One Health Determinants of Escherichia coli Antimicrobial Resistance in Humans in the Community: An Umbrella Review
Abstract
:1. Introduction
2. Results
2.1. Review Characteristics
2.2. Quality Assessment
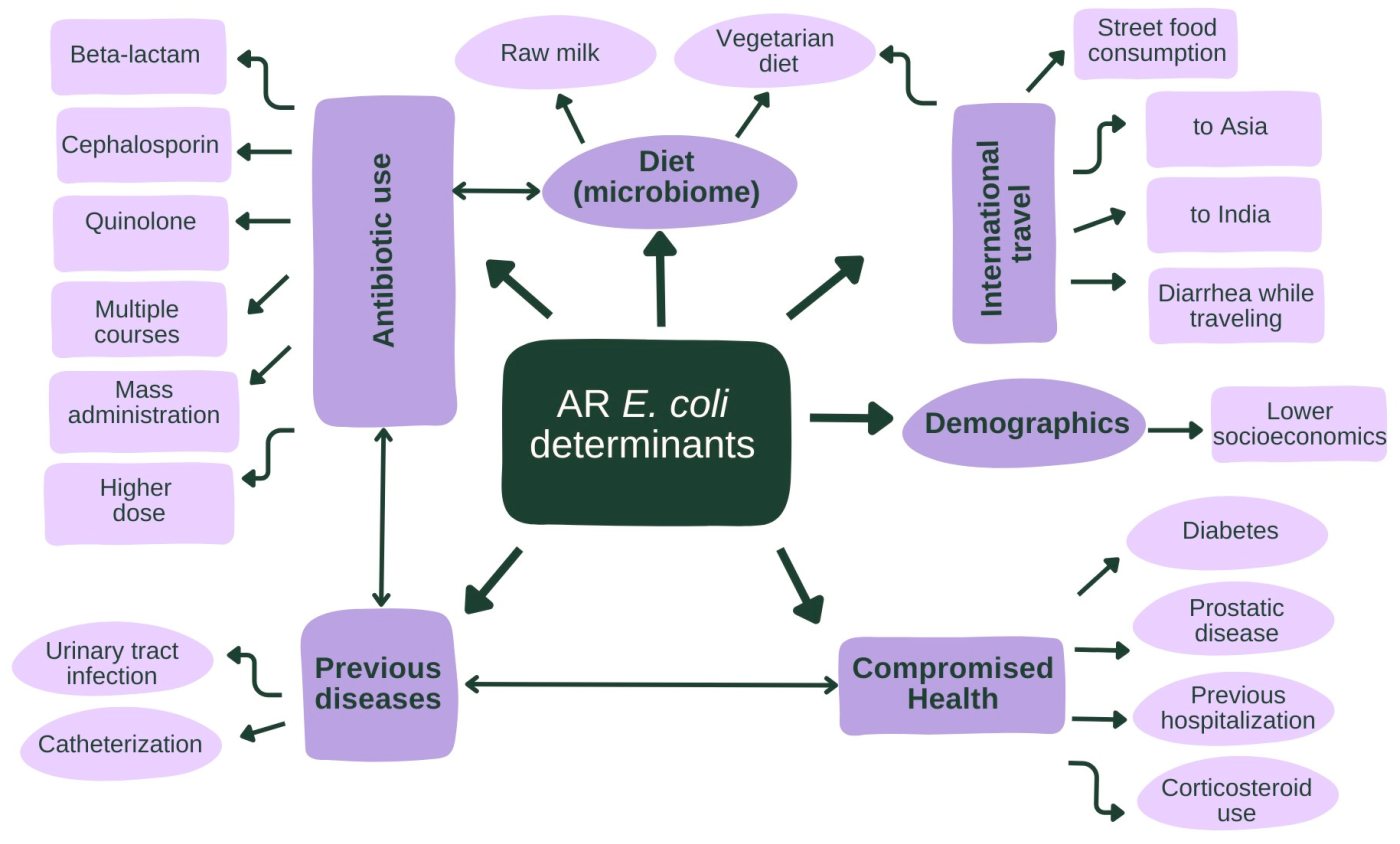
2.3. Human Variables
2.3.1. Antibiotic Use
2.3.2. Comorbidities, Medication Use, and Hospitalization
2.3.3. Diet, Sex, Age, and Living
2.3.4. Travel
2.4. Animal and Environmental Variables
2.5. Temporal Relationship Variable and AMR E. coli
3. Discussion
4. Materials and Methods
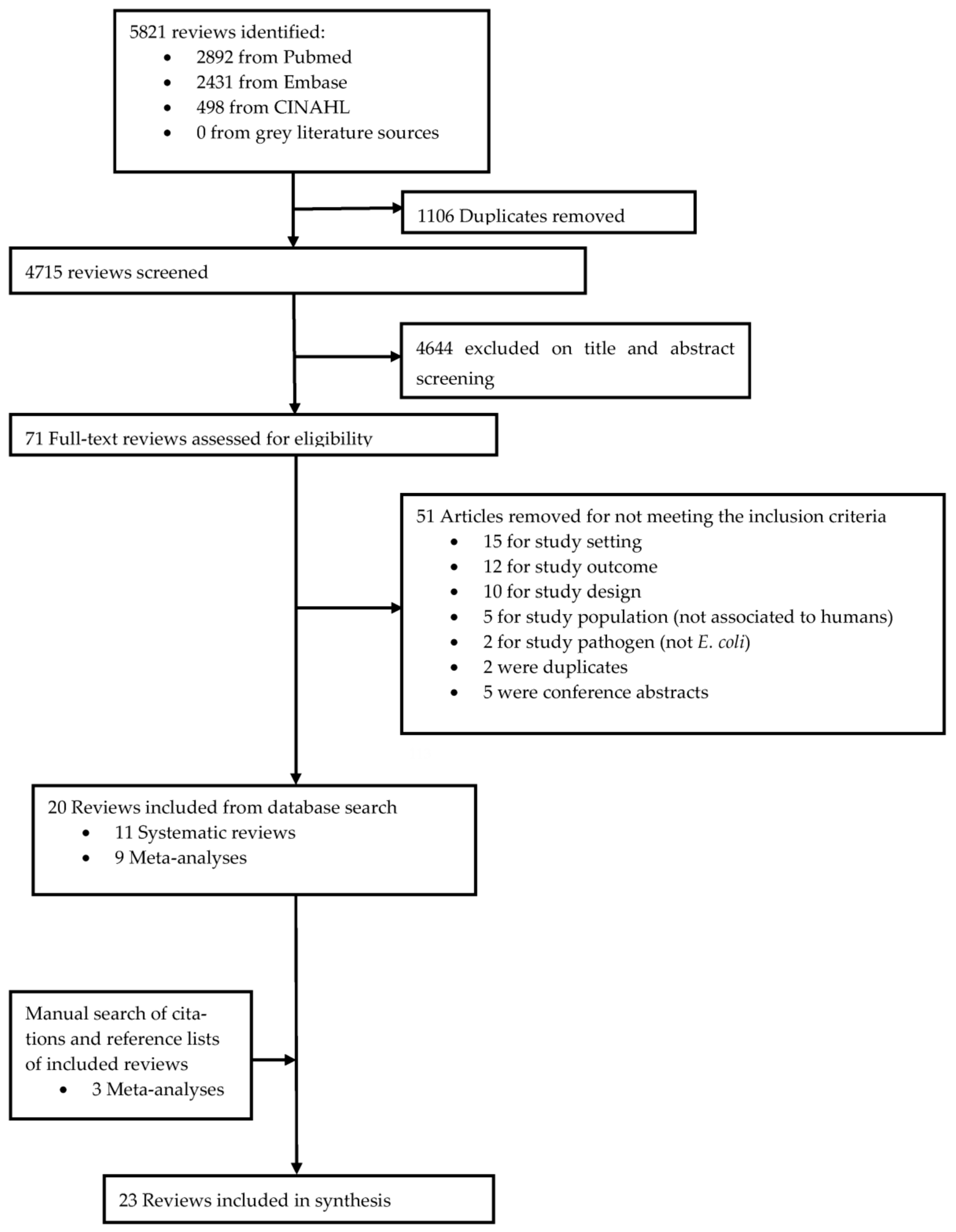
4.1. The Search Strategy and Selection Criteria
4.2. Data Extraction
4.3. Assessment of Methodological Quality
4.4. Data Synthesis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- World Health Organization (WHO). Antibiotic Resistance. Available online: https://www.who.int/news-room/fact-sheets/detail/antibiotic-resistance (accessed on 5 July 2023).
- Holmes, A.H.; Moore, L.S.P.; Sundsfjord, A.; Steinbakk, M.; Regmi, S.; Karkey, A.; Guerin, P.J.; Piddock, L.J.V. Understanding the mechanisms and drivers of antimicrobial resistance. Lancet 2016, 387, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Stanton, I.C.; Bethel, A.; Leonard, A.F.C.; Gaze, W.H.; Garside, R. Existing evidence on antibiotic resistance exposure and transmission to humans from the environment: A systematic map. Environ. Evid. 2022, 11, 8. [Google Scholar] [CrossRef]
- Rahman, M.; Alam, M.-U.; Luies, S.K.; Kamal, A.; Ferdous, S.; Lin, A.; Sharior, F.; Khan, R.; Rahman, Z.; Parvez, S.M.; et al. Contamination of Fresh Produce with Antibiotic-Resistant Bacteria and Associated Risks to Human Health: A Scoping Review. Int. J. Environ. Res. Public Health 2022, 19, 360. [Google Scholar] [CrossRef] [PubMed]
