Effects of Elevated Temperature on Pisum sativum Nodule Development: I—Detailed Characteristic of Unusual Apical Senescence
Abstract
:1. Introduction
2. Results
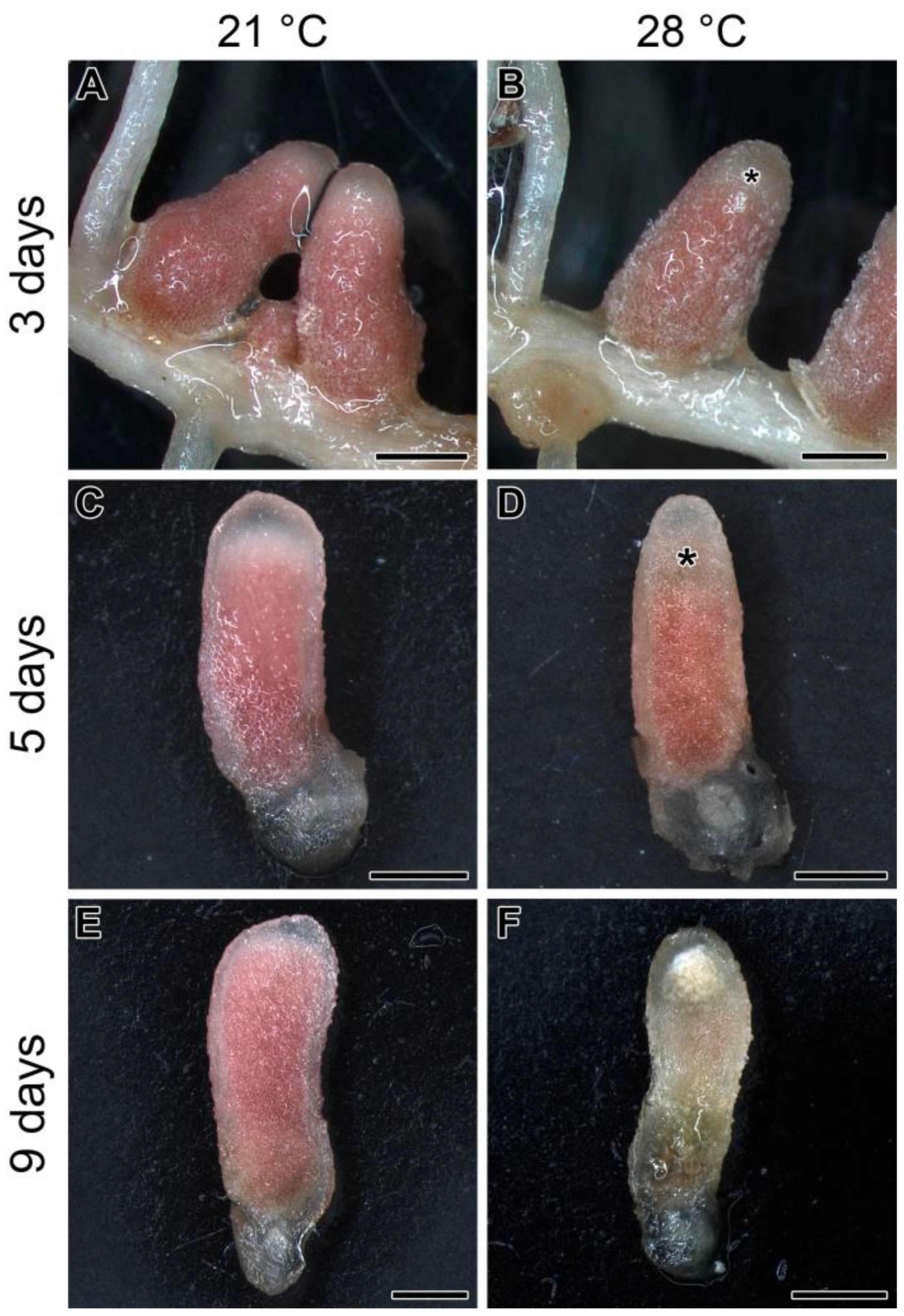
2.1. Elevated Temperature in Pea Line SGE Leads to Apical Nodule Senescence
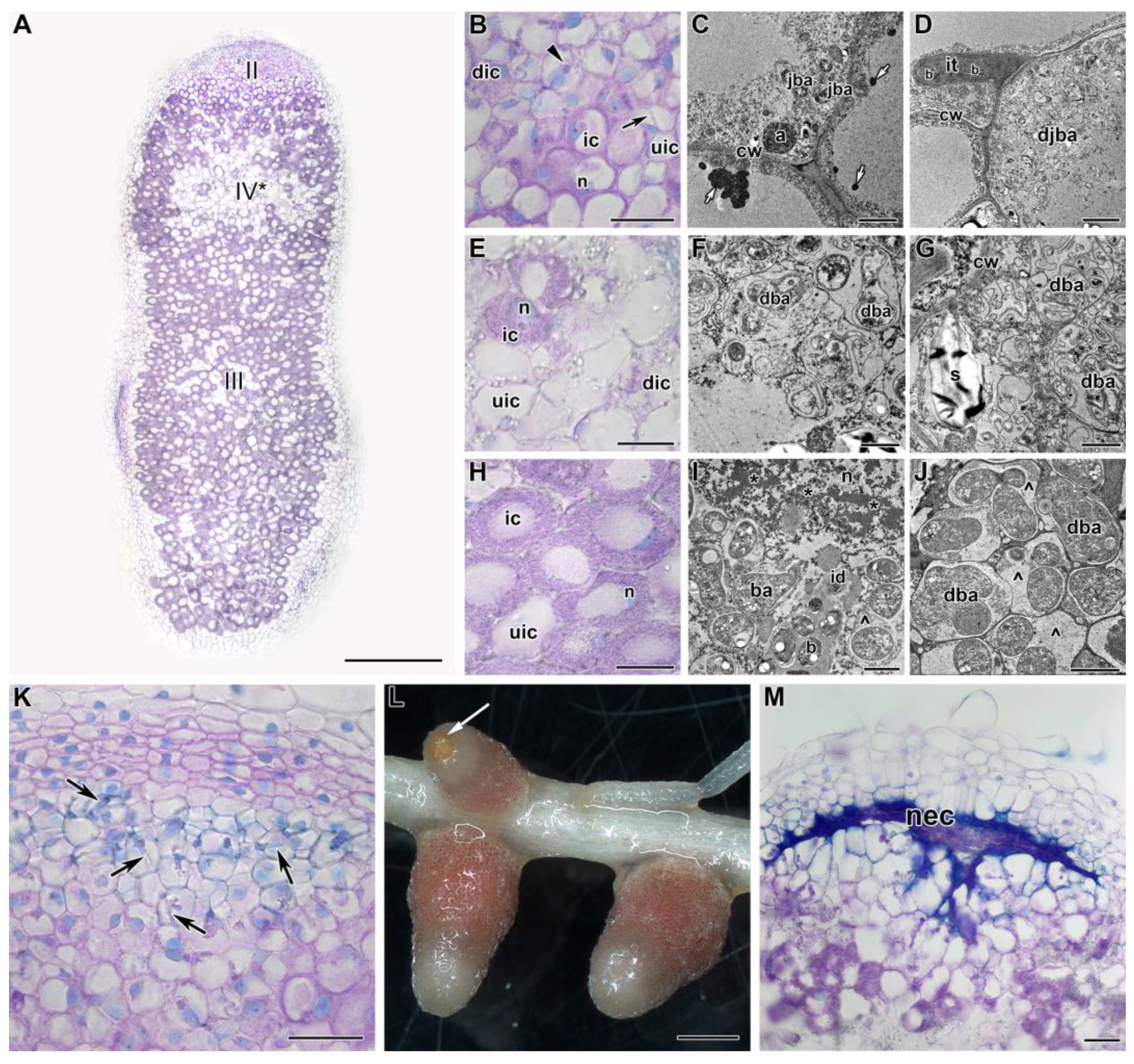
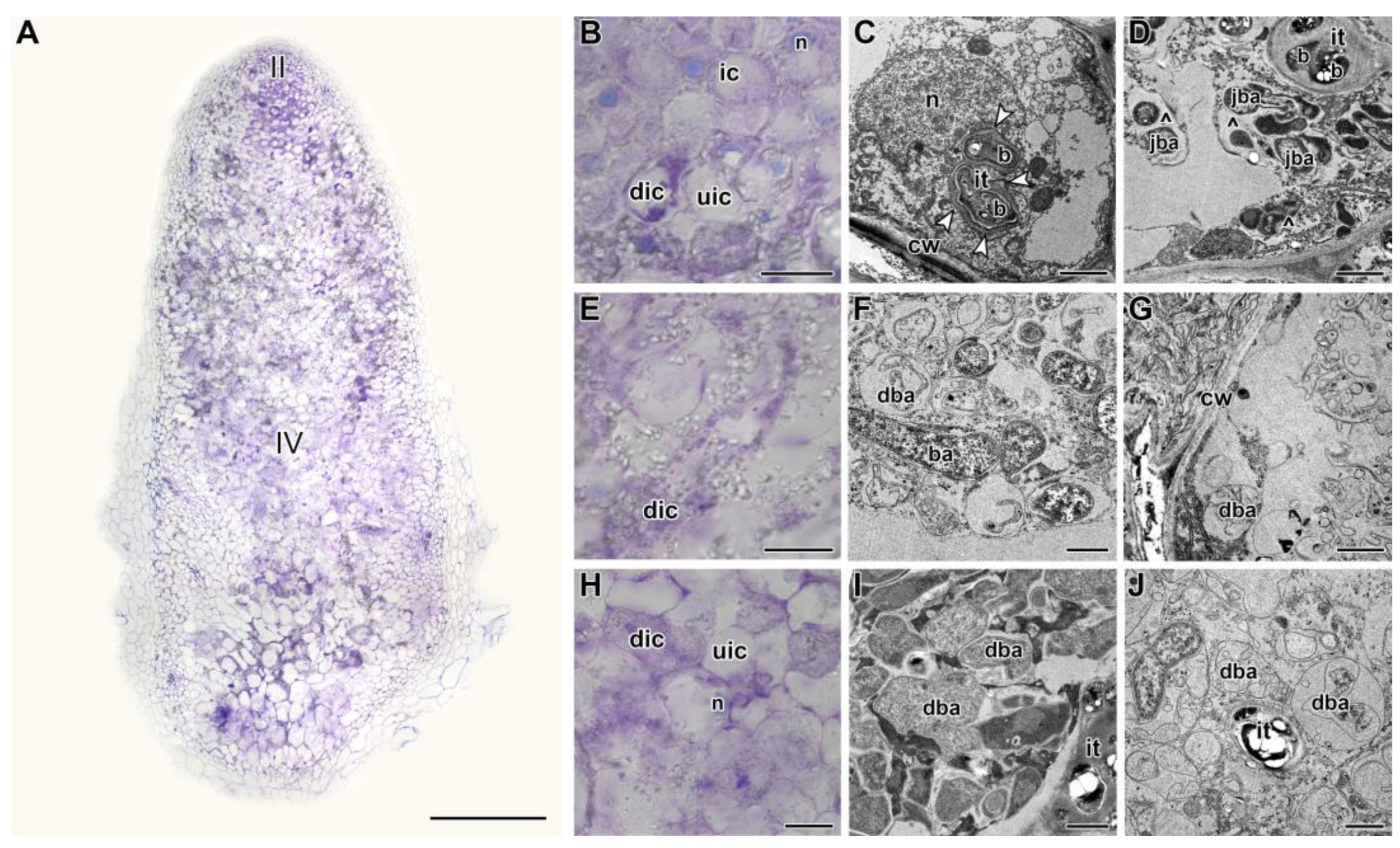
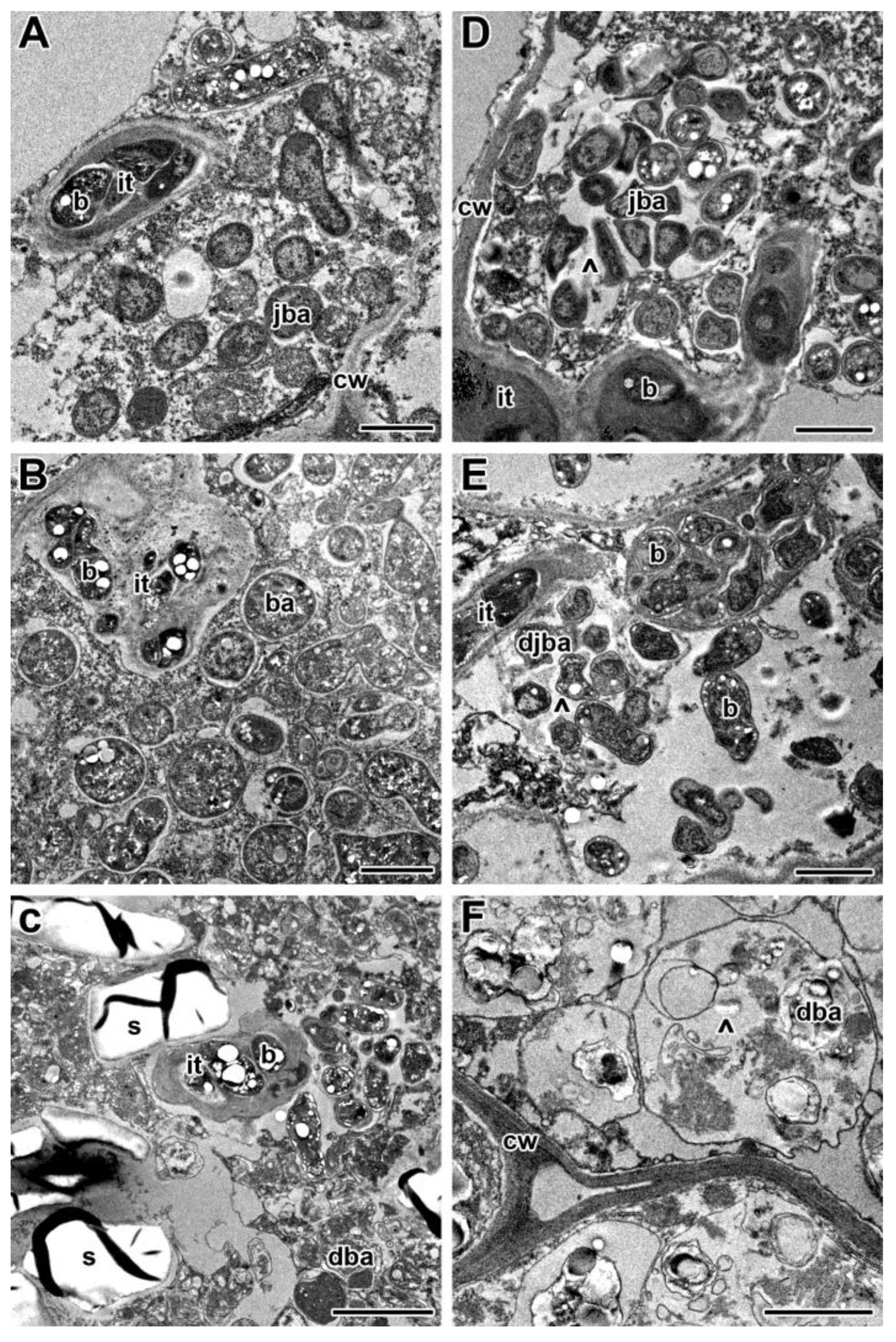
2.2. Histological and Ultrastructural Organization in Heat-Stressed and Heat-Unstressed Nodules in the Line SGE
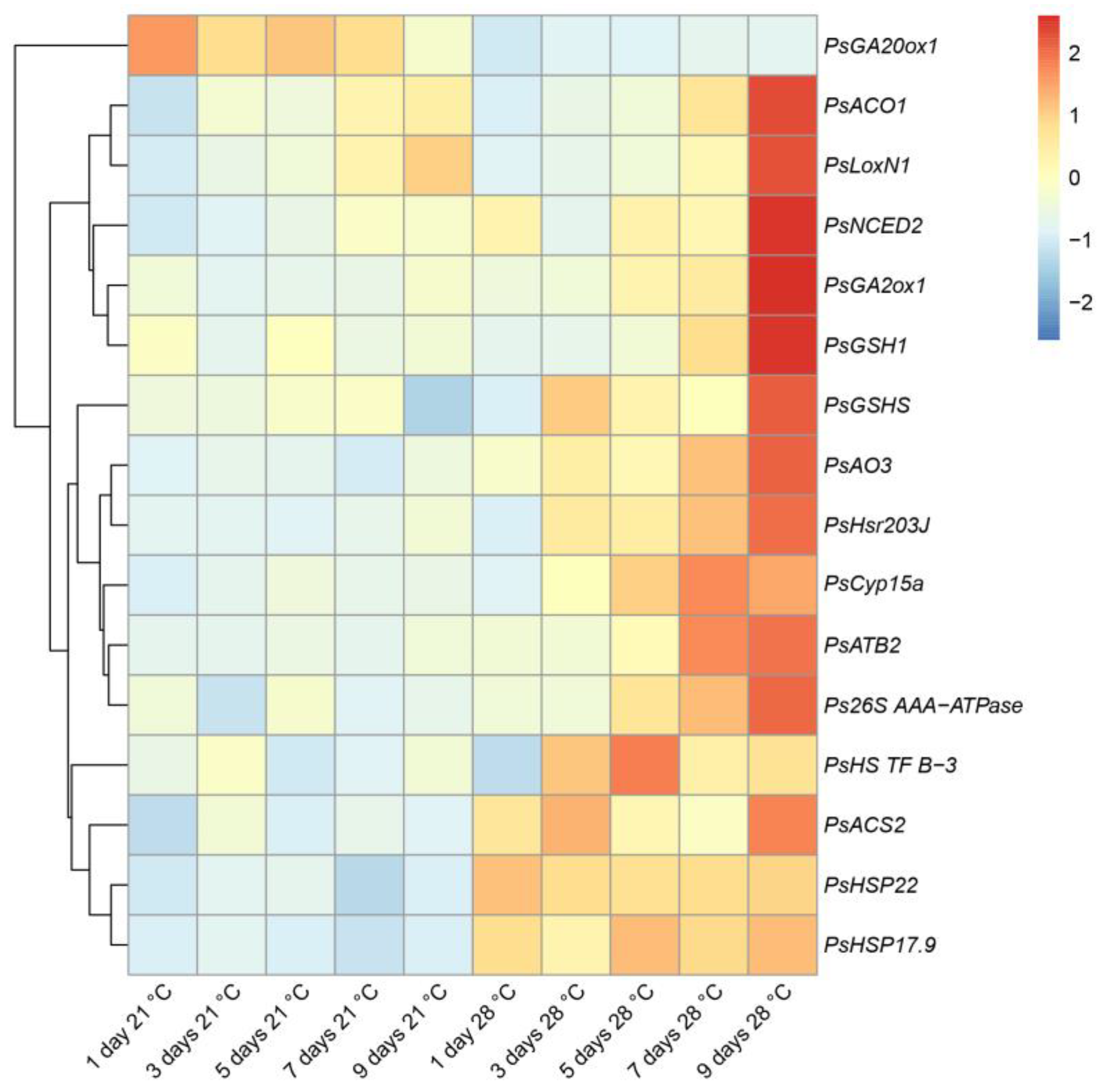
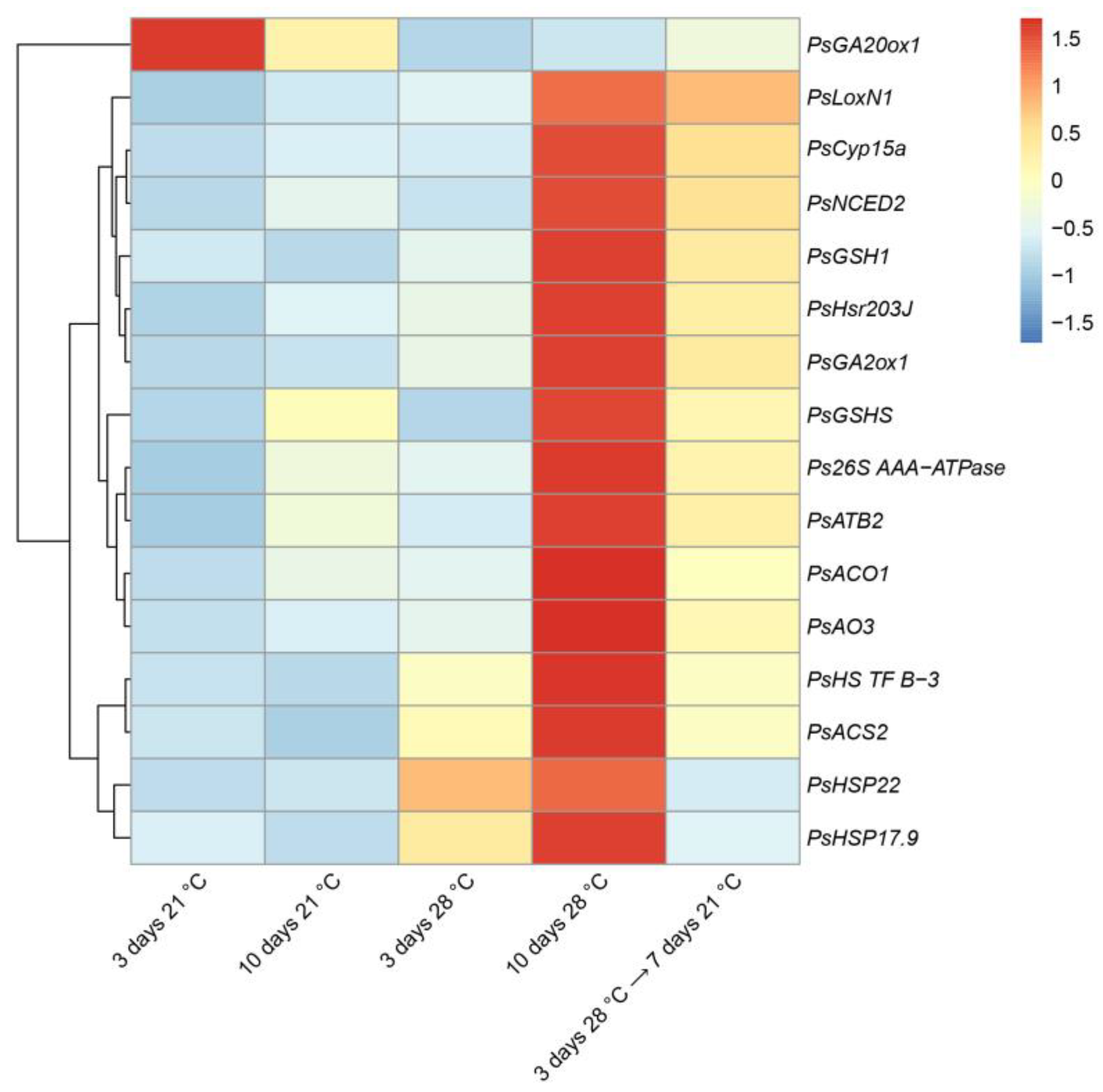
2.3. Expression Analysis of the Genes Associated with Senescence, Heat, and Defense Responses in Heat-Stressed and Heat-Unstressed Nodules in the Line SGE
2.4. The Transcriptomic Analysis of Heat-Stressed and Heat-Unstressed Nodules in the Line SGE
2.5. Analysis of Nodule Recovery in Plants of Line SGE Transferred from Elevated Temperature (28 °C) to Optimal One (21 °C)
2.6. Exposure of Plants of the Line SGE Inoculated with R. leguminosarum bv. viciae 3841 for Some Time to Optimal Temperature Leveled the Negative Effect of Elevated Temperature
3. Discussion
4. Materials and Methods
4.1. Plant Material, Bacterial Strains, and Plant Growth Conditions
4.2. Growing Pea Plants at Elevated Temperature
4.3. Light Microscopy Analysis
4.4. Electron Microscopy Analysis
4.5. Extraction of Total RNA and cDNA Synthesis
4.6. Relative Real-Time PCR Analysis
4.7. Transcriptomic Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IPCC. Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Masson-Delmotte, V., Zhai, P., Pirani, A., Connors, S.L., Péan, C., Berger, S., Caud, N., Chen, Y., Goldfarb, L., Gomis, M.I., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2021; in press. [Google Scholar] [CrossRef]
- Ahuja, I.; de Vos, R.C.H.; Bones, A.M.; Hall, R.D. Plant molecular stress responses face climate change. Trends Plant Sci. 2010, 15, 664–674. [Google Scholar] [CrossRef] [PubMed]
- Gaur, P.M.; Samineni, S.; Krishnamurthy, L.; Varshney, R.K.; Kumar, S.; Ghanem, M.E.; Beebe, S.E.; Rao, I.M.; Chaturvedi, S.K.; Basu, P. High temperature tolerance in grain legumes. Legume Perspect. 2015, 7, 23–24. [Google Scholar]
- Aranjuelo, I.; Aldasoro, J.; Arrese-Igor, C.; Erice, G.; Sanz-Sáez, Á. How does high temperature affect legume nodule symbiotic activity? In Legume Nitrogen Fixation in a Changing Environment: Achievements and Challenges; Sulieman, S., Tran, L.-S.P., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 67–87. [Google Scholar] [CrossRef]
- Jones, F.R.; Tisdale, W.B. Effect of soil temperature upon the development of nodules on the roots of certain legumes. J. Agric. Res. 1921, 22, 17–37. [Google Scholar]
- Meyer, D.R.; Anderson, A.J. Temperature and symbiotic nitrogen fixation. Nature 1959, 183, 61. [Google Scholar] [CrossRef]
- Mes, M.G. Influence of temperature on the symbiotic nitrogen fixation of legumes. Nature 1959, 184, 2032–2033. [Google Scholar] [CrossRef]
- Aranjuelo, I.; Arrese-Igor, C.; Molero, G. Nodule performance within a changing environmental context. J. Plant Physiol. 2014, 171, 1076–1090. [Google Scholar] [CrossRef] [PubMed]
