Collagen Mimetic Peptides Promote Repair of MMP-1-Damaged Collagen in the Rodent Sclera and Optic Nerve Head
Abstract
:1. Introduction
2. Results
2.1. Collagen Mimetic Peptide Restores Stiffness of the PPS after MMP-1
2.2. Collagen Mimetic Peptide Restores Stiffness of the Glial Lamina after MMP-1
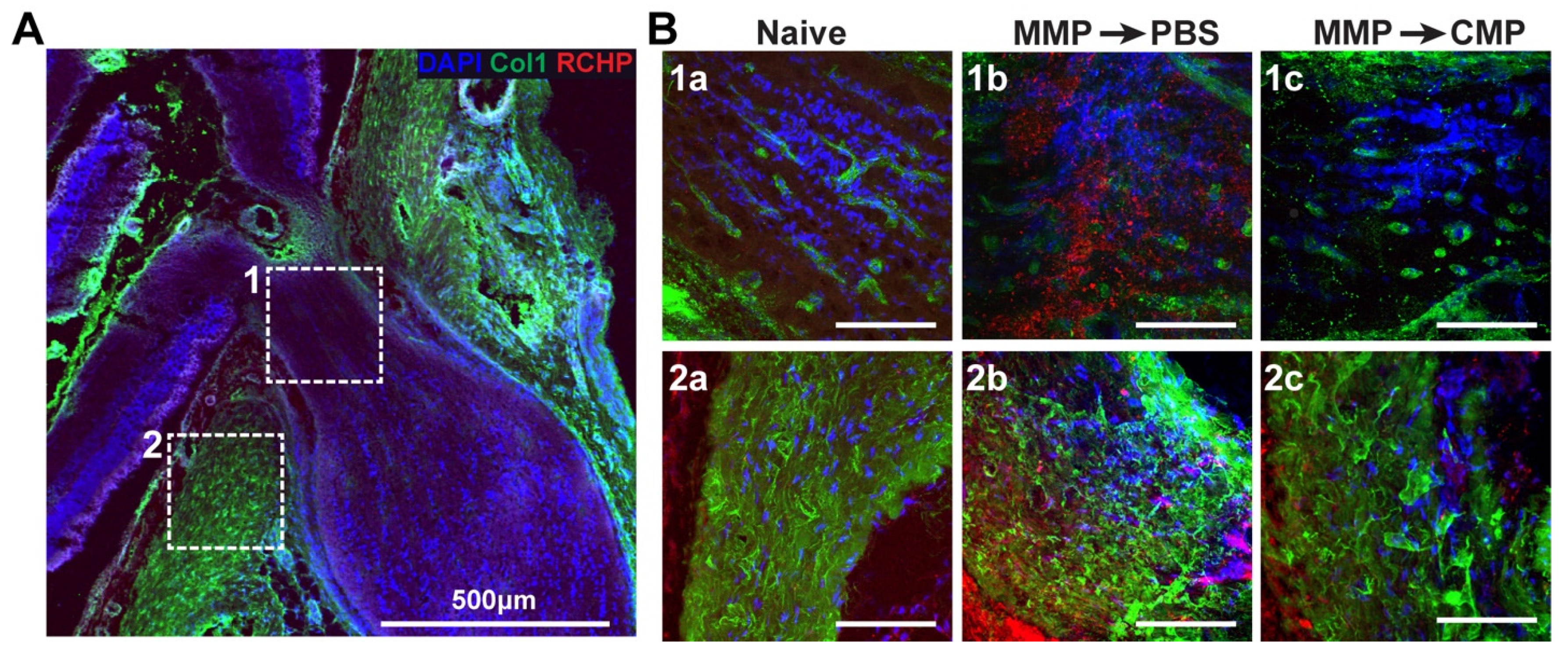
2.3. Collagen Mimetic Peptide Mitigates Collagen Fragmentation
3. Discussion
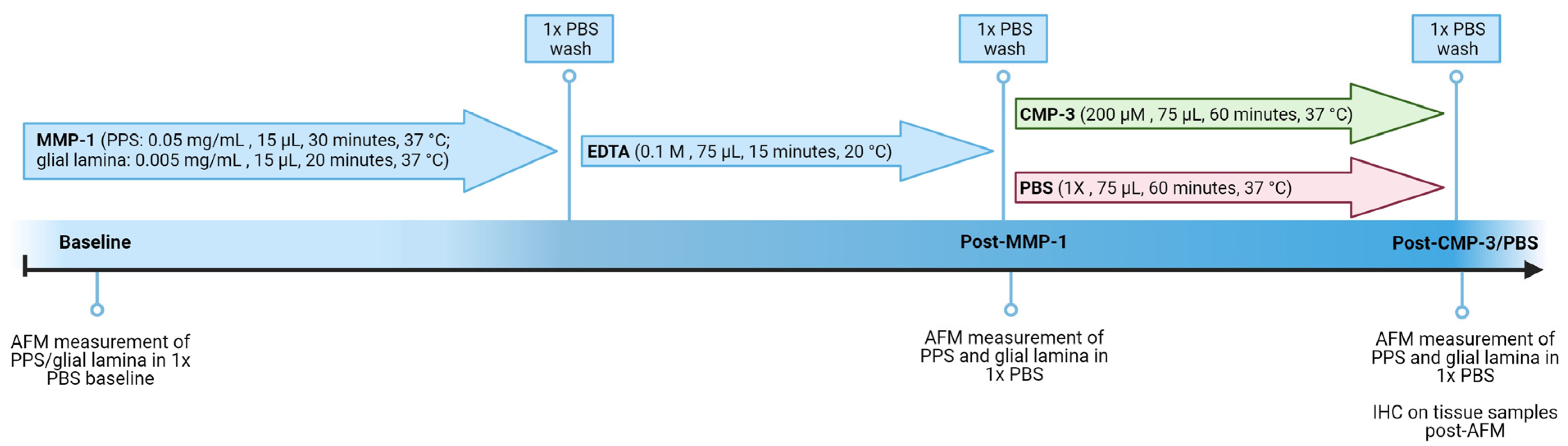
4. Materials and Methods
4.1. Animals
4.2. Tissue Preparation
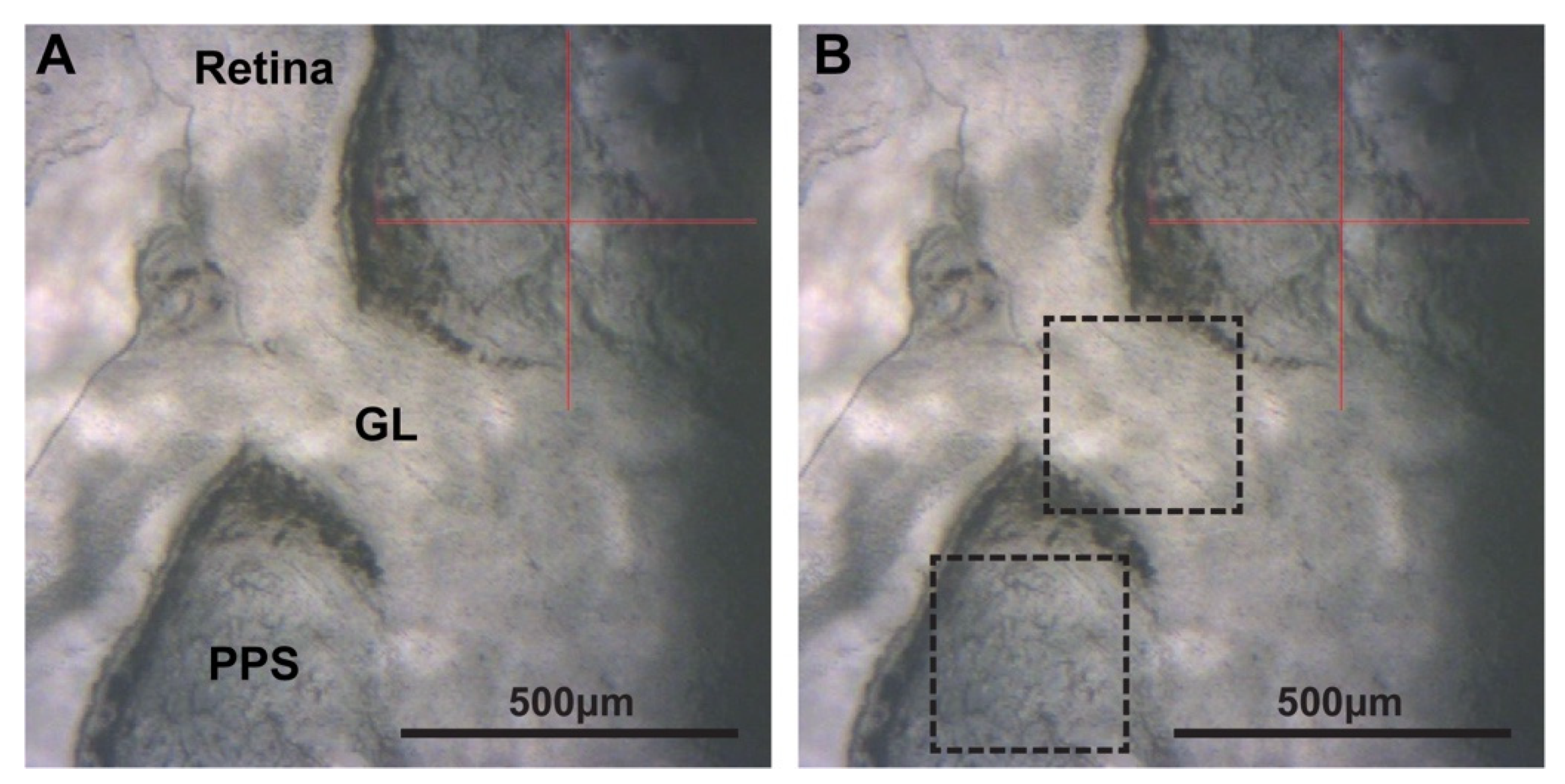
4.3. Atomic Force Microscopy
4.4. Matrix Metalloproteinase Treatment
4.5. Collagen Mimetic Peptide or Vehicle Treatment
4.6. Immunohistochemistry
4.7. Optic Nerve Head Tissue Imaging
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meek, K.M. The Cornea and Sclera. In Collagen: Structure and Mechanics; Fratzl, P., Ed.; Springer: Boston, MA, USA, 2008; pp. 359–396. [Google Scholar]
- Holden, B.A.; Fricke, T.R.; Wilson, D.A.; Jong, M.; Naidoo, K.S.; Sankaridurg, P.; Wong, T.Y.; Naduvilath, T.J.; Resnikoff, S. Global Prevalence of Myopia and High Myopia and Temporal Trends from 2000 through 2050. Ophthalmology 2016, 123, 1036–1042. [Google Scholar] [CrossRef] [PubMed]
- Guggenheim, J.A.; McBrien, N.A. Form-deprivation myopia induces activation of scleral matrix metalloproteinase-2 in tree shrew. Investig. Ophthalmol. Vis. Sci. 1996, 37, 1380–1395. [Google Scholar]
- Morgan, I.G.; French, A.N.; Ashby, R.S.; Guo, X.; Ding, X.; He, M.; Rose, K.A. The epidemics of myopia: Aetiology and prevention. Prog. Retin. Eye Res. 2018, 62, 134–149. [Google Scholar] [CrossRef]
- Morgan, I.G.; Ohno-Matsui, K.; Saw, S.-M. Myopia. Lancet 2012, 379, 1739–1748. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.Y.; Ferreira, A.; Hughes, R.; Carter, G.; Mitchell, P. Epidemiology and Disease Burden of Pathologic Myopia and Myopic Choroidal Neovascularization: An Evidence-Based Systematic Review. Am. J. Ophthalmol. 2014, 157, 9–25.e12. [Google Scholar] [CrossRef] [PubMed]
- McBrien, N.A.; Cornell, L.M.; Gentle, A. Structural and Ultrastructural Changes to the Sclera in a Mammalian Model of High Myopia. Investig. Ophthalmol. Vis. Sci. 2001, 42, 2179–2187. [Google Scholar]