- Acar, J.; Röstel, B. Antimicrobial resistance: An overview. Rev. Sci. Et Tech. (Int. Off. Epizoot.) 2002, 20, 797–810. [Google Scholar] [CrossRef]
- O’Neill, C.B.J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations; Government of the United Kingdom: London, UK, 2016. [Google Scholar]
- Abbas, S.S.; Shorten, T.; Rushton, J. Meanings and Mechanisms of One Health Partnerships: Insights from a Critical Review of Literature on Cross-Government Collaborations. Health Policy Plan. 2022, 37, 385–399. [Google Scholar] [CrossRef]
- Jang, J.; Hur, H.G.; Sadowsky, M.J.; Byappanahalli, M.N.; Yan, T.; Ishii, S. Environmental Escherichia coli: Ecology and public health implications—A review. J. Appl. Microbiol. 2017, 123, 570–581. [Google Scholar] [CrossRef] [PubMed]
- Pouwels, K.B.; Muller-Pebody, B.; Smieszek, T.; Hopkins, S.; Robotham, J.V. Selection and co-selection of antibiotic resistances among Escherichia coli by antibiotic use in primary care: An ecological analysis. PLoS ONE 2019, 14, e0218134. [Google Scholar] [CrossRef]
- World Health Organization (WHO). WHO Integrated Global Surveillance on ESBL-Producing E. coli Using a “One Health” Approach: Implementation and Opportunities. Available online: https://www.who.int/publications/i/item/9789240021402 (accessed on 3 March 2023).
- World Health Organization (WHO). Global Antimicrobial Resistance and Use Surveillance System (GLASS) Report 2022. 2022. Available online: https://www.who.int/publications/i/item/9789240062702 (accessed on 3 March 2023).
