Infrared Spectroscopy: A New Frontier in Hematological Disease Diagnosis
Abstract
:1. Introduction
2. Fundamentals of Infrared Spectroscopy
3. Hematological Diseases
3.1. Anemia
3.2. Leukemia
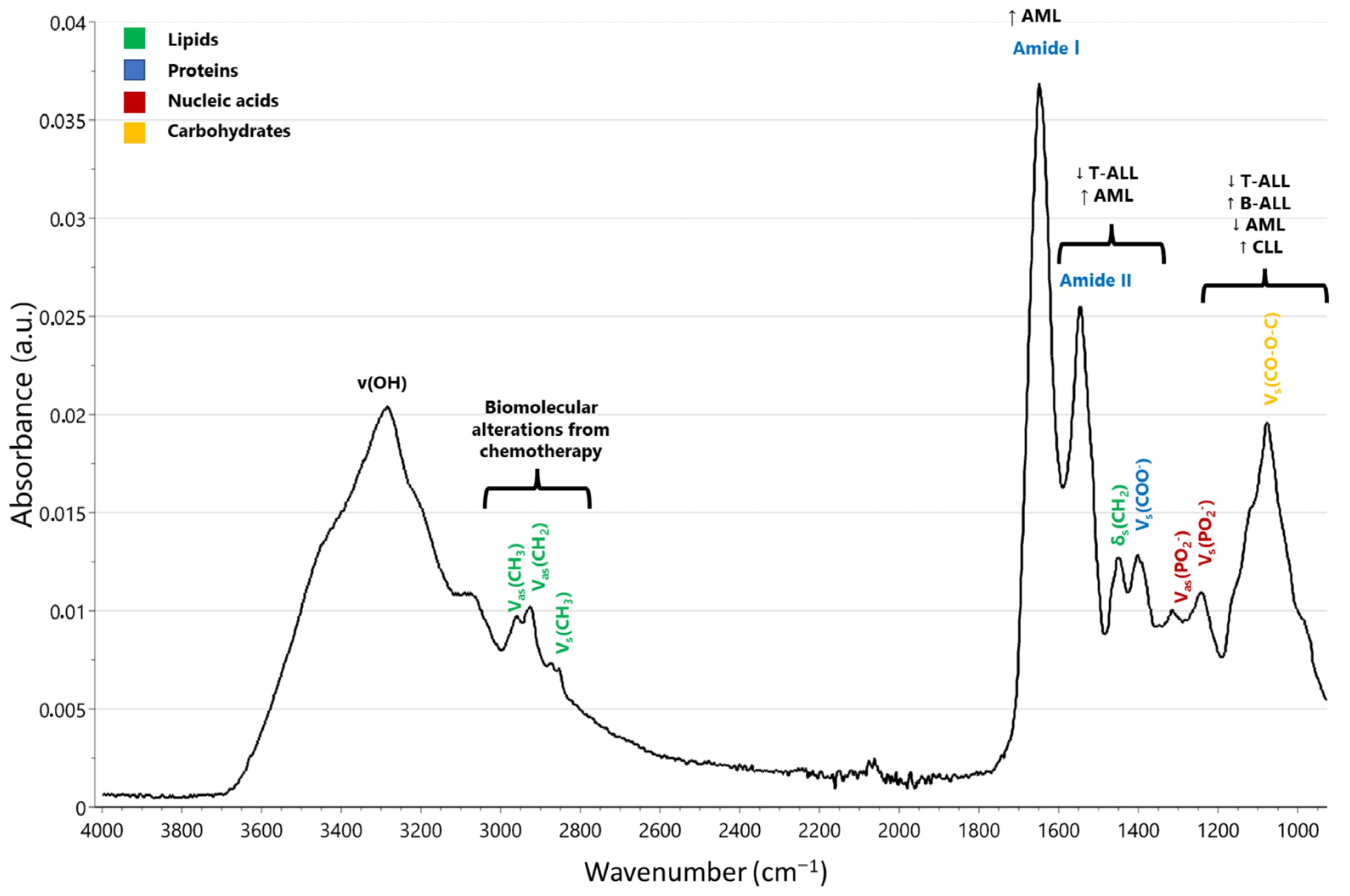
3.2.1. Acute Lymphoblastic Leukemia
| Study Population | Sample and Main Technique | Key Spectral Findings | Ref |
|---|---|---|---|
| Childhood ALL (T-cell and B-cell precursors) | Peripheral lymphocytes Micro-FTIR spectroscopy | - Micro-FTIR analysis distinguished T-cell ALL patients from controls through absorbance alterations in the range of 1300–1600 cm−1. - Notable spectral variations were observed in the 1000–1200 cm−1 region (related to nucleic acids). - Biomolecular alterations resulting from chemotherapy were observed in the higher-wavenumber region spanning from 2800–3000 cm−1. - Notable reduction in absorption at 965 and 1245 cm−1 associated with phosphodiester bonds in nucleic acids. - Reduced integrated absorbance during chemotherapy in B-cell ALL cases may be linked to decreased nucleic acids or phospholipids, with no major alterations in protein content. - Reduced integrated absorbance in T-cell ALL cases prior to treatment could be linked to decreased protein or phospholipid content. - Immediate decrease in DNA and RNA levels following chemotherapy initiation, with corroborated reduction in total phosphate content. | [27] |
| Leukemia patients, patients with “infection” symptoms resembling leukemia, and healthy individuals | PBMCs Micro-FTIR spectroscopy | - Distinct spectral differences related to lipids and proteins in the 3000–2800 cm−1 region among leukemia patients, “infection” patients, and healthy individuals. - Reduced lipid absorption in leukemia patients possibly attributed to altered lipid composition in plasma membrane of blast cells. - Biochemical markers, including DNA, identified and statistically validated for childhood leukemia diagnosis. - Decrease in DNA absorption linked to rapid reduction in blast cells during early chemotherapy and chromatin condensation in apoptotic blasts/PBMCs. | [28] |
| Childhood ALL vs. healthy controls | Bone marrow FTIR spectroscopy | - Evidence of distinct alterations in characteristic bands related to cellular proteins, lipids, and DNA in both healthy and diseased samples. - Structural changes in protein secondary structure with a higher proportion of antiparallel β-sheet protein constituents in ALL samples. - Various absorbance ratios indicating changes in biomolecular structure as potential biomarkers. - Frequency shifts observed at specific wavenumbers in the FTIR spectra. | [36] |
| BCP-ALL patients vs. healthy controls | Serum FTIR spectroscopy | - Significant difference in the peak area ratio at 2965/1645 cm−1 between BCP-ALL patients and healthy controls, indicating distinct structural differences in sera. - Lower average percentage of both β-sheet and β-turn protein structures in sera of BCP-ALL patients. - Development of a predictive model achieving an 85% accuracy rate in classifying individuals as healthy or afflicted with BCP-ALL. - Correlation observed between phase shift of the first derivative in the spectral range of 1050–1042 cm−1 and white blood cell and blast cell count in BCP-ALL patients, potentially providing insights into disease progression and severity. | [37] |
3.2.2. Acute Myeloid Leukemia
3.2.3. Chronic Lymphocytic Leukemia
3.3. Lymphoma
| Study Population | Main Technique | Key Spectral Findings | Ref |
|---|---|---|---|
| Animal model | |||
| Transgenic mice model (VavBcl2/TACI-Ig mice, genetically altered Vav-Bcl2 mice) | MIR microscopy imaging | - Strong correlation between MIR microscopy and tissue characteristics. - Spectral groupings differentiate phenotypes (especially follicular hyperplasia and cancer). - Notable wavenumber shifts observed in amide I (1650 cm−1) and nucleic-acid-related bands. | [54] |
| EL4 mouse model | ATR-FTIR spectroscopy | - Spectral differences between control and tumorous samples. - Variations in protein absorption intensities, with specific wavenumber shifts in the amide I band (1650 cm−1). - Changes in carbohydrate and nucleic acid bands. | [58] |
| Human model | |||
| Follicular lymphomas, diffuse large B-cell lymphomas, reactive lymph nodes | MIR imaging | - Differentiation between lymphoma entities. - Subtyping based on wavenumber shifts. - Follicular lymphoma: higher concentration of amide I proteins and lipids in follicular region (1650 cm−1), but not in the interfollicular area. -Diffuse large B-cell lymphomas: lower amounts of amide I and a higher but more heterogeneously distributed amounts of lipids. - Reactive lymph nodes: high amounts of amide I and lipids in the secondary germinal centers, with lower amounts in the surrounding areas. - The follicles exhibit a distinct polarity, particularly evident in the asymmetric distribution of biochemical components. One side of the secondary follicle displays a significantly lower intensity of the amide I band and lipid-related spectral features compared to the opposite side. This asymmetry aligns with the histological and biological characteristics of secondary follicles. | [55] |
| Human lymphoid tissues, benign and malignant lymphoid tissues | MIR imaging | - Distinguishes benign and malignant lymphoid tissues. - Categorizes lymphoma subtypes based on specific wavenumber shifts. | [57] |
| Human lymphoma (percentage of PD-L1+ cells) | Visible and NIR hyperspectral imaging | - Differentiates lymphoma subtypes based on spectral signatures. - Measures protein expression with specific wavenumber shifts around 1650 cm−1. - Potential for multiplex immunohistochemistry analysis. | [63] |
