparticles using the pair distribution function technique. J. Appl. Crystallogr. 2017, 50, 741–748. [Google Scholar] [CrossRef]
- Siburian, R.; Sihotang, H.; Raja, S.L.; Supeno, M.; Simanjuntak, C. New Route to Synthesize of Graphene Nano Sheets. Orient. J. Chem. 2018, 34, 182–187. [Google Scholar] [CrossRef]
- Warren, B.E. X-Ray Diffraction, 1st ed.; Addisone-Wesley: Reading, UK; Dover: Mineola, NY, USA, 1969; pp. 27–40. ISBN 0486663175. [Google Scholar]
- Myers, H. Introductory Solid State Physics; Taylor & Francis Ltd.: London, UK, 2002; ISBN 0-7484-0660-3. [Google Scholar] [CrossRef]
- Wu, Z.; Han, Y.; Lin, J.; Zhu, W.; He, M.; Xu, S.; Chen, X.; Lu, H.; Ye, W.; Han, T.; et al. Detection of interlayer interaction in few-layer graphene. Phys. Rev. B 2015, 92, 075408. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Basko, D.M. Raman spectroscopy as a versatile tool for studying the properties of graphene. Nat. Nanotechnol. 2013, 8, 235–246. [Google Scholar] [CrossRef]
- Lee, W.S.V.; Leng, M.; Li, M.; Huang, X.L.; Xue, J.M. Sulphur-functionalized graphene towards high performance supercapacitor. Nano Energy 2015, 12, 250–257. [Google Scholar] [CrossRef]
- Gao, H.; Liu, Z.; Song, L.; Guo, W.; Gao, W.; Ci, L.; Rao, A.; Quan, W.; Vajtai, R.; Ajayan, P.M. Synthesis of S-doped graphene by liquid precursor. Nanotechnology 2012, 23, 275605. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.S.; Roth, S.; et al. Raman spectrum of graphene and graphene layers. Phys. Rev. Lett. 2006, 97, 187401. [Google Scholar] [CrossRef] [PubMed]
- Tuinstra, F.; Koenig, J.L. Raman Spectrum of Graphite. J. Chem. Phys. 1970, 53, 1126–1130. [Google Scholar] [CrossRef]
- Magerusan, L.; Pogacean, F.; Coros, M.; Socaci, C.; Pruneanu, S.; Leostean, C.; Pana, I.O. Green methodology for the preparation of chitosan/graphene nanomaterial through electrochemical exfoliation and its applicability in Sunset Yellow detection. Electrochim. Acta 2018, 283, 578–589. [Google Scholar] [CrossRef]
- Magerusan, L.; Pogacean, F.; Pruneanu, S. Eco-friendly synthesis of sulphur-doped graphenes with applicability in caffeic acid electrochemical assay. Bioelectrochemistry 2022, 148, 108228. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Zhang, Y.; Zhuang, X.; Li, S.; Wu, D.; Zhang, F.; Feng, X. Low-temperature synthesis of nitrogen/sulfur co-doped three-dimensional graphene frameworks as efficient metal-free electrocatalyst for oxygen reduction reaction. Carbon 2013, 62, 296–301. [Google Scholar] [CrossRef]
- Wang, J.; Ma, R.; Zhou, Z.; Liu, G.; Liu, Q. Magnesiothermic synthesis of sulfur-doped graphene as an efficient metal-free electrocatalyst for oxygen reduction. Sci. Rep. 2015, 5, srep09304. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Sun, L.; Luo, Y.; Li, M.; Xu, Y.; Hu, G.; Li, X.; Wang, L. Effect of thiophene S on the enhanced ORR electrocatalytic performance of sulfur-doped graphene quantum dot/reduced graphene oxide nanocomposites. RSC Adv. 2018, 8, 19635–19641. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Yao, Z.; Li, G.; Fang, G.; Nie, H.; Liu, Z.; Zhou, X.; Chen, X.; Huang, S. Sulfur-Doped Graphene as an Efficient Metal-free Cathode Catalyst for Oxygen Reduction. ACS Nano 2011, 6, 205–211. [Google Scholar] [CrossRef]
- Brett, C.M.A.; Oliveira-Brett, A.M. Electrochemistry. In Principles, Methods and Applications; Oxford University Press: Oxford, UK, 1993; Chapter 9; pp. 174–185. ISBN 10:0198553889. [Google Scholar] [CrossRef]
- Zoski, C.G. Handbook of Electrochemistry; Elsevier Science: Amsterdam, The Netherlands, 2007; ISBN 978-0-444-51958-0. [Google Scholar] [CrossRef]