- Shen, L.; You, Q.S.; Xu, X.; Gao, F.; Zhang, Z.; Li, B.; Jonas, J.B. Scleral Thickness in Chinese Eyes. Investig. Ophthalmol. Vis. Sci. 2015, 56, 2720–2727. [Google Scholar] [CrossRef] [PubMed]
- Norton, T.T.; Rada, J.A. Reduced extracellular matrix in mammalian sclera with induced myopia. Vis. Res. 1995, 35, 1271–1281. [Google Scholar] [CrossRef]
- Tham, Y.-C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.-Y. Global Prevalence of Glaucoma and Projections of Glaucoma Burden through 2040: A Systematic Review and Meta-Analysis. Ophthalmology 2014, 121, 2081–2090. [Google Scholar] [CrossRef]
- Steinmetz, J.D.; Bourne, R.R.A.; Briant, P.S.; Flaxman, S.R.; Taylor, H.R.B.; Jonas, J.B.; Abdoli, A.A.; Abrha, W.A.; Abualhasan, A.; Abu-Gharbieh, E.G.; et al. Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020: The Right to Sight: An analysis for the Global Burden of Disease Study. Lancet Glob. Health 2021, 9, e144–e160. [Google Scholar] [CrossRef]
- Johnson, E.C.; Morrison, J.C.; Farrell, S.; Deppmeier, L.; Moore, C.G.; McGinty, M.R. The Effect of Chronically Elevated Intraocular Pressure on the Rat Optic Nerve Head Extracellular Matrix. Exp. Eye Res. 1996, 62, 663–674. [Google Scholar] [CrossRef]
- Calkins, D.J. Adaptive responses to neurodegenerative stress in glaucoma. Prog. Retin. Eye Res. 2021, 84, 100953. [Google Scholar] [CrossRef] [PubMed]
- Quigley, H.A.; Dorman-Pease, M.E.; Brown, A.E. Quantitative study of collagen and elastin of the optic nerve head and sclera in human and experimental monkey glaucoma. Curr. Eye Res. 1991, 10, 877–888. [Google Scholar] [CrossRef] [PubMed]
- Pijanka, J.K.; Coudrillier, B.; Ziegler, K.; Sorensen, T.; Meek, K.M.; Nguyen, T.D.; Quigley, H.A.; Boote, C. Quantitative Mapping of Collagen Fiber Orientation in Non-glaucoma and Glaucoma Posterior Human Sclerae. Investig. Ophthalmol. Vis. Sci. 2012, 53, 5258–5270. [Google Scholar] [CrossRef] [PubMed]
