Attempts to Understand Oral Mucositis in Head and Neck Cancer Patients through Omics Studies: A Narrative Review
Abstract
:1. Introduction
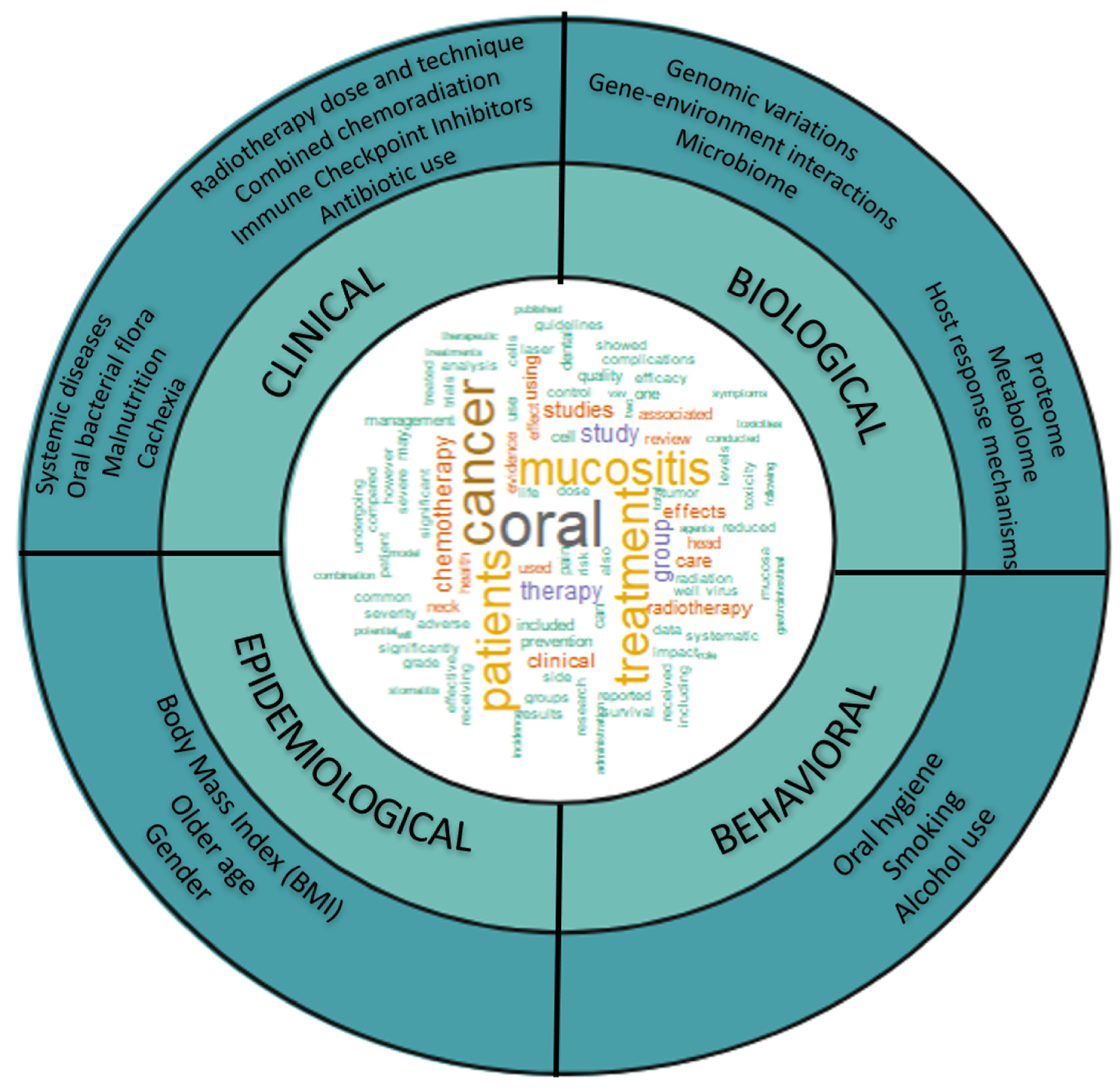
2. “Omics” Approaches May Help in Risk Assessment and Developing Personalized and More Effective Therapies OM
2.1. Genomics
2.1.1. Candidate-Gene Studies
2.1.2. Pathway-Based
| Year | First Author | Approach | Sample Size | Therapy | Sample | Phenotype | Genes | SNPs |
|---|---|---|---|---|---|---|---|---|
| 2022 | Schack [27] | Genome-wide | Discovery = 1183 Danish Cohort Replication = 597 Danish Cohort Validation = 235 Asian Cohort | RTx | Buffy coats | Mucositis 0: no 1: erythema 2: patchy 3: confluent 4:ulceration | STING1 | rs1131769 |
| 2020 | Mlak [23] | Candidate gene | 60 | RTx | Peripheral blood | Mucositis RTOG/EORTC | TNFRS1 A | rs767455 |
| 2020 | Mlak [24] | Candidate gene | 62 | RTx | Peripheral blood | Mucositis RTOG/EORTC | TNF alpha | rs1799964 |
| 2020 | Yang [28] | Genome-wide | 960 560 | RTx | Blood | RTOG/EORTC | TNKS | rs117157809 |
| 2018 | Brzozowska [25] | Candidate gene | 62 | RTx | Peripheral blood | Mucositis RTOG/EORTC | APEH | rs4855883 |
| 2018 | Brzozowska [22] | Candidate gene | 58 | RTx | Peripheral blood | Mucositis RTOG/EORTC | TNFRS1 A | rs4149570 |
| 2018 | Brzozowska [21] | Candidate gene | 65 | RTx | Peripheral blood | Mucositis RTOG/EORTC | GHRL | rs1629816 |
| 2017 | Chen [26] | Candidate gene | 114 | RTx | Peripheral blood | Mucositis RTOG/VRS | XRCC1 | rs25487 |
| 2017 | Reyes-Gibby [20] | Pathway-based | 885 | RTx and/or CTx | Peripheral blood | Oral Mucositis (ICD) | RB1 | rs2227311 |
2.1.3. Genome-Wide Approach
2.2. Transcriptomics and Proteomics
2.3. Metabolomics
| Year | First Author | Phenotype | Samples | Therapy | Sample Size | Methods | Targets | Results |
|---|---|---|---|---|---|---|---|---|
| Metabolomics | ||||||||
| Animal Models | ||||||||
| 2021 | Geng [43] | Mucositis (0–5) | Serum from OM rat model, induced with 5-FU and 10% acetic acid | CTx | 30 rats | UHPLC | Cholic acid, linoleic acid, 4-pyridoxic acid, LysoPC | Shuanghua Baihe tablets improve inflammatory symptoms of oral mucositis. |
| 2020 | Chen [44] | Induced oral ulcers and degree of healing | Serum from OM rat model, induced with 15% chloral hydrate | CTx | 42 rats | LC-QTOF/MS | 5-HT, GABA | Kouyanqing granules attenuate the symptoms of oral ulcers worsened by sleep deprivation through regulation of the neuroimmunoendocrine system, oxidative stress levels, and tryptophan metabolism. |
| Clinical Samples | ||||||||
| 2021 | Yatsuoka [40] | NCI CTCAE | Saliva | CTx/RTx | 9 HNC | CE-TOF-MS | tryptophan, D-glucose, D-glutamate, GABA, 2-AB | Pre-treatment concentrations of gamma-aminobutyric acid and 2-aminobutyric acids were higher in the high-grade OM group. |
| 2021 | Yang [41] | NRS 010 | Peripheral blood | RCTx | 10 NPC | UHPLC-MS/MS | 9-HEPE, 15-HETE | MOM promotes the release of anti-inflammatory lipids to reduce tissue damage; enhancement of 9S-HEPE and 15-HETE in all radiation doses. |
| Microbiomics | ||||||||
| 2020 | Reyes-Gibby [12] | NCI CTCAE | buccal mucosal | RTx and/or CTx | 66 Locoregional HNSCC | 16S rRNA | Cardiobacterium, Granulicatella, Prevotella, Fusobacterium, Streptococcus, Megasphaera, Cardiobacterium | Genera abundance was associated with the hazard for the onset of severe OM. |
