The Emerging Roles of the Metabolic Regulator G6PD in Human Cancers
Abstract
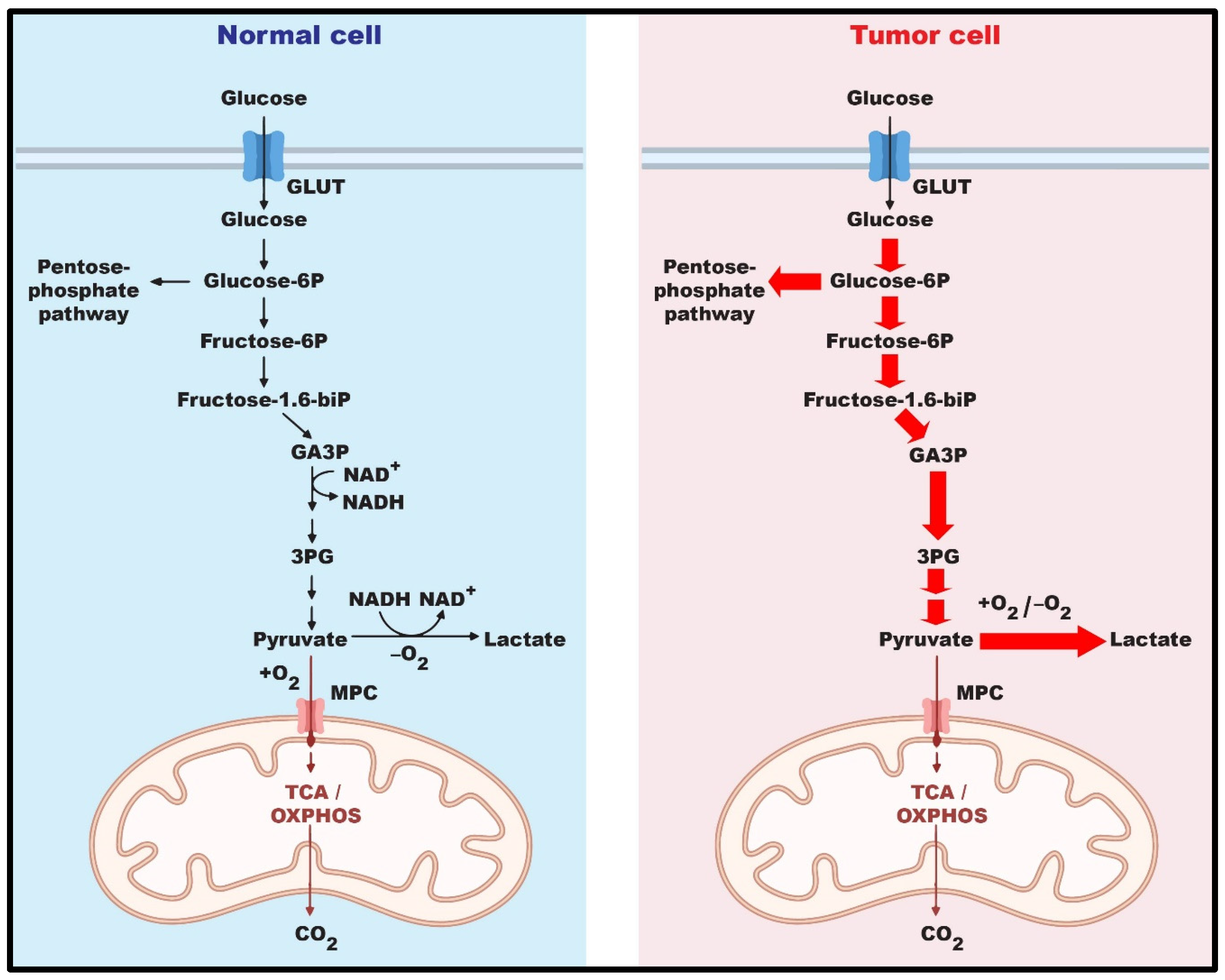
:1. Introduction
2. Structure and Biological Role of G6PD
2.1. Structural Significance of G6PD
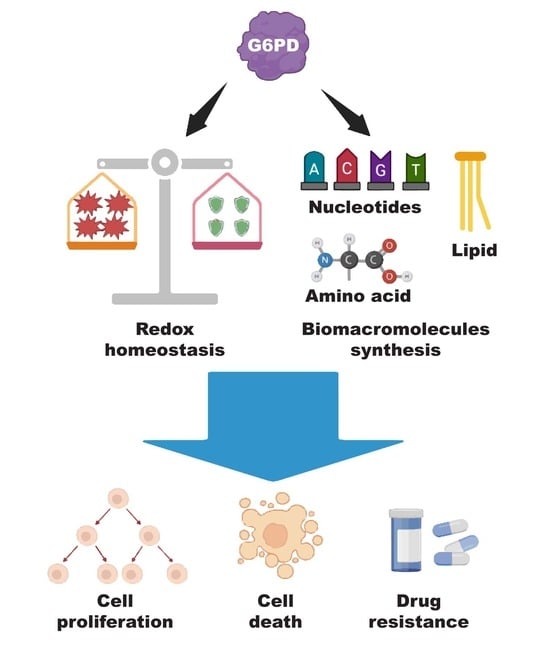
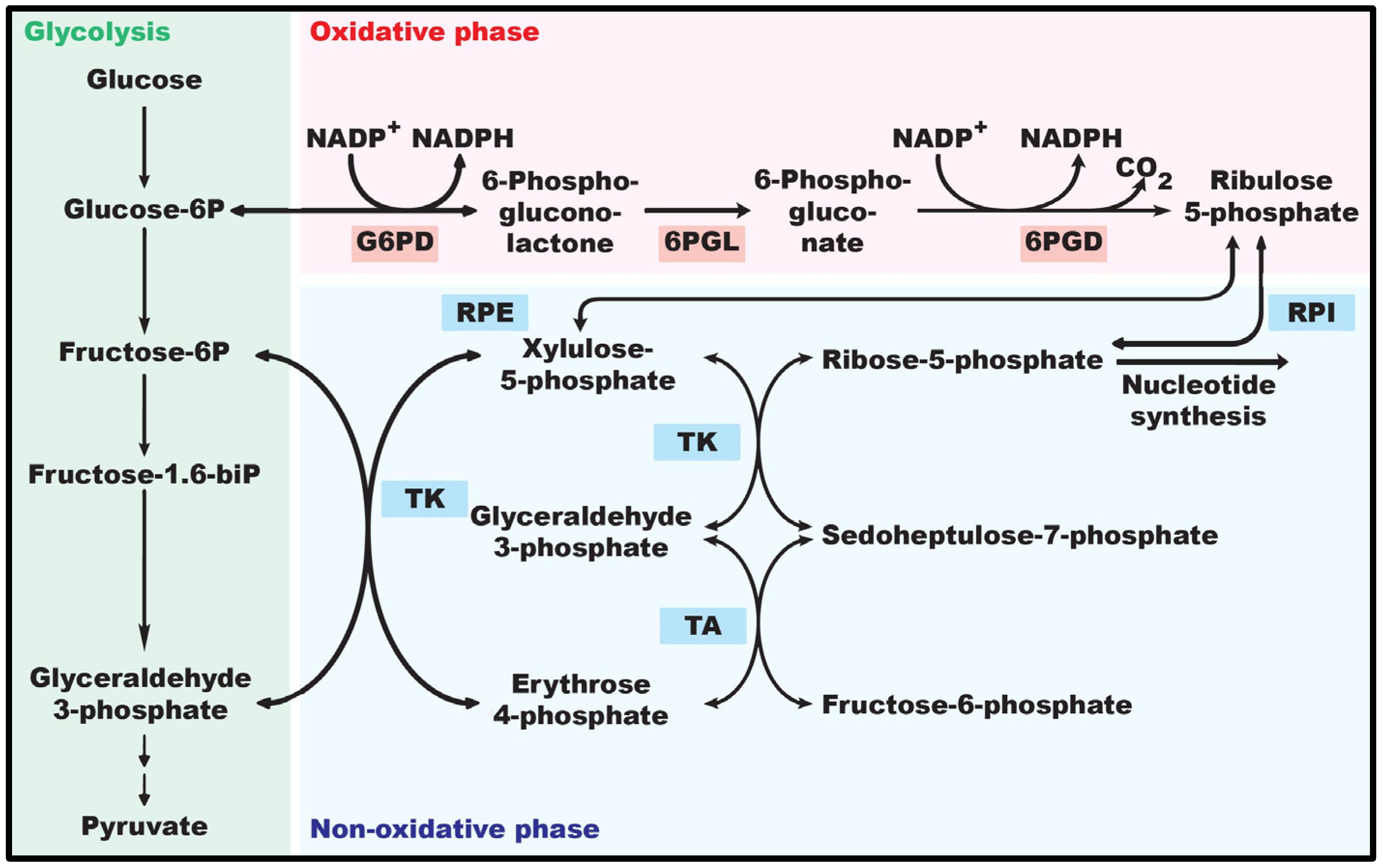
2.2. Role of G6PD in Redox Homeostasis
2.3. Role of G6PD in Biomacromolecule Synthesis
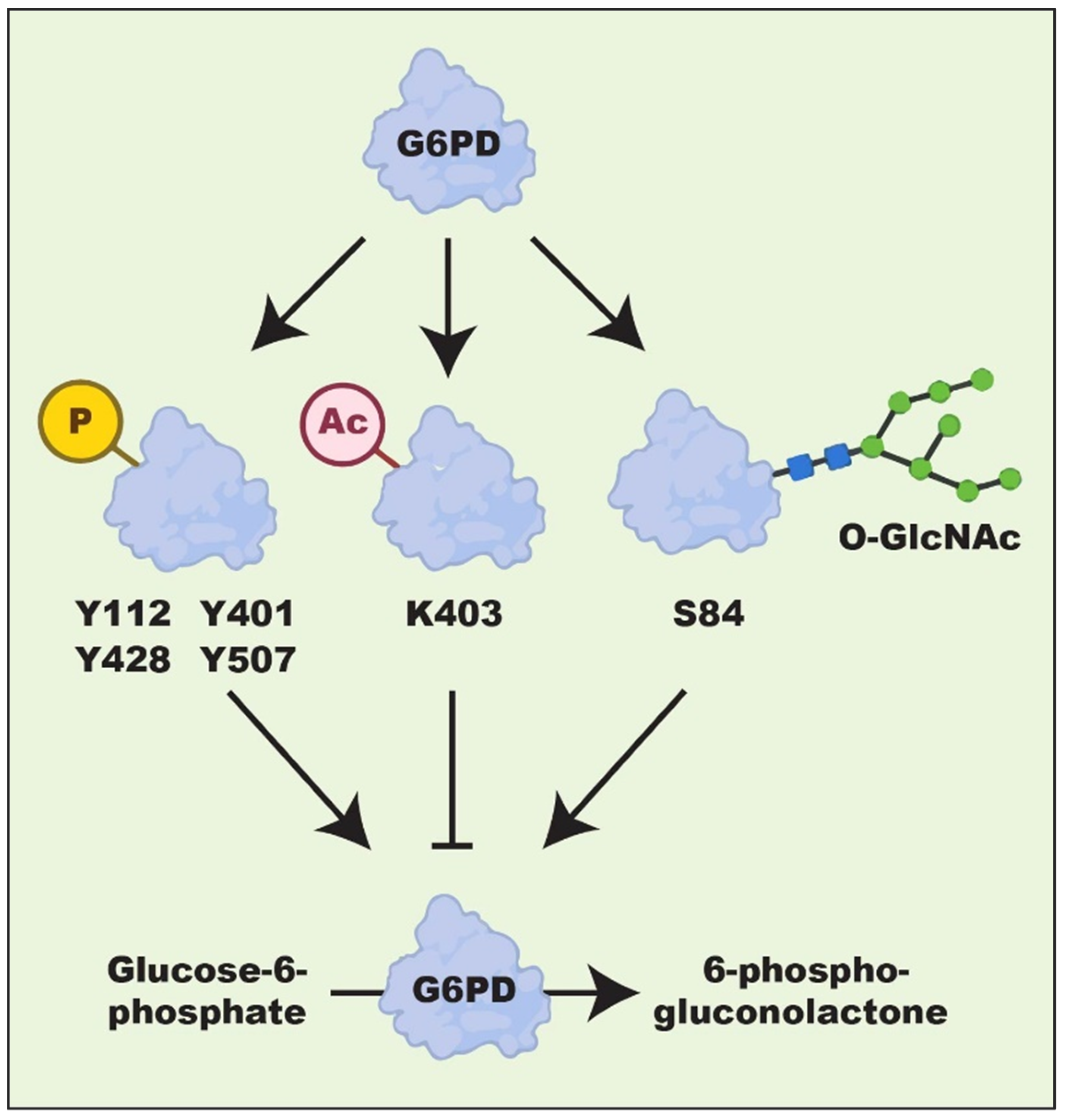
3. Regulation of G6PD Expression
| Factor | Effect on G6PD | Type of Factor | Type of Regulation | Ref. |
|---|---|---|---|---|
| YY1 | Upregulation | Transcription factor | Transcriptional | [20] |
| PBX3 | Upregulation | Transcription factor | Transcriptional | [21] |
| SREBP1 | Upregulation | Transcription factor | Transcriptional | [64,65] |
| VDR | Upregulation | Transcription factor | Transcriptional | [66] |
| c-Myc | Upregulation | Transcription factor | Transcriptional | [70] |
| HMGA1 | Upregulation | Transcription factor | Transcriptional | [71,72] |
| p65 | Upregulation | Transcription factor | Transcriptional | [73] |
| HIF-1α | Upregulation | Transcription factor | Transcriptional | [74] |
| p53 | Downregulation | Transcription factor | Transcriptional | [67] |
| p52-ZER6 | Upregulation | Transcription factor | Transcriptional | [75] |
| Snail | Upregulation | Transcription factor | Transcriptional | [76] |
| Nrf-2 | Upregulation | Transcription factor | Transcriptional | [77] |
| TAp73 | Upregulation | Transcription factor | Transcriptional | [69] |
| NeuroD1 | Upregulation | Transcription factor | Transcriptional | [78] |
| PI3K | Upregulation | Kinase | Post-translational | [79] |
| AMPK | Downregulation | Kinase | Post-translational | [80] |
| c-Src | Upregulation | Kinase | Post-translational | [81] |
| Cyclin D3 | Upregulation | Kinase | Post-translational | [81] |
| PAK4 | Upregulation | Kinase | Post-translational | [82] |
| AKT | Upregulation | Kinase | Post-translational | [79] |
| Plk1 | Upregulation | Kinase | Post-translational | [83] |
| ATM | Upregulation | Kinase | Post-translational | [84] |
| PDIA3P | Upregulation | lncRNA | Transcriptional | [85] |
| PTEN | Downregulation | Phosphatase | Post-translational | [86] |
| mTORC1 | Upregulation | Signaling protein | Transcriptional | [64] |
| ID1 | Upregulation | Signaling protein | Transcriptional | [70] |
4. Role of G6PD in Cancers
4.1. Role of G6PD in Tumor Cell Proliferation
4.2. Role of G6PD in Tumor Cell Death and Survival
4.3. G6PD and Tumor Cell Drug Resistance
4.4. Role of G6PD in Tumor Cell Invasion and Metastasis
5. G6PD as a Potential Target for Anti-Tumor Therapy
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wild, C.; Weiderpass, E.; Stewart, B.W. World Cancer Report: Cancer Research for Cancer Prevention; International Agency for Research on Cancer: Lyon, France, 2020. [Google Scholar]
- Hanahan, D. Hallmarks of cancer: New dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Hsu, P.P.; Sabatini, D.M. Cancer cell metabolism: Warburg and beyond. Cell 2008, 134, 703–707. [Google Scholar] [CrossRef] [PubMed]
- Ball, S.; Colleoni, C.; Cenci, U.; Raj, J.N.; Tirtiaux, C. The evolution of glycogen and starch metabolism in eukaryotes gives molecular clues to understand the establishment of plastid endosymbiosis. J. Exp. Bot. 2011, 62, 1775–1801. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Reyes, I.; Chandel, N.S. Mitochondrial TCA cycle metabolites control physiology and disease. Nat. Commun. 2020, 11, 102. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Xiao, Z.; Chen, T.; Liang, S.H.; Guo, H. Glucose metabolism on tumor plasticity, diagnosis, and treatment. Front. Oncol. 2020, 10, 317. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Vaupel, P.; Schmidberger, H.; Mayer, A. The Warburg effect: Essential part of metabolic reprogramming and central contributor to cancer progression. Int. J. Radiat. Biol. 2019, 95, 912–919. [Google Scholar] [CrossRef]
- Riester, M.; Xu, Q.; Moreira, A.; Zheng, J.; Michor, F.; Downey, R. The Warburg effect: Persistence of stem-cell metabolism in cancers as a failure of differentiation. Ann. Oncol. 2018, 29, 264–270. [Google Scholar] [CrossRef]
- Patra, K.C.; Hay, N. The pentose phosphate pathway and cancer. Trends Biochem. Sci. 2014, 39, 347–354. [Google Scholar] [CrossRef]
- Vaupel, P.; Multhoff, G. Revisiting the Warburg effect: Historical dogma versus current understanding. J. Physiol. 2021, 599, 1745–1757. [Google Scholar] [CrossRef]
- Chen, J.; Wu, H.; Zhang, W.; Mu, W. Ribose-5-phosphate isomerases: Characteristics, structural features, and applications. Appl. Microbiol. Biotechnol. 2020, 104, 6429–6441. [Google Scholar] [CrossRef]
- Ge, T.; Yang, J.; Zhou, S.; Wang, Y.; Li, Y.; Tong, X. The role of the pentose phosphate pathway in diabetes and cancer. Front. Endocrinol. 2020, 11, 365. [Google Scholar] [CrossRef]
- Dentin, R.; Tomas-Cobos, L.; Foufelle, F.; Leopold, J.; Girard, J.; Postic, C.; Ferré, P. Glucose 6-phosphate, rather than xylulose 5-phosphate, is required for the activation of ChREBP in response to glucose in the liver. J. Hepatol. 2012, 56, 199–209. [Google Scholar] [CrossRef]
- Stincone, A.; Prigione, A.; Cramer, T.; Wamelink, M.M.; Campbell, K.; Cheung, E.; Olin-Sandoval, V.; Grüning, N.M.; Krüger, A.; Tauqeer Alam, M. The return of metabolism: Biochemistry and physiology of the pentose phosphate pathway. Biol. Rev. 2015, 90, 927–963. [Google Scholar] [CrossRef]