| Mantle cell lymphoma | S-FTIR microscopy | - Increased absorbance for peaks linked to amide I (1650 cm−1), amide II, and nucleic acids. - Notable wavenumber shifts in DNA vibrations. - Distinguishes classic and aggressive MCL subtypes. | [68] |
| Cutaneous T-cell lymphoma | S-FTIR microscopy | - Higher amide I/RNA and amide II/RNA ratios in mycosis fungoides IIA and IB compared to MF IA and pityriasis lichenoides chronica (around 1650 cm−1). - Distinctions among the three groups based on specific wavenumber shifts. | [70] |
3.4. Thalassemia
3.5. Sickle Cell Anemia
3.6. Myelodysplastic Syndrome
3.7. Myeloproliferative Neoplasms
3.7.1. Primary Myelofibrosis
3.7.2. Essential Thrombocythemia
3.7.3. Chronic Myeloid Leukemia
3.7.4. Polycythemia Vera
4. Future Directions and Potential Developments
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, K.-Z.; Shi, M.-H.; Mantsch, H.H. Molecular and Chemical Characterization of Blood Cells by Infrared Spectroscopy: A New Optical Tool in Hematology. Blood Cells Mol. Dis. 2005, 35, 404–412. [Google Scholar] [CrossRef]
- Sampath, V.; Zhao, X.J.; Caughey, W.S. Characterization of Interactions of Nitric Oxide with Human Hemoglobin A by Infrared Spectroscopy. Biochem. Biophys. Res. Commun. 1994, 198, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Potter, W.T.; Hazzard, J.H.; Kawanishi, S.; Caughey, W.S. Direct Measurement of Carbon Monoxide Bound to Different Subunits of Hemoglobin A in Solution and in Red Cells by Infrared Spectroscopy. Biochem. Biophys. Res. Commun. 1983, 116, 719–725. [Google Scholar] [CrossRef] [PubMed]
- Moh, P.P.; Fiamingo, F.G.; Alben, J.O. Conformational Sensitivity of Beta-93 Cysteine SH to Ligation of Hemoglobin Observed by FT-IR Spectroscopy. Biochemistry 1987, 26, 6243–6249. [Google Scholar] [CrossRef]
- Gregoriou, V.G.; Jayaraman, V.; Hu, X.; Spiro, T.G. FT-IR Difference Spectroscopy of Hemoglobins A and Kempsey: Evidence That a Key Quaternary Interaction Induces Protonation of Asp Beta 99. Biochemistry 1995, 34, 6876–6882. [Google Scholar] [CrossRef]
- Wallace, W.J.; Volpe, J.A.; Maxwell, J.C.; Caughey, W.S. Properties of Hemoglobin A and Hemoglobin Zurich (Beta63 Histidine Replaced by Arginine): Quantitative Evaluation of Functional Abnormalities in Hemoglobins. Biochem. Biophys. Res. Commun. 1976, 68, 1379–1386. [Google Scholar] [CrossRef] [PubMed]
- Dong, A.; Caughey, W.S. Infrared Methods for Study of Hemoglobin Reactions and Structures. Methods Enzymol. 1994, 232, 139–175. [Google Scholar] [CrossRef]
- Dong, A.; Nagai, M.; Yoneyama, Y.; Caughey, W.S. Determination of the Amounts and Oxidation States of Hemoglobins M Boston and M Saskatoon in Single Erythrocytes by Infrared Microspectroscopy. J. Biol. Chem. 1994, 269, 25365–25368. [Google Scholar] [CrossRef]
- Dong, A.C.; Huang, P.; Caughey, B.; Caughey, W.S. Infrared Analysis of Ligand- and Oxidation-Induced Conformational Changes in Hemoglobins and Myoglobins. Arch. Biochem. Biophys. 1995, 316, 893–898. [Google Scholar] [CrossRef]
- Yoo, B.-K.; Kruglik, S.G.; Lamarre, I.; Martin, J.-L.; Negrerie, M. Absorption Band III Kinetics Probe the Picosecond Heme Iron Motion Triggered by Nitric Oxide Binding to Hemoglobin and Myoglobin. J. Phys. Chem. B 2012, 116, 4106–4114. [Google Scholar] [CrossRef]
- Benko, B.; Yu, N.T. Resonance Raman Studies of Nitric Oxide Binding to Ferric and Ferrous Hemoproteins: Detection of Fe(III)—NO Stretching, Fe(III)—N—O Bending, and Fe(II)—N—O Bending Vibrations. Proc. Natl. Acad. Sci. USA 1983, 80, 7042–7046. [Google Scholar] [CrossRef]
- Ohta, T.; Shibata, T.; Kobayashi, Y.; Yoda, Y.; Ogura, T.; Neya, S.; Suzuki, A.; Seto, M.; Yamamoto, Y. A Nuclear Resonance Vibrational Spectroscopic Study of Oxy Myoglobins Reconstituted with Chemically Modified Heme Cofactors: Insights into the Fe–O2 Bonding and Internal Dynamics of the Protein. Biochemistry 2018, 57, 6649–6652. [Google Scholar] [CrossRef]
- Lin, S.-H.; Yu, N.-T.; Gersonde, K. Resonance Raman Evidence for an Unusually Strong Exogenous Ligand—Metal Bond in a Monomeric Nitrosyl Manganese Hemoglobin. FEBS Lett. 1988, 229, 367–371. [Google Scholar] [CrossRef]
- Smulevich, G.; Mauro, J.M.; Fishel, L.A.; English, A.M.; Kraut, J.; Spiro, T.G. Cytochrome c Peroxidase Mutant Active Site Structures Probed by Resonance Raman and Infrared Signatures of the CO Adducts. Biochemistry 1988, 27, 5486–5492. [Google Scholar] [CrossRef]
- Al-Mustafa, J.I. FTIR Investigation of the Conformational Properties of the Cyanide Bound Human Hemoglobin. Vib. Spectrosc. 2002, 30, 139–146. [Google Scholar] [CrossRef]
- Yoshikawa, S.; Caughey, W.S. Infrared Evidence of Cyanide Binding to Iron and Copper Sites in Bovine Heart Cytochrome c Oxidase. Implications Regarding Oxygen Reduction. J. Biol. Chem. 1990, 265, 7945–7958. [Google Scholar] [CrossRef]
- Yoshikawa, S.; O’Keeffe, D.H.; Caughey, W.S. Investigations of Cyanide as an Infrared Probe of Hemeprotein Ligand Binding Sites. J. Biol. Chem. 1985, 260, 3518–3528. [Google Scholar] [CrossRef]
- Al-Mustafa, J.; Kincaid, J.R. Resonance Raman Study of Cyanide-Ligated Horseradish Peroxidase.Detection of Two Binding Geometries and Direct Evidence for the “Push-Pull” Effect. Biochemistry 1994, 33, 2191–2197. [Google Scholar] [CrossRef] [PubMed]
- Pinakoulaki, E.; Vamvouka, M.; Varotsis, C. The Active Site Structure of Heme a3 3+ C≡NCuB2+ of Cytochrome a a 3 Oxidase as Revealed from Resonance Raman Scattering. J. Phys. Chem. B 2003, 107, 9865–9868. [Google Scholar] [CrossRef]
