Modifications of Blood Molecular Components after Treatment with Low Ozone Concentrations
Abstract
:1. Introduction
2. Results
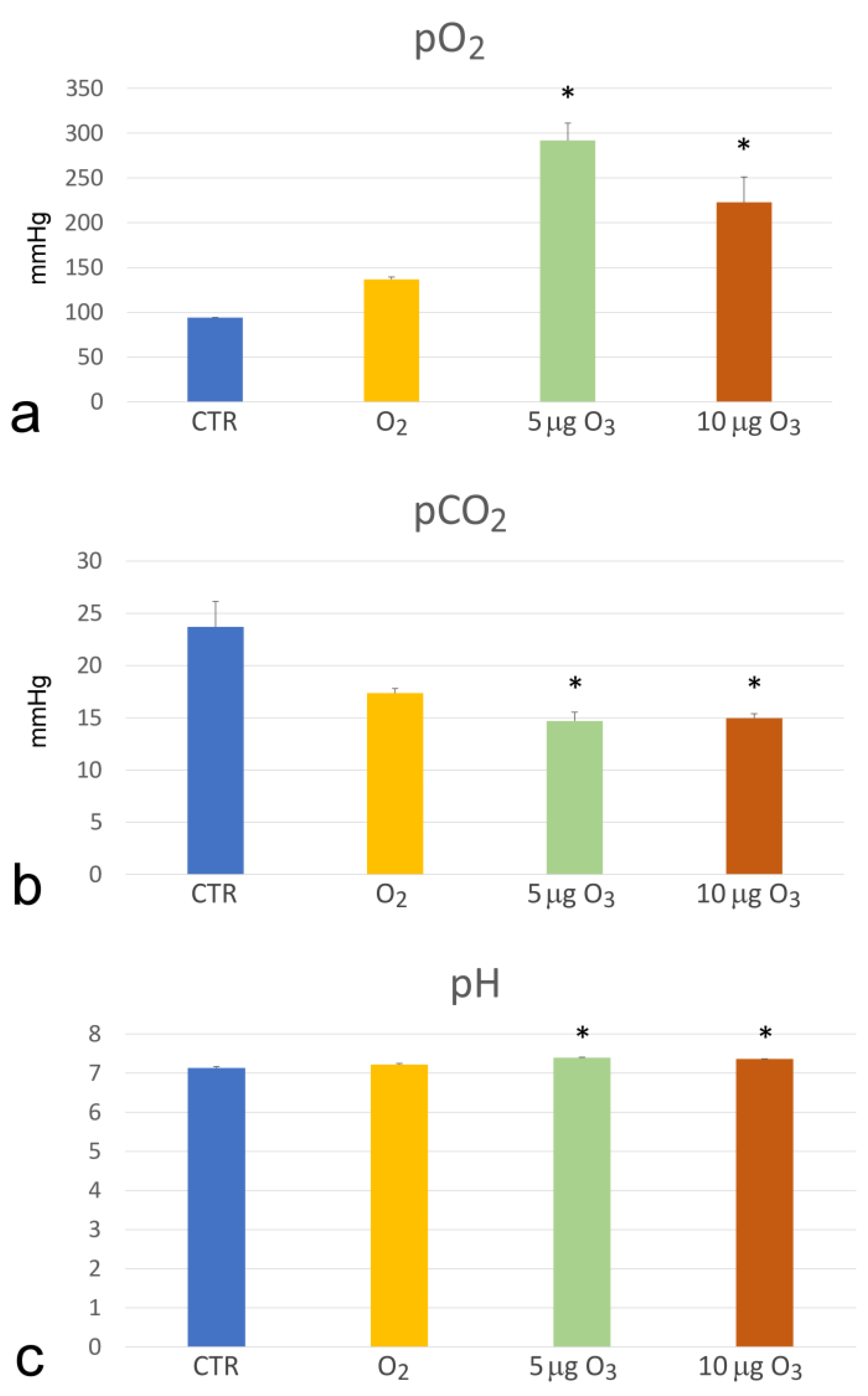
2.1. Haemogas Analysis
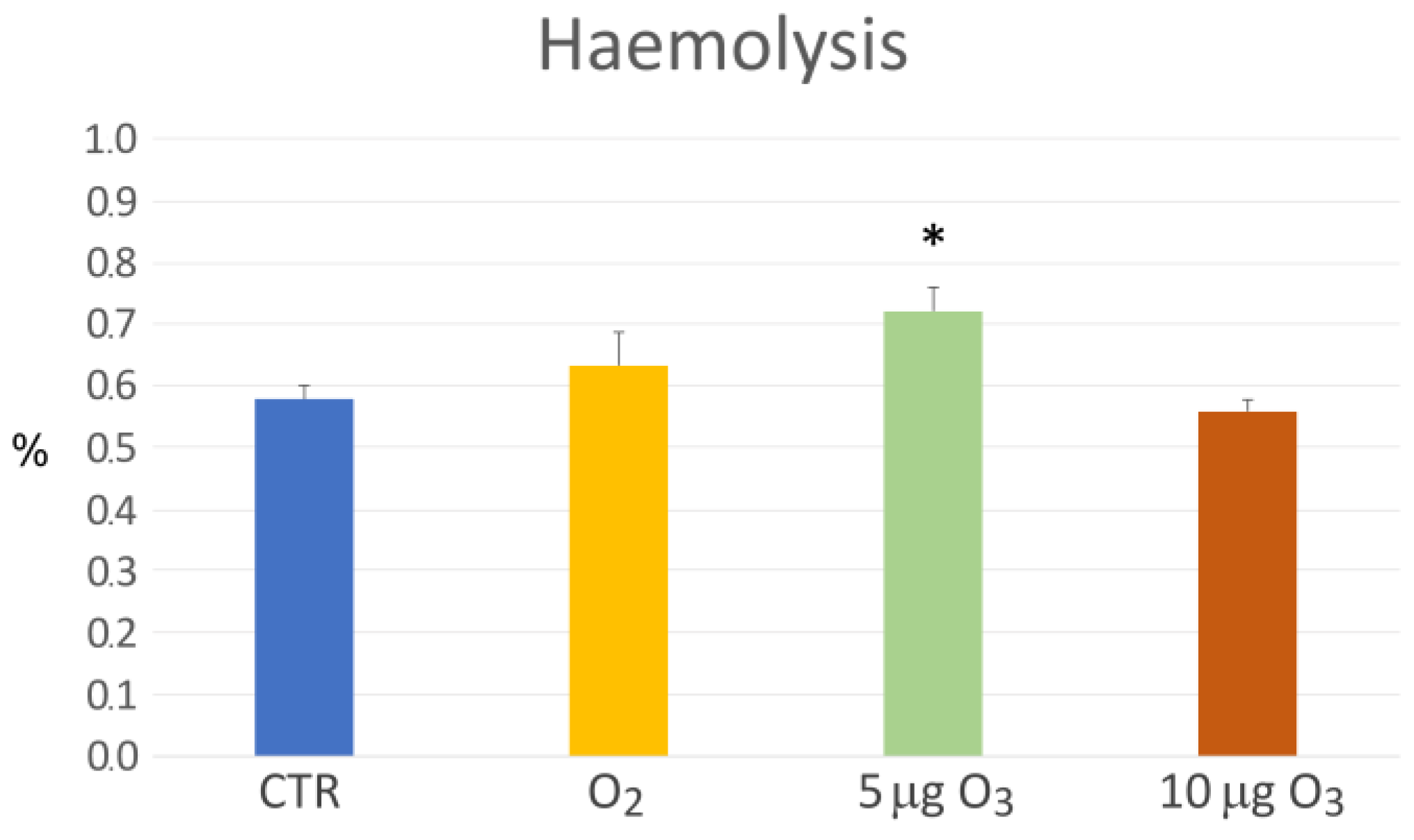
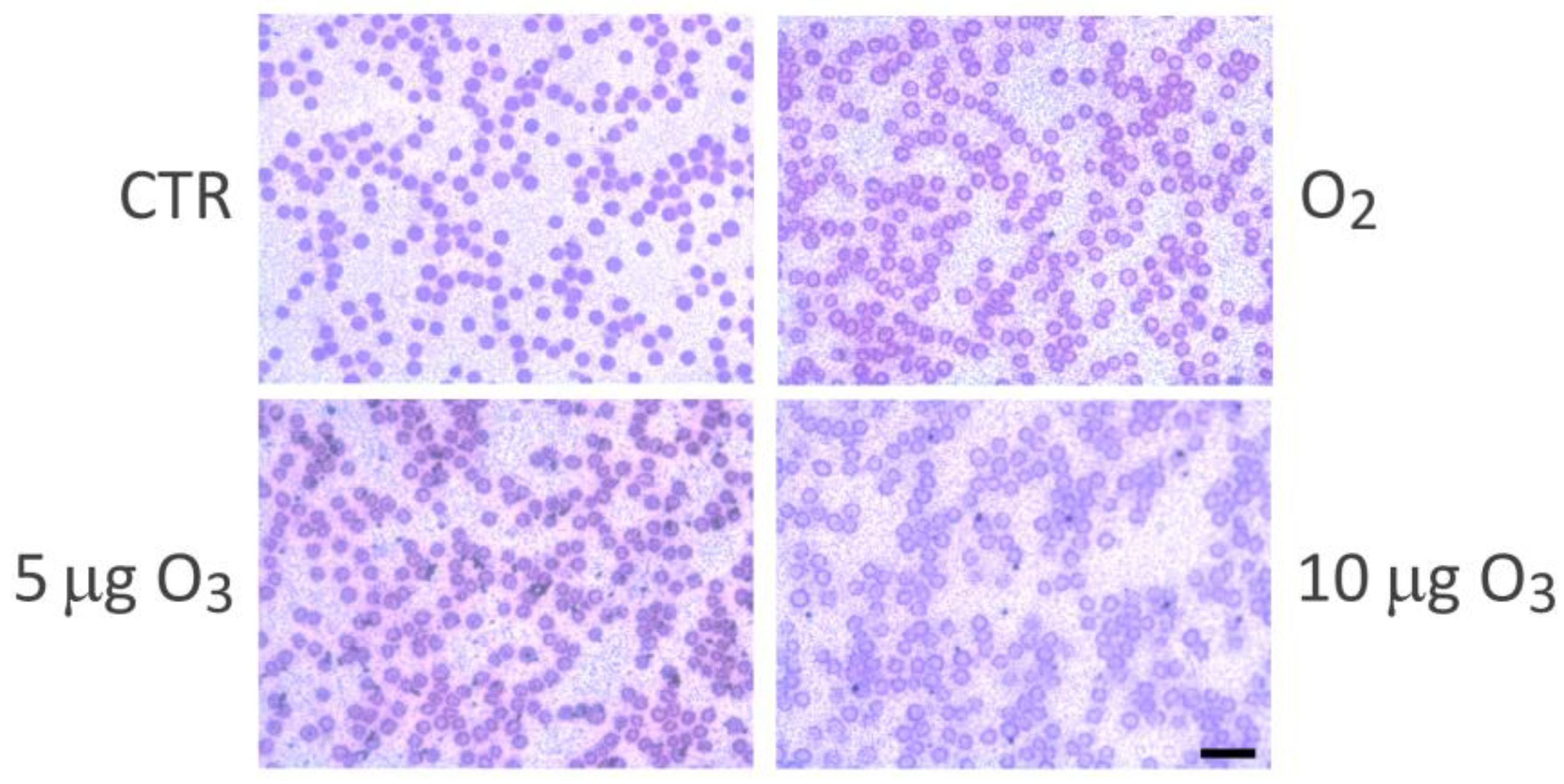
2.2. Haemolysis
2.3. Total Antioxidant Capacity
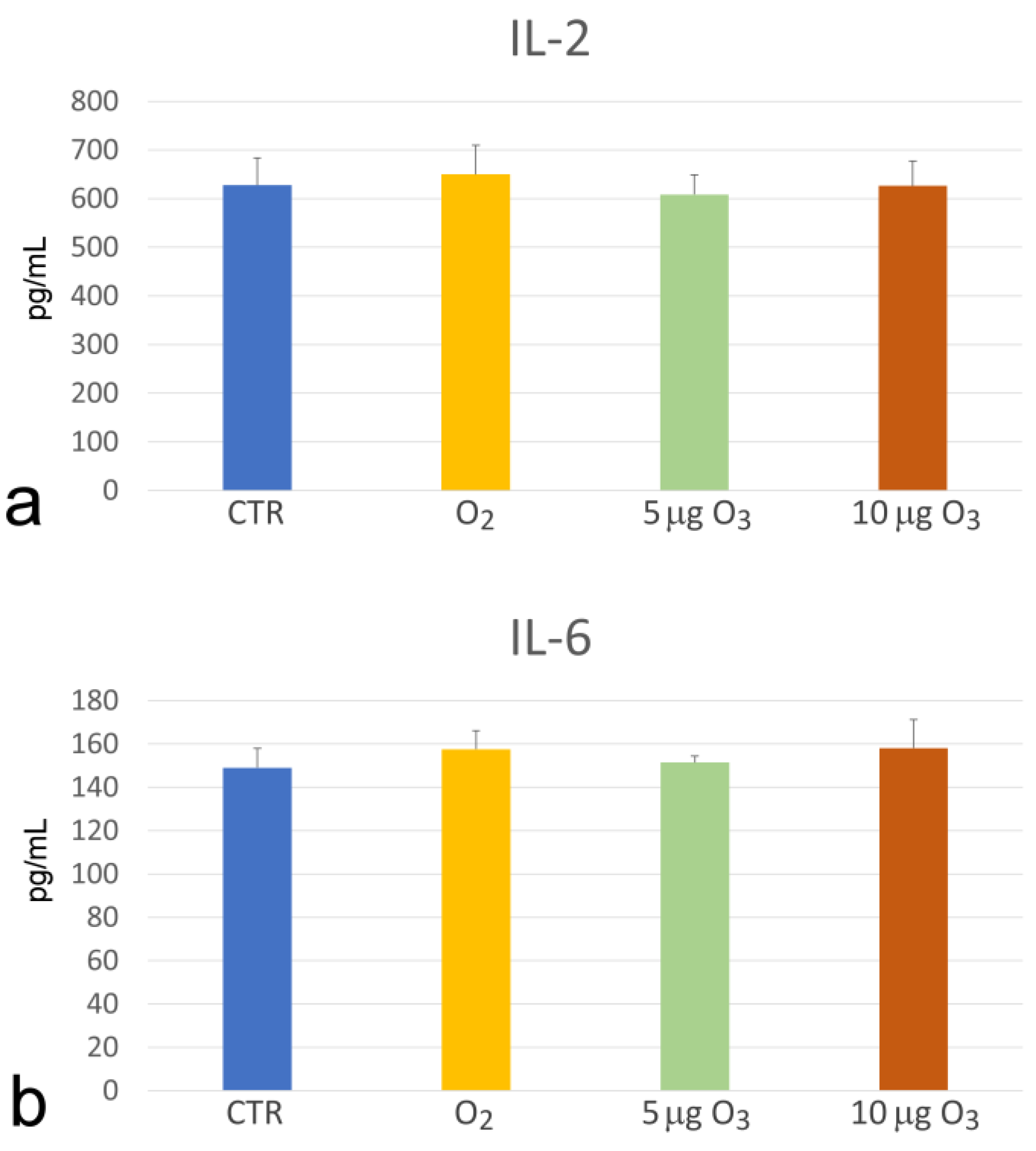
2.4. Interleukins
2.5. Metabolomics
3. Discussion
3.1. The Impact on Blood Oxygenation and Haemolysis
3.2. The Increase in Blood Antioxidant Capacity
3.3. The Unchanged Levels of IL-2 and IL-6
3.4. The Modifications of Amino Acid, Carbohydrate, Lipid and Nucleotide Metabolites
4. Materials and Methods
4.1. Blood Ozonation
4.2. Haemogas Analysis
4.3. Haemolysis Assay
4.4. Total Antioxidant Capacity Assay
4.5. Light Microscopy Analysis
4.6. Interleukin Assessment
4.7. Metabolomics
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Viebahn-Hansler, R. The Use of Ozone in Medicine, 5th ed.; ODREI Publishers: Iezhein, Germany, 2007; pp. 1–170. [Google Scholar]
- Wolff, H.H. Die Behandlung peripherer Durchblutungsstörungen mit Ozon. Erfahr. Hk. 1974, 23, e181–e184. [Google Scholar]
- Bocci, V.; Zanardi, I.; Travagli, V. Ozone: A new therapeutic agent in vascular diseases. Am. J. Cardiovasc. Drugs 2011, 11, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Bocci, V.A.; Zanardi, I.; Travagli, V. Ozone acting on human blood yields a hormetic dose-response relationship. J. Transl. Med. 2011, 9, 66. [Google Scholar] [CrossRef] [PubMed]
- Sagai, M.; Bocci, V. Mechanisms of Action Involved in Ozone Therapy: Is healing induced via a mild oxidative stress? Med. Gas Res. 2011, 1, 29. [Google Scholar] [CrossRef]
- Bocci, V.; Borrelli, E.; Travagli, V.; Zanardi, I. The ozone paradox: Ozone is a strong oxidant as well as a medical drug. Med. Res. Rev. 2009, 29, 646–682. [Google Scholar] [CrossRef]
- Guichardant, M.; Bacot, S.; Molière, P.; Lagarde, M. Hydroxy-alkenals from the peroxidation of n-3 and n-6 fatty acids and urinary metabolites. Prostaglandins Leukot. Essent. Fatty Acids 2006, 75, 179–182. [Google Scholar] [CrossRef]
- Poli, G.; Schaur, R.J.; Siems, W.G.; Leonarduzzi, G. 4-Hydroxynonenal: A membrane lipid oxidation product of medicinal interest. Med. Res. Rev. 2008, 28, 569–631. [Google Scholar] [CrossRef] [PubMed]
- Long, E.K.; Picklo, M.J., Sr. Trans-4-hydroxy-2-hexenal, a product of n-3 fatty acid peroxidation: Make some room HNE. Free Radic. Biol. Med. 2010, 49, 1–8. [Google Scholar] [CrossRef]
- Bocci, V.; Valacchi, G.; Corradeschi, F.; Aldinucci, C.; Silvestri, S.; Paccagnini, E.; Gerli, R. Studies on the biological effects of ozone: 7. Generation of reactive oxygen species (ROS) after exposure of human blood to ozone. J. Biol. Regul. Homeost. Agents 1998, 12, 67–75. [Google Scholar]
- Tricarico, G.; Travagli, V. The Relationship between Ozone and Human Blood in the Course of a Well-Controlled, Mild, and Transitory Oxidative Eustress. Antioxidants 2021, 10, 1946. [Google Scholar] [CrossRef] [PubMed]
