Light-Driven Tetra- and Octa-β-substituted Cationic Zinc(II) Phthalocyanines for Eradicating Fusarium oxysporum Conidia
Abstract
:1. Introduction
2. Results
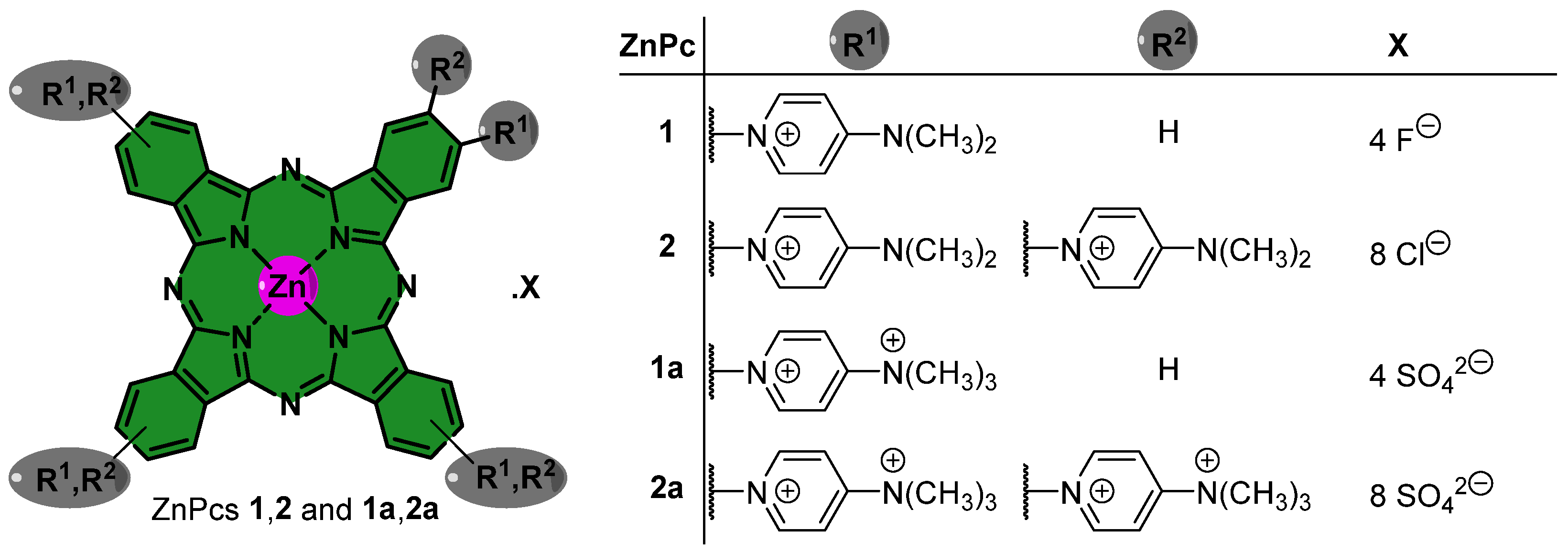
2.1. Synthesis and Photophysical Analysis of Phthalocyanine Dyes
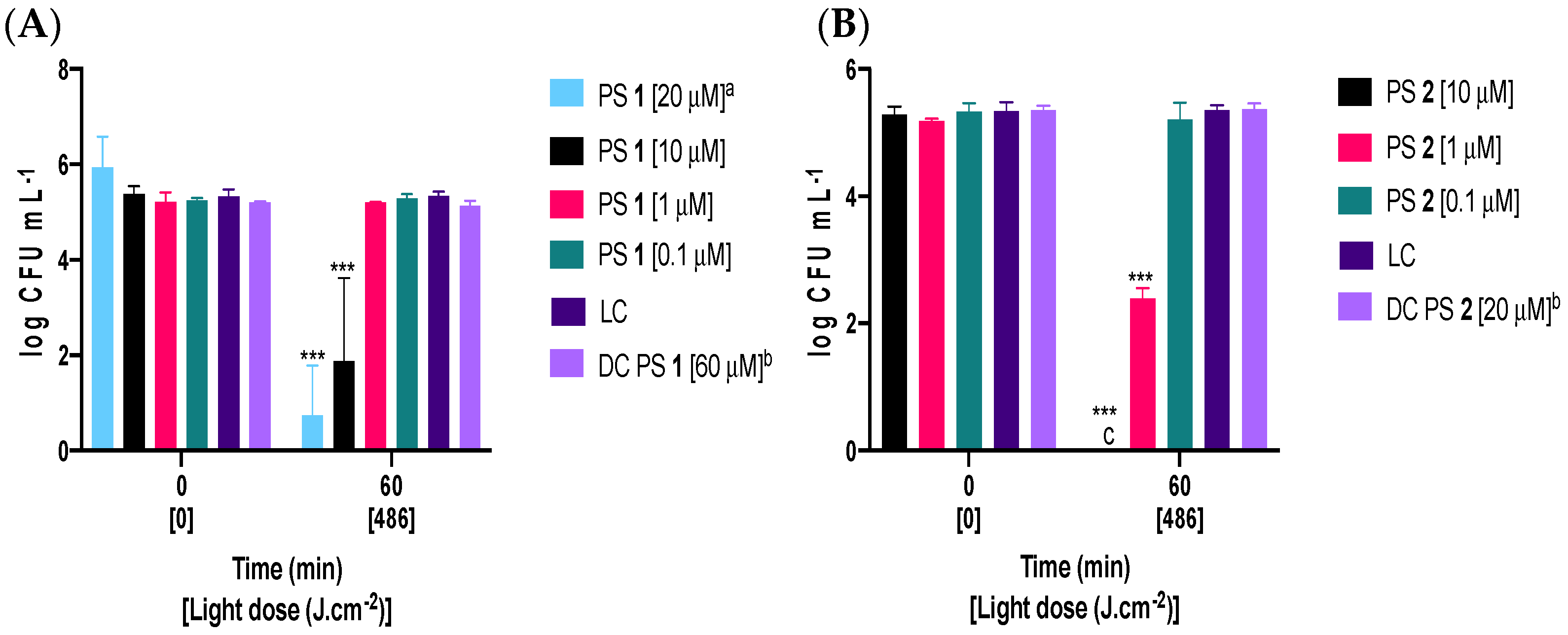
2.2. Photodynamic Inactivation of Fusarium oxysporum Conidia
3. Discussion
4. Materials and Methods
4.1. Synthesis and Photophysical Characterization of the Photosensitizers
4.2. Photosenstizer Stock Solutions
4.3. Light Source
4.4. Preparation of Stock Suspensions of Fusarium oxysporum Conidia
4.5. Photodynamic Inactivation Assays
4.6. Statistical Assessment
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van Diepeningen, A.D.; Feng, P.; Ahmed, S.; Sudhadham, M.; Bunyaratavej, S.; de Hoog, G.S. Spectrum of Fusarium Infections in Tropical Dermatology Evidenced by Multilocus Sequencing Typing Diagnostics. Mycoses 2015, 58, 48–57. [Google Scholar] [CrossRef]
- Ortoneda, M.; Guarro, J.; Madrid, M.P.; Caracuel, Z.; Roncero, M.I.G.; Mayayo, E.; Di Pietro, A. Fusarium oxysporum as a Multihost Model for the Genetic Dissection of Fungal Virulence in Plants and Mammals. Infect. Immun. 2004, 72, 1760–1766. [Google Scholar] [CrossRef]
- Gordon, T.R.; Swett, C.L.; Wingfield, M.J. Management of Fusarium Diseases Affecting Conifers. Crop Prot. 2015, 73, 28–39. [Google Scholar] [CrossRef]
- Wu, C.Y.; Chen, G.S.; Lan, C.C.E. Onychomycosis Caused by Fusarium solani in a Woman with Diabetes. Clin. Exp. Dermatol. 2009, 34, e772–e774. [Google Scholar] [CrossRef]
- Guilhermetti, E.; Takahachi, G.; Shinobu, C.S.; Svidzinski, T.I.E. Fusarium spp. as Agents of Onychomycosis in Immunocompetent Hosts. Int. J. Dermatol. 2007, 46, 822–826. [Google Scholar] [CrossRef]
- Edel-Hermann, V.; Lecomte, C. Current Status of Fusarium oxysporum Formae Speciales and Races. Phytopathology 2019, 109, 512–530. [Google Scholar] [CrossRef]
- Nag, P.; Paul, S.; Shriti, S.; Das, S. Defence Response in Plants and Animals against a Common Fungal Pathogen, Fusarium oxysporum. Curr. Res. Microb. Sci. 2022, 3, 100135. [Google Scholar] [CrossRef]
- João, A.L.; Lencastre, A.; Dutra, E.; Pessoa e Costa, T.; Formiga, A.; Neves, J. Fusarium spp.—An Emerging Pathogen in Chronic Diabetic Ulcer: Case Report and Review of the Literature. Int. J. Low. Extrem. Wounds 2021, 20, 67–72. [Google Scholar] [CrossRef]
