Metabolomics (Non-Targeted) of Induced Type 2 Diabetic Sprague Dawley Rats Comorbid with a Tissue-Dwelling Nematode Parasite
Abstract
1. Introduction
2. Results
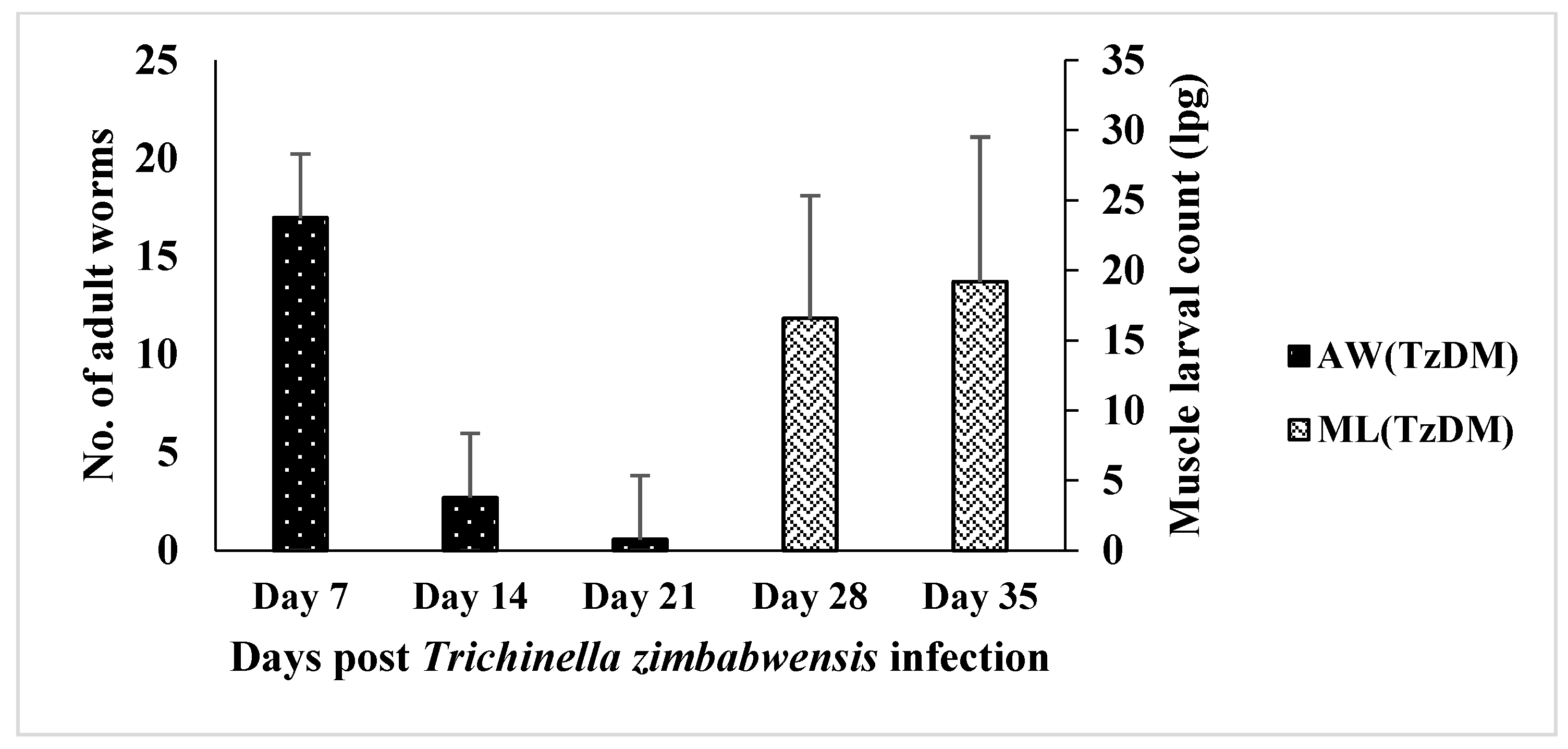
2.1. Trichinella zimbabwensis Burden in Type 2 Diabetic Rats
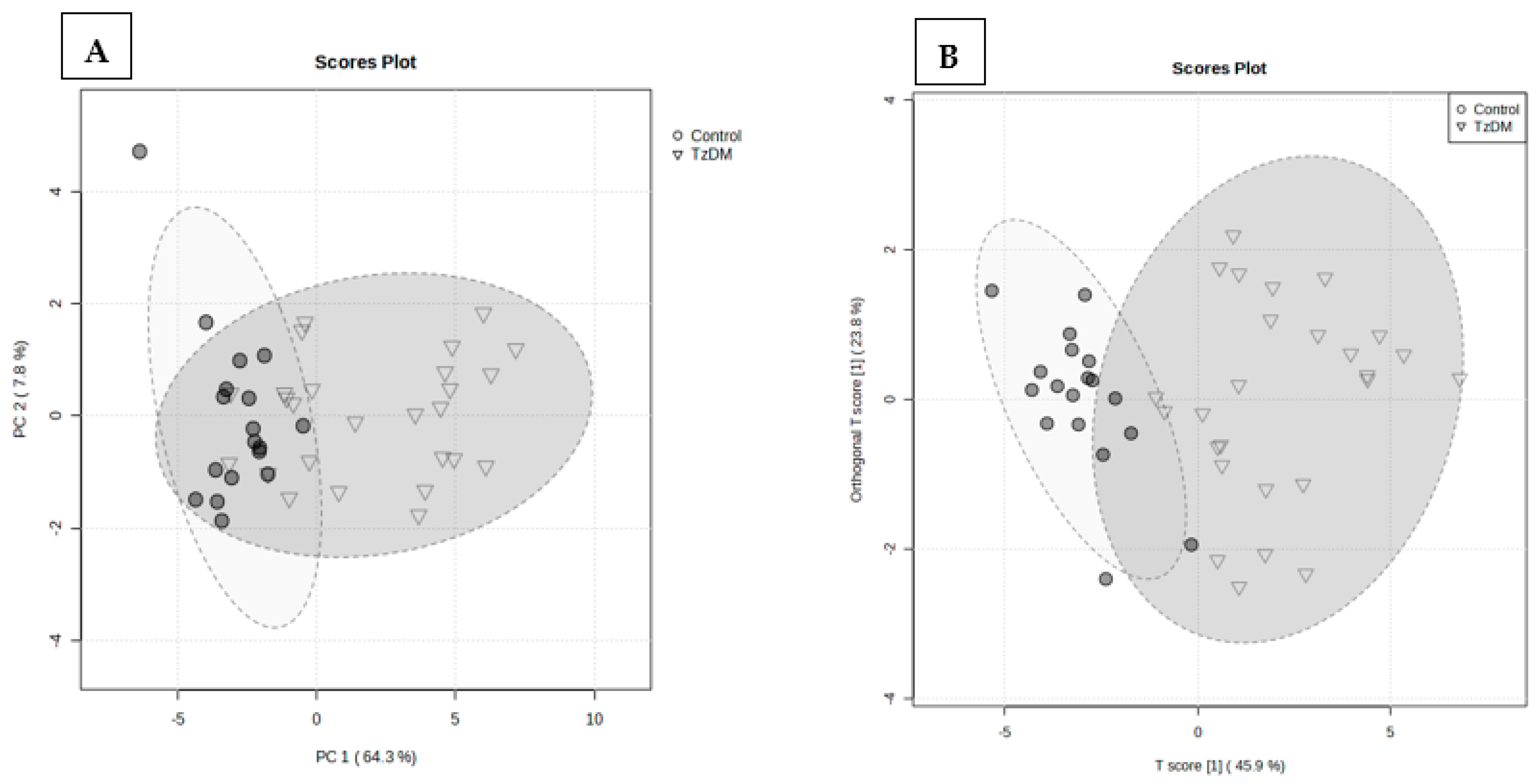
2.2. Natural Discrimination between TzDM Rats and the Control Rats
2.3. Metabolite Changes between the Groups
2.4. Weekly Metabolite Changes Post-Infection
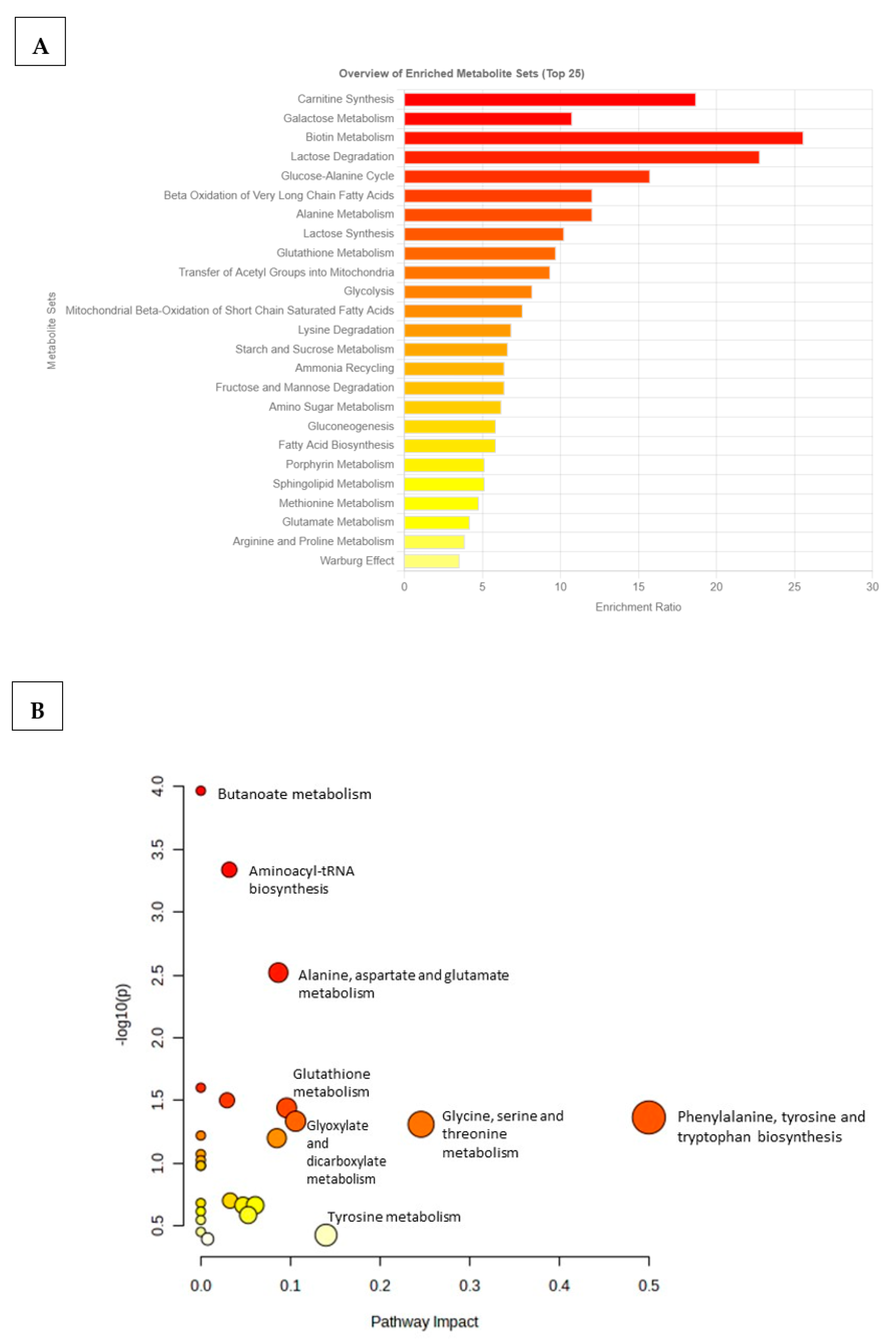
2.5. Pathway Discovery and Impact Analyses
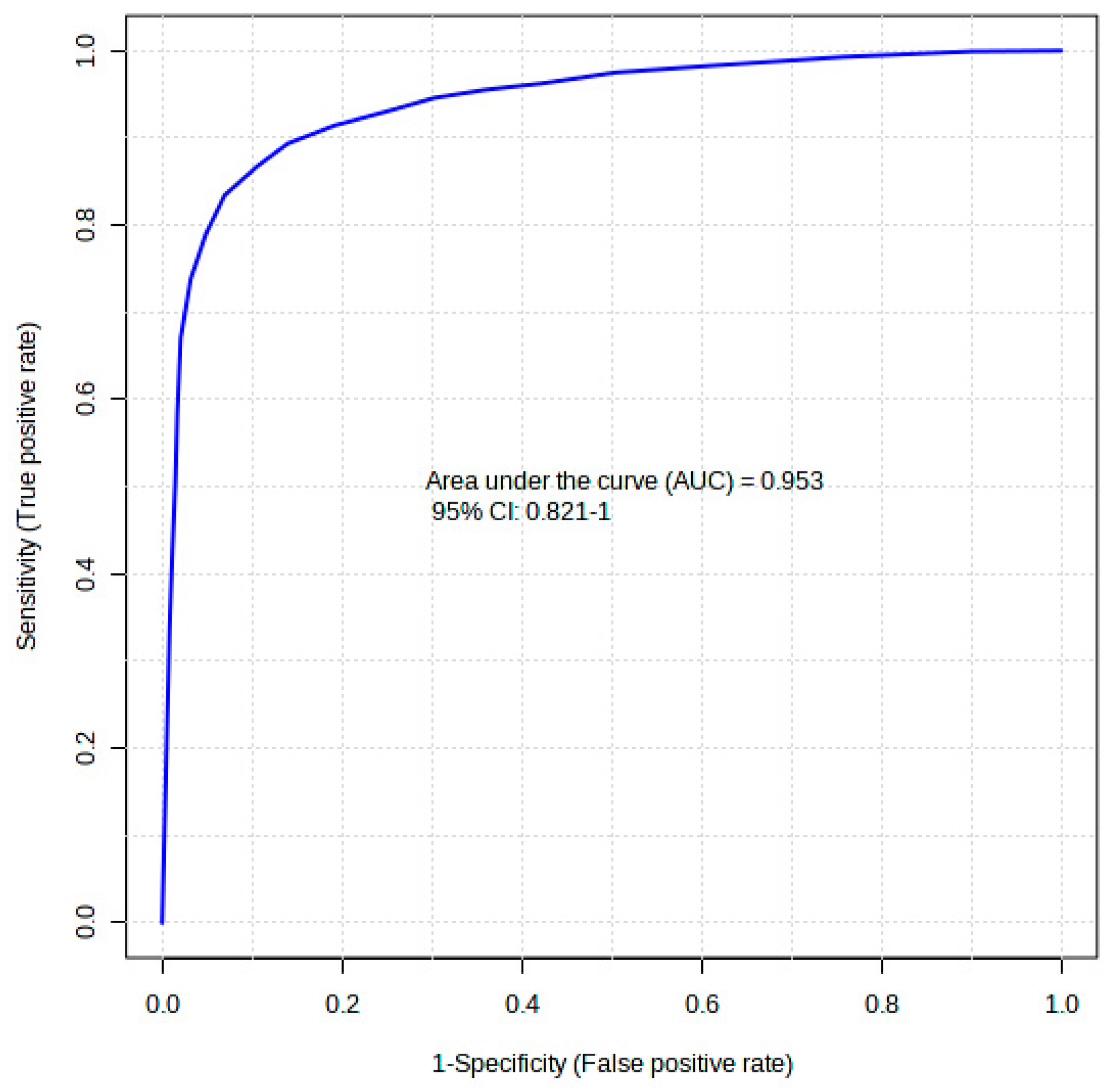
2.6. Diagnostic Accuracy
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Type 2 Diabetes Mellitus Induction
4.3. Trichinella zimbabwensis Infection
4.4. Trichinella zimbabwensis Worm Recovery
4.5. Analysis of Serum Samples
4.6. Untargeted GCxGC-TOFMS Approach
4.7. Peak Identification
4.8. Data Clean-Up
4.9. Identification of Compounds and Pathway Analysis
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moudgil, V.; Rana, R.; Tripathi, P.K.; Farooq, U.; Sehgal, R.; Khan, M.A. Coprevalence of parasitic infections and diabetes in Sub-Himalayan region of Northern India. Int. J. Health Sci. 2019, 13, 19. [Google Scholar]
- Belmokhtar, F.; Belmokhtar, R.; Charef, M. Risk factors associated with type 2 diabetes mellitus in west region of Algeria, Maghnia. J. Diabetes Metab. 2011, 2, 2. [Google Scholar] [CrossRef]
- Cho, N.H.; Shaw, J.; Karuranga, S.; Huang, Y.; da Rocha Fernandes, J.; Ohlrogge, A.; Malanda, B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 2018, 138, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Wild, S.; Roglic, G.; Green, A.; Sicree, R.; King, H. Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care 2004, 27, 1047–1053. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Ley, S.H.; Hu, F.B. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat. Rev. Endocrinol. 2018, 14, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Tuei, V.C.; Maiyoh, G.K.; Ha, C.E. Type 2 diabetes mellitus and obesity in sub-Saharan Africa. Diabetes/Metab. Res. Rev. 2010, 26, 433–445. [Google Scholar] [CrossRef]