- Pijanka, J.K.; Kimball, E.C.; Pease, M.E.; Abass, A.; Sorensen, T.; Nguyen, T.D.; Quigley, H.A.; Boote, C. Changes in Scleral Collagen Organization in Murine Chronic Experimental Glaucoma. Investig. Ophthalmol. Vis. Sci. 2014, 55, 6554–6564. [Google Scholar] [CrossRef] [PubMed]
- Cone-Kimball, E.; Nguyen, C.; Oglesby, E.N.; Pease, M.E.; Steinhart, M.R.; Quigley, H.A. Scleral structural alterations associated with chronic experimental intraocular pressure elevation in mice. Mol. Vis. 2013, 19, 2023–2039. [Google Scholar] [PubMed]
- Nguyen, C.; Cone, F.E.; Nguyen, T.D.; Coudrillier, B.; Pease, M.E.; Steinhart, M.R.; Oglesby, E.N.; Jefferys, J.L.; Quigley, H.A. Studies of Scleral Biomechanical Behavior Related to Susceptibility for Retinal Ganglion Cell Loss in Experimental Mouse Glaucoma. Investig. Ophthalmol. Vis. Sci. 2013, 54, 1767–1780. [Google Scholar] [CrossRef] [PubMed]
- Girard, M.J.A.; Suh, J.-K.F.; Bottlang, M.; Burgoyne, C.F.; Downs, J.C. Biomechanical Changes in the Sclera of Monkey Eyes Exposed to Chronic IOP Elevations. Investig. Ophthalmol. Vis. Sci. 2011, 52, 5656–5669. [Google Scholar] [CrossRef]
- Jonas, R.A.; Holbach, L. Peripapillary border tissue of the choroid and peripapillary scleral flange in human eyes. Acta Ophthalmol. 2020, 98, e43–e49. [Google Scholar] [CrossRef]
- Jonas, J.B.; Jonas, R.A.; Bikbov, M.M.; Wang, Y.X.; Panda-Jonas, S. Myopia: Histology, clinical features, and potential implications for the etiology of axial elongation. Prog. Retin. Eye Res. 2022, 96, 101156. [Google Scholar] [CrossRef]
- Burgoyne, C.F.; Crawford Downs, J.; Bellezza, A.J.; Francis Suh, J.K.; Hart, R.T. The optic nerve head as a biomechanical structure: A new paradigm for understanding the role of IOP-related stress and strain in the pathophysiology of glaucomatous optic nerve head damage. Prog. Retin. Eye Res. 2005, 24, 39–73. [Google Scholar] [CrossRef]
- Sun, D.; Lye-Barthel, M.; Masland, R.H.; Jakobs, T.C. The morphology and spatial arrangement of astrocytes in the optic nerve head of the mouse. J. Comp. Neurol. 2009, 516, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Lim, S.-H. Matrix Metalloproteinases and Glaucoma. Biomolecules 2022, 12, 1368. [Google Scholar] [CrossRef] [PubMed]
