Osteogenic Activities of Trifolirhizin as a Bioactive Compound for the Differentiation of Osteogenic Cells
Abstract
:1. Introduction
2. Results
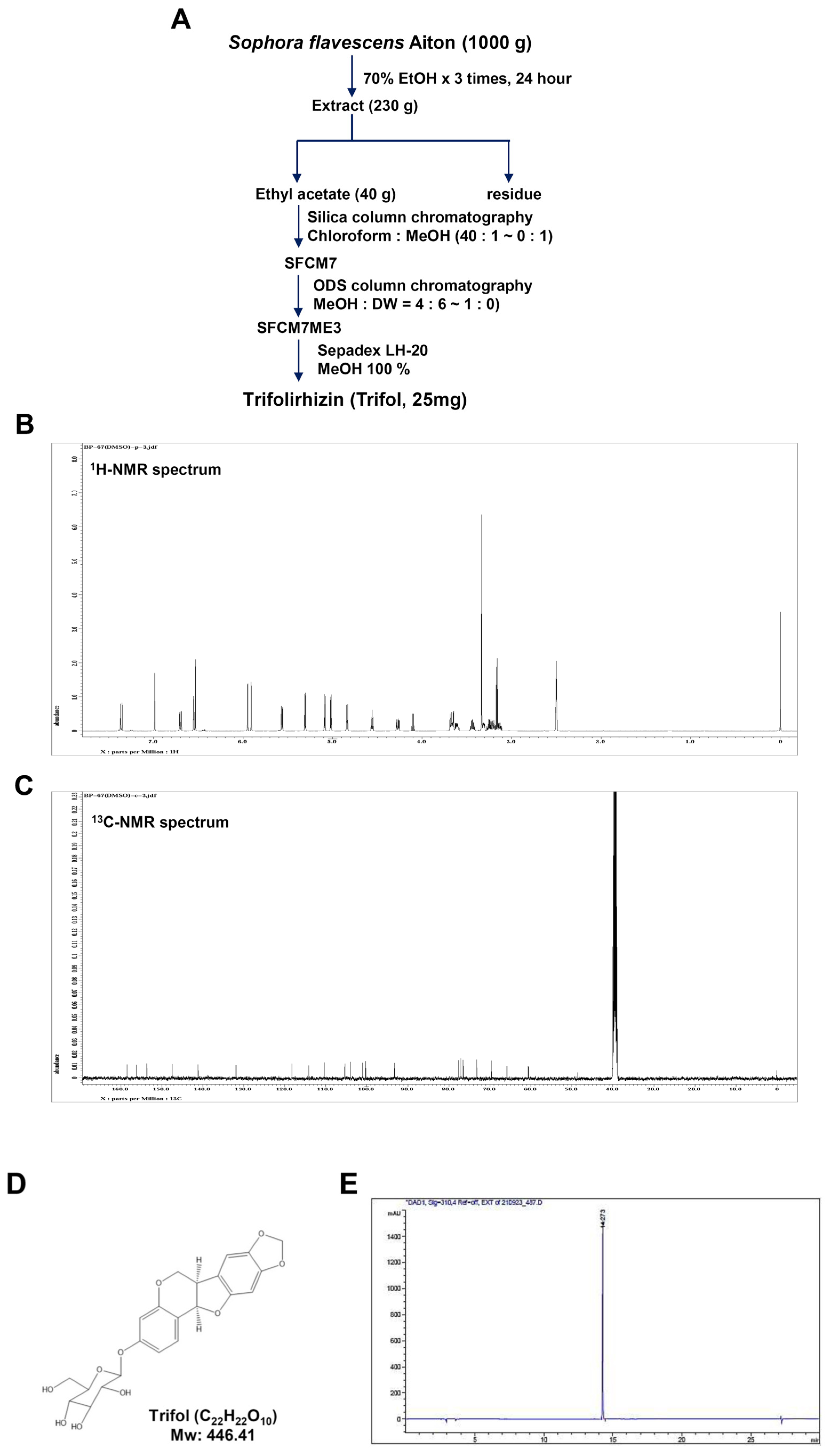
2.1. Isolation and Characterization of Trifol from the Root of Sophora flavescens
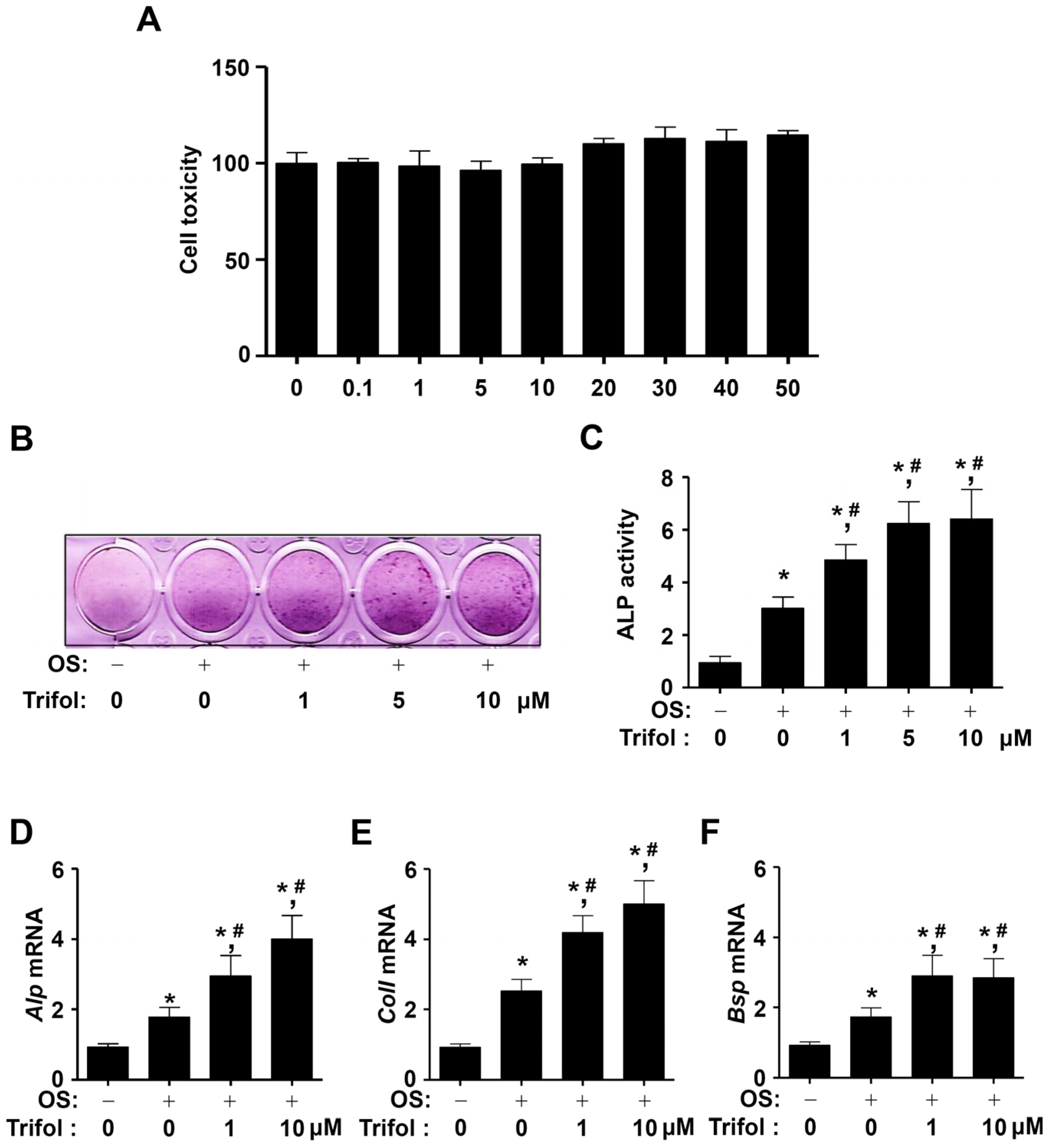
2.2. Trifol Increases Osteoblast Differentiation and the Levels of Osteogenic Marker Genes
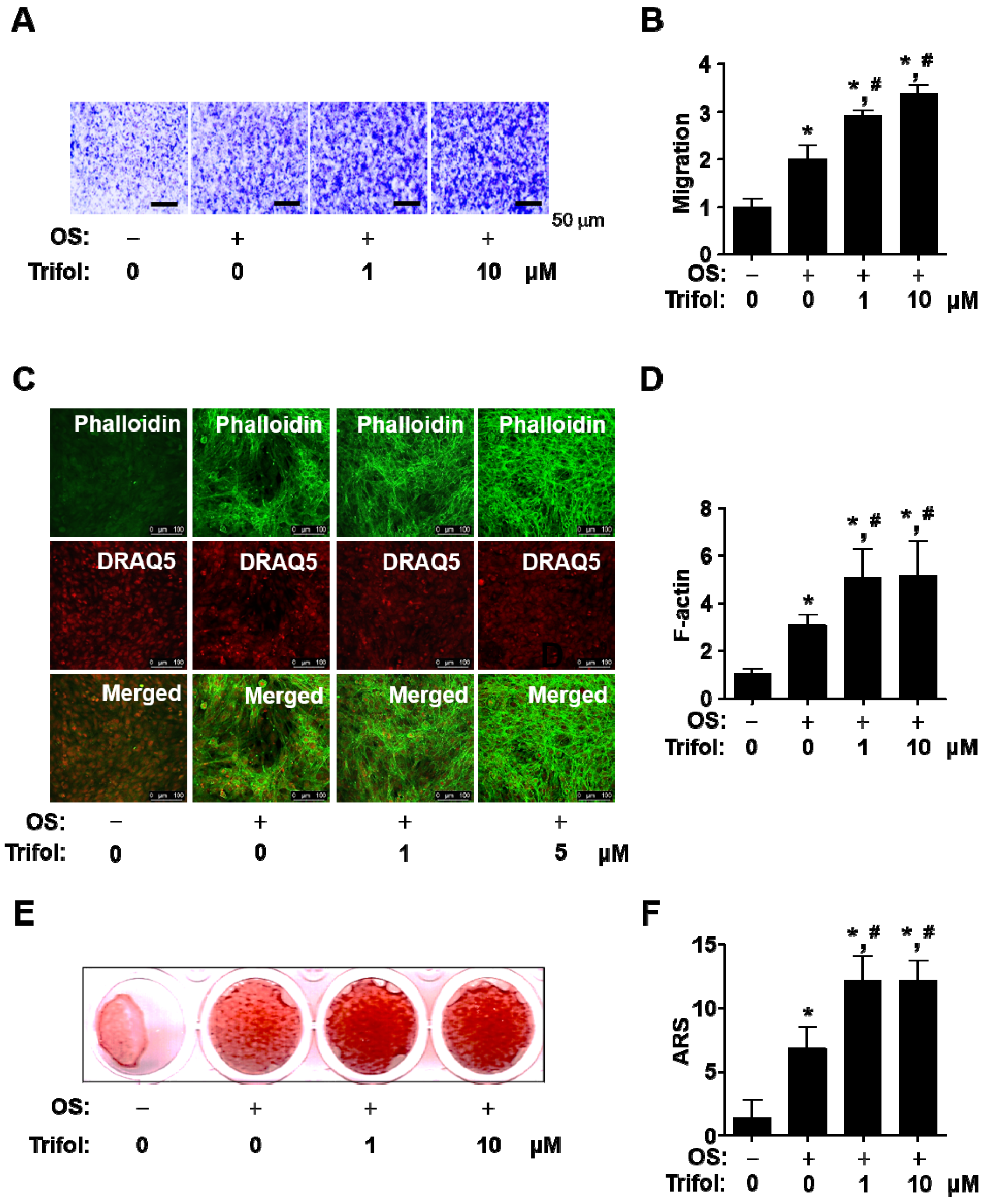
2.3. Trifol Enhances Osteogenic Phenotype and Mineralization in Osteoblast Differentiation
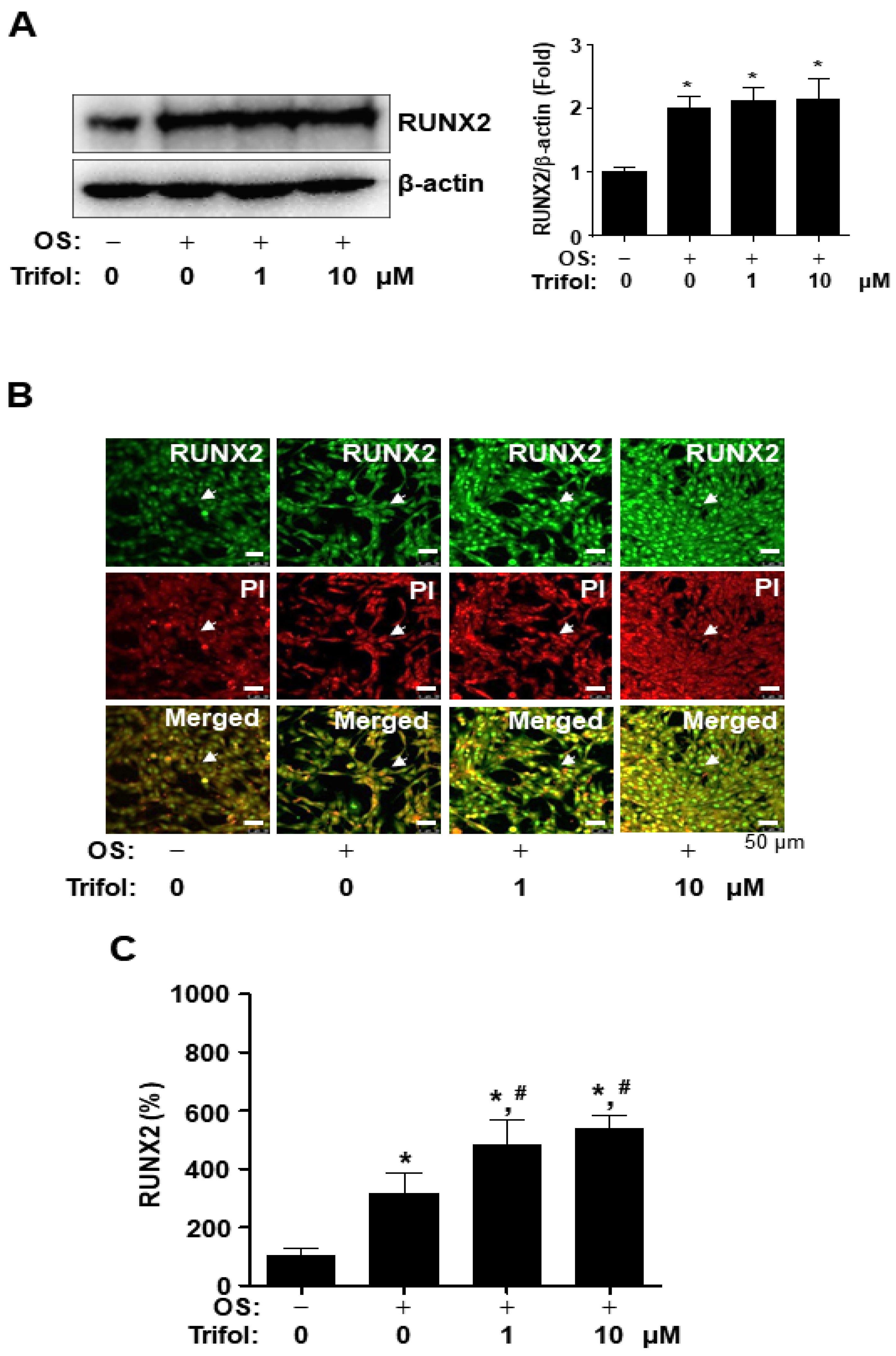
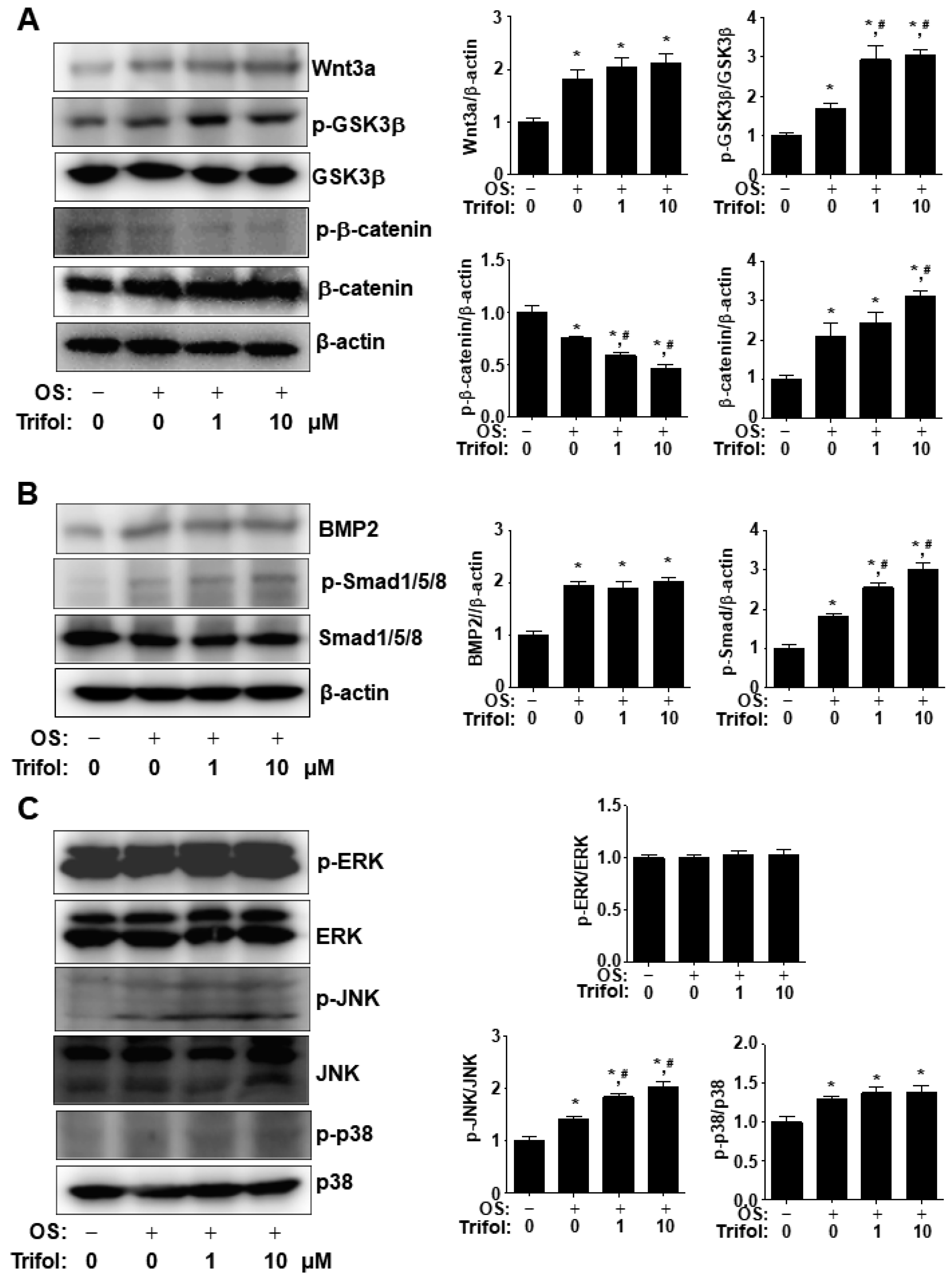
2.4. Trifol Enhances Nuclear RUNX2 Expression and Osteogenic Signaling Proteins in Osteoblast Differentiation
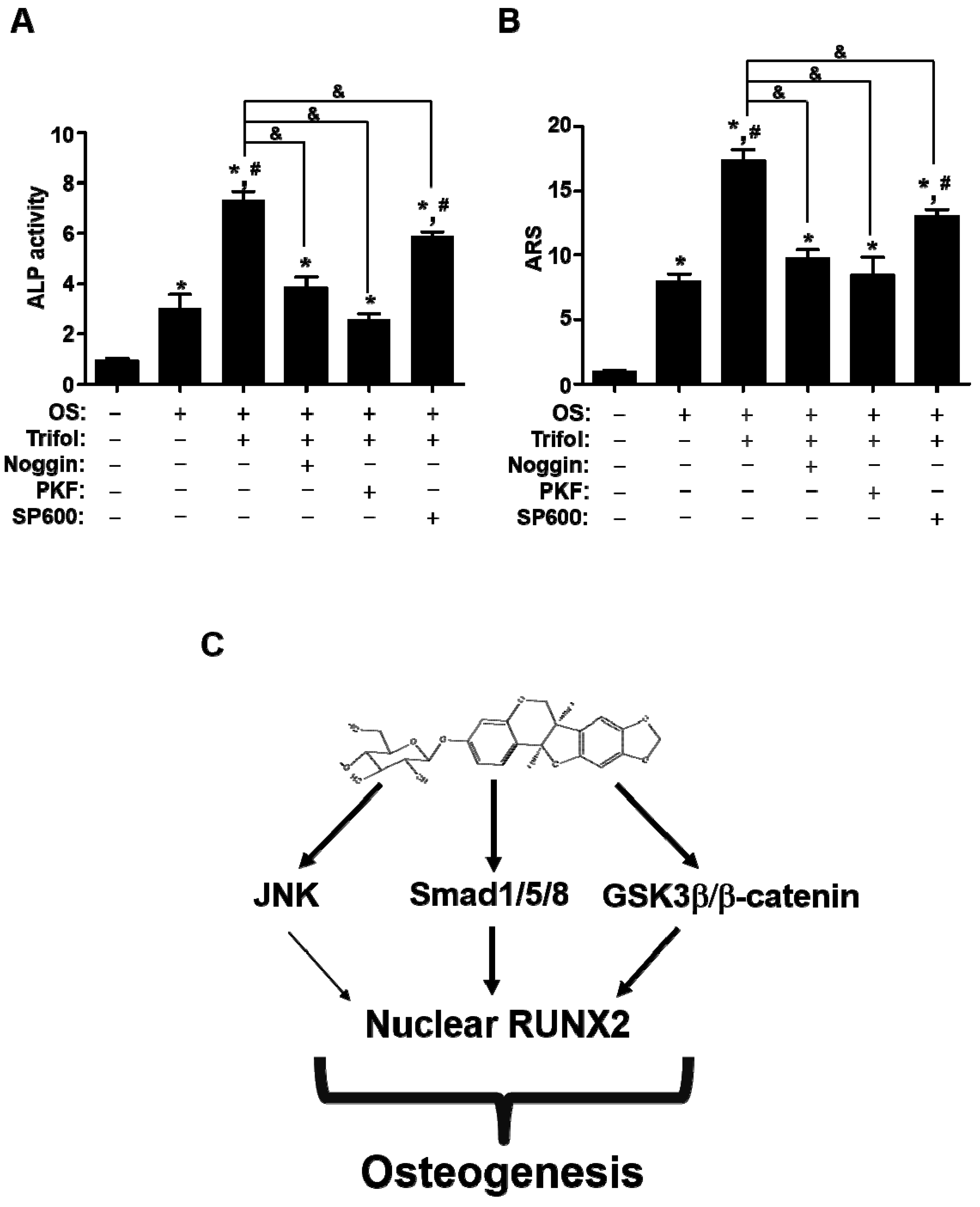
2.5. Trifol Enhances Osteoblast Differentiation through the Stimulation of Smad1/5/8 and β-Catenin
3. Discussion
4. Materials and Methods
4.1. Plant Material and Isolation
4.2. Cell Culture and Trifol Stock Solution
4.3. Cytotoxicity
4.4. ALP Staining and Activity Assays
4.5. Total RNA Isolation and RT-PCR Analysis
4.6. Western Blot Analysis
4.7. Immunofluorescence Assay
4.8. Migration Assay
4.9. Phalloidin and DRAQ5 Staining
4.10. ARS Staining Assay
4.11. Inhibitors
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALP | Alkaline phosphatase |
| ARS | Alizarin Red S. |
| BMP2 | Bone morphogenetic protein 2 |
| BSP | Bone sialoprotein |
| ColI | Collagen Type I |
| DMSO | Dimethyl sulfoxide |
| ECM | Extracellular matrix |
| HPLC | High performance liquid chromatography |
| L-AA | L-ascorbic acid |
| MAPKs | Mitogen-activated protein kinases. |
| MTT | 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide. |
| NMR | Nuclear magnetic resonance |
| OS | Osteogenic supplement medium. |
| RUNX2 | Runt-related transcription factor 2. |
| TBST | Tris buffered saline, with Tween 20 |
| Trifol TriFs | Trifolirhizin Trifloroside |
| Wnt3a | Wnt family member 3a |
References
- Infante, A.; Rodriguez, C.I. Osteogenesis and aging: Lessons from mesenchymal stem cells. Stem Cell Res. Ther. 2018, 9, 244. [Google Scholar] [CrossRef] [PubMed]
- Marie, P.J. Osteoblast dysfunctions in bone diseases: From cellular and molecular mechanisms to therapeutic strategies. Cell Mol. Life Sci. 2015, 72, 1347–1361. [Google Scholar] [CrossRef] [PubMed]
