The Effect of Mesoporous Structure of the Support on the Oxidation of Dibenzothiophene
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of the Catalysts
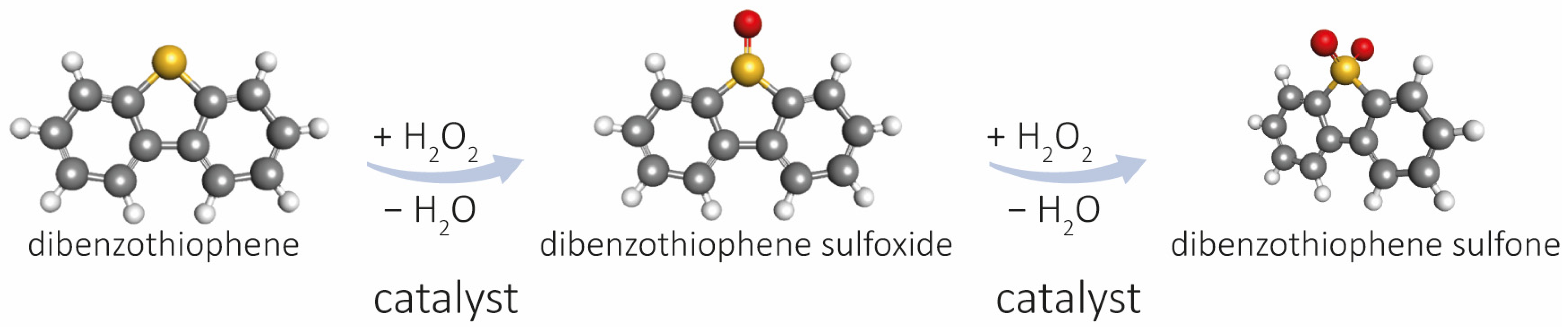
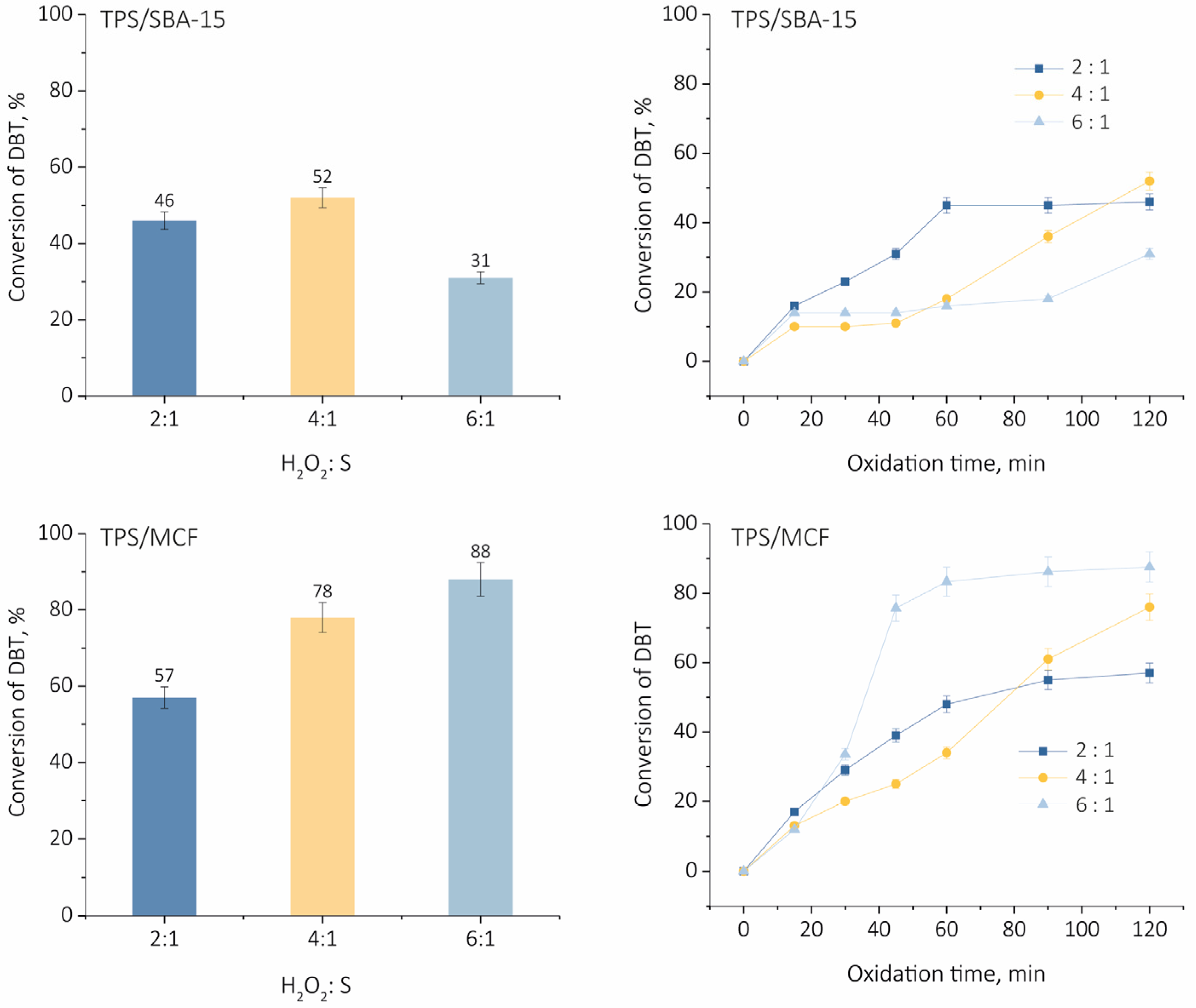
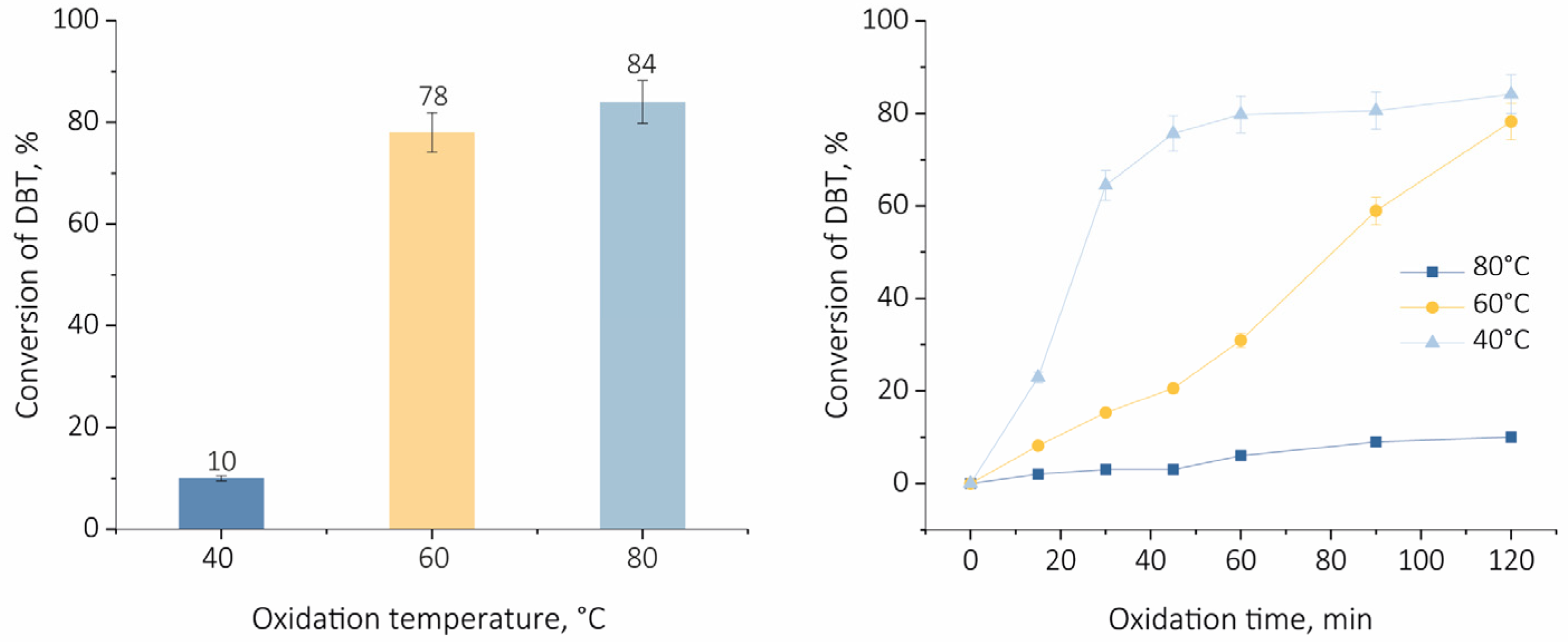
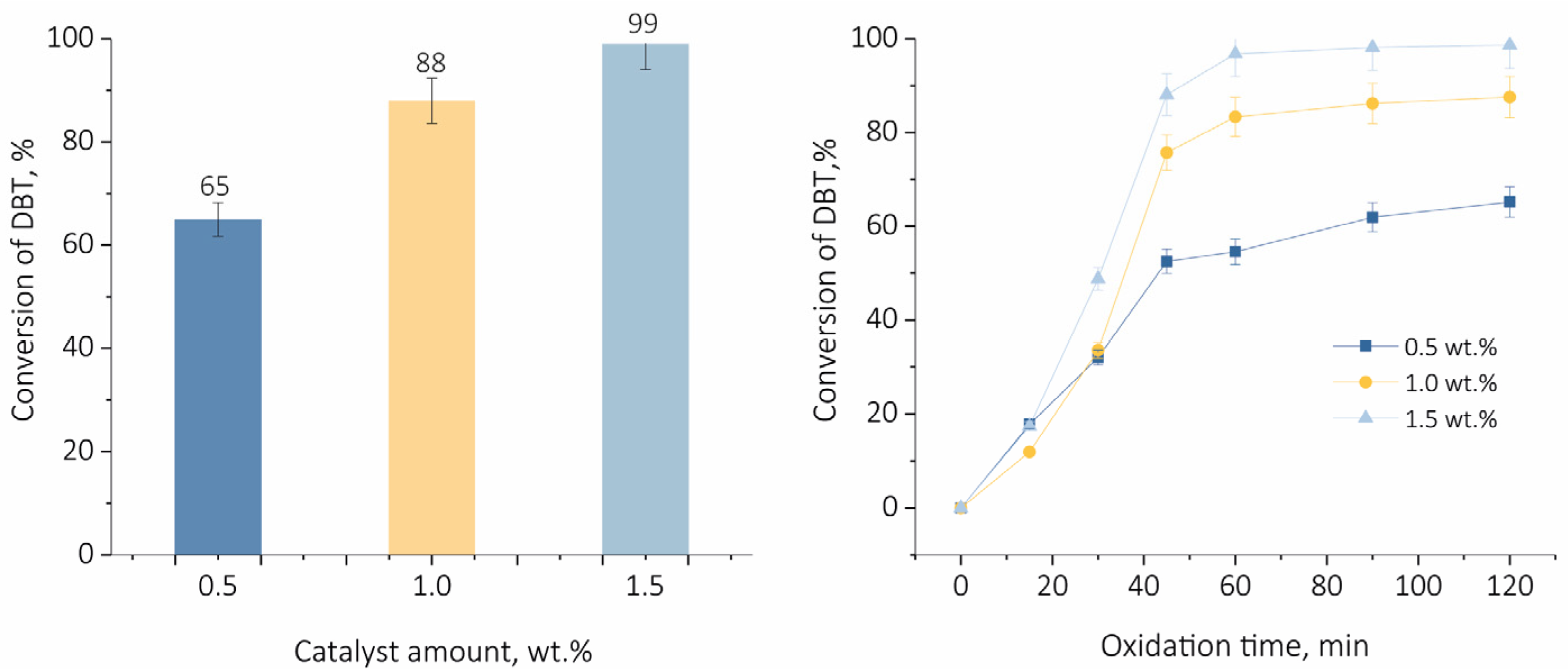
2.2. Oxidative Desulfurization of Dibenzothiophene
3. Materials and Methods
3.1. Materials and Chemicals
3.2. Preparation of Catalysts
3.2.1. Preparation of SBA-15 Support
3.2.2. Preparation of MCF Support
3.2.3. Preparation TPS Containing Catalysts
3.3. Catalyst Characterization
3.4. Catalytic Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ahmadian, M.; Anbia, M. Oxidative Desulfurization of Liquid Fuels Using Polyoxometalate-Based Catalysts: A Review. Energy Fuels 2021, 35, 10347–10373. [Google Scholar] [CrossRef]
- Rajendran, A.; Cui, T.Y.; Fan, H.X.; Yang, Z.F.; Feng, J.; Li, W.Y. A comprehensive review on oxidative desulfurization catalysts targeting clean energy and environment. J. Mater. Chem. A 2020, 8, 2246–2285. [Google Scholar] [CrossRef]
- Boshagh, F.; Rahmani, M.; Rostami, K.; Yousefifar, M. Key Factors Affecting the Development of Oxidative Desulfurization of Liquid Fuels: A Critical Review. Energy Fuels 2022, 36, 98–132. [Google Scholar] [CrossRef]