- Nucci, M.; Anaissie, E. Fusarium Infections in Immunocompromised Patients. Clin. Microbiol. Rev. 2007, 20, 695–704. [Google Scholar] [CrossRef]
- Nelson, P.E.; Dignani, M.C.; Anaissie, E.J. Taxonomy, Biology, and Clinical Aspects of Fusarium Species. Clin. Microbiol. Rev. 1994, 7, 479–504. [Google Scholar] [CrossRef]
- Palmieri, D.; Ianiri, G.; Del Grosso, C.; Barone, G.; De Curtis, F.; Castoria, R.; Lima, G. Advances and Perspectives in the Use of Biocontrol Agents against Fungal Plant Diseases. Horticulturae 2022, 8, 577. [Google Scholar] [CrossRef]
- Jestoi, M. Emerging Fusarium-Mycotoxins Fusaproliferin, Beauvericin, Enniatins, And Moniliformin—A Review. Crit. Rev. Food Sci. Nutr. 2008, 48, 21–49. [Google Scholar] [CrossRef] [PubMed]
- Gurdaswani, V.; Ghag, S.B. Toxins from Fusarium Species and Their Role in Animal and Plant Diseases. In New and Future Developments in Microbial Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2020; pp. 7–27. [Google Scholar]
- Achari, S.R.; Kaur, J.K.; Mann, R.C.; Sawbridge, T.; Summerell, B.A.; Edwards, J. Investigating the Effector Suite Profile of Australian Fusarium oxysporum Isolates from Agricultural and Natural Ecosystems. Plant Pathol. 2021, 70, 387–396. [Google Scholar] [CrossRef]
- de Menezes, H.D.; Massola, N.S.; Flint, S.D.; Silva, G.J.; Bachmann, L.; Rangel, D.E.N.; Braga, G.U.L. Growth under Visible Light Increases Conidia and Mucilage Production and Tolerance to UV-B Radiation in the Plant Pathogenic Fungus Colletotrichum acutatum. Photochem. Photobiol. 2015, 91, 397–402. [Google Scholar] [CrossRef]
- Zhao, B.; He, D.; Wang, L. Advances in Fusarium Drug Resistance Research. J. Glob. Antimicrob. Resist. 2021, 24, 215–219. [Google Scholar] [CrossRef]
- Chitwood-Brown, J.; Vallad, G.E.; Lee, T.G.; Hutton, S.F. Breeding for Resistance to Fusarium Wilt of Tomato: A Review. Genes 2021, 12, 1673. [Google Scholar] [CrossRef]
- Sampaio, A.M.; de Araújo, S.S.; Rubiales, D.; Vaz Patto, M.C. Fusarium Wilt Management in Legume Crops. Agronomy 2020, 10, 1073. [Google Scholar] [CrossRef]
- Koike, S.T.; Gordon, T.R. Management of Fusarium Wilt of Strawberry. Crop Prot. 2015, 73, 67–72. [Google Scholar] [CrossRef]