- Perez-Castro, L.; Garcia, R.; Venkateswaran, N.; Barnes, S.; Conacci-Sorrell, M. Tryptophan and its metabolites in normal physiology and cancer etiology. FEBS J. 2023, 290, 7–27. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Huang, Q.; Ma, Y.; Wang, L.; Rivera, G.O.; Ouyang, Y.; Whitaker, R.; Gibson, R.A.; Kontos, C.D.; Berchuck, A. G6PD inhibition sensitizes ovarian cancer cells to oxidative stress in the metastatic omental microenvironment. Cell Rep. 2022, 39, 111012. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Ling, X.; Sun, Y.; Liu, L.; Liu, L.; Wang, X.; Lu, C.; Ren, C.; Han, X.; Yu, Z. FDX1 enhances endometriosis cell cuproptosis via G6PD-mediated redox homeostasis. Apoptosis 2023, 28, 1128–1140. [Google Scholar] [CrossRef]
- Song, J.; Sun, H.; Zhang, S.; Shan, C. The multiple roles of glucose-6-phosphate dehydrogenase in tumorigenesis and cancer chemoresistance. Life 2022, 12, 271. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Wang, H.; Li, Y.; Xie, Y.; Huang, C.; Zhao, H.; Miyagishi, M.; Kasim, V. Transcription factor YY1 promotes cell proliferation by directly activating the pentose phosphate pathway. Cancer Res. 2018, 78, 4549–4562. [Google Scholar] [CrossRef]
- Luo, X.; Wei, M.; Li, W.; Zhao, H.; Kasim, V.; Wu, S. PBX3 promotes pentose phosphate pathway and colorectal cancer progression by enhancing G6PD expression. Int. J. Biol. Sci. 2023, 19, 4525. [Google Scholar] [CrossRef]
- Mele, L.; Paino, F.; Papaccio, F.; Regad, T.; Boocock, D.; Stiuso, P.; Lombardi, A.; Liccardo, D.; Aquino, G.; Barbieri, A. A new inhibitor of glucose-6-phosphate dehydrogenase blocks pentose phosphate pathway and suppresses malignant proliferation and metastasis in vivo. Cell Death Dis. 2018, 9, 572. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.-C.; Wu, Y.-H.; Yen, W.-C.; Liu, H.-Y.; Hwang, T.-L.; Stern, A.; Chiu, D.T.-Y. The redox role of G6PD in cell growth, cell death, and cancer. Cells 2019, 8, 1055. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Li, X.; Sun, W.; Sun, M.; Li, Z.; Sheng, H.; Xie, F.; Zhang, S.; Shan, C. Targeting G6PD reverses paclitaxel resistance in ovarian cancer by suppressing GSTP1. Biochem. Pharmacol. 2020, 178, 114092. [Google Scholar] [CrossRef] [PubMed]
- Spencer, N.Y.; Stanton, R.C. Glucose 6-phosphate dehydrogenase and the kidney. Curr. Opin. Nephrol. Hypertens. 2017, 26, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Allahverdiyev, A.M.; Bagirova, M.; Elcicek, S.; Koc, R.C.; Ates, S.C.; Baydar, S.Y.; Yaman, S.; Abamor, E.S.; Oztel, O.N. Glucose-6-phosphate dehydrogenase deficiency and malaria: A method to detect primaquine-induced hemolysis in vitro. In Dehydrogenases; IntechOpen: London, UK, 2012. [Google Scholar]
- Persico, M.G.; Viglietto, G.; Martini, G.; Toniolo, D.; Paonessa, G.; Moscatelli, C.; Dono, R.; Vulliamy, T.; Luzzatto, L.; D’Urso, M. Isolation of human glucose-6-pbosphate debydrogenase (G6PD) cDNA clones: Primary structure of the protein and unusual 5’non-coding region. Nucleic Acids Res. 1986, 14, 2511–2522. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Manzo, S.; Marcial-Quino, J.; Ortega-Cuellar, D.; Serrano-Posada, H.; González-Valdez, A.; Vanoye-Carlo, A.; Hernández-Ochoa, B.; Sierra-Palacios, E.; Castillo-Villanueva, A.; Reyes-Vivas, H. Functional and biochemical analysis of glucose-6-phosphate dehydrogenase (G6PD) variants: Elucidating the molecular basis of G6PD deficiency. Catalysts 2017, 7, 135. [Google Scholar] [CrossRef]
- Ravera, S.; Calzia, D.; Morelli, A.; Panfoli, I. Oligomerization studies of Leuconostoc mesenteroides G6PD activity after SDS-PAGE and blotting. Mol. Biol. 2010, 44, 415–419. [Google Scholar] [CrossRef]
- Kotaka, M.; Gover, S.; Vandeputte-Rutten, L.; Au, S.W.; Lam, V.M.; Adams, M.J. Structural studies of glucose-6-phosphate and NADP+ binding to human glucose-6-phosphate dehydrogenase. Acta Crystallogr. Sect. D Biol. Crystallogr. 2005, 61, 495–504. [Google Scholar] [CrossRef]
- Beutler, E.; Hartman, K.; Gelbart, T.; Forman, L. G-6-PD Walter Reed: Possible insight into “structural” NADP in G-6-PD. Am. J. Hematol. 1986, 23, 25–30. [Google Scholar] [CrossRef]
- Carson, P.E.; Flanagan, C.L.; Ickes, C.; Alving, A.S. Enzymatic deficiency in primaquine-sensitive erythrocytes. Science 1956, 124, 484–485. [Google Scholar] [CrossRef]
- Ho, H.; Cheng, M.; Chiu, D. G6PD-an old bottle with new wine. Chang. Gung Med. J. 2005, 28, 606. [Google Scholar]
- Beaconsfield, P.; Rainsbury, R.; Kalton, G. Glucose-6-phosphate dehydrogenase deficiency and the incidence of cancer. Oncology 1965, 19, 11–19. [Google Scholar] [CrossRef]
- Au, S.W.; Gover, S.; Lam, V.M.; Adams, M.J. Human glucose-6-phosphate dehydrogenase: The crystal structure reveals a structural NADP+ molecule and provides insights into enzyme deficiency. Structure 2000, 8, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Sirdah, M.; Reading, N.S.; Vankayalapati, H.; Prchal, J.T. A computational study of structural differences of binding of NADP+ and G6P substrates to G6PD Mediterraneanc. 563T, G6PD A− c. 202A/c. 376G, G6PD Cairoc. 404C and G6PD Gazac. 536A mutations. Blood Cells Mol. Dis. 2021, 89, 102572. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.T.; Chan, T.F.; Lam, V.M.; Engel, P.C. What is the role of the second “structural” NADP+-binding site in human glucose 6-phosphate dehydrogenase? Protein Sci. 2008, 17, 1403–1411. [Google Scholar] [CrossRef] [PubMed]
- Shaw, P.X.; Stiles, T.; Douglas, C.; Ho, D.; Fan, W.; Du, H.; Xiao, X. Oxidative stress, innate immunity, and age-related macular degeneration. AIMS Mol. Sci. 2016, 3, 196. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I. Lifestyle, oxidative stress, and antioxidants: Back and forth in the pathophysiology of chronic diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef] [PubMed]
- García-Sánchez, A.; Miranda-Díaz, A.G.; Cardona-Muñoz, E.G. The role of oxidative stress in physiopathology and pharmacological treatment with pro-and antioxidant properties in chronic diseases. Oxid. Med. Cell. Longev. 2020, 2020, 2082145. [Google Scholar] [CrossRef] [PubMed]