- Almogbel, R.A.; Alhussan, F.A.; Alnasser, S.A.; Algeffari, M.A. Prevalence and risk factors of gastroparesis-related symptoms among patients with type 2 diabetes. Int. J. Health Sci. 2016, 10, 397. [Google Scholar] [CrossRef]
- Rajamanickam, A.; Munisankar, S.; Dolla, C.; Menon, P.A.; Thiruvengadam, K.; Nutman, T.B.; Babu, S. Helminth infection modulates systemic pro-inflammatory cytokines and chemokines implicated in type 2 diabetes mellitus pathogenesis. PLoS Neglected Trop. Dis. 2020, 14, e0008101. [Google Scholar] [CrossRef]
- Htun, N.S.N.; Odermatt, P.; Paboriboune, P.; Sayasone, S.; Vongsakid, M.; Phimolsarn-Nusith, V.; Tran, X.D.; Ounnavong, P.-S.; Andriama-Hefasoa, N.; Senvanpan, N.-D. Association between helminth infections and diabetes mellitus in adults from the Lao People’s Democratic Republic: A cross-sectional study. Infect. Dis. Poverty 2018, 7, 105. [Google Scholar] [CrossRef]
- Farrell, S.H.; Coffeng, L.E.; Truscott, J.E.; Werkman, M.; Toor, J.; de Vlas, S.J.; Anderson, R.M. Investigating the effectiveness of current and modified World Health Organization guidelines for the control of soil-transmitted helminth infections. Clin. Infect. Dis. 2018, 66, S253–S259. [Google Scholar] [CrossRef]
- Macpherson, C.N. Human behaviour and the epidemiology of parasitic zoonoses. Int. J. Parasitol. 2005, 35, 1319–1331. [Google Scholar] [CrossRef] [PubMed]
- Khanna, V.; Lakshmi, K.; Khanna, R.; Verma, S.; Acharya, V. Intestinal parasitic infections among diabetic patients in tertiary care hospital. Adv. Gut Microbiome Res. 2022, 2022, 4829943. [Google Scholar] [CrossRef]
- Tracey, E.F.; McDermott, R.A.; McDonald, M.I. Do worms protect against the metabolic syndrome? A systematic review and meta-analysis. Diabetes Res. Clin. Pract. 2016, 120, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.W.; Lu, Y.; Li, F.; Shen, Z.H.; Xu, M.; Yao, W.F.; Feng, Y.B.; Yun, J.T.; Wang, Y.P.; Ling, W. The potential long-term effect of previous schistosome infection reduces the risk of metabolic syndrome among C hinese men. Parasite Immunol. 2015, 37, 333–339. [Google Scholar] [CrossRef]