| 2020 | Vesty [45] | WHO | saliva and oral swabs | RTx | 19 HNC | NGS | Fusobacterium, Haemophilus, Tannerella, Porphyromonas and Eikenella, Candida | Gram-negative bacteria on the buccal mucosa may influence susceptibility to developing OM. |
| 2019 | Subramaniam and Muthukrishnan [46] | WHO | unstimulated whole saliva | RTx and RCTx | 24 HNSCC | 16S rRNA | Staphylococcus aureus, Staphylococcus epidermidis, Pseudomonas aeruginosa, Escherichia coli, Klebsiella pneumoniae | The bacterial isolates obtained during and at the end of therapy appeared to express a higher level of antibiotic-resistance genes (VIM2, MCR-1, TET[K], blaKPC) than those isolated at the onset of therapy. |
| 2018 | Hou [47] | RTOG | oral swabs | RTx | 19 NPC | 16S rRNA | Prevotella, Fusobacterium, Treponema, Porphyromonas | Prevotella, Fusobacterium, Treponema and Porphyromonas showed dynamic synchronous variations in abundance throughout the course of radiation therapy, frequently coinciding with the onset of severe mucositis. |
| 2017 | Zhu [48] | RTOG | oral or retropharyngeal mucosa swabs | RTx | 41 NPC | 16S rRNA | Firmicutes, Proteobacteria, Bacteroidetes, Fusobacteria, Actinobacteria, Spirochaetes, Cyanobacteria, Verrucomicrobia, Acidobacteria, TM7, Deinococcus-Thermus and SR1 | Oral microbiota changes correlate with the progression and aggravation of radiotherapy-induced mucositis in patients with nasopharyngeal carcinoma. |
| Microbiota | ||||||||
| 2018 | Almstahl [49] | WHO | Swab culture | RTx | 33 HNC | Culture | Neisseria, Fusobacterium, Prevotella, Candida | Levels of Neisseria decreased and mucosal pathogens increased during RT; 2 years post-treatment, Fusobacterium and Prevotella decreased; growth of Candida increased |
| 2018 | Gaetti-Jardim [50] | NCI CTCAE | Supra and subgingival biofilms | RTx | 28 HNC | Culture | Candida, Enterobacteriaceae | Candida and family Enterobacteriaceae showed increased prevalence with RT, and were associated with the occurrence of mucositis and xerostomia |
| Transcriptomics | ||||||||
| Animal Models | ||||||||
| 2021 | Geng [43] | Mucositis (0-5) | Serum from OM rat model, induced with 5-FU and 10% acetic acid | CTx | 30 rats | Whole genome sequencing | ALOX15, CYP2J2, CYP1A1, ALOX15, GATM, ALAS2, PLA2G5 | Shuanghua Baihe tablets improve inflammatory symptoms of oral mucositis. |
| 2021 | Saul-McBeth [51] | Induced oral ulcers, % damage | OM mice model; Induced with head and neck irradiation | RTx | 3 mice | RNA Seq | IL-17RA | IL-17RA provides protection during HNI-induced OM by preventing excess inflammation during ulceration phase of OM. |
| Clinical Samples | ||||||||
| 2018 | Mlak [52] | RTOG/EORTC | Plasma | RTx | 60 HNC | Microarray | RRM1 | RRM1 gene expression in cfRNA allows for estimating risk of severe OM. |
| Proteomics | ||||||||
| 2015 | Jehmlich [53] | NCI CTC v3 | Unstimulated whole saliva | RTx | 50 HNC | MS | RPL18A, C6orf115, PRTN3, RPS20, FGB, ARPC1B, PLBD1, GGH, ANXA6, FGG, ANP32E, CTSG, PTGR1, SERPINA1, MDH2, CORO1A, HSPE1, BAHCC1 CP, MMP9, GCA, PLYRP1, SCGB2A1, GPI, PPIC, QRDL, HIST1H4A, HNRNPA2B1, ATP5B, LTA4H, TIMP1, TKT, RPL10A, AZU1, MMP8, RPLP2, ARPC4, CAT, S100A8, B2M, SERPING1, CYBB, ELANE, C3, CALML5, ITIHRPS15A, ACTR2 | 48 proteins differed significantly between OM group and non-OM group. 17 proteins displayed increased levels and 31 proteins decreased in level in OM. |
2.4. Microbiomics of OM
2.5. Challenges in Integrating a Multi-Omics Approach for OM
