Proteomic Analysis of Fractionated Eimeria tenella Sporulated Oocysts Reveals Involvement in Oocyst Wall Formation
Abstract
:1. Introduction
2. Results
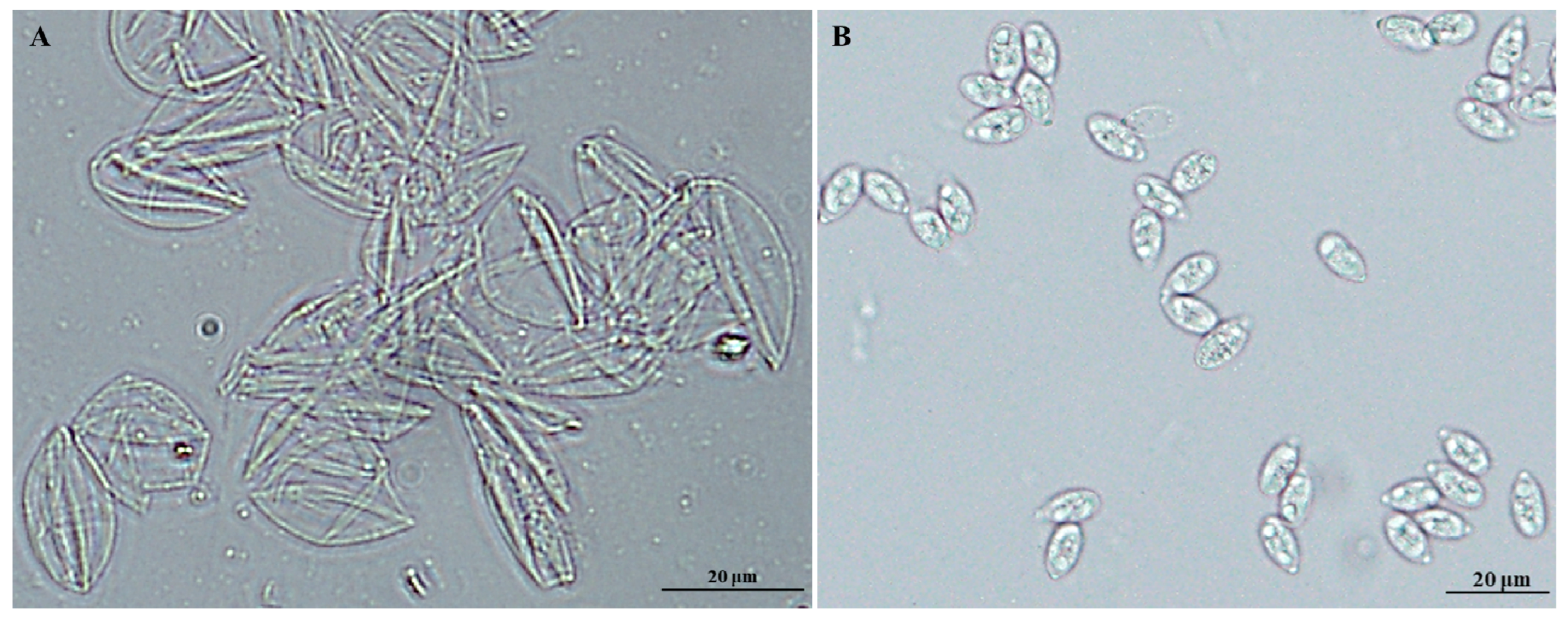
2.1. Identification of Proteins in Sporulated Oocyst Fractions
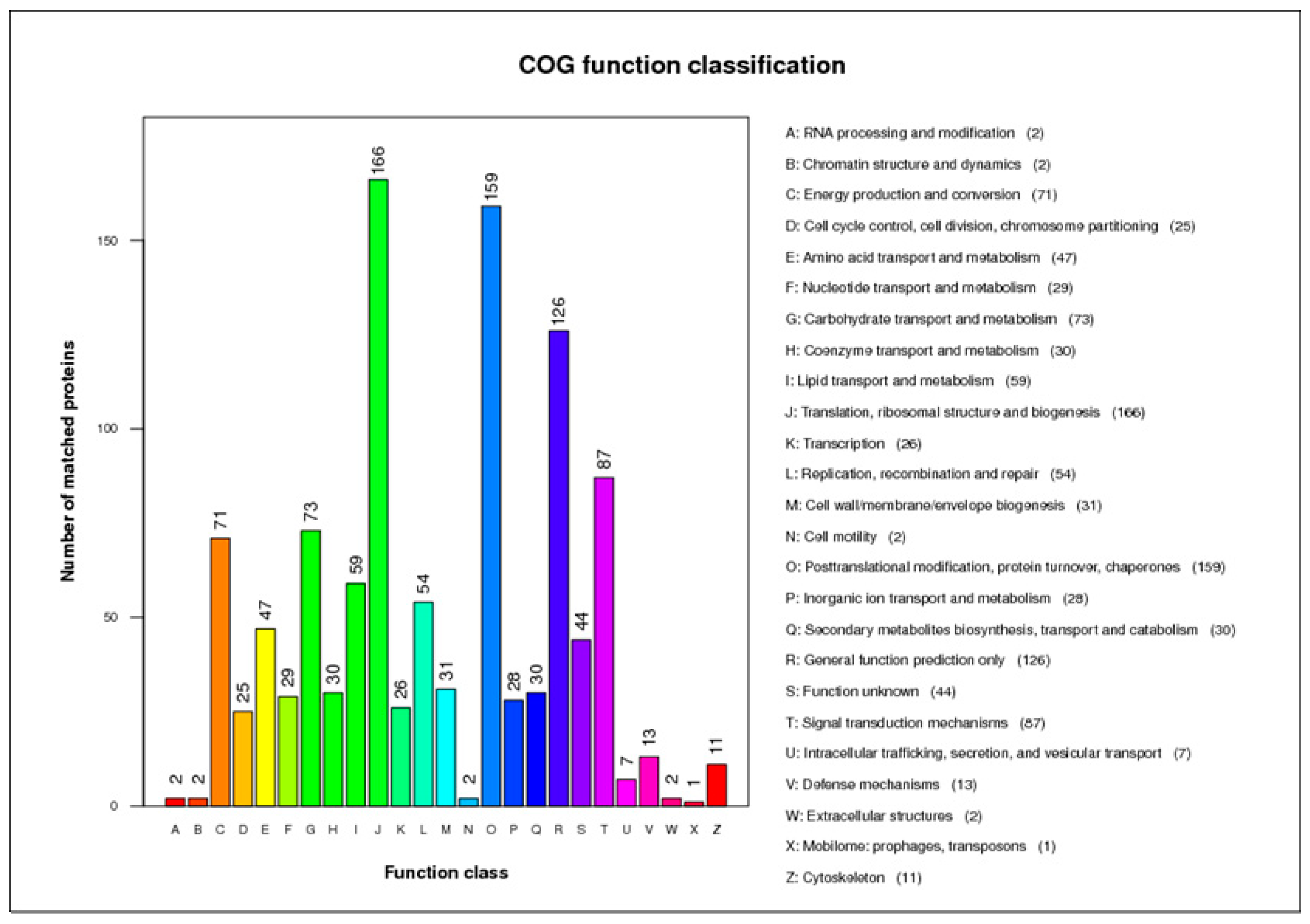
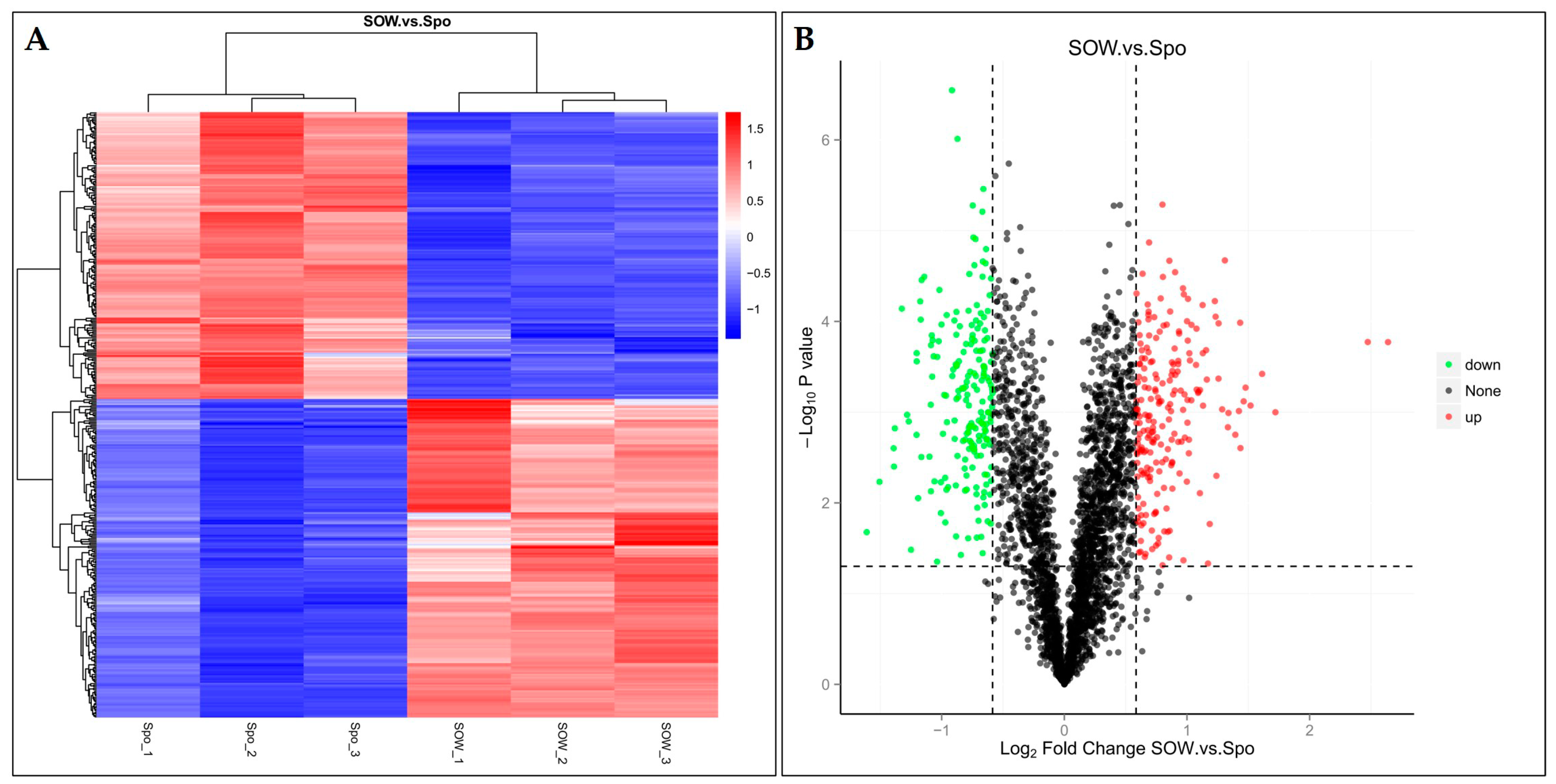
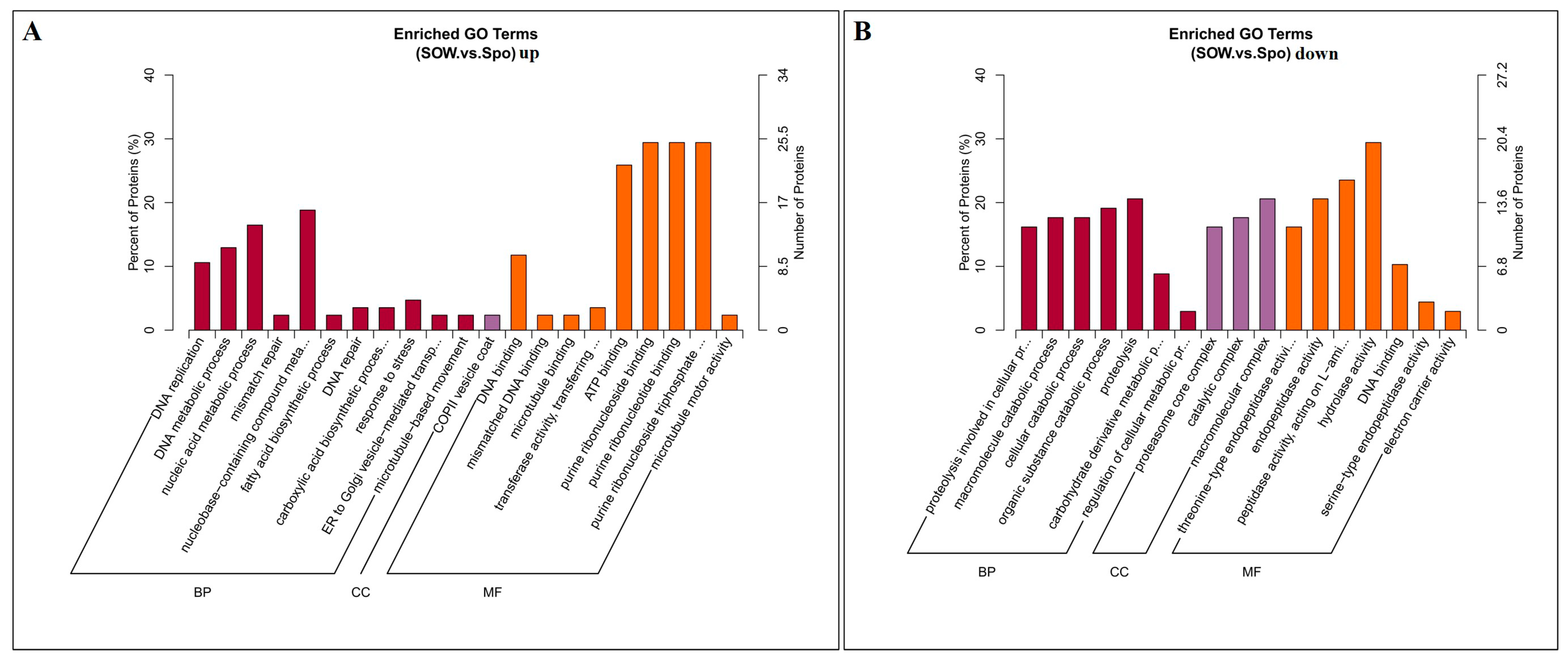
2.2. Identification and Functional Analysis of DEPs
2.3. Most Abundant Up- and Down-Regulated Proteins in Oocyst Walls
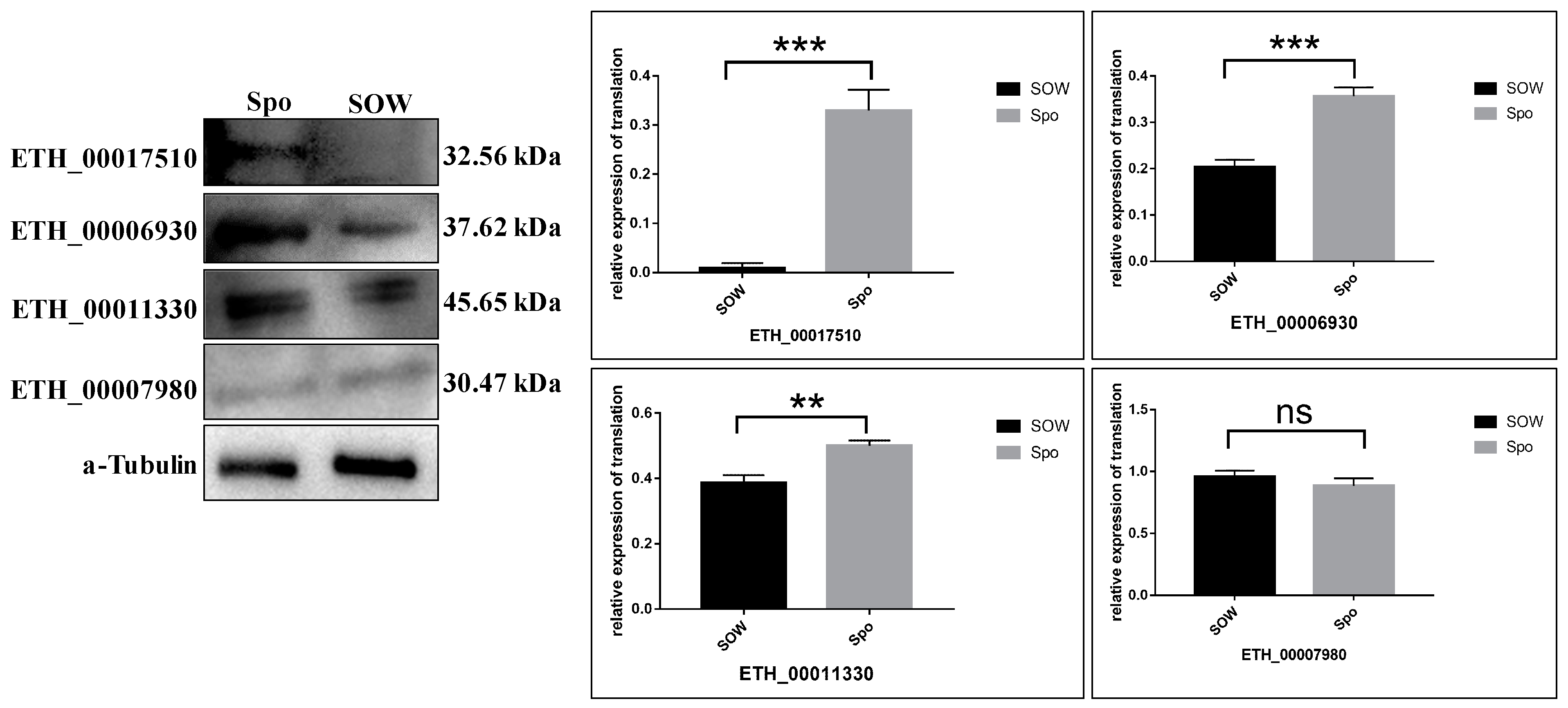
2.4. Validation of TMT Data for Selected Proteins by Western Blot Assay
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Animals and Parasites
4.3. Isolation of Oocyst Walls and Sporocysts
4.4. Protein Extraction and Tandem Mass Tag Labeling
4.5. Separation of Fractions and LC-MS/MS Analysis
4.6. The Identification and Quantification of Proteins
4.7. The Functional Analysis of Proteins and DEPs
4.8. Western Blot
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Su, S.; Hou, Z.; Liu, D.; Jia, C.; Wang, L.; Xu, J.; Tao, J. Comparative transcriptome analysis of Eimeria necatrix third-generation merozoites and gametocytes reveals genes involved in sexual differentiation and gametocyte development. Vet. Parasitol. 2018, 252, 35–46. [Google Scholar] [CrossRef]
- Novaes, J.; Rangel, L.T.; Ferro, M.; Abe, R.Y.; Manha, A.P.; de Mello, J.C.; Varuzza, L.; Durham, A.M.; Madeira, A.M.; Gruber, A. A comparative transcriptome analysis reveals expression profiles conserved across three Eimeria spp. of domestic fowl and associated with multiple developmental stages. Int. J. Parasitol. 2012, 42, 39–48. [Google Scholar] [CrossRef]
- Blake, D.P.; Tomley, F.M. Securing poultry production from the ever-present Eimeria challenge. Trends Parasitol. 2014, 30, 12–19. [Google Scholar] [CrossRef]
- Frölich, S.; Wallach, M. Use of fluorescent nanoparticles to investigate nutrient acquisition by developing Eimeria maxima macrogametocytes. Sci. Rep. 2016, 6, 29030. [Google Scholar] [CrossRef]
- Shirley, M.W.; Ivens, A.; Gruber, A.; Madeira, A.M.; Wan, K.L.; Dear, P.H.; Tomley, F.M. The Eimeria genome projects: A sequence of events. Trends Parasitol. 2004, 20, 199–201. [Google Scholar] [CrossRef]
- Dalloul, R.A.; Lillehoj, H.S. Poultry coccidiosis: Recent advancements in control measures and vaccine development. Expert. Rev. Vaccines 2006, 5, 143–163. [Google Scholar] [CrossRef]
- Smith, T.G.; Walliker, D.; Ranford-Cartwright, L.C. Sexual differentiation and sex determination in the Apicomplexa. Trends Parasitol. 2002, 18, 315–323. [Google Scholar] [CrossRef]
- Liu, J.; Shi, F.; Zhang, Y.; Tang, X.; Wang, C.; Gao, Y.; Suo, J.; Yu, Y.; Chen, L.; Zhang, N.; et al. Evidence of high-efficiency cross fertilization in Eimeria acervulina revealed using two lines of transgenic parasites. Int. J. Parasitol. 2023, 53, 81–89. [Google Scholar] [CrossRef]