- Aravindhan, V.; Mohan, V.; Surendar, J.; Rao, M.M.; Pavankumar, N.; Deepa, M.; Rajagopalan, R.; Kumaraswami, V.; Nutman, T.B.; Babu, S. Decreased prevalence of lymphatic filariasis among diabetic subjects associated with a diminished pro-inflammatory cytokine response (CURES 83). PLoS Negl. Trop. Dis. 2010, 4, e707. [Google Scholar] [CrossRef] [PubMed]
- Taira, K.; Yazawa, R.; Watanabe, A.; Ishikawa, Y.; Okamoto, M.; Takahashi, A.; Asai, F. Syphacia muris infection delays the onset of hyperglycemia in WBN/Kob-Lepr fa rats, a new type 2 diabetes mellitus model. Helminthologia 2015, 52, 58–62. [Google Scholar] [CrossRef]
- Silas, E.; Tshilwane, S.I.; Mukaratirwa, S. Cytokines, Chemokines, Insulin and Haematological Indices in Type 2 Diabetic Male Sprague Dawley Rats Infected with Trichinella zimbabwensis. Appl. Sci. 2022, 12, 7743. [Google Scholar] [CrossRef]
- Camaya, I.; O’Brien, B.; Donnelly, S. How do parasitic worms prevent diabetes? An exploration of their influence on macrophage and β-cell crosstalk. Front. Endocrinol. 2023, 14, 1205219. [Google Scholar] [CrossRef]
- Hu, F.B.; Satija, A.; Manson, J.E. Curbing the diabetes pandemic: The need for global policy solutions. JAMA 2015, 313, 2319–2320. [Google Scholar] [CrossRef]
- Lindström, J.; Tuomilehto, J. The Diabetes Risk Score. A practical tool to predict type 2 diabetes risk. Diabetes Care 2003, 26, 725–731. [Google Scholar] [CrossRef]
- Klein, M.S.; Shearer, J. Metabolomics and type 2 diabetes: Translating basic research into clinical application. J. Diabetes Res. 2016, 2016, 3898502. [Google Scholar] [CrossRef]
- Rennie, C.; Fernandez, R.; Donnelly, S.; McGrath, K.C. The impact of helminth infection on the incidence of metabolic syndrome: A systematic review and meta-analysis. Front. Endocrinol. 2021, 12, 728396. [Google Scholar] [CrossRef]
- Berbudi, A.; Ajendra, J.; Wardani, A.P.; Hoerauf, A.; Hübner, M.P. Parasitic helminths and their beneficial impact on type 1 and type 2 diabetes. Diabetes/Metab. Res. Rev. 2016, 32, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Ndlovu, I.S.; Tshilwane, S.I.; Vosloo, A.; Chaisi, M.; Mukaratirwa, S. Metabolomics of Type 2 Diabetes Mellitus in Sprague Dawley Rats—In Search of Potential Metabolic Biomarkers. Int. J. Mol. Sci. 2023, 24, 12467. [Google Scholar] [CrossRef] [PubMed]