- Poirel, L.; Madec, J.-Y.; Lupo, A.; Schink, A.-K.; Kieffer, N.; Nordmann, P.; Schwarz, S. Antimicrobial Resistance in Escherichia coli. Microbiol. Spectr. 2018, 6, 4. [Google Scholar] [CrossRef]
- Paitan, Y. Current Trends in Antimicrobial Resistance of Escherichia coli. In Escherichia coli, a Versatile Pathogen; Frankel, G., Ron, E.Z., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 181–211. [Google Scholar]
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Matsui, Y.; Riley, L.W. Risk factors for fecal carriage of drug-resistant Escherichia coli: A systematic review and meta-analysis. Antimicrob. Resist. Infect. Control 2020, 9, 31. [Google Scholar] [CrossRef]
- Terlizzi, M.E.; Gribaudo, G.; Maffei, M.E. UroPathogenic Escherichia coli (UPEC) Infections: Virulence Factors, Bladder Responses, Antibiotic, and Non-antibiotic Antimicrobial Strategies. Front. Microbiol. 2017, 8, 1566. [Google Scholar] [CrossRef]
- Nicolle, L.E. Uncomplicated urinary tract infection in adults including uncomplicated pyelonephritis. Urol. Clin. N. Am. 2008, 35, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Flores-Mireles, A.L.; Walker, J.N.; Caparon, M.; Hultgren, S.J. Urinary tract infections: Epidemiology, mechanisms of infection and treatment options. Nat. Rev. Microbiol. 2015, 13, 269–284. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Gao, H.; Zheng, L.; Liu, S.; Cao, Y.; Zhu, S.; Wu, Z.; Ren, H.; Mao, D.; Luo, Y. Antibiotic Resistance and Virulence of Extraintestinal Pathogenic Escherichia coli (ExPEC) Vary According to Molecular Types. Front. Microbiol. 2020, 11, 598305. [Google Scholar] [CrossRef] [PubMed]
- Rozwadowski, M.; Gawel, D. Molecular Factors and Mechanisms Driving Multidrug Resistance in Uropathogenic Escherichia coli—An Update. Genes 2022, 13, 1397. [Google Scholar] [CrossRef]
- Johnston, B.D.; Thuras, P.; Porter, S.B.; Anacker, M.; VonBank, B.; Vagnone, P.S.; Witwer, M.; Castanheira, M.; Johnson, J.R. Global molecular epidemiology of carbapenem-resistant Escherichia coli (2002–2017). Eur. J. Clin. Microbiol. 2021, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Dadashi, M.; Sameni, F.; Bostanshirin, N.; Yaslianifard, S.; Khosravi-Dehaghi, N.; Nasiri, M.J.; Goudarzi, M.; Hashemi, A.; Hajikhani, B. Global prevalence and molecular epidemiology of mcr-mediated colistin resistance in Escherichia coli clinical isolates: A systematic review. J. Glob. Antimicrob. Resist. 2021, 29, 444–461. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Multidrug-Resistant E. coli. 2019. Available online: https://arpsp.cdc.gov/profile/antibiotic-resistance/mdr-ecoli (accessed on 3 March 2023).
- O’Donoghue, G.; Kennedy, A.; Puggina, A.; Aleksovska, K.; Buck, C.; Burns, C.; Cardon, G.; Carlin, A.; Ciarapica, D.; Colotto, M.; et al. Socio-economic determinants of physical activity across the life course: A “DEterminants of DIet and Physical Activity” (DEDIPAC) umbrella literature review. PLoS ONE 2018, 13, e0190737. [Google Scholar] [CrossRef]
- Hayes, A.F. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach; Guilford Press: New York, NY, USA, 2013; pp. 3–22. [Google Scholar]
- Schwaber, M.J.; De-Medina, T.; Carmeli, Y. Epidemiological interpretation of antibiotic resistance studies—What are we missing? Nat. Rev. Microbiol. 2004, 2, 979–983. [Google Scholar] [CrossRef]
- Davison, H.C.; Low, J.C.; Woolhouse, M.E. What is antibiotic resistance and how can we measure it? Trends Microbiol. 2000, 8, 554–559. [Google Scholar] [CrossRef]
- Chatterjee, A.; Modarai, M.; Naylor, N.R.; Boyd, S.E.; Atun, R.; Barlow, J.; Holmes, A.H.; Johnson, A.; Robotham, J.V. Quantifying drivers of antibiotic resistance in humans: A systematic review. Lancet Infect. Dis. 2018, 18, e368–e378. [Google Scholar] [CrossRef] [PubMed]
- Mughini-Gras, L.; Dorado-García, A.; van Duijkeren, E.; van den Bunt, G.; Dierikx, C.M.; Bonten, M.J.M.; Bootsma, M.C.J.; Schmitt, H.; Hald, T.; Evers, E.G.; et al. Attributable sources of community-acquired carriage of Escherichia coli containing beta-lactam antibiotic resistance genes: A population-based modelling study. Lancet Planet. Health 2019, 3, e357–e369. [Google Scholar] [CrossRef] [PubMed]
- Iskandar, K.; Molinier, L.; Hallit, S.; Sartelli, M.; Catena, F.; Coccolini, F.; Hardcastle, T.C.; Roques, C.; Salameh, P. Drivers of antibiotic resistance transmission in low-and middle-income countries from a “one health” perspective—A review. Antibiotics 2020, 9, 372. [Google Scholar] [CrossRef]
- King, T.; Schindler, R.; Chavda, S.; Conly, J. Dimensions of poverty as risk factors for antimicrobial resistant organisms in Canada: A structured narrative review. Antimicrob. Resist. Infect. Control 2022, 11, 18. [Google Scholar] [CrossRef] [PubMed]
- Medina-Pizzali, M.L.; Hartinger, S.M.; Salmon-Mulanovich, G.; Larson, A.; Riveros, M.; Mäusezahl, D. Antimicrobial Resistance in Rural Settings in Latin America: A Scoping Review with a One Health Lens. Int. J. Environ. Res. Public. Health 2021, 18, 9837. [Google Scholar] [CrossRef] [PubMed]
- Aromataris, E.; Fernandez, R.; Godfrey, C.M.; Holly, C.; Khalil, H.; Tungpunkom, P. Summarizing systematic reviews: Methodological development, conduct and reporting of an umbrella review approach. Int. J. Evid. Based Healthc. 2015, 13, 132–140. [Google Scholar] [CrossRef]