- Mai, K.; Sharman, P.A.; Walker, R.A.; Katrib, M.; De Souza, D.; McConville, M.J.; Wallach, M.G.; Belli, S.I.; Ferguson, D.J.; Smith, N.C. Oocyst wall formation and composition in coccidian parasites. Mem. Inst. Oswaldo Cruz. 2009, 104, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Walker, R.A.; Niepceron, A.; Ramakrishnan, C.; Sedano, L.; Hehl, A.B.; Brossier, F.; Smith, N.C. Discovery of a tyrosine-rich sporocyst wall protein in Eimeria tenella. Parasit. Vectors 2016, 9, 124. [Google Scholar] [CrossRef] [PubMed]
- Salman, D.; Okuda, L.H.; Ueno, A.; Dautu, G.; Zhang, F.; Igarashi, M. Evaluation of novel oocyst wall protein candidates of Toxoplasma gondii. Parasitol. Int. 2017, 66, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Belli, S.I.; Smith, N.C.; Ferguson, D.J. The coccidian oocyst: A tough nut to crack! Trends Parasitol. 2006, 22, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Fritz, H.M.; Bowyer, P.W.; Bogyo, M.; Conrad, P.A.; Boothroyd, J.C. Proteomic analysis of fractionated Toxoplasma oocysts reveals clues to their environmental resistance. PLoS ONE 2012, 7, e29955. [Google Scholar] [CrossRef] [PubMed]
- Stotish, R.L.; Wang, C.C.; Meyenhofer, M. Structure and composition of the oocyst wall of Eimeria tenella. J. Parasitol. 1978, 64, 1074–1081. [Google Scholar] [CrossRef] [PubMed]
- Dumètre, A.; Dubey, J.P.; Ferguson, D.J.P. Effect of household bleach on the structure of the sporocyst wall of Toxoplasma gondii. Parasite 2021, 28, 68. [Google Scholar] [CrossRef] [PubMed]
- Belli, S.I.; Wallach, M.G.; Luxford, C.; Davies, M.J.; Smith, N.C. Roles of tyrosine-rich precursor glycoproteins and dityrosine- and 3,4-dihydroxyphenylalanine-mediated protein cross-linking in development of the oocyst wall in the coccidian parasite Eimeria maxima. Eukaryot. Cell 2003, 2, 456–464. [Google Scholar] [CrossRef]
- Ferguson, D.J.; Belli, S.I.; Smith, N.C.; Wallach, M.G. The development of the macrogamete and oocyst wall in Eimeria maxima: Immuno-light and electron microscopy. Int. J. Parasitol. 2003, 33, 1329–1340. [Google Scholar] [CrossRef]
- Belli, S.I.; Witcombe, D.; Wallach, M.G.; Smith, N.C. Functional genomics of gam56: Characterisation of the role of a 56 kilodalton sexual stage antigen in oocyst wall formation in Eimeria maxima. Int. J. Parasitol. 2002, 32, 1727–1737. [Google Scholar] [CrossRef]
- Spano, F.; Puri, C.; Ranucci, L.; Putignani, L.; Crisanti, A. Cloning of the entire COWP gene of Cryptosporidium parvum and ultrastructural localization of the protein during sexual parasite development. Parasitology 1997, 114, 427–437. [Google Scholar] [CrossRef]
- Walker, R.A.; Sharman, P.A.; Miller, C.M.; Lippuner, C.; Okoniewski, M.; Eichenberger, R.M.; Ramakrishnan, C.; Brossier, F.; Deplazes, P.; Hehl, A.B.; et al. RNA Seq analysis of the Eimeria tenella gametocyte transcriptome reveals clues about the molecular basis for sexual reproduction and oocyst biogenesis. BMC Genom. 2015, 16, 94. [Google Scholar] [CrossRef]