- Ndlovu, I.S.; Silas, E.; Tshilwane, S.; Chaisi, M.; Vosloo, A.; Mukaratirwa, S. Preliminary insights on the metabolomics of Trichinella zimbabwensis infection in Sprague Dawley rats using GCxGC-TOF-MS (untargeted approach). Front. Mol. Biosci. 2023, 10, 1128542. [Google Scholar] [CrossRef] [PubMed]
- Dunn, W.B. Diabetes-the role of metabolomics in the discovery of new mechanisms and novel biomarkers. Curr. Cardiovasc. Risk Rep. 2013, 7, 25–32. [Google Scholar] [CrossRef]
- Cummings, J.; Stephen, A. Carbohydrate terminology and classification. Eur. J. Clin. Nutr. 2007, 61, S5–S18. [Google Scholar] [CrossRef]
- Chukwuma, C.I.; Matsabisa, M.G.; Erukainure, O.L.; Ibeji, C.U.; Islam, M.S. D-mannitol modulates glucose uptake ex vivo; suppresses intestinal glucose absorption in normal and type 2 diabetic rats. Food Biosci. 2019, 29, 30–36. [Google Scholar] [CrossRef]
- Tielens, A. Energy generation in parasitic helminths. Parasitol. Today 1994, 10, 346–352. [Google Scholar] [CrossRef]
- Chappell, L.H.; Chappell, L.H. Carbohydrate metabolism and energy production. In Physiology of Parasites; Tertiary Level Biology; Springer: Boston, MA, USA, 1979; pp. 42–62. [Google Scholar]
- Pozio, E.; Foggin, C.; Marucci, G.; La Rosa, G.; Sacchi, L.; Corona, S.; Rossi, P.; Mukaratirwa, S. Trichinella zimbabwensis n. sp. (Nematoda), a new non-encapsulated species from crocodiles (Crocodylus niloticus) in Zimbabwe also infecting mammals. Int. J. Parasitol. 2002, 32, 1787–1799. [Google Scholar] [CrossRef]
- Fredensborg, B.; Poulin, R. Larval helminths in intermediate hosts: Does competition early in life determine the fitness of adult parasites? Int. J. Parasitol. 2005, 35, 1061–1070. [Google Scholar] [CrossRef] [PubMed]
- Ang, B.R.G.; Yu, G. The role of fructose in type 2 diabetes and other metabolic diseases. J. Nutr. Food Sci. 2018, 8, 659. [Google Scholar] [CrossRef]