- Li, X.; Gao, Y.; Xiong, H.; Yang, Z. The electrochemical redox mechanism and antioxidant activity of polyphenolic compounds based on inlaid multi-walled carbon nanotubes-modified graphite electrode. Open Chem. 2021, 19, 961–973. [Google Scholar] [CrossRef]
- Trabelsi, S.K.; Tahar, N.B.; Trabelsi, B.; Abdelhedi, R. Electrochemical Oxidation of Ferulic Acid in Aqueous Solutions at Gold Oxide and Lead Dioxide Electrodes. J. Appl. Electrochem. 2005, 35, 967–973. [Google Scholar] [CrossRef]
- Manaia, M.A.; Diculescu, V.C.; Gil, E.d.S.; Oliveira-Brett, A.M. Guaicolic spices curcumin and capsaicin electrochemical oxidation behaviour at a glassy carbon electrode. J. Electroanal. Chem. 2012, 682, 83–89. [Google Scholar] [CrossRef]
- Tomac, I. Electrochemical Properties of Chlorogenic Acids and Determination of Their Content in Coffee Using Differential Pulse Voltammetry. Int. J. Electrochem. Sci. 2016, 11, 2854–2876. [Google Scholar] [CrossRef]
- da Silva, L.V.; Lopes, C.B.; da Silva, W.C.; de Paiva, Y.G.; dos Santos Silva, F.d.A.; Lima, P.R.; Kubota, L.T.; Goulart, M. Electropolymerization of ferulic acid on multi-walled carbon nanotubes modified glassy carbon electrode as a versatile platform for NADH, dopamine and epinephrine separate detection. Microchem. J. 2017, 133, 460–467. [Google Scholar] [CrossRef]
- Borges, F.; Lima, J.L.F.C.; Pinto, I.; Reis, S.; Siquet, C. Application of a Potentiometric System with Data-Analysis Computer Programs to the Quantification of Metal-Chelating Activity of Two Natural Antioxidants: Caffeic Acid and Ferulic Acid. Helvetica Chim. Acta 2003, 86, 3081–3087. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Yang, Z.; Yang, Y.; Pang, P.; Gao, Y.; Hu, Q. Rapid electrochemical detection of ferulic acid based on a graphene modified glass carbon electrode. Anal. Methods 2013, 5, 3834–3839. [Google Scholar] [CrossRef]
- Erady, V.; Mascarenhas, R.J.; Satpati, A.K.; Detriche, S.; Mekhalif, Z.; Dalhalle, J.; Dhason, A. Sensitive detection of Ferulic acid using multi-walled carbon nanotube decorated with silver nano-particles modified carbon paste electrode. J. Electroanal. Chem. 2017, 806, 22–31. [Google Scholar] [CrossRef]
- Li, G.; Liu, S.; Liu, Y.; Pang, X.; Li, M.; Gong, Y.; Wu, Y.; Guo, X. Au-doped nanostructured TiO2/C material derived from MIL-125 as a highly sensitive electrochemical sensor for ferulic acid. J. Coord. Chem. 2023, 76, 1007–1019. [Google Scholar] [CrossRef]
- Bharathi, T.D.; Anandh, S.P.; Rangarajan, M. Differential Pulse Voltammetric Detection of Ferulic Acid Using RGO-TiO2Electrodes. In Proceedings of the 15th IEEE India Council International Conference (INDICON), Coimbatore, India, 16–18 December 2018; pp. 1–6. [Google Scholar] [CrossRef]
- Buffon, E.; Stradiotto, N.R. A molecularly imprinted polymer on reduced graphene oxide-gold nanoparticles modified screen-printed electrode for selective determination of ferulic acid in orange peels. Microchem. J. 2021, 167, 106339. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Farahmandfar, R.; Hosseinpour, R.; Alizadeh, J.; Abbaspourrad, A. Determination of ferulic acid in the presence of butylated hydroxytoluene as two phenolic antioxidants using a highly conductive food nanostructure electrochemical sensor. Chem. Pap. 2019, 73, 2441–2447. [Google Scholar] [CrossRef]
- Yu, Y.-Y.; Wu, Q.-S.; Wang, X.-G.; Ding, Y.-P. Electrochemical determination of ferulic acid in Chinese traditional medicine Xiao Yao Pills at electrode modified with carbon nanotube. Russ. J. Electrochem. 2009, 45, 170–174. [Google Scholar] [CrossRef]