- Keeley, F.W.; Morin, J.D.; Vesely, S. Characterization of collagen from normal human sclera. Exp. Eye Res. 1984, 39, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, M.R.; Luo, X.X.; Igoe, F.; Neufeld, A.H. Extracellular Matrix of the Human Lamina Cribrosa. Am. J. Ophthalmol. 1987, 104, 567–576. [Google Scholar] [CrossRef]
- Sorokin, L. The impact of the extracellular matrix on inflammation. Nat. Rev. Immunol. 2010, 10, 712–723. [Google Scholar] [CrossRef]
- May, C.A. The optic nerve head region of the aged rat: An immunohistochemical investigation. Curr. Eye Res. 2003, 26, 347–354. [Google Scholar] [CrossRef]
- Last, J.A.; Russell, P.; Nealey, P.F.; Murphy, C.J. The Applications of Atomic Force Microscopy to Vision Science. Investig. Ophthalmol. Vis. Sci. 2010, 51, 6083–6094. [Google Scholar] [CrossRef]
- Dufrêne, Y.F.; Ando, T.; Garcia, R.; Alsteens, D.; Martinez-Martin, D.; Engel, A.; Gerber, C.; Müller, D.J. Imaging modes of atomic force microscopy for application in molecular and cell biology. Nat. Nanotechnol. 2017, 12, 295–307. [Google Scholar] [CrossRef]
- Sun, M.; Zafrullah, N.; Devaux, F.; Hemmavanh, C.; Adams, S.; Ziebarth, N.M.; Koch, M.; Birk, D.E.; Espana, E.M. Collagen XII Is a Regulator of Corneal Stroma Structure and Function. Investig. Ophthalmol. Vis. Sci. 2020, 61, 61. [Google Scholar] [CrossRef]
- Last, J.A.; Thomasy, S.M.; Croasdale, C.R.; Russell, P.; Murphy, C.J. Compliance profile of the human cornea as measured by atomic force microscopy. Micron 2012, 43, 1293–1298. [Google Scholar] [CrossRef] [PubMed]
- John, T.; Patel, A.; Vasavada, A.; Singh, M.; Nath, V.; Cheng, A.M.; Sheha, H. Effect of Trypan Blue on Descemet Membrane Elasticity. Cornea 2016, 35, 1401–1403. [Google Scholar] [CrossRef]
- Huang, J.; Camras, L.J.; Yuan, F. Mechanical analysis of rat trabecular meshwork. Soft Matter 2015, 11, 2857–2865. [Google Scholar] [CrossRef]
- Batchelor, W.M.; Heilman, B.M.; Arrieta-Quintero, E.; Ruggeri, M.; Parel, J.M.; Manns, F.; Cabrera-Ghayouri, S.; Dibas, M.; Ziebarth, N.M. Measuring the effects of postmortem time and age on mouse lens elasticity using atomic force microscopy. Exp. Eye Res. 2021, 212, 108768. [Google Scholar] [CrossRef]