- El Assar, M.; Angulo, J.; Rodríguez-Mañas, L. Oxidative stress and vascular inflammation in aging. Free Radic. Biol. Med. 2013, 65, 380–401. [Google Scholar] [CrossRef]
- Yang, H.-C.; Cheng, M.-L.; Ho, H.-Y.; Chiu, D.T.-Y. The microbicidal and cytoregulatory roles of NADPH oxidases. Microbes Infect. 2011, 13, 109–120. [Google Scholar] [CrossRef]
- Cortese-Krott, M.M.; Koning, A.; Kuhnle, G.G.; Nagy, P.; Bianco, C.L.; Pasch, A.; Wink, D.A.; Fukuto, J.M.; Jackson, A.A.; van Goor, H. The reactive species interactome: Evolutionary emergence, biological significance, and opportunities for redox metabolomics and personalized medicine. Antioxid. Redox Signal. 2017, 27, 684–712. [Google Scholar] [CrossRef]
- Paul, B.D.; Snyder, S.H. H2S: A novel gasotransmitter that signals by sulfhydration. Trends Biochem. Sci. 2015, 40, 687–700. [Google Scholar] [CrossRef] [PubMed]
- Szabo, C. A timeline of hydrogen sulfide (H2S) research: From environmental toxin to biological mediator. Biochem. Pharmacol. 2018, 149, 5–19. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Shen, X.; Kevil, C.G. Beyond a gasotransmitter: Hydrogen sulfide and polysulfide in cardiovascular health and immune response. Antioxid. Redox Signal. 2017, 27, 634–653. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, B.L.; Neumann, C.A. Redoxins as gatekeepers of the transcriptional oxidative stress response. Redox Biol. 2019, 21, 101104. [Google Scholar] [CrossRef] [PubMed]
- Vona, R.; Gambardella, L.; Cittadini, C.; Straface, E.; Pietraforte, D. Biomarkers of oxidative stress in metabolic syndrome and associated diseases. Oxid. Med. Cell. Longev. 2019, 2019, 8267234. [Google Scholar] [CrossRef]
- Chen, P.-H.; Tjong, W.-Y.; Yang, H.-C.; Liu, H.-Y.; Stern, A.; Chiu, D.T.-Y. Glucose-6-Phosphate dehydrogenase, redox homeostasis and embryogenesis. Int. J. Mol. Sci. 2022, 23, 2017. [Google Scholar] [CrossRef] [PubMed]
- TeSlaa, T.; Ralser, M.; Fan, J.; Rabinowitz, J.D. The pentose phosphate pathway in health and disease. Nat. Metab. 2023, 5, 1275–1289. [Google Scholar] [CrossRef]
- Wang, Y.; Branicky, R.; Noë, A.; Hekimi, S. Superoxide dismutases: Dual roles in controlling ROS damage and regulating ROS signaling. J. Cell Biol. 2018, 217, 1915–1928. [Google Scholar] [CrossRef]
- Pei, J.; Pan, X.; Wei, G.; Hua, Y. Research progress of glutathione peroxidase family (GPX) in redoxidation. Front. Pharmacol. 2023, 14, 1147414. [Google Scholar] [CrossRef]
- Seco-Cervera, M.; González-Cabo, P.; Pallardó, F.V.; Romá-Mateo, C.; García-Giménez, J.L. Thioredoxin and glutaredoxin systems as potential targets for the development of new treatments in Friedreich’s ataxia. Antioxidants 2020, 9, 1257. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Choe, S.S.; Choi, A.H.; Kim, K.H.; Yoon, M.J.; Suganami, T.; Ogawa, Y.; Kim, J.B. Increase in glucose-6-phosphate dehydrogenase in adipocytes stimulates oxidative stress and inflammatory signals. Diabetes 2006, 55, 2939–2949. [Google Scholar] [CrossRef] [PubMed]
- Putker, M.; Crosby, P.; Feeney, K.A.; Hoyle, N.P.; Costa, A.S.; Gaude, E.; Frezza, C.; O’Neill, J.S. Mammalian circadian period, but not phase and amplitude, is robust against redox and metabolic perturbations. Antioxid. Redox Signal. 2018, 28, 507–520. [Google Scholar] [CrossRef] [PubMed]
- Rey, G.; Valekunja, U.K.; Feeney, K.A.; Wulund, L.; Milev, N.B.; Stangherlin, A.; Ansel-Bollepalli, L.; Velagapudi, V.; O’Neill, J.S.; Reddy, A.B. The pentose phosphate pathway regulates the circadian clock. Cell Metab. 2016, 24, 462–473. [Google Scholar] [CrossRef]
- Gnocchi, D.; Bruscalupi, G. Circadian rhythms and hormonal homeostasis: Pathophysiological implications. Biology 2017, 6, 10. [Google Scholar] [CrossRef]
- Pareek, V.; Pedley, A.M.; Benkovic, S.J. Human de novo purine biosynthesis. Crit. Rev. Biochem. Mol. Biol. 2021, 56, 1–16. [Google Scholar] [CrossRef]
- Lane, A.N.; Fan, T.W. Regulation of mammalian nucleotide metabolism and biosynthesis. Nucleic Acids Res. 2015, 43, 2466–2485. [Google Scholar] [CrossRef]
- Wasylenko, T.M.; Ahn, W.S.; Stephanopoulos, G. The oxidative pentose phosphate pathway is the primary source of NADPH for lipid overproduction from glucose in Yarrowia lipolytica. Metab. Eng. 2015, 30, 27–39. [Google Scholar] [CrossRef]
- Suagee, J.K.; Corl, B.A.; Crisman, M.V.; Wearn, J.G.; McCutcheon, L.J.; Geor, R.J. De novo fatty acid synthesis and NADPH generation in equine adipose and liver tissue. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2010, 155, 322–326. [Google Scholar] [CrossRef]
- Stanton, R.C. Glucose-6-phosphate dehydrogenase, NADPH, and cell survival. IUBMB Life 2012, 64, 362–369. [Google Scholar] [CrossRef]
- Pittard, J.; Yang, J. Biosynthesis of the aromatic amino acids. EcoSal Plus 2008, 3, 10–1128. [Google Scholar] [CrossRef]
- Düvel, K.; Yecies, J.L.; Menon, S.; Raman, P.; Lipovsky, A.I.; Souza, A.L.; Triantafellow, E.; Ma, Q.; Gorski, R.; Cleaver, S. Activation of a metabolic gene regulatory network downstream of mTOR complex 1. Mol. Cell 2010, 39, 171–183. [Google Scholar] [CrossRef]
- Amemiya-Kudo, M.; Shimano, H.; Hasty, A.H.; Yahagi, N.; Yoshikawa, T.; Matsuzaka, T.; Okazaki, H.; Tamura, Y.; Iizuka, Y.; Ohashi, K. Transcriptional activities of nuclear SREBP-1a,-1c, and-2 to different target promoters of lipogenic and cholesterogenic genes. J. Lipid Res. 2002, 43, 1220–1235. [Google Scholar] [CrossRef] [PubMed]
- Bao, B.Y.; Ting, H.J.; Hsu, J.W.; Lee, Y.F. Protective role of 1α, 25-dihydroxyvitamin D3 against oxidative stress in nonmalignant human prostate epithelial cells. Int. J. Cancer 2008, 122, 2699–2706. [Google Scholar] [CrossRef]