- Crispin, P.; Forwood, K. Near Infrared Spectroscopy in Anemia Detection and Management: A Systematic Review. Transfus. Med. Rev. 2021, 35, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Polli, E.; Semenza, G. Deoxyribonucleic Acids from Normal and Leukaemic Human Leukocyte. Inst. Lomb. Sci. Lett. (Rend. Sci.) 1955, 160–195. [Google Scholar]
- Benedetti, E.; Papineschi, F.; Vergamini, P.; Consolini, R.; Spremolla, G. Analytical Infrared Spectral Differences between Human Normal and Leukaemic Cells (CLL)—I. Leuk. Res. 1984, 8, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, E.; Palatresi, M.P.; Vergamini, P.; Papineschi, F.; Spremolla, G. New Possibilities of Research in Chronic Lymphatic Leukemia by Means of Fourier Transform-Infrared Spectroscopy—II. Leuk. Res. 1985, 9, 1001–1008. [Google Scholar] [CrossRef]
- Schultz, C.P.; Liu, K.Z.; Johnston, J.B.; Mantsch, H.H. Differentiation of Leukemic from Normal Human Lymphocytes by FT-IR Spectroscopy and Cluster Analysis. Leukemia Res. 1996, 20, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Sheng, D.; Liu, X.; Li, W.; Wang, Y.; Chen, X.; Wang, X. Distinction of Leukemia Patients’ and Healthy Persons’ Serum Using FTIR Spectroscopy. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2013, 101, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Su, K.-Y.; Lee, W.-L. Fourier Transform Infrared Spectroscopy as a Cancer Screening and Diagnostic Tool: A Review and Prospects. Cancers 2020, 12, 115. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, J.; Kapelushnik, J.; Mordehai, J.; Moser, A.; Huleihel, M.; Erukhimovitch, V.; Levi, C.; Mordechai, S. Novel Methodology for the Follow-up of Acute Lymphoblastic Leukemia Using FTIR Microspectroscopy. J. Biochem. Biophys. Methods 2002, 51, 251–261. [Google Scholar] [CrossRef]
- Zelig, U.; Mordechai, S.; Shubinsky, G.; Sahu, R.K.; Huleihel, M.; Leibovitz, E.; Nathan, I.; Kapelushnik, J. Pre-Screening and Follow-up of Childhood Acute Leukemia Using Biochemical Infrared Analysis of Peripheral Blood Mononuclear Cells. Biochim. Biophys. Acta 2011, 1810, 827–835. [Google Scholar] [CrossRef]
- Agatha, G.; Häfer, R.; Zintl, F. Fatty Acid Composition of Lymphocyte Membrane Phospholipids in Children with Acute Leukemia. Cancer Lett. 2001, 173, 139–144. [Google Scholar] [CrossRef]
- Gottfried, E.L. Lipids of Human Leukocytes: Relation to Celltype. J. Lipid Res. 1967, 8, 321–327. [Google Scholar] [CrossRef]
- Spiegel, R.J.; Schaefer, E.J.; Magrath, I.T.; Edwards, B.K. Plasma Lipid Alterations in Leukemia and Lymphoma. Am. J. Med. 1982, 72, 775–782. [Google Scholar] [CrossRef]
- Inbar, M.; Goldman, R.; Inbar, L.; Bursuker, I.; Goldman, B.; Akstein, E.; Segal, P.; Ipp, E.; Ben-Bassat, I. Fluidity Difference of Membrane Lipids in Human Normal and Leukemic Lymphocytes as Controlled by Serum Components. Cancer Res. 1977, 37, 3037–3041. [Google Scholar]
- Inbar, M.; Shinitzky, M. Cholesterol as a Bioregulator in the Development and Inhibition of Leukemia. Proc. Natl. Acad. Sci. USA 1974, 71, 4229–4231. [Google Scholar] [CrossRef]
- Lavie, Y.; Fiucci, G.; Czarny, M.; Liscovitch, M. Changes in Membrane Microdomains and Caveolae Constituents in Multidrug-Resistant Cancer Cells. Lipids 1999, 34 (Suppl. S1), S57–S63. [Google Scholar] [CrossRef]
- Zelig, U.; Kapelushnik, J.; Moreh, R.; Mordechai, S.; Nathan, I. Diagnosis of Cell Death by Means of Infrared Spectroscopy. Biophys. J. 2009, 97, 2107–2114. [Google Scholar] [CrossRef] [PubMed]
- Raouf, G.A.; Elkhateeb, W.F.; Toumah, H.; Quari, M.; Jaouni, S.; Elkebba, K.; Kumosani, T.A. Infrared Spectroscopy of Human Bone Marrow: Evidence of Structural Changes during Acute Leukemia. Int. J. Nano Biomater. 2009, 2, 289. [Google Scholar] [CrossRef]
- Chaber, R.; Kowal, A.; Jakubczyk, P.; Arthur, C.; Łach, K.; Wojnarowska-Nowak, R.; Kusz, K.; Zawlik, I.; Paszek, S.; Cebulski, J. A Preliminary Study of FTIR Spectroscopy as a Potential Non-Invasive Screening Tool for Pediatric Precursor B Lymphoblastic Leukemia. Molecules 2021, 26, 1174. [Google Scholar] [CrossRef] [PubMed]
- Musharraf, S.G.; Siddiqui, A.J.; Shamsi, T.; Choudhary, M.I.; Rahman, A.-U. Serum Metabonomics of Acute Leukemia Using Nuclear Magnetic Resonance Spectroscopy. Sci. Rep. 2016, 6, 30693. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Chen, W.-L.; Wang, J.-H.; Li, N.; Li, J.-M.; Mi, J.-Q.; Zhang, W.-N.; Li, Y.; Wu, S.-F.; et al. Rapid Diagnosis and Prognosis of de Novo Acute Myeloid Leukemia by Serum Metabonomic Analysis. J. Proteome Res. 2013, 12, 4393–4401. [Google Scholar] [CrossRef]
- Zeijlemaker, W.; Kelder, A.; Oussoren-Brockhoff, Y.J.M.; Scholten, W.J.; Snel, A.N.; Veldhuizen, D.; Cloos, J.; Ossenkoppele, G.J.; Schuurhuis, G.J. Peripheral Blood Minimal Residual Disease May Replace Bone Marrow Minimal Residual Disease as an Immunophenotypic Biomarker for Impending Relapse in Acute Myeloid Leukemia. Leukemia 2016, 30, 708–715. [Google Scholar] [CrossRef]
- Xie, L.; Wang, J.; Wang, N.; Zhu, J.; Yin, Q.; Guo, R.; Duan, J.; Wang, S.; Hao, C.; Shen, X. Identification of Acute Myeloid Leukemia by Infrared Difference Spectrum of Peripheral Blood. J. Pharm. Biomed. Anal. 2023, 233, 115454. [Google Scholar] [CrossRef] [PubMed]
- Denbigh, J.L.; Perez-Guaita, D.; Vernooij, R.R.; Tobin, M.J.; Bambery, K.R.; Xu, Y.; Southam, A.D.; Khanim, F.L.; Drayson, M.T.; Lockyer, N.P.; et al. Probing the Action of a Novel Anti-Leukaemic Drug Therapy at the Single Cell Level Using Modern Vibrational Spectroscopy Techniques. Sci. Rep. 2017, 7, 2649. [Google Scholar] [CrossRef] [PubMed]
- Chomienne, C.; Ballerini, P.; Balitrand, N.; Amar, M.; Bernard, J.F.; Boivin, P.; Daniel, M.T.; Berger, R.; Castaigne, S.; Degos, L. Retinoic Acid Therapy for Promyelocytic Leukaemia. Lancet 1989, 2, 746–747. [Google Scholar] [CrossRef]