- Fakhry, M.; Hamade, E.; Badran, B.; Buchet, R.; Magne, D. Molecular mechanisms of mesenchymal stem cell differentiation towards osteoblasts. World J. Stem Cells 2013, 5, 136–148. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Kronenberg, H.M. Overview of Skeletal Development. Methods Mol. Biol. 2021, 2230, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, T.; Jiang, M.; Yin, X.; Luo, X.; Sun, H. Effect of the immune responses induced by implants in a integrated three-dimensional micro-nano topography on osseointegration. J. Biomed. Mater. Res. A 2021, 109, 1429–1440. [Google Scholar] [CrossRef]
- Park, K.R.; Kim, S.; Cho, M.; Yun, H.M. Limonoid Triterpene, Obacunone Increases Runt-Related Transcription Factor 2 to Promote Osteoblast Differentiation and Function. Int. J. Mol. Sci. 2021, 22, 2483. [Google Scholar] [CrossRef]
- Rosenberg, N.; Rosenberg, O.; Soudry, M. Osteoblasts in bone physiology-mini review. Rambam Maimonides Med. J. 2012, 3, e0013. [Google Scholar] [CrossRef]
- Khotib, J.; Gani, M.A.; Budiatin, A.S.; Lestari, M.; Rahadiansyah, E.; Ardianto, C. Signaling Pathway and Transcriptional Regulation in Osteoblasts during Bone Healing: Direct Involvement of Hydroxyapatite as a Biomaterial. Pharmaceuticals 2021, 14, 615. [Google Scholar] [CrossRef]
- Park, K.R.; Lee, J.Y.; Cho, M.; Hong, J.T.; Yun, H.M. Biological Mechanisms of Paeonoside in the Differentiation of Pre-Osteoblasts and the Formation of Mineralized Nodules. Int. J. Mol. Sci. 2021, 22, 6899. [Google Scholar] [CrossRef]
- Chen, G.; Deng, C.; Li, Y.P. TGF-beta and BMP signaling in osteoblast differentiation and bone formation. Int. J. Biol. Sci. 2012, 8, 272–288. [Google Scholar] [CrossRef]
- Martiniakova, M.; Babikova, M.; Omelka, R. Pharmacological agents and natural compounds: Available treatments for osteoporosis. J. Physiol. Pharmacol. 2020, 71, 307–320. [Google Scholar] [CrossRef]
- Yuan, H.; Ma, Q.; Ye, L.; Piao, G. The Traditional Medicine and Modern Medicine from Natural Products. Molecules 2016, 21, 559. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.M.; Park, M.H.; Yun, H.M.; Han, S.B.; Oh, K.W.; Son, D.J.; Yun, J.S.; Hong, J.T. CCR5 knockout suppresses experimental autoimmune encephalomyelitis in C57BL/6 mice. Oncotarget 2016, 7, 15382–15393. [Google Scholar] [CrossRef] [PubMed]