- Amini, J.; Sidovich, D. The Effects of Fungicides on Fusarium oxysporum f. sp. Lycopersici Associated with Fusarium Wilt of Tomato. J. Plant Prot. Res. 2010, 50, 172–178. [Google Scholar] [CrossRef]
- Ölmez, H.; Kretzschmar, U. Potential Alternative Disinfection Methods for Organic Fresh-Cut Industry for Minimizing Water Consumption and Environmental Impact. LWT Food Sci. Technol. 2009, 42, 686–693. [Google Scholar] [CrossRef]
- Lykogianni, M.; Bempelou, E.; Karamaouna, F.; Aliferis, K.A. Do Pesticides Promote or Hinder Sustainability in Agriculture? The Challenge of Sustainable Use of Pesticides in Modern Agriculture. Sci. Total Environ. 2021, 795, 148625. [Google Scholar] [CrossRef]
- Ran, X.; Hadiatullah, H.; Yuchi, Z.; Yang, X.; Zhu, X. Sustainable Use of Pesticides. Agriculture 2023, 13, 1393. [Google Scholar] [CrossRef]
- Zafeiriou, E.; Karelakis, C.; Martínez-Zarzoso, I.; Galanopoulos, K.; Gkika, D. Economic Development and Pesticide Use in EU Agriculture: A Nonlinear Panel Data Autoregressive Distributed Lag Approach. Agriculture 2023, 13, 1693. [Google Scholar] [CrossRef]
- Ons, L.; Bylemans, D.; Thevissen, K.; Cammue, B.P.A. Combining Biocontrol Agents with Chemical Fungicides for Integrated Plant Fungal Disease Control. Microorganisms 2020, 8, 1930. [Google Scholar] [CrossRef] [PubMed]
- do Prado-Silva, L.; Brancini, G.T.P.; Braga, G.Ú.L.; Liao, X.; Ding, T.; Sant’Ana, A.S. Antimicrobial Photodynamic Treatment (APDT) as an Innovative Technology to Control Spoilage and Pathogenic Microorganisms in Agri-Food Products: An Updated Review. Food Control 2022, 132, 108527. [Google Scholar] [CrossRef]
- Piksa, M.; Lian, C.; Samuel, I.C.; Pawlik, K.J.; Samuel, I.D.W.; Matczyszyn, K. The Role of the Light Source in Antimicrobial Photodynamic Therapy. Chem. Soc. Rev. 2023, 52, 1697–1722. [Google Scholar] [CrossRef] [PubMed]
- Aroso, R.T.; Schaberle, F.A.; Arnaut, L.G.; Pereira, M.M. Photodynamic Disinfection and Its Role in Controlling Infectious Diseases. Photochem. Photobiol. Sci. 2021, 20, 1497–1545. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-González, R.; Setaro, F.; Gulías, Ò.; Agut, M.; Hahn, U.; Torres, T.; Nonell, S. Cationic Phthalocyanine Dendrimers as Potential Antimicrobial Photosensitisers. Org. Biomol. Chem. 2017, 15, 9008–9017. [Google Scholar] [CrossRef]