- Piha, M.I.; Munns, D.N. Sensitivity of the common bean (Phaseolus vulgaris L.) symbiosis to high soil temperature. Plant Soil 1987, 98, 183–194. [Google Scholar] [CrossRef]
- Herridge, D.F.; Roughley, R.J. Influence of temperature and Rhizobium strain on nodulation and growth of two tropical legumes. Trop. Grassl. 1976, 10, 21–23. [Google Scholar]
- Gibson, A.H. Root temperature and symbiotic nitrogen fixation. Nature 1961, 191, 1080–1081. [Google Scholar] [CrossRef]
- Pate, J.S. Temperature characteristics of bacterial variation in legume symbiosis. Nature 1961, 192, 637–639. [Google Scholar] [CrossRef]
- Gibson, A.H. Physical environment and symbiotic nitrogen fixation I. The effect of root temperature on recently nodulated Trifolium subterraneum L. plants. Aust. J. Biol. Sci. 1963, 16, 28–42. [Google Scholar] [CrossRef]
- Gibson, A.H. Physical environment and symbiotic nitrogen fixation. V. Effect of time of exposure to unfavourable root temperatures. Aust. J. Biol. Sci. 1967, 20, 1105–1118. [Google Scholar] [CrossRef]
- Pankhurst, C.E.; Gibson, A.H. Rhizobium strain influence on disruption of clover nodule development at high root temperature. Microbiology 1973, 74, 219–231. [Google Scholar] [CrossRef]
- Munévar, F.; Wollum II, A.G. Effect of high root temperature and Rhizobium strain on nodulation, nitrogen fixation, and growth of soybeans. Soil Sci. Soc. Am. J. 1981, 45, 1113–1120. [Google Scholar] [CrossRef]
- Montañez, A.; Danso, S.K.A.; Hardarson, G. The effect of temperature on nodulation and nitrogen fixation by five Bradyrhizobium japonicum strains. Appl. Soil Ecol. 1995, 2, 165–174. [Google Scholar] [CrossRef]
- Joffe, A.; Weyer, F.; Saubert, S. The role of root temperature in symbiotic nitrogen fixation. S. Afr. J. Sci. 1961, 57, 265–274. [Google Scholar]
- Frings, J.F.J. The Rhizobium-pea Symbiosis as Affected by High Temperatures. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 1976. [Google Scholar]
- Gogorcena, Y.; Iturbe-Ormaetxe, l.; Escuredo, P.R.; Becana, M. Antioxidant defenses against activated oxygen in pea nodules subjected to water stress. Plant Physiol. 1995, 108, 753–759. [Google Scholar] [CrossRef]
- Gordon, A.J.; Minchin, F.R.; Skot, L.; James, C.L. Stress-induced declines in soybean N2 fixation are related to nodule sucrose synthase activity. Plant Physiol. 1997, 114, 937–946. [Google Scholar] [CrossRef]
- Clement, M.; Lambert, A.; Herouart, D.; Boncompagni, E. Identification of new up-regulated genes under drought stress in soybean nodules. Gene 2008, 426, 15–22. [Google Scholar] [CrossRef]
- Karmarkar, V.M. Transcriptional Regulation of Nodule Development and Senescence in Medicago truncatula. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 2014. [Google Scholar]
- Gogorcena, Y.; Gordon, A.J.; Escuredo, P.R.; Minchin, F.R.; Witty, J.F.; Moran, J.F.; Becana, M. N2 fixation, carbon metabolism, and oxidative damage in nodules of dark-stressed common bean plants. Plant Physiol. 1997, 113, 1193–1201. [Google Scholar] [CrossRef]
- Matamoros, M.A.; Baird, L.M.; Escuredo, P.R.; Dalton, D.A.; Minchin, F.R.; Iturbe-Ormaetxe, I.; Rubio, M.C.; Moran, J.F.; Gordon, A.J.; Becana, M. Stress-induced legume root nodule senescence. Physiological, biochemical, and structural alterations. Plant Physiol. 1999, 121, 97–112. [Google Scholar] [CrossRef]