- Cattel, F.; Giordano, S.; Bertiond, C.; Lupia, T.; Corcione, S.; Scaldaferri, M.; Angelone, L.; De Rosa, F.G. Ozone therapy in COVID-19: A narrative review. Virus Res. 2021, 291, 198207. [Google Scholar] [CrossRef]
- Niño-Sandoval, T.C.; Rocha, N.S.; Sarinho, F.W.; Vasconcelos, C.F.M.; Vasconcelos, A.F.M.; Vasconcelos, B.C. Effect of autohemotherapy in the treatment of viral infections—A systematic review. Public Health 2021, 201, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Tahmasebi, S.; Qasim, M.T.; Krivenkova, M.V.; Zekiy, A.O.; Thangavelu, L.; Aravindhan, S.; Izadi, M.; Jadidi-Niaragh, F.; Ghaebi, M.; Aslani, S.; et al. The effects of oxygen-ozone therapy on regulatory T-cell responses in multiple sclerosis patients. Cell Biol. Int. 2021, 45, 1498–1509. [Google Scholar] [CrossRef]
- An, J.X.; Wu, G.P.; Niu, K.; Wei, Y.P.; Liu, H.; Gao, X.Y.; Wu, J.P.; Wang, Y.; Renz, H.; Williams, J.P. Treatment of Femoral Head Osteonecrosis with Ozone Therapy: Pilot Trial of a New Therapeutic Approach. Pain Physician 2022, 25, E43–E54. [Google Scholar]
- Shen, W.; Liu, N.; Ji, Z.; Fang, H.; Liu, F.; Zhang, W.; Yu, X.; Wang, M.; Zhang, J.; Wang, X. Combining Ozonated Autohemotherapy with Pharmacological Therapy for Comorbid Insomnia and Myofascial Pain Syndrome: A Prospective Randomized Controlled Study. Pain Res. Manag. 2022, 2022, 3562191. [Google Scholar] [CrossRef] [PubMed]
- Serra, M.E.G.; Baeza-Noci, J.; Abdala, C.V.M.; Luvisotto, M.M.; Bertol, C.D.; Anzolin, A.P. Clinical effectiveness of medical ozone therapy in COVID-19: The evidence and gaps map. Med. Gas. Res. 2023, 13, 172–180. [Google Scholar] [CrossRef]
- Bocci, V.; Valacchi, G. Nrf2 activation as target to implement therapeutic treatments. Front. Chem. 2015, 3, 4. [Google Scholar] [CrossRef]
- Bocci, V. Autohaemotherapy after treatment of blood with ozone. A reappraisal. J. Int. Med. Res. 1994, 22, 131–144. [Google Scholar] [CrossRef]
- Bocci, V.; Valacchi, G.; Corradeschi, F.; Fanetti, G. Studies on the biological effects of ozone: 8. Effects on the total antioxidant status and on interleukin-8 production. Mediators Inflamm. 1998, 7, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Viebahn-Hänsler, R.; Fernández, O.S.L.; Fahmy, Z. Ozone in medicine: The low-dose ozone concept—Guidelines and treatment strategies. Ozone Sci. Eng. 2012, 34, 408–424. [Google Scholar] [CrossRef]
- Viebahn-Haensler, R.; Fernández, O.L. Ozone in medicine. The low-dose ozone concept and its basic biochemical mechanisms of action in chronic inflammatory diseases. Int. J. Mol. Sci. 2021, 22, 7890. [Google Scholar] [CrossRef]
- Travagli, V.; Zanardi, I.; Bocci, V. A realistic evaluation of the action of ozone on whole human blood. Int. J. Biol. Macromol. 2006, 39, 317–320. [Google Scholar] [CrossRef] [PubMed]