- Lopes, M.M.; Bartolomeu, M.; Gomes, A.T.P.C.; Figueira, E.; Pinto, R.; Reis, L.; Balcão, V.M.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Almeida, A. Antimicrobial Photodynamic Therapy in the Control of Pseudomonas syringae pv. actinidiae Transmission by Kiwifruit Pollen. Microorganisms 2020, 8, 1022. [Google Scholar] [CrossRef] [PubMed]
- Ndemueda, A.; Pereira, I.; Faustino, M.A.F.; Cunha, Â. Photodynamic Inactivation of the Phytopathogenic Bacterium Xanthomonas citri subsp. Citri. Lett. Appl. Microbiol. 2020, 71, 420–427. [Google Scholar] [CrossRef]
- Garcia, M.; David, B.; Sierra-Garcia, I.N.; Faustino, M.A.F.; Alves, A.; Esteves, A.C.; Cunha, A. Photodynamic Inactivation of Lasiodiplodia theobromae: Lighting the Way towards an Environmentally Friendly Phytosanitary Treatment. Biol. Lett. 2021, 17, 20200820. [Google Scholar] [CrossRef] [PubMed]
- Ambrosini, V.; Issawi, M.; Sol, V.; Riou, C. Photodynamic Inactivation of Botrytis Cinerea by an Anionic Porphyrin: An Alternative Pest Management of Grapevine. Sci. Rep. 2020, 10, 17438. [Google Scholar] [CrossRef]
- Gonzales, J.C.; Brancini, G.T.P.; Rodrigues, G.B.; Silva-Junior, G.J.; Bachmann, L.; Wainwright, M.; Braga, G.Ú.L. Photodynamic Inactivation of Conidia of the Fungus Colletotrichum abscissum on Citrus Sinensis Plants with Methylene Blue under Solar Radiation. J. Photochem. Photobiol. B 2017, 176, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.K.; Shah, M.P.; Parmar, S.; Kumar, A. Fungi Bio-Prospects in Sustainable Agriculture, Environment and Nano-Technology; Elsevier: Amsterdam, The Netherlands, 2021; ISBN 9780128217344. [Google Scholar]
- Sierra-Garcia, I.N.; Cunha, Â.; Lourenço, L.M.O. In Vitro Photodynamic Treatment of Fusarium oxysporum Conidia through the Action of Thiopyridinium and Methoxypyridinium Chlorins. J. Photochem. Photobiol. A Chem. 2022, 432, 114081. [Google Scholar] [CrossRef]
- Ryu, A.-R.; Han, C.-S.; Oh, H.-K.; Lee, M.-Y. Chlorin E6-Mediated Photodynamic Inactivation with Halogen Light against Microbes and Fungus. Toxicol. Environ. Health Sci. 2015, 7, 231–238. [Google Scholar] [CrossRef]