- Haghighi, M.; Gooneh-Farahani, S. Insights to the oxidative desulfurization process of fossil fuels over organic and inorganic heterogeneous catalysts: Advantages and issues. Environ. Sci. Pollut. Res. 2020, 27, 39923–39945. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Tian, Q.; Tian, Y.; Cui, J.; Wang, G. Ultra-Deep Oxidative Desulfurization of Fuel with H2O2 Catalyzed by Mesoporous Silica-Supported Molybdenum Oxide Modified by Ce. Appl. Sci. 2021, 11, 2018. [Google Scholar] [CrossRef]
- Teimouri, A.; Mahmoudsalehi, M.; Salavati, H. Catalytic oxidative desulfurization of dibenzothiophene utilizing molybdenum and vanadium oxides supported on MCM-41. Int. J. Hydrog. Energy 2018, 43, 14816–14833. [Google Scholar] [CrossRef]
- Akopyan, A.; Polikarpova, P.; Gul, O.; Anisimov, A.; Karakhanov, E. Catalysts Based on Acidic SBA-15 for Deep Oxidative Desulfurization of Model Fuels. Energy Fuels 2020, 34, 14611–14619. [Google Scholar] [CrossRef]
- Rivoira, L.P.; Cussa, J.; Martínez, M.L.; Beltramone, A.R. Experimental design optimization of the ODS of DBT using vanadium oxide supported on mesoporous Ga-SBA-15. Catal. Today 2020, 349, 68–80. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, G.; Guan, T.; Xu, F.; Wu, J.; Zhou, E.; Wang, J.; Li, K. Ultra-Deep Oxidative Desulfurization of Model Oil Catalyzed by In Situ Carbon-Supported Vanadium Oxides Using Cumene Hydroperoxide as Oxidant. Chem. Sel. 2020, 5, 2148–2156. [Google Scholar] [CrossRef]
- Ramos, J.M.; Wang, J.A.; Flores, S.O.; Chen, L.; Arellano, U.; Noreña, L.E.; González, J.; Navarrete, J. Ultrasound-Assisted Hydrothermal Synthesis of V2O5/Zr-SBA-15 Catalysts for Production of Ultralow Sulfur Fuel. Catalysts 2021, 11, 408. [Google Scholar] [CrossRef]
- Polikarpova, P.; Akopyan, A.; Shigapova, A.; Glotov, A.; Anisimov, A.; Karakhanov, E. Oxidative Desulfurization of Fuels Using Heterogeneous Catalysts Based on MCM-41. Energy Fuels 2018, 32, 10898–10903. [Google Scholar] [CrossRef]
- Estephane, G.; Lancelot, C.; Blanchard, P.; Dufaud, V.; Chambrey, S.; Nuns, N.; Toufaily, J.; Hamiye, T.; Lamonier, C. W-SBA based materials as efficient catalysts for the ODS of model and real feeds: Improvement of their lifetime through active phase encapsulation. Appl. Catal. A 2019, 571, 42–50. [Google Scholar] [CrossRef]
- Wang, Y.; Du, F.; Wang, C.; Zhao, J.; Sun, H.; Sun, C. The synthesis and oxidation desulfurization performance of Ti-modified hierarchical cheese-like ZSM-5 zeolite. J. Chem. Res. 2022, 46, 1–8. [Google Scholar] [CrossRef]