- Travagli, V.; Zanardi, I.; Silvietti, A.; Bocci, V. A physicochemical investigation on the effects of ozone on blood. Int. J. Biol. Macromol. 2007, 41, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Skorup, P.; Fransson, A.; Gustavsson, J.; Sjöholm, J.; Rundgren, H.; Özenci, V.; Wong, A.Y.W.; Karlsson, T.; Svensén, C.; Günther, M. Evaluation of an extracorporeal ozone-based bactericide system for the treatment of Escherichia coli sepsis. Intensive Care Med. Exp. 2022, 10, 14. [Google Scholar] [CrossRef] [PubMed]
- Bocci, V.; Aldinucci, C. Biochemical modifications induced in human blood by oxygenation-ozonation. J. Biochem. Mol. Toxicol. 2006, 20, 133–138. [Google Scholar] [CrossRef]
- Pecorelli, A.; Bocci, V.; Acquaviva, A.; Belmonte, G.; Gardi, C.; Virgili, F.; Ciccoli, L.; Valacchi, G. NRF2 activation is involved in ozonated human serum upregulation of HO-1 in endothelial cells. Toxicol. Appl. Pharmacol. 2013, 267, 30–40. [Google Scholar] [CrossRef]
- Re, L.; Martínez-Sánchez, G.; Bordicchia, M.; Malcangi, G.; Pocognoli, A.; Morales-Segura, M.A.; Rothchild, J.; Rojas, A. Is ozone pre-conditioning effect linked to Nrf2/EpRE activation pathway in vivo? A preliminary result. Eur. J. Pharmacol. 2014, 742, 158–162. [Google Scholar] [CrossRef]
- Delgado-Roche, L.; Riera-Romo, M.; Mesta, F.; Hernández-Matos, Y.; Barrios, J.M.; Martínez-Sánchez, G.; Al-Dalaien, S.M. Medical ozone promotes Nrf2 phosphorylation reducing oxidative stress and pro-inflammatory cytokines in multiple sclerosis patients. Eur. J. Pharmacol. 2017, 811, 148–154. [Google Scholar] [CrossRef]
- Galiè, M.; Costanzo, M.; Nodari, A.; Boschi, F.; Calderan, L.; Mannucci, S.; Covi, V.; Tabaracci, G.; Malatesta, M. Mild ozonisation activates antioxidant cell response by the Keap1/Nrf2 dependent pathway. Free Radic. Biol. Med. 2018, 124, 114–121. [Google Scholar] [CrossRef]
- Cisterna, B.; Costanzo, M.; Nodari, A.; Galiè, M.; Zanzoni, S.; Bernardi, P.; Covi, V.; Tabaracci, G.; Malatesta, M. Ozone Activates the Nrf2 Pathway and Improves Preservation of Explanted Adipose Tissue In Vitro. Antioxidants 2020, 9, 989. [Google Scholar] [CrossRef]
- Cisterna, B.; Costanzo, M.; Lacavalla, M.A.; Galiè, M.; Angelini, O.; Tabaracci, G.; Malatesta, M. Low Ozone Concentrations Differentially Affect the Structural and Functional Features of Non-Activated and Activated Fibroblasts In Vitro. Int. J. Mol. Sci. 2021, 22, 10133. [Google Scholar] [CrossRef] [PubMed]
- Cappellozza, E.; Costanzo, M.; Calderan, L.; Galiè, M.; Angelini, O.; Tabaracci, G.; Malatesta, M. Low ozone concentrations affect the structural and functional features of Jurkat T cells. Processes 2021, 9, 1030. [Google Scholar] [CrossRef]
- Lacavalla, M.A.; Inguscio, C.R.; Cisterna, B.; Bernardi, P.; Costanzo, M.; Galiè, M.; Scambi, I.; Angelini, O.; Tabaracci, G.; Malatesta, M. Ozone at low concentration modulates microglial activity in vitro: A multimodal microscopy and biomolecular study. Microsc. Res. Tech. 2022, 85, 3777–3792. [Google Scholar] [CrossRef]