3. Conclusions
4. Future Directions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mody, M.D.; Rocco, J.W.; Yom, S.S.; Haddad, R.I.; Saba, N.F. Head and neck cancer. Lancet 2021, 398, 2289–2299. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Cohen, E.E.; LaMonte, S.J.; Erb, N.L.; Beckman, K.L.; Sadeghi, N.; Hutcheson, K.A.; Stubblefield, M.D.; Abbott, D.M.; Fisher, P.S.; Stein, K.D.; et al. American Cancer Society Head and Neck Cancer Survivorship Care Guideline. CA Cancer J. Clin. 2016, 66, 203–239. [Google Scholar] [CrossRef]
- Maria, O.M.; Eliopoulos, N.; Muanza, T. Radiation-Induced Oral Mucositis. Front. Oncol. 2017, 7, 89. [Google Scholar] [CrossRef]
- Pulito, C.; Cristaudo, A.; Porta, C.; Zapperi, S.; Blandino, G.; Morrone, A.; Strano, S. Oral mucositis: The hidden side of cancer therapy. J. Exp. Clin. Cancer Res. 2020, 39, 210. [Google Scholar] [CrossRef]
- Yeh, S.A. Radiotherapy for head and neck cancer. Semin. Plast. Surg. 2010, 24, 127–136. [Google Scholar] [CrossRef]
- Narayan, S.; Lehmann, J.; Coleman, M.A.; Vaughan, A.; Yang, C.C.; Enepekides, D.; Farwell, G.; Purdy, J.A.; Laredo, G.; Nolan, K.; et al. Prospective evaluation to establish a dose response for clinical oral mucositis in patients undergoing head-and-neck conformal radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2008, 72, 756–762. [Google Scholar] [CrossRef]
- Bhide, S.A.; Gulliford, S.; Schick, U.; Miah, A.; Zaidi, S.; Newbold, K.; Nutting, C.M.; Harrington, K.J. Dose-response analysis of acute oral mucositis and pharyngeal dysphagia in patients receiving induction chemotherapy followed by concomitant chemo-IMRT for head and neck cancer. Radiother. Oncol. 2012, 103, 88–91. [Google Scholar] [CrossRef]
- Harris, D.J. Cancer treatment-induced mucositis pain: Strategies for assessment and management. Ther. Clin. Risk Manag. 2006, 2, 251–258. [Google Scholar] [CrossRef]
- Lalla, R.V.; Sonis, S.T.; Peterson, D.E. Management of oral mucositis in patients who have cancer. Dent. Clin. N. Am. 2008, 52, 61–77. [Google Scholar] [CrossRef]
- Bonomi, M.; Batt, K. Supportive Management of Mucositis and Metabolic Derangements in Head and Neck Cancer Patients. Cancers 2015, 7, 1743–1757. [Google Scholar] [CrossRef]
- Reyes-Gibby, C.C.; Wang, J.; Zhang, L.; Peterson, C.B.; Do, K.A.; Jenq, R.R.; Shelburne, S.; Shah, D.P.; Chambers, M.S.; Hanna, E.Y.; et al. Oral microbiome and onset of oral mucositis in patients with squamous cell carcinoma of the head and neck. Cancer 2020, 126, 5124–5136. [Google Scholar] [CrossRef]
- Sunaga, T.; Nagatani, A.; Fujii, N.; Hashimoto, T.; Watanabe, T.; Sasaki, T. The association between cumulative radiation dose and the incidence of severe oral mucositis in head and neck cancers during radiotherapy. Cancer Rep. 2021, 4, e1317. [Google Scholar] [CrossRef]
- de Pauli Paglioni, M.; Faria, K.M.; Palmier, N.R.; Prado-Ribeiro, A.C.; RB, E.D.; da Graça Pinto, H.; Treister, N.S.; Epstein, J.B.; Migliorati, C.A.; Santos-Silva, A.R.; et al. Patterns of oral mucositis in advanced oral squamous cell carcinoma patients managed with prophylactic photobiomodulation therapy-insights for future protocol development. Lasers Med. Sci. 2021, 36, 429–436. [Google Scholar] [CrossRef]
- Chen, S.C.; Lai, Y.H.; Huang, B.S.; Lin, C.Y.; Fan, K.H.; Chang, J.T. Changes and predictors of radiation-induced oral mucositis in patients with oral cavity cancer during active treatment. Eur. J. Oncol. Nurs. 2015, 19, 214–219. [Google Scholar] [CrossRef]