- Jiang, P.; Du, W.; Wang, X.; Mancuso, A.; Gao, X.; Wu, M.; Yang, X. p53 regulates biosynthesis through direct inactivation of glucose-6-phosphate dehydrogenase. Nat. Cell Biol. 2011, 13, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Du, W.; Yang, X. A critical role of glucose-6-phosphate dehydrogenase in TAp73-mediated cell proliferation. Cell Cycle 2013, 12, 3720–3726. [Google Scholar] [CrossRef]
- Du, W.; Jiang, P.; Mancuso, A.; Stonestrom, A.; Brewer, M.D.; Minn, A.J.; Mak, T.W.; Wu, M.; Yang, X. TAp73 enhances the pentose phosphate pathway and supports cell proliferation. Nat. Cell Biol. 2013, 15, 991–1000. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Tang, B.; Li, J.-H.; Wang, Y.; Zhang, L.; Xie, X.-Y.; Zhang, B.-H.; Qiu, S.-J.; Wu, W.-Z.; Ren, Z.-G. ID1 promotes hepatocellular carcinoma proliferation and confers chemoresistance to oxaliplatin by activating pentose phosphate pathway. J. Exp. Clin. Cancer Res. 2017, 36, 166. [Google Scholar] [CrossRef]
- Zhang, R.; Tao, F.; Ruan, S.; Hu, M.; Hu, Y.; Fang, Z.; Mei, L.; Gong, C. The TGFβ1-FOXM1-HMGA1-TGFβ1 positive feedback loop increases the cisplatin resistance of non-small cell lung cancer by inducing G6PD expression. Am. J. Transl. Res. 2019, 11, 6860. [Google Scholar]
- Gong, C.; Qiao, L.; Feng, R.; Xu, Q.; Zhang, Y.; Fang, Z.; Shen, J.; Li, S. IL-6-induced acetylation of E2F1 aggravates oxidative damage of retinal pigment epithelial cell line. Exp. Eye Res. 2020, 200, 108219. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, Z.; Ni, Y.; Bai, H.; Han, Q.; Yi, Z.; Yi, X.; Agbana, Y.L.; Kuang, Y.; Zhu, Y. NF-κB and pSTAT3 synergistically drive G6PD overexpression and facilitate sensitivity to G6PD inhibition in ccRCC. Cancer Cell Int. 2020, 20, 483. [Google Scholar] [CrossRef] [PubMed]
- Yi, W.; Clark, P.M.; Mason, D.E.; Keenan, M.C.; Hill, C.; Goddard III, W.A.; Peters, E.C.; Driggers, E.M.; Hsieh-Wilson, L.C. Phosphofructokinase 1 glycosylation regulates cell growth and metabolism. Science 2012, 337, 975–980. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Li, W.; Qiu, L.; Zhang, X.; Zhang, L.; Miyagishi, M.; Zhao, H.; Wu, S.; Kasim, V. The p52-ZER6/G6PD axis alters aerobic glycolysis and promotes tumor progression by activating the pentose phosphate pathway. Oncogenesis 2023, 12, 17. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.H.; Cha, Y.H.; Lee, J.; Lee, S.-H.; Yang, J.H.; Yun, J.S.; Cho, E.S.; Zhang, X.; Nam, M.; Kim, N. Snail reprograms glucose metabolism by repressing phosphofructokinase PFKP allowing cancer cell survival under metabolic stress. Nat. Commun. 2017, 8, 14374. [Google Scholar] [CrossRef] [PubMed]
- Zimta, A.-A.; Cenariu, D.; Irimie, A.; Magdo, L.; Nabavi, S.M.; Atanasov, A.G.; Berindan-Neagoe, I. The role of Nrf2 activity in cancer development and progression. Cancers 2019, 11, 1755. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; He, Y.; Li, Y.; Li, J.; Zhao, H.; Song, G.; Miyagishi, M.; Wu, S.; Kasim, V. NeuroD1 promotes tumor cell proliferation and tumorigenesis by directly activating the pentose phosphate pathway in colorectal carcinoma. Oncogene 2021, 40, 6736–6747. [Google Scholar] [CrossRef]
- Wagle, A.; Jivraj, S.; Garlock, G.L.; Stapleton, S.R. Insulin regulation of glucose-6-phosphate dehydrogenase gene expression is rapamycin-sensitive and requires phosphatidylinositol 3-kinase. J. Biol. Chem. 1998, 273, 14968–14974. [Google Scholar] [CrossRef]
- Yang, L.; He, Z.; Yao, J.; Tan, R.; Zhu, Y.; Li, Z.; Guo, Q.; Wei, L. Regulation of AMPK-related glycolipid metabolism imbalances redox homeostasis and inhibits anchorage independent growth in human breast cancer cells. Redox Biol. 2018, 17, 180–191. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, S.; Jiang, B.; Huang, L.; Ji, Z.; Li, X.; Zhou, H.; Han, A.; Chen, A.; Wu, Y. c-Src phosphorylation and activation of hexokinase promotes tumorigenesis and metastasis. Nat. Commun. 2017, 8, 13732. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, X.; Li, Y.; Shao, Y.; Xiao, J.; Zhu, G.; Li, F. PAK4 regulates G6PD activity by p53 degradation involving colon cancer cell growth. Cell Death Dis. 2017, 8, e2820. [Google Scholar] [CrossRef]
- Ma, X.; Wang, L.; Huang, D.; Li, Y.; Yang, D.; Li, T.; Li, F.; Sun, L.; Wei, H.; He, K. Polo-like kinase 1 coordinates biosynthesis during cell cycle progression by directly activating pentose phosphate pathway. Nat. Commun. 2017, 8, 1506. [Google Scholar] [CrossRef] [PubMed]
- Cosentino, C.; Grieco, D.; Costanzo, V. ATM activates the pentose phosphate pathway promoting anti-oxidant defence and DNA repair. EMBO J. 2011, 30, 546–555. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Ye, H.; He, M.; Zhou, X.; Sun, N.; Guo, W.; Lin, X.; Huang, H.; Lin, Y.; Yao, R. LncRNA PDIA3P interacts with c-Myc to regulate cell proliferation via induction of pentose phosphate pathway in multiple myeloma. Biochem. Biophys. Res. Commun. 2018, 498, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Hong, X.; Song, R.; Song, H.; Zheng, T.; Wang, J.; Liang, Y.; Qi, S.; Lu, Z.; Song, X.; Jiang, H. PTEN antagonises Tcl1/hnRNPK-mediated G6PD pre-mRNA splicing which contributes to hepatocarcinogenesis. Gut 2014, 63, 1635–1647. [Google Scholar] [CrossRef] [PubMed]
- Meliala, I.T.S.; Hosea, R.; Kasim, V.; Wu, S. The biological implications of Yin Yang 1 in the hallmarks of cancer. Theranostics 2020, 10, 4183. [Google Scholar] [CrossRef] [PubMed]
- Hosea, R.; Hillary, S.; Wu, S.; Kasim, V. Targeting Transcription Factor YY1 for Cancer Treatment: Current Strategies and Future Directions. Cancers 2023, 15, 3506. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.S.; Zhang, Z.G.; Du, G.Y.; Sun, H.L.; Liu, H.Y.; Zhou, Z.; Gou, X.M.; Wu, X.H.; Yu, X.Y.; Huang, Y.H. Nrf2 promotes breast cancer cell migration via up-regulation of G6PD/HIF-1α/Notch1 axis. J. Cell. Mol. Med. 2019, 23, 3451–3463. [Google Scholar] [CrossRef]