- Ding, S.; Duanmu, X.; Xu, L.; Zhu, L.; Wu, Z. Ozone pretreatment alleviates ischemiareperfusion injury-induced myocardial ferroptosis by activating the Nrf2/Slc7a11/Gpx4 axis. Biomed. Pharmacother. 2023, 165, 115185. [Google Scholar] [CrossRef]
- Inguscio, C.R.; Cisterna, B.; Lacavalla, M.A.; Donati, F.; Angelini, O.; Tabaracci, G.; Malatesta, M. Ozone and procaine increase secretion of platelet-derived factors in platelet-rich plasma. Eur. J. Histochem. 2023, 67, 3879. [Google Scholar] [CrossRef] [PubMed]
- Larini, A.; Bocci, V. Effects of ozone on isolated peripheral blood mononuclear cells. Toxicol. In Vitro 2005, 19, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Cataldo, F. Ozone Degradation of Biological Macromolecules: Proteins, Hemoglobin, RNA, and DNA. Ozone Sci. Eng. 2006, 28, 317–328. [Google Scholar] [CrossRef]
- Sharma, V.K.; Graham, N.J.D. Oxidation of Amino Acids, Peptides and Proteins by Ozone: A Review. Ozone Sci. Eng. 2010, 32, 81–90. [Google Scholar] [CrossRef]
- Grosser, N.; Oberle, S.; Berndt, G.; Erdmann, K.; Hemmerle, A.; Schröder, H. Antioxidant action of L-alanine: Heme oxygenase-1 and ferritin as possible mediators. Biochem. Biophys. Res. Commun. 2004, 314, 351–355. [Google Scholar] [CrossRef]
- Reyes-Gonzales, M.C.; Fuentes-Broto, L.; Martínez-Ballarín, E.; Miana-Mena, F.J.; Berzosa, C.; García-Gil, F.A.; Aranda, M.; García, J.J. Effects of tryptophan and 5-hydroxytryptophan on the hepatic cell membrane rigidity due to oxidative stress. J. Membr. Biol. 2009, 231, 93–99. [Google Scholar] [CrossRef]
- Keithahn, C.; Lerchl, A. 5-hydroxytryptophan is a more potent in vitro hydroxyl radical scavenger than melatonin or vitamin C. J. Pineal Res. 2005, 38, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Liu, G.; Fu, C. The Tryptophan Pathway Targeting Antioxidant Capacity in the Placenta. Oxid. Med. Cell Longev. 2018, 2018, 1054797. [Google Scholar] [CrossRef] [PubMed]
- Buyuklu, M.; Kandemir, F.M.; Set, T.; Bakırcı, E.M.; Degirmenci, H.; Hamur, H.; Topal, E.; Kucukler, S.; Turkmen, K. Beneficial Effects of Ozone Therapy on Oxidative Stress, Cardiac Functions and Clinical Findings in Patients with Heart Failure Reduced Ejection Fraction. Cardiovasc. Toxicol. 2017, 17, 426–433. [Google Scholar] [CrossRef]
- Yuan, A.; Gong, L.; Luo, L.; Dang, J.; Gong, X.; Zhao, M.; Li, Y.; Li, Y.; Peng, C. Revealing anti-inflammation mechanism of water-extract and oil of forsythiae fructus on carrageenan-Induced edema rats by serum metabolomics. Biomed. Pharmacother. 2017, 95, 929–937. [Google Scholar] [CrossRef] [PubMed]
- Galiè, M.; Covi, V.; Tabaracci, G.; Malatesta, M. The Role of Nrf2 in the Antioxidant Cellular Response to Medical Ozone Exposure. Int. J. Mol. Sci. 2019, 20, 4009. [Google Scholar] [CrossRef] [PubMed]