- Müller, J.; Boller, T.; Wiemken, A. Trehalose becomes the most abundant non-structural carbohydrate during senescence of soybean nodules. J. Exp. Bot. 2001, 52, 943–947. [Google Scholar] [CrossRef] [PubMed]
- Vauclare, P.; Bligny, R.; Gout, E.; De Meuron, V.; Widmer, F. Metabolic and structural rearrangement during dark-induced autophagy in soybean (Glycine max L.) nodules: An electron microscopy and 31P and 13C nuclear magnetic resonance study. Planta 2010, 231, 1495–1504. [Google Scholar] [CrossRef] [PubMed]
- Pierre, O.; Hopkins, J.; Combier, M.; Baldacci, F.; Engler, G.; Brouquisse, R.; Hérouart, D.; Boncompagni, E. Involvement of papain and legumain proteinase in the senescence process of Medicago truncatula nodules. New Phytol. 2014, 202, 849–863. [Google Scholar] [CrossRef]
- van Heerden, P.D.R.; Kiddle, G.; Pellny, T.K.; Mokwala, P.W.; Jordaan, A.; Strauss, A.J.; de Beer, M.; Schlüter, U.; Kunert, K.J.; Foyer, C.H. Regulation of respiration and the oxygen diffusion barrier in soybean protect symbiotic nitrogen fixation from chilling-induced inhibition and shoots from premature senescence. Plant Physiol. 2008, 148, 316–327. [Google Scholar] [CrossRef] [PubMed]
- Balestrasse, K.B.; Gallego, S.M.; Tomaro, M.L. Cadmium-induced senescence in nodules of soybean (Glycine max L.) plants. Plant Soil 2004, 262, 373–381. [Google Scholar] [CrossRef]
- Tsyganova, A.V.; Seliverstova, E.V.; Tsyganov, V.E. Influence of mutation in pea (Pisum sativum L.) cdt (cadmium tolerance) gene on histological and ultrastructural nodule organization. Ekol. Genet. 2019, 17, 71–80. [Google Scholar] [CrossRef]
- Tsyganov, V.E.; Tsyganova, A.V.; Gorshkov, A.P.; Seliverstova, E.V.; Kim, V.E.; Chizhevskaya, E.P.; Belimov, A.A.; Serova, T.A.; Ivanova, K.A.; Kulaeva, O.A.; et al. Efficacy of a plant-microbe system: Pisum sativum (L.) cadmium-tolerant mutant and Rhizobium leguminosarum strains, expressing pea metallothionein genes PsMT1 and PsMT2, for cadmium phytoremediation. Front. Microbiol. 2020, 11, 15. [Google Scholar] [CrossRef]
- Pérez Guerra, J.C.; Coussens, G.; De Keyser, A.; De Rycke, R.; De Bodt, S.; Van De Velde, W.; Goormachtig, S.; Holsters, M. Comparison of developmental and stress-induced nodule senescence in Medicago truncatula. Plant Physiol. 2010, 152, 1574–1584. [Google Scholar] [CrossRef]
- Serova, T.A.; Tikhonovich, I.A.; Tsyganov, V.E. Analysis of nodule senescence in pea (Pisum sativum L.) using laser microdissection, real-time PCR, and ACC immunolocalization. J. Plant Physiol. 2017, 212, 29–44. [Google Scholar] [CrossRef]
- Serova, T.A.; Tsyganova, A.V.; Tsyganov, V.E. Early nodule senescence is activated in symbiotic mutants of pea (Pisum sativum L.) forming ineffective nodules blocked at different nodule developmental stages. Protoplasma 2018, 255, 1443–1459. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, K.A.; Tsyganova, A.V.; Brewin, N.J.; Tikhonovich, I.A.; Tsyganov, V.E. Induction of host defences by Rhizobium during ineffective nodulation of pea (Pisum sativum L.) carrying symbiotically defective mutations sym40 (PsEFD), sym33 (PsIPD3/PsCYCLOPS) and sym42. Protoplasma 2015, 252, 1505–1517. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, K.A.; Chernova, E.N.; Kulaeva, O.A.; Tsyganova, A.V.; Kusakin, P.G.; Russkikh, I.V.; Tikhonovich, I.A.; Tsyganov, V.E. The regulation of pea (Pisum sativum L.) symbiotic nodule infection and defense responses by glutathione, homoglutathione, and their ratio. Front. Plant Sci. 2022, 13, 843565. [Google Scholar] [CrossRef] [PubMed]