- Rivoira, L.P.; Ledesma, B.C.; Juárez, J.M.; Beltramone, A.R. Novel and simple one-pot method for the synthesis of TiO2 modified-CMK-3 applied in oxidative desulfurization of refractory organosulfur compounds. Fuel 2018, 226, 498–507. [Google Scholar] [CrossRef]
- Shinde, P.S.; Suryawanshi, P.S.; Patil, K.K.; Belekar, V.M.; Sankpal, S.A.; Delekar, S.D.; Jadhav, S.A. A brief overview of recent progress in porous silica as catalyst supports. J. Compos. Sci. 2021, 5, 75. [Google Scholar] [CrossRef]
- Liang, Y. Recent advanced development of metal-loaded mesoporous organosilicas as catalytic nanoreactors. Nanoscale Adv. 2021, 3, 6827–6868. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Huo, Q.; Feng, J.; Chmelka, B.F.; Stucky, G.D. Nonionic Triblock and Star Diblock Copolymer and Oligomeric Surfactant Syntheses of Highly Ordered, Hydrothermally Stable, Mesoporous Silica Structures. J. Am. Chem. Soc. 1998, 120, 6024–6036. [Google Scholar] [CrossRef]
- Lettow, J.S.; Han, Y.J.; Schmidt-Winkel, P.; Yang, P.; Zhao, D.; Stucky, G.D.; Ying, J.Y. Hexagonal to Mesocellular Foam Phase Transition in Polymer-Templated Mesoporous Silicas. Langmuir 2000, 16, 8291–8295. [Google Scholar] [CrossRef]