- Nogawa, H.; Ishibashi, Y.; Ogawa, A.; Masuda, K.; Tsubuki, T.; Kameda, T.; Matsuzawa, S. Carbocisteine can scavenge reactive oxygen species in vitro. Respirology 2009, 14, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zheng, J.P.; Zhu, S.X.; Guan, W.J.; Chen, M.; Zhong, N.S. Carbocisteine attenuates hydrogen peroxide-induced inflammatory injury in A549 cells via NF-κB and ERK1/2 MAPK pathways. Int. Immunopharmacol. 2015, 24, 306–313. [Google Scholar] [CrossRef]
- Krishnan, N.; Dickman, M.B.; Becker, D.F. Proline modulates the intracellular redox environment and protects mammalian cells against oxidative stress. Free Radic. Biol. Med. 2008, 44, 671–681. [Google Scholar] [CrossRef]
- Vettore, L.A.; Westbrook, R.L.; Tennant, D.A. Proline metabolism and redox; maintaining a balance in health and disease. Amino Acids 2021, 53, 1779–1788. [Google Scholar] [CrossRef]
- Bocci, V.; Zanardi, I.; Huijberts, M.S.; Travagli, V. Diabetes and chronic oxidative stress. A perspective based on the possible usefulness of ozone therapy. Diabetes Metab. Syndr. 2011, 5, 45–49. [Google Scholar] [CrossRef]
- Tang, X.; Liu, J.; Dong, W.; Li, P.; Li, L.; Lin, C.; Zheng, Y.; Hou, J.; Li, D. The cardioprotective effects of citric Acid and L-malic Acid on myocardial ischemia/reperfusion injury. Evid. Based Complement. Alternat. Med. 2013, 2013, 820695. [Google Scholar] [CrossRef]
- Koriem, K.M.M.; Tharwat, H.A.K. Malic Acid Improves Behavioral, Biochemical, and Molecular Disturbances in the Hypothalamus of Stressed Rats. J. Integr. Neurosci. 2023, 22, 98. [Google Scholar] [CrossRef]
- Jonsson, P.; Antti, H.; Späth, F.; Melin, B.; Björkblom, B. Identification of Pre-Diagnostic Metabolic Patterns for Glioma Using Subset Analysis of Matched Repeated Time Points. Cancers 2020, 12, 3349. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, M.; Yin, A.; Chen, M.; Tan, N.; Wang, M.; Zhang, Y.; Ye, H.; Zhang, X.; Zhou, W. Serum metabolomics reveals the effect of electroacupuncture on urinary leakage in women with stress urinary incontinence. J. Pharm. Biomed. Anal. 2020, 190, 113513. [Google Scholar] [CrossRef]
- Bennett, M.J.; Ragni, M.C.; Hood, I.; Hale, D.E. Azelaic and pimelic acids: Metabolic intermediates or artefacts? J. Inherit. Metab. Dis. 1992, 15, 220–223. [Google Scholar] [CrossRef]
- Fatima, T.; Hashmi, S.; Iqbal, A.; Siddiqui, A.J.; Sami, S.A.; Basir, N.; Bokhari, S.S.; Sharif, H.; Musharraf, S.G. Untargeted metabolomic analysis of coronary artery disease patients with diastolic dysfunction show disturbed oxidative pathway. Metabolomics 2019, 15, 98. [Google Scholar] [CrossRef]