- Kusakin, P.G.; Serova, T.A.; Gogoleva, N.E.; Gogolev, Y.V.; Tsyganov, V.E. Laser microdissection of Pisum sativum L. nodules followed by RNA-seq analysis revealed crucial transcriptomic changes during infected cell differentiation. Agronomy 2021, 11, 2504. [Google Scholar] [CrossRef]
- Gorshkov, A.P.; Kusakin, P.G.; Borisov, Y.G.; Tsyganova, A.V.; Tsyganov, V.E. Effect of triazole fungicides Titul Duo and Vintage on the development of pea (Pisum sativum L.) symbiotic nodules. Int. J. Mol. Sci. 2023, 24, 8646. [Google Scholar] [CrossRef]
- Gorshkov, A.P.; Tsyganova, A.V.; Vorobiev, M.G.; Tsyganov, V.E. The fungicide tetramethylthiuram disulfide negatively affects plant cell walls, infection thread walls, and symbiosomes in pea (Pisum sativum L.) symbiotic nodules. Plants 2020, 9, 1488. [Google Scholar] [CrossRef]
- Virtanen, A.I.; Miettinen, J.K. Formation of biliverdin from legcholeglobin, the green pigment in leguminous root nodules. Acta Chem. Scand. 1949, 3, 17–21. [Google Scholar] [CrossRef]
- Becana, M.; Klucas, R.V. Oxidation and reduction of leghemoglobin in root nodules of leguminous plants. Plant Physiol. 1992, 98, 1217–1221. [Google Scholar] [CrossRef]
- Van de Velde, W.; Guerra, J.C.P.; Keyser, A.D.; De Rycke, R.; Rombauts, S.; Maunoury, N.; Mergaert, P.; Kondorosi, E.; Holsters, M.; Goormachtig, S. Aging in legume symbiosis. A molecular view on nodule senescence in Medicago truncatula. Plant Physiol. 2006, 141, 711–720. [Google Scholar] [CrossRef]
- Musiałek, M.W.; Deckert, J.; Rybaczek, D. Hydroxyurea and caffeine impact pRb-like protein-dependent chromatin architecture profiles in interphase cells of Vicia faba. Int. J. Mol. Sci. 2021, 22, 4572. [Google Scholar] [CrossRef]
- Zhao, Y.; Simon, M.; Seluanov, A.; Gorbunova, V. DNA damage and repair in age-related inflammation. Nat. Rev. Immunol. 2022, 23, 75–89. [Google Scholar] [CrossRef] [PubMed]
- Borisov, A.Y.; Rozov, S.M.; Tsyganov, V.E.; Morzhina, E.V.; Lebsky, V.K.; Tikhonovich, I.A. Sequential functioning of Sym-13 and Sym-31, two genes affecting symbiosome development in root nodules of pea (Pisum sativum L). Mol. Gen. Genet. 1997, 254, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Sherrier, D.J.; Borisov, A.Y.; Tikhonovich, I.A.; Brewin, N.J. Immunocytological evidence for abnormal symbiosome development in nodules of the pea mutant line Sprint-2Fix− (sym31). Protoplasma 1997, 199, 57–68. [Google Scholar] [CrossRef]
- Novák, K.; Pešina, K.; Nebesářová, J.; Škrdleta, V.; Lisá, L.; Našinec, V. Symbiotic tissue degradation pattern in the ineffective nodules of three nodulation mutants of pea (Pisum sativum L.). Ann. Bot. 1995, 76, 303–313. [Google Scholar] [CrossRef]
- Tsyganov, V.E.; Morzhina, E.V.; Stefanov, S.Y.; Borisov, A.Y.; Lebsky, V.K.; Tikhonovich, I.A. The pea (Pisum sativum L.) genes sym33 and sym40 control infection thread formation and root nodule function. Mol. Gen. Genet. 1998, 259, 491–503. [Google Scholar] [CrossRef]