- Walker, R.A.; Ferguson, D.J.; Miller, C.M.; Smith, N.C. Sex and Eimeria: A molecular perspective. Parasitology 2013, 140, 1701–1717. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, D.J.; Hutchison, W.M.; Siim, J.C. The ultrastructural development of the macrogamete and formation of the oocyst wall of Toxoplasma gondii. Acta Pathol. Microbiol. Scand. B 1975, 83, 491–505. [Google Scholar] [CrossRef] [PubMed]
- Templeton, T.J.; Lancto, C.A.; Vigdorovich, V.; Liu, C.; London, N.R.; Hadsall, K.Z.; Abrahamsen, M.S. The Cryptosporidium oocyst wall protein is a member of a multigene family and has a homolog in Toxoplasma. Infect Immun. 2004, 72, 980–987. [Google Scholar] [CrossRef] [PubMed]
- Jonscher, E.; Erdbeer, A.; Günther, M.; Kurth, M. Two COWP-like cysteine rich proteins from Eimeria nieschulzi (coccidia, apicomplexa) are expressed during sporulation and involved in the sporocyst wall formation. Parasit. Vectors 2015, 8, 395. [Google Scholar] [CrossRef] [PubMed]
- Possenti, A.; Cherchi, S.; Bertuccini, L.; Pozio, E.; Dubey, J.P.; Spano, F. Molecular characterisation of a novel family of cysteine-rich proteins of Toxoplasma gondii and ultrastructural evidence of oocyst wall localisation. Int. J. Parasitol. 2010, 40, 1639–1649. [Google Scholar] [CrossRef] [PubMed]
- Mai, K.; Smith, N.C.; Feng, Z.P.; Katrib, M.; Slapeta, J.; Slapetova, I.; Wallach, M.G.; Luxford, C.; Davies, M.J.; Zhang, X.; et al. Peroxidase catalysed cross-linking of an intrinsically unstructured protein via dityrosine bonds in the oocyst wall of the apicomplexan parasite, Eimeria maxima. Int. J. Parasitol. 2011, 41, 1157–1164. [Google Scholar] [CrossRef] [PubMed]
- Bushkin, G.G.; Motari, E.; Carpentieri, A.; Dubey, J.P.; Costello, C.E.; Robbins, P.W.; Samuelson, J. Evidence for a structural role for acid-fast lipids in oocyst walls of Cryptosporidium, Toxoplasma, and Eimeria. mBio 2013, 4, e00387-13. [Google Scholar] [CrossRef]
- Frölich, S.; Johnson, M.; Robinson, M.; Entzeroth, R.; Wallach, M. The spatial organization and extraction of the wall-forming bodies of Eimeria maxima. Parasitology 2013, 140, 876–887. [Google Scholar] [CrossRef]
- Samuelson, J.; Bushkin, G.G.; Chatterjee, A.; Robbins, P.W. Strategies to discover the structural components of cyst and oocyst walls. Eukaryot Cell. 2013, 12, 1578–1587. [Google Scholar] [CrossRef]
- Freppel, W.; Ferguson, D.J.P.; Shapiro, K.; Dubey, J.P.; Puech, P.H.; Dumètre, A. Structure, composition, and roles of the Toxoplasma gondii oocyst and sporocyst walls. Cell Surf. 2018, 5, 100016. [Google Scholar] [CrossRef]
- Thompson, S.N.; Lee, R.W. Metabolic fate of alanine in an insect Manduca sexta: Effects of starvation and parasitism. Biochim. Biophys. Acta 1993, 1157, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Zhu, S.; Chen, T.; Zhao, Q.; Dong, H.; Huang, B.; Yao, Y.; Liu, Z.; Yu, Y.; Han, H. Molecular characterization of glyceraldehyde-3-phosphate dehydrogenase from Eimeria tenella. Parasitol. Res. 2022, 121, 1749–1760. [Google Scholar] [CrossRef]