- Elliott, S.S.; Keim, N.L.; Stern, J.S.; Teff, K.; Havel, P.J. Fructose, weight gain, and the insulin resistance syndrome. Am. J. Clin. Nutr. 2002, 76, 911–922. [Google Scholar] [CrossRef] [PubMed]
- Basciano, H.; Federico, L.; Adeli, K. Fructose, insulin resistance, and metabolic dyslipidemia. Nutr. Metab. 2005, 2, 5. [Google Scholar] [CrossRef]
- Kolderup, A.; Svihus, B. Fructose metabolism and relation to atherosclerosis, type 2 diabetes, and obesity. J. Nutr. Metab. 2015, 2015, 823081. [Google Scholar] [CrossRef]
- Anuradha, C.V. Aminoacid support in the prevention of diabetes and diabetic complications. Curr. Protein Pept. Sci. 2009, 10, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Sun, W.-W.; Chen, J.-C.; Zhang, H.-L.; Liu, J.; Lin, Y.; Lin, P.-C.; Wu, B.-X.; An, Y.-P.; Huang, L. Phenylalanine impairs insulin signaling and inhibits glucose uptake through modification of IRβ. Nat. Commun. 2022, 13, 4291. [Google Scholar] [CrossRef]
- Koeck, T.; Corbett, J.A.; Crabb, J.W.; Stuehr, D.J.; Aulak, K.S. Glucose-modulated tyrosine nitration in beta cells: Targets and consequences. Arch. Biochem. Biophys. 2009, 484, 221–231. [Google Scholar] [CrossRef]
- Tinker, L.F.; Sarto, G.E.; Howard, B.V.; Huang, Y.; Neuhouser, M.L.; Mossavar-Rahmani, Y.; Beasley, J.M.; Margolis, K.L.; Eaton, C.B.; Phillips, L.S. Biomarker-calibrated dietary energy and protein intake associations with diabetes risk among postmenopausal women from the Women’s Health Initiative. Am. J. Clin. Nutr. 2011, 94, 1600–1606. [Google Scholar] [CrossRef]
- Scholl, M.T. Synthesis, Structural Characterization and Applications of Hyperbranched Polymers Based on L-Lysine; EPFL: Lausanne, Switzerland, 2007; p. 274. [Google Scholar] [CrossRef]
- Ren, W.; Rajendran, R.; Zhao, Y.; Tan, B.; Wu, G.; Bazer, F.W.; Zhu, G.; Peng, Y.; Huang, X.; Deng, J. Amino acids as mediators of metabolic cross talk between host and pathogen. Front. Immunol. 2018, 9, 319. [Google Scholar] [CrossRef]
- Ranasinghe, P.; Jayawardena, R.; Chandrasena, L.; Noetzel, V.; Burd, J. Effects of Lysulin™ supplementation on pre-diabetes: Study protocol for a randomized controlled trial. Trials 2019, 20, 171. [Google Scholar] [CrossRef]