- Gamelas, S.R.D.; Sierra-Garcia, I.N.; Tomé, A.C.; Cunha, Â.; Lourenço, L.M.O. In Vitro Photoinactivation of Fusarium oxysporum Conidia with Light-Activated Ammonium Phthalocyanines. Int. J. Mol. Sci. 2023, 24, 3922. [Google Scholar] [CrossRef] [PubMed]
- Prandini, J.A.; Castro, K.A.D.F.; Biazzotto, J.C.; Brancini, G.T.P.; Tomé, J.P.C.; Lourenço, L.M.O.; Braga, G.Ú.L.; da Silva, R.S. Thiopyridinium Phthalocyanine for Improved Photodynamic Efficiency against Pathogenic Fungi. J. Photochem. Photobiol. B 2022, 231, 112459. [Google Scholar] [CrossRef]
- Ribeiro, C.P.S.; Lourenço, L.M.O. Overview of Cationic Phthalocyanines for Effective Photoinactivation of Pathogenic Microorganisms. J. Photochem. Photobiol. C Photochem. Rev. 2021, 48, 100422. [Google Scholar] [CrossRef]
- van Leeuwen, M.; Beeby, A.; Fernandes, I.; Ashworth, S.H. The Photochemistry and Photophysics of a Series of Alpha Octa(Alkyl-Substituted) Silicon, Zinc and Palladium Phthalocyanines. Photochem. Photobiol. Sci. 2014, 13, 62–69. [Google Scholar] [CrossRef]
- Al-Asmari, F.; Mereddy, R.; Sultanbawa, Y. A Novel Photosensitization Treatment for the Inactivation of Fungal Spores and Cells Mediated by Curcumin. J. Photochem. Photobiol. B 2017, 173, 301–306. [Google Scholar] [CrossRef]
- Yaakoub, H.; Mina, S.; Calenda, A.; Bouchara, J.-P.; Papon, N. Oxidative Stress Response Pathways in Fungi. Cell. Mol. Life Sci. 2022, 79, 333. [Google Scholar] [CrossRef]
- Ziental, D.; Mlynarczyk, D.T.; Czarczynska-Goslinska, B.; Lewandowski, K.; Sobotta, L. Photosensitizers Mediated Photodynamic Inactivation against Fungi. Nanomaterials 2021, 11, 2883. [Google Scholar] [CrossRef]
- Calzavara-Pinton, P.G.; Venturini, M.; Sala, R. A Comprehensive Overview of Photodynamic Therapy in the Treatment of Superficial Fungal Infections of the Skin. J. Photochem. Photobiol. B 2005, 78, 1–6. [Google Scholar] [CrossRef]
- Lourenço, L.M.O.; Rocha, D.M.G.C.; Ramos, C.I.V.; Gomes, M.C.; Almeida, A.; Faustino, M.A.F.; Almeida Paz, F.A.; Neves, M.G.P.M.S.; Cunha, Â.; Tomé, J.P.C. Photoinactivation of Planktonic and Biofilm Forms of Escherichia coli through the Action of Cationic Zinc(II) Phthalocyanines. ChemPhotoChem 2019, 3, 251–260. [Google Scholar] [CrossRef]
- Beveridge, A.C.; Bench, B.A.; Gorun, S.M.; Diebold, G.J. Evaluation of Photodynamic Therapy Agents through Transient Grating Measurements. J. Phys. Chem. A 2003, 107, 5138–5143. [Google Scholar] [CrossRef]