- Liu, M.-J.; Wang, Z.; Wu, R.-C.; Sun, S.-Q.; Wu, Q.-Y. Monitoring All-Trans-Retinoic Acid-Induced Differentiation of Human Acute Promyelocytic Leukemia NB4 Cells by Fourier-Transform Infrared Spectroscopy. Leukemia 2003, 17, 1670–1674. [Google Scholar] [CrossRef]
- O’Brien, S.; del Giglio, A.; Keating, M. Advances in the Biology and Treatment of B-Cell Chronic Lymphocytic Leukemia. Blood 1995, 85, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Schultz, C.P. The Potential Role of Fourier Transform Infrared Spectroscopy and Imaging in Cancer Diagnosis Incorporating Complex Mathematical Methods. Technol. Cancer Res. Treat. 2002, 1, 95–104. [Google Scholar] [CrossRef]
- Spremolla, G.; Benedetti, E.; Vergamini, P.; Andreucci, M.C.; Macchia, P. An Investigation of Acute Lymphoblastic Leukemia (ALL) in Children by Means of Infrared Spectroscopy. Part IV. Haematologica 1988, 73, 21–24. [Google Scholar]
- Juliusson, G.; Oscier, D.G.; Fitchett, M.; Ross, F.M.; Stockdill, G.; Mackie, M.J.; Parker, A.C.; Castoldi, G.L.; Guneo, A.; Knuutila, S.; et al. Prognostic Subgroups in B-Cell Chronic Lymphocytic Leukemia Defined by Specific Chromosomal Abnormalities. N. Engl. J. Med. 1990, 323, 720–724. [Google Scholar] [CrossRef]
- Erukhimovitch, V.; Talyshinsky, M.; Souprun, Y.; Huleihel, M. FTIR Spectroscopy Examination of Leukemia Patients Plasma. Vib. Spectrosc. 2006, 40, 40–46. [Google Scholar] [CrossRef]
- Liu, K.Z.; Schultz, C.P.; Johnston, J.B.; Lee, K.; Mantsch, H.H. Comparison of Infrared Spectra of CLL Cells with Their Ex Vivo Sensitivity (MTT Assay) to Chlorambucil and Cladribine. Leuk. Res. 1997, 21, 1125–1133. [Google Scholar] [CrossRef]
- Liu, K.Z.; Schultz, C.P.; Mohammad, R.M.; Al-Katib, A.M.; Johnston, J.B.; Mantsch, H.H. Similarities between the Sensitivity to 2-Chlorodeoxyadenosine of Lymphocytes from CLL Patients and Bryostatin 1-Treated WSU-CLL Cells: An Infrared Spectroscopic Study. Cancer Lett. 1998, 127, 185–193. [Google Scholar] [CrossRef]
- Liu, K.Z.; Schultz, C.P.; Johnston, J.B.; Beck, F.W.; Al-Katib, A.M.; Mohammad, R.M.; Mantsch, H.H. Infrared Spectroscopic Study of Bryostatin 1-Induced Membrane Alterations in a B-CLL Cell Line. Leukemia 1999, 13, 1273–1280. [Google Scholar] [CrossRef]
- Swerdlow, S.H.; Campo, E.; Pileri, S.A.; Harris, N.L.; Stein, H.; Siebert, R.; Advani, R.; Ghielmini, M.; Salles, G.A.; Zelenetz, A.D.; et al. The 2016 Revision of the World Health Organization Classification of Lymphoid Neoplasms. Blood 2016, 127, 2375–2390. [Google Scholar] [CrossRef]
- Woess, C.; Drach, M.; Villunger, A.; Tappert, R.; Stalder, R.; Pallua, J.D. Application of Mid-Infrared (MIR) Microscopy Imaging for Discrimination between Follicular Hyperplasia and Follicular Lymphoma in Transgenic Mice. Analyst 2015, 140, 6363–6372. [Google Scholar] [CrossRef]
- Willenbacher, E.; Brunner, A.; Zelger, B.; Unterberger, S.H.; Stalder, R.; Huck, C.W.; Willenbacher, W.; Pallua, J.D. Application of Mid-infrared Microscopic Imaging for the Diagnosis and Classification of Human Lymphomas. J. Biophotonics 2021, 14, e202100079. [Google Scholar] [CrossRef]
- Schwickert, T.A.; Lindquist, R.L.; Shakhar, G.; Livshits, G.; Skokos, D.; Kosco-Vilbois, M.H.; Dustin, M.L.; Nussenzweig, M.C. In Vivo Imaging of Germinal Centres Reveals a Dynamic Open Structure. Nature 2007, 446, 83–87. [Google Scholar] [CrossRef]
- Zelger, P.; Brunner, A.; Zelger, B.; Willenbacher, E.; Unterberger, S.H.; Stalder, R.; Huck, C.W.; Willenbacher, W.; Pallua, J.D. Deep Learning Analysis of Mid-Infrared Microscopic Imaging Data for the Diagnosis and Classification of Human Lymphomas. J. Biophotonics 2023, 16, e202300015. [Google Scholar] [CrossRef]
- Ghimire, H.; Venkataramani, M.; Bian, Z.; Liu, Y.; Perera, A.G.U. ATR-FTIR Spectral Discrimination between Normal and Tumorous Mouse Models of Lymphoma and Melanoma from Serum Samples. Sci. Rep. 2017, 7, 16993. [Google Scholar] [CrossRef] [PubMed]
- Hands, J.R.; Clemens, G.; Stables, R.; Ashton, K.; Brodbelt, A.; Davis, C.; Dawson, T.P.; Jenkinson, M.D.; Lea, R.W.; Walker, C.; et al. Brain Tumour Differentiation: Rapid Stratified Serum Diagnostics via Attenuated Total Reflection Fourier-Transform Infrared Spectroscopy. J. Neuro-Oncol. 2016, 127, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Cameron, J.M.; Butler, H.J.; Smith, B.R.; Hegarty, M.G.; Jenkinson, M.D.; Syed, K.; Brennan, P.M.; Ashton, K.; Dawson, T.; Palmer, D.S.; et al. Developing Infrared Spectroscopic Detection for Stratifying Brain Tumour Patients: Glioblastoma Multiforme vs. Lymphoma. Analyst 2019, 144, 6736–6750. [Google Scholar] [CrossRef]
- Cameron, J.M.; Rinaldi, C.; Butler, H.J.; Hegarty, M.G.; Brennan, P.M.; Jenkinson, M.D.; Syed, K.; Ashton, K.M.; Dawson, T.P.; Palmer, D.S.; et al. Stratifying Brain Tumour Histological Sub-Types: The Application of ATR-FTIR Serum Spectroscopy in Secondary Care. Cancers 2020, 12, 1710. [Google Scholar] [CrossRef]
- Theakstone, A.G.; Brennan, P.M.; Jenkinson, M.D.; Goodacre, R.; Baker, M.J. Investigating Centrifugal Filtration of Serum-Based FTIR Spectroscopy for the Stratification of Brain Tumours. PLoS ONE 2023, 18, e0279669. [Google Scholar] [CrossRef]
- Brunner, A.; Willenbacher, E.; Willenbacher, W.; Zelger, B.; Zelger, P.; Huck, C.W.; Pallua, J.D. Visible- and near-Infrared Hyperspectral Imaging for the Quantitative Analysis of PD-L1+ Cells in Human Lymphomas: Comparison with Fluorescent Multiplex Immunohistochemistry. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2023, 285, 121940. [Google Scholar] [CrossRef]