- Beck, J.S.; Vartuli, J.C.; Roth, W.J.; Leonowicz, M.E.; Kresge, C.T.; Schmitt, K.D.; Chu, C.T.W.; Olson, D.H.; Sheppard, E.W.; McCullen, S.B.; et al. A New Family of Mesoporous Molecular Sieves Prepared with Liquid Crystal Templates. J. Am. Chem. Soc. 1992, 114, 10834–10843. [Google Scholar] [CrossRef]
- Castanheiro, J.E.; Vital, J.; Fonseca, I.M.; Ramos, A.M. Acetalization of glycerol with hexanal in the presence of SBA-15 with sulfonic acid groups. Catal. Today 2022, 384, 2–11. [Google Scholar] [CrossRef]
- Cabrera-Munguia, D.; González, H.; Tututi-Ríos, E.; Gutiérrez-Alejandre, A.; Rico, J. Acid properties of M-SBA-15 and M-SBA-15-SO3H (M = Al, Ti) materials and their role on esterification of oleic acid. J. Mater. Res. 2018, 33, 3634–3645. [Google Scholar] [CrossRef]
- Paniagua, M.; Cuevas, F.; Morales, G.; Melero, J.A. Sulfonic Mesostructured SBA-15 Silicas for the Solvent-Free Production of Bio-Jet Fuel Precursors via Aldol Dimerization of Levulinic Acid. ACS Sustain. Chem. Eng. 2021, 9, 5952–5962. [Google Scholar] [CrossRef]
- Testa, M.L.; Tummino, M.L.; Venezia, A.M.; Russo, M. Interesterification of Glyceryl Trioctanoate Catalyzed by Sulfonic Silica-Based Materials: Insight into the Role of Catalysts on the Reaction Mechanism. Materials 2023, 16, 5121. [Google Scholar] [CrossRef] [PubMed]