- Blango, M.G.; Kniemeyer, O.; Brakhage, A.A. Conidial Surface Proteins at the Interface of Fungal Infections. PLoS Pathog. 2019, 15, e1007939. [Google Scholar] [CrossRef] [PubMed]
- Ogunsipe, A.; Nyokong, T. Photophysical and Photochemical Studies of Sulphonated Non-Transition Metal Phthalocyanines in Aqueous and Non-Aqueous Media. J. Photochem. Photobiol. A Chem. 2005, 173, 211–220. [Google Scholar] [CrossRef]
- Sharma, M.; Visai, L.; Bragheri, F.; Cristiani, I.; Gupta, P.K.; Speziale, P. Toluidine Blue-Mediated Photodynamic Effects on Staphylococcal Biofilms. Antimicrob. Agents Chemother. 2008, 52, 299–305. [Google Scholar] [CrossRef]
- Beer, D.D.E.; Srinivasan, R.; Stewart, P.S. Direct Measurement of Chlorine Penetration into Biofilms during Disinfection. Appl. Environ. Microbiol. 1994, 60, 4339–4344. [Google Scholar] [CrossRef]
- Billings, N.; Millan, M.; Caldara, M.; Rusconi, R.; Tarasova, Y.; Stocker, R.; Ribbeck, K. The Extracellular Matrix Component Psl Provides Fast-Acting Antibiotic Defense in Pseudomonas Aeruginosa Biofilms. PLoS Pathog. 2013, 9, e1003526. [Google Scholar] [CrossRef] [PubMed]
- Cidlina, A.; Novakova, V.; Miletin, M.; Zimcik, P. Peripheral Substitution as a Tool for Tuning Electron-Accepting Properties of Phthalocyanine Analogs in Intramolecular Charge Transfer. Dalton Trans. 2015, 44, 6961–6971. [Google Scholar] [CrossRef]
- Costa, T.P.C.; Rodrigues, E.M.; Dias, L.P.; Pupin, B.; Ferreira, P.C.; Rangel, D.E.N. Different Wavelengths of Visible Light Influence the Conidial Production and Tolerance to Ultra-Violet Radiation of the Plant Pathogens Colletotrichum Acutatum and Fusarium fujikuroi. Eur. J. Plant Pathol. 2021, 159, 105–115. [Google Scholar] [CrossRef]
- Avalos, J.; Estrada, A.F. Regulation by Light in Fusarium. Fungal Genet. Biol. 2010, 47, 930–938. [Google Scholar] [CrossRef]
- Li, C.; Jia, X.; Bian, Y.; Qi, D.; Wu, J. Different Susceptibility of Spores and Hyphae of Trichophyton rubrum to Methylene Blue Mediated Photodynamic Treatment in Vitro. Mycoses 2021, 64, 48–54. [Google Scholar] [CrossRef]
- Magyar, D.; Vass, M.; Li, D.-W. Dispersal Strategies of Microfungi. In Biology of Microfungi; Springer: Berlin/Heidelberg, Germany, 2016; pp. 315–371. [Google Scholar]