- Fabricant, D.S.; Farnsworth, N.R. The value of plants used in traditional medicine for drug discovery. Environ. Health Perspect. 2001, 109 (Suppl. S1), 69–75. [Google Scholar] [CrossRef]
- Sun, P.; Zhao, W.; Wang, Q.; Chen, L.; Sun, K.; Zhan, Z.; Wang, J. Chemical diversity, biological activities and Traditional uses of and important Chinese herb Sophora. Phytomedicine 2022, 100, 154054. [Google Scholar] [CrossRef]
- Wei, G.; Chen, Y.; Guo, X.; Wei, J.; Dong, L.; Chen, S. Biosyntheses characterization of alkaloids and flavonoids in Sophora flavescens by combining metabolome and transcriptome. Sci. Rep. 2021, 11, 7388. [Google Scholar] [CrossRef]
- Zhang, J.H.; Zhao, Y.Y.; Liu, Q.X.; Ye, X.J. Studies on the chemical constituents from Sophora flavescens ait. Zhongguo Zhong Yao Za Zhi 2000, 25, 37–39. [Google Scholar]
- Zhang, L.; Xu, L.; Xiao, S.S.; Liao, Q.F.; Li, Q.; Liang, J.; Chen, X.H.; Bi, K.S. Characterization of flavonoids in the extract of Sophora flavescens Ait. by high-performance liquid chromatography coupled with diode-array detector and electrospray ionization mass spectrometry. J. Pharm. Biomed. Anal. 2007, 44, 1019–1028. [Google Scholar] [CrossRef]
- Huang, R.; Liu, Y.; Zhao, L.L.; Chen, X.X.; Wang, F.; Cai, W.; Chen, L. A new flavonoid from Sophora flavescens Ait. Nat. Prod. Res. 2017, 31, 2228–2232. [Google Scholar] [CrossRef]
- Huang, X.B.; Yuan, L.W.; Shao, J.; Yang, Y.; Liu, Y.; Lu, J.J.; Chen, L. Cytotoxic effects of flavonoids from root of Sophora flavescens in cancer cells. Nat. Prod. Res. 2021, 35, 4317–4322. [Google Scholar] [CrossRef]
- Jin, J.H.; Kim, J.S.; Kang, S.S.; Son, K.H.; Chang, H.W.; Kim, H.P. Anti-inflammatory and anti-arthritic activity of total flavonoids of the roots of Sophora flavescens. J. Ethnopharmacol. 2010, 127, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yang, J.; Xu, C.; Huang, M.; Zhou, Q.; Lv, J.; Ma, X.; Ke, C.; Ye, Y.; Shu, G.; et al. Antidiabetic effects of flavonoids from Sophora flavescens EtOAc extract in type 2 diabetic KK-ay mice. J. Ethnopharmacol. 2015, 171, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zeng, W.; Ma, C.; Wang, Z.; Wang, C.; Li, S.; He, W.; Zhang, Q.; Xu, J.; Zhou, C. Maackiain dampens osteoclastogenesis via attenuating RANKL-stimulated NF-kappaB signalling pathway and NFATc1 activity. J. Cell Mol. Med. 2020, 24, 12308–12317. [Google Scholar] [CrossRef]