- Paget, T.; Haroune, N.; Bagchi, S.; Jarroll, E. Metabolomics and protozoan parasites. Acta Parasitol. 2013, 58, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Fujita, Y.; Matsuoka, H.; Hirooka, K. Regulation of fatty acid metabolism in bacteria. Mol. Microbiol. 2007, 66, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Swale, C.; Bougdour, A.; Gnahoui-David, A.; Tottey, J.; Georgeault, S.; Laurent, F.; Palencia, A.; Hakimi, M.A. Metal-captured inhibition of pre-mRNA processing activity by CPSF3 controls Cryptosporidium infection. Sci. Transl. Med. 2019, 11, eaax7161. [Google Scholar] [CrossRef]
- Lendner, M.; Daugschies, A. Cryptosporidium infections: Molecular advances. Parasitology 2014, 141, 1511–1532. [Google Scholar] [CrossRef]
- Muñoz, C.; San Francisco, J.; Gutiérrez, B.; González, J. Role of the Ubiquitin-Proteasome Systems in the Biology and Virulence of Protozoan Parasites. Biomed. Res. Int. 2015, 2015, 141526. [Google Scholar] [CrossRef]
- Péroval, M.; Péry, P.; Labbé, M. The heat shock protein 90 of Eimeria tenella is essential for invasion of host cell and schizont growth. Int. J. Parasitol. 2006, 36, 1205–1215. [Google Scholar] [CrossRef]
- Carruthers, V.B.; Tomley, F.M. Microneme proteins in apicomplexans. Subcell. Biochem. 2008, 47, 33–45. [Google Scholar]
- Ramly, N.Z.; Dix, S.R.; Ruzheinikov, S.N.; Sedelnikova, S.E.; Baker, P.J.; Chow, Y.P.; Tomley, F.M.; Blake, D.P.; Wan, K.L.; Nathan, S.; et al. The structure of a major surface antigen SAG19 from Eimeria tenella unifies the Eimeria SAG family. Commun. Biol. 2021, 4, 376. [Google Scholar] [CrossRef]
- Lee, Y.; Park, I.; Wickramasuriya, S.S.; Arous, J.B.; Koziol, M.E.; Lillehoj, H.S. Co-administration of chicken IL-7 or NK-lysin peptide 2 enhances the efficacy of Eimeria elongation factor-1α vaccination against Eimeria maxima infection in broiler chickens. Poult. Sci. 2022, 101, 102013. [Google Scholar] [CrossRef] [PubMed]
- Brecht, S.; Carruthers, V.B.; Ferguson, D.J.P.; Giddings, O.K.; Wang, G.; Jackle, U.; Sibley, L.D.; Soldati, D. The Toxoplasma microneme protein MIC4 is an adhesin composed of six conserved apple domains. J. Biol. Chem. 2001, 276, 4119–4127. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, D.J.; Brecht, S.; Soldati, D. The microneme protein MIC4, or an MIC4-like protein, is expressed within the macrogamete and associated with oocyst wall formation in Toxoplasma gondii. Int. J. Parasitol. 2000, 30, 1203–1209. [Google Scholar] [CrossRef]
- Tordai, H.; Bányai, L.; Patthy, L. The PAN module: The N-terminal domains of plasminogen and hepatocyte growth factor are homologous with the apple domains of the prekallikrein family and with a novel domain found in numerous nematode proteins. FEBS Lett. 1999, 461, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.J.; Gill, A.C.; Nugent, P.G.; McVey, J.H.; Tomley, F.M. Domains of invasion organelle proteins from apicomplexan parasites are homologous with the Apple domains of blood coagulation factor XI and plasma pre-kallikrein and are members of the PAN module superfamily. FEBS Lett. 2001, 497, 31–38. [Google Scholar] [CrossRef]