- Willenbacher, E.; Brunner, A.; Willenbacher, W.; Zelger, B.; Wolf, D.; Rogge, D.; Tappert, M.; Pallua, J.D. Visible and Near-Infrared Hyperspectral Imaging Techniques Allow the Reliable Quantification of Prognostic Markers in Lymphomas: A Pilot Study Using the Ki67 Proliferation Index as an Example. Exp. Hematol. 2020, 91, 55–64. [Google Scholar] [CrossRef]
- Pérez-Galán, P.; Dreyling, M.; Wiestner, A. Mantle Cell Lymphoma: Biology, Pathogenesis, and the Molecular Basis of Treatment in the Genomic Era. Blood 2011, 117, 26–38. [Google Scholar] [CrossRef]
- Campo, E.; Rule, S. Mantle Cell Lymphoma: Evolving Management Strategies. Blood 2015, 125, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Dreyling, M.; Campo, E.; Hermine, O.; Jerkeman, M.; Le Gouill, S.; Rule, S.; Shpilberg, O.; Walewski, J.; Ladetto, M. Newly Diagnosed and Relapsed Mantle Cell Lymphoma: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2017, 28, iv62–iv71. [Google Scholar] [CrossRef]
- Kolodziej, M.; Jesionek-Kupnicka, D.; Braun, M.; Atamaniuk, V.; Sloniec, S.; Cebulski, J.; Cholewa, M.; Kopczynski, J.; Heraud, P.; Tobin, M.J.; et al. Classification of Aggressive and Classic Mantle Cell Lymphomas Using Synchrotron Fourier Transform Infrared Microspectroscopy. Sci. Rep. 2019, 9, 12857. [Google Scholar] [CrossRef]
- Luanpitpong, S.; Janan, M.; Thumanu, K.; Poohadsuan, J.; Rodboon, N.; Klaihmon, P.; Issaragrisil, S. Deciphering the Elevated Lipid via CD36 in Mantle Cell Lymphoma with Bortezomib Resistance Using Synchrotron-Based Fourier Transform Infrared Spectroscopy of Single Cells. Cancers 2019, 11, 576. [Google Scholar] [CrossRef] [PubMed]
- El Bedewi, A.; El Anany, G.; El Mofty, M.; Kretlow, A.; Park, S.; Miller, L.M. The Use of Synchrotron Infrared Microspectroscopy in the Assessment of Cutaneous T-Cell Lymphoma vs. Pityriasis Lichenoides Chronica. Photodermatol. Photoimmunol. Photomed. 2010, 26, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Andrus, P.G. Cancer Monitoring by FTIR Spectroscopy. Technol. Cancer Res. Treat. 2006, 5, 157–167. [Google Scholar] [PubMed]
- Pan, T.; Liu, J.; Chen, J.; Zhang, G.; Zhao, Y. Rapid Determination of Preliminary Thalassaemia Screening Indicators Based on Near-Infrared Spectroscopy with Wavelength Selection Stability. Anal. Methods 2013, 5, 4355. [Google Scholar] [CrossRef]
- Chen, J.; Peng, L.; Han, Y.; Yao, L.; Zhang, J.; Pan, T. A Rapid Quantification Method for the Screening Indicator for β-Thalassemia with near-Infrared Spectroscopy. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 193, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Aksoy, C.; Guliyev, A.; Kilic, E.; Uckan, D.; Severcan, F. Bone Marrow Mesenchymal Stem Cells in Patients with Beta Thalassemia Major: Molecular Analysis with Attenuated Total Reflection-Fourier Transform Infrared Spectroscopy Study as a Novel Method. Stem Cells Dev. 2012, 21, 2000–2011. [Google Scholar] [CrossRef] [PubMed]
- Long, X.; Liu, G.; Pan, T.; Chen, J. Waveband Selection of Reagent-Free Determination for Thalassemia Screening Indicators Using Fourier Transform Infrared Spectroscopy with Attenuated Total Reflection. J. Biomed. Opt. 2014, 19, 087004. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.-H.; Tian, P.-L.; Yin, H.; Han, Y.; Wei, X.-C.; Pan, T. A Preliminary Evaluation of Attenuated Total Reflection Fourier Transform Infrared Spectroscopy for the Hematological Analysis of Thalassemias. Clin. Biochem. 2013, 46, 128–132. [Google Scholar] [CrossRef]
- Liu, K.-Z.; Tsang, K.S.; Li, C.K.; Shaw, R.A.; Mantsch, H.H. Infrared Spectroscopic Identification of Beta-Thalassemia. Clin. Chem. 2003, 49, 1125–1132. [Google Scholar] [CrossRef]
- Waltz, X.; Hardy-Dessources, M.-D.; Lemonne, N.; Mougenel, D.; Lalanne-Mistrih, M.-L.; Lamarre, Y.; Tarer, V.; Tressières, B.; Etienne-Julan, M.; Hue, O.; et al. Is There a Relationship between the Hematocrit-to-Viscosity Ratio and Microvascular Oxygenation in Brain and Muscle? Clin. Hemorheol. Microcirc. 2015, 59, 37–43. [Google Scholar] [CrossRef]
- Waltz, X.; Pichon, A.; Lemonne, N.; Mougenel, D.; Lalanne-Mistrih, M.-L.; Lamarre, Y.; Tarer, V.; Tressières, B.; Etienne-Julan, M.; Hardy-Dessources, M.-D.; et al. Normal Muscle Oxygen Consumption and Fatigability in Sickle Cell Patients despite Reduced Microvascular Oxygenation and Hemorheological Abnormalities. PLoS ONE 2012, 7, e52471. [Google Scholar] [CrossRef]
- Waltz, X.; Pichon, A.; Mougenel, D.; Lemonne, N.; Lalanne-Mistrih, M.-L.; Sinnapah, S.; Tarer, V.; Tressières, B.; Lamarre, Y.; Etienne-Julan, M.; et al. Hemorheological Alterations, Decreased Cerebral Microvascular Oxygenation and Cerebral Vasomotion Compensation in Sickle Cell Patients. Am. J. Hematol. 2012, 87, 1070–1073. [Google Scholar] [CrossRef] [PubMed]
- Tavakkoli, F.; Nahavandi, M.; Wyche, M.Q.; Castro, O. Effects of Hydroxyurea Treatment on Cerebral Oxygenation in Adult Patients with Sickle Cell Disease: An Open-Label Pilot Study. Clin. Ther. 2005, 27, 1083–1088. [Google Scholar] [CrossRef]
- Nahavandi, M.; Nichols, J.P.; Hassan, M.; Gandjbakhche, A.; Kato, G.J. Near-Infrared Spectra Absorbance of Blood from Sickle Cell Patients and Normal Individuals. Hematology 2009, 14, 46–48. [Google Scholar] [CrossRef] [PubMed]
- Raj, A.; Bertolone, S.J.; Mangold, S.; Edmonds, H.L. Assessment of Cerebral Tissue Oxygenation in Patients with Sickle Cell Disease: Effect of Transfusion Therapy. J. Pediatr. Hematol. Oncol. 2004, 26, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Quinn, C.T.; Dowling, M.M. Cerebral Tissue Hemoglobin Saturation in Children with Sickle Cell Disease. Pediatr. Blood Cancer 2012, 59, 881–887. [Google Scholar] [CrossRef] [PubMed]
- Barriteau, C.M.; Chiu, A.; Rodeghier, M.; Liem, R.I. Cerebral and Skeletal Muscle Tissue Oxygenation during Exercise Challenge in Children and Young Adults with Sickle Cell Anaemia. Br. J. Haematol. 2022, 196, 179–182. [Google Scholar] [CrossRef]