- Luo, L.; Wang, X.; Li, Q.; Ding, Y.; Jia, J.; Deng, D. Voltammetric Determination of Ferulic Acid by Didodecyldimethyl-ammonium Bromide/Nafion Composite Film-modified Carbon Paste Electrode. Anal. Sci. 2010, 26, 907–911. [Google Scholar] [CrossRef]
- Goujot, D.; Cuvelier, M.-E.; Soto, P.; Courtois, F. A stoichio-kinetic model for a DPPH∙ -ferulic acid reaction. Talanta 2019, 196, 284–292. [Google Scholar] [CrossRef]

| Electrode | Linear Range (µM) | Detection Limit (µM) | Technique | Reference |
| ERGO/GCE ERGO—electrochemically reduced graphene oxide GCE—glassy carbon electrode | 0.08–38.9 | 0.02 | DPV | [35] |
| PDDA-G/GCE PDDA-G—poly(diallyldimethylammoniumchloride) functionalized graphene GCE—glassy carbon electrode | 0.09–52.5 | 0.02 | DPV | [32] |
| PAA/GCE PAA—poly-aspartic acid GCE—glassy carbon electrode | 0.91–3000 | 0.3 | DPV | [33] |
| TiO2/C/Au/GCE TiO2/C/Au—Au-doped nanostructured TiO2/C material GCE—glassy carbon electrode | 0–20.0 | 0.016 | DPV | [71] |
| rGO-TiO2/GCE rGO-TiO2-TiO2 nanoparticles-decorated, chemically reduced graphene oxide | 0.1–1.0 | 0.01 | DPV | [72] |
| rGO-AuNPs-MIP/SPE rGO-AuNPs-MIP—molecularly imprinted polymer on reduced graphene oxide–gold nanoparticles SPE—screen-printed electrode | 0.01–1.0 | 0.031 | DPV | [73] |
| MBIBr/NiO-SWCNTs/CPE MBIBr/NiO-SWCNTs—NiO-embedded single-wall carbon nanotube nanocomposite and n-methyl-3-butylimidazolium bromide CPE—carbon paste electrode | 0.06–900.0 | 0.02 | SWV | [74] |
| PPy-MWCNTs/GCE PPy-MWCNTs—polypyrrole multi-walled carbon nanotube GCE—glassy carbon electrode | 3.32–25.9 | 1.17 | SWV | [34] |
| MWCNT/GCE MWCNT—multi-walled carbon nanotube | 10–5000 | 1.17 | CV | [75] |
| MWCNTs-Ag/CPE MWCNTs-Ag—multi-walled carbon nanotube decorated with silver nanoparticle-modified CPE—carbon paste electrodes | 0.04–1000 | 0.03 | CV | [69] |
| DDAB/nafion/CPE DDAB/nafion—Didodecyldimethyl-ammonium Bromide/Nafion Composite Film CPE—carbon paste electrode | 2–1200 | 0.39 | CV | [76] |
| GOs/MWCNTs/GCE GOs/MWCNTs—graphene oxide sheets (GOs) and multi-walled carbon nanotubes (MWCNTs) nanocomposite GCE—glassy carbon electrode | 0.24–32 88–1230 | 0.08 | CV | [31] |
| GN/GCE GN—graphene nanosheet GCE—glassy carbon electrode | 0.5–50 | 0.2 | CV | [68] |
| exf-SGR/GCE | 0.1–100 | 0.0303 | CV | current work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mǎgeruşan, L.; Pogǎcean, F.; Soran, M.-L.; Pruneanu, S.-M. Graphene-Based Electrochemical Sensing Platform for Rapid and Selective Ferulic Acid Quantification. Int. J. Mol. Sci. 2023, 24, 16937. https://doi.org/10.3390/ijms242316937
Mǎgeruşan L, Pogǎcean F, Soran M-L, Pruneanu S-M. Graphene-Based Electrochemical Sensing Platform for Rapid and Selective Ferulic Acid Quantification. International Journal of Molecular Sciences. 2023; 24(23):16937. https://doi.org/10.3390/ijms242316937
Chicago/Turabian StyleMǎgeruşan, Lidia, Florina Pogǎcean, Maria-Loredana Soran, and Stela-Maria Pruneanu. 2023. "Graphene-Based Electrochemical Sensing Platform for Rapid and Selective Ferulic Acid Quantification" International Journal of Molecular Sciences 24, no. 23: 16937. https://doi.org/10.3390/ijms242316937
APA StyleMǎgeruşan, L., Pogǎcean, F., Soran, M.-L., & Pruneanu, S.-M. (2023). Graphene-Based Electrochemical Sensing Platform for Rapid and Selective Ferulic Acid Quantification. International Journal of Molecular Sciences, 24(23), 16937. https://doi.org/10.3390/ijms242316937