- Sinharoy, S.; Torres-Jerez, I.; Bandyopadhyay, K.; Kereszt, A.; Pislariu, C.I.; Nakashima, J.; Benedito, V.A.; Kondorosi, E.; Udvardi, M.K. The C2H2 transcription factor REGULATOR OF SYMBIOSOME DIFFERENTIATION represses transcription of the secretory pathway gene VAMP721a and promotes symbiosome development in Medicago truncatula. Plant Cell 2013, 25, 3584–3601. [Google Scholar] [CrossRef]
- MacKenzie, C.R.; Jordan, D.C. Ultrastructure of root nodules formed by ineffective strains of Rhizobium meliloti. Can. J. Microbiol. 1974, 20, 755–758. [Google Scholar] [CrossRef]
- Tsyganova, A.V.; Tsyganov, V.E. Organization of the endoplasmic reticulum in cells of effective and ineffective pea nodules (Pisum sativum L.). Ekol. Genet. 2019, 17, 5–14. [Google Scholar] [CrossRef]
- Serova, T.A.; Tsyganova, A.V.; Tikhonovich, I.A.; Tsyganov, V.E. Gibberellins inhibit nodule senescence and stimulate nodule meristem bifurcation in pea (Pisum sativum L.). Front. Plant Sci. 2019, 10, 285. [Google Scholar] [CrossRef]
- Kardailsky, I.V.; Brewin, N.J. Expression of cysteine protease genes in pea nodule development and senescence. Mol. Plant Microbe Interact. 1996, 9, 689–695. [Google Scholar] [CrossRef]
- Cabeza, R.; Koester, B.; Liese, R.; Lingner, A.; Baumgarten, V.; Dirks, J.; Salinas-Riester, G.; Pommerenke, C.; Dittert, K.; Schulze, J. An RNA sequencing transcriptome analysis reveals novel insights into molecular aspects of the nitrate impact on the nodule activity of Medicago truncatula. Plant Physiol. 2014, 164, 400–411. [Google Scholar] [CrossRef] [PubMed]
- Chungopast, S.; Hirakawa, H.; Sato, S.; Handa, Y.; Saito, K.; Kawaguchi, M.; Tajima, S.; Nomura, M. Transcriptomic profiles of nodule senescence in Lotus japonicus and Mesorhizobium loti symbiosis. Plant Biotechnol. 2014, 31, 345–349. [Google Scholar] [CrossRef]
- Wang, C.; Yu, H.; Luo, L.; Duan, L.; Cai, L.; He, X.; Wen, J.; Mysore, K.S.; Li, G.; Xiao, A.; et al. NODULES WITH ACTIVATED DEFENSE 1 is required for maintenance of rhizobial endosymbiosis in Medicago truncatula. New Phytol. 2016, 212, 176–191. [Google Scholar] [CrossRef]
- Deng, J.; Zhu, F.; Liu, J.; Zhao, Y.; Wen, J.; Wang, T.; Dong, J. Transcription factor bHLH2 represses CYSTEINE PROTEASE77 to negatively regulate nodule senescence. Plant Physiol. 2019, 181, 1683–1703. [Google Scholar] [CrossRef]
- D’haeseleer, K.; De Keyser, A.; Goormachtig, S.; Holsters, M. Transcription factor MtATB2: About nodulation, sucrose and senescence. Plant Cell Physiol. 2010, 51, 1416–1424. [Google Scholar] [CrossRef]
- Ullah, I.; Magdy, M.; Wang, L.; Liu, M.; Li, X. Genome-wide identification and evolutionary analysis of TGA transcription factors in soybean. Sci. Rep. 2019, 9, 11186 . [Google Scholar] [CrossRef]
- Yang, Y.; Yu, T.-F.; Ma, J.; Chen, J.; Zhou, Y.-B.; Chen, M.; Ma, Y.-Z.; Wei, W.-L.; Xu, Z.-S. The soybean bZIP transcription factor gene GmbZIP2 confers drought and salt resistances in transgenic plants. Int. J. Mol. Sci. 2020, 21, 670. [Google Scholar] [CrossRef]
- Tronchet, M.; Ranty, B.; Marco, Y.; Roby, D. HSR203 antisense suppression in tobacco accelerates development of hypersensitive cell death. Plant J. 2001, 27, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Wang, X.; Zhang, L. Structural and functional dynamics of dehydrins: A plant protector protein under abiotic stress. Int. J. Mol. Sci. 2018, 19, 3420. [Google Scholar] [CrossRef]