- Chiou, W.F.; Lee, C.H.; Liao, J.F.; Chen, C.C. 8-Prenylkaempferol accelerates osteoblast maturation through bone morphogenetic protein-2/p38 pathway to activate Runx2 transcription. Life Sci. 2011, 88, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Tao, W.; Zhang, F.; Shen, W.; Tan, J.; Li, L.; Meng, Q.; Chen, Y.; Yang, Y.; Cheng, H. Trifolirhizin induces autophagy-dependent apoptosis in colon cancer via AMPK/mTOR signaling. Signal Transduct. Target. Ther. 2020, 5, 174. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, S.; Ji, S. Trifolirhizin regulates the balance of Th17/Treg cells and inflammation in the ulcerative colitis mice through inhibiting the TXNIP-mediated activation of NLRP3 inflammasome. Clin. Exp. Pharmacol. Physiol. 2022, 49, 787–796. [Google Scholar] [CrossRef]
- Zhou, H.; Lutterodt, H.; Cheng, Z.; Yu, L.L. Anti-Inflammatory and antiproliferative activities of trifolirhizin, a flavonoid from Sophora flavescens roots. J. Agric. Food Chem. 2009, 57, 4580–4585. [Google Scholar] [CrossRef]
- Aratanechemuge, Y.; Hibasami, H.; Katsuzaki, H.; Imai, K.; Komiya, T. Induction of apoptosis by maackiain and trifolirhizin (maackiain glycoside) isolated from sanzukon (Sophora Subprostrate Chen et T. Chen) in human promyelotic leukemia HL-60 cells. Oncol. Rep. 2004, 12, 1183–1188. [Google Scholar] [CrossRef]
- Hyun, S.K.; Lee, W.H.; Jeong, D.M.; Kim, Y.; Choi, J.S. Inhibitory effects of kurarinol, kuraridinol, and trifolirhizin from Sophora flavescens on tyrosinase and melanin synthesis. Biol. Pharm. Bull. 2008, 31, 154–158. [Google Scholar] [CrossRef]
- Histing, T.; Stenger, D.; Kuntz, S.; Scheuer, C.; Tami, A.; Garcia, P.; Holstein, J.H.; Klein, M.; Pohlemann, T.; Menger, M.D. Increased osteoblast and osteoclast activity in female senescence-accelerated, osteoporotic SAMP6 mice during fracture healing. J. Surg. Res. 2012, 175, 271–277. [Google Scholar] [CrossRef]
- Zayzafoon, M. Calcium/calmodulin signaling controls osteoblast growth and differentiation. J. Cell Biochem. 2006, 97, 56–70. [Google Scholar] [CrossRef] [PubMed]
- Broz, A.; Ukraintsev, E.; Kromka, A.; Rezek, B.; Hubalek Kalbacova, M. Osteoblast adhesion, migration, and proliferation variations on chemically patterned nanocrystalline diamond films evaluated by live-cell imaging. J. Biomed. Mater. Res. A 2017, 105, 1469–1478. [Google Scholar] [CrossRef] [PubMed]