- Jozi, F.; Kheiripour, N.; Taheri, M.A.; Ardjmand, A.; Ghavipanjeh, G.; Nasehi, Z.; Shahaboddin, M.E. L-lysine ameliorates diabetic nephropathy in rats with streptozotocin-induced diabetes mellitus. BioMed Res. Int. 2022, 2022, 4547312. [Google Scholar] [CrossRef]
- Jafarnejad, A.; Bathaie, S.; Nakhjavani, M.; Hassan, M.; Banasadegh, S. The improvement effect of L-Lys as a chemical chaperone on STZ-induced diabetic rats, protein structure and function. Diabetes/Metab. Res. Rev. 2008, 24, 64–73. [Google Scholar] [CrossRef]
- Meléndez, R.R. Importance of biotin metabolism. Rev. Investig. Clin. 2000, 52, 194–199. [Google Scholar]
- Kalogeropoulou, D.; LaFave, L.; Schweim, K.; Gannon, M.C.; Nuttall, F.Q. Lysine ingestion markedly attenuates the glucose response to ingested glucose without a change in insulin response. Am. J. Clin. Nutr. 2009, 90, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Onkoba, N.; Chimbari, M.; Kamau, J.; Mukaratirwa, S. Metabolic and adaptive immune responses induced in mice infected with tissue-dwelling nematode Trichinella zimbabwensis. Open Vet. J. 2016, 6, 178–184. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Thabet, H.S.; Saleh, N.K.; Thabet, S.S.; Abdel-Aziz, M.; Kalleny, N.K. Decreased basal non-insulin-stimulated glucose uptake by diaphragm in streptozotocin-induced diabetic mice infected with Schistosoma mansoni. Parasitol. Res. 2008, 103, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Granado-Casas, M.; Mauricio, D. Oleic acid in the diet and what it does: Implications for diabetes and its complications. In Bioactive Food as Dietary Interventions for Diabetes; Elsevier: Amsterdam, The Netherlands, 2019; pp. 211–229. [Google Scholar] [CrossRef]
- Vassiliou, E.K.; Gonzalez, A.; Garcia, C.; Tadros, J.H.; Chakraborty, G.; Toney, J.H. Oleic acid and peanut oil high in oleic acid reverse the inhibitory effect of insulin production of the inflammatory cytokine TNF-α both in vitro and in vivo systems. Lipids Health Dis. 2009, 8, 25. [Google Scholar] [CrossRef] [PubMed]