- Sun, M.; Sheng, H.; Wu, T.; Song, J.; Sun, H.; Wang, Y.; Wang, J.; Li, Z.; Zhao, H.; Tan, J. PIKE-A promotes glioblastoma growth by driving PPP flux through increasing G6PD expression mediated by phosphorylation of STAT3. Biochem. Pharmacol. 2021, 192, 114736. [Google Scholar] [CrossRef]
- Lu, C.; Yang, D.; Klement, J.D.; Colson, Y.L.; Oberlies, N.H.; Pearce, C.J.; Colby, A.H.; Grinstaff, M.W.; Liu, Z.; Shi, H. H3K9me3 represses G6PD expression to suppress the pentose phosphate pathway and ROS production to promote human mesothelioma growth. Oncogene 2022, 41, 2651–2662. [Google Scholar] [CrossRef]
- Song, C.; Liu, X.; Lin, W.; Lai, K.; Pan, S.; Lu, Z.; Li, D.; Li, N.; Geng, Q. Systematic analysis of histone acetylation regulators across human cancers. BMC Cancer 2023, 23, 733. [Google Scholar] [CrossRef]
- Wang, E.; Aifantis, I. RNA splicing and cancer. Trends Cancer 2020, 6, 631–644. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Kou, J.; Qin, J.; Li, L.; Zhang, Z.; Pan, Y.; Xue, Y.; Du, W. NADPH levels affect cellular epigenetic state by inhibiting HDAC3–Ncor complex. Nat. Metab. 2021, 3, 75–89. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Hu, J.; Yan, L.; Yang, Y.; He, M.; Guo, S.; Wu, M.; Li, Q.; Gong, W.; Yang, Y. High glucose-induced ubiquitylation of G6PD leads to the injury of podocyte. bioRxiv 2018. [Google Scholar] [CrossRef]
- Wang, Y.P.; Zhou, L.S.; Zhao, Y.Z.; Wang, S.W.; Chen, L.L.; Liu, L.X.; Ling, Z.Q.; Hu, F.J.; Sun, Y.P.; Zhang, J.Y. Regulation of G 6 PD acetylation by SIRT2 and KAT9 modulates NADPH homeostasis and cell survival during oxidative stress. EMBO J. 2014, 33, 1304–1320. [Google Scholar] [PubMed]
- Xu, S.-N.; Wang, T.-S.; Li, X.; Wang, Y.-P. SIRT2 activates G6PD to enhance NADPH production and promote leukaemia cell proliferation. Sci. Rep. 2016, 6, 32734. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Gao, F.; Ai, H.; Wang, S.; Song, Z.; Zheng, L.; Wang, G.; Sun, Y.; Bao, Y. TSP50 promotes hepatocyte proliferation and tumour formation by activating glucose-6-phosphate dehydrogenase (G6PD). Cell Prolif. 2021, 54, e13015. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Yang, Z.; Agbana, Y.L.; Bai, H.; Wang, L.; Yang, L.; Yi, Z.; Cheng, J.; Zhang, Q.; Kuang, Y. Silent information regulator 2 promotes clear cell renal cell carcinoma progression through deacetylation and small ubiquitin-related modifier 1 modification of glucose 6-phosphate dehydrogenase. Cancer Sci. 2021, 112, 4075–4086. [Google Scholar] [CrossRef]
- Raza, H.; John, A.; Benedict, S. Acetylsalicylic acid-induced oxidative stress, cell cycle arrest, apoptosis and mitochondrial dysfunction in human hepatoma HepG2 cells. Eur. J. Pharmacol. 2011, 668, 15–24. [Google Scholar] [CrossRef]
- Ai, G.; Dachineni, R.; Kumar, D.R.; Alfonso, L.F.; Marimuthu, S.; Bhat, G.J. Aspirin inhibits glucose-6-phosphate dehydrogenase activity in HCT 116 cells through acetylation: Identification of aspirin-acetylated sites. Mol. Med. Rep. 2016, 14, 1726–1732. [Google Scholar] [CrossRef]
- Ma, H.; Zhang, F.; Zhou, L.; Cao, T.; Sun, D.; Wen, S.; Zhu, J.; Xiong, Z.; Tsau, M.-T.; Cheng, M.-L. c-Src facilitates tumorigenesis by phosphorylating and activating G6PD. Oncogene 2021, 40, 2567–2580. [Google Scholar] [CrossRef]
- Pan, S.; World, C.J.; Kovacs, C.J.; Berk, B.C. Glucose 6-phosphate dehydrogenase is regulated through c-Src–mediated tyrosine phosphorylation in endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 895–901. [Google Scholar] [CrossRef]
- Mattè, A.; Lupo, F.; Tibaldi, E.; Di Paolo, M.L.; Federti, E.; Carpentieri, A.; Pucci, P.; Brunati, A.M.; Cesaro, L.; Turrini, F. Fyn specifically Regulates the activity of red cell glucose-6-phosphate-dehydrogenase. Redox Biol. 2020, 36, 101639. [Google Scholar] [CrossRef] [PubMed]
- Rao, X.; Duan, X.; Mao, W.; Li, X.; Li, Z.; Li, Q.; Zheng, Z.; Xu, H.; Chen, M.; Wang, P.G. O-GlcNAcylation of G6PD promotes the pentose phosphate pathway and tumor growth. Nat. Commun. 2015, 6, 8468. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Luo, Z.; Ying, W.; Cao, Q.; Huang, H.; Dong, J.; Wu, Q.; Zhao, Y.; Qian, X.; Dai, J. 2-Hydroxyisobutyrylation on histone H4K8 is regulated by glucose homeostasis in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 2017, 114, 8782–8787. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Yruela, C.; Zhang, D.; Wei, W.; Bæk, M.; Liu, W.; Gao, J.; Danková, D.; Nielsen, A.L.; Bolding, J.E.; Yang, L. Class I histone deacetylases (HDAC1–3) are histone lysine delactylases. Sci. Adv. 2022, 8, eabi6696. [Google Scholar] [CrossRef] [PubMed]
- Sabari, B.R.; Zhang, D.; Allis, C.D.; Zhao, Y. Metabolic regulation of gene expression through histone acylations. Nat. Rev. Mol. Cell Biol. 2017, 18, 90–101. [Google Scholar] [CrossRef]
- Zhang, D.; Tang, Z.; Huang, H.; Zhou, G.; Cui, C.; Weng, Y.; Liu, W.; Kim, S.; Lee, S.; Perez-Neut, M. Metabolic regulation of gene expression by histone lactylation. Nature 2019, 574, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Wang, F.; Sun, R.; Chen, X.; Zhang, M.; Xu, Q.; Wang, Y.; Wang, S.; Xiong, Y.; Guan, K.L. SIRT 5 promotes IDH 2 desuccinylation and G6 PD deglutarylation to enhance cellular antioxidant defense. EMBO Rep. 2016, 17, 811–822. [Google Scholar] [CrossRef]
- Meng, Q.; Zhang, Y.; Hao, S.; Sun, H.; Liu, B.; Zhou, H.; Wang, Y.; Xu, Z.-X. Recent findings in the regulation of G6PD and its role in diseases. Front. Pharmacol. 2022, 13, 932154. [Google Scholar] [CrossRef]