- Alividza, V.; Mariano, V.; Ahmad, R.; Charani, E.; Rawson, T.M.; Holmes, A.H.; Castro-Sanchez, E. Investigating the impact of poverty on colonization and infection with drug-resistant organisms in humans: A systematic review. Infect. Dis. Poverty 2018, 7, 76. [Google Scholar] [CrossRef]
- Bakhit, M.; Hoffmann, T.; Scott, A.M.; Beller, E.; Rathbone, J.; Del Mar, C. Resistance decay in individuals after antibiotic exposure in primary care: A systematic review and meta-analysis. BMC Med. 2018, 16, 126. [Google Scholar] [CrossRef]
- Bell, B.G.; Schellevis, F.; Stobberingh, E.; Goossens, H.; Pringle, M. A systematic review and meta-analysis of the effects of antibiotic consumption on antibiotic resistance. BMC Infect. Dis. 2014, 14, 13. [Google Scholar] [CrossRef]
- Bhate, K.; Lin, L.Y.; Barbieri, J.S.; Leyrat, C.; Hopkins, S.; Stabler, R.; Shallcross, L.; Smeeth, L.; Francis, N.; Mathur, R.; et al. Is there an association between long-term antibiotics for acne and subsequent infection sequelae and antimicrobial resistance? A systematic review. BJGP Open 2021, 5, BJGPO.2020.0181. [Google Scholar] [CrossRef] [PubMed]
- Bryce, A.; Costelloe, C.; Hawcroft, C.; Wootton, M.; Hay, A.D. Faecal carriage of antibiotic resistant Escherichia coli in asymptomatic children and associations with primary care antibiotic prescribing: A systematic review and meta-analysis. BMC Infect. Dis. 2016, 16, 359. [Google Scholar] [CrossRef]
- Bryce, A.; Hay, A.D.; Lane, I.F.; Thornton, H.V.; Wootton, M.; Costelloe, C. Global prevalence of antibiotic resistance in paediatric urinary tract infections caused by Escherichia coli and association with routine use of antibiotics in primary care: Systematic review and meta-analysis. BMJ 2016, 352, i939. [Google Scholar] [CrossRef] [PubMed]
- Butcher, C.R.; Rubin, J.; Mussio, K.; Riley, L.W. Risk Factors Associated with Community-Acquired Urinary Tract Infections Caused by Extended-Spectrum β-Lactamase-Producing Escherichia coli: A Systematic Review. Curr. Epidemiol. Rep. 2019, 6, 300–309. [Google Scholar] [CrossRef]
- Chan, Y.Q.; Chen, K.; Chua, G.T.; Wu, P.; Tung, K.T.; Tsang, H.W.; Lung, D.; Ip, P.; Chui, C.S. Risk factors for carriage of antimicrobial-resistant bacteria in community dwelling-children in the Asia-Pacific region: A systematic review and meta-analysis. JAC-Antimicrob. Resist. 2022, 4, dlac036. [Google Scholar] [CrossRef] [PubMed]
- Costelloe, C.; Metcalfe, C.; Lovering, A.; Mant, D.; Hay, A.D. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: Systematic review and meta-analysis. BMJ 2010, 340, c2096. [Google Scholar] [CrossRef] [PubMed]
- Furuya-Kanamori, L.; Stone, J.; Yakob, L.; Kirk, M.; Collignon, P.; Mills, D.J.; Lau, C.L. Risk factors for acquisition of multidrug-resistant Enterobacterales among international travellers: A synthesis of cumulative evidence. J. Travel. Med. 2020, 27, taz083. [Google Scholar] [CrossRef] [PubMed]
- Hackmann, C.; Gastmeier, P.; Schwarz, S.; Lübke-Becker, A.; Bischoff, P.; Leistner, R. Pet husbandry as a risk factor for colonization or infection with MDR organisms: A systematic meta-analysis—authors’ response. J. Antimicrob. Chemother. 2022, 77, 2043. [Google Scholar] [CrossRef] [PubMed]
- Hassing, R.J.; Alsma, J.; Arcilla, M.S.; van Genderen, P.J.; Stricker, B.H.; Verbon, A. International travel and acquisition of multidrug-resistant Enterobacteriaceae: A systematic review. Euro Surveill. 2015, 20, 30074. [Google Scholar] [CrossRef]
- Karanika, S.; Karantanos, T.; Arvanitis, M.; Grigoras, C.; Mylonakis, E. Fecal Colonization with Extended-spectrum Beta-lactamase–Producing Enterobacteriaceae and Risk Factors Among Healthy Individuals: A Systematic Review and Metaanalysis. Clin. Infect. Dis. 2016, 63, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Köck, R.; Daniels-Haardt, I.; Becker, K.; Mellmann, A.; Friedrich, A.W.; Mevius, D.; Schwarz, S.; Jurke, A. Carbapenem-resistant Enterobacteriaceae in wildlife, food-producing, and companion animals: A systematic review. Clin. Microbiol. Infect. 2018, 24, 1241–1250. [Google Scholar] [CrossRef]
- Larramendy, S.; Deglaire, V.; Dusollier, P.; Fournier, J.P.; Caillon, J.; Beaudeau, F.; Moret, L. Risk factors of extended-spectrum beta-lactamases-producing Escherichia coli community acquired urinary tract infections: A systematic review. Infect. Drug Resist. 2020, 13, 3945–3955. [Google Scholar] [CrossRef] [PubMed]
- Lazarus, B.; Paterson, D.L.; Mollinger, J.L.; Rogers, B.A. Do human extraintestinal Escherichia coli infections resistant to expanded-spectrum cephalosporins originate from food-producing animals? A systematic review. Clin. Infect. Dis. 2015, 60, 439–452. [Google Scholar] [CrossRef]
- Messina, N.L.; Williamson, D.A.; Robins-Browne, R.; Bryant, P.A.; Curtis, N. Risk Factors for Carriage of Antibiotic-resistant Bacteria in Healthy Children in the Community: A Systematic Review. Pediatr. Infect. Dis. J. 2020, 39, 397–405. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, K.S.; Emerson, P.; Hooper, P.J.; Reingold, A.L.; Dennis, E.G.; Keenan, J.D.; Lietman, T.M.; Oldenburg, C.E. Antimicrobial resistance following mass azithromycin distribution for trachoma: A systematic review. Lancet Infect. Dis. 2019, 19, e14–e25. [Google Scholar] [CrossRef] [PubMed]