- Ament, Z.; Bevers, M.B.; Wolcott, Z.; Kimberly, W.T.; Acharjee, A. Uric acid and gluconic acid as predictors of hyperglycemia and cytotoxic injury after stroke. Transl. Stroke Res. 2021, 12, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Samocha-Bonet, D.; Chisholm, D.J.; Gribble, F.M.; Coster, A.C.; Carpenter, K.H.; Jones, G.R.; Holst, J.J.; Greenfield, J.R. Glycemic effects and safety of L-Glutamine supplementation with or without sitagliptin in type 2 diabetes patients—A randomized study. PLoS ONE 2014, 9, e113366. [Google Scholar] [CrossRef]
- Islam, M.S.; Wilson, R.D. Experimentally induced rodent models of type 2 diabetes. Anim. Models Diabetes Res. 2012, 933, 161–174. [Google Scholar]
- Mukaratirwa, S.; Nkulungo, E.; Matenga, E.; Bhebhe, E. Effect of host age in the distribution of adult Trichinella zimbabwensis in the small intestines of golden hamsters (Mesocricetus auratus) and Balb C mice. Onderstepoort J. Vet. Res. 2003, 70, 169–173. [Google Scholar] [PubMed]
- Du Preez, I.; Loots, D. New sputum metabolite markers implicating adaptations of the host to Mycobacterium tuberculosis, and vice versa. Tuberculosis 2013, 93, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Alonso, A.; Marsal, S.; Julià, A. Analytical methods in untargeted metabolomics: State of the art in 2015. Front. Bioeng. Biotechnol. 2015, 3, 23. [Google Scholar] [CrossRef]
- Sun, H.; Zhao, J.; Zhong, D.; Li, G. Potential serum biomarkers and metabonomic profiling of serum in ischemic stroke patients using UPLC/Q-TOF MS/MS. PLoS ONE 2017, 12, e0189009. [Google Scholar] [CrossRef]
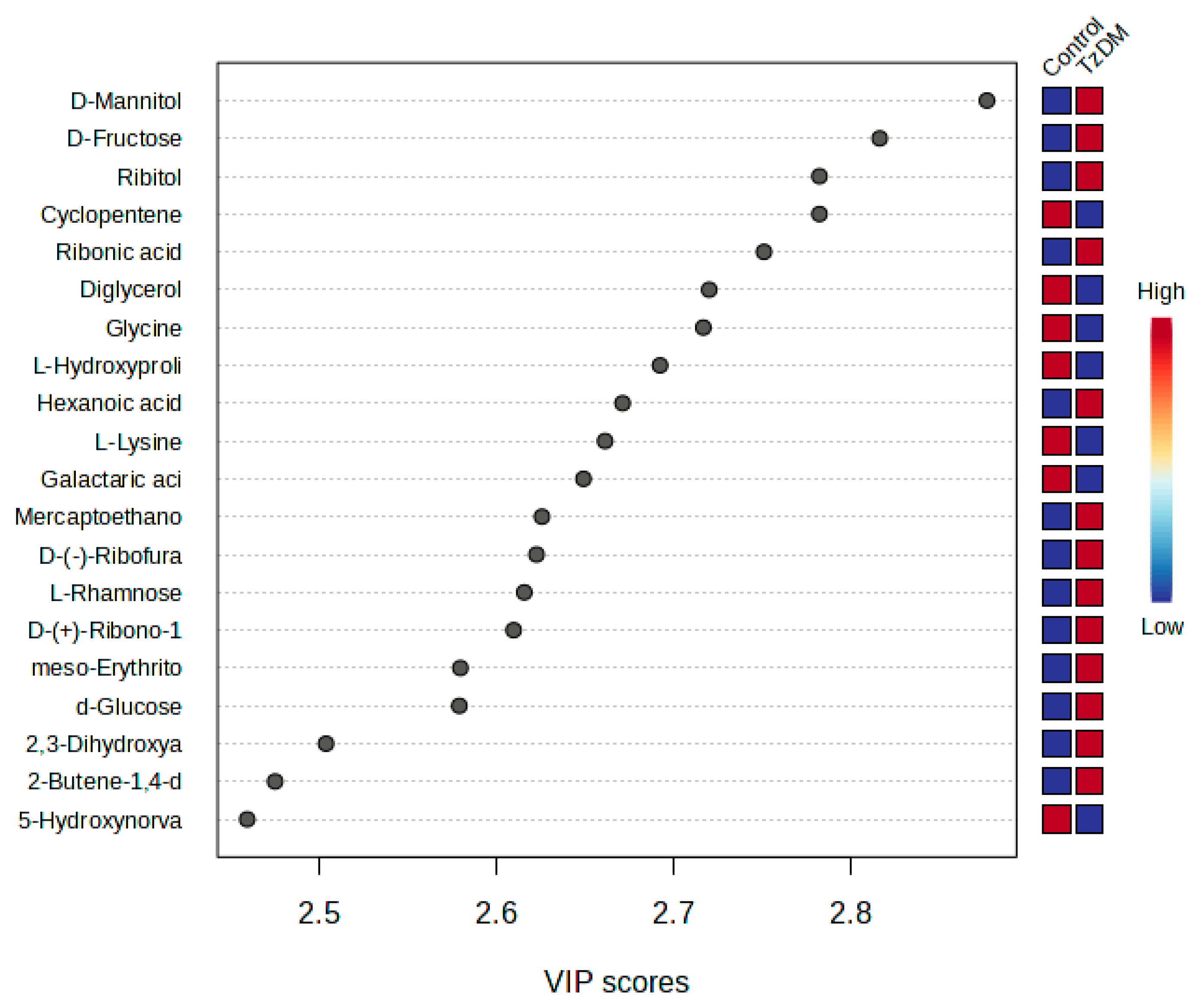

| Metabolite | Group | AUC | VIP | Log2 FC | Variation |
|---|---|---|---|---|---|
| D-Mannitol | Carbohydrate | 0.837 | 2.877 | 1.326 | UP |
| D-Fructose | Carbohydrate | 0.858 | 2.8165 | 1.0025 | Up |
| Ribitol | Carbohydrate | 0.846 | 2.7825 | −0.773 | Down |
| Cyclopentene | Xenobiotic | 0.863 | 2.7825 | 0.620 | Up |
| Ribonic acid | Organic compound | 0.861 | 2.7512 | ||
| Diglycerol | Amino acid | 0.869 | 2.7203 | 0.966 | Up |
| Glycine | Amino acid | 0.837 | 2.717 | 0.437 | Up |
| L-Hydroxyproline | Amino acid | 0.850 | 2.6925 | −2.976 | Down |
| Hexanoic acid | Fatty acid | 0.869 | 2.6714 | 1.0446 | Up |