- Kathagen-Buhmann, A.; Schulte, A.; Weller, J.; Holz, M.; Herold-Mende, C.; Glass, R.; Lamszus, K. Glycolysis and the pentose phosphate pathway are differentially associated with the dichotomous regulation of glioblastoma cell migration versus proliferation. Neuro-Oncol. 2016, 18, 1219–1229. [Google Scholar] [CrossRef]
- Debeb, B.G.; Lacerda, L.; Larson, R.; Wolfe, A.R.; Krishnamurthy, S.; Reuben, J.M.; Ueno, N.T.; Gilcrease, M.; Woodward, W.A. Histone deacetylase inhibitor-induced cancer stem cells exhibit high pentose phosphate pathway metabolism. Oncotarget 2016, 7, 28329. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Ke, M.; Qi, M.; Han, Z.; Cao, Y.; Deng, Z.; Qian, J.; Yang, Y.; Gu, C. G6PD promotes cell proliferation and dexamethasone resistance in multiple myeloma via increasing anti-oxidant production and activating Wnt/β-catenin pathway. Exp. Hematol. Oncol. 2022, 11, 77. [Google Scholar] [CrossRef] [PubMed]
- Mele, L.; la Noce, M.; Paino, F.; Regad, T.; Wagner, S.; Liccardo, D.; Papaccio, G.; Lombardi, A.; Caraglia, M.; Tirino, V. Glucose-6-phosphate dehydrogenase blockade potentiates tyrosine kinase inhibitor effect on breast cancer cells through autophagy perturbation. J. Exp. Clin. Cancer Res. 2019, 38, 160. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Duan, Z.; Nugent, Z.; Zou, J.X.; Borowsky, A.D.; Zhang, Y.; Tepper, C.G.; Li, J.J.; Fiehn, O.; Xu, J. Reprogramming metabolism by histone methyltransferase NSD2 drives endocrine resistance via coordinated activation of pentose phosphate pathway enzymes. Cancer Lett. 2016, 378, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Thakor, P.; Subramanian, R.B.; Thakkar, S.S.; Ray, A.; Thakkar, V.R. Phytol induces ROS mediated apoptosis by induction of caspase 9 and 3 through activation of TRAIL, FAS and TNF receptors and inhibits tumor progression factor Glucose 6 phosphate dehydrogenase in lung carcinoma cell line (A549). Biomed. Pharmacother. 2017, 92, 491–500. [Google Scholar] [CrossRef]
- Kong, D.-H.; Li, S.; Du, Z.-X.; Liu, C.; Liu, B.-Q.; Li, C.; Zong, Z.-H.; Wang, H.-Q. BAG3 elevation inhibits cell proliferation via direct interaction with G6PD in hepatocellular carcinomas. Oncotarget 2016, 7, 700. [Google Scholar] [CrossRef]
- Lu, M.; Lu, L.; Dong, Q.; Yu, G.; Chen, J.; Qin, L.; Wang, L.; Zhu, W.; Jia, H. Elevated G6PD expression contributes to migration and invasion of hepatocellular carcinoma cells by inducing epithelial-mesenchymal transition. Acta Biochim. Biophys. Sin. 2018, 50, 370–380. [Google Scholar] [CrossRef]
- Chen, X.; Xu, Z.; Zhu, Z.; Chen, A.; Fu, G.; Wang, Y.; Pan, H.; Jin, B. Modulation of G6PD affects bladder cancer via ROS accumulation and the AKT pathway in vitro. Int. J. Oncol. 2018, 53, 1703–1712. [Google Scholar] [CrossRef]
- Poulain, L.; Sujobert, P.; Zylbersztejn, F.; Barreau, S.; Stuani, L.; Lambert, M.; Palama, T.; Chesnais, V.; Birsen, R.; Vergez, F. High mTORC1 activity drives glycolysis addiction and sensitivity to G6PD inhibition in acute myeloid leukemia cells. Leukemia 2017, 31, 2326–2335. [Google Scholar] [CrossRef]
- Ye, H.; Huang, H.; Cao, F.; Chen, M.; Zheng, X.; Zhan, R. HSPB1 enhances SIRT2-mediated G6PD activation and promotes glioma cell proliferation. PLoS ONE 2016, 11, e0164285. [Google Scholar] [CrossRef]
- Cheng, J.; Huang, Y.; Zhang, X.; Yu, Y.; Wu, S.; Jiao, J.; Tran, L.; Zhang, W.; Liu, R.; Zhang, L. TRIM21 and PHLDA3 negatively regulate the crosstalk between the PI3K/AKT pathway and PPP metabolism. Nat. Commun. 2020, 11, 1880. [Google Scholar] [CrossRef]
- Cheung, E.C.; Vousden, K.H. The role of ROS in tumour development and progression. Nat. Rev. Cancer 2022, 22, 280–297. [Google Scholar] [CrossRef]
- Shan, F.; Yang, R.; Ji, T.; Jiao, F. Vitamin C inhibits aggravated eryptosis by hydrogen peroxide in glucose-6-phosphated dehydrogenase deficiency. Cell. Physiol. Biochem. 2016, 39, 1453–1462. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhang, Z.; Hu, J.; Stillman, I.E.; Leopold, J.A.; Handy, D.E.; Loscalzo, J.; Stanton, R.C. Glucose-6-phosphate dehydrogenase-deficient mice have increased renal oxidative stress and increased albuminuria. FASEB J. 2010, 24, 609. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liew, C.W.; Handy, D.E.; Zhang, Y.; Leopold, J.A.; Hu, J.; Guo, L.; Kulkarni, R.N.; Loscalzo, J.; Stanton, R.C. High glucose inhibits glucose-6-phosphate dehydrogenase, leading to increased oxidative stress and β-cell apoptosis. FASEB J. 2010, 24, 1497. [Google Scholar] [CrossRef]
- Yang, H.; Chen, T.; Wu, Y.; Cheng, K.; Lin, Y.; Cheng, M.; Ho, H.; Lo, S.; Chiu, D.T. Glucose 6-phosphate dehydrogenase deficiency enhances germ cell apoptosis and causes defective embryogenesis in Caenorhabditis elegans. Cell Death Dis. 2013, 4, e616. [Google Scholar] [CrossRef]
- Lin, C.-J.; Ho, H.-Y.; Cheng, M.-L.; Cheng, T.-H.; Yu, J.-S.; Chiu, D.T.-Y. Impaired dephosphorylation renders G6PD-knockdown HepG2 cells more susceptible to H2O2-induced apoptosis. Free Radic. Biol. Med. 2010, 49, 361–373. [Google Scholar] [CrossRef]
- Jiang, X.; Stockwell, B.R.; Conrad, M. Ferroptosis: Mechanisms, biology and role in disease. Nat. Rev. Mol. Cell Biol. 2021, 22, 266–282. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, J.; Li, Z.; Wei, M.; Zhao, H.; Miyagishi, M.; Wu, S.; Kasim, V. Homeostasis imbalance of YY2 and YY1 promotes tumor growth by manipulating ferroptosis. Adv. Sci. 2022, 9, 2104836. [Google Scholar] [CrossRef]
- Zhu, L.; Yang, F.; Wang, L.; Dong, L.; Huang, Z.; Wang, G.; Chen, G.; Li, Q. Identification the ferroptosis-related gene signature in patients with esophageal adenocarcinoma. Cancer Cell Int. 2021, 21, 124. [Google Scholar] [CrossRef]