- Charlot, K.; Antoine-Jonville, S.; Moeckesch, B.; Jumet, S.; Romana, M.; Waltz, X.; Divialle-Doumdo, L.; Hardy-Dessources, M.-D.; Petras, M.; Tressières, B.; et al. Cerebral and Muscle Microvascular Oxygenation in Children with Sickle Cell Disease: Influence of Hematology, Hemorheology and Vasomotion. Blood Cells Mol. Dis. 2017, 65, 23–28. [Google Scholar] [CrossRef]
- Connes, P.; Verlhac, S.; Bernaudin, F. Advances in Understanding the Pathogenesis of Cerebrovascular Vasculopathy in Sickle Cell Anaemia. Br. J. Haematol. 2013, 161, 484–498. [Google Scholar] [CrossRef]
- Waltz, X.; Romana, M.; Lalanne-Mistrih, M.-L.; Machado, R.F.; Lamarre, Y.; Tarer, V.; Hardy-Dessources, M.-D.; Tressières, B.; Divialle-Doumdo, L.; Petras, M.; et al. Hematologic and Hemorheological Determinants of Resting and Exercise-Induced Hemoglobin Oxygen Desaturation in Children with Sickle Cell Disease. Haematologica 2013, 98, 1039–1044. [Google Scholar] [CrossRef]
- Bertuglia, S.; Colantuoni, A.; Coppini, G.; Intaglietta, M. Hypoxia- or Hyperoxia-Induced Changes in Arteriolar Vasomotion in Skeletal Muscle Microcirculation. Am. J. Physiol. 1991, 260, H362–H372. [Google Scholar] [CrossRef]
- Kislukhin, V.V. Stochasticity of Flow through Microcirculation as a Regulator of Oxygen Delivery. Theor. Biol. Med. Model. 2010, 7, 29. [Google Scholar] [CrossRef] [PubMed]
- Stotesbury, H.; Kawadler, J.M.; Hales, P.W.; Saunders, D.E.; Clark, C.A.; Kirkham, F.J. Vascular Instability and Neurological Morbidity in Sickle Cell Disease: An Integrative Framework. Front. Neurol. 2019, 10, 871. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.Y.; Wang, H.Y.; Wang, H.Y.; Hua, W.; Zhao, M.S.; Li, P.; Zhao, L.N. The value of intraoperative cerebral oxygen saturation in predicting postoperative neurocognitive dysfunction in elderly patients with mild cognitive impairment. Zhonghua Yi Xue Za Zhi 2020, 100, 3224–3229. [Google Scholar] [CrossRef] [PubMed]
- Fields, M.E.; Mirro, A.E.; Guilliams, K.P.; Binkley, M.M.; Gil Diaz, L.; Tan, J.; Fellah, S.; Eldeniz, C.; Chen, Y.; Ford, A.L.; et al. Functional Connectivity Decreases with Metabolic Stress in Sickle Cell Disease. Ann. Neurol. 2020, 88, 995–1008. [Google Scholar] [CrossRef]
- Guilliams, K.P.; Fields, M.E.; Ragan, D.K.; Eldeniz, C.; Binkley, M.M.; Chen, Y.; Comiskey, L.S.; Doctor, A.; Hulbert, M.L.; Shimony, J.S.; et al. Red Cell Exchange Transfusions Lower Cerebral Blood Flow and Oxygen Extraction Fraction in Pediatric Sickle Cell Anemia. Blood 2018, 131, 1012–1021. [Google Scholar] [CrossRef] [PubMed]
- Rooks, C.R.; Thom, N.J.; McCully, K.K.; Dishman, R.K. Effects of Incremental Exercise on Cerebral Oxygenation Measured by Near-Infrared Spectroscopy: A Systematic Review. Prog. Neurobiol. 2010, 92, 134–150. [Google Scholar] [CrossRef]
- Ravelojaona, M.; Féasson, L.; Oyono-Enguéllé, S.; Vincent, L.; Djoubairou, B.; Ewa’Sama Essoue, C.; Messonnier, L.A. Evidence for a Profound Remodeling of Skeletal Muscle and Its Microvasculature in Sickle Cell Anemia. Am. J. Pathol. 2015, 185, 1448–1456. [Google Scholar] [CrossRef]
- Heaney, M.L.; Golde, D.W. Myelodysplasia. N. Engl. J. Med. 1999, 340, 1649–1660. [Google Scholar] [CrossRef]
- Arber, D.A.; Orazi, A.; Hasserjian, R.; Thiele, J.; Borowitz, M.J.; Le Beau, M.M.; Bloomfield, C.D.; Cazzola, M.; Vardiman, J.W. The 2016 Revision to the World Health Organization Classification of Myeloid Neoplasms and Acute Leukemia. Blood 2016, 127, 2391–2405. [Google Scholar] [CrossRef]
- Ma, X.; Does, M.; Raza, A.; Mayne, S.T. Myelodysplastic Syndromes: Incidence and Survival in the United States. Cancer 2007, 109, 1536–1542. [Google Scholar] [CrossRef]
- Valent, P.; Orazi, A.; Büsche, G.; Schmitt-Gräff, A.; George, T.I.; Sotlar, K.; Streubel, B.; Beham-Schmid, C.; Cerny-Reiterer, S.; Krieger, O.; et al. Standards and Impact of Hematopathology in Myelodysplastic Syndromes (MDS). Oncotarget 2010, 1, 483–496. [Google Scholar] [CrossRef]
- Komrokji, R.S.; Zhang, L.; Bennett, J.M. Myelodysplastic Syndromes Classification and Risk Stratification. Hematol. Oncol. Clin. North Am. 2010, 24, 443–457. [Google Scholar] [CrossRef]
- Malins, D.C.; Anderson, K.M.; Polissar, N.L.; Ostrander, G.K.; Knobbe, E.T.; Green, V.M.; Gilman, N.K.; Spivak, J.L. Models of Granulocyte DNA Structure Are Highly Predictive of Myelodysplastic Syndrome. Proc. Natl. Acad. Sci. USA 2004, 101, 5008–5011. [Google Scholar] [CrossRef]
- Brewer, S.H.; Allen, A.M.; Lappi, S.E.; Chasse, T.L.; Briggman, K.A.; Gorman, C.B.; Franzen, S. Infrared Detection of a Phenylboronic Acid Terminated Alkane Thiol Monolayer on Gold Surfaces. Langmuir 2004, 20, 5512–5520. [Google Scholar] [CrossRef]
- Mughal, T.I.; Vaddi, K.; Sarlis, N.J.; Verstovsek, S. Myelofibrosis-Associated Complications: Pathogenesis, Clinical Manifestations, and Effects on Outcomes. Int. J. Gen. Med. 2014, 7, 89–101. [Google Scholar] [CrossRef]
- Guleken, Z.; Ceylan, Z.; Aday, A.; Bayrak, A.G.; Hindilerden, İ.Y.; Nalçacı, M.; Jakubczyk, P.; Jakubczyk, D.; Depciuch, J. Application of Fourier Transform InfraRed Spectroscopy of Machine Learning with Support Vector Machine and Principal Components Analysis to Detect Biochemical Changes in Dried Serum of Patients with Primary Myelofibrosis. Biochim. Biophys. Acta Gen. Subj. 2023, 1867, 130438. [Google Scholar] [CrossRef]
- Guleken, Z.; Ceylan, Z.; Aday, A.; Bayrak, A.G.; Hindilerden, İ.Y.; Nalçacı, M.; Jakubczyk, P.; Jakubczyk, D.; Depciuch, J. FTIR- Based Serum Structure Analysis in Molecular Diagnostics of Essential Thrombocythemia Disease. J. Photochem. Photobiol. B 2023, 245, 112734. [Google Scholar] [CrossRef] [PubMed]