- Ziebarth, N.M.; Wojcikiewicz, E.P.; Manns, F.; Moy, V.T.; Parel, J.M. Atomic force microscopy measurements of lens elasticity in monkey eyes. Mol. Vis. 2007, 13, 504–510. [Google Scholar]
- Zhuola, Z.; Barrett, S.; Kharaz, Y.A.; Akhtar, R. Nanostructural and mechanical changes in the sclera following proteoglycan depletion. Model. Artif. Intell. Ophthalmol. 2018, 2, 14–17. [Google Scholar] [CrossRef]
- Grant, C.A.; Thomson, N.H.; Savage, M.D.; Woon, H.W.; Greig, D. Surface characterisation and biomechanical analysis of the sclera by atomic force microscopy. J. Mech. Behav. Biomed. Mater. 2011, 4, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Wareham, L.K.; Kuchtey, J.; Wu, H.-J.; Krystofiak, E.; Wu, Y.; Reinhart-King, C.A.; Kuchtey, R.W. Lysyl oxidase-like 1 deficiency alters ultrastructural and biomechanical properties of the peripapillary sclera in mice. Matrix Biol. Plus 2022, 16, 100120. [Google Scholar] [CrossRef]
- Liu, L.; Liu, Y.; Li, T.; Li, L.; Qian, X.; Liu, Z. A feasible method for independently evaluating the mechanical properties of glial LC and RGC axons by combining atomic force microscopy measurement with image segmentation. J. Mech. Behav. Biomed. Mater. 2022, 126, 105041. [Google Scholar] [CrossRef]
- Braunsmann, C.; Hammer, C.M.; Rheinlaender, J.; Kruse, F.E.; Schäffer, T.E.; Schlötzer-Schrehardt, U. Evaluation of lamina cribrosa and peripapillary sclera stiffness in pseudoexfoliation and normal eyes by atomic force microscopy. Investig. Ophthalmol. Vis. Sci. 2012, 53, 2960–2967. [Google Scholar] [CrossRef]
- McGrady, N.R.; Pasini, S.; Baratta, R.O.; Del Buono, B.J.; Schlumpf, E.; Calkins, D.J. Restoring the Extracellular Matrix: A Neuroprotective Role for Collagen Mimetic Peptides in Experimental Glaucoma. Front. Pharmacol. 2021, 12, 764709. [Google Scholar] [CrossRef] [PubMed]
- Baratta, R.O.; Del Buono, B.J.; Schlumpf, E.; Ceresa, B.P.; Calkins, D.J. Collagen Mimetic Peptides Promote Corneal Epithelial Cell Regeneration. Front. Pharmacol. 2021, 12, 705623. [Google Scholar] [CrossRef] [PubMed]