- Elad, S.; Zadik, Y. Chronic oral mucositis after radiotherapy to the head and neck: A new insight. Support. Care Cancer 2016, 24, 4825–4830. [Google Scholar] [CrossRef]
- Kwon, J.M.; Goate, A.M. The candidate gene approach. Alcohol. Res. Health 2000, 24, 164–168. [Google Scholar]
- Guo, Y.F.; Li, J.; Chen, Y.; Zhang, L.S.; Deng, H.W. A new permutation strategy of pathway-based approach for genome-wide association study. BMC Bioinform. 2009, 10, 429. [Google Scholar] [CrossRef]
- Uffelmann, E.; Huang, Q.Q.; Munung, N.S.; de Vries, J.; Okada, Y.; Martin, A.R.; Martin, H.C.; Lappalainen, T.; Posthuma, D. Genome-wide association studies. Nat. Rev. Methods Primers 2021, 1, 59. [Google Scholar] [CrossRef]
- Reyes-Gibby, C.C.; Melkonian, S.C.; Wang, J.; Yu, R.K.; Shelburne, S.A.; Lu, C.; Gunn, G.B.; Chambers, M.S.; Hanna, E.Y.; Yeung, S.J.; et al. Identifying novel genes and biological processes relevant to the development of cancer therapy-induced mucositis: An informative gene network analysis. PLoS ONE 2017, 12, e0180396. [Google Scholar] [CrossRef]
- Brzozowska, A.; Homa-Mlak, I.; Mlak, R.; Golebiowski, P.; Mazurek, M.; Ciesielka, M.; Malecka-Massalska, T. Polymorphism of regulatory region of GHRL gene (-2531C>T) as a promising predictive factor for radiotherapy-induced oral mucositis in patients with head neck cancer. Head. Neck 2018, 40, 1799–1811. [Google Scholar] [CrossRef]
- Brzozowska, A.; Powrozek, T.; Homa-Mlak, I.; Mlak, R.; Ciesielka, M.; Golebiowski, P.; Malecka-Massalska, T. Polymorphism of Promoter Region of TNFRSF1A Gene (-610 T > G) as a Novel Predictive Factor for Radiotherapy Induced Oral Mucositis in HNC Patients. Pathol. Oncol. Res. 2018, 24, 135–143. [Google Scholar] [CrossRef]
- Mlak, R.; Powrozek, T.; Brzozowska, A.; Homa-Mlak, I.; Mazurek, M.; Golebiowski, P.; Korzeb, D.; Rahnama-Hezavah, M.; Malecka-Massalska, T. Polymorphism of TNFRSF1 A may act as a predictor of severe radiation-induced oral mucositis and a prognosis factor in patients with head and neck cancer. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. 2020, 130, 283–291.e282. [Google Scholar] [CrossRef]
- Mlak, R.; Powrozek, T.; Brzozowska, A.; Homa-Mlak, I.; Mazurek, M.; Golebiowski, P.; Sobieszek, G.; Malecka-Massalska, T. The relationship between TNF-alpha gene promoter polymorphism (-1211 T > C), the plasma concentration of TNF-alpha, and risk of oral mucositis and shortening of overall survival in patients subjected to intensity-modulated radiation therapy due to head and neck cancer. Support. Care Cancer 2020, 28, 531–540. [Google Scholar] [CrossRef]
- Brzozowska, A.; Mlak, R.; Homa-Mlak, I.; Golebiowski, P.; Mazurek, M.; Ciesielka, M.; Malecka-Massalska, T. Polymorphism of regulatory region of APEH gene (c.-521G>C, rs4855883) as a relevant predictive factor for radiotherapy induced oral mucositis and overall survival in head neck cancer patients. Oncotarget 2018, 9, 29644–29653. [Google Scholar] [CrossRef]
- Chen, H.; Wu, M.; Li, G.; Hua, L.; Chen, S.; Huang, H. Association between XRCC1 single-nucleotide polymorphism and acute radiation reaction in patients with nasopharyngeal carcinoma: A cohort study. Medicine 2017, 96, e8202. [Google Scholar] [CrossRef]