- Paniagua, C.; Bilkova, A.; Jackson, P.; Dabravolski, S.; Riber, W.; Didi, V.; Houser, J.; Gigli-Bisceglia, N.; Wimmerova, M.; Budínská, E.; et al. Dirigent proteins in plants: Modulating cell wall metabolism during abiotic and biotic stress exposure. J. Exp. Bot. 2017, 68, 3287–3301. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Wang, M.-M.; Jiang, Y.; Bao, Y.-M.; Huang, X.; Sun, H.; Xu, D.-Q.; Lan, H.-X.; Zhang, H.-S. Expression analysis of rice A20/AN1-type zinc finger genes and characterization of ZFP177 that contributes to temperature stress tolerance. Gene 2008, 420, 135–144. [Google Scholar] [CrossRef]
- Vieira, P.; Mowery, J.; Eisenback, J.D.; Shao, J.; Nemchinov, L.G. Cellular and transcriptional responses of resistant and susceptible cultivars of alfalfa to the root lesion nematode, Pratylenchus penetrans. Front. Plant Sci. 2019, 10, 971. [Google Scholar] [CrossRef]
- Williamson-Benavides, B.A.; Sharpe, R.M.; Nelson, G.; Bodah, E.T.; Porter, L.D.; Dhingra, A. Identification of root rot resistance QTLs in pea using Fusarium solani f. sp. pisi-responsive differentially expressed genes. Front. Genet. 2021, 12, 629267. [Google Scholar] [CrossRef]
- Hua, J. Modulation of plant immunity by light, circadian rhythm, and temperature. Curr. Opin. Plant Biol. 2013, 16, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Huot, B.; Castroverde, C.D.M.; Velásquez, A.C.; Hubbard, E.; Pulman, J.A.; Yao, J.; Childs, K.L.; Tsuda, K.; Montgomery, B.L.; He, S.Y. Dual impact of elevated temperature on plant defence and bacterial virulence in Arabidopsis. Nat. Commun. 2017, 8, 1808. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, J.; Kang, B.-C. Current views on temperature-modulated R gene-mediated plant defense responses and tradeoffs between plant growth and immunity. Curr. Opin. Plant Biol. 2019, 50, 9–17. [Google Scholar] [CrossRef] [PubMed]
- van Wyk, S.G.; Du Plessis, M.; Cullis, C.A.; Kunert, K.J.; Vorster, B.J. Cysteine protease and cystatin expression and activity during soybean nodule development and senescence. BMC Plant Biol. 2014, 14, 294. [Google Scholar] [CrossRef] [PubMed]
- Kunert, K.J.; van Wyk, S.G.; Cullis, C.A.; Vorster, B.J.; Foyer, C.H. Potential use of phytocystatins in crop improvement, with a particular focus on legumes. J. Exp. Bot. 2015, 66, 3559–3570. [Google Scholar] [CrossRef] [PubMed]
- Atkins, C.; Smith, P.; Mann, A.; Thumfort, P. Localization of carbonic anhydrase in legume nodules. Plant Cell Environ. 2001, 24, 317–326. [Google Scholar] [CrossRef]
- De La Peña, T.C.; Frugier, F.; McKhann, H.I.; Bauer, P.; Brown, S.; Kondorosi, A.; Crespi, M. A carbonic anhydrase gene is induced in the nodule primordium and its cell-specific expression is controlled by the presence of Rhizobium during development. Plant J. 1997, 11, 407–420. [Google Scholar] [CrossRef]
- Escudero, V.; Abreu, I.; del Sastre, E.; Tejada-Jiménez, M.; Larue, C.; Novoa-Aponte, L.; Castillo-González, J.; Wen, J.; Mysore, K.S.; Abadía, J.; et al. Nicotianamine synthase 2 is required for symbiotic nitrogen fixation in Medicago truncatula nodules. Front. Plant Sci. 2020, 10, 1780. [Google Scholar] [CrossRef] [PubMed]