- Wisniewska, J.; Dziedzic, I.; Ziolek, M. A platinum promoted Ag/SBA-15 catalyst effective in selective oxidation of methanol design and surface characterization. RSC Adv. 2020, 10, 14570–14580. [Google Scholar] [CrossRef] [PubMed]
- Polikarpova, P.; Akopyan, A.; Shlenova, A.; Anisimov, A. New mesoporous catalysts with Brønsted acid sites for deep oxidative desulfurization of model fuels. Catal. Commun. 2020, 146, 106123. [Google Scholar] [CrossRef]
- Habeche, F.; Hachemaoui, M.; Mokhtar, A.; Chikh, K.; Benali, F.; Mekki, A.; Zaoui, F.; Cherifi, Z.; Boukoussa, B. Recent Advances on the Preparation and Catalytic Applications of Metal Complexes Supported-Mesoporous Silica MCM-41 (Review). J. Inorg. Organomet. Polym. Mater. 2020, 30, 4245–4268. [Google Scholar] [CrossRef]
- Trejda, M.; Drobnik, M.; Nurwita, A. Application of microwave radiation in the grafting of acidic sites on SBA-15 type material. J. Porous Mater. 2021, 28, 1261–1267. [Google Scholar] [CrossRef]
- Trejda, M.; Kaszuba, A.; Nurwita, A.; Ziolek, M. Towards Efficient Acidic Catalysts via Optimization of SO3H-Organosilane Immobilization on SBA-15 under Increased Pressure: Potential Applications in Gas and Liquid Phase Reactions. Materials 2021, 14, 7226. [Google Scholar] [CrossRef]