- Schack, L.M.H.; Naderi, E.; Fachal, L.; Dorling, L.; Luccarini, C.; Dunning, A.M.; The Head and Neck Group of the Radiogenomics Consortium; The Danish Head and Neck Cancer Group (DAHANCA); Ong, E.H.W.; Chua, M.L.K.; et al. A genome-wide association study of radiotherapy induced toxicity in head and neck cancer patients identifies a susceptibility locus associated with mucositis. Br. J. Cancer 2022, 126, 1082–1090. [Google Scholar] [CrossRef]
- Yang, D.W.; Wang, T.M.; Zhang, J.B.; Li, X.Z.; He, Y.Q.; Xiao, R.; Xue, W.Q.; Zheng, X.H.; Zhang, P.F.; Zhang, S.D.; et al. Genome-wide association study identifies genetic susceptibility loci and pathways of radiation-induced acute oral mucositis. J. Transl. Med. 2020, 18, 224. [Google Scholar] [CrossRef]
- Zhang, R.; Kang, R.; Tang, D. The STING1 network regulates autophagy and cell death. Signal Transduct. Target. Ther. 2021, 6, 208. [Google Scholar] [CrossRef]
- Patel, S.; Jin, L. TMEM173 variants and potential importance to human biology and disease. Genes Immun. 2019, 20, 82–89. [Google Scholar] [CrossRef]
- Motwani, M.; Pesiridis, S.; Fitzgerald, K.A. DNA sensing by the cGAS–STING pathway in health and disease. Nat. Rev. Genet. 2019, 20, 657–674. [Google Scholar] [CrossRef]
- Barber, G.N. STING: Infection, inflammation and cancer. Nat. Rev. Immunol. 2015, 15, 760–770. [Google Scholar] [CrossRef]
- Dregalla, R.C.; Zhou, J.; Idate, R.R.; Battaglia, C.L.; Liber, H.L.; Bailey, S.M. Regulatory roles of tankyrase 1 at telomeres and in DNA repair: Suppression of T-SCE and stabilization of DNA-PKcs. Aging 2010, 2, 691–708. [Google Scholar] [CrossRef]
- Gutierrez-Camino, A.; Oosterom, N.; den Hoed, M.A.H.; Lopez-Lopez, E.; Martin-Guerrero, I.; Pluijm, S.M.F.; Pieters, R.; de Jonge, R.; Tissing, W.J.E.; Heil, S.G.; et al. The miR-1206 microRNA variant is associated with methotrexate-induced oral mucositis in pediatric acute lymphoblastic leukemia. Pharmacogenet Genom. 2017, 27, 303–306. [Google Scholar] [CrossRef]
- Tao, J.; Fan, M.; Zhou, D.; Hong, Y.; Zhang, J.; Liu, H.; Sharma, S.; Wang, G.; Dong, Q. miR-200c Modulates the Pathogenesis of Radiation-Induced Oral Mucositis. Oxid. Med. Cell Longev. 2019, 2019, 2352079. [Google Scholar] [CrossRef]
- Kiyomi, A.; Yoshida, K.; Arai, C.; Usuki, R.; Yamazaki, K.; Hoshino, N.; Kurokawa, A.; Imai, S.; Suzuki, N.; Toyama, A.; et al. Salivary inflammatory mediators as biomarkers for oral mucositis and oral mucosal dryness in cancer patients: A pilot study. PLoS ONE 2022, 17, e0267092. [Google Scholar] [CrossRef]
- Jehmlich, N.; Stegmaier, P.; Golatowski, C.; Salazar, M.G.; Rischke, C.; Henke, M.; Volker, U. Proteome data of whole saliva which are associated with development of oral mucositis in head and neck cancer patients undergoing radiotherapy. Data Brief. 2016, 8, 501–505. [Google Scholar] [CrossRef]
- Clish, C.B. Metabolomics: An emerging but powerful tool for precision medicine. Cold Spring Harb. Mol. Case Stud. 2015, 1, a000588. [Google Scholar] [CrossRef]
- Segers, K.; Declerck, S.; Mangelings, D.; Heyden, Y.V.; Eeckhaut, A.V. Analytical techniques for metabolomic studies: A review. Bioanalysis 2019, 11, 2297–2318. [Google Scholar] [CrossRef]
- Yatsuoka, W.; Ueno, T.; Miyano, K.; Enomoto, A.; Ota, S.; Sugimoto, M.; Uezono, Y. Time-Course of Salivary Metabolomic Profiles during Radiation Therapy for Head and Neck Cancer. J. Clin. Med. 2021, 10, 2631. [Google Scholar] [CrossRef]