- Tome, M.; Lopez-Romero, P.; Albo, C.; Sepulveda, J.C.; Fernandez-Gutierrez, B.; Dopazo, A.; Bernad, A.; Gonzalez, M.A. miR-335 orchestrates cell proliferation, migration and differentiation in human mesenchymal stem cells. Cell Death Differ. 2011, 18, 985–995. [Google Scholar] [CrossRef] [PubMed]
- Deng, T.; Zhang, W.; Zhang, Y.; Zhang, M.; Huan, Z.; Yu, C.; Zhang, X.; Wang, Y.; Xu, J. Thyroid-stimulating hormone decreases the risk of osteoporosis by regulating osteoblast proliferation and differentiation. BMC Endocr. Disord. 2021, 21, 49. [Google Scholar] [CrossRef]
- Shalehin, N.; Hosoya, A.; Takebe, H.; Hasan, M.R.; Irie, K. Boric acid inhibits alveolar bone loss in rat experimental periodontitis through diminished bone resorption and enhanced osteoblast formation. J. Dent. Sci. 2020, 15, 437–444. [Google Scholar] [CrossRef]
- Mishra, B.B.; Tiwari, V.K. Natural products: An evolving role in future drug discovery. Eur. J. Med. Chem. 2011, 46, 4769–4807. [Google Scholar] [CrossRef]
- An, J.; Yang, H.; Zhang, Q.; Liu, C.; Zhao, J.; Zhang, L.; Chen, B. Natural products for treatment of osteoporosis: The effects and mechanisms on promoting osteoblast-mediated bone formation. Life Sci. 2016, 147, 46–58. [Google Scholar] [CrossRef]
- Soelaiman, I.N.; Das, S.; Shuid, A.N.; Mo, H.; Mohamed, N. Use of medicinal plants and natural products for treatment of osteoporosis and its complications. Evid. Based Complement. Alternat Med. 2013, 2013, 764701. [Google Scholar] [CrossRef]
- Liang, J.; Bao, A.L.; Ma, H.Y.; Dong, W.; Li, W.H.; Wu, X.; Li, H.Y.; Hou, H.Y.; Chen, Y.Q.; Fu, J.L.; et al. Prevention of polycystic ovary syndrome and postmenopausal osteoporosis by inhibiting apoptosis with Shenling Baizhu powder compound. PeerJ 2022, 10, e13939. [Google Scholar] [CrossRef]
- Xue, C.; Pan, W.; Lu, X.; Guo, J.; Xu, G.; Sheng, Y.; Yuan, G.; Zhao, N.; Sun, J.; Guo, X.; et al. Effects of compound deer bone extract on osteoporosis model mice and intestinal microflora. J. Food Biochem. 2021, 45, e13740. [Google Scholar] [CrossRef]