- Luan, J.-C.; Zeng, T.-Y.; Zhang, Q.-J.; Xia, D.-R.; Cong, R.; Yao, L.-Y.; Song, L.-B.; Zhou, X.; Zhou, X.; Chen, X. A novel signature constructed by ferroptosis-associated genes (FAGs) for the prediction of prognosis in bladder urothelial carcinoma (BLCA) and associated with immune infiltration. Cancer Cell Int. 2021, 21, 414. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wei, G.; Ma, J.; Cheng, S.; Jia, L.; Song, X.; Zhang, M.; Ju, M.; Wang, L.; Zhao, L. Identification of the prognostic value of ferroptosis-related gene signature in breast cancer patients. BMC Cancer 2021, 21, 645. [Google Scholar] [CrossRef] [PubMed]
- Niu, B.; Liao, K.; Zhou, Y.; Wen, T.; Quan, G.; Pan, X.; Wu, C. Application of glutathione depletion in cancer therapy: Enhanced ROS-based therapy, ferroptosis, and chemotherapy. Biomaterials 2021, 277, 121110. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Sun, S.; Johnson, T.; Qi, R.; Zhang, S.; Zhang, J.; Yang, K. The glutathione peroxidase Gpx4 prevents lipid peroxidation and ferroptosis to sustain Treg cell activation and suppression of antitumor immunity. Cell Rep. 2021, 35, 109235. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; He, Z.; Yao, F.; Liao, S.; Sun, K.; Sun, S.; Li, Z.; Wang, Z. Role of Escin in breast cancer therapy: Potential mechanism for inducing ferroptosis and synergistic antitumor activity with cisplatin. Apoptosis 2023, 28, 1154–1167. [Google Scholar] [CrossRef]
- Kroemer, G.; Levine, B. Autophagic cell death: The story of a misnomer. Nat. Rev. Mol. Cell Biol. 2008, 9, 1004–1010. [Google Scholar] [CrossRef] [PubMed]
- Dodson, M.; Darley-Usmar, V.; Zhang, J. Cellular metabolic and autophagic pathways: Traffic control by redox signaling. Free Radic. Biol. Med. 2013, 63, 207–221. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, Y.; Mishra, P.K. Autophagy-Mediated Cell Survival and Death in Disease Progression and Treatment. Front. Cell Dev. Biol. 2022, 10, 916347. [Google Scholar] [CrossRef]
- Dalby, K.; Tekedereli, I.; Lopez-Berestein, G.; Ozpolat, B. Targeting the pro-death and pro-survival functions of autophagy as novel therapeutic strategies in cancer. Autophagy 2010, 6, 322–329. [Google Scholar] [CrossRef]
- Jenwitheesuk, A.; Nopparat, C.; Mukda, S.; Wongchitrat, P.; Govitrapong, P. Melatonin regulates aging and neurodegeneration through energy metabolism, epigenetics, autophagy and circadian rhythm pathways. Int. J. Mol. Sci. 2014, 15, 16848–16884. [Google Scholar] [CrossRef]
- Galadari, S.; Rahman, A.; Pallichankandy, S.; Thayyullathil, F. Reactive oxygen species and cancer paradox: To promote or to suppress? Free Radic. Biol. Med. 2017, 104, 144–164. [Google Scholar] [CrossRef]
- Polimeni, M.; Voena, C.; Kopecka, J.; Riganti, C.; Pescarmona, G.; Bosia, A.; Ghigo, D. Modulation of doxorubicin resistance by the glucose-6-phosphate dehydrogenase activity. Biochem. J. 2011, 439, 141–149. [Google Scholar] [CrossRef]
- Luo, M.; Fu, A.; Wu, R.; Wei, N.; Song, K.; Lim, S.; Luo, K.Q. High expression of G6PD increases doxorubicin resistance in triple negative breast cancer cells by maintaining GSH level. Int. J. Biol. Sci. 2022, 18, 1120. [Google Scholar] [CrossRef]
- Wang, J.; Yuan, W.; Chen, Z.; Wu, S.; Chen, J.; Ge, J.; Hou, F.; Chen, Z. Overexpression of G6PD is associated with poor clinical outcome in gastric cancer. Tumor Biol. 2012, 33, 95–101. [Google Scholar] [CrossRef]
- Jahani, M.; Azadbakht, M.; Norooznezhad, F.; Mansouri, K. L-arginine alters the effect of 5-fluorouracil on breast cancer cells in favor of apoptosis. Biomed. Pharmacother. 2017, 88, 114–123. [Google Scholar] [CrossRef]
- Bachur, N.R.; Gordon, S.L.; Gee, M.V.; Kon, H. NADPH cytochrome P-450 reductase activation of quinone anticancer agents to free radicals. Proc. Natl. Acad. Sci. USA 1979, 76, 954–957. [Google Scholar] [CrossRef]
- Brabletz, T.; Kalluri, R.; Nieto, M.A.; Weinberg, R.A. EMT in cancer. Nat. Rev. Cancer 2018, 18, 128–134. [Google Scholar] [CrossRef]
- Bakir, B.; Chiarella, A.M.; Pitarresi, J.R.; Rustgi, A.K. EMT, MET, plasticity, and tumor metastasis. Trends Cell Biol. 2020, 30, 764–776. [Google Scholar] [CrossRef] [PubMed]
- Koperniku, A.; Garcia, A.A.; Mochly-Rosen, D. Boosting the discovery of small molecule inhibitors of glucose-6-phosphate dehydrogenase for the treatment of cancer, infectious diseases, and inflammation. J. Med. Chem. 2022, 65, 4403–4423. [Google Scholar] [CrossRef] [PubMed]
- Köhler, E.; Barrach, H.-J.; Neubert, D. Inhibition of NADP dependent oxidoreductases by the 6-aminonicotinamide analogue of NADP. FEBS Lett. 1970, 6, 225–228. [Google Scholar] [CrossRef] [PubMed]
- Varshney, R.; Dwarakanath, B.; Jain, V. Radiosensitization by 6-aminonicotinamide and 2-deoxy-D-glucose in human cancer cells. Int. J. Radiat. Biol. 2005, 81, 397–408. [Google Scholar] [CrossRef]
- Sharma, P.K.; Bhardwaj, R.; Dwarakanath, B.S.; Varshney, R. Metabolic oxidative stress induced by a combination of 2-DG and 6-AN enhances radiation damage selectively in malignant cells via non-coordinated expression of antioxidant enzymes. Cancer Lett. 2010, 295, 154–166. [Google Scholar] [CrossRef]
- Arbe, M.F.; Agnetti, L.; Breininger, E.; Glikin, G.C.; Finocchiaro, L.M.E.; Villaverde, M.S. Glucose 6-phosphate dehydrogenase inhibition sensitizes melanoma cells to metformin treatment. Transl. Oncol. 2020, 13, 100842. [Google Scholar] [CrossRef]
- Preuss, J.; Richardson, A.D.; Pinkerton, A.; Hedrick, M.; Sergienko, E.; Rahlfs, S.; Becker, K.; Bode, L. Identification and characterization of novel human glucose-6-phosphate dehydrogenase inhibitors. J. Biomol. Screen. 2013, 18, 286–297. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wu, G.; Cao, G.; Yang, L.; Xu, H.; Huang, J.; Hou, J. Zoledronic acid inhibits the pentose phosphate pathway through attenuating the Ras-TAp73-G6PD axis in bladder cancer cells. Mol. Med. Rep. 2015, 12, 4620–4625. [Google Scholar] [CrossRef] [PubMed]