- Yang, G.; Feng, D.; Li, F.; Luo, B.; Zhu, J.; Yang, Q.; Zheng, L.; Dong, Q.; Chen, M.; Xu, Z.; et al. A randomized, controlled phase II trial of maxillofacial and oral massage in attenuating severe radiotherapy-induced oral mucositis and lipid metabolite changes in nasopharyngeal carcinoma. Radiother. Oncol. 2021, 163, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, W.; Yao, L.; Zhang, X.; Zhang, X.; Ye, C.; Jiang, H.; He, J.; Zhu, Y.; Ai, D. Hydroxyeicosapentaenoic acids and epoxyeicosatetraenoic acids attenuate early occurrence of nonalcoholic fatty liver disease. Br. J. Pharmacol. 2017, 174, 2358–2372. [Google Scholar] [CrossRef]
- Geng, Q.S.; Liu, R.J.; Shen, Z.B.; Wei, Q.; Zheng, Y.Y.; Jia, L.Q.; Wang, L.H.; Li, L.F.; Li, J.; Xue, W.H. Transcriptome sequencing and metabolome analysis reveal the mechanism of Shuanghua Baihe Tablet in the treatment of oral mucositis. Chin. J. Nat. Med. 2021, 19, 930–943. [Google Scholar] [CrossRef]
- Chen, W.; Li, C.; Jin, D.; Shi, Y.; Zhang, M.; Bo, M.; Qian, D.; Wang, M.; Li, G. Metabolomics Combined with Network Pharmacology-Based Strategy to Reveal the Underlying Mechanism of Zhenhuang Submicron Emulsion in Treating Oropharyngeal Mucositis Complications of Radiation Therapy for Head and Neck Cancer. Drug Des. Devel Ther. 2022, 16, 3169–3182. [Google Scholar] [CrossRef]
- Vesty, A.; Gear, K.; Biswas, K.; Mackenzie, B.W.; Taylor, M.W.; Douglas, R.G. Oral microbial influences on oral mucositis during radiotherapy treatment of head and neck cancer. Support. Care Cancer 2020, 28, 2683–2691. [Google Scholar] [CrossRef]
- Subramaniam, N.; Muthukrishnan, A. Oral mucositis and microbial colonization in oral cancer patients undergoing radiotherapy and chemotherapy: A prospective analysis in a tertiary care dental hospital. J. Investig. Clin. Dent. 2019, 10, e12454. [Google Scholar] [CrossRef]
- Hou, J.; Zheng, H.; Li, P.; Liu, H.; Zhou, H.; Yang, X. Distinct shifts in the oral microbiota are associated with the progression and aggravation of mucositis during radiotherapy. Radiother. Oncol. 2018, 129, 44–51. [Google Scholar] [CrossRef]
- Zhu, X.X.; Yang, X.J.; Chao, Y.L.; Zheng, H.M.; Sheng, H.F.; Liu, H.Y.; He, Y.; Zhou, H.W. The Potential Effect of Oral Microbiota in the Prediction of Mucositis During Radiotherapy for Nasopharyngeal Carcinoma. EBioMedicine 2017, 18, 23–31. [Google Scholar] [CrossRef]
- Almstahl, A.; Finizia, C.; Carlen, A.; Fagerberg-Mohlin, B.; Alstad, T. Mucosal microflora in head and neck cancer patients. Int. J. Dent. Hyg. 2018, 16, 459–466. [Google Scholar] [CrossRef]
- Gaetti-Jardim, E., Jr.; Jardim, E.C.G.; Schweitzer, C.M.; da Silva, J.C.L.; Oliveira, M.M.; Masocatto, D.C.; Dos Santos, C.M. Supragingival and subgingival microbiota from patients with poor oral hygiene submitted to radiotherapy for head and neck cancer treatment. Arch. Oral. Biol. 2018, 90, 45–52. [Google Scholar] [CrossRef]
- Saul-McBeth, J.; Dillon, J.; Lee, A.; Launder, D.; Kratch, J.M.; Abutaha, E.; Williamson, A.A.; Schroering, A.G.; Michalski, G.; Biswas, P.; et al. Tissue Damage in Radiation-Induced Oral Mucositis Is Mitigated by IL-17 Receptor Signaling. Front. Immunol. 2021, 12, 687627. [Google Scholar] [CrossRef]
- Mlak, R.; Powrozek, T.; Brzozowska, A.; Homa-Mlak, I.; Mazurek, M.; Malecka-Massalska, T. RRM1 gene expression evaluated in the liquid biopsy (blood cfRNA) as a non-invasive, predictive factor for radiotherapy-induced oral mucositis and potential prognostic biomarker in head and neck cancer patients. Cancer Biomark. 2018, 22, 657–667. [Google Scholar] [CrossRef] [PubMed]