- Björkblom, B.; Wibom, C.; Jonsson, P.; Mörén, L.; Andersson, U.; Johannesen, T.B.; Langseth, H.; Antti, H.; Melin, B. Metabolomic screening of pre-diagnostic serum samples identifies association between α- and γ-tocopherols and glioblastoma risk. Oncotarget 2016, 7, 37043–37053. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.S.; Zhang, P.; Liu, Y.H.; Xu, Q.; Zhang, Y.; Li, H.Z.; Liu, L.; Liu, Y.M.; Yang, X.Y.; Xue, C.Y. Caprylic Acid Improves Lipid Metabolism, Suppresses the Inflammatory Response and Activates the ABCA1/p-JAK2/p-STAT3 Signaling Pathway in C57BL/6J Mice and RAW264.7 Cells. Biomed. Environ. Sci. 2022, 35, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Orešič, M.; Hyötyläinen, T.; Herukka, S.K.; Sysi-Aho, M.; Mattila, I.; Seppänan-Laakso, T.; Julkunen, V.; Gopalacharyulu, P.V.; Hallikainen, M.; Koikkalainen, J.; et al. Metabolome in progression to Alzheimer’s disease. Transl. Psychiatry 2011, 1, e57. [Google Scholar] [CrossRef] [PubMed]
- Shao, X.; Wang, K.; Liu, X.; Gu, C.; Zhang, P.; Xie, J.; Liu, W.; Sun, L.; Chen, T.; Li, Y. Screening and verifying endometrial carcinoma diagnostic biomarkers based on a urine metabolomic profiling study using UPLC-Q-TOF/MS. Clin. Chim. Acta 2016, 463, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Levy, E.; Rizwan, Y.; Thibault, L.; Lepage, G.; Brunet, S.; Bouthillier, L.; Seidman, E. Altered lipid profile, lipoprotein composition, and oxidant and antioxidant status in pediatric Crohn disease. Am. J. Clin. Nutr. 2000, 71, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Ragino, Y.I.; Shramko, V.S.; Stakhneva, E.M.; Chernyak, E.I.; Morozov, S.V.; Shakhtshneider, E.V.; Polonskaya, Y.V.; Shcherbakova, L.V.; Chernyavskiy, A.M. Changes in the blood fatty-acid profile associated with oxidative-antioxidant disturbances in coronary atherosclerosis. J. Med. Biochem. 2020, 39, 46–53. [Google Scholar] [CrossRef]
- Chen, S.J.; Chuang, L.T.; Chen, S.N. Incorporation of eicosatrienoic acid exerts mild anti-inflammatory properties in murine RAW264.7 cells. Inflammation 2015, 38, 534–545. [Google Scholar] [CrossRef] [PubMed]
- Vilakazi, H.; Olasehinde, T.A.; Olaniran, A.O. Chemical Characterization, Antiproliferative and Antioxidant Activities of Polyunsaturated Fatty Acid-Rich Extracts from Chlorella sp. S14. Molecules 2021, 26, 4109. [Google Scholar] [CrossRef]
- Parcheta, M.; Świsłocka, R.; Świderski, G.; Matejczyk, M.; Lewandowski, W. Spectroscopic Characterization and Antioxidant Properties of Mandelic Acid and Its Derivatives in a Theoretical and Experimental Approach. Materials 2022, 15, 5413. [Google Scholar] [CrossRef] [PubMed]
- Crout, R.J.; Gilbertson, J.R.; Gilbertson, J.D.; Platt, D.; Langkamp, H.H.; Connamacher, R.H. Effect of linolenyl alcohol on the in-vitro growth of the oral bacterium Streptococcus mutans. Arch. Oral Biol. 1982, 27, 1033–1037. [Google Scholar] [CrossRef]
- Kobori, M.; Nakayama, H.; Fukushima, K.; Ohnishi-Kameyama, M.; Ono, H.; Fukushima, T.; Akimoto, Y.; Masumoto, S.; Yukizaki, C.; Hoshi, Y.; et al. Bitter gourd suppresses lipopolysaccharide-induced inflammatory responses. J. Agric. Food Chem. 2008, 56, 4004–4011. [Google Scholar] [CrossRef]