- Movasaghi, Z.; Rehman, S.; Ur Rehman, I., Dr. Fourier Transform Infrared (FTIR) Spectroscopy of Biological Tissues. Appl. Spectrosc. Rev. 2008, 43, 134–179. [Google Scholar] [CrossRef]
- Naumann, D.; Helm, D.; Labischinski, H. Microbiological Characterizations by FT-IR Spectroscopy. Nature 1991, 351, 81–82. [Google Scholar] [CrossRef] [PubMed]
- Ellis, D.I.; Goodacre, R. Metabolic Fingerprinting in Disease Diagnosis: Biomedical Applications of Infrared and Raman Spectroscopy. Analyst 2006, 131, 875. [Google Scholar] [CrossRef]
- Baran, Y.; Ceylan, C.; Camgoz, A. The Roles of Macromolecules in Imatinib Resistance of Chronic Myeloid Leukemia Cells by Fourier Transform Infrared Spectroscopy. Biomed. Pharmacother. 2013, 67, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.H.; Kim, T.; Kim, E.; Park, J.K.; Park, S.-J.; Joo, H.; Kim, H.J. The Mitochondrial Warburg Effect: A Cancer Enigma. Interdiscip. Bio. Central 2009, 1, 1–7. [Google Scholar] [CrossRef]
- Papineschi, F.; Bucalossi, A.; Capochiani, E.; Benedetti, E.; Bramanti, E.; Dastoli, G.; Dispensa, E.; Benedetti, E.; Spremolla, G. Recombinant Alpha 2a Interferon and Polycythemia Vera: Clinical Results and Biological Evaluation by Means of Fourier-Transform Infrared Microspectroscopy. Eur. J. Haematol. 1994, 53, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.-Y.; Yoo, S.-J.; Bang, S.-M.; Park, P.-W.; Seo, Y.-H.; Shin, D.-B.; Lee, J.-H. JAK2 V617F Mutation in Korean Patients with Essential Thrombocythemia. Ann. Lab. Med. 2007, 27, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Mora, B.; Passamonti, F. Developments in Diagnosis and Treatment of Essential Thrombocythemia. Expert. Rev. Hematol. 2019, 12, 159–171. [Google Scholar] [CrossRef]
- Zaidi, U.; Sufaida, G.; Rashid, M.; Kaleem, B.; Maqsood, S.; Mukry, S.N.; Khan, R.Z.A.; Munzir, S.; Borhany, M.; Shamsi, T.S. A Distinct Molecular Mutational Profile and Its Clinical Impact in Essential Thrombocythemia and Primary Myelofibrosis Patients. BMC Cancer 2020, 20, 205. [Google Scholar] [CrossRef]
- Hoffman, R.; Prchal, J.T.; Samuelson, S.; Ciurea, S.O.; Rondelli, D. Philadelphia Chromosome–Negative Myeloproliferative Disorders: Biology and Treatment. Biol. Blood Marrow Transplant. 2007, 13, 64–72. [Google Scholar] [CrossRef]
- Waggoner, M. Polycythemia Vera: Thinking Beyond the Hematocrit. J. Adv. Pract. Oncol. 2023, 14, 405–413. [Google Scholar] [CrossRef]


| Study Population | Sample and Main Technique | Key Spectral Findings | Ref |
|---|---|---|---|
| CLL patients | CLL cells vs. normal cells Micro-FTIR spectroscopy | - Main spectral shifts in leukemic cells in the range between 1000 and 1200 cm−1, correlated with nucleic acids (DNA/RNA). - Consistent correlation between the decline in nucleic acid content and the decrease in blast percentage post-chemotherapy in both B- and T-cell patients. | [27] |
| CLL patients | CLL cells vs. normal cells FTIR spectroscopy | - Higher DNA content (observed in spectral range 900–1300 cm−1) and lower lipid content in CLL cells. - Segregation of CLL cells into three distinct subgroups based on spectral variations. - Increased DNA content associated with chromosomal abnormalities. | [24] |
| CLL patients vs. healthy individuals | Human plasma Micro-FTIR spectroscopy | - Spectral peaks at 1056 cm−1 (carbohydrates), 1270 cm−1 (amide III band), and 1592 cm−1 (δ(NH2): amino acids) exhibited significant reductions in patient samples. - Effective classification of healthy and patient samples based on these spectral changes. | [49] |
| Study Population | Main Technique | Key Spectral Findings | Ref |
|---|---|---|---|
| Patients with beta-thalassemia major | ATR-FTIR spectroscopy and ELISA | - Increased content of macromolecules. - Marked reduction post-transplant. - Varied erythropoietin and GDF-15 levels. | [74] |
| Individuals screened for thalassemia | ATR-FTIR spectroscopy | - Optimal wavebands: 1722–1504 cm−1 for Hb, 1653–901 cm−1 for MCH, and 1562–964 cm−1 for MCV. - Alternative bands: 1717–1510 cm−1 for Hb, and 1562–901 cm−1 for MCH and MCV. | [75,76] |
| β-thalassemia patients vs. controls | ATR-FTIR spectroscopy | - Reduction in α-helix content (1657 cm−1), increase in β-sheets (1640 cm−1 and 1680 cm−1), alterations in tyrosine ring absorption (1517 cm−1), and heightened intensity in bands associated with cysteine SH groups (2550 cm−1). | [77] |
| Study Population | Main Technique | Key Spectral Findings | Ref |
|---|---|---|---|
| Individuals with sickle cell anemia and sickle cell hemoglobin C disease | NIR spectroscopy | - Reduction in TOI and cerebral/muscle microvascular oxygenation. - Elevated cerebral vasomotion activity in SS individuals as a possible adaptive response to chronic cerebral hypoxemia. | [78,79,80,81,82,83,84,85,86,87,88] |
| Children and young adults with sickle cell anemia vs. control subjects | NIR spectroscopy | - Lower cerebral StO2 levels in patients during all exercise stages. - More pronounced declines in cerebral StO2 as exercise progressed. - A trend toward reduced quadriceps StO2 levels in patients. | [85,91,92,93,94,95] |
| Comparative study involving children and adolescents with SS and SC genotypes | NIR spectroscopy | - Significantly lower microvascular oxygenation in cerebral and muscle tissues in SS individuals. - Correlation between cerebral TOI, hematocrit levels, red blood cell deformability, and SpO2 in SS individuals. | [86] |
| Study Population | Main Technique | Key Spectral Findings | Ref |
|---|---|---|---|