- Di, Y.; Wasan, E.K.; Cawthray, J.; Syeda, J.; Ali, M.; Cooper, D.M.L.; Al-Dissi, A.; Ashjaee, N.; Cheng, W.; Johnston, J.; et al. Evaluation of La(XT), a novel lanthanide compound, in an OVX rat model of osteoporosis. Bone Rep. 2021, 14, 100753. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Shi, X.L.; Xu, C.; Wu, L.G.; He, B.; Li, Y.H.; Liang, B.C. Mechanism action of Chinese herbal compound and target network pharmacology of Yougui (YG) pill for the treatment of osteoporosis. Zhongguo Gu Shang 2020, 33, 933–937. [Google Scholar] [CrossRef] [PubMed]
- Bernardini, S.; Tiezzi, A.; Laghezza Masci, V.; Ovidi, E. Natural products for human health: An historical overview of the drug discovery approaches. Nat. Prod. Res. 2018, 32, 1926–1950. [Google Scholar] [CrossRef] [PubMed]
- Orimo, H. The mechanism of mineralization and the role of alkaline phosphatase in health and disease. J. Nippon. Med. Sch. 2010, 77, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Wennberg, C.; Hessle, L.; Lundberg, P.; Mauro, S.; Narisawa, S.; Lerner, U.H.; Millan, J.L. Functional characterization of osteoblasts and osteoclasts from alkaline phosphatase knockout mice. J. Bone Miner. Res. 2000, 15, 1879–1888. [Google Scholar] [CrossRef]
- Lin, X.; Patil, S.; Gao, Y.G.; Qian, A. The Bone Extracellular Matrix in Bone Formation and Regeneration. Front. Pharmacol. 2020, 11, 757. [Google Scholar] [CrossRef]
- Ogata, Y. Bone sialoprotein and its transcriptional regulatory mechanism. J. Periodontal Res. 2008, 43, 127–135. [Google Scholar] [CrossRef]
- Amarasekara, D.S.; Kim, S.; Rho, J. Regulation of Osteoblast Differentiation by Cytokine Networks. Int. J. Mol. Sci. 2021, 22, 2851. [Google Scholar] [CrossRef]
- Schroeder, T.M.; Jensen, E.D.; Westendorf, J.J. Runx2: A master organizer of gene transcription in developing and maturing osteoblasts. Birth Defects Res. C Embryo Today 2005, 75, 213–225. [Google Scholar] [CrossRef]
- Komori, T. Regulation of bone development and extracellular matrix protein genes by RUNX2. Cell Tissue Res. 2010, 339, 189–195. [Google Scholar] [CrossRef]
- Huang, R.L.; Yuan, Y.; Tu, J.; Zou, G.M.; Li, Q. Opposing TNF-alpha/IL-1beta- and BMP-2-activated MAPK signaling pathways converge on Runx2 to regulate BMP-2-induced osteoblastic differentiation. Cell Death Dis. 2014, 5, e1187. [Google Scholar] [CrossRef] [PubMed]