- Serhan, C.N.; Dalli, J.; Colas, R.A.; Winkler, J.W.; Chiang, N. Protectins and maresins: New pro-resolving families of mediators in acute inflammation and resolution bioactive metabolome. Biochim. Biophys. Acta 2015, 1851, 397–413. [Google Scholar] [CrossRef]
- Harayama, T.; Shimizu, T. Roles of polyunsaturated fatty acids, from mediators to membranes. J. Lipid Res. 2020, 61, 1150–1160. [Google Scholar] [CrossRef]
- Jantwal, A.; Durgapal, S.; Upadhyay, J.; Joshi, T.; Kumar, A. Tartaric acid. In Antioxidants Effects in Health—The Bright and the Dark Side, 1st ed.; Nabavi, S.M., Sanches Silva, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 485–492. [Google Scholar] [CrossRef]
- Santa-María, C.; López-Enríquez, S.; Montserrat-de la Paz, S.; Geniz, I.; Reyes-Quiroz, M.E.; Moreno, M.; Palomares, F.; Sobrino, F.; Alba, G. Update on Anti-Inflammatory Molecular Mechanisms Induced by Oleic Acid. Nutrients 2023, 15, 224. [Google Scholar] [CrossRef]
- Lee, S.I.; Kang, K.S. Function of capric acid in cyclophosphamide-induced intestinal inflammation, oxidative stress, and barrier function in pigs. Sci. Rep. 2017, 7, 16530. [Google Scholar] [CrossRef] [PubMed]
- Jayaraj, R.L.; Beiram, R.; Azimullah, S.; Mf, N.M.; Ojha, S.K.; Adem, A.; Jalal, F.Y. Valeric Acid Protects Dopaminergic Neurons by Suppressing Oxidative Stress, Neuroinflammation and Modulating Autophagy Pathways. Int. J. Mol. Sci. 2020, 21, 7670. [Google Scholar] [CrossRef]
- Denu, J.M. Vitamins and aging: Pathways to NAD+ synthesis. Cell 2007, 129, 453–454. [Google Scholar] [CrossRef] [PubMed]
- Xie, N.; Zhang, L.; Gao, W.; Huang, C.; Huber, P.E.; Zhou, X.; Li, C.; Shen, G.; Zou, B. NAD+ metabolism: Pathophysiologic mechanisms and therapeutic potential. Signal Transduct. Target Ther. 2020, 5, 227. [Google Scholar] [CrossRef] [PubMed]
- Kamat, J.P.; Devasagayam, T.P. Nicotinamide (vitamin B3) as an effective antioxidant against oxidative damage in rat brain mitochondria. Redox Rep. 1999, 4, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Hirvonen, O.P.; Kyröläinen, H.; Lehti, M.; Kainulainen, H. Randomized Trial: D-Glyceric Acid Activates Mitochondrial Metabolism in 50–60- Year-Old Healthy Humans. Front. Aging 2021, 2, 752636. [Google Scholar] [CrossRef] [PubMed]
- Hira, H.S.; Samal, P.; Kaur, A.; Kapoor, S. Plasma level of hypoxanthine/xanthine as markers of oxidative stress with different stages of obstructive sleep apnea syndrome. Ann. Saudi Med. 2014, 34, 308–313. [Google Scholar] [CrossRef]
- Krylova, I.B.; Bulion, V.V.; Selina, E.N.; Mironova, G.D.; Sapronov, N.S. Effect of uridine on energy metabolism, LPO, and antioxidant system in the myocardium under conditions of acute coronary insufficiency. Bull. Exp. Biol. Med. 2012, 153, 644–646. [Google Scholar] [CrossRef]
- Khezri, M.K.; Turkkan, A.; Koc, C.; Salman, B.; Levent, P.; Cakir, A.; Kafa, I.M.; Cansev, M.; Bekar, A. Anti-Apoptotic and Anti-Oxidant Effects of Systemic Uridine Treatment in an Experimental Model of Sciatic Nerve Injury. Turk. Neurosurg. 2021, 31, 373–378. [Google Scholar] [CrossRef]