- Katrib, M.; Ikin, R.J.; Brossier, F.; Robinson, M.; Slapetova, I.; Sharman, P.A.; Walker, R.A.; Belli, S.I.; Tomley, F.M.; Smith, N.C. Stage-specific expression of protease genes in the apicomplexan parasite, Eimeria tenella. BMC Genom. 2012, 13, 685. [Google Scholar] [CrossRef] [PubMed]
- Han, H.Y.; Lin, J.J.; Zhao, Q.P.; Dong, H.; Jiang, L.L.; Xu, M.Q.; Zhu, S.H.; Huang, B. Identification of differentially expressed genes in early stages of Eimeria tenella by suppression subtractive hybridization and cDNA microarray. J. Parasitol. 2010, 96, 95–102. [Google Scholar] [CrossRef]
- Liang, S.; Zhao, Q.; Ye, Y.; Zhu, S.; Dong, H.; Yu, Y.; Huang, B.; Han, H. Characteristics analyses of Eimeria tenella 14-3-3 protein and verification of its interaction with calcium-dependent protein kinase 4. Eur. J. Protistol. 2022, 85, 125895. [Google Scholar] [CrossRef]
- Wiśniewski, J.R.; Zougman, A.; Nagaraj, N.; Mann, M. Universal sample preparation method for proteome analysis. Nat. Methods 2009, 6, 359–362. [Google Scholar] [CrossRef]
- Kachuk, C.; Stephen, K.; Doucette, A. Comparison of sodium dodecyl sulfate depletion techniques for proteome analysis by mass spectrometry. J. Chromatogr. A 2015, 1418, 158–166. [Google Scholar] [CrossRef]
- Gillette, M.A.; Satpathy, S.; Cao, S.; Dhanasekaran, S.M.; Vasaikar, S.V.; Krug, K.; Petralia, F.; Li, Y.; Liang, W.W.; Reva, B.; et al. Clinical Proteomic Tumor Analysis Consortium. Proteogenomic Characterization Reveals Therapeutic Vulnerabilities in Lung Adenocarcinoma. Cell 2020, 182, 200–225.e35. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.; Binns, D.; Chang, H.Y.; Fraser, M.; Li, W.; McAnulla, C.; McWilliam, H.; Maslen, J.; Mitchell, A.; Nuka, G.; et al. InterProScan 5: Genome-scale protein function classification. Bioinformatics 2014, 30, 1236–1240. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Bioinformatics enrichment tools: Paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009, 37, 1–13. [Google Scholar] [CrossRef] [PubMed]

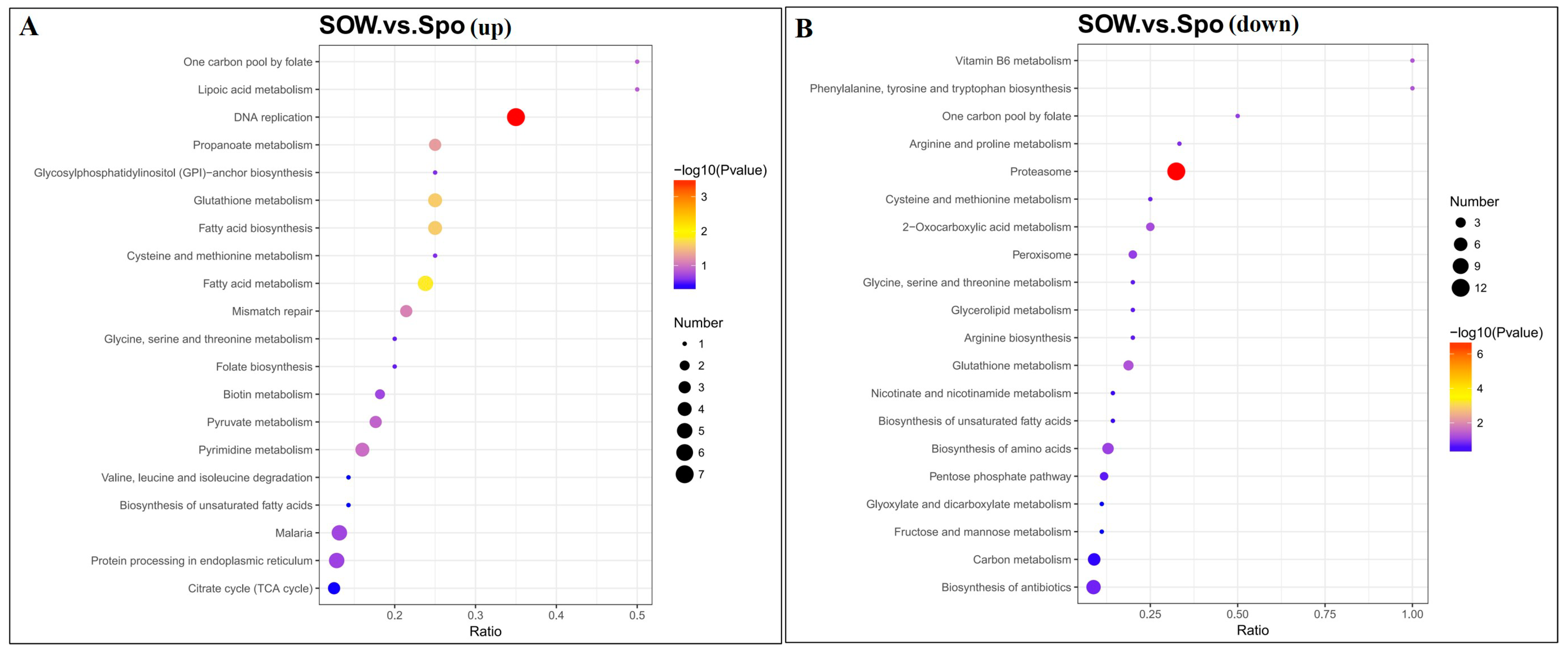
| Gene | Description | Average Abundance 1 | p Value | UP.DOWN |
|---|---|---|---|---|
| ETH_00015095 | alanine dehydrogenase | 5415 | 0.00158 | up |
| ETH_00030295 | glycan synthetase | 3486.767 | 2.87 × 10−5 | up |
| ETH_00000160 | acetyltransferase domain-containing protein | 3142.4 | 6.51 × 10−5 | up |
| ETH_00015875 | Peptidyl-prolyl cis-trans isomerase d-like protein | 3116.1 | 0.000129 | up |
| ETH_00041205 | dihydrolipoyl dehydrogenase protein | 3070.6 | 0.000281 | up |
| ETH_00037325 | protein disulfide-isomerase | 1961.8 | 0.000482 | up |
| ETH_00040975 | histidyl-tRNA synthetase | 1749.767 | 0.000109 | up |
| ETH_00028630 | CMGC kinase, CK2 family | 1739.9 | 0.000105 | up |