- Ai, G.; Hagen, F.K.; Gunaje, J.B. Aspirin acetylates glucose 6 phosphate dehydrogenase and inhibits its activity in colon cancer cells. Cancer Res. 2013, 73, 3681. [Google Scholar] [CrossRef]
- Roshanzadeh, A.; Kang, H.; You, S.-H.; Park, J.; Khoa, N.D.; Lee, D.-H.; Kim, G.-J.; Kim, E.-S. Real-time monitoring of NADPH levels in living mammalian cells using fluorescence-enhancing protein bound to NADPHs. Biosens. Bioelectron. 2019, 146, 111753. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhang, Z.; Hoshino, A.; Zheng, H.D.; Morley, M.; Arany, Z.; Rabinowitz, J.D. NADPH production by the oxidative pentose-phosphate pathway supports folate metabolism. Nat. Metab. 2019, 1, 404–415. [Google Scholar] [CrossRef]
- Ju, H.; Lu, Y.; Wu, Q.; Liu, J.; Zeng, Z.; Mo, H.; Chen, Y.; Tian, T.; Wang, Y.; Kang, T. Disrupting G6PD-mediated Redox homeostasis enhances chemosensitivity in colorectal cancer. Oncogene 2017, 36, 6282–6292. [Google Scholar] [CrossRef]
- Ma, L.; Cheng, Q. Inhibiting 6-phosphogluconate dehydrogenase reverses doxorubicin resistance in anaplastic thyroid cancer via inhibiting NADPH-dependent metabolic reprogramming. Biochem. Biophys. Res. Commun. 2018, 498, 912–917. [Google Scholar] [CrossRef]
- Gnocchi, D.; Sabbà, C.; Massimi, M.; Mazzocca, A. Metabolism as a New Avenue for Hepatocellular Carcinoma Therapy. Int. J. Mol. Sci. 2023, 24, 3710. [Google Scholar] [CrossRef] [PubMed]
- Gnocchi, D.; Nikolic, D.; Paparella, R.R.; Sabbà, C.; Mazzocca, A. Cellular Adaptation Takes Advantage of Atavistic Regression Programs during Carcinogenesis. Cancers 2023, 15, 3942. [Google Scholar] [CrossRef] [PubMed]
- Pirola, L.; Fröjdö, S. Resveratrol: One molecule, many targets. IUBMB Life 2008, 60, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Massimi, M.; Tomassini, A.; Sciubba, F.; Sobolev, A.P.; Devirgiliis, L.C.; Miccheli, A. Effects of resveratrol on HepG2 cells as revealed by 1H-NMR based metabolic profiling. Biochim. Biophys. Acta BBA Gen. Subj. 2012, 1820, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Stine, Z.E.; Schug, Z.T.; Salvino, J.M.; Dang, C.V. Targeting cancer metabolism in the era of precision oncology. Nat. Rev. Drug Discov. 2022, 21, 141–162. [Google Scholar] [CrossRef]
- Werelusz, P.; Galiniak, S.; Mołoń, M. Molecular functions of moonlighting proteins in cell metabolic processes. Biochim. Biophys. Acta BBA Mol. Cell Res. 2024, 1871, 119598. [Google Scholar] [CrossRef]
- Kim, J.-W.; Dang, C.V. Glycolysis. Trends Biochem. Sci. 2005, 3, 142–150. [Google Scholar] [CrossRef]
| Tumor | Cell Lines | Phenotypes | Mechanisms | Ref. |
|---|---|---|---|---|
| Breast | MCF7 | Increased lapatinib resistance | Reduced ER-stress-mediated autophagy | [115] |
| SUM149, SUM159, T47D, and MCF7 | Enhanced proliferation | Histone deacetylase inhibition | [113] | |
| MCF7, ZR-75-1, T47D, and 293T | Increased tamoxifen resistance | G6PD promoter demethylation | [116] | |
| MCF7 and MDA-MB-231 | Enhanced proliferation, migration, and invasion | Notch-1-mediated EMT activation | [89] | |
| Lung | A549 | Reduced cell death | Suppression of ROS-induced apoptosis | [117] |
| 293T, A549, MCF7, H661, SKOV-3, A375, and U2OS | Enhanced proliferation | G6PD protein O-GlcNAcylation | [105] | |
| Colorectal | HCT116 and HT-29 | Enhanced proliferation | G6PD protein acetylation | [101] |
| HCT116 | Enhanced proliferation | YY1-induced G6PD transcriptional activation | [20] | |
| HCT116 | Enhanced proliferation | p52-ZER6-induced G6PD transcriptional activation | [75] | |
| HCT116 | Enhanced proliferation | PBX3-induced G6PD transcriptional activation | [21] | |
| HCT116 | Enhanced proliferation | NeuroD1-induced G6PD transcriptional activation | [78] | |
| HCT116 | Enhanced proliferation | Enhanced G6PD dimerization | [67] | |
| HCC | HepG2 | Enhanced proliferation | Enhanced G6PD dimerization | [118] |
| HepG2, Huh7, MHCC-97H, HCC-LM3, and L02 | Enhanced migration and invasion | STAT3-mediated EMT activation | [119] | |
| Bladder | 5637, T24, TCCSUP, and SV-HUC-1 | Enhanced proliferation | Suppression of AKT pathway | [120] |
| Leukemia | MOLM-14 | Increased cytarabine resistance | Upregulation of mTORC1 | [121] |
| HL-60 | Enhanced proliferation | SIRT2-mediated G6PD deacetylation | [97] | |
| Glioma | U87-MG and U373-MG | Enhanced proliferation and reduced cell death | SIRT2-mediated G6PD deacetylation | [122] |
| Cervical | HeLa | Enhanced cell proliferation | Enhanced G6PD dimerization | [83] |
| Prostate | PC3 | Enhanced cell proliferation | Enhanced G6PD protein stabilization | [123] |
| Cancer | Model | Inhibitor | Drug Concentration | Ref. |
|---|---|---|---|---|
| Breast | MCF7 | Polydatin | 30 μM | [115] |
| MDA-MB-231 | DHEA | 200 μM | [145] | |
| Colorectal | HCT116 and HT-29 | Aspirin | 0.25–2.5 mM | [101] |
| Bladder | 5637, T24, TCCSUP, SV-HUC-1 | 6-AN | 10 μM | [120] |
| T24, 293T | Zoledronic acid | 200 μM | [157] | |
| Leukemia | Mouse | 6-AN | 5 mg/kg | [121] |
| Prostate | Mouse | 6-AN | 1 mg/kg | [123] |
| HCC | Rat | 6-AN | 5–10 mg/kg | [151] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahamed, A.; Hosea, R.; Wu, S.; Kasim, V. The Emerging Roles of the Metabolic Regulator G6PD in Human Cancers. Int. J. Mol. Sci. 2023, 24, 17238. https://doi.org/10.3390/ijms242417238
Ahamed A, Hosea R, Wu S, Kasim V. The Emerging Roles of the Metabolic Regulator G6PD in Human Cancers. International Journal of Molecular Sciences. 2023; 24(24):17238. https://doi.org/10.3390/ijms242417238
Chicago/Turabian StyleAhamed, Alfar, Rendy Hosea, Shourong Wu, and Vivi Kasim. 2023. "The Emerging Roles of the Metabolic Regulator G6PD in Human Cancers" International Journal of Molecular Sciences 24, no. 24: 17238. https://doi.org/10.3390/ijms242417238