- Mishur, R.J.; Khan, M.; Munkácsy, E.; Sharma, L.; Bokov, A.; Beam, H.; Radetskaya, O.; Borror, M.; Lane, R.; Bai, Y.; et al. Mitochondrial metabolites extend lifespan. Aging Cell 2016, 15, 336–348. [Google Scholar] [CrossRef]
- Barberis, E.; Amede, E.; Tavecchia, M.; Marengo, E.; Cittone, M.G.; Rizzi, E.; Pedrinelli, A.R.; Tonello, S.; Minisini, R.; Pirisi, M.; et al. Understanding protection from SARS-CoV-2 using metabolomics. Sci. Rep. 2021, 11, 13796. [Google Scholar] [CrossRef] [PubMed]


| 5 μg O3 | 10 μg O3 | 5 μg O3 and 10 μg O3 |
|---|---|---|
| (8Z,11Z,14Z)-Icosa-8,11,14-trienoate (↓) | 1-Deoxypentitol (↓) | 2-Butenedioic acid (↑) |
| Aceturic acid (↑) | 2,4-Pyridinedicarboxylic acid (↑) | 5-Hydroxytryptophan (↑) |
| D-Glucose (↓) | 2-Hydroxy-3-methylbutyric acid (↑) | 9(E),11(E)-Conjugated linoleic acid, trimethylsilyl ester (↑) |
| Erythrono-1,4-lactone (↑) | 3-Octenoic acid (↑) | Adenine (↓) |
| 4-Hydroxybenzeneacetic acid (↓) | Arabinofuranose, 1,2,3,5-tetrakis-O- (↓) | |
| 9H-Purin-6-ol (↓) | Benzenepropanoic acid, 3,5-bis(1,1-dimethylethyl)-4-hydroxy-, methyl ester (↓) | |
| 9-Octadecen-1-ol (↓) | L-Glutamic acid, bis(trimethylsilyl) ester (↑) | |
| Arachidic acid (↓) | Malic acid (↑) | |
| Behenic acid (↓) | Niacinamide (↓) | |
| Butanoic acid, 2,4-bis[(trimethylsilyl)oxy]-, trimethylsilyl ester (↑) | Ornithine (↑) | |
| Campesterol (↓) | Pentanedioic acid (↓) | |
| Decanoic acid (↑) | Picolinic acid (↑) | |
| Glucuronolactone, trisO-(trimethylsilyl)- (↓) | Pyrrole-2-carboxylic acid (↑) | |
| Glyceric acid (↑) | Stearic Acid (↓) | |
| 3-Indoleacetic acid (↑) | Tartaric acid (↓) | |
| Indole-3-lactic acid (↑) | ||
| Lanopalmitic acid (↓) | ||
| L-Proline (↑) | ||
| L-Threonine (↑) | ||
| L-Tyrosine (↓) | ||
| N-Acetyl-L-alanine (↑) | ||
| N-Isobutyrylglycine (↑) | ||
| Oleic acid (↑) | ||
| Pentanoic acid (↑) | ||
| Pimelic acid (↑) | ||
| S-Carboxymethyl-L-cysteine (↑) | ||
| Uridine (↓) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inguscio, C.R.; Cisterna, B.; Carton, F.; Barberis, E.; Manfredi, M.; Malatesta, M. Modifications of Blood Molecular Components after Treatment with Low Ozone Concentrations. Int. J. Mol. Sci. 2023, 24, 17175. https://doi.org/10.3390/ijms242417175
Inguscio CR, Cisterna B, Carton F, Barberis E, Manfredi M, Malatesta M. Modifications of Blood Molecular Components after Treatment with Low Ozone Concentrations. International Journal of Molecular Sciences. 2023; 24(24):17175. https://doi.org/10.3390/ijms242417175
Chicago/Turabian StyleInguscio, Chiara Rita, Barbara Cisterna, Flavia Carton, Elettra Barberis, Marcello Manfredi, and Manuela Malatesta. 2023. "Modifications of Blood Molecular Components after Treatment with Low Ozone Concentrations" International Journal of Molecular Sciences 24, no. 24: 17175. https://doi.org/10.3390/ijms242417175