| ETH_00011615 | pseudouridylate synthase 1 | 1613 | 0.002113 | up |
| ETH_00020990 | pyruvate dehydrogenase | 1611.6 | 0.000588 | up |
| ETH_00036640 | alkyl sulfatase | 1519.633 | 0.011512 | up |
| ETH_00005785 | PKSN polyketide synthase for alternapyrone biosynthesis protein | 1288.9 | 0.000935 | up |
| ETH_00021530 | ribonucleotide-diphosphate reductase, small subunit | 714.9333 | 0.002011 | up |
| ETH_00001040 | AGC kinase | 694.7667 | 0.002582 | up |
| ETH_00026125 | bifunctional dihydrofolate reductase/thymidylate synthase | 668.4667 | 0.000152 | up |
| ETH_00035460 | Lipoyl synthase, chloroplastic | 551.5667 | 0.003634 | up |
| ETH_00023085 | SNF2 family helicase | 508.3 | 0.000468 | up |
| ETH_00028615 | AGC kinase | 465.6333 | 0.000302 | up |
| ETH_00005380 | CMGC kinase, MAPK family | 459.8333 | 0.000614 | up |
| ETH_00011460 | cytochrome c oxidase subunit | 449.5333 | 0.001913 | up |
| ETH_00019830 | Phosphorylase family protein | 389.9333 | 0.000733 | up |
| ETH_00006545 | N2,N2-dimethylguanosine tRNA methyltransferase | 357.8333 | 5.98 × 10−5 | up |
| ETH_00033505 | D-3-phosphoglycerate dehydrogenase | 290.8667 | 0.000928 | up |
| ETH_00029870 | 4-nitrophenylphosphatase | 143.8333 | 0.001741 | up |
| ETH_00002930 | gamma-glutamylcysteine synthetase | 80.03333 | 0.008645 | up |
| ETH_00035130 | Serine/threonine protein kinase | 10,300.33 | 0.000169 | up |
| ETH_00016690 | ribonucleoside-diphosphate reductase subunit M2 | 1412.4 | 0.00039 | up |
| ETH_00042280 | 5’-3’ exoribonuclease 2 | 528.5667 | 0.014544 | up |
| Gene | Description | Average Abundance 1 | Function |
|---|---|---|---|
| ETH_00005950 | Subtilisin-like protein | 4694.6 | serine-type endopeptidase activity, proteolysis |
| ETH_00002580 | ubiquitin carboxyl-terminal hydrolase isozyme L5 | 1556.633333 | thiol-dependent ubiquitin-specific protease activity |
| ETH_00006825 | Whole genome shotgun assembly, reference scaffold old set, scaffold scaffold_12 (subtilisin 4) | 896.4666667 | serine-type endopeptidase activity, proteolysis |
| ETH_00032295 | opine dehydrogenase | 6142.566667 | oxidoreductase activity |
| ETH_00027970 | TPR domain-containing protein | 6142.566667 | oxidoreductase activity |
| ETH_00041205 | dihydrolipoyl dehydrogenase protein | 3070.6 | oxidoreductase activity |
| ETH_00015485 | hypothetical protein | 3908.533333 | Flavin-dependent oxidoreductase, luciferase family |
| ETH_00033360 | oxidoreductase | 1971.266667 | oxidoreductase |
| ETH_00015480 | Equisetin synthetase | 113,994.1667 | Fatty acid metabolism |
| ETH_00009260 | acetyl-CoA carboxylase | 14,813.36667 | Fatty acid metabolism |
| ETH_00005790 | hypothetical protein | 10,591.33333 | Fatty acid metabolism |
| ETH_00002595 | 3-oxoacyl-(acyl-carrier-protein) synthase III family protein | 1044.933333 | Fatty acid metabolism |
| ETH_00005790 | 3-oxoacyl-[acyl-carrier-protein] synthase II | 10,591.33 | Fatty acid metabolism |