- Ramblière, L.; Guillemot, D.; Delarocque-Astagneau, E.; Huynh, B.T. Impact of mass and systematic antibiotic administration on antibiotic resistance in low- and middle-income countries? A systematic review. Int. J. Antimicrob. Agents 2021, 58, 106364. [Google Scholar] [CrossRef] [PubMed]
- Truong, R.; Tang, V.; Grennan, T.; Tan, D.H.S. A systematic review of the impacts of oral tetracycline class antibiotics on antimicrobial resistance in normal human flora. JAC Antimicrob. Resist. 2022, 4, dlac009. [Google Scholar] [CrossRef]
- Voor In ’t Holt, A.F.; Mourik, K.; Beishuizen, B.; van der Schoor, A.S.; Verbon, A.; Vos, M.C.; Severin, J.A. Acquisition of multidrug-resistant Enterobacterales during international travel: A systematic review of clinical and microbiological characteristics and meta-analyses of risk factors. Antimicrob. Resist. Infect. Control 2020, 9, 71. [Google Scholar] [CrossRef]
- Willems, R.P.J.; van Dijk, K.; Ket, J.C.F.; Vandenbroucke-Grauls, C. Evaluation of the Association Between Gastric Acid Suppression and Risk of Intestinal Colonization with Multidrug-Resistant Microorganisms: A Systematic Review and Meta-analysis. JAMA Intern. Med. 2020, 180, 561–571. [Google Scholar] [CrossRef]
- Campos-Madueno, E.I.; Moradi, M.; Eddoubaji, Y.; Shahi, F.; Moradi, S.; Bernasconi, O.J.; Moser, A.I.; Endimiani, A. Intestinal colonization with multidrug-resistant Enterobacterales: Screening, epidemiology, clinical impact, and strategies to decolonize carriers. Eur. J. Clin. Microbiol. Infect. Dis. 2023, 42, 229–254. [Google Scholar] [CrossRef]
- Manaia, C.M.; Donner, E.; Vaz-Moreira, I.; Hong, P. Antibiotic Resistance in the Environment: A Worldwide Overview; Springer Nature: Cham, Switzerland, 2020; Volume 91. [Google Scholar]
- Meinen, A.; Tomczyk, S.; Wiegand, F.N.; Abu Sin, M.; Eckmanns, T.; Haller, S. Antimicrobial resistance in Germany and Europe—A systematic review on the increasing threat accelerated by climate change. J. Health Monit. 2023, 8, 93–108. [Google Scholar] [CrossRef]
- Leonard, A.F.C.; Zhang, L.; Balfour, A.J.; Garside, R.; Hawkey, P.M.; Murray, A.K.; Ukoumunne, O.C.; Gaze, W.H. Exposure to and colonisation by antibiotic-resistant E. coli in UK coastal water users: Environmental surveillance, exposure assessment, and epidemiological study (Beach Bum Survey). Environ. Int. 2018, 114, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Søraas, A.; Sundsfjord, A.; Sandven, I.; Brunborg, C.; Jenum, P.A. Risk Factors for Community-Acquired Urinary Tract Infections Caused by ESBL-Producing Enterobacteriaceae–A Case–Control Study in a Low Prevalence Country. PLoS ONE 2013, 8, e69581. [Google Scholar] [CrossRef] [PubMed]
- Meier, H.; Spinner, K.; Crump, L.; Kuenzli, E.; Schuepbach, G.; Zinsstag, J. State of Knowledge on the Acquisition, Diversity, Interspecies Attribution and Spread of Antimicrobial Resistance between Humans, Animals and the Environment: A Systematic Review. Antibiotics 2023, 12, 73. [Google Scholar] [CrossRef] [PubMed]
- Van Nieuwkoop, C. Antibiotic treatment of urinary tract infection and its impact on the gut microbiota. Lancet Infect. Dis. 2022, 22, 307–309. [Google Scholar] [CrossRef] [PubMed]
- Bezabih, Y.M.; Bezabih, A.; Dion, M.; Batard, E.; Teka, S.; Obole, A.; Dessalegn, N.; Enyew, A.; Roujeinikova, A.; Alamneh, E.; et al. Comparison of the global prevalence and trend of human intestinal carriage of ESBL-producing Escherichia coli between healthcare and community settings: A systematic review and meta-analysis. JAC-Antimicrob. Resist. 2022, 4, dlac048. [Google Scholar] [CrossRef] [PubMed]
- Ioannidis, J.P. Integration of evidence from multiple meta-analyses: A primer on umbrella reviews, treatment networks and multiple treatments meta-analyses. CMAJ 2009, 181, 488–493. [Google Scholar] [CrossRef]
- Smith, V.; Devane, D.; Begley, C.M.; Clarke, M. Methodology in conducting a systematic review of systematic reviews of healthcare interventions. BMC Med. Res. Methodol. 2011, 11, 15. [Google Scholar] [CrossRef]
- Smit, C.; Lambert, M.; Rogers, K.; Djordjevic, S.; van Oijen, A.M.; Keighley, C.; Taxis, K.; Robertson, H.; Pont, L. Determinants Associated with Antimicrobial Resistance of Escherichia coli in the Community: An Umbrella Review. 2022. Available online: https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=316431, (accessed on 14 November 2023).
- Google. Google Scholar. Available online: https://scholar.google.com (accessed on 23 September 2022).
- Janssens, A.C.J.W.; Gwinn, M.; Brockman, J.E.; Powell, K.; Goodman, M. Novel citation-based search method for scientific literature: A validation study. BMC Med. Res. Methodol. 2020, 20, 25. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control (ECDC). 2005–2022. Available online: https://www.ecdc.europa.eu/en (accessed on 23 September 2022).
- ReAct Group. Action on Antibiotic Resistance. 2005. Available online: https://www.reactgroup.org/ (accessed on 23 September 2022).
- University of Oxford. Study Designs. Resources: Evidence-Based Medicine Tools. Centre for Evidence-Based Medicine. 2023. Available online: https://www.cebm.ox.ac.uk/resources/ebm-tools/study-designs (accessed on 23 September 2022).
- Covidence. Covidence Systematic Review Software. Available online: https://www.covidence.org/ (accessed on 23 September 2022).