- Stawicka, K.; Sobczak, I.; Trejda, M.; Sulikowski, B.; Ziolek, M. Organosilanes affecting the structure and formation of mesoporous cellular foams. Microporous Mesoporous Mater. 2012, 155, 143–152. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Zholobenko, V.; Freitas, C.; Jendrlin, M.; Bazin, P.; Travert, A.; Thibault-Starzyk, F. Probing the acid sites of zeolites with pyridine: Quantitative AGIR measurements of the molar absorption coefficients. J. Catal. 2020, 385, 52–60. [Google Scholar] [CrossRef]
- Caero, L.C.; Hernández, E.; Pedraza, F.; Murrieta, F. Oxidative desulfurization of synthetic diesel using supported catalysts: Part I. Study of the operation conditions with a vanadium oxide-based catalyst. Catal. Today 2005, 107, 564–569. [Google Scholar] [CrossRef]
- Akopyan, A.V.; Polikarpova, P.D.; Arzyaeva, N.; Anisimov, A.V.; Maslova, O.; Senko, O.; Efremenko, E.N. Model Fuel Oxidation in the Presence of Molybdenum-Containing Catalysts Based on SBA-15 with Hydrophobic Properties. ACS Omega 2021, 6, 26932–26941. [Google Scholar] [CrossRef] [PubMed]