- Fedorova, M.; Tikhonovich, I.A.; Vance, C.P. Expression of C-assimilating enzymes in pea (Pisum sativum L.) root nodules. In situ localization in effective nodules. Plant Cell Environ. 1999, 22, 1249–1262. [Google Scholar] [CrossRef]
- Reynolds, P.H.S.; Blevins, D.G.; Boland, M.J.; Schubert, K.R.; Randall, D.D. Enzymes of ammonia assimilation in legume nodules: A comparison between ureide- and amide-transporting plants. Physiol. Plant. 1982, 55, 255–260. [Google Scholar] [CrossRef]
- Delgado, M.J.; Garrido, J.M.; Ligero, F.; Lluch, C. Nitrogen fixation and carbon metabolism by nodules and bacteroids of pea plants under sodium chloride stress. Physiol. Plant. 1993, 89, 824–829. [Google Scholar] [CrossRef]
- Mhadhbi, H.; Fotopoulos, V.; Mylona, P.V.; Jebara, M.; Elarbi Aouani, M.; Polidoros, A.N. Antioxidant gene–enzyme responses in Medicago truncatula genotypes with different degree of sensitivity to salinity. Physiol. Plant. 2010, 141, 201–214. [Google Scholar] [CrossRef]
- Becana, M.; Aparicio-Tejo, P.; Peña, J.; Aguirreolea, J.; Sánchez-Díaz, M. N2 fixation (C2H2-reducing activity) and leghaemoglobin content during nitrate- and water-stress-induced senescence of Medicago sativa root nodules. J. Exp. Bot. 1986, 37, 597–605. [Google Scholar] [CrossRef]
- Scott, D.B.; Farnden, K.J.F.; Robertson, J.G. Ammonia assimilation in lupin nodules. Nature 1976, 263, 703–705. [Google Scholar] [CrossRef]
- Vincze, E.; Reeves, J.M.; Lamping, E.; Farnden, K.J.F.; Reynolds, P.H.S. Repression of the L-asparaginase gene during nodule development in Lupinus angustifolius. Plant Mol. Biol. 1994, 26, 303–311. [Google Scholar] [CrossRef]
- Kitaeva, A.B.; Demchenko, K.N.; Tikhonovich, I.A.; Timmers, A.C.J.; Tsyganov, V.E. Comparative analysis of the tubulin cytoskeleton organization in nodules of Medicago truncatula and Pisum sativum: Bacterial release and bacteroid positioning correlate with characteristic microtubule rearrangements. New Phytol. 2016, 210, 168–183. [Google Scholar] [CrossRef]
- Giordano, W.; Hirsch, A.M. The expression of MaEXP1, a Melilotus alba expansin gene, is upregulated during the sweetclover–Sinorhizobium meliloti interaction. Mol. Plant Microbe Interact. 2004, 17, 613–622. [Google Scholar] [CrossRef]
- Li, X.; Zhao, J.; Tan, Z.; Zeng, R.; Liao, H. GmEXPB2, a cell wall β-expansin, affects soybean nodulation through modifying root architecture and promoting nodule formation and development. Plant Physiol. 2015, 169, 2640–2653. [Google Scholar] [CrossRef]
- Timmers, A.C.J.; Soupène, E.; Auriac, M.-C.; de Billy, F.; Vasse, J.; Boistard, P.; Truchet, G. Saprophytic intracellular rhizobia in alfalfa nodules. Mol. Plant Microbe Interact. 2000, 13, 1204–1213. [Google Scholar] [CrossRef]
- Zhang, H.; Lang, Z.; Zhu, J.-K. Dynamics and function of DNA methylation in plants. Nat. Rev. Mol. Cell Biol. 2018, 19, 489–506. [Google Scholar] [CrossRef] [PubMed]
- Pecrix, Y.; Sallet, E.; Moreau, S.; Bouchez, O.; Carrere, S.; Gouzy, J.; Jardinaud, M.F.; Gamas, P. DNA demethylation and hypermethylation are both required for late nodule development in Medicago. Nat. Plants 2022, 8, 741–749. [Google Scholar] [CrossRef] [PubMed]