| ETH_00013640 | fatty acyl-CoA desaturase | 731.6666667 | Fatty acid metabolism |
| Gene | Description | Average Abundance 1 | p Value | UP.DOWN |
|---|---|---|---|---|
| ETH_00015095 | alanine dehydrogenase | 5415 | 0.00158 | up |
| ETH_00030295 | glycan synthetase | 3486.767 | 2.87 × 10−5 | up |
| ETH_00000160 | acetyltransferase domain-containing protein | 3142.4 | 6.51 × 10−5 | up |
| ETH_00015875 | Peptidyl-prolyl cis-trans isomerase d-like protein | 3116.1 | 0.000129 | up |
| ETH_00041205 | dihydrolipoyl dehydrogenase protein | 3070.6 | 0.000281 | up |
| ETH_00037325 | protein disulfide-isomerase | 1961.8 | 0.000482 | up |
| ETH_00040975 | histidyl-tRNA synthetase | 1749.767 | 0.000109 | up |
| ETH_00028630 | CMGC kinase, CK2 family | 1739.9 | 0.000105 | up |
| ETH_00011615 | pseudouridylate synthase 1 | 1613 | 0.002113 | up |
| ETH_00020990 | pyruvate dehydrogenase | 1611.6 | 0.000588 | up |
| ETH_00036640 | alkyl sulfatase | 1519.633 | 0.011512 | up |
| ETH_00005785 | PKSN polyketide synthase for alternapyrone biosynthesis protein | 1288.9 | 0.000935 | up |
| ETH_00021530 | ribonucleotide-diphosphate reductase, small subunit | 714.9333 | 0.002011 | up |
| ETH_00001040 | AGC kinase | 694.7667 | 0.002582 | up |
| ETH_00026125 | bifunctional dihydrofolate reductase / thymidylate synthase | 668.4667 | 0.000152 | up |
| ETH_00035460 | Lipoyl synthase, chloroplastic | 551.5667 | 0.003634 | up |
| ETH_00023085 | SNF2 family helicase | 508.3 | 0.000468 | up |
| ETH_00028615 | AGC kinase | 465.6333 | 0.000302 | up |
| ETH_00005380 | CMGC kinase, MAPK family | 459.8333 | 0.000614 | up |
| ETH_00011460 | cytochrome c oxidase subunit | 449.5333 | 0.001913 | up |
| ETH_00019830 | Phosphorylase family protein | 389.9333 | 0.000733 | up |
| ETH_00006545 | N2,N2-dimethylguanosine tRNA methyltransferase | 357.8333 | 5.98 × 10−5 | up |
| ETH_00033505 | D-3-phosphoglycerate dehydrogenase | 290.8667 | 0.000928 | up |
| ETH_00029870 | 4-nitrophenylphosphatase | 143.8333 | 0.001741 | up |
| ETH_00002930 | gamma-glutamylcysteine synthetase | 80.03333 | 0.008645 | up |
| ETH_00035130 | Serine/threonine protein kinase | 10300.33 | 0.000169 | up |
| ETH_00016690 | ribonucleoside-diphosphate reductase subunit M2 | 1412.4 | 0.00039 | up |
| ETH_00042280 | 5’-3’ exoribonuclease 2 | 528.5667 | 0.014544 | up |
| Gene | Description | Average Abundance 1 | p Value | UP.DOWN |
|---|---|---|---|---|
| ETH_00040825 | heat shock protein 90kDa beta | 8598.533 | 0.004669 | up |
| ETH_00028350 | GRA9 protein | 4837.967 | 0.000294 | up |
| ETH_00028100 | Eukaryotic translation initiation factor 3 subunit A | 2394.167 | 0.001605 | up |
| ETH_00019335 | protein antigen | 2165.633 | 0.000389 | up |
| ETH_00013135 | SAG family member | 81.63333 | 0.00222 | up |
| ETH_00014565 | elongation factor 1-alpha | 54.3 | 0.042969 | up |
| ETH_00012815 | hypothetical protein (AN/Apple domain) | 543.6333 | 0.002164 | up |
| ETH_00027460 | PAN domain-containing protein | 2479.267 | 0.001279 | up |