- Stawicka, K.; Decyk, P.; Wojtaszek-Gurdak, A.; Ziolek, M. Comparative study of acid-basic properties of MCF impregnated with niobium and cerium species. Catal. Today 2019, 325, 2–10. [Google Scholar] [CrossRef]
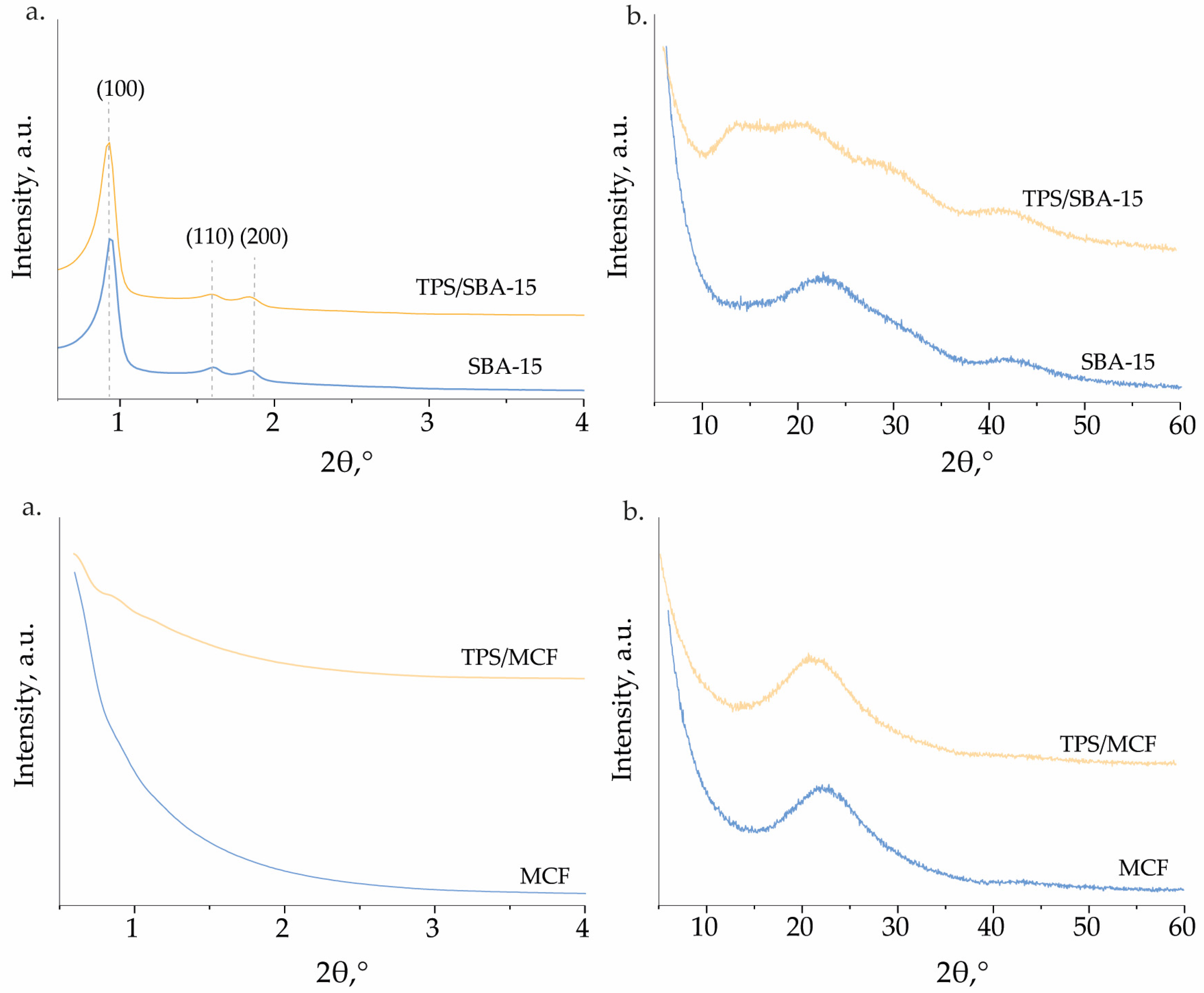
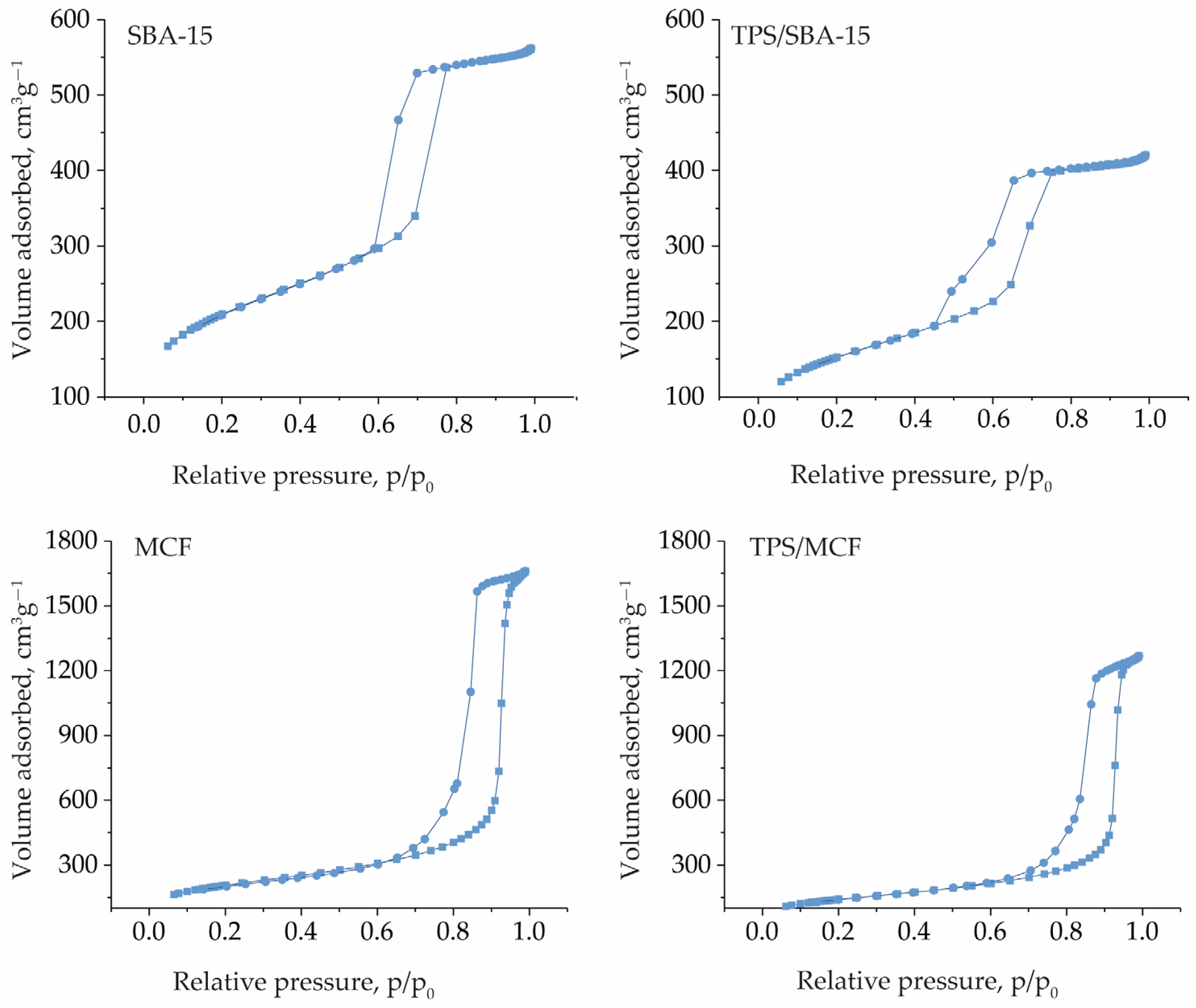


| Catalyst | SBET (m2 g−1) a | Pore Size (nm) | VTotal (cm3 g−1) e | S Content, (wt.%) |
|---|---|---|---|---|
| SBA-15 | 762 | 10.1 b | 0.83 | - |
| TPS/SBA-15 | 554 | 8.3 b | 0.64 | 2.2 f |
| MCF | 753 | 23.7 c; 12.0 d | 2.21 | - |
| TPS/MCF | 517 | 24.0 c; 11.6 d | 1.72 | 2.4 f |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nurwita, A.; Trejda, M. The Effect of Mesoporous Structure of the Support on the Oxidation of Dibenzothiophene. Int. J. Mol. Sci. 2023, 24, 16957. https://doi.org/10.3390/ijms242316957
Nurwita A, Trejda M. The Effect of Mesoporous Structure of the Support on the Oxidation of Dibenzothiophene. International Journal of Molecular Sciences. 2023; 24(23):16957. https://doi.org/10.3390/ijms242316957
Chicago/Turabian StyleNurwita, Ardian, and Maciej Trejda. 2023. "The Effect of Mesoporous Structure of the Support on the Oxidation of Dibenzothiophene" International Journal of Molecular Sciences 24, no. 23: 16957. https://doi.org/10.3390/ijms242316957