- Jehmlich, N.; Stegmaier, P.; Golatowski, C.; Salazar, M.G.; Rischke, C.; Henke, M.; Volker, U. Differences in the whole saliva baseline proteome profile associated with development of oral mucositis in head and neck cancer patients undergoing radiotherapy. J. Proteom. 2015, 125, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Trotti, A.; Garden, A.; Warde, P.; Symonds, P.; Langer, C.; Redman, R.; Pajak, T.F.; Fleming, T.R.; Henke, M.; Bourhis, J.; et al. A multinational, randomized phase III trial of iseganan HCl oral solution for reducing the severity of oral mucositis in patients receiving radiotherapy for head-and-neck malignancy. Int. J. Radiat. Oncol. Biol. Phys. 2004, 58, 674–681. [Google Scholar] [CrossRef]
- Sonis, S.T. The Chicken or the Egg? Changes in Oral Microbiota as Cause or Consequence of Mucositis During Radiation Therapy. EBioMedicine 2017, 18, 7–8. [Google Scholar] [CrossRef] [PubMed]
- Bruno, J.S.; Al-Qadami, G.H.; Laheij, A.; Bossi, P.; Fregnani, E.R.; Wardill, H.R. From Pathogenesis to Intervention: The Importance of the Microbiome in Oral Mucositis. Int. J. Mol. Sci. 2023, 24, 8274. [Google Scholar] [CrossRef] [PubMed]
- Hong, B.Y.; Sobue, T.; Choquette, L.; Dupuy, A.K.; Thompson, A.; Burleson, J.A.; Salner, A.L.; Schauer, P.K.; Joshi, P.; Fox, E.; et al. Chemotherapy-induced oral mucositis is associated with detrimental bacterial dysbiosis. Microbiome 2019, 7, 66. [Google Scholar] [CrossRef]
- Xia, C.; Jiang, C.; Li, W.; Wei, J.; Hong, H.; Li, J.; Feng, L.; Wei, H.; Xin, H.; Chen, T. A Phase II Randomized Clinical Trial and Mechanistic Studies Using Improved Probiotics to Prevent Oral Mucositis Induced by Concurrent Radiotherapy and Chemotherapy in Nasopharyngeal Carcinoma. Front. Immunol. 2021, 12, 618150. [Google Scholar] [CrossRef]
- Jiang, D.; Armour, C.R.; Hu, C.; Mei, M.; Tian, C.; Sharpton, T.J.; Jiang, Y. Microbiome Multi-Omics Network Analysis: Statistical Considerations, Limitations, and Opportunities. Front. Genet. 2019, 10, 995. [Google Scholar] [CrossRef]
- Vernocchi, P.; Gili, T.; Conte, F.; Del Chierico, F.; Conta, G.; Miccheli, A.; Botticelli, A.; Paci, P.; Caldarelli, G.; Nuti, M.; et al. Network Analysis of Gut Microbiome and Metabolome to Discover Microbiota-Linked Biomarkers in Patients Affected by Non-Small Cell Lung Cancer. Int. J. Mol. Sci. 2020, 21, 8730. [Google Scholar] [CrossRef]
- Wang, Y.; Mao, M.; Li, F.; Deng, W.; Shen, S.; Jiang, X. Predicting microbial interactions from time series data with network information. Int. J. Data Min. Bioinform. 2017, 17, 97–114. [Google Scholar] [CrossRef]
- El Saie, A.; Fu, C.; Grimm, S.L.; Robertson, M.J.; Hoffman, K.; Putluri, V.; Ambati, C.S.R.; Putluri, N.; Shivanna, B.; Coarfa, C.; et al. Metabolome and microbiome multi-omics integration from a murine lung inflammation model of bronchopulmonary dysplasia. Pediatr. Res. 2022, 92, 1580–1589. [Google Scholar] [CrossRef] [PubMed]
- Peterson, C.B.; Saha, S.; K-A, D. Analysis of microbiome data. Annu. Rev. Stat. Appl. 2023. [Google Scholar] [CrossRef]
- Sonis, S.T. Precision medicine for risk prediction of oral complications of cancer therapy-The example of oral mucositis in patients receiving radiation therapy for cancers of the head and neck. Front. Oral. Health 2022, 3, 917860. [Google Scholar] [CrossRef] [PubMed]