- Husaini, A.M.; Sakina, A.; Cambay, S.R. Host–Pathogen Interaction in Fusarium oxysporum Infections: Where Do We Stand? Mol. Plant-Microbe Interact. 2018, 31, 889–898. [Google Scholar] [CrossRef]
- de Menezes, H.D.; Rodrigues, G.B.; de Teixeira, S.P.; Massola, N.S.; Bachmann, L.; Wainwright, M.; Braga, G.U.L. In Vitro Photodynamic Inactivation of Plant-Pathogenic Fungi Colletotrichum acutatum and Colletotrichum gloeosporioides with Novel Phenothiazinium Photosensitizers. Appl. Environ. Microbiol. 2014, 80, 1623–1632. [Google Scholar] [CrossRef]
- Hamminger, C.; Glueck, M.; Fefer, M.; Ckurshumova, W.; Liu, J.; Tenhaken, R.; Plaetzer, K. Photodynamic Inactivation of Plant Pathogens Part II: Fungi. Photochem. Photobiol. Sci. 2022, 21, 195–207. [Google Scholar] [CrossRef]
- Armarego, W.L.F. (Ed.) Purification of Laboratory Chemicals; Elsevier: Oxford, UK, 2017; ISBN 9780128054574. [Google Scholar]
- Gordon, T.R. Fusarium oxysporum and the Fusarium Wilt Syndrome. Annu. Rev. Phytopathol. 2017, 55, 23–39. [Google Scholar] [CrossRef]

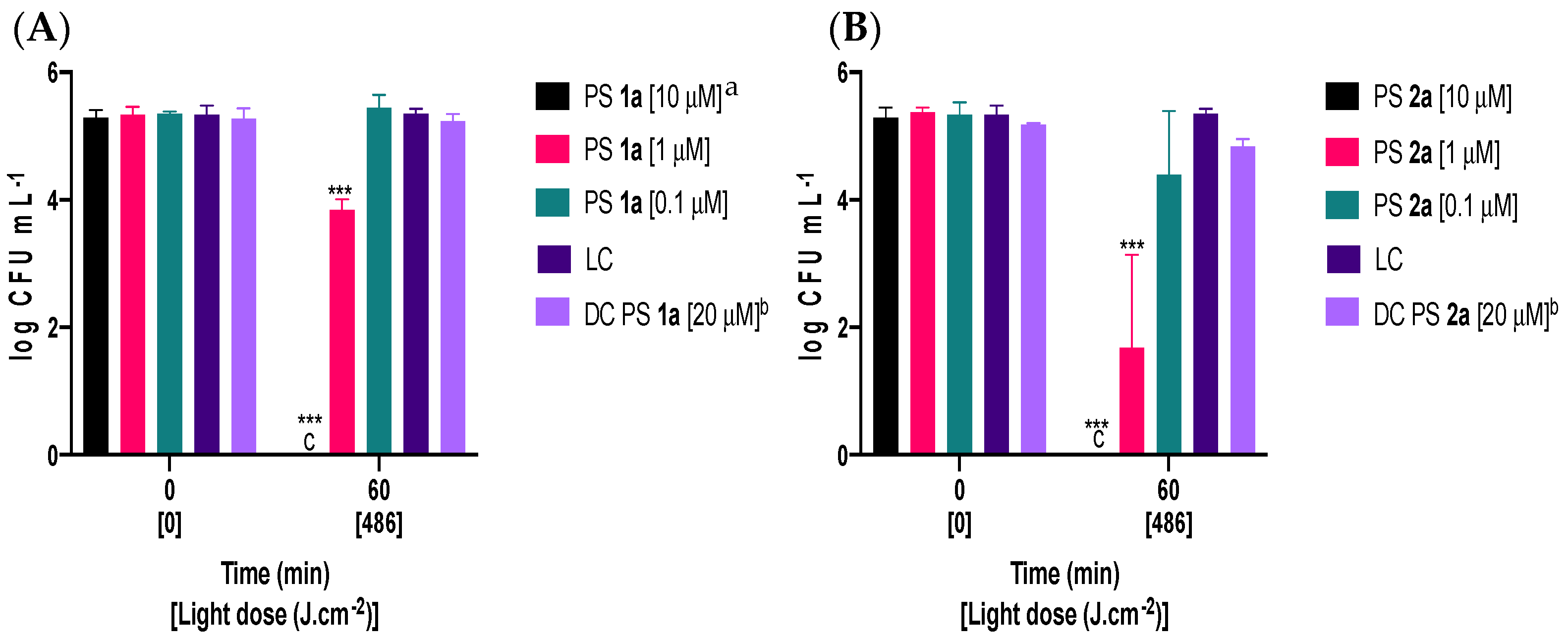
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lourenço, L.M.O.; Cunha, Â.; Sierra-Garcia, I.N. Light-Driven Tetra- and Octa-β-substituted Cationic Zinc(II) Phthalocyanines for Eradicating Fusarium oxysporum Conidia. Int. J. Mol. Sci. 2023, 24, 16980. https://doi.org/10.3390/ijms242316980
Lourenço LMO, Cunha Â, Sierra-Garcia IN. Light-Driven Tetra- and Octa-β-substituted Cationic Zinc(II) Phthalocyanines for Eradicating Fusarium oxysporum Conidia. International Journal of Molecular Sciences. 2023; 24(23):16980. https://doi.org/10.3390/ijms242316980
Chicago/Turabian StyleLourenço, Leandro M. O., Ângela Cunha, and Isabel N. Sierra-Garcia. 2023. "Light-Driven Tetra- and Octa-β-substituted Cationic Zinc(II) Phthalocyanines for Eradicating Fusarium oxysporum Conidia" International Journal of Molecular Sciences 24, no. 23: 16980. https://doi.org/10.3390/ijms242316980
APA StyleLourenço, L. M. O., Cunha, Â., & Sierra-Garcia, I. N. (2023). Light-Driven Tetra- and Octa-β-substituted Cationic Zinc(II) Phthalocyanines for Eradicating Fusarium oxysporum Conidia. International Journal of Molecular Sciences, 24(23), 16980. https://doi.org/10.3390/ijms242316980