- Yun, H.M.; Park, K.R.; Quang, T.H.; Oh, H.; Hong, J.T.; Kim, Y.C.; Kim, E.C. 2,4,5-Trimethoxyldalbergiquinol promotes osteoblastic differentiation and mineralization via the BMP and Wnt/beta-catenin pathway. Cell Death Dis. 2015, 6, e1819. [Google Scholar] [CrossRef] [PubMed]
- Phimphilai, M.; Zhoa, Z.R.; Boules, H.; Roca, H.; Franceschi, R.T. BMP signaling is required for RUNX2-dependent induction of the osteoblast phenotype. J. Bone Miner. Res. 2006, 21, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Granero-Molto, F.; Weis, J.A.; Miga, M.I.; Landis, B.; Myers, T.J.; O’Rear, L.; Longobardi, L.; Jansen, E.D.; Mortlock, D.P.; Spagnoli, A. Regenerative effects of transplanted mesenchymal stem cells in fracture healing. Stem Cells 2009, 27, 1887–1898. [Google Scholar] [CrossRef] [PubMed]
- Delaisse, J.M. The reversal phase of the bone-remodeling cycle: Cellular prerequisites for coupling resorption and formation. Bonekey Rep. 2014, 3, 561. [Google Scholar] [CrossRef]
- Kalbacova, M.; Broz, A.; Kong, J.; Kalbac, M. Graphene substrates promote adherence of human osteoblasts and mesenchymal stromal cells. Carbon 2010, 48, 4323–4329. [Google Scholar] [CrossRef]
- Aryaei, A.; Jayatissa, A.H.; Jayasuriya, A.C. The effect of graphene substrate on osteoblast cell adhesion and proliferation. J. Biomed. Mater. Res. Part A 2014, 102, 3282–3290. [Google Scholar] [CrossRef]
- Tong, Z.; Liu, Y.; Xia, R.; Chang, Y.; Hu, Y.; Liu, P.; Zhai, Z.; Zhang, J.; Li, H. F-actin Regulates Osteoblastic Differentiation of Mesenchymal Stem Cells on TiO2 Nanotubes Through MKL1 and YAP/TAZ. Nanoscale Res. Lett. 2020, 15, 183. [Google Scholar] [CrossRef]
- Xue, X.; Hong, X.; Li, Z.; Deng, C.X.; Fu, J. Acoustic tweezing cytometry enhances osteogenesis of human mesenchymal stem cells through cytoskeletal contractility and YAP activation. Biomaterials 2017, 134, 22–30. [Google Scholar] [CrossRef]
- Wozney, J.M.; Rosen, V.; Celeste, A.J.; Mitsock, L.M.; Whitters, M.J.; Kriz, R.W.; Hewick, R.M.; Wang, E.A. Novel regulators of bone formation: Molecular clones and activities. Science 1988, 242, 1528–1534. [Google Scholar] [CrossRef]
- Krishnan, V.; Bryant, H.U.; MacDougald, O.A. Regulation of bone mass by Wnt signaling. J. Clin. Investig. 2006, 116, 1202–1209. [Google Scholar] [CrossRef] [PubMed]
- Canalis, E.; Economides, A.N.; Gazzerro, E. Bone morphogenetic proteins, their antagonists, and the skeleton. Endocr. Rev. 2003, 24, 218–235. [Google Scholar] [CrossRef] [PubMed]