| L-Lysine | Amino acid | 0.829 | 2.6615 | 0.618 | Down |
| Galactaric acid | Organic compound | 0.850 | 2.6493 | 0.735 | Up |
| Mercaptoethanol | Xenobiotic | 0.825 | 2.6259 | 1.437 | Down |
| D-(−)-Rib furanose | Carbohydrate | 0.835 | 2.6228 | 1.165 | Up |
| L-Rhamnose | Carbohydrate | 0.826 | 2.6159 | 0.851 | Up |
| D-(+)-Ribono-1,4-lactone | Organic compound | 0.856 | 2.6099 | 1.098 | |
| meso-Erythritol | Organic compound | 0.831 | 2.5799 | 0.262 | Up |
| d-Glucose | Carbohydrate | 0.835 | 2.5793 | 0.966 | Up |
| 2,3-Dihydroxyacrylic-Acid | Organic compound | 0.8365 | 2.504 | 0.652 | Up |
| 2-Butene-1,4-diol | Xenobiotic | 0.826 | 2.4753 | 0.876 | Up |
| 5-Hydroxynorvaline | Amino acids | 0.825 | 2.877 | 0.784 | Down |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ndlovu, I.S.; Tshilwane, S.I.; Ngcamphalala, P.I.; Vosloo, A.; Chaisi, M.; Mukaratirwa, S. Metabolomics (Non-Targeted) of Induced Type 2 Diabetic Sprague Dawley Rats Comorbid with a Tissue-Dwelling Nematode Parasite. Int. J. Mol. Sci. 2023, 24, 17211. https://doi.org/10.3390/ijms242417211
Ndlovu IS, Tshilwane SI, Ngcamphalala PI, Vosloo A, Chaisi M, Mukaratirwa S. Metabolomics (Non-Targeted) of Induced Type 2 Diabetic Sprague Dawley Rats Comorbid with a Tissue-Dwelling Nematode Parasite. International Journal of Molecular Sciences. 2023; 24(24):17211. https://doi.org/10.3390/ijms242417211
Chicago/Turabian StyleNdlovu, Innocent Siyanda, Selaelo Ivy Tshilwane, Philile Ignecious Ngcamphalala, Andre’ Vosloo, Mamohale Chaisi, and Samson Mukaratirwa. 2023. "Metabolomics (Non-Targeted) of Induced Type 2 Diabetic Sprague Dawley Rats Comorbid with a Tissue-Dwelling Nematode Parasite" International Journal of Molecular Sciences 24, no. 24: 17211. https://doi.org/10.3390/ijms242417211
APA StyleNdlovu, I. S., Tshilwane, S. I., Ngcamphalala, P. I., Vosloo, A., Chaisi, M., & Mukaratirwa, S. (2023). Metabolomics (Non-Targeted) of Induced Type 2 Diabetic Sprague Dawley Rats Comorbid with a Tissue-Dwelling Nematode Parasite. International Journal of Molecular Sciences, 24(24), 17211. https://doi.org/10.3390/ijms242417211