- Shea, B.J.; Reeves, B.C.; Wells, G.; Thuku, M.; Hamel, C.; Moran, J.; Moher, D.; Tugwell, P.; Welch, V.; Kristjansson, E.; et al. AMSTAR 2: A critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017, 358, j4008. [Google Scholar] [CrossRef]
- Cars, O.; Chandy, S.J.; Mpundu, M.; Peralta, A.Q.; Zorzet, A.; So, A.D. Resetting the agenda for antibiotic resistance through a health systems perspective. Lancet Glob. Health 2021, 9, E1022. [Google Scholar] [CrossRef]
- Sleddens, E.F.; Kroeze, W.; Kohl, L.F.; Bolten, L.M.; Velema, E.; Kaspers, P.; Kremers, S.P.; Brug, J. Correlates of dietary behavior in adults: An umbrella review. Nutr. Rev. 2015, 73, 477–499. [Google Scholar] [CrossRef]
- Nyadanu, S.D.; Dunne, J.; Tessema, G.A.; Mullins, B.; Kumi-Boateng, B.; Lee Bell, M.; Duko, B.; Pereira, G. Prenatal exposure to ambient air pollution and adverse birth outcomes: An umbrella review of 36 systematic reviews and meta-analyses. Environ. Pollut. 2022, 306, 119465. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, C.J. An Effect Size Primer: A Guide for Clinicians and Researchers. Prof. Psychol. Res. Pract. 2009, 40, 532–538. [Google Scholar] [CrossRef]
| Study ID | Total Number of Studies That Fulfilled Inclusion Criteria in Umbrella Review (Total Number of Studies in Review) | One Health Category | Population | Continent(s) |
|---|---|---|---|---|
| Alividza 2018 [34] | 11 (19) | Human | Any age group | Asia, Europe, South America |
| Bakhit 2018 [35] | 3 (25) | Human | Any age group | Europe, South America |
| Bell 2014 [36] | 243 (243) | Human | Any age group | Europe, North America, Other 1 |
| Bhate 2021 [37] | 2 (5) | Human | Aged ≥ 8 years with acne | Europe |
| Bryce 2016 [38] | 6 (34) | Human | Children and adolescents (0–17 years old) | Asia, North America, South America |
| Bryce 2016 [39] | 5 (58) | Human | Children and adolescents (0–17 years old) | Asia, Europe, North America |
| Butcher 2019 [40] | 15 (34) | Human | Any age group | Asia, Europe, South America |
| Chan 2022 [41] | 25 (25) | Human | Children and adolescents (0–18 years old) | Asia |
| Costelloe 2010 [42] | 8 (24) | Human | Any age group | NR |
| Furuya-Kanamori 2020 [43] | 20 (20) | Human | International travelers | Asia, Europe, North America, Oceania |
| Hackmann 2021 [44] | 23 (23) | Animal | Any pet | Asia, Europe, North America, South America |
| Hassing 2015 [45] | 11 (11) | Human | Asymptomatic travelers | Europe, North America, Oceania |
| Hu 2020 [15] | 15 (15) | Human | Healthy population aged 18–65 | Africa, Asia, Europe |
| Karanika 2016 [46] | 66 (66) | Human | Healthy individuals | Africa, Asia, Europe, North America, South America, Oceania |
| Köck 2018 [47] | 2 (68) | Animal | Wildlife, food-producing, and companion animals | Asia, Africa |
| Larramendy 2020 [48] | 16 (16) | Human and Environment | Any age group | Africa, Asia, Europe, South America |
| Lazarus 2015 [49] | 34 (34) | Animal | Food-producing animals | Global 2 |
| Messina 2020 [50] | 4 (30) | Human | Healthy children and adolescents (0–21 years old) | Asia, Europe, North America, Oceania |
| O’Brien 2019 [51] | 3 (19) | Human | Children (1 month to 5 years old) | Africa |
| Ramblière 2021 [52] | 3 (36) | Human | Children (0–15 months old) exposed to HIV and HIV-infected adults | Africa |
| Truong 2022 [53] | 2 (7) | Human | Oral daily tetracycline users | Africa, Asia |
| Voor In ‘t Holt 2020 [54] | 22 (22) | Human | Travelers without infection | Asia, Europe, North America, Oceania |
| Willems 2020 [55] | 4 (26) | Human | Acid suppressant users | Europe |
| Variable | Subcategory | Number of Participants (Number of Studies Investigating Variable) | Magnitude of Association OR (95% CI) | Importance Rating * |
|---|---|---|---|---|
| Antibiotic use | General antibiotic use | 6 studies (NR) | 1.51 (1.17–1.94) [15] | + |
| 1528 (6 studies) | 1.58 ** (1.16–2.16) [46] | |||
| 1297 (5 studies) | 1.63 ** (1.19–2.24) [46] | |||
| 449 (1 study) | 1.8 (1.0–3.1) [48] | |||
| 88 studies (NR) | 2.33 (2.19–2.49) [36] | |||
| NR (5 studies) | 2.65 (1.70–4.12) [41] | |||
| 172 (1 study) | 3.1 (1.4–6.7) [48] | |||
| 484 (1 study) | 4.0 (1.6–10.0) [48] | |||
| 300 (1 study) | 4.6 (1.9–11.0) [48] | |||
| 140 (1 study) | 5.6 (2.1–14.8) [48] | |||
| Trimethoprim and β-lactams | 179 (2 studies) | 3.2 (0.9–10.8) [35] | 0 | |
| Beta-Lactam | 290 (1 study) | 4.5 (1.8–11.0) [48] | +++ | |
| 510 (1 study) | 4.6 (2.0–10.7) [48] | |||
| (Fluoro)Quinolone | 449 (1 study) | 2.1 (0.6–7.3) [48] | + | |
| 200 (1 study) | 2.6 (1.3–5.1) [48] | |||
| 140 (1 study) | 9.9 (2.2–44.6) [48] | |||
| 290 (1 study) | 19.0 (3.3–111.4) [48] | |||
| Penicillin | 7170 (1 study) | 0.9 (0.5–1.7) [48] | 0 | |
| 408 (1 study) | 2.7 (1.2–6.3) [48] | |||
| Cephalosporin | 74 (1 study) | 1.5 (5.4–85.2) [48] | + | |
| 200 (1 study) | 2.2 (1.01–5.0) [48] | |||
| 408 (1 study) | 2.2 (1.1–4.5) [48] | |||
| 200 (1 study) | 3.9 (1.8–8.5) [48] | |||
| Macrolides | 7170 (1 study) | 1.5 (1.1–2.2) [48] | 0 | |
| Nitrofurantoin | 7170 (1 study) | 1.54 (1.1–2.3) [48] | 0 | |