- Marx, H.; Minogue, C.E.; Jayaraman, D.; Richards, A.L.; Kwiecien, N.W.; Siahpirani, A.F.; Rajasekar, S.; Maeda, J.; Garcia, K.; Del Valle-Echevarria, A.R.; et al. A proteomic atlas of the legume Medicago truncatula and its nitrogen-fixing endosymbiont Sinorhizobium meliloti. Nat. Biotechnol. 2016, 34, 1198–1205. [Google Scholar] [CrossRef]
- Rafferty, J.P. Heat Wave. Available online: https://www.britannica.com/science/heat-wave-meteorology (accessed on 25 November 2022).
- Kosterin, O.E.; Rozov, S.M. Mapping of the new mutation blb and the problem of integrity of linkage group I. Pisum Genet. 1993, 25, 27–31. [Google Scholar]
- Glenn, A.R.; Poole, P.S.; Hudman, J.F. Succinate uptake by free-living and bacteroid forms of Rhizobium leguminosarum. Microbiology 1980, 119, 267–271. [Google Scholar] [CrossRef]
- Die, J.V.; Román, B.; Nadal, S.; Dita, M.Á.; González-Verdejo, C.I. Expression analysis of Pisum sativum putative defence genes during Orobanche crenata infection. Crop Pasture Sci. 2009, 60, 490–498. [Google Scholar] [CrossRef]
- Weston, D.E.; Elliott, R.C.; Lester, D.R.; Rameau, C.; Reid, J.B.; Murfet, I.C.; Ross, J.J. The pea DELLA proteins LA and CRY are important regulators of gibberellin synthesis and root growth. Plant Physiol. 2008, 147, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsova, E.; Seddas-Dozolme, P.M.A.; Arnould, C.; Tollot, M.; van Tuinen, D.; Borisov, A.; Gianinazzi, S.; Gianinazzi-Pearson, V. Symbiosis-related pea genes modulate fungal and plant gene expression during the arbuscule stage of mycorrhiza with Glomus intraradices. Mycorrhiza 2010, 20, 427–443. [Google Scholar] [CrossRef] [PubMed]
- Kolde, R. pheatmap: Pretty Heatmaps, R package version 1.0.12. 2019.
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Pantano, L. DEGreport: Report of DEG Analysis, R package version 1.24.1. 2020.
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar] [CrossRef]
- Alexa, A.; Rahnenfuhrer, J. topGO: Enrichment Analysis for Gene Ontology, R package version 2.44.0. 2021.



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serova, T.A.; Kusakin, P.G.; Kitaeva, A.B.; Seliverstova, E.V.; Gorshkov, A.P.; Romanyuk, D.A.; Zhukov, V.A.; Tsyganova, A.V.; Tsyganov, V.E. Effects of Elevated Temperature on Pisum sativum Nodule Development: I—Detailed Characteristic of Unusual Apical Senescence. Int. J. Mol. Sci. 2023, 24, 17144. https://doi.org/10.3390/ijms242417144
Serova TA, Kusakin PG, Kitaeva AB, Seliverstova EV, Gorshkov AP, Romanyuk DA, Zhukov VA, Tsyganova AV, Tsyganov VE. Effects of Elevated Temperature on Pisum sativum Nodule Development: I—Detailed Characteristic of Unusual Apical Senescence. International Journal of Molecular Sciences. 2023; 24(24):17144. https://doi.org/10.3390/ijms242417144
Chicago/Turabian StyleSerova, Tatiana A., Pyotr G. Kusakin, Anna B. Kitaeva, Elena V. Seliverstova, Artemii P. Gorshkov, Daria A. Romanyuk, Vladimir A. Zhukov, Anna V. Tsyganova, and Viktor E. Tsyganov. 2023. "Effects of Elevated Temperature on Pisum sativum Nodule Development: I—Detailed Characteristic of Unusual Apical Senescence" International Journal of Molecular Sciences 24, no. 24: 17144. https://doi.org/10.3390/ijms242417144