| ETH_00006825 | Whole genome shotgun assembly, reference scaffold old set, scaffold scaffold_12 (EGF-like calcium-binding domain) | 845.3667 | 0.001102 | up |
| ETH_00029115 | UDP-N-acetyl-D-galactosamine:polypeptide N-acetylgalactosaminyltransferase T2 (lectin domain) | 5568.333 | 0.000111 | up |
| Gene | Description | Average Abundance 1 | p Value | UP.DOWN |
|---|---|---|---|---|
| ETH_00008685 | SAG family member | 6059.2 | 9.77 × 10−5 | down |
| ETH_00017510 | heat shock protein | 4203.9 | 0.000845 | down |
| ETH_00021655 | Micronemal protein MIC4 | 66,229.83 | 0.000583 | down |
| ETH_00026625 | microneme protein 2 | 25,170.7 | 0.000718 | down |
| ETH_00024085 | Microneme protein 4 | 25,612.6 | 0.001731 | down |
| ETH_00021010 | microneme protein | 14,041.6 | 0.002008 | down |
| ETH_00006930 | Microneme protein Etmic-2 | 19,872.93 | 0.008696 | down |
| ETH_00007745 | apical membrane antigen-1 | 5813.8 | 8.97 × 10−5 | down |
| ETH_00017540 | microneme protein MIC3 | 2875.933 | 0.002346 | down |
| ETH_00000645 | thrombospondin type 1 domain-containing protein | 361.0667 | 0.006292 | down |
| ETH_00027625 | serine/threonine protein phosphatase | 1545.967 | 0.001134 | down |
| ETH_00024540 | calcium-dependent protein kinase | 7274.733 | 0.000642 | down |
| ETH_00011830 | Superoxide dismutase | 7096.533 | 0.00051 | down |
| ETH_00028715 | CMGC kinase, GSK family TgPK3 | 4870.3 | 0.000163 | down |
| Gene | Description | SOW.vs. Spo UP.DOWN |
|---|---|---|
| ETH_00006930 | Microneme protein Etmic-2 | down |
| ETH_00011330 | SERPIN1 protein | down |
| ETH_00007980 | 14-3-3 protein | NA |
| ETH_00017510 | heat shock protein | down |
| ETH_00009260 | acetyl-CoA carboxylase | up |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, L.; Zhao, Q.; Zhu, S.; Han, H.; Zhao, H.; Yu, Y.; Yang, J.; Dong, H. Proteomic Analysis of Fractionated Eimeria tenella Sporulated Oocysts Reveals Involvement in Oocyst Wall Formation. Int. J. Mol. Sci. 2023, 24, 17051. https://doi.org/10.3390/ijms242317051
Jia L, Zhao Q, Zhu S, Han H, Zhao H, Yu Y, Yang J, Dong H. Proteomic Analysis of Fractionated Eimeria tenella Sporulated Oocysts Reveals Involvement in Oocyst Wall Formation. International Journal of Molecular Sciences. 2023; 24(23):17051. https://doi.org/10.3390/ijms242317051
Chicago/Turabian StyleJia, Liushu, Qiping Zhao, Shunhai Zhu, Hongyu Han, Huanzhi Zhao, Yu Yu, Jia Yang, and Hui Dong. 2023. "Proteomic Analysis of Fractionated Eimeria tenella Sporulated Oocysts Reveals Involvement in Oocyst Wall Formation" International Journal of Molecular Sciences 24, no. 23: 17051. https://doi.org/10.3390/ijms242317051
APA StyleJia, L., Zhao, Q., Zhu, S., Han, H., Zhao, H., Yu, Y., Yang, J., & Dong, H. (2023). Proteomic Analysis of Fractionated Eimeria tenella Sporulated Oocysts Reveals Involvement in Oocyst Wall Formation. International Journal of Molecular Sciences, 24(23), 17051. https://doi.org/10.3390/ijms242317051