| Individuals with primary myelofibrosis | FTIR spectroscopy | - Elevated levels of phospholipids and proteins; reduction in H-O=H vibrations; ratio of α-helix to β-sheet structures in proteins is 1.5 times higher; significant alterations in vibrations associated with the C–O bond and the amide III region of proteins. | [105] |
| Patients with essential thrombocythemia vs. control subjects | FTIR spectroscopy with machine learning techniques | - Decreased protein and increased lipid levels. - Spectroscopic markers: CH2 bending, amide II, and C-O vibrations; elevated presence of amide I and amide III vibrations, reduced level of amide II; FTIR peaks at 1079 cm−1, 1241 cm−1, 1307 cm−1, 1453 cm−1, 1537 cm−1, 1637 cm−1, 2865 cm−1, 2928 cm−1, and 2964 cm−1 representing vibrations from DNA, RNA, proteins, lipids, and carbohydrates. | [106,107,108,109] |
| Original and imatinib-resistant K562/IMA-3 cells (chronic myeloid leukemia) | FTIR spectroscopy | - Reduction in glycogen levels (1155 cm−1); heightened membrane order (2959 cm−1); increase in unsaturated lipids (3015 cm−1); frequency alterations in nucleic acid bands (1239 cm−1, 1086 cm−1, 971 cm−1); proteomic alterations in resistant cells with variations in amide bands (3300 cm−1 for amide I and 3061 cm−1 for amide II); structure changes in antiparallel beta-sheets (1690 cm−1), alpha-helix (1653 cm−1), beta-sheets (1637 cm−1), random coils (1648 cm−1), and turns. | [110,111] |
| Polycythemia vera patients undergoing α2a-IFN therapy | Micro-FTIR | Spectroscopic metric (A₁/A₂) based on integrated areas of bands at 1080 cm−1 (nucleic acids) and 1540 cm−1 (protein components). | [112] |
| Hematological Disease | Spectral Fingerprint Features | Explanation of Features | Ref |
|---|---|---|---|
| Leukemia | - DNA marker bands at 966 cm−1 and 530 cm−1. - H2959 cm−1/H2931 cm−1 ratio. - RNA/DNA ratios at 1115 cm−1/1028 cm−1. | - Characteristic of lymphoid leukemia. - Indicates significant differences between leukemia patients and healthy individuals. | [23] |
| ALL | - Reduction in protein content (amide II band changes). - Variations in nucleic acids (1000–1200 cm−1 region). - Lipid and protein changes (2800–3000 cm−1 region). | - Signifies alteration in lymphocyte composition. - Reflects biochemical alterations from chemotherapy and disease progression. | [27] |
| AML | - Changes in protein structures (α-helices at 1657 cm−1 and 1650 cm−1; β-sheets at 1686 cm−1 and 1635 cm−1). - Amino acid alterations. | - Indicates a reduction in α-helical protein structures and an increase in β-sheets. - Reflects specific biochemical changes associated with AML. | [41] |
| CLL | - Higher DNA content and lower lipid content. - Spectral peaks at 1056 cm−1, 1270 cm−1, and 1592 cm−1. | - Highlights differences in cellular composition of CLL cells compared to normal cells. | [49] |
| CML | - Reduction in glycogen levels (1155 cm−1). - Heightened membrane order (2959 cm−1). - Increase in unsaturated lipids (3015 cm−1). - Proteomic alterations (3300 cm−1 for amide I, 3061 cm−1 for amide II). | - Indicates metabolic and structural changes in imatinib-resistant cells. - Suggests alterations in lipid composition and protein structure. | [110,111] |
| Lymphoma | - Variations in amide I band and lipid distribution. - Specific changes in protein secondary structure (β-sheet protein constituents at 1688 cm−1). | - Differentiates between follicular lymphomas and DLBCL. - Indicates changes in cellular protein composition. | [36] |
| Thalassemia | - Increase in macromolecules in bone marrow mesenchymal stem cells. - Changes in Hb secondary structure (α-helix reduction at 1657 cm−1, β-sheets increase at 1640 and 1680 cm−1). | - Reflects increased cell growth and bone marrow activity. - Indicates alterations in hemoglobin structure. | [77] |
| MDS | - Structural variances in DNA spectra. - Peaks at 1651 cm−1, 1230 cm−1, and 1084 cm−1. | - Indicates changes in nucleotide bases and backbone, differentiating MDS from normal cells. | [103] |
| Essential thrombocythemia | - Decreased protein and increased lipid levels. - Amide I (1637 cm−1) and amide III vibrations. - Reduced level of amide II (1537 cm−1). - Peaks related to DNA and RNA (1079 cm−1), peptide backbone (1241 cm−1), CH3 groups (1307 cm−1), CH2 bending (1453 cm−1), and C-H stretching (2865 cm−1, 2928 cm−1, 2964 cm−1). | - Reflects biochemical changes in the serum of patients. - Indicates alterations in protein and lipid composition, possibly resulting from mutations. | [106,107,108,109] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delrue, C.; Speeckaert, R.; Oyaert, M.; Kerre, T.; Rottey, S.; Coopman, R.; Huvenne, W.; De Bruyne, S.; Speeckaert, M.M. Infrared Spectroscopy: A New Frontier in Hematological Disease Diagnosis. Int. J. Mol. Sci. 2023, 24, 17007. https://doi.org/10.3390/ijms242317007
Delrue C, Speeckaert R, Oyaert M, Kerre T, Rottey S, Coopman R, Huvenne W, De Bruyne S, Speeckaert MM. Infrared Spectroscopy: A New Frontier in Hematological Disease Diagnosis. International Journal of Molecular Sciences. 2023; 24(23):17007. https://doi.org/10.3390/ijms242317007
Chicago/Turabian StyleDelrue, Charlotte, Reinhart Speeckaert, Matthijs Oyaert, Tessa Kerre, Sylvie Rottey, Renaat Coopman, Wouter Huvenne, Sander De Bruyne, and Marijn M. Speeckaert. 2023. "Infrared Spectroscopy: A New Frontier in Hematological Disease Diagnosis" International Journal of Molecular Sciences 24, no. 23: 17007. https://doi.org/10.3390/ijms242317007
APA StyleDelrue, C., Speeckaert, R., Oyaert, M., Kerre, T., Rottey, S., Coopman, R., Huvenne, W., De Bruyne, S., & Speeckaert, M. M. (2023). Infrared Spectroscopy: A New Frontier in Hematological Disease Diagnosis. International Journal of Molecular Sciences, 24(23), 17007. https://doi.org/10.3390/ijms242317007