- Wareham, L.K.; Holden, J.M.; Bossardet, O.L.; Baratta, R.O.; Del Buono, B.J.; Schlumpf, E.; Calkins, D.J. Collagen mimetic peptide repair of the corneal nerve bed in a mouse model of dry eye disease. Front. Neurosci. 2023, 17, 1148950. [Google Scholar] [CrossRef]
- Kadler, K.E.; Baldock, C.; Bella, J.; Boot-Handford, R.P. Collagens at a glance. J. Cell Sci. 2007, 120, 1955–1958. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, S.; Raines, R.T. Collagen-based biomaterials for wound healing. Biopolymers 2014, 101, 821–833. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Guthrie, K.M.; Teixeira, L.; Murphy, C.J.; Dubielzig, R.R.; McAnulty, J.F.; Raines, R.T. Anchoring a cytoactive factor in a wound bed promotes healing. J. Tissue Eng. Regen. Med. 2016, 10, 1012–1020. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Murphy, C.J.; McAnulty, J.F.; Raines, R.T. Peptides that anneal to natural collagen in vitro and ex vivo. Org. Biomol. Chem. 2012, 10, 5892. [Google Scholar] [CrossRef]
- Panwar, P.; Butler, G.S.; Jamroz, A.; Azizi, P.; Overall, C.M.; Brömme, D. Aging-associated modifications of collagen affect its degradation by matrix metalloproteinases. Matrix Biol. 2018, 65, 30–44. [Google Scholar] [CrossRef]
- Kazaili, A.; Abdul-Amir Al-Hindy, H.; Madine, J.; Akhtar, R. Nano-Scale Stiffness and Collagen Fibril Deterioration: Probing the Cornea Following Enzymatic Degradation Using Peakforce-QNM AFM. Sensors 2021, 21, 1629. [Google Scholar] [CrossRef]
- Moeendarbary, E.; Weber, I.P.; Sheridan, G.K.; Koser, D.E.; Soleman, S.; Haenzi, B.; Bradbury, E.J.; Fawcett, J.; Franze, K. The soft mechanical signature of glial scars in the central nervous system. Nat. Commun. 2017, 8, 14787. [Google Scholar] [CrossRef]
- Tang, V.W. Collagen, stiffness, and adhesion: The evolutionary basis of vertebrate mechanobiology. Mol. Biol. Cell 2020, 31, 1823–1834. [Google Scholar] [CrossRef]
- Hu, Y.; Huang, G.; Tian, J.; Qiu, J.; Jia, Y.; Feng, D.; Wei, Z.; Li, S.; Xu, F. Matrix stiffness changes affect astrocyte phenotype in an in vitro injury model. NPG Asia Mater. 2021, 13, 35. [Google Scholar] [CrossRef]
- Grytz, R. Scleral remodeling in myopia. In Biomechanics of the Eye; Kugler Publications: Amsterdam, The Netherlands, 2018; p. 383. [Google Scholar]