- Tahir, U.A.; Gerszten, R.E. Omics and Cardiometabolic Disease Risk Prediction. Annu. Rev. Med. 2020, 71, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Stingo, F.C.; Chen, Y.A.; Vannucci, M.; Barrier, M.; Mirkes, P.E. A Bayesian Graphical Modeling Approach to Microrna Regulatory Network Inference. Ann. Appl. Stat. 2010, 4, 2024–2048. [Google Scholar] [CrossRef]
- Wang, W.; Baladandayuthapani, V.; Morris, J.S.; Broom, B.M.; Manyam, G.; Do, K.-A. iBAG: Integrative Bayesian analysis of high-dimensional multiplatform genomics data. Bioinformatics 2012, 29, 149–159. [Google Scholar] [CrossRef]
- Srivastava, S.; Wang, W.; Manyam, G.; Ordonez, C.; Baladandayuthapani, V. Integrating multi-platform genomic data using hierarchical Bayesian relevance vector machines. EURASIP J. Bioinform. Syst. Biol. 2013, 2013, 9. [Google Scholar] [CrossRef]
- Chekouo, T.; Stingo, F.C.; Doecke, J.D.; Do, K.A. miRNA-target gene regulatory networks: A Bayesian integrative approach to biomarker selection with application to kidney cancer. Biometrics 2015, 71, 428–438. [Google Scholar] [CrossRef]
- Daemen, A.; Gevaert, O.; Bie, T.D.; Debucquoy, A.; Machiels, J.-P.; Moor, B.D.; Haustermans, K. Integrating microarray and proteomics data to predict the response of cetuximab in patients with rectal cancer. In Proceedings of the Pacific Symposium on Biocomputing, Kohala Coast, HI, USA, 4–8 January 2008; pp. 166–177. [Google Scholar]
- Wu, S.; Xu, Y.; Feng, Z.; Yang, X.; Wang, X.; Gao, X. Multiple-platform data integration method with application to combined analysis of microarray and proteomic data. BMC Bioinform. 2012, 13, 320. [Google Scholar] [CrossRef]
- Chekouo, T.; Stingo, F.C.; Doecke, J.D.; Do, K.A. A Bayesian integrative approach for multi-platform genomic data: A kidney cancer case study. Biometrics 2017, 73, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Zhou, F.; Ren, J.; Li, X.; Jiang, Y.; Ma, S. A Selective Review of Multi-Level Omics Data Integration Using Variable Selection. High. Throughput 2019, 8, 4. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Gibby, C.C.; Wu, X.; Spitz, M.; Kurzrock, R.; Fisch, M.; Bruera, E.; Shete, S. Molecular epidemiology, cancer-related symptoms, and cytokines pathway. Lancet Oncol. 2008, 9, 777–785. [Google Scholar] [CrossRef] [PubMed]
- National Institutes of Health. NIDCR Prospective Observational or Biomarker Validation Study Cooperative Agreement. Available online: https://grants.nih.gov/grants/guide/pa-files/PAR-20-060.html (accessed on 13 April 2023).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
San Valentin, E.M.D.; Do, K.-A.; Yeung, S.-C.J.; Reyes-Gibby, C.C. Attempts to Understand Oral Mucositis in Head and Neck Cancer Patients through Omics Studies: A Narrative Review. Int. J. Mol. Sci. 2023, 24, 16995. https://doi.org/10.3390/ijms242316995
San Valentin EMD, Do K-A, Yeung S-CJ, Reyes-Gibby CC. Attempts to Understand Oral Mucositis in Head and Neck Cancer Patients through Omics Studies: A Narrative Review. International Journal of Molecular Sciences. 2023; 24(23):16995. https://doi.org/10.3390/ijms242316995
Chicago/Turabian StyleSan Valentin, Erin Marie D., Kim-Anh Do, Sai-Ching J. Yeung, and Cielito C. Reyes-Gibby. 2023. "Attempts to Understand Oral Mucositis in Head and Neck Cancer Patients through Omics Studies: A Narrative Review" International Journal of Molecular Sciences 24, no. 23: 16995. https://doi.org/10.3390/ijms242316995