- Reya, T.; Clevers, H. Wnt signalling in stem cells and cancer. Nature 2005, 434, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Gaur, T.; Lengner, C.J.; Hovhannisyan, H.; Bhat, R.A.; Bodine, P.V.; Komm, B.S.; Javed, A.; van Wijnen, A.J.; Stein, J.L.; Stein, G.S.; et al. Canonical WNT signaling promotes osteogenesis by directly stimulating Runx2 gene expression. J. Biol. Chem. 2005, 280, 33132–33140. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Ma, J.; Qiu, H.; Yang, L.; Cao, L.; Shen, J. Anti-proliferation effects of trifolirhizin on MKN45 cells and possible mechanism. Oncol. Rep. 2016, 36, 2785–2792. [Google Scholar] [CrossRef]
- Yun, H.M.; Kim, B.; Park, J.E.; Park, K.R. Trifloroside Induces Bioactive Effects on Differentiation, Adhesion, Migration, and Mineralization in Pre-Osteoblast MC3T3E-1 Cells. Cells 2022, 11, 3887. [Google Scholar] [CrossRef]
- Yun, H.M.; Kim, B.; Jeong, Y.H.; Hong, J.T.; Park, K.R. Suffruticosol A elevates osteoblast differentiation targeting BMP2-Smad/1/5/8-RUNX2 in pre-osteoblasts. Biofactors 2023, 49, 127–139. [Google Scholar] [CrossRef]
- Park, K.R.; Park, J.E.; Kim, B.; Kwon, I.K.; Hong, J.T.; Yun, H.M. Calycosin-7-O-beta-Glucoside Isolated from Astragalus membranaceus Promotes Osteogenesis and Mineralization in Human Mesenchymal Stem Cells. Int. J. Mol. Sci. 2021, 22, 11362. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yun, H.-M.; Cho, M.H.; Jeong, H.; Kim, S.H.; Jeong, Y.H.; Park, K.-R. Osteogenic Activities of Trifolirhizin as a Bioactive Compound for the Differentiation of Osteogenic Cells. Int. J. Mol. Sci. 2023, 24, 17103. https://doi.org/10.3390/ijms242317103
Yun H-M, Cho MH, Jeong H, Kim SH, Jeong YH, Park K-R. Osteogenic Activities of Trifolirhizin as a Bioactive Compound for the Differentiation of Osteogenic Cells. International Journal of Molecular Sciences. 2023; 24(23):17103. https://doi.org/10.3390/ijms242317103
Chicago/Turabian StyleYun, Hyung-Mun, Mi Hyeon Cho, Hoibin Jeong, Soo Hyun Kim, Yun Hee Jeong, and Kyung-Ran Park. 2023. "Osteogenic Activities of Trifolirhizin as a Bioactive Compound for the Differentiation of Osteogenic Cells" International Journal of Molecular Sciences 24, no. 23: 17103. https://doi.org/10.3390/ijms242317103