- Liu, H.H.; Kenning, M.S.; Jobling, A.I.; McBrien, N.A.; Gentle, A. Reduced Scleral TIMP-2 Expression Is Associated with Myopia Development: TIMP-2 Supplementation Stabilizes Scleral Biomarkers of Myopia and Limits Myopia Development. Investig. Ophthalmol. Vis. Sci. 2017, 58, 1971–1981. [Google Scholar] [CrossRef] [PubMed]
- Gentle, A.; Liu, Y.; Martin, J.E.; Conti, G.L.; McBrien, N.A. Collagen gene expression and the altered accumulation of scleral collagen during the development of high myopia. J. Biol. Chem. 2003, 278, 16587–16594. [Google Scholar] [CrossRef] [PubMed]
- McBrien, N.A.; Norton, T.T. Prevention of collagen crosslinking increases form-deprivation myopia in tree shrew. Exp. Eye Res. 1994, 59, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Corpuz, C.C.C. Effects of scleral cross-linking using genipin on the process of form-deprivation myopia in the guinea pig: A randomized controlled experimental study. BMC Ophthalmol. 2015, 15, 89. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P.; Hourihan, F.; Sandbach, J.; Jin Wang, J. The relationship between glaucoma and myopia: The blue mountains eye study. Ophthalmology 1999, 106, 2010–2015. [Google Scholar] [CrossRef] [PubMed]
- Powell, S.; Irnaten, M.; O’Brien, C. Glaucoma—‘A Stiff Eye in a Stiff Body’. Curr. Eye Res. 2023, 48, 152–160. [Google Scholar] [CrossRef]
- Kimball, E.C.; Nguyen, C.; Steinhart, M.R.; Nguyen, T.D.; Pease, M.E.; Oglesby, E.N.; Oveson, B.C.; Quigley, H.A. Experimental scleral cross-linking increases glaucoma damage in a mouse model. Exp. Eye Res. 2014, 128, 129–140. [Google Scholar] [CrossRef]
- Coudrillier, B.; Campbell, I.C.; Read, A.T.; Geraldes, D.M.; Vo, N.T.; Feola, A.; Mulvihill, J.; Albon, J.; Abel, R.L.; Ethier, C.R. Effects of Peripapillary Scleral Stiffening on the Deformation of the Lamina Cribrosa. Investig. Ophthalmol. Vis. Sci. 2016, 57, 2666–2677. [Google Scholar] [CrossRef]
- Thornton, I.L.; Dupps, W.J.; Sinha Roy, A.; Krueger, R.R. Biomechanical effects of intraocular pressure elevation on optic nerve/lamina cribrosa before and after peripapillary scleral collagen cross-linking. Investig. Ophthalmol. Vis. Sci. 2009, 50, 1227–1233. [Google Scholar] [CrossRef] [PubMed]