| Longer duration of course (>7 days vs. <7 days amoxicillin and trimethoprim) | 1521 (2 studies) | 1.50 (0.76–2.92) [42] | 0 | |
| 1521 (2 studies) | 2.89 (1.44–5.78) [42] | |||
| Multiple courses (>3 courses vs. 1 course, trimethoprim, amoxicillin, trimethoprim) | 1521 (2 studies) | 0.4 (0.12–1.31) [42] | ++ | |
| 1521 (2 studies) | 3.95 (1.06–14.72) [42] | |||
| 1521 (2 studies) | 3.62 (1.25–10.48) [42] | |||
| Mass administration | NR (1 study) | 3.64 (2.38–5.78) [51] | +++ | |
| NR (5 studies) | 7.8 (3.0–20.2) [52] | |||
| NR (5 studies) | 10.2 (5.9–17.8) [52] | |||
| NR (5 studies) | 17.1 (2.3–127.7) [52] | |||
| Higher dose (each 200 mg trimethoprim tablet extra and 500 mg instead of 250 mg amoxicillin) | 1521 (2 studies) | 1.01 (1.01–1.02) [42] | + | |
| 1521 (2 studies) | 2.26 (1.13–4.55) [42] | |||
| Comorbidities | Previous/recurrent UTI | 7170 (1 study) | 1.3 (1.01–1.6) [48] | ++ |
| 408 (1 study) | 3.4 (1.8–6.7) [48] | |||
| 510 (1 study) | 3.8 (1.8–8.1) [48] | |||
| Previous/recurrent pyelonephritis | 300 (1 study) | 1.7 (0.7–3.9) [48] | − | |
| Previous catheterization | 408 (1 study) | 3.3 (1.7–6.6) [48] | + | |
| Diarrhea symptoms | 5144 (7 studies) | 1.53 (1.27–1.84) [15] | 0 | |
| Diabetes | 300 (1 study) | 1.7 (0.8–3.4) [48] | ++ | |
| 290 (1 study) | 3.7 (1.1–12.7) [48] | |||
| 484 (1 study) | 3.0 (1.1–8.0) [48] | |||
| Recurrent acute pyelonephritis and a history of diabetes | 300 (1 study) | 4.2 (1.3–16.9) [48] | + | |
| Renal or urological disorder | 7170 (1 study) | 1.6 (1.0–2.5) [48] | − | |
| 484 (1 study) | 3.5 (1.0–11.5) [48] | |||
| History prostatic disease | 510 (1 study) | 9.6 (2.1–44.8) [48] | + | |
| Chronic disease | 2323 (3 studies) | 0.91 (0.13–6.53) [15] | − | |
| Medication use | Immunosuppressive therapy | 7170 (1 study) | 1.5 (1.1–2.1) [48] | 0 |
| Corticosteroids | 172 (1 study) | 24.3 (2.4–246.9) [48] | + | |
| Acid suppressants | 4111 (3 studies) | 1.31 (0.11–15.5) [15] | 0 | |
| NR (4 studies) | 1.41 (1.07–1.87) [55] | |||
| Hospitalization | Previous hospitalization | 1379 (5 studies) | 1.18 ** (0.78–1.81) [46] | + |
| 1163 (4 studies) | 1.28 ** (0.82–2.03) [46] | |||
| 7170 (1 study) | 1.7 (1.3–2.3) [48] | |||
| 172 (1 study) | 2.9 (1.3–6.6) [48] | |||
| 7170 (1 study) | 3.9 (2.6–5.8) [48] | |||
| 449 (1 study) | 3.9 (1.2–12.7) [48] | |||
| Prior surgery | 172 (1 study) | 2.8 (1.9–8.0) [48] | 0 | |
| Diet | Vegetarian | 6802 (5 studies) | 1.60 (1.0043–2.5587) [15] | 0 |
| Raw milk | 226 (1 study) | 7.54 (2.41–23.45) [15] | + | |
| Fish | 290 (1 study) | 0.6 (0.5–0.9) [48] | 0 | |
| Sex and age | Older age | 300 (1 study) | 2.0 (1.02–3.5) [48] | 0 |
| Male sex | NR (9 studies) | 0.96 (0.74–1.24) [41] | 0 | |
| 7170 (1 study) | 1.6 (1.2–2.1) [48] |
| Variable | Subcategory | Number of Participants (Number of Studies Investigating Variable) | Magnitude of Association OR (95% CI) | Importance Rating * |
|---|---|---|---|---|
| Living standards | Lower socioeconomic status | 2775 (1 study) | 1.33 (1.07–1.75) [34] | + |
| 2775 (1 study) | 2.47 (1.08–5.66) [34] | |||
| Day-care attendance | NR (6 studies) | 1.49 (1.17–1.91) [41] | 0 | |
| Living in Northern vs. Southern Europe | 7170 (1 study) | 0.4 (0.2–0.7) [48] | 0 | |
| Travel | International travel | 1887 (6 studies) | 4.06 ** (1.33–2.41) [46] | +++ |
| 834 (1 study) | 21 (4.5–97) [48] | |||
| To Asia | NR (4 studies) | 1.78 (0.64–4.98) [15] | ++ | |
| NR (12 studies) | 14.16 (5.50–36.45) [54] | |||
| 370 (1 study) | 30.0 (6.3–147.2) [45] | |||
| To Africa | NR (3 studies) | 0.94 ** (0.14–6.17) [46] | − | |
| To India | 182 (3 studies) | 2.4 ** (1.26–4.58) [46] | + | |
| NR (3 studies) | 3.80 (2.23–6.47) [15] | |||
| Health while traveling | Inflammatory bowel disease | 5253 (20 studies) | 2.09 (1.16–3.77) [43] | 0 |
| Diarrhea | NR (4 studies) | 1.65 (1.02–2.68) [15] | + | |
| 5253 (20 studies) | 1.69 (1.25–2.30) [43] | |||
| NR (12 studies) | 2.02 (1.45–2.81) [54] | |||
| 430 (1 study) | 31.0 (2.7–358.1) [45] | |||
| Contact with healthcare while traveling | 5253 (20 studies) | 1.53 (1.09–2.15) [43] | 0 | |
| Antibiotic use | 5253 (20 studies) | 2.38 (1.88–3.00) [43] | + | |
| NR (12 studies) | 2.78 (1.76–4.39) [54] | |||
| NR (4 studies) | 2.81 (1.47–5.36) [15] | |||
| 99 (1 study) | 3.0 (1.4–6.7) [45] | |||
| 99 (1 study) | 5.0 (1.1–26.2) [45] | |||
| Traveler demographics | Backpackers compared to other travelers | 5253 (20 studies) | 1.46 (1.20–1.78) [43] | 0 |
| Vegetarian diet | 5253 (20 studies) | 1.41 (1.01–1.96) [43] | + | |
| NR (3 studies) | 1.92 (1.13–3.26) [15] | |||
| Diet associated with risk (pastry, meals from stalls, etc.) | NR (12 studies) | 1.27 (0.67–2.41) [54] | − | |
| Street food consumption | NR (2 studies) | 0.92 (0.49–1.74) [15] | + | |
| NR (2 studies) | 1.37 (1.08–1.73) [15] | |||
| NR (2 studies) | 2.09 (1.30–3.38) [15] | |||
| Raw vegetable consumption | NR (2 studies) | 0.34 (0.12–0.93) [15] | − | |
| NR (2 studies) | 0.58 (0.33–1.07) [15] | |||
| NR (2 studies) | 2.18 (1.29–3.68) [15] | |||