- Hutter, J.L.; Bechhoefer, J. Calibration of atomic-force microscope tips. Rev. Sci. Instrum. 1993, 64, 1868–1873. [Google Scholar] [CrossRef]
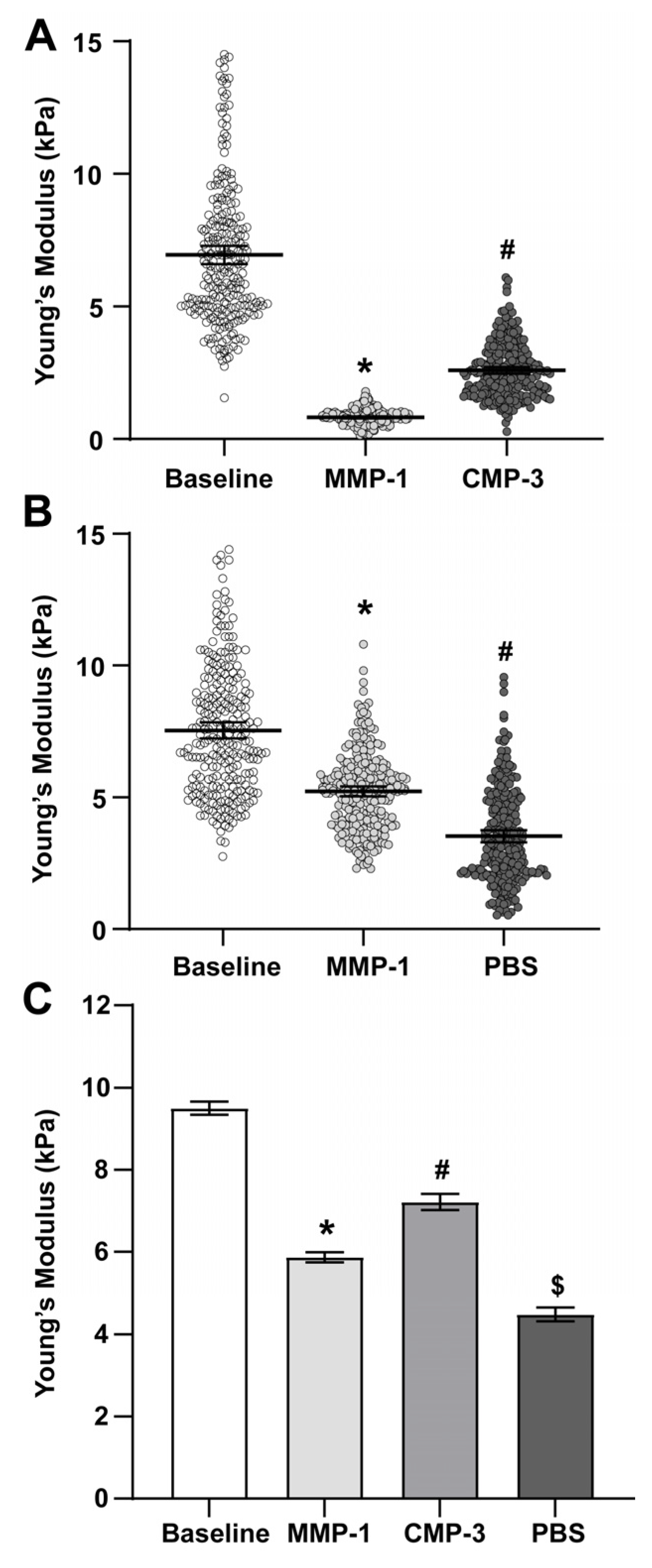
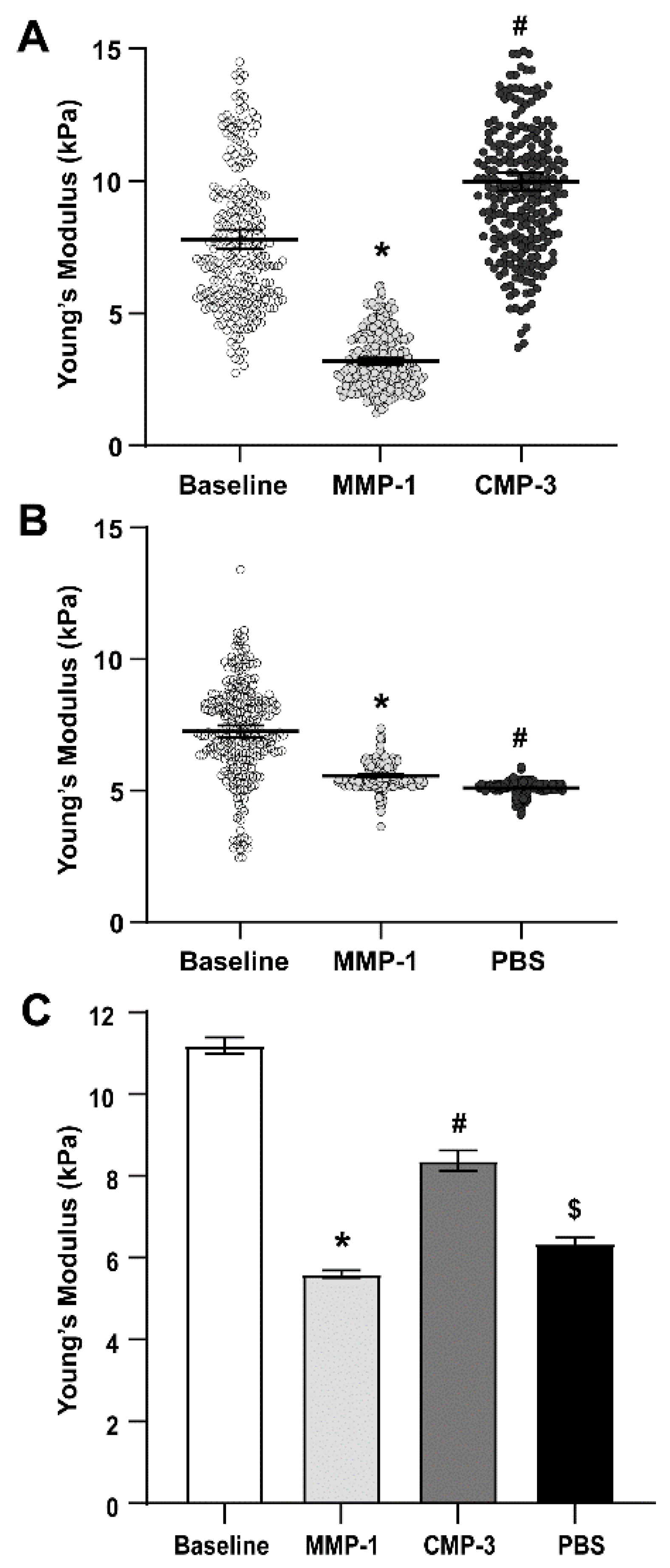
| Sample Type | Mean Young’s Modulus (kPa) | 95% C.I. | n |
|---|---|---|---|
| Baseline | 9.500 | 0.161 | 6656 |
| MMP-1 | 5.865 | 0.124 | 5120 |
| CMP-3 | 7.214 | 0.101 | 2938 |
| 1x PBS (vehicle) | 4.345 | 0.323 | 1536 |
| Sample Type | Mean Young’s Modulus (kPa) | 95% C.I. | n |
|---|---|---|---|
| Baseline | 13.096 | 0.248 | 9082 |
| MMP-1 | 5.602 | 0.090 | 2304 |
| CMP-3 | 8.373 | 0.253 | 2229 |
| 1x PBS (vehicle) | 6.342 | 0.151 | 1024 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bou Ghanem, G.O.; Koktysh, D.; Baratta, R.O.; Del Buono, B.J.; Schlumpf, E.; Wareham, L.K.; Calkins, D.J. Collagen Mimetic Peptides Promote Repair of MMP-1-Damaged Collagen in the Rodent Sclera and Optic Nerve Head. Int. J. Mol. Sci. 2023, 24, 17031. https://doi.org/10.3390/ijms242317031
Bou Ghanem GO, Koktysh D, Baratta RO, Del Buono BJ, Schlumpf E, Wareham LK, Calkins DJ. Collagen Mimetic Peptides Promote Repair of MMP-1-Damaged Collagen in the Rodent Sclera and Optic Nerve Head. International Journal of Molecular Sciences. 2023; 24(23):17031. https://doi.org/10.3390/ijms242317031
Chicago/Turabian StyleBou Ghanem, Ghazi O., Dmitry Koktysh, Robert O. Baratta, Brian J. Del Buono, Eric Schlumpf, Lauren K. Wareham, and David J. Calkins. 2023. "Collagen Mimetic Peptides Promote Repair of MMP-1-Damaged Collagen in the Rodent Sclera and Optic Nerve Head" International Journal of Molecular Sciences 24, no. 23: 17031. https://doi.org/10.3390/ijms242317031
APA StyleBou Ghanem, G. O., Koktysh, D., Baratta, R. O., Del Buono, B. J., Schlumpf, E., Wareham, L. K., & Calkins, D. J. (2023). Collagen Mimetic Peptides Promote Repair of MMP-1-Damaged Collagen in the Rodent Sclera and Optic Nerve Head. International Journal of Molecular Sciences, 24(23), 17031. https://doi.org/10.3390/ijms242317031