| Protective measures while traveling | Consuming bottled water | 5253 (20 studies) | 1.29 (0.50–3.34) [43] | − |
| General protective measures (disposable gloves, bottled water, etc.) | NR (12 studies) | 0.83 (0.61–1.13) [54] | − | |
| Meticulous hand hygiene | 5253 (20 studies) | 1.10 (0.81–1.49) [43] | − | |
| Probiotics | 5253 (20 studies) | 1.06 (0.78–1.45) [43] | − |
| Animal | Subcategory | Number of Studies Investigating Variable (Number of Participants) | Magnitude of Association OR (95% CI) | Importance of Rating * |
|---|---|---|---|---|
| Pets | Pet owner | 963 (5 studies) | 1.39 ** (0.89–2.18) [44,46] | − |
| 9403 (12 studies) | 1.18 ** (0.83–1.68) [44] | |||
| 5159 (4 studies) | 1.15 (0.33–4.06) [15] | |||
| Dog owner | 9403 (12 studies) | 0.88 ** (0.56–1.40) [44] | − | |
| Cat owner | 9403 (12 studies) | 1.16 ** (0.58–2.34) [44] | − | |
| Rodent owner | 9403 (12 studies) | 1.34 ** (0.43–4.18) [44] | − | |
| Bird owner | 9403 (12 studies) | 0.91 ** (0.38–2.18) [44] | − | |
| Environment | ||||
| Freshwater | Swimming | 290 (1 study) | 2.1 (1.02–4.3) [48] | 0 |
| Variable | Subcategory | Number of Studies Investigating Variable (Number of Participants) | Magnitude of Association OR (95% CI) | Importance of Rating * |
|---|---|---|---|---|
| Time after antibiotic use | One week | 129 (2 studies) | 7.1 (4.2–12) [35] | 0 |
| Two weeks | NR (6 studies) | 1.08 (0.6–1.96) [38] | + | |
| NR (1 study) | 6.12 (3.18–11.76) [39] | |||
| One month | NR (6 studies) | 1.38 (1.16–1.64) [38] | ++ | |
| 93 (1 study) | 1.8 (0.9–3.6) [35] | |||
| NR (1 study) | 6.20 (2.14–15.96) [39] | |||
| NR (2 studies) | 8.38 (2.84–24.77) [39] | |||
| 1208 (3 studies) | 11.21 (7.13–17.63) [51] | |||
| Two months | 14,348 (5 studies) | 2.5 (2.1–2.9) [42] | + | |
| NR (1 study) | 5.08 (2.70–9.56) [38] | |||
| Three months | NR (6 studies) | 1.65 (1.36–2.0) [38] | ++ | |
| NR (1 study) | 3.38 (2.05–5.55) [39] | |||
| 1208 (3 studies) | 10.64 (3.79–29.92) [51] | |||
| Six months | NR (1 study) | 3.16 (1.65–6.06) [39] | +++ | |
| 1208 (3 studies) | 4.76 (1.52–14.90) [51] | |||
| NR (1 study) | 13.23 (7.84–22.31) [39] | |||
| 12 months 11, 51, 54, 59, 60 | 14,348 (5 studies) | 1.33 (1.2–1.5) [42] | + | |
| NR (1 study) | 0.94 (0.57–1.56) [39] | |||
| 10,079 (13 studies) | 1.84 (1.35–2.51) [15] | |||
| NR (1 study) | 1.89 (1.04–3.42) [39] | |||
| Over 12 months | NR (1 study) | 0.94 (0.57–1.56) [39] | − | |
| Time after return from travel | Six weeks | 290 (1 study) | 16.4 (3.4–78.8) [48] | + |
| Between six weeks and two years | 290 (1 study) | 2.2 (1.1–4.3) [48] | 0 |
| Category of Importance of Variable | Grading Criteria * |
|---|---|
| Very strong association: +++ | The variable is associated with AMR E. coli in all reviews, without exception. More than one study included in the review(s) needed to show a significant association and a moderate effect size of OR/RR ** ≥ 3.0. |
| Strong association: ++ | One study, or more than 50% of the studies included in the review(s), showed a significant association between the variable and AMR E. coli with a moderate effect size of OR/RR ≥ 3.0. |
| Moderate association: + | The variable is associated with AMR E. coli in a single study or in ≤ 50% of studies in the review(s) with a significant moderate effect size of OR/RR ≥ 3.0. Or the variable is associated with AMR E. coli in >50% of the studies with a small significant effect size of OR/RR < 3.0. |
| Weak association: 0 | The variable is associated with AMR E. coli in a single study or in ≤50% of studies in the review(s) with a moderate significant effect size of OR/RR < 3.0. Or the variable is associated with AMR E. coli in >50% of the studies with a moderate nonsignificant effect size of OR/RR ≥ 3.0. |
| No association: − | One study, or more than 50% of the studies included in the review(s), showed an association between the variable and AMR E. coli with a small nonsignificant effect size of OR/RR < 3.0. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smit, C.C.H.; Lambert, M.; Rogers, K.; Djordjevic, S.P.; Van Oijen, A.M.; Keighley, C.; Taxis, K.; Robertson, H.; Pont, L.G. One Health Determinants of Escherichia coli Antimicrobial Resistance in Humans in the Community: An Umbrella Review. Int. J. Mol. Sci. 2023, 24, 17204. https://doi.org/10.3390/ijms242417204
Smit CCH, Lambert M, Rogers K, Djordjevic SP, Van Oijen AM, Keighley C, Taxis K, Robertson H, Pont LG. One Health Determinants of Escherichia coli Antimicrobial Resistance in Humans in the Community: An Umbrella Review. International Journal of Molecular Sciences. 2023; 24(24):17204. https://doi.org/10.3390/ijms242417204
Chicago/Turabian StyleSmit, Chloé C. H., Maarten Lambert, Kris Rogers, Steven P. Djordjevic, Antoine M. Van Oijen, Caitlin Keighley, Katja Taxis, Hamish Robertson, and Lisa G. Pont. 2023. "One Health Determinants of Escherichia coli Antimicrobial Resistance in Humans in the Community: An Umbrella Review" International Journal of Molecular Sciences 24, no. 24: 17204. https://doi.org/10.3390/ijms242417204





